Abstract
Polyamines (PAs) are positively charged molecules known to mitigate drought stress; however, little is known about their mechanism of alleviating drought stress. We investigated the effects of PAs exogenously applied as a seed primer and as a foliar spray on the growth, membrane stability (MS), electrolyte leakage (EL), Na+ and K+ cations, reactive oxygen species (ROS), catalase (CAT; EC 1.11.1.6) and guaiacol peroxidase (GPX; EC 1.11.1.7) activity and chloroplast ultra-structure in wheat (Triticum aestivum L.; cv. Sakha-94) under drought stress. Three PA solutions, namely, putrescine, spermine and a mixture of the two (Mix), were each applied at a concentration of 100 µM. Our study demonstrated that the retardation of chlorophyll loss and elevation of Rubisco levels were involved in PA-enhanced growth under drought stress. These relationships were mainly reflected in elevated fresh weight and dry weight in response to foliar spraying with all PA solutions and seed priming with the Mix solution. The elevated growth seemed to be due to increased photosynthetic pigments, protein and Rubisco. In contrast, drought decreased growth, photosynthetic pigments, protein and Rubisco. MS was enhanced by PAs applied as a seed primer or foliar spray, as shown by clear reductions in EL %, malondialdehyde (MDA) content and the Na+/K+ ratio as well as reduced ROS markers and elevated CAT (but not GPX) activity. Further study showed that the Mix solution of PAs, applied either during seed priming or as a foliar spray, improved chloroplast ultra-structure, suggesting that improvements in Rubisco and photosynthetic pigments were involved in PA maintenance of chloroplast stability. Therefore, the present study showed that elevated CAT activity is the main mechanism through which PAs reduce ROS and MDA, thereby improving MS and protecting mesophyll cells structurally and functionally under drought stress in wheat.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00744-7) contains supplementary material, which is available to authorized users.
Keywords: Chloroplast, Drought, Membrane injury, Oxidative damage, Polyamines, Wheat
Introduction
Wheat is an important cereal crop worldwide. Subjecting wheat to water shortages during certain stages such as flowering and grain filling results in yield loss (Liu et al. 2016; Ebeed et al. 2017). The problem of yield reduction is worsened by global climate change and a reduction in the amount of precipitation falling in many areas (Nielsen et al. 2010). Therefore, increasing wheat yield is a major strategy for coping with the great demand for food, particularly under the expected worsening of climate change (Hassan et al. 2015a).
A reduction in yield reflects many physiological and metabolic disturbances. These disturbances include changes in proteins, pigments, ions or free radicals (Foyer and Noctor 2005; Ebeed et al. 2018) as well as a reduction in photosynthesis due to chlorophyll (CHL) degradation or a decline in Rubisco activity (Feller et al. 2007; Hassan et al. 2015a). Rubisco is a stroma-localized protein that is responsible for CO2 fixation in the Calvin cycle. Rubisco is the most abundant protein in leaves of C3 plants and may contribute up to 50% to the soluble leaf proteins and 20–30% of total leaf nitrogen (Spreitzer and Salvucci 2002) and exists as a holoenzyme composed of eight large subunits (LSUs; 55 kDa) and eight small subunits (SSUs; 15–18 kDa) (Spreitzer 1993). A further disturbance is damage to cellular membranes and cellular structures as a direct result of membrane lipid peroxidation, which is elevated by the production of higher levels of reactive oxygen species (ROS) under drought (Neill et al. 2002). These ROS, such as singlet oxygen, superoxide, hydrogen peroxide (H2O2) and hydroxyl radicals, are generated under steady-state conditions as a by-product of major metabolic processes such as the C2 cycle or photorespiration. ROS production is accelerated by stress conditions, but ROS may play a beneficial role as signalling molecules if they do not exceed the toxic level (Choudhury et al. 2013). Therefore, the production and scavenging of ROS take place in synchrony to maintain redox homeostasis in plants by developing protection mechanisms through enzymatic and non-enzymatic detoxification of excess ROS (Mittler 2002). Catalase (CAT) and peroxidase (POD) are among the enzymatic antioxidants. CAT scavenges H2O2 to form H2O and O·−2 and it is the most efficient scavenger of H2O2 (Asada and Takahashi 1987). POD reduces H2O2 using several reductants, such as ascorbate, guaiacol and phenolic compounds (Apel and Hirt 2004). Carotenoids are non-enzymatic antioxidants that are embedded in thylakoids and protect CHL from photooxidative damage (Botella-Pavía and Rodríguez-Concepción 2006).
Plants can be stimulated to adapt to adverse environmental conditions and to improve their physiological performance under stress in several ways. One of the most promising methods for this stimulation is the exogenous application of chemical compounds either by seed priming or foliar spraying. Polyamines (PAs) are low-molecular-weight nitrogenous compounds that are ubiquitous and widely involved in several physiological processes (Silveira et al. 2013) and auspicious chemicals that have been found to play a role in enhancing the tolerance of crops to stresses. The diamine putrescine (Put), triamine spermidine (Spd) and tetraamine spermine (Spm) are the major PAs in plants. At least one of these three PAs significantly increases under stress conditions (Ebeed et al. 2017).
Although several reports have revealed the enhancement of drought tolerance in plants by PAs, the precise role and mechanism of exogenously applied PAs in mitigating drought stress have not been well defined. Additionally, most of the studies on drought cascades have focused on physiological and biochemical changes, while very few papers have addressed these changes at the ultra-structural level. PAs are responsible for accumulating osmolytes and endogenous PAs, which could be involved in the stabilizing of membranes (Ebeed et al. 2017), in addition to an increase in ROS markers under drought stress in wheat (Hassan et al. 2015b) and alterations in chloroplasts under adverse environmental conditions (Grigorova et al. 2012). In this context, our proposed study was carried out to investigate how exogenous application of PAs by foliar spraying or seed priming improves membrane stability (MS) and whether this improvement enhances chloroplast ultra-structure and conserves stromal protein; Rubisco, and photosynthetic pigments to collectively improve wheat growth and tolerance to drought stress.
Materials and methods
Experimental materials and treatments
The bread wheat cultivar Sakha-94 was provided by the Egyptian Agricultural Research Centre (ARC). Grains were sterilized by washing with 1% sodium hypochlorite followed by thorough washing with distilled water. PAs concentration has been optimized as indicated in our previous study (Ebeed et al. 2017). 100 µM Put, Spm and a mixture of Put and Spm (Mix) were applied as seed primers or sprayed on leaves in a pot experiment. The experiment had five subgroups; one as control (normal irrigation two times per week throughout the entire time of the experiment) and four drought subgroups. (1) drought without PAs treatment (irrigation stopped at 10 days after sowing “DAS”), (2) drought with supplemental 100 µM Put, (3) drought with supplemental 100 µM Spm and (4) drought with a supplemental mixture of Put and Spm 100 µM each (Mix). After seed sterilization, half of the seeds was primed with water or the appropriate PA solutions as discussed in (Ebeed et al. 2017). Then, seeds were sown in pots filled with compost and perlite mixtures at a ratio of 2:1 and after germination, seedlings were watered with equal amount of tap water two times per week to maintain soil moisture at 60% field capacity equal in all pots for all subgroups until developing the second leaf at 10 DAS and then irrigation was discontinued for all drought subgroups and continued for control subgroup only until the end of the experiment (21 DAS).
