Abstract
Salicylic acid (SA), an essential secondary messenger for plant defence responses, plays a role in maintaining a balance (trade‐off) between plant growth and resistance induction, but the detailed mechanism has not been explored. Because the SA mimic benzothiadiazole (BTH) is a more stable inducer of plant defence than SA after exogenous application, we analysed expression profiles of defence genes after BTH treatment to better understand SA‐mediated immune induction. Transcript levels of the salicylic acid glucosyltransferase (SAGT) gene were significantly lower in BTH‐treated Nicotiana tabacum (Nt) plants than in SA‐treated Nt control plants, suggesting that SAGT may play an important role in SA‐related host defence responses. Treatment with BTH followed by SA suppressed SAGT transcription, indicating that the inhibitory effect of BTH is not reversible. In addition, in BTH‐treated Nt and Nicotiana benthamiana (Nb) plants, an early high accumulation of SA and SA 2‐O‐β‐d‐glucoside was only transient compared to the control. This observation agreed well with the finding that SAGT‐overexpressing (OE) Nb lines contained less SA and jasmonic acid (JA) than in the Nb plants. When inoculated with a virus, the OE Nb plants showed more severe symptoms and accumulated higher levels of virus, while resistance increased in SAGT‐silenced (IR) Nb plants. In addition, the IR plants restricted bacterial spread to the inoculated leaves. After the BTH treatment, OE Nb plants were slightly larger than the Nb plants. These results together indicate that SAGT has a pivotal role in the balance between plant growth and SA/JA‐mediated defence for optimum plant fitness.
Keywords: benzothiadiazole (BTH), defence, fitness, growth, immune induction, salicylic acid, salicylic acid glucosyltransferase
SAGT, whose transcript levels can be suppressed by BTH, plays an important role in balancing plant growth and defence for optimum plant fitness.

1. INTRODUCTION
The trade‐off between growth and defence in plants has been thought to be regulated by signal pathways that are mediated by plant hormones (Huot et al., 2014). The phytohormone salicylic acid (SA) is involved in plant development and responses to biotic and abiotic stresses (Rivas‐San Vicente and Plasencia, 2011). Most SA in cells is glucosylated and/or methylated (Rivas‐San Vicente and Plasencia, 2011). The conversion of methyl salicylate (MeSA) to SA can be catalysed by SA‐binding protein 2 (SABP2) to induce systemic acquired resistance (SAR) (Forouhar et al., 2005; Park et al., 2007; Tripathi et al., 2010), a key mechanism in the innate immune system of plants (Canet et al., 2010). On the contrary, SA can be converted into SA 2‐O‐β‐d‐glucoside (SAG) (Pastor et al., 2013). The glucosylation of SA is mediated by uridine diphosphate (UDP)‐glucosyltransferase, also known as SA glucosyltransferase (SAGT) (Vlot et al., 2009), which is generally not required to induce SAR (Loake and Grant, 2007). Seto et al. (2011) reported that SAGT of tobacco also catalyses the glucosylation of tuberonic acid (12‐hydroxyjasmonic acid; TA), which is a derivative of jasmonic acid (JA), and its expression can be induced by mechanical wounding. SAGT expression is induced by exogenous SA application or pathogen attack, indicating a crucial role for SAGT in regulating a balance between SA and SAG. Chivasa and Carr (1998) previously reported that SA‐induced resistance was greatly reduced by the expression of NahG, which converts SA to catechol, in a transgenic Nicotiana tabacum line (NahG Nt), indicating that the resistance depends on the concentration of endogenous SA. The effect of exogenously applied SA on plant growth is often affected by plant species and growth stage, and varies with frequent changes in endogenous SA levels controlled by SAGT (Vlot et al., 2009; Klessig et al., 2018).
Chemically induced immune responses have been well studied and documented for developing strategies to protect plants from pathogen attack. Although the effectiveness of resistance‐inducing chemicals varies widely depending on the combination of host and pathogen, such immune induction is generally accompanied by up‐regulation of defence genes associated with SAR (Ryals et al., 1996; Oostendorp et al., 2001; Gozzo and Faoro, 2013; Faoro and Gozzo, 2015; Dempsey and Klessig, 2017). For synthetic immune induction, benzothiadiazole (BTH), also known as 1,2,3‐benzothiadiazole‐7‐thiocarboxylic acid‐S‐methyl‐ester (ASM), is one of the most commonly used chemical inducers. BTH can induce host resistance against a wide range of pathogens, including plant viruses (Ishii et al., 1999; Narusaka et al., 1999a, 1999b; Anfoka, 2000; Pappu et al., 2000; Oostendorp et al., 2001; Cools and Ishii, 2002; Smith‐Becker et al., 2003; Mandal et al., 2008; Lin and Ishii, 2009; Takeshita et al., 2013; Frąckowiak et al., 2019). This immune induction by BTH is correlated with an increase in gene expression of resistance‐related genes, including the pathogenesis‐related protein 1 gene (PR1), a well‐known marker of SA‐induced SAR. Tripathi et al. (2010) reported that SABP2 catalyses conversion of BTH into acibenzolar and that acibenzolar is required for SAR induction in tobacco.
Friedrich et al. (1996) showed that BTH increased the transcript level of PR1a in a dose‐dependent manner, even in NahG Nt, and did not directly induce SA accumulation in the plants. These results indicate that BTH can elicit the resistance‐related pathway downstream of SA accumulation. BTH can induce resistance to Peronospora tabacina and tobacco mosaic virus (TMV) in NahG Nt, suggesting that it can activate defence responses without SA accumulation (Friedrich et al., 1996). Lawton et al. (1996) first reported that BTH can induce host resistance in NahG Arabidopsis plants but not in the Nim1 (NPR1; NON‐EXPRESSOR of PATHOGENESIS‐RELATED GENES 1) mutant, suggesting that BTH can activate the SAR‐associated pathway between SA accumulation and NPR1 expression. SA has been found to bind NPR1, which has a slightly higher affinity for BTH than for SA (Wu et al., 2012).
These findings raise the question: How can BTH induce the immune response more effectively than SA? We first analysed an inhibitory effect of BTH on viral infection and then analysed the changes in transcript level of the genes associated with host basal resistance. We eventually focused on the changes in transcript level of salicylic acid glucosyltransferase (SAGT) as the potential cause of the difference in action between BTH and SA. Here, we found that BTH suppressed SAGT expression to enhance immune induction and that SAGT can discriminate between SA and BTH to induce resistance. In addition, we propose that SAGT regulates not only SA but also JA, two major plant hormones controlling plant defence. We conclude that SAGT is a key factor that modulates the balance (trade‐off) between plant growth and defence.
2. RESULTS
2.1. Prior treatment with BTH suppresses CMV‐inducing symptoms
When wild‐type (Wt) N. tabacum (Nt) and Nicotiana benthamiana (Nb) plants were treated with BTH 2 days before inoculation with cucumber mosaic virus (CMV), the plants developed very mild symptoms (Figure 1a,c). CMV accumulated to a much lower level in the BTH‐treated Nt plants than in the control at 9 days post‐inoculation (dpi) (Figure 1b), and to less than one‐third the level of the control in BTH‐treated Nb plants at 5 dpi (Figure 1d). These results suggest that BTH is certainly effective in suppressing viral spread to upper leaves. Treatments with BTH did not induce any abnormal development in the Nt and Nb plants.
Figure 1.
