Abstract
In order to ascertain the regulatory mechanism of fruit development in Isatis indigotica Fortune, the complementary DNA (cDNA) sequence of the SHATTERPROOF 2 (SHP2) orthologous gene was identified by Rapid Amplification of cDNA Ends technology and the corresponding gene was named IiSHP2. The expression pattern of IiSHP2 was determined by quantitative reverse transcription-polymerase chain reaction and wild-type Col-0 Arabidopsis plants were transformed with the IiSHP2 gene using Agrobacterium tumefaciens and the floral-dip method. Expression analyses indicated that IiSHP2 was highly expressed in flowers, silicles and seeds. Compared to wild-type plants, IiSHP2 transgenic lines bolted earlier. Detailed phenotypic observations showed that the size of the rosette and cauline leaves in transgenic lines was reduced and the cauline leaves of the transgenic lines were incurved and displayed a funnel-like shape. During the reproductive growth stage, IiSHP2 transgenic plants produced shortened sepals and the flower buds were not encapsulated completely. Moreover, the petals of the transgenic lines were converted into stamineous tissues, accompanied by exposed stamens, short malformed siliques and wrinkled valves, indicating a severe decline in fertility. These experimental conclusions are valuable as a reference for the breeding of medicinal plants.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00745-6) contains supplementary material, which is available to authorized users.
Keywords: Isatis indigotica Fortune, IiSHP2, MADS-box gene, Floral differentiation, Silique development
Introduction
As one of the evidence types in taxonomy, the flower is the most complicated structure arising in the process of plant growth (APG 2003). The floral transition is composed of three stages, including formation of inflorescence meristems, production of floral meristems and genesis of floral organs, and is highly regulated by the genetic background of plants and environmental factors (Fornara et al. 2010). In research about auto-regulation signals of plants, it was found that the transcriptional factors of MADS (MCM1, AGAMOUS, DEFICIENS and SRF) family played important roles in the floral transition process. The transcriptional factors of MADS family were classified into two categories: Type I and Type II. Up to now, most reports about the plant proteins in this family are related to the Type II MADS transcriptional factors containing M, I, K and C functional domains.
SHATTERPROOF (SHP) genes are members of the MADS-box family. In the model plant Arabidopsis, two SHP genes were identified. They were named as SHATTERPROOF 1 (SHP1) and SHATTERPROOF 2 (SHP2), and were formerly known as AGL1 (AGAMOUS-LIKE 1) and AGL5 (AGAMOUS-LIKE 5). The ABCDE model of flowers has confirmed that AG (AGAMOUS), SHP1 and SHP2 are C-class floral homeotic genes, while STK (AGAMOUS-LIKE 11, SEEDSTICK) is a D-class floral homeotic gene (Kater et al. 2006). Both the euAG-lineage and the PLE-lineage of floral homeotic genes are derived from C-class precursor genes. SHP1 and SHP2 belong to PLE-lineage (Kramer et al. 2004).
In the stage of flowering, SHP1 and SHP2 are mainly involved in regulation of the development of pistil, carpels, ovaries and ovules. They are also the important transcriptional factors in regulation of silique dehiscence in the stage of ripeness (Liljegren et al. 2000). Moreover, SHP1 and SHP2 act redundantly to specify the valve margin and are known as the markers of this region. The siliques of shp1 shp2 double mutant lack the dehiscence zone and are indehiscent. By constitutive expression of SHP1 and SHP2, it was confirmed that these two genes were responsible for the differentiation of dehiscence zone and the lignification of the adjacent cells (Liljegren et al. 2000). Previous research indicated that STK, SHP1 and SHP2 were responsible for the development of ovules (Pinyopich et al. 2003; Battaglia et al. 2006). In the stk shp1 shp2 triple mutant, integuments are converted into carpelloid tissues and ovules are transformed into leaf-like or carpel-like structures, illustrating that SHP2 is also associated with controlling ovule identity (Brambilla et al. 2007). Overexpression of any one of these three genes would result in production of ovules on sepals (Ferrándiz et al. 2000; Battaglia et al. 2006). At the same time, SHP1 and SHP2 were found to be involved in the regulation of mucilage production in seeds. shp1 shp2 seeds generated a thinner mucilage layer with an uneven distribution of pectins (Ehlers et al. 2016).
The biological functions of SHP homologous genes are conserved. BnSHP1, BnSHP2a and BnSHP2b are the SHP homologous genes identified in oilseed rape. When the expression level of BnSHP1 was downregulated by RNA interference technology, the pods of oilseed rape showed an indehiscent phenotype (Kord et al. 2015). Overexpression of some D-class genes also produced phenotypes similar to overexpression of SHP1 and SHP2 in transgenic Arabidopsis thaliana, including PeMADS7 cloned from Phalaenopsis equestris, BdMADS2 and BdMADS4 cloned from Brachypodium distachyon (Chen et al. 2012; Wei et al. 2013). These results demonstrate partial functional redundancy of C-class and D-class genes. However, the SHP homologous genes of different plant species also exhibit obvious variations in function, which might be seen as the result of the influences of geographical conditions and environmental factors on evolution.
Woad (Isatis indigotica Fortune) is a biennial herb and is distributed in many places of China. It possesses important medicinal value and its efficacies include heat-clearing, detoxifying, blood-cooling and sore-throat relieving. As a cruciferous plant species, woad produces terminal racemes and its flowers contain four intact whorls of floral organs, including four sepals, four petals, six stamens (tetradynamous stamens) and one pistil. After pollination, the pistils develop into fruits. The fruits of woad are oval-shaped, laterally compressed, winged, and indehiscent after maturation, and are referred to as silicles in taxology. Owing to the higher medicinal value, protection and innovation of the germplasm resources by cross breeding or genetic improvement to woad are of great significance. Compared to model plant Arabidopsis, woad has a longer growth cycle and its requirements for growth conditions are more complex. Woad is usually planted in the spring of the first year. After vernalization by the low temperature in the winter, woad plants would flower and bear fruits in the next year.