The other half of the non-primed seeds was used for foliar spray experiment. Seeds were sown in pots filled with the same previous potting mixture and irrigated until the same time point (10 DAS) then, irrigation was continued only for the control subgroup and stopped for all the remaining subgroups. Foliar sprays with 100 µM each of PA solution was applied twice per week after withholding water while distilled water was used for spraying control as discussed in (Ebeed et al. 2017). The experiment was performed in a growth chamber under controlled conditions (23 ± 2 °C, 60% relative humidity (RH), 16 h photoperiod, and 250 μmol m−2 s−1 light intensity). Shoot samples of three plants were collected at 21 DAS to determine fresh and dry weight (FW and DW, respectively). Then, leaves were stored at − 70 °C for further analysis. Three replicates from each treatment were used for each analysis.
Measurements of plant growth, photosynthetic pigments, and protein and in-gel quantification of Rubisco
Twenty-one DAS, three plants from different pots in each treatment were collected. The whole shoots were harvested and immediately weighed for determination of FW (g) and then dried in an oven at 80 °C for 48 h and weighed for determination of DW (g). Three plants from each treatment were used to measure plant height and leaf area. Plant height was measured with a ruler. Leaf area (LA) was measured by scanning leaves using a computer scanner and then measured with ImageJ software. The relative growth rate (RGR) was measured for shoot using the following formula: RGR = (lnWf − lnWi)/Δt (Hoffmann and Poorter 2002) where: Wf = final shoot dry weight (at the end of the experiment 21 DAS), Wi = initial shoot dry weight (at the beginning of the treatment 10 DAS) and Δt = time is elapsed between the two measurements (i.e. 11 days) three replicates were used for each measurement.
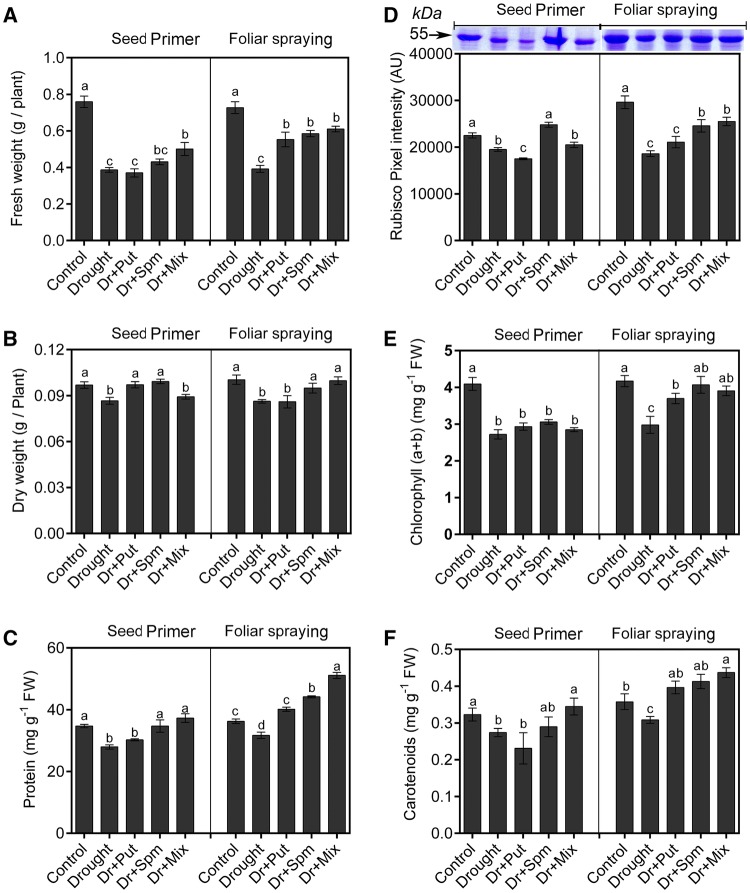
Liquid nitrogen-frozen leaf discs were homogenized in 50 mM potassium phosphate buffer (pH of 7.0) containing 2 mM ethylenediaminetetraacetic acid (EDTA), 5 mM β-mercaptoethanol and 4% (w/v) polyvinylpyrrolidone-40 and centrifuged at 12000 ×g for 20 min at 4 °C. The supernatants were used for protein determination and enzyme activity assays. The protein concentration was determined using Bradford assay (1976). For in-gel quantification of Rubisco protein (the large subunit), ten micrograms of protein from each sample were loaded into each lane and one lane was loaded with the PageRuler Plus prestained protein ladder, Thermo Scientific (#26630) with nine standard protein bands ranging from 10 to 250 kDa. The gels were resolved as described by Laemmli (1970) in a 12% acrylamide resolving gel and 5% acrylamide stacking gel at 200 V by using a Bio-Rad Mini-Protean 3 (Bio-Rad Laboratories, Hercules, CA, USA). The gels were stained with coomassie brilliant blue (CBB) and destained with methanol, then the gels were scanned. Rubisco LSU bands appeared at approximately 55 kDa as estimated from the protein ladder, then quantified by measuring band colour intensity using ImageJ 1.49v software. Three replicates were made for each treatment.
The fully emerged leaves were weighed, used for pigment extraction with 80% v/v acetone, and centrifuged at 5000×g for 10 min. Then, the supernatants were used to obtain absorbances with an ultraviolet (UV)-visible spectrophotometer at 663, 644, and 452.5 nm to calculate CHL a, CHL b and carotenoids, respectively, on an FW basis according to Metzner et al. (1965).
Determination of electrolyte leakage (EL) % and Na+ and K+ contents
MS was determined indirectly by measuring the electrical conductivity (Ec) of leaf pieces following the protocol of Ghoulam et al. (2002). Leaf samples of equal weights (10 mm × 10 mm pieces) were added to 20 mL of double-distilled water and kept at 10 °C for 24 h with shaking. The initial Ec (E1) was recorded after bringing the sample to 25 °C; then, the sample was autoclaved at 0.1 MPa for 10 min and cooled to 25 °C, at which point the final Ec (E2) was recorded. Leaf MS was estimated as EL % = (E1/E2) × 100.
For cation analysis, oven-dried leaf materials were extracted with diluted acid as described by Hunt (1982) with modification. Approximately 50 mg of oven-dried leaves was homogenized with 3 ml of 0.5 M hydrochloric acid. The samples were heated in a water bath at 70 °C for 30 min and then centrifuged at 8000 ×g for 20 min. The supernatants were then used to measure Na+ and K+ by a flame photometer (PFP7, Jenway, UK).
Determination of lipid peroxidation, H2O2 content, and antioxidant enzymes
The malondialdehyde (MDA) content was measured as a product of lipid peroxidation using thiobarbituric acid (TBA) according to Dhindsa and Matowe (1981). Then, 1.0 g of fresh leaves was homogenized in liquid nitrogen, extraction buffer containing 20% (w/v) trichloroacetic acid (TCA) and 0.5% TBA was added, and the mixture was heated at 95 °C for 30 min. The reaction mixture was placed in ice or at − 20 °C for 30 min followed by centrifugation at 10,000 ×g for 10 min. The MDA content was measured by observing the difference in absorbance at 532 nm using an extinction coefficient (ε) of 155 mM−1 cm−1 and expressed as nmol MDA g FW−1 (Heath and Packer 1968).