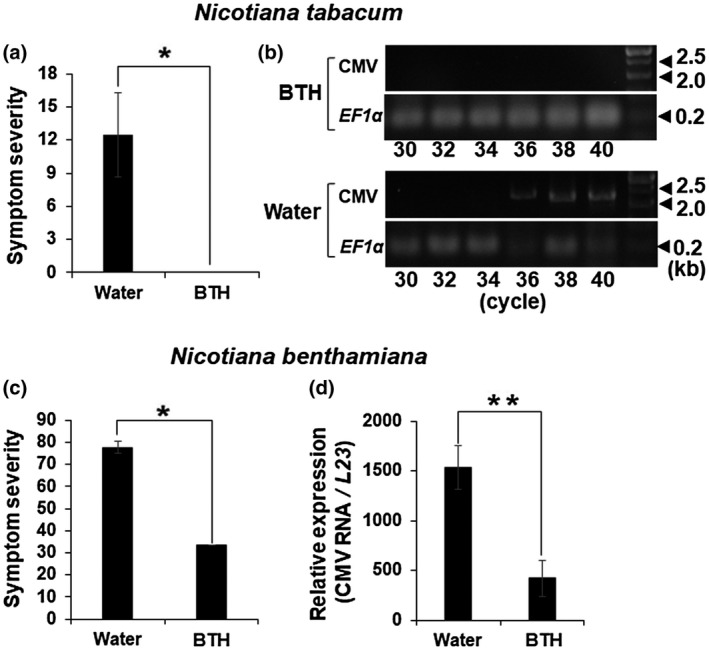
Mean disease severity and relative levels of cucumber mosaic virus strain Y (CMV‐Y) RNA in benzothiadiazole (BTH)‐treated and water‐treated plants. (a) and (c) Mean symptom severity (±SE), (b) relative levels of CMV‐Y RNA3, and (d) those of CMV‐Y RNA3 and RNA4 in the wild‐type (Wt) Nicotiana tabacum (Nt) and Wt N. benthamiana (Nb) plants. BTH (0.12 mM) was applied 2 days before inoculation with CMV. Symptoms on the second and third upper, noninoculated leaf tissues were evaluated and then the leaves were collected at 9 days post‐inoculation (dpi) for Nt and 5 dpi for Nb. Relative accumulation levels of viral RNA were measured (b) by reverse transcription (RT)‐semiquantitative PCR for Nt and (d) by RT‐quantitative PCR for Nb using total RNAs from the leaves of the plants. Severity levels (in arbitrary units) and CMV RNA levels (in arbitrary units) are for individual Nt (n = 6) and Nb (n = 3) plants. *Significant difference (p < .05) and **significant difference (p < .01) between the BTH‐treated and the water‐treated controls, according to Student's t test, respectively
2.2. BTH and SA differ in their mechanism of action in immune induction
To characterize the chemical induction of immunity by BTH, we analysed the expression of several marker genes involved in SA‐, jasmonic acid (JA)‐, and ethylene (ET)‐signalling, SA accumulation, and RNA silencing in BTH‐treated leaf tissues of Wt Nt and NahG Nt plants. The transcript levels of these genes were measured using quantitative reverse transcription PCR (RT‐qPCR) between 6 hr post‐treatment (hpt) and 288 hpt. Accumulation of PR1a and PR1b transcripts in BTH‐treated plants was significantly higher than in water‐treated plants at 12 hpt (Figure S1a,b), but other host genes (SAGT, Coi1, PDF 1.2, EREBP1, EREBP2, ERF1, PAL, and ICS) were not up‐regulated by the BTH treatment compared with those in the water‐treated controls (Figures 2 and S1c–i). Furthermore, the relative levels of the RDR1 and RDR6 transcripts were not consistently elevated by the BTH treatment (Figure S1j,k). Obvious differences in gene expression were not found between the NahG Nt and the Wt Nt plants (Figure [Link], [Link], [Link]a–l). These results suggest that the host responses activated by BTH in the SA‐mediated signal transduction pathway are regulated downstream of SA biosynthesis.
Figure 2.
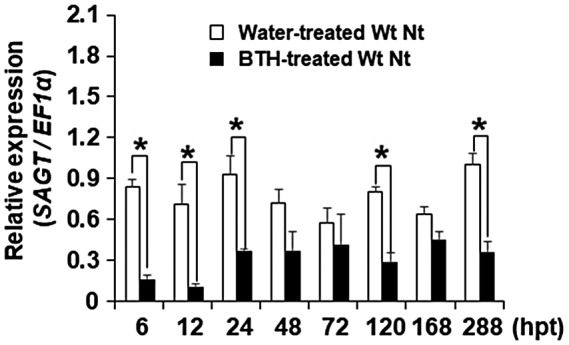
SAGT transcript levels in benzothiadiazole (BTH)‐treated wild‐type Nicotiana tabacum plants (Wt Nt) as determined by quantitative reverse transcription PCR. Leaf samples were harvested from plants in a greenhouse at 6–288 hr post‐treatment (hpt) with BTH (0.12 mM). Black bar: BTH‐treated, white bar: water‐treated. Levels (in arbitrary units) are for individual plants (n = 3). *Significant difference (p < .05) between the BTH‐treated and water‐treated leaves according to Student's t test. Bars indicate standard errors (±SE)
Focusing on the genes with a significant change in transcript levels during the 12 days of observation, we noticed that only SAGT transcription was continuously down‐regulated in the BTH‐treated Wt Nt plants in the assay (Figure 2). We thus decided to elucidate the role of SAGT in the BTH‐induced immunity. SAGT catalyses the conversion of SA to SAG, which does not stimulate plant defence responses (Lee and Raskin, 1998). The transcript level of SAGT in the BTH‐treated Wt Nt plants compared to the level in the water‐treated plants did not differ at 6 hpt (Figure 3a) but was lower at 12 and 48 hpt (Figure 3b,c). On the contrary, SAGT in the SA‐treated Wt Nt plants was greatly up‐regulated at 6 hpt (Figure 3a), then down‐regulated to levels equivalent to those in the water‐treated plants at 12 and 48 hpt (Figure 3b,c). We also used Wt Nb in the same experiments to determine whether the SAGT expression profile in response to BTH is similar in other Nicotiana species. The results showed that SAGT expression was very similar between the two species except that the SAGT transcript level in the BTH‐treated Wt Nb plants was almost equivalent to that in the water‐treated controls at 12 hpt, indicating that the initial effect of BTH in Nb can last longer than in Nt (Figure 3d–f). In addition to SAGT, we measured the transcript levels of PR1a, Coi1, PDF 1.2, and RDR6. The transcript levels of PR1a in the BTH‐treated Wt Nt plants were equivalent to those in the water‐treated controls at 6 hpt (Figure 3g) but were significantly higher at 12 and 48 hpt (Figure 3h,i). On the contrary, PR1a in the SA‐treated Wt Nt plants was greatly up‐regulated at 6 hpt (Figure 3g), then quickly down‐regulated to the levels equivalent to those in the water‐treated controls after 12 hpt (Figure 3h,i). These results suggest that the resistance induced by BTH lasts longer than that by SA. In the BTH‐ and the SA‐treated Wt Nt plants, Coi1, PDF 1.2, and RDR6 had similar expression profiles (Figure 3j–r).
Figure 3.
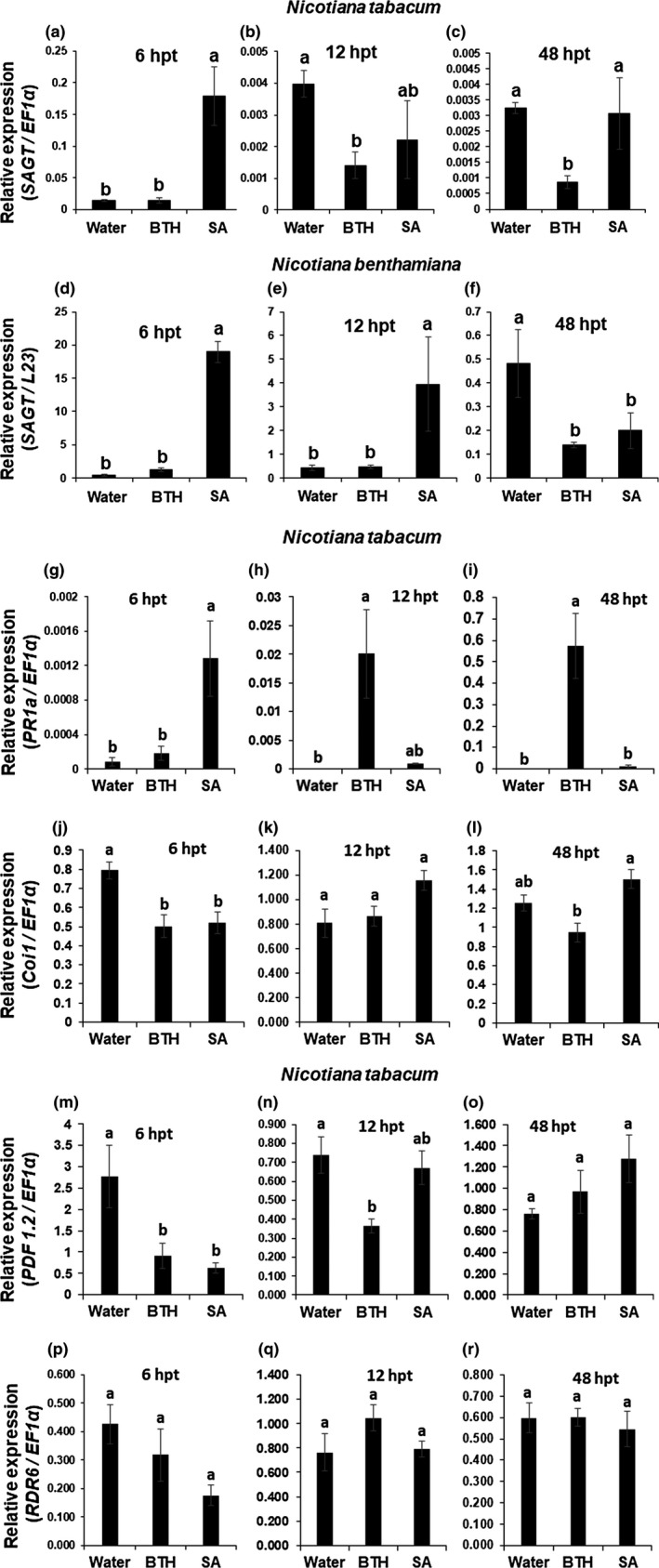
Transcript levels of several resistance‐related genes in benzothiadiazole (BTH)‐treated wild‐type Nicotiana tabacum and N. benthamiana plants. (a)–(c), (g)–(r) Wild‐type (Wt) N. tabacum, (d)–(f) Wt N. benthamiana plants. (a)–(f) SAGT, (g)–(i) PR1a, (j)–(l) Coi1, (m)–(o) PDF1.2, (p)–(r) RDR6. Leaf samples were harvested at 6, 12, and 48 hr post‐treatment (hpt) with BTH (0.12 mM) or salicylic acid (SA) (1 mM). Mean relative transcript levels (±SE) of the host genes were measured by quantitative reverse transcription PCR. Levels (in arbitrary units) are for individual plants (n = 4). Different letters denote statistically significant differences among the BTH‐, SA‐ and water‐treated leaves (Tukey–Kramer method; p < .05). Bars indicate standard errors (±SE)
2.3. BTH decreases the level of SAGT transcripts and SAG
We then conducted pre‐BTH + subsequent‐SA applications to investigate whether BTH can reduce the SAGT expression level compared to the SAGT induction by exogenously applied SA. As shown in Figure 4a,b, even a relatively low level of BTH (0.12 mM) efficiently cancelled the SAGT induction by a higher level of SA (1 mM). We therefore assume that BTH can directly and strongly control SAGT expression regardless of the concentration of endogenous SA.