At present, research about identification of gene function in woad is concentrated on the genes related to the synthesis of medicinal components, and similar research on MADS-box genes in woad are very few. Our laboratory has isolated the coding sequence, analyzed the expression patterns and determined the functions of FRUITFULL of I. indigotica (IiFUL) (Ma et al. 2017). In order to further investigate the function of woad genes in MADS-box family, the coding sequence of SHATTERPROOF 2 of I. indigotica (IiSHP2) was isolated with the inflorescences as materials in the present work. Subsequently, analyses of bioinformatic features, expression patterns and biological functions were carried out. The results of this work can provide references for research about the regulatory network of woad genes associated with the development of flowers and fruits.
Materials and methods
Plant materials
The flowers of biennial woad plants grown in greenhouse were used as materials and were stored in − 80 °C freezer after quick freezing with liquid nitrogen.
Identification of the cDNA sequence of IiSHP2 and bioinformatics analysis
In previous works of our laboratory, degenerate primers were designed according to the coding sequences of the MADS domains in the MADS transcriptional factors of other plant species and a number of the conserved fragments of the MADS-box family genes in woad were amplified (Ma et al. 2017). Sequencing results showed that one fragment was closely analogous to SHP2 in Arabidopsis and the corresponding gene was named as IiSHP2. In the present work, specific primers were designed in accordance with the sequence of the conserved fragment (Table 1) and the cDNA (complementary DNA) sequence of IiSHP2 was ascertained by Rapid Amplification of cDNA Ends (RACE) method. Total RNA was extracted from woad flowers with Trizol reagent (Invitrogen). cDNA used in 5′-RACE or 3′-RACE was prepared with the reverse transcriptase according to the protocol provided by SMARTer RACE cDNA Amplification Kit (Takara). Touchdown PCR (Polymerase Chain Reaction) was carried out with adapter primers and gene specific primers. After separation on a 1.2% agarose gel, the amplification products were ligated with pMD-18T vector (Takara) and competent cells of Escherichia coli strain DH5α were transformed. Positive clones were sequenced after verification by colony PCR. The cDNA sequence of IiSHP2 was assembled with the sequences obtained in 5′-RACE and 3′-RACE.
Table 1.
The sequences of the primers used in RACE, quantitative RT-PCR and construction of expression vector
| Primer name | Primer sequence (5′→3′) |
|---|---|
| IiSHP2-5′RACE | GCGTCACACAAGACAGAGAGCTCATAAGC |
| IiSHP2-3′RACE | TGGGTTGATATACATGGAGGGTGGTGCG |
| qRTIiSHP2(F) | CATCAGTCGTGTCCGCTCCAA |
| qRTIiSHP2(R) | GCTGCTGTAGTCCCGCTCTTT |
| qRTIiActin(F) | ACGAGCTACCTGATGGACAAGT |
| qRTIiActin(R) | TTGAACCACCACTGAGGACGAT |
| IiSHP2-GFP(F) | CGCCCATGGAGGGTGGTGCGAGTAATGAAG |
| IiSHP2-GFP(R) | CGCACTAGTAACAAGTTGGAGAGGTGGTTG |
Homology searches and multiple alignments of the amino acid sequence encoded by IiSHP2 were conducted with Protein Basic Local Alignment Search Tool (BLAST) in National Center of Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/). The amino acid sequences of IiSHP2 and its corresponding orthologous proteins were also aligned with DNAman in DNAStar, The phylogenetic tree of MADS-box genes in different species was constructed using the MEGA7 software (Kumar et al. 2016). Molecular weight, isoelectric point and physicochemical property of IiSHP2 were analyzed with ProtParam online tools (https://web.expasy.org/protparam/).
Analyses of the expression patterns of IiSHP2
Total RNA was extracted from root, stem, leaf, inflorescence, flower, sepal, petal, stamen, pistil, silicle, fruit wing, valve and seed with Trizol reagent. The process of silicle development was subdivided into four stages. In the first stage, the fertilized pistil began to grow and no significant shrinkage could be observed in the other three whorls of floral organs. In the second stage, the growing silicles obviously protruded out of other floral organs which were partially withered. In the third stage, silicles elongated evidently and fruit wing grew quickly. In the fourth stage, the length of the silicles was stable and the colour of the silicles was changed from green to tawny gradually. In analyses of the mRNA abundance of IiSHP2 in fruit wing, valve and seed, the silicles at the third stage were used as materials. At this stage, other floral organs have fallen off completely from the green silicles.
Fluorogenic quantitative RT-PCR primers, qRTIiSHP2(F) and qRTIiSHP2(R), were designed according to the coding sequence of IiSHP2 (Table 1). Actin (AY870652.1) of woad was used as a reference gene and the primers were qRTIiActin(F) and qRTIiActin(R) (Table 1). Two-step quantitative RT-PCR was carried out with FastStart Essential DNA Green Master Kit (Roche). In the first step, template was denatured at 95 °C for 10 min. In the second step, 40 cycles of 95 °C for 10 s and 60 °C for 30 s were conducted. The data from three biological repetitions were processed with 2−ΔΔCt method. Root was used as the normalization sample. ΔΔCt = (CtIiSHP2 − CtIiActin)Sample − (CtIiSHP2 − CtIiActin)Root.