H2O2 was measured according to Yu et al. (2003). Approximately 0.5 g of fresh leaves was homogenized in liquid nitrogen and then extracted in 3 ml of 100 mM phosphate buffer (pH of 6.5), followed by centrifugation at 10,000×g. Then, 1% TiCl4 in 20% H2SO4 (v/v) was added, and the resulting solution was spectrophotometrically measured at 410 nm to determine the H2O2 content (ε = 0.28 μM−1 cm−1) and expressed as nmol H2O2 g FW−1.
Catalase (CAT; EC 1.11.1.6) and guaiacol peroxidase (GPX; EC 1.11.1.7) activities were assayed using the protein extract mentioned above. CAT activity was determined according to Hasanuzzaman and Fujita (2011). The assay mixture contained 50 mM potassium phosphate buffer (pH of 7.0), 15 mM H2O2 and enzyme solution. The decrease in absorbance resulting from decomposition of H2O2 was recorded at 240 nm for 1 min. The activity was calculated using an ε of 39.4 M−1 cm−1. GPX activity was assayed in a reaction mixture that consisted of 50 mM potassium phosphate buffer (pH of 6.4), 0.3 mM guaiacol, and 0.14 mM H2O2. Enzymatic activity was determined by measuring the increase in absorbance at 420 nm by its ability to convert guaiacol to tetra-guaiacol and calculated using an ε of 26.6 mM−1 cm−1 according to Chance and Maehly (1955).
Histochemical detection of ROS markers
The production of O·−2 in leaves in situ was spotted according to Chen et al. (2010). A 0.1% nitro blue tetrazolium chloride (NBT) solution was used as the incubation solution for 12 h at 25 °C. Then, the incubated leaves were decolorized by immersion in boiling ethanol (90%) for 15 min to visualize the dark blue spots of O·−2. Spots of H2O2 were detected according to Thordal-Christensen et al. (1997). Leaves were immersed in a solution containing 1 mg ml−1 3′,3′-diaminobenzidine (DAB) at 25 °C for 12 h. Then, the leaves were decolorized in ethanol (90%) for 15 min to visualize the reddish-brown spots of H2O2.
Ultra-microscopic examination of mesophyll cells by transmission electron microscopy (TEM)
At the end of the experiment, leaves were sampled for ultra-microscopic observation. The collected samples were cut into 1-mm segments and immediately placed in 2.5% (w/v) glutaraldehyde. The segments were then fixed under a vacuum for 4 h and washed in 50 mM phosphate buffer (pH of 7.4). The samples were fixed again in 1% (w/v) osmium tetroxide for 1 h, followed by immersion in 50%, 70%, 90%, 95%, and 100% ethanol (30 min each) for rapid dehydration. The specimens were then prepared by placing them in epoxy resin, and 2-μm-thick sections were cut with an LKB-V microtome from each sample. The sections were mounted on microscope slides and stained with toluidine blue. The slides were then photographed using an optical microscope (Olympus CX31, Japan) equipped with a digital camera. For TEM, 80-nm sections were stained with uranyl acetate/lead citrate and then examined at 80 kV under a transmission electron microscope (JEM-2100, JEOL, Japan) at the EM Unit, Mansoura University, Egypt.
Statistical analysis
Three replicates at least were used for all measurements. Analysis of variance (ANOVA) was performed using SPSS v16.0 with a significance level of P ≤ 0.05. To investigate the significant effects, the least significant difference (LSD) test used for multiple comparisons between the treatments.
Results
Effect of PAs on plant growth, protein, Rubisco and photosynthetic pigments
To study how PAs improve wheat growth under drought, we first measured shoot length, leaf area, RGR, shoot FW and DW. Shoot length and RGR of the drought-stressed plants was significantly lower than that in the control sub-group plants, however, leaf area of the control and stressed plants either treated or not with PAs was not significantly different (Table 1). Neither any of seed-priming solutions could increase shoot length whereas, foliar spray with PAs significantly increased the shoot length compared with drought-stressed plants. Leaf area of drought-stressed plants that treated with foliar Spm / Mix solution was higher than the leaf area of drought-stressed plants which primed with Spm / Mix solution, respectively. Only seed-priming with Mix solution and foliar spray with Spm and Mix solution caused a significant increase in the RGR compared with drought-stressed plants. Interestingly, the RGR of the stressed plants and foliar sprayed with Spm was significantly higher than RGR of the stressed plants and seed-primed with Spm. Shoot FW was significantly lower under drought conditions than under control conditions. Only seed priming with the Mix solution and foliar spraying with all PAs caused a significant increase in shoot FW compared with drought-stressed plants (Fig. 1a). Shoot DW was significantly lower in drought-stressed plants than in the control. However, seed priming with Put and Spm and foliar spraying with Spm and Mix increased the shoot DW relative to the control value (Fig. 1b).
Table 1.
Changes in the shoot length, relative growth rate and leaf area in wheat cultivar Sakha-94 under drought by withholding water and PAs applications (each of 100 µM)
| Method of PAs application | Group | Shoot length (cm) | Leaf area (cm2) | Relative growth rate |
|---|---|---|---|---|
| Seed priming | Control | 31.56 ± 0.51a | 7.96 ± 0.05a | 0.10 ± 0.00a |
| Drought (Dr) | 24.23 ± 0.37b | 6.81 ± 0.33a | 0.05 ± 0.00c | |
| Dr + Put | 26.56 ± 0.91b | 6.53 ± 0.13a | 0.06 ± 0.00bc | |
| Dr + Spm | 25.96 ± 0.93b | 6.59 ± 0.43a | 0.07 ± 0.00bc | |
| Dr + Mix | 27.53 ± 0.23b | 6.09± 0.79a | 0.08 ± 0.00ab | |
| Foliar spray | Control | 35.10 ± 1.00a | 8.02 ± 0.96a | 0.11 ± 0.00a |
| Drought (Dr) | 24.56 ± 0.20b | 7.43 ± 1.02a | 0.06 ± 0.01b | |
| Dr + Put | 36.86 ± 1.03a† | 7.99 ± 1.01a | 0.08 ± 0.02ab | |
| Dr + Spm | 37.40 ± 0.18a† | 8.78 ± 0.37a† | 0.10 ± 0.00a† | |
| Dr + Mix | 38.06 ± 0.90a† | 9.05 ± 0.18a† | 0.10 ± 0.01a | |
| LSD interaction at P < 0.05 | 2.80 | 1.92 | 0.03 |
Put = Putrescine, Spm = Spermine and Mix = Put + Spm. Values represent means (± SE) and different letters indicate significant differences between samples in each group according to analysis of variance (two-way ANOVA) statistical analysis with three different replicates (LSD = Least Significant Difference, P < 0.05)
†Indicates significant differences between samples with the same treatment but in different groups (with a different method of PAs application)
Fig. 1.