Figure 4.
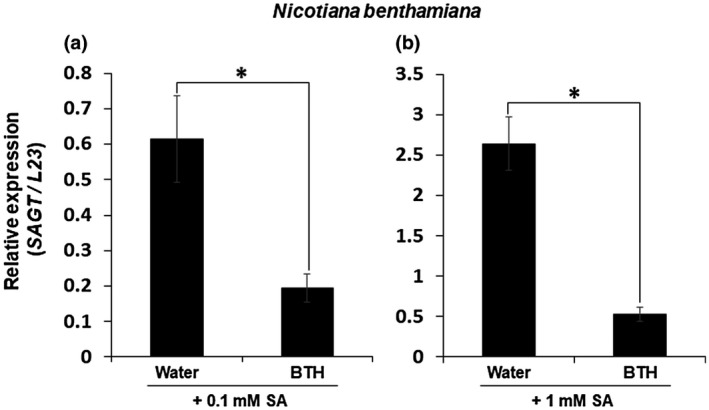
Transcript levels of SAGT in wild‐type Nicotiana benthamiana plants treated with salicylic acid (SA) after benzothiadiazole (BTH) as determined by quantitative reverse transcription PCR. Leaf tissues were treated with (a) SA (0.1 mM) or (b) SA (1.0 mM) at 42 hr after BTH treatment (0.12 mM), then collected at 6 hr after SA treatment. Levels (in arbitrary units) are for individual plants (n = 8). *Significant difference (p < .05) between BTH plus SA‐treated and water plus SA‐treated leaves, according to Student's t test. Bars indicate standard errors (±SE)
Next, the levels of SA and SAG were measured in the Wt Nt and Wt Nb plants (Figures 5 and S3). In the Nt plants, both the SA and BTH treatments led to a temporal increase in SA at 6 hpt, but SA levels declined to the same levels as in the water‐treated control by 12 hpt. On the contrary, only the SA‐treated plants had much higher SAG levels than in the water‐treated controls at 6, 12, and 48 hpt. The results in the Nb plants were essentially similar except that higher levels of SA seemed to persist longer than in the Nt plants (Figure S3). These results together suggest that the dynamics of SAGT regulation by BTH and SA are intrinsically different.
Figure 5.
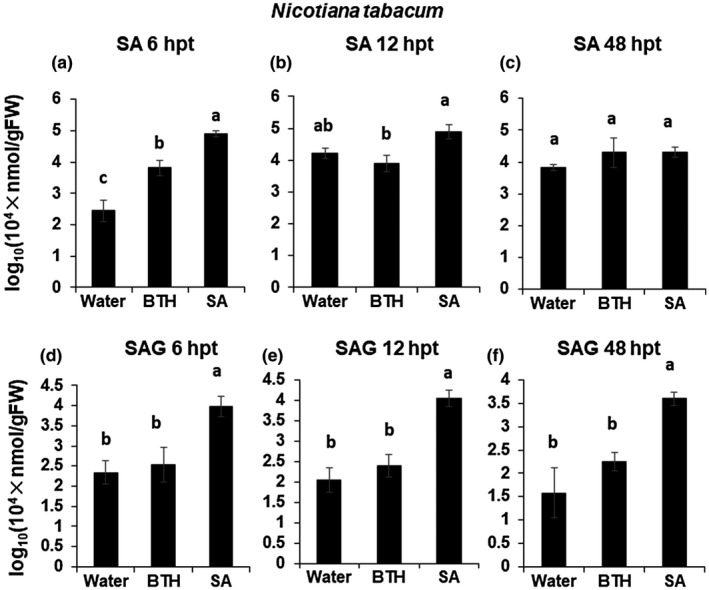
Mean relative levels of salicylic acid (SA) and SA 2‐O‐β‐D‐glucoside (SAG) in BTH‐ (or SA‐) treated wild‐type Nicotiana tabacum plants. (a)–(f) Wild‐type N. tabacum (Wt Nt) plants. Leaf tissues were collected at 6, 12, and 48 hr after treatment with BTH (0.12 mM) or SA (0.1 mM; measurable concentration). Levels (in arbitrary units) are for individual plants (n = 4). Different letters denote statistically significant differences among the BTH‐, SA‐, and water‐treated leaves (Tukey–Kramer method; p < .05). Bars indicate standard errors (±SE)
2.4. SAGT stimulates biomass production and negatively regulates SA and JA levels
Because BTH treatment has been previously reported to lead to decreased plant biomass (Canet et al., 2010), we generated SAGT‐overexpressing (OE1 and OE2) and SAGT‐silenced (IR1 and IR2) Nb transgenic lines to examine whether the SAGT accumulation levels, which are suppressed by BTH, alter plant growth. After treatment with BTH, the OE1 and OE2 Nb lines produced higher levels of SAGT transcripts and SAGT (Figure 6a,b). As we expected, the transgenic lines also accumulated significantly lower levels of SA and higher levels of SAG than in Wt control plants (Figure 6c,d). Curiously, OE2 plants had less JA and TA and accumulated more tuberonic acid glucoside (TAG) than in the Wt control plants (Figure 6e–g). These observations suggest that JA is converted to TAG via TA by SAGT‐mediated glucosylation, thus reducing JA accumulation (Figure 6e–g), in agreement with Seto et al. (2011), who described that SAGT can convert TA into TAG. OE1 plants sprayed with BTH also had slightly more biomass than the Wt control plants, and OE2 plants had significantly more (Figure 6h,i). In contrast, the biomass of IR1 and IR2 plants, which contained lower levels of SAGT transcripts (Figure S5), was not significantly greater than that of the Wt control plants after treatment with BTH (Figure 6h,i). These results together indicate that SAGT has a key role in the trade‐off between plant defence and biomass production.
Figure 6.
