Abstract
Alteration in atmospheric carbon dioxide concentration and other environmental factors are the significant cues of global climate change. Environmental factors affect the most fundamental biological process including photosynthesis and different metabolic pathways. The feeding of the rapidly growing world population is another challenge which imposes pressure to improve productivity and quality of the existing crops. C4 plants are considered the most productive, containing lower photorespiration, and higher water-use & N-assimilation efficiencies, compared to C3 plants. Besides, the C4-photosynthetic genes not only play an important role in carbon assimilation but also modulate abiotic stresses. In this review, fundamental three metabolic processes (C4, C3, and CAM) of carbon dioxide assimilation, the evolution of C4-photosynthetic genes, effect of elevated CO2 on photosynthesis, and overexpression of C4-photosynthetic genes for higher photosynthesis were discussed. Kranz-anatomy is considered an essential prerequisite for the terrestrial C4 carbon assimilation, but single-celled C4 plant species changed this well-established paradigm. C4 plants are insensitive to an elevated CO2 stress condition but performed better under stress conditions. Overexpression of essential C4-photosynthetic genes such as PEPC, PPDK, and NADP-ME in C3 plants like Arabidopsis, tobacco, rice, wheat, and potato not only improved photosynthesis but also provided tolerance to various environmental stresses, especially drought. The review provides useful information for sustainable productivity and yield under elevated CO2 environment, which to be explored further for CO2 assimilation and also abiotic stress tolerance. Additionally, it provides a better understanding to explore C4-photosynthetic gene(s) to cope with global warming and prospective adverse climatic changes.
Keywords: Abiotic stress, C3, C4, CAM, Carbon assimilation, Global warming, Photosynthesis
Introduction
Earth is facing two major threats, first is global warming, which reaches an alarming stage, and the second is world-population that is also expanding rapidly. Global warming leads to climatic changes, an increase of the atmospheric CO2 concentration and other greenhouse gases (GHGs) are a primary source (Nowak et al. 2004). In the atmosphere, CO2 is the most prominent GHG which associated with an increase in atmospheric temperature. Furthermore, it is directly linked with the development and productivity of terrestrial plants system. Enhanced photosynthesis of crop plants leads to the CO2 assimilation and also productivity, therefore considered the most promising approach to solve both problems. According to IPCC (2013) and NASA (2014), the pre-industrial level of global CO2 concentration 285 μmol mol−1 which is now increased to the present level 400 μmol mol−1. About 1.35% increment is predicted annually for global CO2 concentration, and about 936 μmol mol−1 CO2 concentration is speculated with the rise of global atmospheric temperature of 6.4 °C by the year 2100. These changes would severely affect the world flora and fauna including crop plant development and productivity. Researchers have a profound interest to mitigate the adverse effects of global climate changes by improving the atmospheric abundance of CO2 and other GHGs. In the Kyoto protocol, enhanced biological carbon sequestration is one of the most essential, instantly valid, natural and cost-effective approach to alleviate the effects of increased CO2 concentration from atmosphere (Ruan et al. 2012), which leads to improve the storage capacity of ecosystem through plant photosynthesis.
The photosynthesis, one of the fundamental biological process, follows three metabolic strategies to assimilate CO2 from the environment, and plants are categorized into three groups; C3, C4, and CAM, accordingly. These plants have different photosynthesis carbon assimilation, morphological and physiological characteristics. Most of the terrestrial plants, including prominent crops such as rice, soybean, wheat and potato assimilate atmospheric CO2 as a three-carbon compound, 3-phosphoglycerate (3-PGA), and follows Calvin–Benson cycle, also commonly known as C3 photosynthesis pathway (Matsuoka et al. 2001). In C3 photosynthesis metabolic process, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) play a major role to assimilate atmospheric CO2, and under a stressful condition, this enzyme has low affinity to differentiate O2 from the CO2. Resultant, O2 competes with CO2 at the active site of RuBisCO, which leads to about 50% loss of carbon from metabolic sites. Therefore, photosynthesis decreases and another metabolic process such as photorespiration increases because of oxygenase activity (Miyao 2003). During the evolution, hot environments were developed, and plants adapted to stress condition including inadequate water supply up to an extent (Edwards et al. 2001). Plants, evolved with C4 carbon assimilation, release CO2 at the proximity of RuBisCO, which promotes carboxylation, respectively over the oxygenation (Hatch 1987). This assimilation process prevents the possible loss of CO2 which is commonly occurred through photorespiration and also improves the water and nitrogen-use efficiencies, compared to C3 plants (Sage and Pearcy 1987). The Crassulacean Acid Metabolism (CAM) is evolved in some plants which are adapted to arid conditions (Winter and Holtum 2014). In these (CAM) plants, stomata remain closed during the day to diminish evapotranspiration but open in the night to assimilate atmospheric CO2. Both, CAM and C4 carbon assimilation process have evolved from C3 ancestors, independently. In total, about 3% of flowering plants are C4 (Sage et al. 2012) whereas about 6% represent CAM plants (Silvera et al. 2010).
Many essential crops (rice and wheat) possessing C3 photosynthesis pathway compared to other crops (maize and sorghum) harboring C4 pathway are relatively inefficient for CO2 uptake and further assimilation. Consequently, they (C3 crops) have a lower yield, water, and nitrogen-use efficiency. The genetic transformation and recombinant DNA technology may provide a set of accessible and practical tools for enhancing carbon assimilation in crops by introgression C4 characteristics into C3 plants by genetic manipulation (Matsuoka et al. 2001). A number of endeavors have been made to overexpress C4-specific photosynthesis enzymes encoding genes such as phosphoenolpyruvate carboxylase (PEPC), pyruvate, phosphate dikinase (PPDK), NADP-malic enzyme (NADP-ME), nicotinamide adenine dinucleotide phosphate-malate dehydrogenase (NADP-MDH) or phosphoenolpyruvate carboxykinase (PCK) into C3 plants using genetic engineering and transgenic technologies for increasing the efficiency of photosynthesis.
In this review, detailed characteristics of biological approaches for CO2 assimilation in C4 plants, types of C4 pathways, and the molecular evolution of C4 photosynthetic genes will be discussed followed by the effect of enhanced CO2 concentration in C4 plants. The primary focus of the review is the overexpression of C4 photosynthetic gene(s) in C3 plants using genetic engineering. The development of C3 plants with C4 features is an ambitious objective, and it would provide great revolutions for the crop improvement to feed a rapidly growing world population. Studies on the molecular characterization of C4 assimilation and its introgression to C3 plants will enhance our perception and provide novel information that can be explored further for developing crop plants with high photosynthesis traits and abiotic stress tolerance.
C4 photosynthesis: an overview
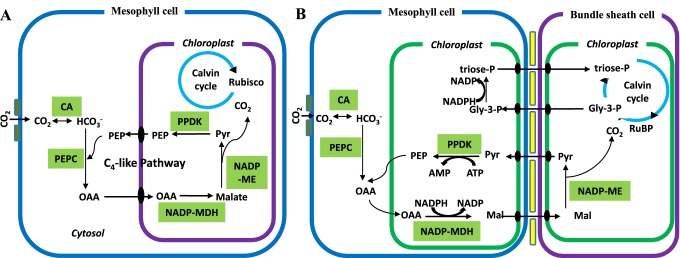
The C4 photosynthetic plants are considered one of the most efficient CO2 sequester, productive and biomass producer on the planet. In the C4 pathway, PEPC acts as an acceptor of atmospheric CO2 in the cytosol of the mesophyll cells (O’Leary 1982), primarily fixes the carbon-di-oxide in the bicarbonate form in mesophylls, and subsequent forms oxaloacetate (OAA) a four-carbon compound, which is converted to malate or aspartate. Aspartate then translocates to the specialized bundle sheath cells where CO2 is decarboxylated by the decarboxylating enzymes (Furbank 2016) and re-released metabolic CO2 re-fixes in the bundle sheath to a three-carbon compound 3-PGA by RuBisCo (Fig. 1) same as C3 (Calvin–Benson) pathway (Hatch 1987). The spatial separation of mesophyll cells and bundle sheath cells has been supposed to be a precondition for an efficient CO2 assimilation mechanism.
Fig. 1.
Schematic diagram of C4 photosynthesis pathway. a Single-cell C4 photosynthesis pathway and b Karnz-anatomy C4 photosynthesis pathway (NADP-ME type). CA carbonic anhydrases; OAA oxaloacetic acid, Mal malate, Pyr pyruvate, Gly-3-P glycerol-3-phosphate
Photorespiration usually occurs in C3 plants, consumes ATP and NADPH, and leads to loss of CO2 from bundle sheath which decreases the efficiency of CO2 sequestration (Leegood et al. 1995). The physical separation between RuBisCO and oxygen-generating light reaction decreases photorespiration and promotes CO2 fixation. In the plant system, C4 photosynthesis acts as a CO2 pump which increases the intracellular availability of CO2 to RuBisCO and thus inhibits the oxygenation reaction (Hatch 1987). This approach avoids significant losses of CO2 by photorespiration, compared to C3 plants.