Changes in growth parameters and pigments in the wheat cv. Sakha-94 21 DAS under drought with or without 100 µM PA application. a FW, b DW, c protein content, d Rubisco content, e leaf CHL (a + b) and f leaf carotenoids. Dr drought, Put putrescine, Spm spermine, and Mix mixture of Put and Spm. Data are the means ± SEs of 3 replicates. Bars labelled with different letters are significantly different at P < 0.05
To further study how PAs improve wheat growth under drought stress, we measured protein, Rubisco and photosynthetic pigments. Drought stress resulted in a significantly lower protein content than the control condition (Fig. 1c). Seed priming with either Spm or Mix and foliar spraying with the three PA solutions elevated the protein content to a level comparable to that of the control and exceeding that under foliar spraying with Spm or Mix. The large subunit of Rubisco is easily detectable on stained gels from C3 or C4 plants (Feller et al. 2007), so Rubisco was quantified by measuring the intensity of the distinctive large subunit of Rubisco band in the gel (Fig. 1d). The Rubisco activity was clearly inhibited by drought stress. Only seed priming with Spm and foliar spraying by Spm or Mix were able to increase Rubisco under drought compared to the non-amended plants under drought.
Drought stress also significantly reduced the CHL (a + b) content (Fig. 1e). None of the PA seed priming treatments significantly changed the CHL (a + b) content compared with the non-amended drought-stress treatment; however, foliar treatment with all PAs significantly recovered the CHL (a + b) content under stress to a level comparable to that of the control. The carotenoid content was significantly lower under drought stress than the control value (Fig. 1f). Seed priming with Mix and foliar spraying with all of the PAs under drought conditions significantly increased the carotenoid content compared to that in non-amended drought plants and even the control.
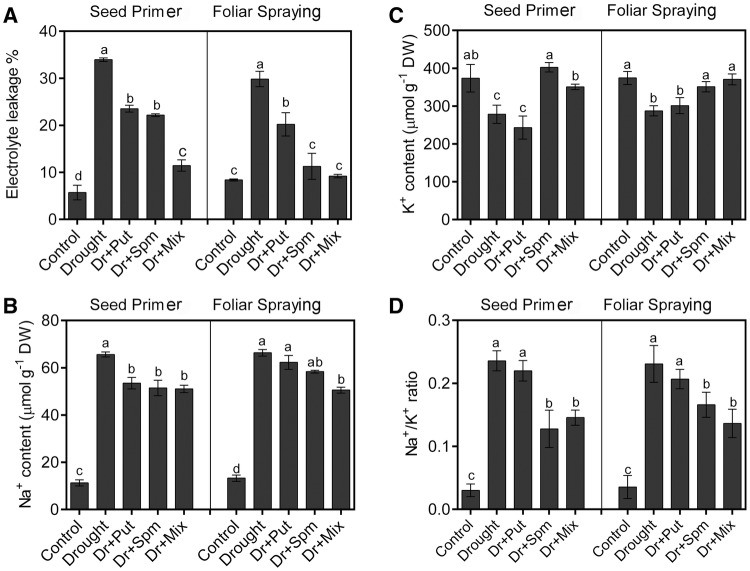
Effect of PAs on EL % and cations
Since PAs can promote the growth of wheat and protect both Rubisco and photosynthetic pigments under drought stress, PAs may improve MS characteristics. MS significantly decreased in response to drought stress, as indicated by the higher EL % (Fig. 2a). Applying PAs by both methods reduced the EL % compared to the value under drought alone. The Mix solution was the best solution for reducing EL %. Compared to the well-irrigated plants, drought-exposed plants exhibited an increased Na+ content, regardless of whether they were amended with PAs (Fig. 2b). However, PA treatment via seed priming with the three PA solutions and foliar spraying with Mix significantly reduced the Na+ content compared to drought alone, but the values were still much higher than the control value. In contrast, the K+ content was significantly lower under drought stress than in the control (Fig. 2c). Spm and Mix treatment by both methods of application significantly increased the K+ content. The ratio of Na+/ K+ significantly increased in response to the drought treatment and decreased in response to PAs, particularly Spm or Mix applied as a seed primer/foliar spray (Fig. 2d).
Fig. 2.
Changes in EL % and cations in the leaves of the wheat cv. Sakha-94 21 DAS under drought with or without 100 µM PA application. a EL %, b sodium cation content, c potassium cation content and d sodium/potassium ratio. Dr drought, Put putrescine, Spm spermine, and Mix mixture of Put and Spm. Data are the means ± SEs of 3 replicates. Bars labelled with different letters are significantly different at P < 0.05
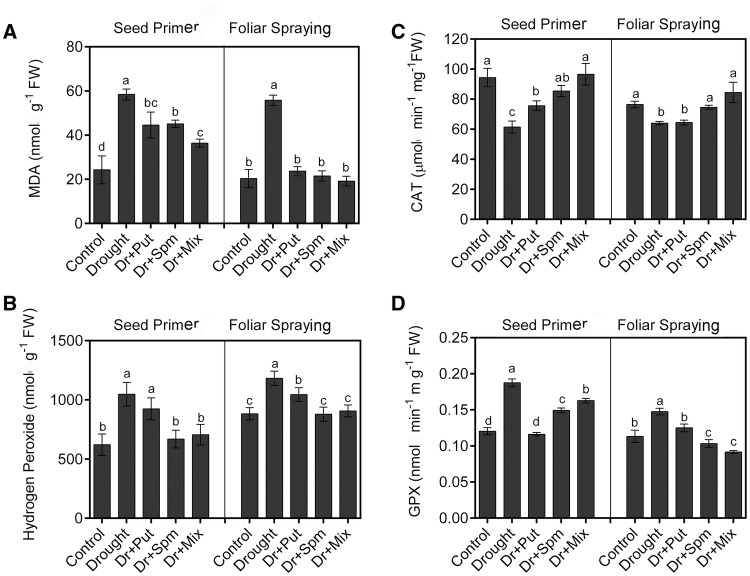
Effect of PAs on lipid peroxidation, H2O2 and enzyme activity of CAT and GPX
Generally, ROS levels and lipid peroxidation affect MS; thus, we evaluated the effect of PAs on lipid peroxidation and H2O2 as well as the H2O2-scavenging enzymes CAT and GPX. Under drought conditions, the MDA content was significantly higher than the control value (Fig. 3a). All exogenous PA applications, either as a seed primer or foliar spray, ameliorated MDA levels under drought conditions. Moreover, in response to drought conditions, plants produced high levels of H2O2 (Fig. 3b). However, drought combined with Spm or Mix amendment by both methods of application decreased H2O2 to the control level. CAT activity was significantly inhibited by drought stress compared with the control (Fig. 3c). Seed priming/foliar spraying with all of the PAs except the Put foliar spray recovered the CAT activity under drought stress. In contrast to the pattern observed for CAT activity, GPX activity increased significantly due to drought stress compared to the control (Fig. 3d). Seed priming/foliar spraying with all PAs readjusted the GPX activity levels compared to those in the drought treatment alone.
Fig. 3.