Cloning of the coding sequence of IiSHP2 and construction of expression vector
pCAMBIA1302 carrying a hygromycin resistance gene was used in construction of plant binary expression vector and DH5α strain of E. coli was used as host in cloning of the coding sequence of IiSHP2. In addition, GV3101 strain of Agrobacterium tumefaciens was used in genetic transformation of Arabidopsis. Fresh woad flowers were ground into powder in liquid nitrogen and total RNA was extracted with Trizol reagent. cDNA was synthesized with reverse transcriptase using Primescript 1st Strand cDNA Synthesis Kit (Takara). Primers were designed according to 5′-terminus and 3′-terminus of the ORF of IiSHP2. Restriction site and protective bases were added to the 5′-end of the primers. In detail, the cutting site of Nco I was added to the 5′-end of the forward primer IiSHP2-GFP(F) and the cutting site of Spe I was added to the 5′-end of the reverse primer IiSHP2-GFP(R). The coding sequence of IiSHP2 was amplified with high-fidelity PrimeSTAR GXL DNA Polymerase (Takara) and was ligated into pCAMBIA1302 after restriction digestion. The recombinant plasmid validated by sequencing was introduced into the competent cells of GV3101 strain by freeze-thaw method.
Screening and phenotypic observation of IiSHP2 transgenic Arabidopsis lines
The 35S∷IiSHP2-GFP fusion gene was transformed into the genome of wild-type Col-0 Arabidopsis by floral dip method (Clough and Bent 1998). The seeds of wild-type Col-0 Arabidopsis were scattered on the surface of the well-watered mixed matrix of nutrient soil, perlite and vermiculite (2:1:1), and were grown under 12 h light/12 h dark, 22 °C in the day/16 °C at night and 50% humidity. GV3101 strain of A. tumefaciens carrying the expression vector of IiSHP2 was propagated at 28 °C in YEB medium (beef extract 5 g/L, yeast extract 1 g/L, peptone 5 g/L, sucrose 5 g/L, MgSO4·7H2O 0.5 g/L, pH 7.0). Bacterial cells were precipitated from suspension cultures with an OD600 of 0.8–1.0 and were resuspended in 50 mL infiltration solution (half strength hormone-free MS medium supplemented with 50 g/L sucrose) (Murashige and Skoog 1962) containing 10 μL silwet L-77 (Lehle Seeds). Flowers were removed from wild-type Col-0 Arabidopsis plants. The aerial parts of the Col-0 wild-type plants were immersed into the bacterial suspension for 5 min under vacuum condition (P = 0.05 MPa). The treated plants were grown overnight in the dark and the environment humidity was maintained in 30% RH. On the following day, the treated plants were transferred to normal illumination conditions and were grown to set seeds. Mature siliques were collected and seeds were screened on selective medium containing hygromycin. The phenotypic changes of IiSHP2 transgenic plants were recorded and the functions of IiSHP2 were investigated.
Results
Determination of the cDNA sequence of IiSHP2
A cDNA fragment between 250 to 500 bp and a cDNA fragment of about 1000 bp were amplified in 5′-RACE and 3′-RACE, respectively. Sequencing results showed that the length of the 5′-end cDNA fragment was 298 bp and the length of the 3′-end cDNA fragment was 920 bp (data not shown). By sequence assembly, the full-length IiSHP2 cDNA of 1020 bp in size was determined. In the cDNA sequence, a complete ORF of 741 bp encoding a 246-amino-acid protein could be found (Supplemental Fig. 1). Homology search in NCBI showed that the cDNA of IiSHP2 was closely related to SHP2 (AGL5), a MADS-box gene in Arabidopsis, and the identity between IiSHP2 cDNA and SHP2 cDNA reached up to 89%. The identity of the entire amino acid sequences between IiSHP2 and SHP2 was 93.17%, indicating SHP homologous proteins were highly conserved in the process of evolution (Fig. 1). Protein BLAST search of the amino acid sequence in NCBI illustrated that IiSHP2 was constituted by M (1–73 aa), I (74–100 aa), K (101–185 aa) and C (186–246 aa) domains from N-terminus to C-terminus (Fig. 1). Predictive analysis of conserved domains also displayed that IiSHP2 contained MADS domain and K-box domain which were specific to the Type-II transcriptional factors in MADS family. Analysis of the physicochemical property showed that IiSHP2 was a basic protein and the isoelectric point was 9.23.
Fig. 1.
Alignment of the amino acid sequences of SHP2 homologous proteins. Green indicates identical amino acids, pink and orange indicate different amino acids, black line shows MADS domain, purple line shows K domain. The AG motif I and AG motif II in the C-terminal region are framed with black boxes. SHP2, Arabidopsis thaliana (NP_850377); EsAGL5, Eutrema salsugineum (XP_024016862.1); RsAGL5, Raphanus sativus (XP_018483390.1); LcSHP2, Lepidium campestre (CBY05405.1) (color figure online)
Multiple alignment of the amino acid sequences and construction of phylogenetic tree
The amino acid sequences of IiSHP2 and SHP homologous proteins in different plant species were used for constructing the diagrams of multiple alignment and phylogenetic tree. Multiple alignment of amino acid sequence of IiSHP2 with SHP-like proteins from Arabidopsis, Eutrema salsugineum, Raphanus sativus and Lepidium campestre indicated that these proteins share high similarity throughout the entire M, I, K and C regions. Furthermore, AG motif I and AG motif II were found in the C-terminus of IiSHP2 containing hydrophobic and polar residues (Fig. 1). The phylogenetic relationship shows that IiSHP2 could be grouped into the PLE-lineage of MADS-box genes (Fig. 2). These results verified that IiSHP2 was a C-class floral homeotic gene and that it was the orthologous gene of SHP2 from Arabidopsis.
Fig. 2.