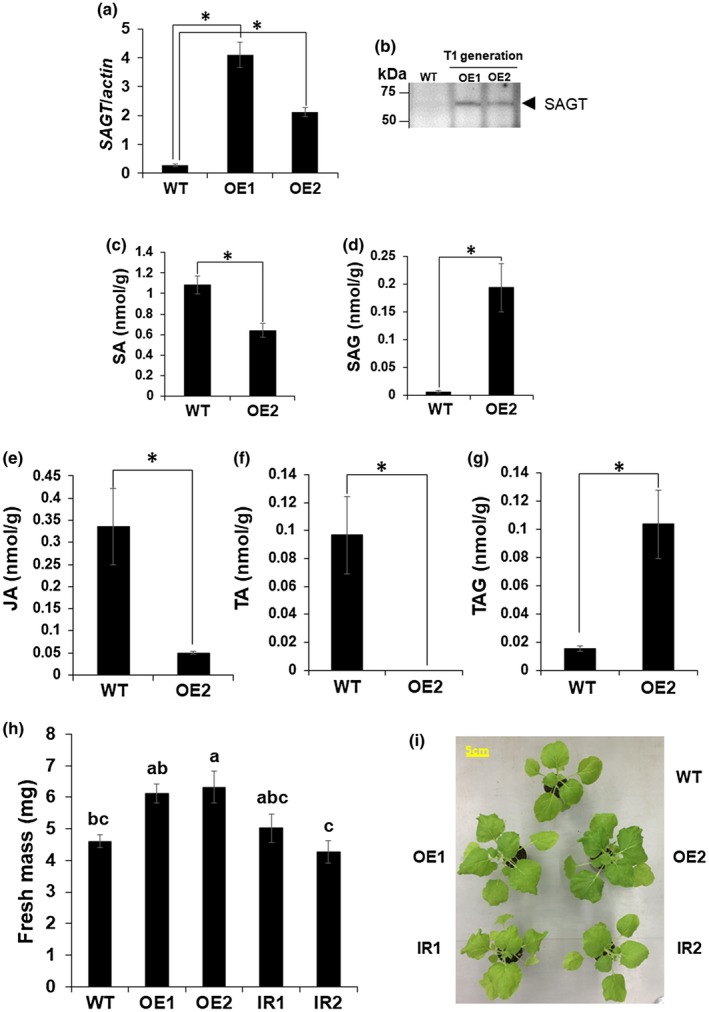
Validation of SAGT‐overexpression and SAGT‐silenced Nicotiana benthamiana (Nb) lines. WT, wild‐type Nb plants. (a) Mean relative transcript levels (±SE) of SAGT in SAGT‐overexpression (OE1 and OE2) Nb lines were measured by quantitative reverse transcription PCR. Levels (in arbitrary units) are for individual plants (n = 4). *Significant difference between the OE1 (or OE2) and the WT Nb plants, according to Student's t test (p < .05). (b) Western blot analysis of SAGT protein overexpressed in OE1‐ and OE2‐transgenic Nb lines. SAGT was detected using anti‐SAGT peptide antibody. (c)–(g) Mean relative levels of (c) salicylic acid (SA), (d) SA 2‐O‐β‐D‐glucoside (SAG), (e) jasmonic acid (JA), (f) tuberonic acid (TA), and (g) tuberonic acid glucoside (TAG) (±SE) in OE2 Nb plants. Levels (in arbitrary units) are for WT and OE2 plants (n = 4). *Significant difference between OE2 Nb line and the WT Nb plants, according to Student's t test (p < .05). (h) Biomass of wild‐type (WT), SAGT‐overexpressing Nb lines (OE1 and OE2), and SAGT‐suppressing Nb lines (IR1 and IR2) at 14 days post‐treatment (dpt). Different letters denote statistically significant differences among the WT and the transgenic Nb lines (Tukey–Kramer method; p < .05, n = 7). (i) WT, OE lines and IR lines at 14 dpt. Whole plants were sprayed with benzothiadiazole (BTH) (0.12 mM)
2.5. A role of SAGT in plant immunity
To confirm the direct involvement of SAGT in host defensive responses, we inoculated OE2 and IR2 plants with CMV. The OE2 plants developed chlorosis and yellow mosaic more consistently than the control Wt plants did (Figures 7a,c and S6), whereas the symptoms on IR2 plants were somewhat attenuated compared to the severe mosaic on the control at 21 dpi (Figures 7b,c and S6). Similarly, after the inoculation with Pseudomonas syringae, the IR1 and IR2 lines expressed a hypersensitive response in the infiltrated areas, indicating that SA‐related host resistance had been activated in the IR plants (Figure 7d–f). CMV accumulation levels were approximately 3‐fold higher in the OE2 plants than those of WT, and one‐quarter the level of WT in the IR2 plants (Figure 7g). We further confirmed that SAGT transcript levels were stably up‐regulated in OE2 plants and down‐regulated in IR2 plants at 21 dpi (Figure 7h,i). Other transgenic lines (OE1 and IR1) showed similar results (Figure S7). Taken together, these results demonstrate that SAGT has an important role in host defensive responses.
Figure 7.
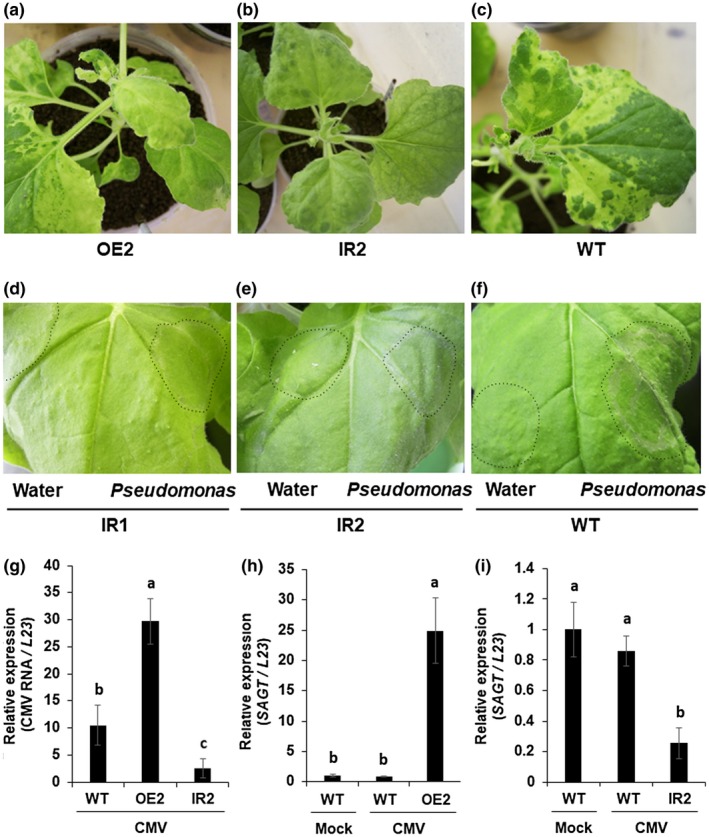
Symptoms, mean relative viral RNA, and transcript levels of SAGT in SAGT‐overexpressing and the SAGT‐silenced Nicotiana benthamiana (Nb) lines inoculated with cucumber mosaic virus strain Y (CMV‐Y) and Pseudomonas syringae. (a)–(c) Symptoms developed on the Nb transgenic plants (OE2 and IR2 lines) at 21 days post‐inoculation (dpi). (d), (e) Suppression and (f) development of necrosis in the SAGT‐suppressing Nb lines (IR1 and IR2) and wild‐type (WT) Nb at 20 hr post‐infiltration of P. syringae. Area infiltrated with bacterial suspension or water is indicated by a broken line. (g)–(i) Mean relative levels (±SE) of CMV‐Y RNA3 and RNA4, and of SAGT transcripts in the upper, noninoculated leaf tissues of the Nb plants at 21 dpi. Relative accumulation levels of viral RNA and SAGT transcripts were measured by quantitative reverse transcription PCR using total RNAs from leaves of the Nb plants. CMV RNA levels (in arbitrary units) and SAGT transcript levels (in arbitrary units) are for individual plants. Different letters denote statistically significant differences among the healthy WT Nb, CMV‐inoculated WT Nb, and transgenic Nb plants (Tukey–Kramer method; p < .05, n = 7)
3. DISCUSSION
3.1. Induction of immunity by BTH does not induce SA synthesis and RNA silencing
In contrast to the strong induction of PR1a and PR1b gene expression by treatment with BTH, the other five JA/ET‐mediated resistance‐related genes, Coi1, PDF1.2, EREBP1, EREBP2, and ERF1, were not up‐regulated (Figure [Link], [Link], [Link]). This result is consistent with the reports for Arabidopsis thaliana mutants that host resistance‐related genes involved in the JA/ET signal transduction pathways are not key players in BTH‐induced resistance (Lawton et al., 1996). In addition, the results from the comparative quantification of transcript levels of ICS, PAL, RDR1, and RDR6 suggest that induction of immunity by BTH did not significantly induce biosynthesis of SA and RNA silencing (Figure [Link], [Link], [Link]). Then, what is the key factor for BTH‐induced resistance? What is responsible for the difference between BTH and SA? We thus further analysed the role of SAGT in the BTH‐mediated resistance, considering that SAGT may be responsible for the difference between BTH and SA (Figure 2).