Changes in MDA, H2O2 content, and CAT and GPX enzyme activity in the leaves of the wheat cv. Sakha-94 21 DAS under drought with or without 100 µM PA application. a MDA content, b H2O2 content, c CAT activity and d GPX activity. Dr drought, Put putrescine, Spm spermine, and Mix mixture of Put and Spm. Data are the means ± SEs of 3 replicates. Bars labelled with different letters are significantly different at P < 0.05
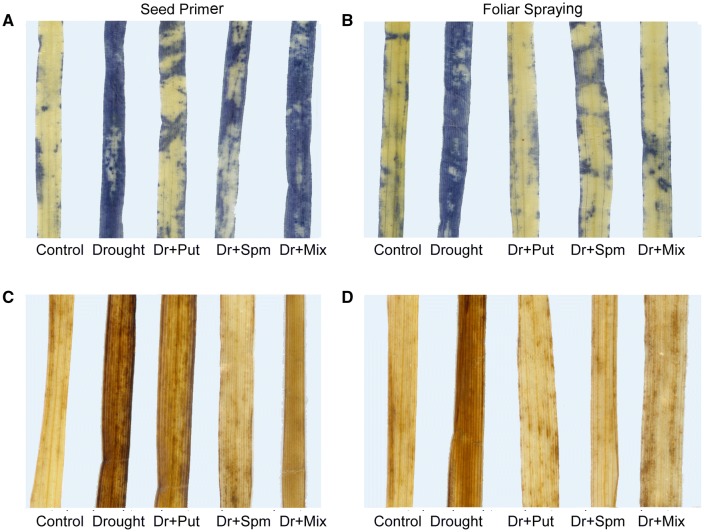
Effect of PAs on histochemical localization of ROS markers in wheat under drought stress
To further examine the histochemical localization of O·−2 in wheat leaves, NBT dye was used. More intense blue patches were observed in drought-stressed leaves than in control leaves (Fig. 4a, b). The intensity and number of spots decreased in the leaves of the seedlings amended with PAs compared with the leaves of drought-stressed plants. Seed priming with Put was the most effective PA treatment, whereas foliar application of all PAs reduced the production of O·−2, in contrast to the seed priming treatment. To visualize the generation of H2O2 in the leaves, DAB was used. Drought-stressed leaves showed more intense dark brown-stained spots than the control, indicating a greater accumulation of H2O2 in the former (Fig. 4c, d). Applying PAs, particularly as a foliar spray, to drought-stressed plants reduced the spots of H2O2 compared with drought alone.
Fig. 4.
Histochemical detection of ROS markers in the leaves of the wheat cv. Sakha-94 21 DAS under drought with or without 100 µM PA application. a, b Histochemical detection of O·−2, c, d histochemical detection of H2O2. Dr drought, Put putrescine, Spm spermine, and Mix mixture of Put and Spm
Effect of PAs on mesophyll cell ultra-structure in wheat under drought stress
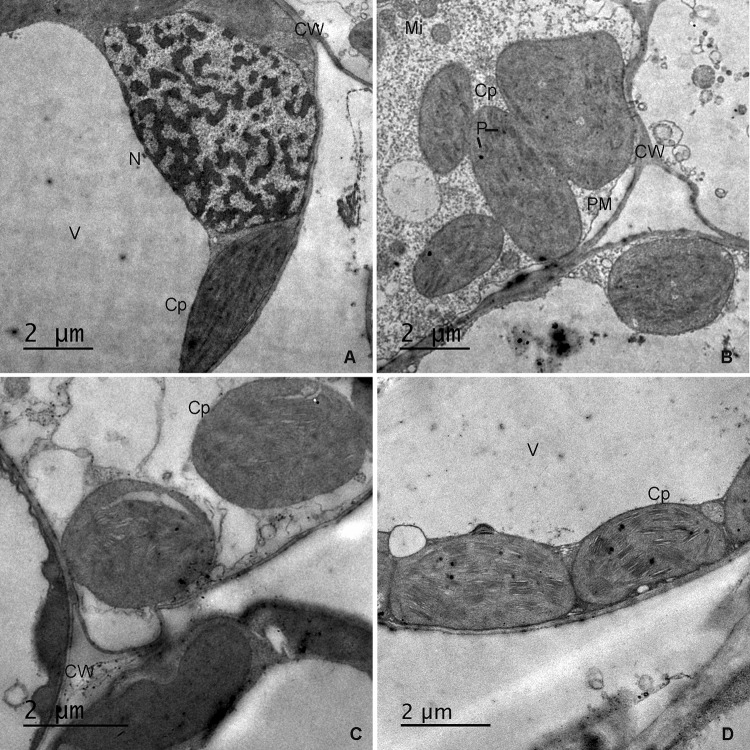
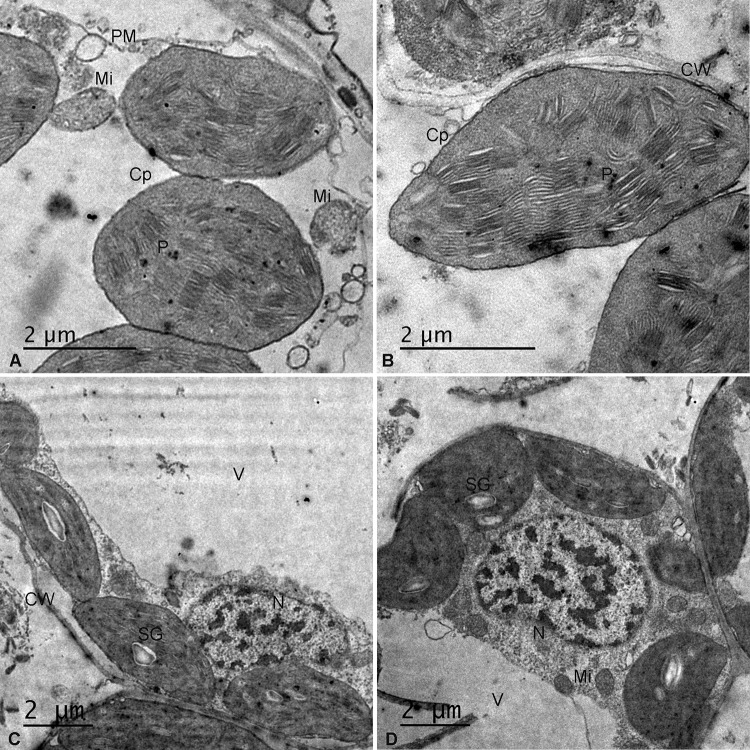
As shown in the previous results, PAs protected the stromal protein (Rubisco) and photosynthetic pigments and improved MS under drought stress in wheat. Thus, PAs may improve mesophyll ultra-structure. Therefore, we examined mesophyll cells by TEM. The mesophyll cells in control plants exhibited large intercellular spaces and contained chloroplasts closely arranged along the cell plasma membrane and cell wall (see Supplementary Figure S1A), as observed in semi-thin sections. Ultra-thin sections examined by TEM showed integrated ellipsoidal chloroplasts with a well-developed membrane system with very dense stacks of grana, integral lamellae and chloroplast-bound membranes (Fig. 5a). Drought stress applied alone induced twisted mesophyll cell walls, and the cells appeared to be linked together without spaces and moved towards the centre of the cell (Figure S1B). Furthermore, spherical or ellipsoidal chloroplasts with swollen thylakoids and a disorganized membrane system were observed in drought-stressed cells (Fig. 5b). Seed priming with Put resulted in less invagination of mesophyll cell walls, large intercellular spaces (Figure S1C), spherical chloroplasts with a disintegrated membrane system and the breakdown of thylakoids (Fig. 5c). Foliar spraying with Put under drought resulted in large intercellular spaces, integrated chloroplasts with minimal cell wall twisting (Figure S1D) and thicker cell walls, and intact chloroplasts that were closely associated with the cell wall. The chloroplasts showed an organized membrane system (Fig. 5d). Seed priming with Spm under drought treatment did not ameliorate the adverse effect of drought stress on mesophyll cell structure (Figure S1E) and resulted in invaginated cell membranes and spherical chloroplasts separated from the cell membrane with the disappearance of lamellar stacking (Fig. 6a). Foliar spraying with Spm under drought stress resulted in intact mesophyll cells with large intercellular spaces and less invaginated walls (S1F) and elliptical chloroplasts with disorganized and swollen lamellae (Fig. 6b). Plants subjected to seed priming and foliar spraying with Mix showed intact mesophyll cells (Figure S1G and S1H) and integrated chloroplasts with compactly arranged thylakoids and well-compartmentalized grana stacks (Fig. 6c, d).