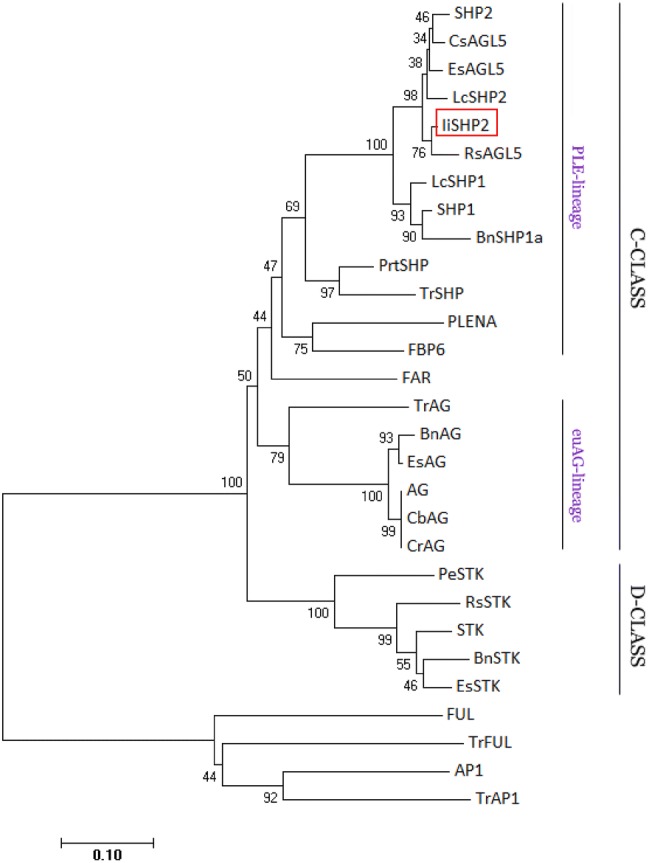
Phylogenetic tree of MADS proteins from different plant species. The tree was constructed with neighbor-joining algorithm in MEGA 7. IiSHP2 is framed in red box. SHP1, NP_001190130.1; SHP2, NP_850377.1; CsAGL5, XP_010506124.1; EsAGL5, XP_024016862.1; LcSHP1, CBY05404.1; LcSHP2, CBY05405.1; RsAGL5, XP_018483390.1; BnSHP1, NP_001302702.1; PrtSHP, AEI01160.1; TrSHP, ABB59995.1; PLENA, AAB25101.1; FAR, BAI68392.1; AG, NP_567569.3; TrAG, ABB59994.1; BnAG, XP_013719485.1; EsAG, XP_024005545.1; CbAG, ACD76828.1; CrAG, XP_023633847.1; STK, NP_001078364.1; PeAGL11, XP_011031538.1; RsAGL11, XP_018473770.1; BnAGL11, XP_013732865.1; EsAGL11, XP_006397127.1; FUL, NP_568929.1; TrFUL, ABB59991.1; AP1, NP_177074.1; TrAP1, ABB59990.1
Expression patterns of IiSHP2 in woad
The expression levels of IiSHP2 in different tissues and organs of woad were analyzed by real-time quantitative PCR. The results showed that the accumulation of IiSHP2 mRNA in root, stem, leaf and inflorescence of the woad plants at the reproductive stage was very low (Fig. 3a). On the contrary, the transcript abundance of IiSHP2 in flowers was very high (Fig. 3a). Besides, the transcriptional abundance of IiSHP2 increased gradually along with the development of flowers and silicles. In four types of floral organs, the difference of IiSHP2 expression was insignificant in sepals and petals (Fig. 3b). The abundance of IiSHP2 mRNA in pistils was the highest, and in stamens the expression level was also significantly higher than that in sepals and petals (Fig. 3b). In silicles, the expression of IiSHP2 at stage 1 was very weak (Fig. 3c). In contrast, the expression levels of IiSHP2 at the third and the fourth stages were increased significantly and were maintained persistently (Fig. 3c). Analysis of different parts of silicles at stage 3 confirmed that the expression level of IiSHP2 in seeds was higher than that in fruit wings and valves, and the fruit wings exhibited the lowest abundance of IiSHP2 transcript (Fig. 3c). These results suggest that IiSHP2 gene in woad is not only involved in the development of pistil, carpel and silicle, but also might be directly or indirectly involved in the specialization of stamens.
Fig. 3.
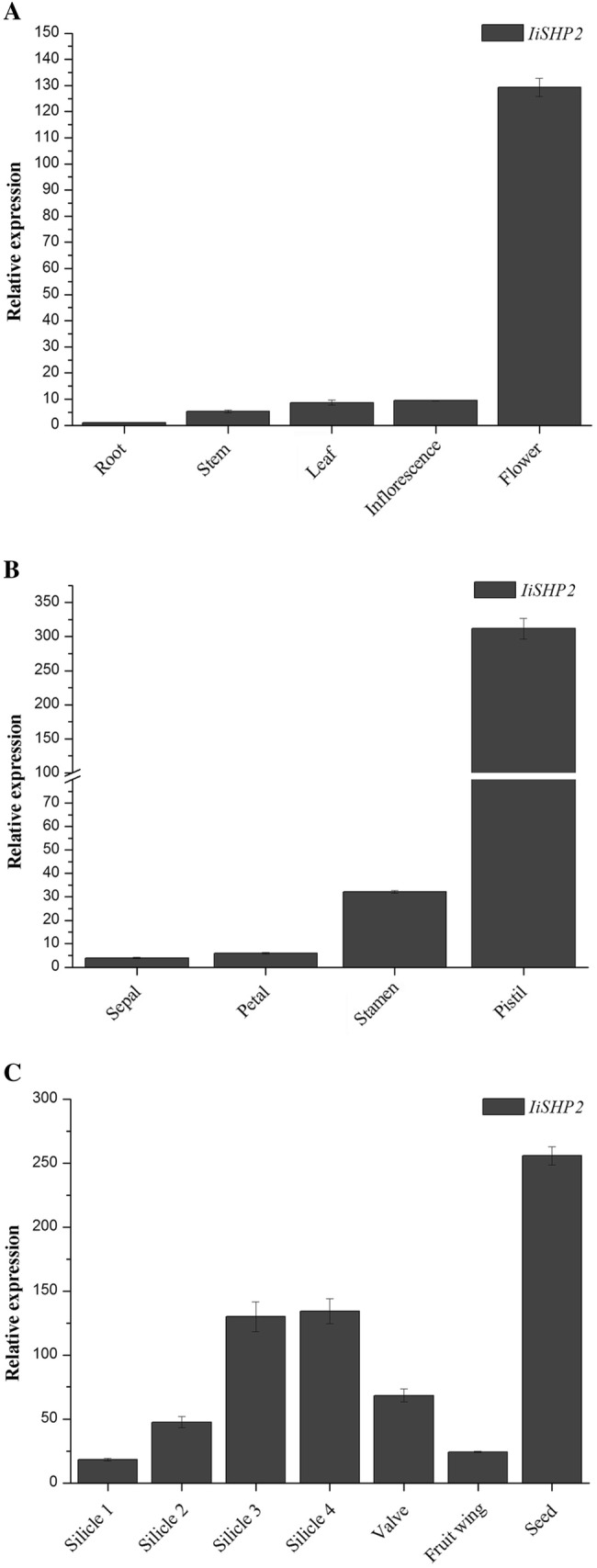
Expression pattern of IiSHP2 in woad in different tissues (a), different floral organs (b), the silicles at different development stages and different parts of the silicles in stage 3 (c). Error bars represent the standard deviation
Phenotypic variations of IiSHP2 transgenic Arabidopsis plants
In order to investigate the biological functions of IiSHP2, an IiSHP2-GFP fusion gene controlled by the Cauliflower Mosaic Virus (CaMV) 35S promoter was introduced into the genome of wild-type Arabidopsis. By Agrobacterium-mediated genetic transformation and selection with hygromycin, twenty-one transgenic lines were obtained and all the lines showed severe phenotypic changes.