Most of the C4 plants are grasses, and the photosynthetic reactions are compartmentalized between two morphologically distinct cell types which are arranged in concentric circles veins or wreath-like structure (Nelson and Langdale 1992). This anatomical barrier, in which vascular bundle is surrounded by a thick layer of bundle sheath cells, which in turns surrounded by a thin layer of mesophyll cells is known as the Kranz-anatomy. It has dimorphic chloroplast: chloroplasts present in mesophyll cells are of the typical type whereas chloroplasts, an inhabitant of bundle sheath are without granum (agranulated). About 15 discrete categories of Kranz anatomy have been identified in different monocot and dicot lineages of C4 photosynthesis (Dengler and Nelson 1999). Subsequently, based on the decarboxylating enzymes, C4 photosynthesis has been classified into three subtypes (Table 1; Fig. 2). Generally, during the C4 cycle, CO2 is released either by a chloroplastic NADP-dependent malic enzyme (NADP-ME), mitochondrial NAD-dependent malic enzyme (NAD-ME) or cytosolic phosphoenolpyruvate carboxykinase (PEPCK) (Leegood 2002).
Table 1.
Subtypes of C4 photosynthesis based on decarboxylation mechanism
| Physiological characteristics | Subtypes | References | ||
|---|---|---|---|---|
| NADP-ME | NAD-ME | PEPCK | ||
| Enzyme location | Chloroplast | Mitochondria | Cytosol | Von Caemmerer and Evans (2010), Pick et al. (2011) |
| Decarboxylase | NADP-ME | NAD-ME | PCK | Pick et al. (2011) |
| Evolution | Gene duplication from C3 ancestor | Existing mitochondrial NAD-ME | Maier et al. (2011) | |
| Physiology | Higher photosynthesis and nitrogen use efficiency | Higher nitrogen use efficiency | Enhance plant adaption to various environmental conditions | Pinto et al. (2016), Furbank (2016), Rao and Dixon (2016) |
| C4 acid in bundle sheath cells (decarboxylated acid) | Malate | Aspartate | Aspartate | Pick et al. (2011) |
| C3 acid returning to mesophyll cells | Pyruvate | Alanine | Pyruvate and alanine | Pick et al. (2011) |
| C4 plastid transporter (Pyruvate transport in chloroplasts of mesophyll cells) | Proton: pyruvate co-transporter | Sodium: pyruvate co-transporter | Weber and von Caemmerer (2010), Furumoto et al. (2011) | |
| Examples | Maize, sugarcane, and sorghum | Switchgrass, pearl millet, and amaranth | Chinese rye grass, and seashore dropseed | Edwards and Walker (1983) |
Fig. 2.
Schematic diagram of three subtypes of C4 photosynthesis. a NADP-ME subtype, b NAD-ME subtype, and c PEPCK subtype. Ala-amino alanine aminotransferase, Asp-amino aspartate aminotransferase
Earlier, it was stated that NADP-ME and NAD-ME subtypes are distinct biochemical pathways, work further co-operation of the PEPCK pathway. Many crop plants like maize, sugarcane, sorghum, and other biofuel crops belong to NADP-ME subtype, and switch-grass, pearl millet, Amaranthaceae, Bristlegrasses and Portulaca oleracea belong to NAD-ME subtype (Edwards and Walker 1983). The NADP-ME and NAD-ME represent almost equal numbers of genera in the dicots, but NADP-ME dominates in monocot families (Sage et al. 2012). Most of the C4 grasses have shown that the NADP-ME subtypes present predominantly in annual precipitation area whereas the NAD-ME subtype presents more in arid regions (Hattersley 1992). Three main paradigms have been suggested to describe the divergence of the evolutionary transformation between NADP-ME and NAD-ME from C4 subtypes: the first one suggested that NAD-ME is the primary subtype of the ancestral C4 type and NADP-ME was evolved from it (Washburn et al. 2015). The second model proposed that the NADP-ME and NAD-ME subtypes share some level of a C4 ancestor after that predominated individually in different lineages. However, the third model suggested that both, NADP-ME, and NAD-ME subtypes evolved individually from C3–C4 intermediate and considered the most recent common ancestor (Washburn et al. 2015).
It is well established that CO2 assimilation rate of the C4 plant is much higher than C3 plants along with water and nitrogen-use efficiencies (Ghannoum et al. 2010). However, the nitrogen content per unit leaf area is found lower in C4 compared to C3 plants, but the concentration of nitrogen per unit dry weight is almost similar. Both, C3 and C4 plants invest the same fraction of leaf nitrogen into photosynthetic components, C4 plants dispense low nitrogen to RuBisCO and more to thylakoids components. In C3 plants, about 30% of leaf nitrogen supplies to RuBisCO compared to 4–21% leaf-nitrogen distribution in C4 plants (Sage et al. 1987). Consequentially, the C4 photosynthesis required less nitrogen for CO2 assimilation process, which significantly increases the metabolic CO2 concentration in the chloroplast because of low gas permeability of bundle-sheath cells, followed by suppression of RuBisCO oxygenase activity and almost eliminating photorespiration. The advantages of higher water- and nitrogen-use-efficiency of C4 compared to C3 photosynthesis is fully appreciated at high temperature and light, where oxygenase reaction increases. At higher temperature, C4 photosynthesis is more efficient than C3. Therefore the worldwide distribution of C4 grasses is positively correlated with the temperate growing season. Although some C4 plants show cold adaptation, they still require warm daytime to exist in a cold environment. Thus, C4 species are poorly distributed in cold climates compared to C3 plants (Sage and McKown 2006). Overall, the current hypothesis is that at a higher temperature, C4 photosynthesis is more efficient than C3 photosynthesis.
Most C4 photosynthesis plant has Kranz-anatomy in which mesophyll and bundle-sheath cells, provides the spatial separation of the two carboxylase reactions, which is crucial for the terrestrial C4 photosynthesis. Eventually discovery of some terrestrial species within the Chenopodiaceae (now Amaranthaceae) family; Suaeda aralocaspica, Suaeda monoica and Bienertia sinuspersici, where C4 photosynthesis operates within an individual cell, has changed the established paradigm that the Kranz-anatomy is essential for terrestrial C4 carbon assimilation (Voznesenskaya et al. 2001). C4 photosynthesis was determined by using gas exchange studies and carbon isotope perception of a single cell and Kranz-anatomy of C4 plants (Leegood 2002). Further, it concluded that both types of anatomy showed similar CO2 assimilation rate and same values of leakiness at high light (King et al. 2012). Nevertheless, an efficiency of single cell C4 photosynthesis remains in question because they lack the cell wall and plasma membrane compartment for CO2 diffusion which is found in Kranz-anatomy of C4 plants. A comparative study of the photosynthesis efficiency of single-cell C4 species (B. sinuspersici and S. aralocaspica) and a Kranz type C4 species (S. eltonica) revealed that both (single-cell and Kranz type) C4 plants have similar photosynthesis capacity (King et al. 2012). Single cell type of C4 photosynthesis was also discovered in some aquatic plants Sagittaria subulata (Alismataceae), green algae, diatoms, and aquatic monocots (Hydrocharitaceae: Egeria densa and Hydrilla verticillata; Von Caemmerer et al. 2014; Reinfelder et al. 2000; Johnston et al. 2001). These terrestrial and aquatic single-celled systems, exhibit the remarkable plasticity inherent in the plant cell, they provide the exquisite control of subcellular mechanism that could be applied to achieve very complex biochemical features. The discovery of single-cell C4 photosynthesis, would be an alternative approach to engineering C3 plants to execute Kranz-type C4 photosynthesis without having a dual-cell system.
Furthermore, to concentrate CO2, C4 photosynthesis required at least two (NADP-ME and NAD-ME type) or one (PEPCK type) extra ATP molecules per CO2 fixation as compared to C3 photosynthesis without the need of additional reduction equivalents. Consequently, the demands of ATP is relative to NADPH in C4 photosynthesis are higher than in C3 photosynthesis, because C3 plants photosynthesis requires both ATP and NADPH (Edwards and Walker 1983). However, in C4 plants, increase in ATP to NADPH ratio is compensated by improved cyclic electron flow nearby PSI, which delivers additional ATP without concurrently generating NADPH (Ishikawa et al. 2016).
Evolution of C4 photosynthetic genes
The evolution of C4 photosynthesis has been achieved through some divergences of pre-existing C4 photosynthesis and represented by about 60 individual origins from 19 families of angiosperms (Bräutigam et al. 2017). A significant step of photosynthesis evolution is the up-regulation of a C4 metabolic cycle, but the precise reason is remains unclear due to unavailability of methods for measuring in situ C4 cycles in C3–C4 intermediates species (Sage 2016). Environmental obligations influenced C4 evolution which led by several alterations of the anatomic, biochemical and genetic pre-conditions. It is hypothesized that the C4 plants evolved under high photorespiration rates, low atmospheric CO2 concentration and limited water supply in a warm environment (Ehleringer and Monson 1993). However, C4 photosynthesis described as an adaptation to hot and dry environments. A combination of high salinity, drought, and temperature (but low humidity) promotes photorespiration, and CO2 deficiency (Ehleringer and Monson 1993). The high efficiency of C4 photosynthesis under low atmospheric CO2, high heat, drought, and saline environment suggested that abiotic stress conditions were the essential driving force of C4 evolution.