3.2. BTH‐mediated immune induction is enhanced through the suppression of SAGT expression
Exogenous application of BTH not only induces resistance against viral infection, but also suppresses symptom development in the Wt Nt and Nb plants (Figure 1). To obtain a clue for the operation site of BTH in the resistance induction, we next analysed comparative changes in expression of the several defence‐related genes in comparison with the case of SA. Among the examined host genes, PR1a showed a longer‐lasting induction in the BTH‐treated Wt Nt plants. The result was consistent with prominent up‐regulation of PR1a in A. thaliana on the basis of microarray analyses (Gruner et al., 2013). We then noticed that BTH, but not SA, specifically suppressed SAGT transcription during plant immune induction (Figure 3), providing a feasible explanation for the phenomenon that BTH is more efficient than SA in induction of the SA‐dependent host immune system. The inhibition of the conversion of SA into SAG could lead to high levels of SA accumulation under biotic and abiotic stresses. The SAGT transcript levels in NahG Nt plants treated with BTH were equivalent to those in the water‐treated NahG Nt plants, implying that initial accumulation of SA may be necessary for the down‐regulation of SAGT by BTH (Figure [Link], [Link], [Link]). As shown in Figure 5, SA seemed to be induced to some extent at 6 hpt by BTH, although BTH did not seem to induce SAG accumulation in either Wt Nt or Wt Nb plants (Figures 5 and S3). In support of our results, Friedrich et al. (1996) previously reported that their BTH treatment elicited host resistance pathways downstream of SA accumulation, not by directly enhancing SA accumulation. We here conclude that one of the crucial roles of BTH is the suppression of SAGT transcript levels to inhibit the conversion of SA to SAG. The observation that BTH is a stronger inducer of resistance than SA can be at least partially explained by the finding that BTH has a slightly higher affinity than SA for NPR1 (Wu et al., 2012); NPR1 is central to the activation of the SA‐mediated signalling pathway. Our hypothesis based on SAGT provides an additional explanation for the BTH‐mediated immune induction. We thus believe that BTH can function not only as a simple SA mimic, but also as an efficient suppressor of SAGT transcription.
3.3. SAGT contributes to the trade‐off between plant growth and defence
The effect of SA on plant growth varies among plant species. Exogenous application of SA promotes growth of soybean plants (Gutiérrez‐Coronado et al., 1998) and larger ears of wheat (Shakirova et al., 2003). A low level (50 µM) of SA has a positive effect on growth of chamomile plants, but a high level (250 µM) has negative effect (Kovácik et al., 2009). In addition, the exogenous SA application (100 µM and 1 mM) suppressed the development of trichomes in A. thaliana (Traw and Bergelcon, 2003). Exogenous SA also causes a change in hormonal balance to affect photosynthesis, transpiration, and opening and closure of stomata (Shakirova et al., 2003; Stevens et al., 2006; Abreu and Munné‐Bosch, 2009). An appropriate level of SA may be indispensable to properly regulate plant growth rate; SA is even involved in flowering and senescence (Khurana and Cleland, 1992; Morris et al., 2000). We here hypothesize that SAGT is a key player in the regulation of SA level and thus in plant growth.
The SAGT‐overexpressing Nb lines, OE1 and OE2, had increased SAG levels and plant fresh mass, whereas the SAGT‐silenced Nb lines, IR1 and IR2, did not (Figure 6). These results suggest that SAGT has a crucial role in optimizing plant fitness by allocating resources for resistance activation and biomass production by regulating SA and SAG levels. In a study of the effects of the SA‐mediated signal transduction pathway on biomass production using BTH, Canet et al. (2010) reported that plant fresh mass of A. thaliana was reduced after BTH treatment, which they concluded to be due to the stimulation of the SA‐mediated signal transduction pathway, not to BTH phytotoxicity. In addition, SA and BTH also inhibited the auxin‐mediated signal transduction pathway, and AXR3, the auxin‐inducible AUX/IAA transcription regulator, played a key role as the sensor for SA and BTH in controlling the balance between disease resistance and plant growth (Canet et al., 2010).
Canet et al. (2010) further described that the biomass of BTH‐treated A. thaliana plants was reduced in a dose‐dependent manner. Acibenzolar, a converted form of BTH, itself thus seems to be maintained at a constant level without conversion to SAG in cells. Although endogenous SA in the BTH‐treated plants was lower than in the SA‐treated plants, the level had increased to some extent by 6 hpt (Figure 5). Because BTH suppresses transcription of SAGT in the BTH‐treated plants (Figures 3 and 4), leading to an increase in endogenous SA, an additive effect of the elevated endogenous SA and exogenously supplied BTH may cause growth inhibition.
In contrast to an increase in SAG levels, the SA levels in the OE Nb lines were reduced by half by overexpression of SAGT. BTH treatment of SAGT‐overexpressing Nb plants led to a slight increase in biomass compared with the Wt Nb plants (Figure 6). In the transgenic Nb plants, the inhibitory effect of BTH on SAGT expression must have been hampered by the overexpression of SAGT. On the contrary, the biomass of the Wt Nb plants will be reduced by the additive effect of BTH and endogenous SA. Considering the results together, we attribute the difference in biomass to the growth reduction in the Wt Nb plants as a result of low levels of SAGT, not to growth enhancement by high levels of SAGT in the SAGT‐overexpressing Nb plants.
We found that BTH can down‐regulate SAGT expression and thus reduce SAG accumulation, unlike SA, as illustrated in our hypothetical model in Figure 8a,b. In this model, we postulate that BTH can up‐regulate endogenous levels of both SA and JA by suppressing SAGT synthesis, although a high level of endogenous SA eventually inhibits JA‐mediated gene expression (Caarls et al., 2015) for approximately 10 days as estimated from Figure S1a,b, resulting in induction of the SA‐related defence responses and subsequently lower biomass (Figure 8a). On the contrary, exogenously applied SA can up‐regulate the expression level of SAGT, which converts SA to SAG, and also TA to TAG, leading to the inhibition of both SA‐ and JA‐mediated resistance (Figure 8b) and eventually transient defence responses for approximately a couple of days as estimated from Figure 3g–i. Considering that SAGT is the key factor in the control of SA and JA levels, the use of BTH for studies on plant defence responses will be an excellent tool to understand the trade‐off between plant defence and biomass production for optimum fitness.
Figure 8.
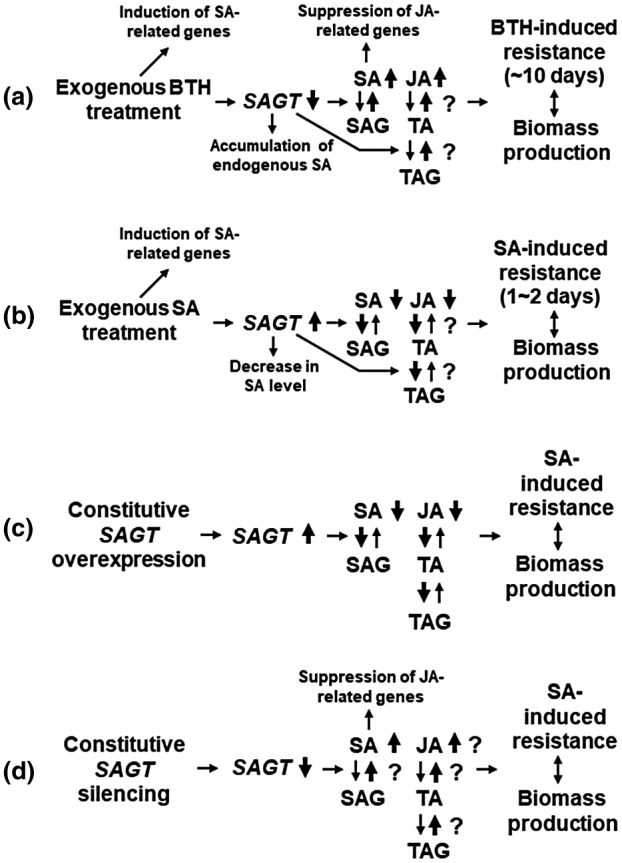
Model for important roles of SAGT in plant responses to benzothiadiazole (BTH) and salicylic acid (SA). Bold vertical arrows indicate the direction of regulation. (a) Exogenous BTH treatment for plants. (b) Exogenous SA treatment for plants. (c) Constitutive SAGT expression in transgenic plants. (d) Constitutive SAGT silencing in transgenic plants
Using the SAGT‐overexpressing and ‐silencing Nb lines, we demonstrated that SAGT regulates the balance between plant defence and growth (Figure 8c,d). In this model, the constitutive overexpression of SAGT can simultaneously down‐regulate both SA‐ and JA‐mediated resistances, resulting in greater biomass production (Figure 8c). On the contrary, the constitutive silencing of SAGT can up‐regulate SA‐mediated resistance but reduce biomass production (Figure 8d). Although the involvement of JA in biomass production has not been intensively discussed before, unlike SA, there are still some findings in connection with this. For example, Campos et al. (2016) previously reported that attenuated growth of A. thaliana associated with anti‐insect resistance could be caused by the JA‐mediated signalling. Given the high relevance of SAGT to the SA/JA‐mediated defence, it is possible that SAGT has roles not only in pathogen‐induced but also in herbivory‐induced resistance, both of which are perhaps associated with reduced plant growth. However, further information on the relationship between SAGT and JA would be necessary to clearly understand how SA (BTH)/JA controls the balance between plant growth and defence.