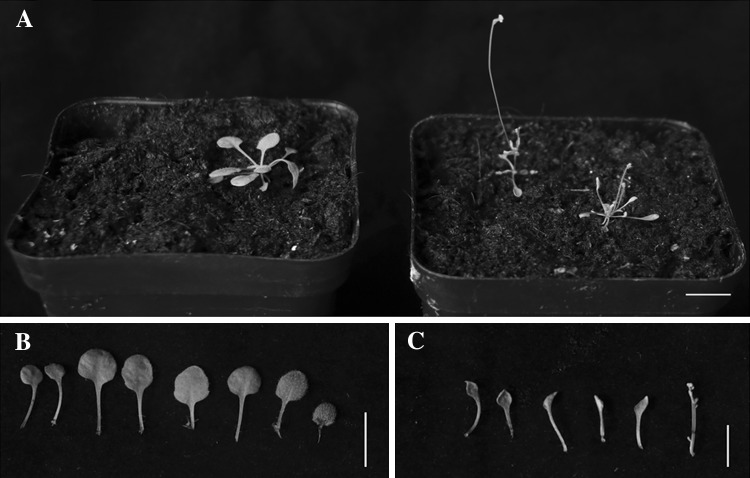
IiSHP2 in transgenic lines 2, 4 and 8 was identified by qRT-PCR and it showed high expression levels (Fig. 4). The overexpressors produce 5–6 rosette leaves, while wild-type plants typically produce 10 rosette leaves (Fig. 5a–c). Moreover, the surface area of rosette leaves was significantly reduced in transgenic plants (Fig. 5b, c). Statistical data analysis also showed significant differences in the number of rosette leaves (Fig. 6).
Fig. 4.
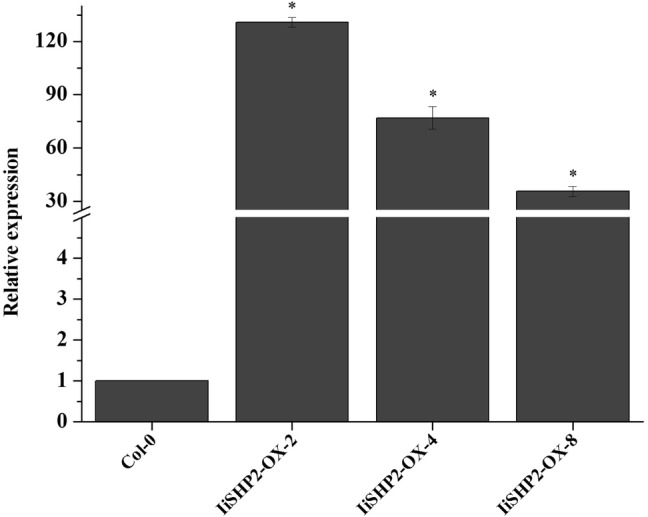
Expression analysis of IiSHP2 in transgenic Arabidopsis by qRT-PCR. Error bars represent the standard deviation. Asterisks indicate a significant difference (P < 0.05) between transgenic lines and wild-type Col-0 plants
Fig. 5.
Comparison of phenotypic characteristics and the number of rosette leaves between wild-type Col-0 and IiSHP2 transgenic plants. a The 35S::IiSHP2 plants (shown at right) flowered after producing 5–6 rosette leaves, while the wild-type plant (shown at left) did not flower until the eighth leaf was produced. b The rosette leaves of a wild-type Col-0. c The rosette leaves of an 35S::IiSHP2 plant. Bar = 1 cm
Fig. 6.