The pre-existing analogous genes of ancestral C3 photosynthesis may evolve the C4 photosynthesis genes and modified the expression level to control the kinetic properties of respective enzymes. Functions of C4 photosynthetic genes/enzymes are found exclusive to C4 plants, they may also present in C3 plants, but activities of the corresponding enzymes are almost undetectable (Hatch 1987). C4 photosynthetic genes and their homologs from C3 plants are considered as C4-specific and C4-like genes, respectively (Miyao 2003). Besides these (C4-like or C4-specific) genes, both C3 and C4 plants possessed other homologs with a housekeeping function and designated as C3-like genes. The isoform of C4 photosynthetic genes may also originate from a pre-existing, non-photosynthetic gene of C3 ancestral species.
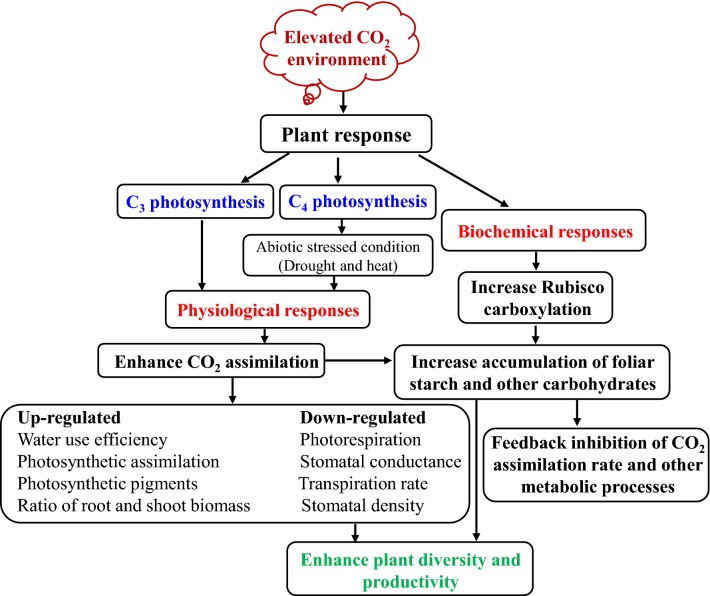
Effect of elevated CO2 on C4 photosynthesis
The elevated CO2 concentration in the atmosphere has both positive and negative impacts and, the study of the plant response to predicted climatic changes is of the immense importance (Fig. 3). Elevated CO2 was demonstrated to mitigate the detrimental effects of abiotic stress conditions on plants (AbdElgawad et al. 2015). Plant-response to elevated CO2 depends on the metabolic stages of a plant and response may vary species to species or plant to plants. Furthermore, C3 and C4 plants behave differently under the elevated CO2 environment (Srinivasarao et al. 2016). The ratio of oxygenation and carboxylation of RuBP depends on the relative concentration of CO2 and O2 at the site of RuBisCO, and a high CO2 increases carbon assimilation by reducing photorespiration. Earlier, it was proven that doubling of current atmospheric CO2 concentration will induce the C4 plant growth by 10–20% whereas about 40–50% of C3 plants (Bowes 1993). C3 plants have potential to regulate the CO2 pump during carbon assimilation under an elevated CO2 condition, and therefore, respond positively due to the substrate specificity of RuBisCO in current atmospheric CO2 concentration (Leakey et al. 2009).
Fig. 3.
Physiological and biochemical response of plants under an elevated CO2 environment condition
C4 photosynthesis is considered near-saturated at current atmospheric CO2, and a further increase of CO2 may not be productive. However, some C4 plants showed positive growth response under elevated CO2 condition (Yadav et al. 2018). It was found that some photosynthetic enzymes, RuBisCO, PPDK and NADP-MDH activities were up-regulated in sugarcane under the elevated CO2 plants, whereas PEPC and NADP-ME enzyme activities were unaffected. Elevated CO2 up-regulated the essential enzymes of photosynthetic and sucrose metabolism, reduced stomatal conductance and transpiration, and finally improved leaf water use efficiency with better plant water status (Jiang et al. 2016). Elevated CO2 also significantly mitigates the negative impact of drought and heat condition such as photosynthesis inhibition, biomass reduction, H2O2 production, chlorophyll fluorescence declination, and protein oxidation (AbdElgawad et al. 2015). Similarly, elevated CO2 may alleviate the impairment of oxidative stress from various abiotic stresses including drought, heat, salinity, and ozone, by amelioration the antioxidant defenses systems of enzymatic and non-enzymatic, including ascorbate peroxidase (APX), superoxide dismutase (SOD), peroxidase (POX), glutathione reductase (GR), glutathione peroxidase (GPX), catalase (CAT), dehydroascorbate reductase (DHAR), and non-enzymatic compounds such as glutathione (GSH), ascorbate (ASC), alkaloids and phenolic compounds (Gill and Tuteja 2010).
Elevated CO2 may also enhance plant diversity and productivity by decreasing stomatal conductance, transpiration rate, stomatal density and consequently increasing water use efficiency, photosynthetic pigments, ratio of root/shoot biomass and soil water availability (Jiang et al. 2016). A comparative study, performed with C3 (sunflower) and C4 (maize) crops under elevated CO2 and different stress conditions revealed interesting results (Vanaja et al. 2011). Under elevated CO2 condition, sunflower (a C3 plant) showed enhanced growth under both normal and drought stress conditions, while maize (a C4 plant) showed better growth response under drought stress condition only. The stomatal conductance, leaf water potential and transpiration decrease in both (C3 and C4) crops under drought stress. However, an ameliorative effect was noticed in elevated CO2 condition which promotes higher photosynthesis in sunflower under drought stress treatment compared to drought stress in control (normal CO2) condition. Similarly, a comparative study of two different kinds of grass and legumes exposed to drought, elevated temperature and elevated CO2 showed that drought inhibits plant growth, photosynthesis assimilation rate, stomatal conductance, but induces osmolytes followed by antioxidants in all species, whereas elevated CO2 ameliorated the detrimental effects of stresses (AbdElgawad et al. 2015). The enhanced CO2 concentration improved salt resistant in C3 species Chenopodium quinoa as well as in the C4 species Atriplex nummularia (Geissler et al. 2015). Overall, it is hypothesized that C3 plants perform better and produce more biomass (Hussin et al. 2017), than C4 plants under elevated CO2 stress condition. However, under the same elevated CO2 condition, an increase in the growth, biomass, and yield of C4 plants was found under drought stress conditions because of higher carbon assimilation and improved photosynthesis efficiency.
Overexpression of C4 photosynthetic genes
The productivity and biomass yield of crop plants are significantly correlated with the carbon assimilation of a particular plant and about 90–95% of dry biomass from plant photosynthesis. However, many researches confirmed that the high photosynthesis is essential for high biomass production. As already discussed, C4 plants thoroughly reduce the deleterious effects of photorespiration by concentrating CO2 in vicinity of RuBisCO of a chloroplast. However, bundle-sheath cells act as a barrier or protecting layer to avoid the leakage of CO2, which leads to improvements in radiation use efficiency and productivity of plants (Mitchell and Sheehy 2006). Based on the advantages of C4 carbon assimilation over the limited factors of C3 plants, introgression of C4 photosynthetic enzymes in C3 plants are a reliable, cost-effective and prospective approach to enhance the productivity and biomass to improve the photosynthesis of C3 plants and also biological CO2 sequestration (Edwards et al. 2001; Leegood 2002). The C4 photosynthetic genes encoding for enzymes PEPC, PPDK, or NADP-ME were overexpressed in tobacco (Laporte et al. 2002; Müller et al. 2018), potato (Ishimaru et al. 1998; Häusler et al. 2001), rice (Taniguchi et al. 2008; Gu et al. 2013; Shen et al. 2015), wheat (Kershanskaya and Teixeira da Silva 2010; Peng et al. 2018;) and Arabidopsis (Fahnenstich et al. 2007; Kandoi et al. 2016).