In the pathogen inoculation experiments, we found that the CMV accumulation levels were higher in the SAGT‐overexpressing plants and lower in the SAGT‐silencing plants, which can be explained in our model (Figure 7g–i). In addition, after Pseudomonas inoculation, expanding necrotic lesions developed on the inoculated leaves of Wt Nb plants, while necrotic lesions were not obvious on the SAGT‐silenced Nb plants at 12 hpi (Figure 7d–f). Numerous papers support our observations. For example, Song et al. (2008) showed that transgenic A. thaliana plants overexpressing AtSGT1 were more susceptible to P. syringae than the wild type. Noutoshi et al. (2012) reported that inhibitors of SAGT actually enhanced resistance against P. syringae. Yao et al. (2007) also demonstrated that transgenic tobacco overexpressing the β‐glucosidase gene from Butyrivibrio fibrisolvens H17c increased SA accumulation through the conversion of SAG into SA, leading to enhancement of host resistance to TMV.
On the contrary, this model does not seem to fit the case of rice. Umemura et al. (2009) previously documented that RNAi suppression of OsSGT1, a probenazole‐responsive UDP‐glucose:SA glucosyltransferase of rice, impaired probenazole‐dependent disease resistance against blast disease in rice plants, indicating that the role of the ratio of SA/SAG in rice seems to be different from that in other plants. In fact, SAG in rice can induce resistance as SA does (Bundó and Coca, 2016). In rice, SA‐mediated resistance is activated not only by SA but also by SAG, and SAG is differentially induced, suggesting that SAG is a key factor for SA‐mediated rice resistance rather than SA per se (Bundó and Coca, 2016). We therefore presume that the role of SAG in rice is unique compared to other plants.
Gruner et al. (2013) previously reported that biologically induced SAR and the immunity induced by exogenously applied BTH shared a common signal transduction system for immune response but with some differences. According to their analyses, some UDP‐glucosyltransferase (SAGT synonym) genes of A. thaliana were up‐regulated and others were down‐regulated by either BTH or SA treatment; the individual genes were thus differentially expressed in response to BTH and SA. We here assume that a certain SAGT among the isoforms actively and specifically responds to pathogen infection; the induction of each SAGT gene would therefore differ depending on the pathogen. For example, Lee and Raskin (1999) demonstrated that tobacco SAGT was specifically induced after inoculation with avirulent viral and bacterial pathogens. Taken together, we conclude that the SAGT genes at least in two Nicotiana species play an important role in the trade‐off between plant defence and growth.
4. EXPERIMENTAL PROCEDURES
4.1. Plants, pathogens, and chemical treatment
N. tabacum and N. benthamiana plants were grown and maintained in an air‐conditioned greenhouse at 25/20 °C (day/night) (Figures 2, [Link], [Link], [Link], and [Link], [Link], [Link]) or in a growth chamber at 25 °C with a 12‐hr photoperiod. The NahG‐expressing transgenic N. tabacum line was generated essentially using the protocol of Bi et al. (1995), and the N. benthamiana line was described by Asai et al. (2010). Wild‐type CMV‐Y (Suzuki et al., 1991) was propagated and purified as described by Takeshita et al. (2012). The largest leaf on a plant of N. benthamiana was infiltrated with a bacterial suspension of P. syringae according to Krzymowska et al. (2007), and kept at 25 °C.
The largest leaf on plants of N. tabacum and N benthamiana was dipped into BTH (Syngenta Japan K. K., Tokyo, Japan) or SA (1.0 or 0.1 mM) for 5 s without a water rinse after treatment. In the biomass assay, the whole plants of the transgenic N. benthamiana lines were sprayed with BTH (Figure 6). BTH was used at 25 ppm (0.12 mM) because PR1 transcript accumulation in tobacco sprayed with 0.036 mM BTH can peak at levels equivalent to those with 1.2 mM BTH even at 7 days after treatment (Friedrich et al., 1996), and some of the tobacco leaves treated with BTH at 100 ppm (0.476 mM) hardened slightly in our preliminary experiments. SA, whose methylated derivative (MeSA) is volatile and is excreted from plants (Kumar, 2014), was also used at a higher concentration (1 mM) in some experiments (Figures 3 and 4) as done by Lewzey and Carr (2009), Naylor et al. (1998), and Zhou et al. (2014).
4.2. RNA extraction and RT‐qPCR analysis
Total RNA was extracted from leaf tissue with RNAiso Plus (Takara Bio) according to the user manual. Six biological replicates per experimental plot were used to validate gene expression by comparative reverse transcription‐quantitative PCR (RT‐qPCR). First‐strand cDNA was synthesized using 50–200 ng of total RNA and ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo), which contains oligo(dT) primer and random hexamer primer. A SAGT‐specific primer was used for efficient reverse transcription of SAGT. Comparative qPCR targeting of a host gene and CMV RNA was performed by using THUNDERBIRD SYBR qPCR Mix (Toyobo) and the Thermal Cycler Dice RealTime System Single TP850 (Takara Bio) according to the procedure of the manufacturer and Takeshita et al. (2013). Each reaction mixture (total 20 μl) was prepared using 10 μl of THUNDERBIRD SYBR qPCR Mix, 1 μl of 10 pmol of each forward and reverse primer, 1 μl of cDNA template, and 7 μl of water. Primers used in RT‐qPCR are listed in Table S1. Samples were analysed by relative RT‐qPCR as done by Takeshita et al. (2013). Transcript accumulation of each target gene was normalized by the quantification of EF1α from N. tabacum and L23 from N. benthamiana. The EF1α and L23 were selected as normalizers after analyses by geNorm essentially according to Takeshita et al. (2013). These values were calculated according to the ΔΔC t method (Pfaffl et al., 2002) and plotted with standard errors. The data were obtained from each assay repeated independently (n = 3–8).
4.3. Measurement of phytohormones
Leaf tissues were weighed immediately after harvest, then frozen in liquid nitrogen and stored at –80 °C until measurement of SA and SAG using ultraperformance liquid chromatography‐tandem mass spectrometry (UPLC‐MSMS). Leaf samples were prepared and analysed with UPLC‐MSMS as done by Matsuura et al. (2009) and Fujiwara et al. (2016).
4.4. Construction of SAGT‐overexpressing and SAGT‐silenced transgenic plants
For the SAGT‐overexpressing transgenic Nb lines, the PCR‐amplified SAGT ORF (1,436 bp) sequence was amplified using primer pair SAGT‐F and SAGT‐R (Table S1). The fragment was cloned into pENTR/D‐TOPO (Thermo Fisher Scientific). The entry clone was integrated into the binary vector pBI‐OX‐GW (Inplanta Innovations, Inc.), using the GATEWAY system (Thermo Fisher Scientific). The recombinant pBI‐SAGT binary plasmid was transferred into Agrobacterium tumefaciens LBA4404. Nb plants were transformed by the conventional leaf disk transformation method and selected on kanamycin‐containing Murashige‐Skoog medium as done by Fukuzawa et al. (2018). Two SAGT‐overexpressing lines (OE1 and OE2) were selected from 38 T1 lines regenerated from T0 generation. The T1 lines of OE1 and OE2 were confirmed to be homozygous.
For the SAGT‐silenced transgenic Nb lines, plasmid pBI‐IR‐SAGT (Figure S4) containing an inverted repeat of the 600‐nt SAGT sequence was constructed. The PCR‐amplified 600‐nt SAGT sequence was amplified using primer pair SAGT‐210F and SAGT‐809R (Table S1). The fragment was then cloned into pENTR/D‐TOPO (Invitrogen). The entry clone was integrated into the binary vector, pBI‐sense, antisense‐GW (Inplanta Innovations Inc.) using the GATEWAY system. The recombinant pBI‐IR‐SAGT vector was transferred into A. tumefaciens LBA4404. Nb plants were transformed as described above. Two independent SAGT‐silenced lines (IR1 and IR2) were selected from 113 regenerated lines. Reduced transcript levels of SAGT in the two lines at the T0 generation stage were verified by RT‐qPCR (Figure S5). Seeds from the two selected T0 transgenic lines were collected, and homozygous T1 lines were used for the subsequent experiments.
4.5. Nucleotide sequencing
The nucleotide sequences of the RT‐qPCR product and transgene were verified using the Big Dye Terminator DNA Sequencing Kit v. 3.1 (Applied Biosystems) and the ABI Prism 310 Genetic Analyzer. The sequence was analysed using the program GENETYX‐Win v. 10 (GENETYX Corp.).