Fig. 5.
Transmission electron micrographs of leaf mesophyll cells of the wheat cv. Sakha-94 grown under normal irrigation (a), drought alone (b), the combined application of drought and 100 µM Put as a seed primer (c) and the combined application of drought and 100 µM Put as a foliar spray (d). V vacuole, CW cell wall, SG starch grain, N nucleus, Cp chloroplast, P plastoglobuli, PM plasma membrane, Mi mitochondria
Fig. 6.
Transmission electron micrographs of leaf mesophyll cells of the wheat cv. Sakha-94 grown under the combined application of drought and 100 µM Spm as a seed primer (a), the combined application of drought and 100 µM Spm as a foliar spray (b), the combined application of drought and 100 µM Mix as a seed primer (c) and the combined application of drought and 100 µM Mix as a foliar spray (d). V vacuole, CW cell wall, SG starch grain, N nucleus, Cp chloroplast, P plastoglobuli, PM plasma membrane, Mi mitochondria
Discussion
Involvement of CHL loss retardation and Rubisco level elevation in PA-enhanced growth
The adverse effect of drought stress on the growth of plants has been widely reported and discussed in numerous researches (Zivcak et al. 2013; Hassan et al. 2015b; Ebeed et al. 2019). The reduction in growth by drought stress reflects many cellular, structural and metabolic disturbances which in turn reduce plant productivity (Basu et al. 2016; Kaur and Asthir 2017). Therefore, researchers in several tries aimed to ameliorate the stress adversities on plants by using an exogenous additive to plants under the stressful conditions with substances like hormones or chemical compounds (Hassan et al. 2011; Osman 2015; Hasanuzzaman et al. 2018). PAs is one of the promising materials that has been used to enhance plant growth and physiological performance under stress conditions (Nahar et al. 2016). However, limited researches have been conducted to study the effect of exogenous PAs on wheat under drought stress by using PAs either as seed soaking or foliar treatment moreover, neither of these studies examined the effect of PAs on the ultra-structural levels of chloroplast (Gupta et al. 2012; Liu et al. 2016; Yang et al. 2016). In this study, we used PAs as seed-priming material and as foliar spraying solution to study the effect of both methods on improving wheat growth under drought stress.
The current results showed a negative impact of drought stress on wheat growth in terms of FW, DW, shoot length and RGR, however, subjecting to drought stress did not significantly change leaf area compared to control plants. In a previous study, researchers found that drought stress reduced leaf area as a mechanism to reduce water loss which is in turn decline photosynthesis and dry matter accumulation (Basu et al. 2016) but in this experiment, drought stress did not reduce the leaf area which may suggests that the reduction in leaf area by drought stress depends on the stress level, stress duration, and genotype used. The present results also showed a decline in the total protein, Rubisco, CHL (a + b) and carotenoid contents as a result of applying drought stress. The growth-inhibiting effects of drought stress may be correlated with a reduction in photosynthesis, which could be partially attributed to the decreased photosynthetic pigment (CHL a + b and carotenoids) content and the decrease in Rubisco (Kaur and Asthir 2017).
The current experiment revealed that PAs supplementation combined with drought stress differentially improved growth parameters and contents of protein, Rubisco, CHL (a + b) and carotenoids. These results are in agreement with results of Nahar et al. (2016) who revealed that exogenous PAs alleviated the adverse effect of salt stress on mung bean seedling by improving growth and restoring the photosynthetic pigments. In previous studies, the content of CHL decreased by increase chlorophyllase activity which degrades CHL or reduction in CHL synthase (Mihailović et al. 1997) whereas PAs treatment protects the photosynthetic apparatus from the adverse effect of stresses through entering chloroplast rapidly (He et al. 2002). Therefore, the positive impact of PAs on growth under drought may be attributed to retardation of CHL loss and elevation of Rubisco levels, which most likely enhanced photosynthesis. Seed-priming with Mix solution improved RGR, FW, and increased contents of protein and carotenoids under drought conditions whereas all PAs solutions or at least Spm and Mix solutions that used as foliar sprays improved all tested characteristics under drought stress. So, we can gather from these results that Mix solution of (Put+Spm) was more efficient than other PAs solutions in improving wheat growth, photosynthetic pigments, protein and Rubisco under drought by both methods, but foliar spray application was more efficient than seed priming. The greater efficiency of foliar spraying than of seed priming may have been related to the improvement of water status in epidermal and underlying cells by direct contact between PAs and the leaf surface (Farooq et al. 2009b).
Scavenging ROS plays an important role in PA-improved membrane stability under drought stress in wheat
An influx of Na+ and activation of the K+ efflux, are one of the plant responses exposed to stress (Shabala et al. 2007). The present results revealed that drought stress elevated the EL % of membranes in wheat leaves, and this elevation was accompanied by an increase in the Na+ content and the Na+/K+ ratio. PA application by seed priming or foliar spraying in the current experiment declined EL % and Na+/K+ ratio. Improving Na+/K+ ratio could be due to retention of intracellular K+, Na+ exclusion or replacement of Na+ by K+ (Shabala et al. 2007). An increase in K+ and a decrease in Na+ level in PAs-supplemented drought-stressed plants indicated the influential roles of PAs in reducing Na toxicity. Nahar et al. (2016) revealed that PAs increased K uptake, reduced Na uptake and improved Na+/K+ homeostasis under salt stress.
The increased levels of MDA, the main product of lipid peroxidation is a main type of damage to membranes which increase the leakiness of the membranes; and damage membrane proteins and enzymes (Farooq et al. 2009a). The current results showed an increase in MDA and ROS production as indicated from the higher H2O2 content as well as the increase in brown patches and blue spots in drought-affected wheat leaves. It has been observed that the increase of the MDA is a consequence of the generation of ROS. The elevated ROS levels could be attributed to reduced ROS-scavenging efficiency, as indicated by the decreases in the non-enzymatic antioxidant (carotenoids) and CAT activity. These results are consistent with the results of (Hasanuzzaman and Fujita 2011), which showed a sharp increase of H2O2 and MDA under drought stress and referred this to the insufficient defense system. However, the exogenous applying of PAs in drought-stressed plants significantly lowered the levels of ROS and MDA compared with drought stress alone. Our results indicate the protective role of PAs in lowering the levels of H2O2 and MDA under drought stress conditions in wheat.