Overexpression of C4 PEPC enzyme-encoding gene in C3 plants
Phosphoenolpyruvate carboxylase (PEPC: EC 4.1.1.31) catalyzes the primary atmospheric CO2 fixation step by C4 carbon assimilation. Besides, its non-photosynthetic isoforms are also involved in carbon–nitrogen interactions, seed germination, and maturation, stomatal opening and regulation of pH in cytosol, additionally play an important role in stress tolerance (Cheng et al. 2016). It is well known that the PEPC gene up-regulated under drought and salinity stress conditions in C3 and C4 plants (Zhou et al. 2011; Kandoi et al. 2016). Overexpression of C4PEPC gene in C3 plants showed an improve photosynthesis and also enhance drought stress tolerance (Zhou et al. 2011) (Table 2). Overexpression of the PEPC gene, isolated from maize and transformed to a model plant tobacco showed about twofold higher PEPC activity in leaves in transgenic lines compared to wild-type, without any significant deviation in the photosynthetic rate of transgenic plants (Hudspeth et al. 1992). However, overexpressing the maize C4PEPC transgenic rice exhibited reduced O2 inhibition of photosynthesis and photosynthetic rate comparable to wild-type (Ku et al. 2000). Similarly, an increase of the PEPC enzyme activity and the photosynthetic rate was detected under high temperature in rice plant by improving stomatal conductance and elevated internal CO2 concentration (Suzuki et al. 2006; Bandyopadhyay et al. 2007). Similar results were observed with transgenic wheat and rice overexpressing maize and sugarcane C4-specific PEPC gene, respectively (Lian et al. 2014; Shen et al. 2015). The overexpression of Zea mays PEPC gene in Arabidopsis thaliana, and wheat plants showed high PEPC activity and improved photosynthesis, growth, and yield under salinity and low nitrogen conditions by modulating carbon metabolism and nitrogen assimilation (Kandoi et al. 2016; Peng et al. 2018). Earlier, it was noticed that overexpression of maize PEPC gene in rice reduces the carbon assimilation rate (7.3%) but improves photorespiration in transgenic plants (Fukayama et al. 2003).
Table 2.
Overexpression of C4 phosphoenolpyruvate carboxylase enzyme encoding PEPC gene in C3 plants
| Host plant (C4) | Recipient plant (C3) | Results | References |
|---|---|---|---|
| Maize | Tobacco | Twofold higher PEPC activity but no physiological changes in the photosynthesis rate or CO2 assimilation | Hudspeth et al. (1992) |
| Maize | Rice | Two–three-fold higher PEPC activity under CO2 atmospheric condition | Ku et al. (2000) |
| Maize | Rice | Two–three-fold higher PEPC activity in the leaves of some transgenic lines | Agarie et al. (2002) |
| Maize | Rice | Slightly lower CO2 assimilation rate (7.3%) | Fukayama et al. (2003) |
| Maize | Rice | 14–60 fold higher PEPC activity | Suzuki et al. (2006) |
| Maize | Indica rice | Enhanced photosynthesis under high-temperature | Bandyopadhyay et al. (2007) |
| Maize | Rice | Higher (22%) grain production | Jiao (2008) |
| Maize | Wheat | Higher (25–50%) grain yield | Kershanskaya and Teixeira da Silva (2010) |
| Maize | Rice | Enhanced drought tolerance | Zhou et al. (2011) |
| Sugarcane | Indica rice | Higher grain filling and total grain numbers but the grain filling percent and 1000-grain weights remained unchanged | Lian et al. (2014) |
| Maize | Rice | Improved drought tolerance | Shen et al. (2015) |
| Maize | Arabidopsis | About Seven–ten-fold increase in PEPC activity, higher chlorophyll content, enhanced electron transport rate (ETR), lower nonphotochemical quenching of chlorophyll a fluorescence, and a higher performance index | Kandoi et al. (2016) |
| Maize | Rice | About 12% increase in grain yield | Sen et al. (2017) |
| Maize | Wheat | Enhanced growth, and yield under low N condition | Peng et al. (2018) |
Some researchers demonstrated that the overexpression of a single C4 photosynthesis enzyme can significantly change the carbon assimilation in C3 plants. However, researcher also overexpressed multiple C4 photosynthesis enzymes, including combinations of NADP-ME and PEPC in C3 plants for enhanced photosynthesis (Häusler et al. 2001) as well as PEPC and PPDK (Matsuoka et al. 2000; Jiao 2008). Four enzymes; maize C4-specific PPDK and PEPC, rice C3-like NADP-ME and sorghum C4-specific NADP-MDH were overexpressed in the mesophyll cells of rice plants individually or in combination (Taniguchi et al. 2008). However, individual overexpression of C4-specific genes did not alter the CO2 assimilation.
Overexpression of C4 PPDK enzyme-encoding gene in C3 plants
PPDK (EC 2.7.9.1) is one of the rate-limiting enzyme of C4 photosynthesis, which is abundantly present in the chloroplast of mesophyll cells (Hatch 1987). It catalyzes the reversible Pi- and ATP-dependent interconversion of pyruvate to PEP (phosphoenolpyruvate), the first acceptor of atmospheric CO2 in C4 plants. PPDK enzyme is also used by some bacteria and unicellular parasitic protists, while they are absent in insects or vertebrates and also a remarkable objective for the antiparasitic and antimicrobial drug besides C4-specific herbicides (Minges and Groth 2017). PPDK enzyme could also be involved in some abiotic stress caused by drought (Wang et al. 2018) and biotic stress induced by viral infection (Lu et al. 2019). It is already described that the relations of PPDK to stress, in stressed environment like abscisic acid, drought stress caused by polyethylene glycol, salts, submergence, low-oxygen, and cold stress, evidently induce the activity of PPDK enzyme (Wang and Li 2008). PPDK enzyme-encoding gene from C4 plants has been successfully introduced and expressed in C3 plants (Table 3). Transgenic potato lines overexpressing ZmPPDK showed higher enzyme activity, but negligible effects were noticed in photosynthetic characteristics compared to control plants (Ishimaru et al. 1998). Similarly, Echinochloa PPDK gene, introduced in H65 rice plant, showed higher enzyme activity without any changes in carbon assimilation (Wang and Li 2008). Whereas, overexpression of maize chloroplastic PPDK gene showed high photosynthesis in transgenic rice than wild-type plants due to an elevated internal CO2 concentration and improved stomatal conductance (Ku et al. 2000; Gu et al. 2013). Higher photosynthesis was also noticed in a model plant Arabidopsis thaliana overexpressing C4-PPDK gene (Wang et al. 2012).
Table 3.
Overexpression of C4 pyruvate phosphate dikinase enzyme encoding PPDK gene in C3 plants
| Host plant (C4) | Recipient plant (C3) | Results | References |
|---|---|---|---|
| Maize | Potato | Four–five-fold higher enzyme activity | Ishimaru et al. (1998) |
| Mesembryanthemum | Tobacco | Higher seed production per capsule | Sheriff et al. (1998) |
| Maize | Rice | Higher photosynthetic rates (up to 35%) | Ku et al. (2000) |
| Maize | Rice | 40-fold higher PPDK activity in leaves | Fukayama et al. (2001) |
| Maize | Rice | High nitrogen content in leaves | Zhang et al. (2007) |
| Echinochloa | Rice | Higher enzyme activity | Wang and Li (2008) |
| Maize | Arabidopsis | Higher photosynthesis rate (123%) | Wang et al. (2012) |
| Maize | Rice | Higher photosynthesis with enhanced drought tolerance | Gu et al. (2013) |
| Maize | Rice | Higher grain yield (12%), increased leaf blade size, root biomass, and plant height with anatomical changes such as greater leaf vein number, bundle sheath cells, and bulliform cells | Sen et al. (2017) |
Overexpression of C4 NADP-ME enzyme-encoding gene in C3 plants
A subtype of C4 plants harbors NADP-ME in the chloroplast of bundle sheath cells (Furbank 2016). During the decarboxylation, NADP-ME helps to enrich the CO2 around RuBisCO and slow down photorespiration to improve the net photosynthesis. The activity of NADP-ME showed contrary correlated with the photorespiration and also regulate stomatal opening (Müller et al. 2018). However, introgression of NADP-ME in C3 plants might be lowered photorespiration and improved CO2 assimilation in C3 plants (Table 4). Overexpression of NADP-ME gene cloned from sorghum in rice showed elevated activity about 1–7 folds. Though high photoinhibition was observed in transgenic rice under high light intensity but no significant change was observed in the carbon assimilation rate (Chi et al. 2004). Similarly, maize NADP-ME gene, overexpressed in transgenic tobacco lines, showed abnormal stomatal behavior due to alteration of malate metabolism in guard cells (Laporte et al. 2002). Some negative results such as immature chlorophyll and abnormal chloroplast with agranal thylakoid membranes were observed in transgenic rice lines harboring maize NADP-ME (Takeuchi et al. 2000). Overexpression of ZmNADP-ME, transformed to the guard and vascular companion cells of the tobacco plant, led to enhance net carbon assimilation rate, higher biomass, more water use efficiency, earlier flowering, and shorter life cycle (Müller et al. 2018).
Table 4.