Supporting information
FIGURE S1 Mean relative transcript levels of resistance‐related genes in BTH‐treated and water‐treated Nicotiana tabacum plants
FIGURE S2 Mean relative transcript levels of resistance‐related genes in BTH‐treated and water‐treated NahG‐transgenic Nicotiana tabacum plants (NahG Nt)
FIGURE S3 Mean relative levels of SA and SAG in BTH‐ (or SA‐) treated wild‐type Nicotiana benthamiana plants
FIGURE S4 Schematic representation of pBI‐IR‐SAGT plasmid used to construct SAGT‐silenced transgenic Nicotiana tabacum lines
FIGURE S5 Validation of SAGT‐silenced Nicotiana benthamiana lines
FIGURE S6 CMV‐Y‐induced symptoms on SAGT‐overexpressing (OE2) and ‐silenced (IR2) Nicotiana benthamiana lines
FIGURE S7 Symptoms, mean relative viral RNA and transcript levels of SAGT in SAGT‐overexpressing (OE1) and the SAGT‐silenced (IR1) Nicotiana benthamiana lines infected with CMV‐Y
TABLE S1 Oligonucleotide primers used in RT‐qPCR analyses and construction of transgenic plants
ACKNOWLEDGEMENTS
We thank Syngenta Japan K. K. for providing BTH (ASM). We also thank Prof. Kenichi Tsuchiya and Prof. Naruto Furuya, Kyushu University for suggestions on this work and Dr. Hoang Hoa Long, Agricultural Genetics Institute, Vietnam, for sharing primer information. This work was partially supported by JSPS KAKENHI grant numbers 24580067 and 18K05655. The authors declare no conflict of interest.
Kobayashi Y, Fukuzawa N, Hyodo A, et al. Role of salicylic acid glucosyltransferase in balancing growth and defence for optimum plant fitness. Molecular Plant Pathology. 2020;21:429–442. 10.1111/mpp.12906
Yudai Kobayashi, Noriho Fukuzawa, and Ayaka Hyodo equally contributed.
DATA ACCESSIBILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abreu, M.E. and Munné‐Bosch, S. (2009) Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana . Journal of Experimental Botany, 60, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfoka, G. (2000) Benzo‐(1,2,3)‐thiadiazole‐7‐carbothioic acid S‐methyl ester induces systemic resistance in tomato (Lycopersicon esculentum Mill cv. Vollendung) to Cucumber mosaic virus . Crop Protection, 19, 401–405. [Google Scholar]
- Asai, S. , Mase, K. and Yoshioka, H. (2010) Role of nitric oxide and reactive oxygen species in disease resistance to necrotrophic pathogens. Plant Signaling & Behavior, 5, 872–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, Y. , Kenton, P. , Mur, L. , Darby, R. and Draper, J. (1995) Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. The Plant Journal, 8, 235–245. [DOI] [PubMed] [Google Scholar]
- Bundó, M. and Coca, M. (2016) Enhancing blast disease resistance by overexpression of the calcium‐dependent protein kinase OsCPK4 in rice. Plant Biotechnology Journal, 14, 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, M.L. , Yoshida, Y. , Major, I.T. , de Oliveira Ferreira, D. , Weraduwage, S.M. , Froehlich, J.E. et al (2016) Rewiring of jasmonate and phytochrome B signalling uncouples plant growth‐defense tradeoffs. Nature Communications, 7, 12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet, J.V. , Dobón, A. , Ibáñez, F. , Perales, L. and Tornero, P. (2010) Resistance and biomass in Arabidopsis: a new model for salicylic acid perception. Plant Biotechnology Journal, 8, 126–141. [DOI] [PubMed] [Google Scholar]
- Caarls, L. , Pieterse, C.M. and Van Wees, S.C. (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Frontiers in Plant Science, 25, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa, S. and Carr, J.P. (1998) Cyanide restores N gene–mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. The Plant Cell, 10, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools, H.J. and Ishii, H. (2002) Pre‐treatment of cucumber plants with acibenzolar‐S‐methyl systemically primes a phenylalanine ammonia lyase gene (PAL1) for enhanced expression upon attack with a pathogenic fungus. Physiological and Molecular Plant Pathology, 61, 273–280. [Google Scholar]
- Dempsey, D.A. and Klessig, D.F. (2017) How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biology, 15, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro, F. and Gozzo, F. (2015) Is modulating virus virulence by induced systemic resistance realistic? Plant Science, 234, 1–13. [DOI] [PubMed] [Google Scholar]
- Forouhar, F. , Yang, Y. , Kumar, D. , Chen, Y. , Fridman, E. , Park, S.W. et al (2005) Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proceedings of the National Academy of Sciences of the United States of America, 102, 1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frąckowiak, P. , Pospieszny, H. , Smiglak, M. and Obrępalska‐Stęplowska, A. (2019) Assessment of the efficacy and mode of action of benzo(1,2,3)‐thiadiazole‐7‐carbothioic acid S‐methyl ester (BTH) and its derivatives in plant protection against viral disease. International Journal of Molecular Sciences, 20, E1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich, L. , Lawton, K. , Ruess, W. , Masner, P. , Specker, N. , Gut‐Rella, M. et al (1996) A benzothiadiazole derivative induces systemic acquired resistance in tobacco. The Plant Journal, 10, 61–70. [Google Scholar]
- Fujiwara, A. , Togawa, S. , Hikawa, T. , Matsuura, H. , Masuta, C. and Inukai, T. (2016) Ascorbic acid accumulates as a defense response to Turnip mosaic virus in resistant Brassica rapa cultivars. Journal of Experimental Botany, 67, 4391–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa, N. , Masuta, C. and Matsumura, T. (2018) Rapid transient protein production by the coat protein‐deficient cucumber mosaic virus vector: non‐packaged CMV system, NoPaCS. Plant Cell Reports, 37, 1513–1522. [DOI] [PubMed] [Google Scholar]
- Gozzo, F. and Faoro, F. (2013) Systemic acquired resistance (50 years after discovery): moving from the lab to the field. Journal of Agriculture and Food Chemistry, 61, 12473–12491. [DOI] [PubMed] [Google Scholar]
- Gruner, K. , Griebel, T. , Návarová1, H. , Attaran, E. and Zeier, J. (2013) Reprogramming of plants during systemic acquired resistance. Frontiers in Plant Science, 4, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Coronado, M.A. , Trejo‐López, C. and Larqué‐Saavedra, A. (1998) Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiology and Biochemistry, 36, 563–565. [Google Scholar]
- Huot, B. , Yao, J. , Montgomery, B.L. and He, S.Y. (2014) Growth‐defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant Pathology, 7, 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, H. , Tomita, Y. , Horio, T. , Narusaka, Y. , Nakazawa, Y. , Nishimura, K. et al (1999) Induced resistance of acibenzolar‐S‐methyl (CGA 245704) to cucumber and Japanese pear diseases. European Journal of Plant Pathology, 105, 77–85. [Google Scholar]
- Khurana, J.P. and Cleland, C.F. (1992) Role of salicylic acid and benzoid acid in flowering of a photoperiod‐insensitive strain, Lemna paucicostata LP6. Plant Physiology, 100, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig, D.F. , Choi, H.W. and Dempsey, D.A. (2018) Systemic acquired resistance and salicylic acid: past, present, and future. Molecular Plant‐Microbe Interactions, 31, 871–888. [DOI] [PubMed] [Google Scholar]
- Kovácik, J. , Grúz, J. , Backor, M. , Strnad, M. and Repcák, M. (2009) Salicylic acid‐induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Reports, 28, 135–143. [DOI] [PubMed] [Google Scholar]
- Krzymowska, M. , Konopka‐Postupolska, D. , Sobczak, M. , Macioszek, V. , Ellis, B.E. and Hennig, J. (2007) Infection of tobacco with different Pseudomonas syringae pathovars leads to distinct morphotypes of programmed cell death. The Plant Journal, 50, 253–264. [DOI] [PubMed] [Google Scholar]
- Kumar, D. (2014) Salicylic acid signaling in disease resistance. Plant Science, 228, 127–134. [DOI] [PubMed] [Google Scholar]
- Lawton, K. , Friedrich, L. , Hunt, M. , Weymann, K. , Delaney, T. , Kessmann, H. et al (1996) Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. The Plant Journal, 10, 71–82. [DOI] [PubMed] [Google Scholar]
- Lee, H.I. and Raskin, I. (1998) Glucosylation of salicylic acid in Nicotiana tabacum cv. Xanthi‐nc. Phytopathology, 88, 692–697. [DOI] [PubMed] [Google Scholar]
- Lee, H.I. and Raskin, I. (1999) Purification, cloning, and expression of a pathogen inducible UDP‐glucose: salicylic acid glucosyltransferase from tobacco. Journal of Biological Chemistry, 274, 36637–36642. [DOI] [PubMed] [Google Scholar]