Our results showed a decrease in CAT activity under drought stress. Our results corroborated with findings of Hasanuzzaman and Fujita (2011). This decrease in CAT activity might be due to its inactivation by the elevated H2O2 induced by water stress. However, nearly all of the used PAs, namely, Put, Spm and Mix, increased activity of CAT, which in turn reduced the H2O2 content, spots of ROS markers and lipid peroxidation. Notably in this study, CAT was more efficient than GPX in ROS-scavenging, as it was substantially induced by PAs under drought, whereas GPX activity was enhanced by drought and lower after treatment with PAs. MS most likely resulted from the reduction of H2O2 by CAT in PA-treated plants. PAs form complexes with antioxidant enzymes to function more efficiently (Alcázar et al. 2010). This finding was consistent with that of Ali et al. (2009), who proposed that improved stress tolerance was accompanied by high levels of antioxidant enzyme activities (Cvikrová et al. 2013), which revealed a controversial relationship between ROS and PAs. Therefore, the present study implied that elevating CAT activity is the main mechanism of ROS and lipid peroxidation reduction under drought by PAs in wheat.
PA application by seed priming or foliar spraying in the current experiment improved MS in drought-stressed wheat plants by promoting ROS elimination, which was reflected in the MDA levels, EL % and Na+/K+ ratio. Foliar application was clearly more efficient than seed priming, particularly in reducing MDA levels and EL %. These findings agree with the results of Farooq et al. (2009a, b), who found that foliar spraying was more efficient than seed priming in improving drought tolerance in rice.
The role of PA amendment in stabilizing chloroplast ultra-structure under drought stress in wheat
Photosynthesis is mainly localized in the thylakoid membranes of chloroplasts (Kirchhoff et al. 2007). Chloroplasts contain two membranous domains: the grana thylakoids and the stromal lamellae. The integrity and stability of the chloroplast structure are correlated with the normal functioning of photosynthesis. However, chloroplast integrity and photosynthesis are disturbed by environmental stressors and are most likely reflected in yield (Grigorova et al. 2012). Drought stress in the present study led to the appearance of spherical chloroplasts with swollen thylakoids. Chloroplast membrane alterations may affect chloroplast/thylakoid-related processes, such as light harvesting, electron transport, and ion movement (Dondini et al. 2003). According to our results, the alteration of chloroplast structure by drought stress was positively correlated with excessive lipid peroxidation of membranes and high levels of ROS.
PAs play a role in stabilizing chloroplast ultra-structure under adverse environmental conditions (Grigorova et al. 2012). However, a limited number of studies have considered the effect of PAs on chloroplast ultra-structure in response to drought stress. The present results revealed that foliar spraying with Put resulted in intact chloroplasts. Additionally, Mix solution amendment (seed priming/foliar spraying) combined with drought was able to eliminate drought-induced damage to the chloroplast and caused integrated chloroplasts with compactly arranged thylakoids and well-compartmentalized grana stacks. Therefore, applying Put and Spm in Mix either by seed priming or foliar spraying was able to counterbalance the deleterious effects of drought stress on chloroplasts, probably by doubling the ability to scavenge ROS. Another possible role of PAs in maintaining chloroplasts and photosynthetic function under drought stress, in addition to their membrane and cell-wall-stabilizing abilities, is conjugation with stromal and thylakoid proteins that are involved in photosynthesis (Zheleva et al. 1994). This role will help maintain photosynthetic efficiency, which will be reflected in growth. Concomitantly, our results reflected a high in-gel relative intensity of Rubisco protein and increased levels of CHL (a + b) and carotenoids, particularly in plants that were subjected to drought combined with PA amendment compared with those treated with drought only. Similarly, Zheleva et al. (1994) revealed the retention of photosynthetic pigments by PAs and attributed this retention to the interaction of PAs with the thylakoid membrane. Therefore, PA amendment under drought stress likely protects chloroplasts functionally and structurally by scavenging ROS directly or indirectly or reducing them, thereby protecting CHL and Rubisco from degradation and reducing lipid peroxidation to protect chloroplast membrane structure. Moreover, Put and Spm were complementary in the Mix solution as they improved MS under drought stress by scavenging ROS or by activating the CAT enzyme, hence reducing lipid peroxidation and EL and improving the Na+/K+ ratio. This effect was reflected in the structural and functional protection of wheat mesophyll cells under drought stress.
Conclusion
Although several studies have reported that PAs are involved in abiotic stress responses, the detailed function of PAs under drought stress has not been fully elucidated. The results of the present study revealed that exogenous PA application, particularly foliar spraying with all of the PA solutions and seed priming with Mix, enhanced the drought stress tolerance of wheat plants. Improved drought tolerance was associated with the elimination of oxidative injury and the maintenance of cellular MS and the photosynthetic apparatus. These changes were probably caused by enhanced activity of the CAT enzyme, which directly scavenges ROS; thus, the lipid peroxidation, membrane leakage and Na+/K+ ratio were reduced, and the chloroplast structure was maintained relatively intact, which in turn contributed to protecting the photosynthetic pigments and Rubisco from degradation. All of these processes were reflected in wheat growth under stress. Since that omic tools are significant to understand the biological network of abiotic stress responses (Ebeed 2019). Prospective work using omics approaches would be essential to further understanding the machinery by which PAs protect membranes and ultra-structure of the chloroplast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Prof. Zakaria A. Baka for providing help with the ultra-structure analysis. This work was performed at Damietta University, Egypt. Scholarship from Sabha University (A.A), Libya (No. 393), supported this research.