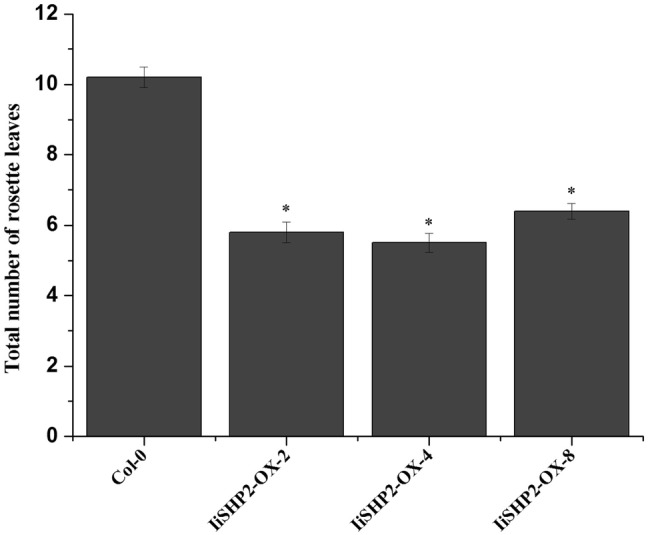
Statistical analysis of rosette leaf number between 35S::IiSHP2 transgenic plants and wild-type Col-0 plants (n = 10). Error bars represent the standard deviation. Asterisks indicate a significant difference (P < 0.05) between transgenic lines and wild-type Col-0 plants
The morphological development of flowers was also affected by IiSHP2. In wild-type Col-0 Arabidopsis plants, the flowers possess four normal whorls of floral organs (Fig. 7a). At the early development stage of flower buds, the petals are wrapped by sepals. Along with the growth of the flowers, the sepals will open and the petals will be exposed, accompanied by the development of stamens and pistil (Fig. 7a, b). However, the structure of the floral organs was changed notably in IiSHP2 overexpressing plants (Fig. 7c–g). Compared to the flower buds of the wild-type plants at the same stage (stage 13 of the flower development in Arabidopsis), the sepals in IiSHP2 overexpressing plants opened ahead of time, the petals were exposed prematurely (Fig. 7c). There was also a change in the orientation of the sepals in transgenic plants. In normal plants, the sepals clinged closely to the petals, incurved slightly and did not splay outwards. On the contrary, the sepals of the overexpressing plants stretched outwardly (Fig. 7d). The white petals of the wild-type Arabidopsis plants were wide and were arranged in a staggered pattern with the sepals. Unlike this, the petals of the transgenic plants were abnormal in development, their size became smaller, the colour of the top was yellowish, the shape looked like a palm-leaf fan and the filamentous base was similar to the staminal column (Fig. 7c, e, f). Moreover, apetalous flowers were also found in certain transgenic plants (Fig. 7d, g).
Fig. 7.
Influence of IiSHP2 overexpression on morphological development of flowers. a, b Flower buds and flowers with four normal whorls of floral organs in wild-type Col-0 plants. c–g Flowers with four whorls of floral organs produced by IiSHP2 transgenic Arabidopsis plants. h–m Abnormal inflorescences and floral organs produced by IiSHP2 transgenic Arabidopsis plants. Bar = 1 mm
Simultaneously, it was found that the development of the apical inflorescence of Arabidopsis could be influenced by overexpression of IiSHP2 (Fig. 7h–m). Under the stereomicroscope, incomplete development of pistil could be observed in a number of flowers. These flowers only possessed one open carpel with exposed ovules and lacked the other carpel (Fig. 7i, k). At the same time, normal pollination could not occur in numerous flowers owing to the abnormal growth of stamens (Fig. 7j). In situation like this, the ovules were infertile and normal seeds could not be produced (Fig. 7k). It should be explained that the pistils without loss of carpel could not receive the pollen from ectopic stamens, and were unable to accomplish normal development as well (Fig. 7m).
The development of siliques and seeds were also obviously affected by IiSHP2. The flower buds of wild-type Arabidopsis plants produce only one pistil. After pollination, the pistil develops into a smooth-skinned silique containing well-developed seeds (Fig. 8a). In IiSHP2 transgenic plants, the length of the siliques became shorter and the diameter of the siliques became smaller (Figs. 8a, 9). Seed abortion was found in siliques due to hypoplasia of pericarp and ovule. Compared to wild-type Arabidopsis, the regions of the siliques encapsulating the well-developed seeds bulged out and wrinkled valves were produced (Fig. 8b, c, e–g). Furthermore, at stage 17 of silique growth, the perianth of wild-type Arabidopsis plants falls off, but the valves are still dark green in colour. However, the perianth of the transgenic Arabidopsis plants did not fall off, the siliques entered a senescent state prematurely and the valve turned yellow gradually (Fig. 8a–g). In addition, siliques curved in varying degrees could be found in many transgenic plants (Fig. 8e–g).
Fig. 8.
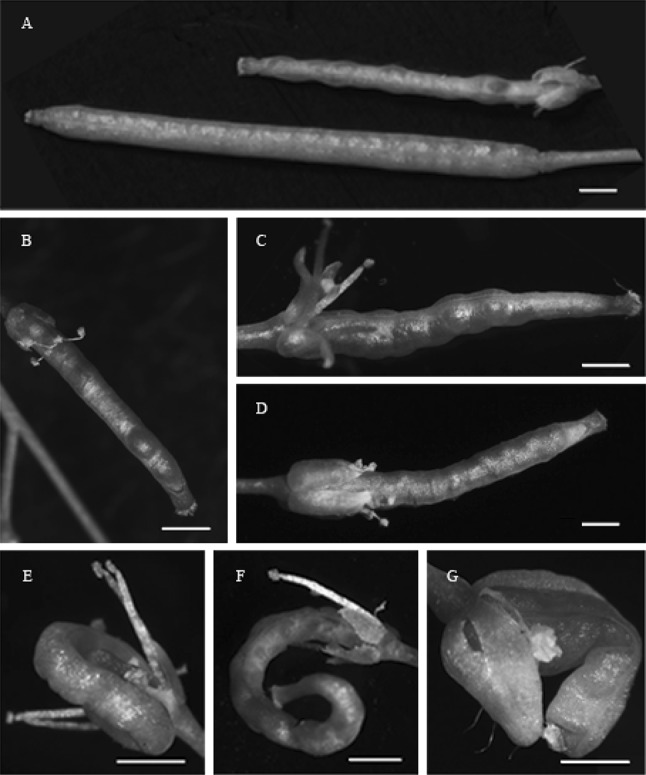
Influence of IiSHP2 overexpression on silique and seed development of Arabidopsis. a Siliques of a wild-type Col-0 plant (lower) and an IiSHP2 transgenic plant (upper). b–g Siliques of IiSHP2 transgenic plants, with non-shedding perianth, wrinkled valves or curled shape. Bar = 1 mm
Fig. 9.