- Lewsey, M.G. and Carr, J.P. (2009) Effects of DICER‐like proteins 2, 3 and 4 on cucumber mosaic virus and tobacco mosaic virus infections in salicylic acid‐treated plants. Journal of General Virology, 90, 3010–3014. [DOI] [PubMed] [Google Scholar]
- Lin, T.C. and Ishii, H. (2009) Accumulation of H2O2 in xylem fluids of cucumber stems during ASM‐induced systemic acquired resistance (SAR) involves increased LOX activity and transient accumulation of shikimic acid. European Journal of Plant Pathology, 125, 119–130. [Google Scholar]
- Loake, G. and Grant, M. (2007) Salicylic acid in plant defence – the players and protagonists. Current Opinion in Plant Biology, 10, 466–472. [DOI] [PubMed] [Google Scholar]
- Mandal, B. , Mandal, S. , Csinos, A.S. , Martinez, N. , Culbreath, A.K. and Pappu, H.R. (2008) Biological and molecular analyses of the acibenzolar‐S‐methyl‐induced systemic acquired resistance in flue‐cured tobacco against Tomato spotted wilt virus . Phytopathology, 98, 196–204. [DOI] [PubMed] [Google Scholar]
- Matsuura, H. , Aoi, A. , Satou, C. , Nakaya, M. , Masuta, C. and Nabeta, K. (2009) Simultaneous UPLC MS/MS analysis of endogenous jasmonic acid, salicylic acid, and their related compounds. Plant Growth Regulation, 57, 293–301. [Google Scholar]
- Morris, K. , Mackerness, S.A.H. , Page, T. , John, C.F. , Murphy, A.M. , Carr, J.P. et al (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. The Plant Journal, 23, 677–685. [DOI] [PubMed] [Google Scholar]
- Narusaka, Y. , Narusaka, M. , Horio, T. and Ishii, H. (1999a) Comparison of local and systemic induction of acquired disease resistance in cucumber plants treated with benzothiadiazoles or salicylic acid. Plant and Cell Physiology, 40, 388–395. [DOI] [PubMed] [Google Scholar]
- Narusaka, Y. , Narusaka, M. , Horio, T. and Ishii, H. (1999b) Induction of disease resistance in cucumber by acibenzolar‐S‐methyl and expression of resistance‐related genes. Annals of the Phytopathological Society of Japan, 65, 116–122. [Google Scholar]
- Naylor, M. , Murphy, A.M. , Berry, J.O. and Carr, J.P. (1998) Salicylic acid can induce resistance to plant virus movement. Molecular Plant‐Microbe Interactions, 11, 860–868. [Google Scholar]
- Noutoshi, Y. , Okazaki, M. , Kida, T. , Nishina, Y. , Morishita, Y. , Ogawa, T. et al (2012) Novel plant immune‐priming compounds identified via high‐throughput chemical screening target salicylic‐acid glucosyltransferases in Arabidopsis . The Plant Cell, 24, 3795–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp, M. , Kunz, W. , Dietrich, B. and Staub, T. (2001) Induced disease resistance in plants by chemicals. European Journal of Plant Pathology, 107, 19–28. [Google Scholar]
- Pappu, H.R. , Csinos, A.S. , McPherson, R.M. , Jones, D.C. and Stephenson, M.G. (2000) Effect of acibenzolar‐S‐methyl and imidacloprid on suppression of tomato spotted wilt tospovirus in flue‐cured tobacco. Crop Protection, 19, 349–354. [Google Scholar]
- Park, S.W. , Kaimoyo, E. , Kumar, D. , Mosher, S. and Klessig, D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science, 318, 113–116. [DOI] [PubMed] [Google Scholar]
- Pastor, V. , Luna, E. , Mauch‐Mani, B. , Ton, J. and Flors, V. (2013) Primed plants do not forget. Environmental and Experimental Botany, 94, 46–56. [Google Scholar]
- Pfaffl, M.W. , Horgan, G.W. and Dempfle, L. (2002) Relative expression software tool (REST) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Research, 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐San Vicente, M. and Plasencia, J. (2011) Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany, 62, 3321–3338. [DOI] [PubMed] [Google Scholar]
- Ryals, J.A. , Neuenschwander, U.H. , Willits, M.G. , Molina, A. , Steiner, H.Y. and Hunt, M.D. (1996) Systemic acquired resistance. The Plant Cell, 8, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, Y. , Hamada, S. , Ito, H. , Masuta, C. , Matsui, H. , Nabeta, K. et al (2011) Tobacco salicylic acid glucosyltransferase is active toward tuberonic acid (12‐hydroxyjasmonic acid) and is induced by mechanical wounding stress. Bioscience, Biotechnology, and Biochemistry, 75, 2316–2320. [DOI] [PubMed] [Google Scholar]
- Shakirova, F.M. , Sakhabutdinova, A.R. , Bezrukova, V. , Fatkhutdinova, R.A. and Fatkhutdinova, D.R. (2003) Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science, 164, 317–322. [Google Scholar]
- Smith‐Becker, J. , Keen, N.T. and Becker, J.O. (2003) Acibenzolar‐S‐methyl induces resistance to Colletotrichum lagenarium and cucumber mosaic virus in cantaloupe. Crop Protection, 22, 769–774. [Google Scholar]
- Song, J.T. , Koo, Y.J. , Seo, H.S. , Kim, M.C. , Choi, Y.D. and Kim, J.H. (2008) Overexpression of AtSGT1, an Arabidopsis salicylic acid glucosyltransferase, leads to increased susceptibility to Pseudomonas syringae . Phytochemistry, 69, 1128–1134. [DOI] [PubMed] [Google Scholar]
- Stevens, J. , Senaratna, T. and Sivasithamparam, K. (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regulation, 49, 77–83. [Google Scholar]
- Suzuki, M. , Kuwata, S. , Kataoka, J. , Masuta, C. , Nitta, N. and Takanami, Y. (1991) Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology, 183, 106–113. [DOI] [PubMed] [Google Scholar]
- Takeshita, M. , Koizumi, E. , Noguchi, M. , Sueda, K. , Shimura, H. , Ishikawa, N. et al (2012) Infection dynamics in viral spread and interference under the synergism between Cucumber mosaic virus and Turnip mosaic virus . Molecular Plant‐Microbe Interactions, 25, 18–27. [DOI] [PubMed] [Google Scholar]
- Takeshita, M. , Okuda, M. , Okuda, S. , Hyodo, A. , Hamano, K. , Furuya, N. et al (2013) Induction of antiviral responses by acibenzolar‐s‐methyl against cucurbit chlorotic yellows virus in Melon. Phytopathology, 103, 960–965. [DOI] [PubMed] [Google Scholar]
- Traw, M.B. and Bergelson, J. (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiology, 133, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, D. , Jiang, Y.L. and Kumar, D. (2010) SABP2, a methyl salicylate esterase is required for the systemic acquired resistance induced by acibenzolar‐S‐methyl in plants. FEBS Letters, 584, 3458–3463. [DOI] [PubMed] [Google Scholar]
- Umemura, K. , Satou, J. , Iwata, M. , Uozumi, N. , Koga, J. , Kawano, T. et al (2009) Contribution of salicylic acid glucosyltransferase, OsSGT1, to chemically induced disease resistance in rice plants. The Plant Journal, 57, 463–472. [DOI] [PubMed] [Google Scholar]
- Vlot, A.C. , Dempsey, D.A. and Klessig, D.F. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology, 47, 177–206. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Zhang, D. , Chu, J.Y. , Boyle, P. , Wang, Y. , Brindle, I.D. et al (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Reports, 1, 639–647. [DOI] [PubMed] [Google Scholar]
- Yao, J. , Huot, B. , Foune, C. , Doddapaneni, H. and Enyedi, A. (2007) Expression of a β‐glucosidase gene results in increased accumulation of salicylic acid in transgenic Nicotiana tabacum cv. Xanthi‐nc NN genotype. Plant Cell Reports, 26, 291–301. [DOI] [PubMed] [Google Scholar]
- Zhou, T. , Murphy, A.M. , Lewsey, M.G. , Westwood, J.H. , Zhang, H.M. , González, I. et al (2014) Domains of the cucumber mosaic virus 2b silencing suppressor protein affecting inhibition of salicylic acid‐induced resistance and priming of salicylic acid accumulation during infection. Journal of General Virology, 95, 1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Mean relative transcript levels of resistance‐related genes in BTH‐treated and water‐treated Nicotiana tabacum plants
FIGURE S2 Mean relative transcript levels of resistance‐related genes in BTH‐treated and water‐treated NahG‐transgenic Nicotiana tabacum plants (NahG Nt)
FIGURE S3 Mean relative levels of SA and SAG in BTH‐ (or SA‐) treated wild‐type Nicotiana benthamiana plants
FIGURE S4 Schematic representation of pBI‐IR‐SAGT plasmid used to construct SAGT‐silenced transgenic Nicotiana tabacum lines
FIGURE S5 Validation of SAGT‐silenced Nicotiana benthamiana lines
FIGURE S6 CMV‐Y‐induced symptoms on SAGT‐overexpressing (OE2) and ‐silenced (IR2) Nicotiana benthamiana lines
FIGURE S7 Symptoms, mean relative viral RNA and transcript levels of SAGT in SAGT‐overexpressing (OE1) and the SAGT‐silenced (IR1) Nicotiana benthamiana lines infected with CMV‐Y
TABLE S1 Oligonucleotide primers used in RT‐qPCR analyses and construction of transgenic plants
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