Overexpression of C4 NADP-ME enzyme encoding gene in C3 plants
| Host plant (C4) | Recipient plant (C3) | Results | References |
|---|---|---|---|
| Maize | Rice | 20–70 fold higher activity causing aberrant chloroplast with agranal thylakoid membranes | Takeuchi et al. (2000) |
| Flaveria | Potato | No changes in enzyme activity | Häusler et al. (2001) |
| Maize | Rice | Enhanced photoinhibition, led to bleaching of leaf color and retarded growth under natural light | Tsuchida et al. (2001) |
| Maize | Tobacco | Altered stomatal behavior and water relations | Laporte et al. (2002) |
| Sorghum | Rice | No significant changes in carbon assimilation, though photoinhibition increased under high light intensity | Chi et al. (2004) |
| Maize | Arabidopsis | Did not show any differences in morphology and development when grown in long days. However, dark-induced senescence progressed more rapidly in transgenic plants | Fahnenstich et al. (2007) |
| Maize | Tobacco | Increased CO2 fixation, enhanced water use efficiency, earlier flowering and shorter life cycle | Müller et al. (2018) |
Overexpressing C4 photosynthetic gene(s) improves abiotic stress tolerance
Photosynthesis is a metabolic process which is highly susceptible to environmental stresses. Overexpression of an individual gene encoding C4 photosynthetic enzyme could modify the carbon assimilation in C3 plants but up to an extent, therefore, a number of genes encoding different C4 pathway enzymes were attempted towards improvement of net photosynthetic efficiency along with abiotic stress tolerance (Matsuoka et al. 2001; Häusler et al. 2001; Gu et al. 2013; Qi et al. 2017). It is a well-known fact that adverse climatic conditions (which will increase soon) including high-temperature, drought, salinity, and cold affect the crop productivity. There is an imperative need to improve plant defense system to combat with abiotic stresses, and improved photosynthesis may serve the purpose, additionally will lead to CO2 assimilation and production of higher biomass.
Many C3 crops such as wheat have lower photosynthetic efficiency at the optimal growth temperature, whereas C4 crop such as sorghum and maize have higher biomass production, greater photosynthesis and improved abiotic stress tolerance compared to C3 plants (Shen et al. 2015). The maize PEPC gene transformed into transgenic wheat conferred drought tolerance and increase grain yield. Lines with improved drought tolerance showed extensive root system as well as higher photosynthetic capability during stress compared to control (wild-type) plants and control (unstressed) condition (Qin et al. 2016). Transgenic wheat overexpressing ZmPEPC gene showed significantly higher photosynthetic rate under high temperature (Qi et al. 2017). Transformation of gene encoding C4 PEPC enzyme is considered a promising strategy for improving rice crop production under drought stress (Zhou et al. 2011; Zai-Song et al. 2012; Gu et al. 2013; Shen et al. 2015). These study suggested that the overexpression of PEPC enzyme not only improve the photosynthetic rate but also enhanced involved in drought resistance in transgenic plants (Zhou et al. 2011). Under drought stress condition, overexpressed PEPC gene in transgenic rice did not show any change under high PAR higher than 1200 μmol m−2 s−1, while, untransformed wild-type rice plant showed a dramatic decrease in the net photosynthetic rate (Zai-Song et al. 2012).
Challenges and limitations
There are lots of inconsistencies in the performance of the transgenic C3 plants expressing C4 enzymes. One of the enormous challenges is the complexity of C4 photosynthesis pathway and some inherent incompatibility between C3 species and C4 photosynthesis. Although, factors including, phylogenetic distance (Häusler et al. 2002), gene silencing (Chandler and Vaucheret 2001), splicing (Goodall and Filipowicz 1991), integration pattern (Gelvin 1998), rearrangement of transgene during transformation protocol (Karami 2008) which create inconsistency in the performance and efficacy of overexpressed enzyme. Inappropriate initiation and termination of transcription, along with incorrect splicing can also take place when enzymes from monocots are overexpressed into dicots (Goodall and Filipowicz 1991). A similar observation was also seen when the C4-specific PEPC gene from maize was not significantly expressed in leaves of tobacco, because of incorrect transcription initiation (Hudspeth et al. 1992). Matsuoka et al. 2001, also suggested that the introgression of a recombinant gene comprising of cDNA for a C4 gene, attached to an active promoter alone or with enhancer sequences, could enhance the activity of C4 enzymes in C3 plants.
Conclusion and prospects
Global warming is one of the primary constraints for the production of biomass and yield of a plant. Researchers have been concerned about improving the productivity and quality of crop plants. C4 plants contain lower photorespiration, higher water, and N assimilation efficiencies compared to C3 plants. Different C4 pathway enzymes encoding genes were overexpressed into C3 plants (Arabidopsis, tobacco, rice, and potato) to improve the carbon assimilation. RuBisCO enzyme is ubiquitous, and it will not be limiting in future climatic conditions in C3 plants, but other photosynthetic enzymes are prone to restrict photosynthesis performance under adverse conditions. Simultaneously, C4 plants are insensitive to enhance CO2 condition, although, enzyme activity will affect only in a stressed condition. In addition, the C4 photosynthetic genes not only play an important role in carbon assimilation but also induce drought stress tolerance. C4 photosynthetic enzymes are of researcher’s choice for improving the carbon assimilation and stress tolerance in C3 plants through genetic transformation. Although, this approach is reasonable regarding output because these C4 enzymes are also expressed a very low level in leaves and non-photosynthetic tissues of C3 plants. Furthermore, overexpression of some single-cell C4 pathway has the potential to support C3 photosynthesis under drought stress. In C4 photosynthesis, PEPC, PPDK, and NADP-ME enzymes are essential for carbon assimilation, and their non-photosynthetic isoforms play an important role in carbon fixing, nitrogen assimilation and pH regulation under various abiotic stress conditions. However, the influence of this employment on the photosynthesis and biomass production of the plants in elevated CO2 stress condition has not yet been tested.
Overexpression of C4 photosynthesis genes in C3 plants approaches has intended at improving the highest potential activity of the particular enzymes in C3 plants. However, it is also obligatory to select many transgenic lines to acquire the preferred expression level of C4 enzyme and its position in C3 plants. In addition, it is also necessary to engineer C4 photosynthetic enzymes with respective promoters to be restored acclimatized in a specific environment of C3 plants to carry out C4 mechanism. Thus, it is further investigated that the overexpression of individual C4 gene encoding enzyme not sufficient or more than one genes are required to achieve an overall improvement in photosynthesis and yield of C3 plants. Therefore, some previous results indicated that a single C4 enzyme did not increase photosynthesis in rice, by inhibited photosynthesis through reduced Rubisco activity and stimulation of respiration in the light. In addition, it is crucial for manipulations of carbon assimilation in C3 plants to find out an alternative approach to reduce photorespiration and comprehend the complexity of evolutionary anatomical development of different cell types in C4 plants. It is obvious that engineering of C4 requires introgression of additional genes in C3 plants (Yadav and Mishra 2019). It is also hypothesised that some genes that suppress C4 cycle should be removed from the recipient (C3) plant. Genome editing by CRISPR/Cas9 would be a very useful technology to knock-out such repressing C3 genes (Schuler et al. 2016; Patel and Mishra 2019).
Furthermore, for the improvement of carbon assimilation by photosynthesis and abiotic stress tolerance in C3 plants, scientists have also explored halophytes for arid, semi-arid and marginal lands where other plants are not able to survive. Although, they also provide better nutritional quality food and biofuel, having good water saver, reduce CO2 emission with higher photosynthetic rate and biomass producer. Halophytes were explored comprehensively for the genes (Jha et al. 2011; Chaturvedi et al. 2012, 2014; Joshi et al. 2013; Singh et al. 2014a; Udawat et al. 2014, 2016; Tiwari et al. 2015) and promoters (Tiwari et al. 2014, 2016, 2019; Singh et al. 2016) that enhanced photosynthesis (Patel et al. 2015; Jha et al 2019) and also provide abiotic stress tolerance (Singh et al. 2014b; Pandey et al. 2016; Udawat et al. 2017; Mishra and Tanna 2017). Many significant abiotic stress tolerant plants with prospective interest for agriculture and environmental administration have already been identified, such as Suaeda monoica (Yadav et al. 2018), Atriplex halimus (Lutts et al. 2004), and Salicornia bigelovii (Zerai et al. 2010). However, most of the halophytes plants have not been identified nor compared to conventional crop with known genetic information for genetic transformation. In future, halophytes would be used as an alternative approach to improvement of carbon assimilation and stress tolerance of C3 crop plants using genetic engineering.