Abbreviations
- CAT
Catalase
- CHL a
Chlorophyll a
- CHL b
Chlorophyll b
- DAB
3′,3′-Diaminobenzidine
- DAS
Days after sowing
- DW
Dry weight
- Ec
Electrical conductivity
- FW
Fresh weight
- GPX
Guaiacol peroxidase
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
- MS
Membrane stability
- NBT
Nitro blue tetrazolium chloride
- PAs
Polyamines
- POD
Peroxidase
- Put
Putrescine
- ROS
Reactive oxygen species
- Spd
Spermidine
- Spm
Spermine
- TBA
Thiobarbituric acid
- TCA
Trichloroacetic acid
- TEM
Transmission electron microscopy
Authors’ Contributions
NMH supervised the experiments and critically revised the manuscript; HTE conceived and designed the research, supervised the experiments, performed the TEM photography, analysed the data statistically, interpreted the results and wrote the manuscript; and AMA performed the plant growth experiments and analysed the physiological parameters.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alcázar R, Altabella T, Marco F, et al. Polyamines : molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–1249. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- Ali Q, Ashraf M, Anwar F. Physico-chemical attributes of seed oil from drought stressed sunflower (Helianthus annuus L.) plants. Grasas Aceites. 2009;60:475–481. doi: 10.3989/gya.021009. [DOI] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osborne CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 227–287. [Google Scholar]
- Basu S, Ramegowda V, Kumar A, Pereira A. Plant adaptation to drought stress. F1000Research. 2016;5:1554. doi: 10.12688/f1000research.7678.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella-Pavía P, Rodríguez-Concepción M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant. 2006;126:369–381. doi: 10.1111/j.1399-3054.2006.00632.x. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly SK. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- Chen F, Wang F, Wu F, et al. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem. 2010;48:663–672. doi: 10.1016/j.plaphy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 2013;8:e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvikrová M, Gemperlová L, Martincová O, Vanková R. Plant Physiology and Biochemistry Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol Biochem. 2013;73:7–15. doi: 10.1016/j.plaphy.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Matowe W. Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot. 1981;32:79–91. doi: 10.1093/jxb/32.1.79. [DOI] [Google Scholar]
- Dondini L, Del Duca S, Dall’Agata L, et al. Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates. Planta. 2003;217:84–95. doi: 10.1007/s00425-003-0998-3. [DOI] [PubMed] [Google Scholar]
- Ebeed Heba T. Wheat Production in Changing Environments. Singapore: Springer Singapore; 2019. Omics Approaches for Developing Abiotic Stress Tolerance in Wheat; pp. 443–463. [Google Scholar]
- Ebeed HT, Hassan NM, Aljarani AM. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol Biochem. 2017;118:438–448. doi: 10.1016/j.plaphy.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Ebeed HT, Stevenson SR, Cuming AC, Baker A. Conserved and differential transcriptional responses of peroxisome associated pathways to drought, dehydration and ABA. J Exp Bot. 2018;69:4971–4985. doi: 10.1093/jxb/ery266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeed HT, Hassan NM, Keshta MM, Hassanin OS. Comparative analysis of seed yield and biochemical attributes in different sunflower genotypes under different levels of irrigation and salinity. Egypt J Bot. 2019;59:339–355. doi: 10.21608/ejbo.2019.5043.1205. [DOI] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, et al. Plant drought stress : effects, mechanisms and management. Agron Sustain Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Farooq M, Wahid ÆA, Lee D. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant. 2009;31:937–945. doi: 10.1007/s11738-009-0307-2. [DOI] [Google Scholar]
- Feller U, Anders I, Mae T. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot. 2007;59:1615–1624. doi: 10.1093/jxb/erm242. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;28:1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x. [DOI] [Google Scholar]
- Ghoulam C, Foursy A, Fares K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot. 2002;47:39–50. doi: 10.1016/S0098-8472(01)00109-5. [DOI] [Google Scholar]
- Grigorova B, Vassileva V, Klimchuk D, et al. Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J Plant Interact. 2012;7:204–213. doi: 10.1080/17429145.2011.654134. [DOI] [Google Scholar]
- Gupta S, Agarwal VP, Gupta NK. Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.) Physiol Mol Biol Plants. 2012 doi: 10.1007/s12298-012-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Fujita M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res. 2011;143:1758–1776. doi: 10.1007/s12011-011-8998-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Rahman A, et al. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants. 2018;24:993–1004. doi: 10.1007/s12298-018-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan NM, El-Sayed AKA, Ebeid HT, Nemat Alla MM. Molecular aspects in elevation of sunflower tolerance to drought by boron and calcium foliar sprays. Acta Physiol Plant. 2011 doi: 10.1007/s11738-010-0585-8. [DOI] [Google Scholar]
- Hassan N, El-bastawisy Z, Ebeed H, Nemat Alla M. Role of defense enzymes, proteins, solutes and Δ1-pyrroline-5-carboxylate synthase in wheat tolerance to drought. Rend Lincei. 2015 doi: 10.1007/s12210-015-0429-y. [DOI] [Google Scholar]
- Hassan NM, El-Bastawisy ZM, El-Sayed AK, et al. Roles of dehydrin genes in wheat tolerance to drought stress. J Adv Res. 2015 doi: 10.1016/j.jare.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Nada K, Kasukabe Y, Tachibana S. Enhanced susceptibility of photosynthesis to low-temperature photoinhibition due to interruption of chill-induced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.) Plant Cell Physiol. 2002;43:196–206. doi: 10.1093/pcp/pcf021. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann WA, Poorter H. Avoiding bias in calculations of relative growth rate. Ann Bot. 2002;90:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. Dilute hydrochloric acid extraction of plant material for routine cation analysis. Commun Soil Sci Plant Anal. 1982;13:49–55. doi: 10.1080/00103628209367243. [DOI] [Google Scholar]
- Kaur G, Asthir B. Molecular responses to drought stress in plants. Biol Plant. 2017;61:201–209. doi: 10.1007/s10535-016-0700-9. [DOI] [Google Scholar]
- Kirchhoff H, Haase W, Haferkamp S, et al. Structural and functional self-organization of Photosystem II in grana thylakoids. Biochim Biophys Acta (BBA)-Bioenergetics. 2007;1767:1180–1188. doi: 10.1016/j.bbabio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang H, Lv X, et al. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem. 2016;100:113–129. doi: 10.1016/j.plaphy.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu H, Wen X, Liao Y. Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric. 2016;15:2759–2774. doi: 10.1016/S2095-3119(16)61366-7. [DOI] [Google Scholar]
- Metzner H, Rau H, Senger H. Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella (Studies on synchronization of some pigment-deficient Chlorella mutants) Planta. 1965;65:186–194. doi: 10.1007/BF00384998. [DOI] [Google Scholar]
- Mihailović N, Lazarević M, Dželetović Z, et al. Chlorophyllase activity in wheat, Triticum aestivum L. leaves during drought and its dependence on the nitrogen ion form applied. Plant Sci. 1997;129:141–146. doi: 10.1016/S0168-9452(97)00189-1. [DOI] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Rahman A, et al. Polyamines confer salt tolerance in Mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002;128:13–16. doi: 10.1104/pp.010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DC, Halvorson AD, Vigil MF. Critical precipitation period for dryland maize production. Field Crops Res. 2010;118:259–263. doi: 10.1016/j.fcr.2010.06.004. [DOI] [Google Scholar]
- Osman HS. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann Agric Sci. 2015;60:389–402. doi: 10.1016/j.aoas.2015.10.004. [DOI] [Google Scholar]
- Shabala S, Cuin TA, Pottosin I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007;581:1993–1999. doi: 10.1016/j.febslet.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Silveira V, de Vita AM, Macedo AF, et al. Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tissue Organ Cult. 2013;114:351–364. doi: 10.1007/s11240-013-0330-2. [DOI] [Google Scholar]
- Spreitzer RJ. Genetic dissection of Rubisco structure and function. Annu Rev Plant Biol. 1993;44:411–434. doi: 10.1146/annurev.pp.44.060193.002211. [DOI] [Google Scholar]
- Spreitzer RJ, Salvucci ME. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol. 2002;53:449–475. doi: 10.1146/annurev.arplant.53.100301.135233. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Yu C-W, Murphy TM, Lin C-H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol. 2003;30:955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- Zheleva D, Tsonev T, Sergiev I, Karanov E. Protective effect of exogenous polyamines against atrazine in pea plants. J Plant Growth Regul. 1994;13:203. doi: 10.1007/BF00226038. [DOI] [Google Scholar]
- Zivcak M, Brestic M, Balatova Z, et al. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res. 2013;117:529–546. doi: 10.1007/s11120-013-9885-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.