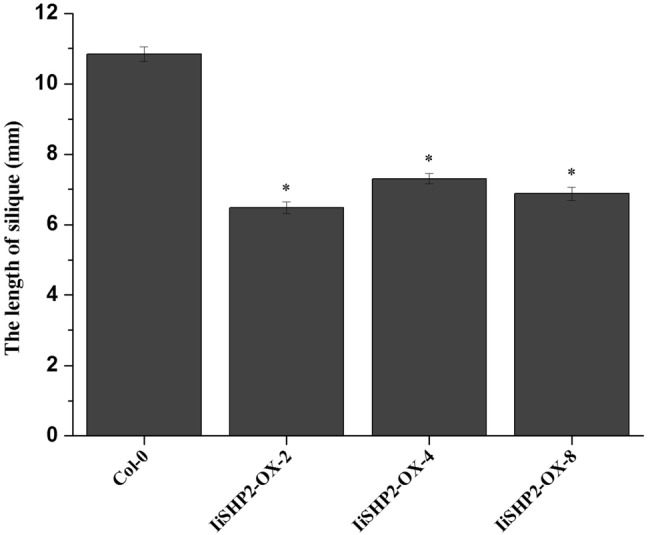
Statistical analysis of the silique length of wild-type Col-0 and 35S::IiSHP2 transgenic Arabidopsis. Error bars represent the standard deviation. Asterisks indicate a significant difference (P < 0.05) between transgenic lines and wild-type Col-0 plants
IiSHP2 can also affect the development of cauline leaves. In wild-type Arabidopsis plants, the cauline leaves are found under the lateral branches, the blades are generally larger and form a flattened elliptic shape. Under normal circumstances, the wild-type cauline leaves are green and are in an expanded state with slight wrinkles (Fig. 10a). In IiSHP2 transgenic Arabidopsis plants, the blade of the cauline leaves curled inwards along the stem in a longitudinal way (Fig. 10b–g). The blade of the cauline leaves under the fully-developed lateral branches curled inwards and were funnel-shaped (Fig. 10b–d, f), whereas the blade of cauline leaves at the positions producing the sprouts of lateral branches curled into a cylindrical shape (Fig. 10e, g). Compared to the wild-type Arabidopsis plants, the length-width ratio of the cauline leaves in transgenic lines decreased substantially (Fig. 10h).
Fig. 10.

Influence of IiSHP2 overexpression on cauline leaf development of Arabidopsis. a Fully-developed cauline leaf of wild-type Arabidopsis plant. b–d, f Fully-developed cauline leaves in IiSHP2 transgenic Arabidopsis plants. e, g The cauline leaves at an earlier stage of production of lateral branches. h Cauline leaves from wild-type (left) and transgenic (right) plants. In a–g, bar = 1 mm. In H, bar = 1 cm
Discussion
As upstream transcriptional factors, MADS proteins participate in all stages of plant growth and development. In the ABCDE model of floral development, the role of MADS-box genes is irreplaceable. C-class floral homeotic genes, such as AG, play an important role in maintenance of the identities of stamens and pistil, whereas the D-class floral homeotic genes, such as STK, control ovule development (Theissen 2001; Rijpkema et al. 2010).
In the present work, the coding sequence of a SHP2 orthologous gene is isolated from I. indigotica. The amino acid sequence of IiSHP2 showed high similarity (93.17%) with SHP2. This means that IiSHP2 is likely to possess the same functions as SHP2. The result of multiple alignment illustrates that IiSHP2 is highly conserved with homologous proteins of other species, such as SHP2, LcSHP2, EsAGL5 and so on (Figs. 1, 2). In phylogenetic analysis, IiSHP2 is grouped into PLE-lineage of C-class floral homeotic genes (Kramer et al. 2004). Moreover, two conserved motifs, AG motif I and AG motif II, are clearly found in the C-terminus of IiSHP2 (Fig. 1), further confirming the identity of IiSHP2 as a C-class MADS-box gene (Kramer et al. 2004).
Expression analysis showed that the transcript abundance of IiSHP2 was very low in vegetative tissues and was high in pistils (Fig. 3a). During fruit growth, the transcript abundance of IiSHP2 increased continuously and was maintained at a high level during later stages of silicle growth (Fig. 3b). These data indicated that IiSHP2 has similar functions with SHP2 in pistil, ovule and fruit development (Fig. 3). In Arabidopsis, expression analysis suggests that the transcripts of SHP1 and SHP2 can be detected obviously in valve margin before and after fertilization, and also in gynoecium primordium, ovule, septum, nectary and style (Flanagan et al. 1996; Favaro et al. 2003; Colombo et al. 2010). Unlike SHP2, the expression level of IiSHP2 in stamens is about 25 times higher than in sepals and petals, indicating it is possibly involved in controlling stamen identity, because the expression patterns of genes often reflect their function (Lü et al. 2007).
Among the other plant species, including Arabidopsis, C-class and D-class genes retain not only their previous functions, but also may evolve new functions. For example, MAwuAG, an euAG-lineage gene, was restricted to reproductive organs, including stamens and carpels (Wu et al. 2012). TrSHP, a PLE-lineage gene, was mainly expressed in stamens, carpels and ovules, and TrAG, an euAG-lineage gene, was mainly expressed in floral meristems, stamens, carpels and ovules (Lü et al. 2007). Clearly, the expression pattern of IiSHP2 is more analogous to that of TrSHP. STK from Arabidopsis is a typical D-class floral homeotic gene. It is mainly expressed in ovules and seed coats, but not in stamens and pistils. Unlike STK, DichSTK is expressed only in pistils (Mizzotti et al. 2012). Although the functions of STK-like genes of different species overlap with those of C-class genes in regulating seed development, these genes do not regulate the development of stamens and pistils under normal circumstances. According to the results of phylogenetic analysis, expression determination and genetic transformation, the function of IiSHP2 is consistent with the C-class genes in PLE-lineage.