Acknowledgements
CSIR-CSMCRI Communication No.: PRIS-170/2018. CSIR-Young Scientist (YSP-02/2016-17) and SERB-DST (EMR/2016/000538) projects are thankfully acknowledged.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AbdElgawad H, Farfan-Vignolo ER, De Vos D, Asard H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015;231:1–10. doi: 10.1016/j.plantsci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Agarie S, Miura A, Sumikura R, Tsukamoto S, Nose A, Arima S, Miyao-Tokutomi M. Overexpression of C4 PEPC caused O2-insensitive photosynthesis in transgenic rice plants. Plant Sci. 2002;162(2):257–265. [Google Scholar]
- Bandyopadhyay A, Datta K, Zhang J, Yang W, Raychaudhuri S, Miyao M, Datta SK. Enhanced photosynthesis rate in genetically engineered indica rice expressing PEPC gene cloned from maize. Plant Sci. 2007;172(6):1204–1209. [Google Scholar]
- Bowes G. Facing the inevitable: plants and increasing atmospheric CO2. Annu Rev Plant Biol. 1993;44:309–332. [Google Scholar]
- Bräutigam A, Schlüter U, Eisenhut M, Gowik U. On the evolutionary origin of CAM photosynthesis. Plant Physiol. 2017;174(2):473–477. doi: 10.1104/pp.17.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler VL, Vaucheret H. Gene activation and gene silencing. Plant Physiol. 2001;125:145–148. doi: 10.1104/pp.125.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Mishra A, Tiwari V, Jha B. Cloning and transcript analysis of type 2 metallothionein gene (SbMT-2) from extreme halophyte Salicornia brachiata and its heterologous expression in E. coli. Gene. 2012;499:280–287. doi: 10.1016/j.gene.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Patel MK, Mishra A, Tiwari V, Jha B. The SbMT-2 gene from a halophyte confers abiotic stress tolerance and modulates ROS scavenging in transgenic tobacco. PLoS ONE. 2014;9:e111379. doi: 10.1371/journal.pone.0111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Wang L, Lan H. Cloning of PEPC-1 from a C4 halophyte Suaeda aralocaspica without Kranz anatomy and its recombinant enzymatic activity in responses to abiotic stresses. Enzym Microb Technol. 2016;83:57–67. doi: 10.1016/j.enzmictec.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Chi W, Zhou JS, Zhang F, Wu NH. Photosynthetic features of transgenic rice expressing sorghum C4 type NADP-ME. Acta Bot Sin. 2004;46(7):873–882. [Google Scholar]
- Dengler NG, Nelson T (1999) Leaf structure and development in C4 plants. In: Sage RF, Monson RK (eds) C4 plant biology. San Diego, pp 133–172
- Edwards G, Walker D. C3, C4: mechanisms, cellular and environmental regulation, of photosynthesis. Berkeley: University of California Press; 1983. [Google Scholar]
- Edwards GE, Furbank RT, Hatch MD, Osmond CB. What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol. 2001;125(1):46–49. doi: 10.1104/pp.125.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Evol Syst. 1993;24(1):411–439. [Google Scholar]
- Fahnenstich H, Saigo M, Niessen M, Zanor MI, Andreo CS, Fernie AR, Maurino VG. Alteration of organic acid metabolism in Arabidopsis overexpressing the maize C4 NADP-malic enzyme causes accelerated senescence during extended darkness. Plant Physiol. 2007;145(3):640–652. doi: 10.1104/pp.107.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama H, Tsuchida H, Agarie S, Nomura M, Onodera H, Ono K, Makino A. Significant accumulation of C4-specific pyruvate, orthophosphate dikinase in a C3 plant, rice. Plant Pathol. 2001;127(3):1136–1146. [PMC free article] [PubMed] [Google Scholar]
- Fukayama H, Hatch MD, Tamai T, Tsuchida H, Sudoh S, Furbank RT, Miyao M. Activity regulation and physiological impacts of maize C4-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants. Photosynth Res. 2003;77(2–3):227–239. doi: 10.1023/A:1025861431886. [DOI] [PubMed] [Google Scholar]
- Furbank RT. Walking the C4 pathway: past, present, and future. J Exp Bot. 2016;67(14):4057–4066. doi: 10.1093/jxb/erw161. [DOI] [PubMed] [Google Scholar]
- Furumoto T, Yamaguchi T, Ohshima-Ichie Y, Nakamura M, Tsuchida-Iwata Y, Shimamura M, Bräutigam A. A plastidial sodium-dependent pyruvate transporter. Nature. 2011;476(7361):472. doi: 10.1038/nature10250. [DOI] [PubMed] [Google Scholar]
- Geissler N, Hussin S, Mervat MM, El-Far Koyro HW. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ Exp Bot. 2015;118:67–77. [Google Scholar]
- Gelvin SB. The introduction and expression of transgenes in plants. Curr Opin Biotechnol. 1998;9:227–232. doi: 10.1016/s0958-1669(98)80120-1. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, Conroy JP, Tissue DT. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric (CO2) and temperature. Plant Cell Environ. 2010;33(10):1671–1681. doi: 10.1111/j.1365-3040.2010.02172.x. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Goodall GJ, Filipowicz W. Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot and dicot plants. EMBO J. 1991;10:2625–2644. doi: 10.1002/j.1460-2075.1991.tb07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JF, Qiu M, Yang JC. Enhanced tolerance to drought in transgenic rice plants overexpressing C4 photosynthesis enzymes. Crop J. 2013;1(2):105–114. [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique elend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta Bioenerg. 1987;895(2):81–106. [Google Scholar]
- Hattersley PW. C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In: Chapman GP, editor. Desertified grasslands: their biology and management. Linnean Society Symposium Series. London: Academic Press; 1992. pp. 181–212. [Google Scholar]
- Häusler RE, Rademacher T, Li J, Lipka V, Fischer KL, Schubert S, Hirsch HJ. Single and double overexpression of C4-cycle genes had differential effects on the pattern of endogenous enzymes, attenuation of photorespiration and on contents of UV protectants in transgenic potato and tobacco plants. J Exp Bot. 2001;52(362):1785–1803. doi: 10.1093/jexbot/52.362.1785. [DOI] [PubMed] [Google Scholar]
- Häusler RE, Hirsch HJ, Kreuzaler F, Peterhänsel C. Overexpression of C4-cycle enzymes in transgenic C3 plants: a biotechnological approach to improve C3-photosynthesis. J Exp Bot. 2002;53:591–607. doi: 10.1093/jexbot/53.369.591. [DOI] [PubMed] [Google Scholar]
- Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MS. Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco: effects on biochemistry and physiology. Plant Physiol. 1992;98(2):458–464. doi: 10.1104/pp.98.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussin S, Geissler N, El-Far MM, Koyro HW. Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol Biochem. 2017;118:178–186. doi: 10.1016/j.plaphy.2017.06.017. [DOI] [PubMed] [Google Scholar]
- IPCC. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Midgley PM. Ssummary for policymakers in climate change: the physical science basis, contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2013. p. 1535. [Google Scholar]
- Ishikawa N, Takabayashi A, Noguchi K, Tazoe Y, Yamamoto H, von Caemmerer S, Endo T. NDH-mediated cyclic electron flow around photosystem I is crucial for C4 photosynthesis. Plant Cell Physiol. 2016;57(10):2020–2028. doi: 10.1093/pcp/pcw127. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Ohkawa Y, Ishige T, Tobias DJ, Ohsugi R. Elevated pyruvate, orthophosphate dikinase (PPDK) activity alters carbon metabolism in C3 transgenic potatoes with a C4 maize PPDK gene. Physiol Plant. 1998;103(3):340–346. [Google Scholar]
- Jha B, Sharma A, Mishra A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol Biol Rep. 2011;38:4823–4832. doi: 10.1007/s11033-010-0625-x. [DOI] [PubMed] [Google Scholar]
- Jha RK, Patel J, Mishra A, Jha B. Introgression of halophytic salt stress-responsive genes for developing stress tolerance in crop plants. In: Hasanuzzaman M, Shabala S, Fujita M, editors. Halophytes and climate change: adaptive mechanisms and potential uses. Wallingford: CABI; 2019. pp. 288–299. [Google Scholar]
- Jiang Y, Xu Z, Zhou G, Liu T. Elevated CO2 can modify the response to a water status gradient in a steppe grass: from cell organelles to photosynthetic capacity to plant growth. BMC Plant Biol. 2016;16(1):157. doi: 10.1186/s12870-016-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao DM (2008) Redesigning C4 rice from limited C4 photosynthesis. In: Charting new pathways to C4 rice, pp 145–162
- Johnston AM, Raven JA, Beardall J, Leegood RC. Carbon fixation: Photosynthesis in a marine diatom. Nature. 2001;412(6842):40. doi: 10.1038/35083694. [DOI] [PubMed] [Google Scholar]
- Joshi M, Jha A, Mishra A, Jha B. Developing transgenic Jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS ONE. 2013;8:e71136. doi: 10.1371/journal.pone.0071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoi D, Mohanty S, Tripathy BC. Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res. 2016;130(1-3):47–72. doi: 10.1007/s11120-016-0224-3. [DOI] [PubMed] [Google Scholar]
- Karami O. Factors affecting Agrobacterium-mediated transformation of plants. Transgenic Plant J. 2008;3:127–137. [Google Scholar]
- Kershanskaya OI, Teixeira da Silva JA. Photosynthetic basis for wheat crop improvement: genetic modification of photosynthesis. Khazakistan plant science and biotechnology. Asian-Australas J Biosci Biotechnol. 2010;4:27–34. [Google Scholar]
- King JL, Edwards GE, Cousins AB. The efficiency of the CO2-concentrating mechanism during single-cell C4 photosynthesis. Plant Cell Environ. 2012;35(3):513–523. doi: 10.1111/j.1365-3040.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- Ku MSB, Cho D, Ranade U, Hsu TP, Li X, Jiao DM, Ehleringer J, Miyao M, Matsuoka M. Photosynthetic performance of transgenic rice plants overexpressing maize C4 photosynthesis enzymes. In: Sheehy JE, Mitchell PL, Hardy B, editors. Redesigning rice photosynthesis to increase yield. Amsterdam: Elsevier; 2000. pp. 193–204. [Google Scholar]
- Laporte MM, Shen B, Tarczynski MC. Engineering for drought avoidance: expression of maize NADP-malic enzyme in tobacco results in altered stomatal function. J Exp Bot. 2002;53(369):699–705. doi: 10.1093/jexbot/53.369.699. [DOI] [PubMed] [Google Scholar]
- Leakey AD, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60(10):2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Leegood RC. C4 photosynthesis: principles of CO2 concentration and prospects for its introduction into C3 plants. J Exp Bot. 2002;53(369):581–590. doi: 10.1093/jexbot/53.369.581. [DOI] [PubMed] [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Häusler RE. The regulation and control of photorespiration. J Exp Bot. 1995;46:1397–1414. [Google Scholar]
- Lian L, Wang X, Zhu Y, He W, Cai Q, Xie H, Zhang J. Physiological and photosynthetic characteristics of indica Hang2 expressing the sugarcane PEPC gene. Mol Biol Rep. 2014;41(4):2189–2197. doi: 10.1007/s11033-014-3070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZS, Chen QS, Zheng QX, Shen JJ, Luo ZP, Fan K, Liu PP. Proteomic and phosphoproteomic analysis in tobacco mosaic virus-infected tobacco (Nicotiana tabacum) Biomolecules. 2019;9(2):39. doi: 10.3390/biom9020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutts S, Lefèvre I, Delpérée C, Kivits S, Dechamps C, Robledo A, Correal E. Heavy metal accumulation by the halophyte species Mediterranean saltbush. J Environ Qual. 2004;33:1271–1279. doi: 10.2134/jeq2004.1271. [DOI] [PubMed] [Google Scholar]
- Maier A, Zell MB, Maurino VG. Malate decarboxylases: evolution and roles of NAD (P)-ME isoforms in species performing C4 and C3 photosynthesis. J Exp Bot. 2011;62(9):3061–3069. doi: 10.1093/jxb/err024. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Fukayama H, Tsuchida H, Nomura M, Agarie S, Ku MSB, Miyao M. How to express some C4 photosynthesis genes at high levels in rice. Stud Plant Sci. 2000;7:167–175. [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M. Molecular engineering of C4 photosynthesis. Annu Rev Plant Biol. 2001;52(1):297–314. doi: 10.1146/annurev.arplant.52.1.297. [DOI] [PubMed] [Google Scholar]
- Minges A, Groth G. Small-molecule inhibition of pyruvate phosphate dikinase targeting the nucleotide binding site. PLoS ONE. 2017;12(7):e0181139. doi: 10.1371/journal.pone.0181139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Tanna B. Halophytes: potential resources for salt stress tolerance genes and promoters. Front Plant Sci. 2017;8:829. doi: 10.3389/fpls.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PL, Sheehy JE. Super charging rice photosynthesis to increase yield. New Phytol. 2006;171(4):688–693. doi: 10.1111/j.1469-8137.2006.01855.x. [DOI] [PubMed] [Google Scholar]
- Miyao M. Molecular evolution and genetic engineering of C4 photosynthetic enzymes. J Exp Bot. 2003;53(381):179–189. doi: 10.1093/jxb/erg026. [DOI] [PubMed] [Google Scholar]
- Müller GL, Lara MV, Oitaven P, Andreo CS, Maurino VG, Drincovich MF. Improved water use efficiency and shorter life cycle of Nicotiana tabacum due to modification of guard and vascular companion cells. Sci Rep. 2018;8(1):4380. doi: 10.1038/s41598-018-22431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASA (2014) Global climate change: vital signs of the planet. https://climate.nasa.gov/400ppmquotes. Accessed 4 Dec 2014
- Nelson T, Langdale JA. Developmental genetics of C4 photosynthesis. Annu Rev Plant Biol. 1992;43(1):25–47. [Google Scholar]
- Nowak RS, Ellsworth DS, Smith SD. Functional responses of plants to elevated atmospheric CO2-do photosynthetic and productivity data from FACE experiments support early predictions. New Phytol. 2004;162(2):253–280. [Google Scholar]
- O'Leary MH. Phosphoenolpyruvate carboxylase: an enzymologist's view. Annu Rev Plant Physiol. 1982;33(1):297–315. [Google Scholar]
- Pandey S, Patel MK, Mishra A, Jha B. In planta transformed cumin (Cuminum cyminum L.) plants, overexpressing the SbNHX1 gene showed enhanced salt endurance. PLoS ONE. 2016;11:e0159349. doi: 10.1371/journal.pone.0159349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Mishra A. Genome editing: advances and prospects. In: Khurana S, Gaur R, editors. Plant biotechnology: progress in genomic era. Singapore: Springer; 2019. pp. 147–174. [Google Scholar]
- Patel MK, Joshi M, Mishra A, Jha B. Ectopic expression of SbNHX1 gene in transgenic castor (Ricinus communis L.) enhances salt stress by modulating physiological process. Plant Cell Tiss Organ Cult. 2015;122:477–490. doi: 10.1007/s11240-015-0785-4. [DOI] [Google Scholar]
- Peng C, Xu W, Hu L, Li Y, Qi X, Wang H, Zhao M. Effects of the maize C4 phosphoenolpyruvate carboxylase (ZmPEPC) gene on nitrogen assimilation in transgenic wheat. Plant Growth Regul. 2018;84(1):191–205. [Google Scholar]
- Pick TR, Bräutigam A, Schlüter U, Denton AK, Colmsee C, Scholz U, Weber AP. Systems analysis of a maize leaf developmental gradient redefines the current C4 model and provides candidates for regulation. Plant Cell. 2011;23(12):4208–4220. doi: 10.1105/tpc.111.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto H, Powell JR, Sharwood RE, Tissue DT, Ghannoum O. Variations in nitrogen use efficiency reflect the biochemical subtype while variations in water use efficiency reflect the evolutionary lineage of C4 grasses at inter-glacial CO2. Plant Cell Environ. 2016;39(3):514–526. doi: 10.1111/pce.12636. [DOI] [PubMed] [Google Scholar]
- Qi X, Xu W, Zhang J, Guo R, Zhao M, Hu L, Li Y. Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma. 2017;254(2):1017–1030. doi: 10.1007/s00709-016-1010-y. [DOI] [PubMed] [Google Scholar]
- Qin N, Xu W, Hu L, Li Y, Wang H, Qi X, Hua X. Drought tolerance and proteomics studies of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene. Protoplasma. 2016;253(6):1503–1512. doi: 10.1007/s00709-015-0906-2. [DOI] [PubMed] [Google Scholar]
- Rao X, Dixon RA. The differences between NAD-ME and NADP-ME subtypes of C4 photosynthesis: more than decarboxylating enzymes. Front Plant Sci. 2016;7:1525. doi: 10.3389/fpls.2016.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfelder JR, Kraepiel AM, Morel FM. Unicellular C4 photosynthesis in a marine diatom. Nature. 2000;407(6807):996–999. doi: 10.1038/35039612. [DOI] [PubMed] [Google Scholar]
- Ruan CJ, Shao HB, Teixeira da Silva JA. A critical review on the improvement of photosynthetic carbon assimilation in C3 plants using genetic engineering. Crit Rev Biotechnol. 2012;32(1):1–21. doi: 10.3109/07388551.2010.533119. [DOI] [PubMed] [Google Scholar]
- Sage RF. Tracking the evolutionary rise of C4 metabolism. J Exp Bot. 2016;67(10):2919–2922. doi: 10.1093/jxb/erw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, McKown AD. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? J Exp Bot. 2006;57:303–317. doi: 10.1093/jxb/erj040. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW. The nitrogen use efficiency of C3 and C4 plants: I. leaf nitrogen, growth, and biomass partitioning in Chenopodium album (L.) and Amaranthus retroflexus (L.) Plant Physiol. 1987;84(3):954–958. doi: 10.1104/pp.84.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylatingenzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.) Plant Physiol. 1987;85(2):355–359. doi: 10.1104/pp.85.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. Photorespiration and the evolution of C4 photosynthesis. Kocacinar, photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol. 2012;63:19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- Schuler ML, Mantegazza O, Weber AP. Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J. 2016;87(1):51–65. doi: 10.1111/tpj.13155. [DOI] [PubMed] [Google Scholar]
- Sen P, Ghosh S, Sarkar SN, Chanda P, Mukherjee AS, Datta K, Datta K. Pyramiding of three C4 specific genes towards yield enhancement in rice. Plant Cell Tissue Organ Cult. 2017;128(1):145–160. [Google Scholar]
- Shen WJ, Chen GX, Xu JG, Jiang Y, Liu L, Gao ZP, Lv CF. Overexpression of maize phosphoenolpyruvate carboxylase improves drought tolerance in rice by stabilization the function and structure of thylakoid membrane. Photosynthetica. 2015;53(3):436–446. [Google Scholar]
- Sheriff A, Meyer H, Riedel E, Schmitt JM, Lapke C. The influence of plant pyruvate, orthophosphate dikinase on a C3 plant with respect to the intracellular location of the enzyme. Plant Sci. 1998;136(1):43–57. [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC. Evolution along the crassulacean acid metabolism continuum. Funct Plant Biol. 2010;37:995–1010. [Google Scholar]
- Singh N, Mishra A, Jha B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar Biotechnol. 2014;16:321–332. doi: 10.1007/s10126-013-9548-6. [DOI] [PubMed] [Google Scholar]
- Singh N, Mishra A, Jha B. Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea) Gene. 2014;547:119–125. doi: 10.1016/j.gene.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Singh VK, Mishra A, Haque I, Jha B. A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci Rep. 2016;6:31686. doi: 10.1038/srep3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasarao C, Kundu S, Shanker AK, Naik RP, Vanaja M, Venkanna K, Rao VUM. Continuous cropping under elevated CO2: differential effects on C4 and C3 crops, soil properties and carbon dynamics in semi-arid alfisols. Agr Ecosyst Environ. 2016;218:73–86. [Google Scholar]
- Suzuki S, Murai N, Kasaoka K, Hiyoshi T, Imaseki H, Burnell JN, Arai M. Carbon metabolism in transgenic rice plants that express phosphoenolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant Sci. 2006;170:1010–1019. [Google Scholar]
- Takeuchi Y, Akagi H, Kamasawa N, Osumi M, Honda H. Aberrant chloroplasts in transgenic rice plants expressing a high level of maize NADP-dependent malic enzyme. Planta. 2000;211(2):265–274. doi: 10.1007/s004250000282. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Ohkawa H, Masumoto C, Fukuda T, Tamai T, Lee K, Miyao M. Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J Exp Bot. 2008;59(7):1799–1809. doi: 10.1093/jxb/ern016. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chaturvedi AK, Mishra A, Jha B. The transcriptional regulatory mechanism of the peroxisomal ascorbate peroxidase (pAPX) gene cloned from an extreme halophyte Salicornia brachiata. Plant Cell Physiol. 2014;55:1471–1774. doi: 10.1093/pcp/pct172. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chaturvedi AK, Mishra A, Jha B. Introgression of the SbASR−1 gene cloned from a halophyte Salicornia brachiata enhances salinity and drought endurance in transgenic groundnut (Arachis hypogaea) and acts as a transcription factor. PLoS ONE. 2015;10:e0135541. doi: 10.1371/journal.pone.0131567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Patel MK, Chaturvedi AK, Mishra A, Jha B. Functional characterization of the tau class Glutathione-S-Transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic Stress. PLoS ONE. 2016;11:e0148494. doi: 10.1371/journal.pone.0148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Patel MK, Chaturvedi AK, Mishra A, Jha B. Cloning and functional characterization of the Na+/H+ antiporter (NHX1) gene promoter from an extreme halophyte Salicornia brachiata. Gene. 2019;683:233–242. doi: 10.1016/j.gene.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Tsuchida H, Tamai T, Fukayama H, Agarie S, Nomura M, Onodera H, Toki S. High level expression of C4-specific NADP-malic enzyme in leaves and impairment of photoautotrophic growth in a C3 plant, rice. Plant Cell Physiol. 2001;42(2):138–145. doi: 10.1093/pcp/pce013. [DOI] [PubMed] [Google Scholar]
- Udawat P, Mishra A, Jha B. Heterologous expression of an uncharacterized universal stress protein gene (SbUSP) from the extreme halophyte, Salicornia brachiata, which confers salt and osmotic tolerance to E. coli. Gene. 2014;536:163–170. doi: 10.1016/j.gene.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Udawat P, Jha RK, Sinha D, Mishra A, Jha B. Overexpression of a cytosolic abiotic stress responsive universal stress protein (SbUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front Plant Sci. 2016;7:518. doi: 10.3389/fpls.2016.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udawat P, Jha RK, Mishra A, Jha B. Overexpression of a plasma membrane-localized SbSRP-like protein enhances salinity and osmotic stress tolerance in transgenic tobacco. Front Plant Sci. 2017;8:582. doi: 10.3389/fpls.2017.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja M, Yadav SK, Archana G, Jyothi Lakshmi N, Ram Reddy PR, Vagheera P, Abdul Razak SK, Maheswari M, Venkateswarlu B. Response of C4 (maize) and C3 (sunflower) crop plants to drought stress and enhanced carbon dioxide concentration. Plant Soil Environ. 2011;57(5):207–215. [Google Scholar]
- Von Caemmerer S, Evans JR. Enhancing C3 photosynthesis. Plant Physiol. 2010;154(2):589–592. doi: 10.1104/pp.110.160952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Caemmerer S, Edwards GE, Koteyeva N, Cousins AB. Single cell C4 photosynthesis in aquatic and terrestrial plants: a gas exchange perspective. Aquat Bot. 2014;118:71–80. [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414:543. doi: 10.1038/35107073. [DOI] [PubMed] [Google Scholar]
- Wang J, Li R. Integration of C4-specific ppdk gene of Echinochloa to C3 upland rice and its photosynthesis characteristics analysis. Afr J Biotechnol. 2008;7(6):783–787. [Google Scholar]
- Wang YM, Xu WG, Hu L, Zhang L, Li Y, Du XH. Expression of maize gene encoding C4-pyruvate orthophosphate dikinase (PPDK) and C4-phosphoenolpyruvate carboxylase (PEPC) in transgenic Arabidopsis. Plant Mol Biol Rep. 2012;30(6):1367–1374. [Google Scholar]
- Wang H, Liu C, Ma PA, Lu C, Li K, Wang W. Functional characterization of cytosolic pyruvate phosphate dikinase gene (MecyPPDK) and Promoter (MecyPPDKP) of cassava in response to abiotic stress in transgenic tobacco. Crop Sci. 2018;58:2002–2009. [Google Scholar]
- Washburn JD, Schnable JC, Davidse G, Pires JC. Phylogeny and photosynthesis of the grass tribe Paniceae. Am J Bot. 2015;102:1493–1505. doi: 10.3732/ajb.1500222. [DOI] [PubMed] [Google Scholar]
- Weber AP, Von Caemmerer S. Plastid transport and metabolism of C3 and C4 plants comparative analysis and possible biotechnological exploitation. Curr Opin Plant Biol. 2010;13(3):256–264. doi: 10.1016/j.pbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JA. Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. J Exp Bot. 2014;65(13):3425–3441. doi: 10.1093/jxb/eru063. [DOI] [PubMed] [Google Scholar]
- Yadav S, Mishra A. Introgression of C4 pathway gene(s) in C3 plants to improve photosynthetic carbon assimilation for crop improvement: a biotechnological approach. In: Ahmad P, Ahanger MA, Alyemeni MN, Alam P, editors. Photosynthesis, productivity, and environmental stress. Hoboken: Wiley; 2019. pp. 267–282. [Google Scholar]
- Yadav S, Mishra A, Jha B. Elevated CO2 leads to carbon sequestration by modulating C4 photosynthesis pathway enzyme (PPDK) in Suaeda monoica and S. fruticose. J Photochem Photobiol. 2018;178:310–315. doi: 10.1016/j.jphotobiol.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Zai-Song DING, Bao-Yuan ZHOU, Xue-Fang SUN, Ming ZHAO. High light tolerance is enhanced by overexpressed PEPC in rice under drought stress. Acta Agron Sin. 2012;38(2):285–292. [Google Scholar]
- Zerai DB, Glenn EP, Chatervedi R, Lu Z, Mamood AN, Nelson SG, Ray DT. Potential for improvement of Salicornia bigelovii through selective breeding. Ecol Eng. 2010;36:730–739. [Google Scholar]
- Zhang J, Datta SK, Wang G, Xie H. Integration of C4-specific PPDK gene of maize to C3 rice and its characteristics in relation to photosynthesis. Front Agric China. 2007;1(3):243–249. [Google Scholar]
- Zhou BY, Ding ZS, Zhao M. Alleviation of drought stress inhibition on photosynthesis by overexpression of PEPC in rice. Acta Agron Sin. 2011;37(1):112. [Google Scholar]