Constitutive expression of IiSHP2 in Arabidopsis is the best way to further research its functions. 35S::IiSHP2 transgenic plants produced plentiful phenotypic changes in the first, the second and the fourth whorls of floral organs, and in leaves (Figs. 5, 7, 8, 10). Early flowering is thought to be common in all kinds of transgenic Arabidopsis plants overexpressing MADS-box genes (Kotoda et al. 2002; Thiruvengadam et al. 2012; Hong et al. 2013). Our experimental results showed that the petals were converted into stamineous tissues in transgenic Arabidopsis plants overexpressing IiSHP2 and ovules appeared in the wrong area of carpels. Overexpression of AG, a C-class gene in euAG-lineage, or SHP1 and SHP2, two C-class genes in PLE-lineage, produced similar results (Mizukami and Ma 1992; Favaro et al. 2003). The activity of AG could be substituted partially by ectopic expression of SHP genes, and introduction of 35S::SHP2 into the ag single mutant was sufficient to rescue the deficiency in stamens and carpels (Pinyopich et al. 2003). Apparently, the functions of euAG-lineage and PLE-lineage are partially redundant (Kramer et al. 2004). In addition, misexpression of AG in the second whorl of floral organs mimics the phenotypic changes of apetala2 mutant (ap2) of Arabidopsis (Jack et al. 1997). In IiSHP2 transgenic plants, we found that the surface area of petals was reduced, the shape and the colour of petals was similar to stamens. At the same time, some plants could not produce petals. These results strongly suggest that IiSHP2, as a C-class gene, was able to regulate and control the development of stamens and carpels in woad. In addition, the changes of petals could also be caused by antagonizing A-class genes, such as IiAP1 and IiAP2.
The phenotypic changes of siliques and ovules were also given great attention in our research. In Arabidopsis, the specific expression of SHP2 in valve margins endowed the dehiscence phenotype to siliques (Mühlhausen et al. 2013). SHP1 and SHP2 can regulate the lignification of valve margin cells redundantly and their single mutants are normal in phenotype (Flanagan et al. 1996; Smyth 2000). The siliques of shp1 shp2 double mutant are indehiscent owing to disappearance of the dehiscence zone on the surface of siliques. All the internal cells of the valve were lignified in SHP1 and SHP2 overexpressing plants driven by CaMV 35S promoter and the perianth did not shed when the siliques were ripe (Liljegren et al. 2000). In comparison with the wild-type plants, the diameter of the siliques in SHP1 and SHP2 overexpressing Arabidopsis plants was smaller and precracked valves were found on the siliques (Liljegren et al. 2000; Mühlhausen et al. 2013). Significantly, siliques of smaller diameter were also observed in 35S::IiSHP2 overexpressing transgenic plants and the perianth also did not shed (Fig. 6). Short-sized siliques were found in 35S::IiSHP2 plants, whereas this phenomenon was not found in 35S::SHP1 and 35S::SHP2 transgenic Arabidopsis. In addition, the early cracking siliques were also not found in IiSHP2 transgenic plants. STK, SHP1 and SHP2 are necessary for the development of ovules (Liljegren et al. 2000; Pinyopich et al. 2003). Previous experimental results confirmed that STK is a D-class gene and AG can promote SHP and STK expression in ovules (Pinyopich et al. 2003; Rodríguez-Cazorla et al. 2018). In IiSHP2 overexpressing plants, some ovules have been aborted and normal seeds did not develop, which caused a decline in seed yield. These results indicate that IiSHP2 is similar to SHP1/SHP2 in regulation of the growth of siliques, ovules and seeds. However, some phenotypic differences may be due to the fact that the genes have retained their original functions and undergone slight changes due to differences in the evolutionary process between different plants.
CURLY LEAF (CLF) is involved in control of leaf morphogenesis and is necessary for stable repression of C-class floral homeotic gene AG (Goodrich et al. 1997). The clf mutant showed curled rosette and cauline leaves (Krizek et al. 2006). The phenotype of curled leaves in 35S::IiSHP2 transgenic Arabidopsis indicated that ectopic expression of IiSHP2 in rosette and cauline leaves probably could inhibit the activity of CLF.
Conclusions
IiSHP2 is specifically expressed in stamens, carpels and ovules. Multiple alignment and phylogenetic analysis indicated that IiSHP2 is a putative C-class PLE-lineage gene. Genetic transformation of Arabidopsis showed that IiSHP2 could change the morphology of floral organs in the first, second and fourth whorls. These results confirmed that IiSHP2 was involved in regulation of the development of stamens, pistils and seeds in I. indigotica Fortune. IiSHP2 is highly similar to Arabidopsis SHP, but shows slight changes in function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 The cDNA sequence of IiSHP2 and the amino acid sequence of the encoded product. (DOCX 16 kb)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30870194, J1210063), the Research Project of Provincial Key Laboratory of Shaanxi (15JS111), and the Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University), Ministry of Education.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng-Xin Lu and Dian-Zhen Li have contributed equally to this work.
References
- APG An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc. 2003;141:399–436. doi: 10.1046/j.1095-8339.2003.t01-1-00158.x. [DOI] [Google Scholar]
- Battaglia R, Brambilla V, Colombo L, Stuitje AR, Kater MM. Functional analysis of MADS-box genes controlling ovule development in Arabidopsis using the ethanol-inducible alc gene-expression system. Mech Dev. 2006;123:267–276. doi: 10.1016/j.mod.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Brambilla V, Battaglia R, Colombo M, Masiero S, Bencivenga S, Kater MM, Colombo L. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell. 2007;19:2544–2556. doi: 10.1105/tpc.107.051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Lee PF, Hsiao YY, Wu WL, Pan ZJ, Lee YI, Liu KW, Chen LJ, Liu ZJ, Tsai WC. C- and D-class MADS-box genes from Phalaenopsis equestris (Orchidaceae) display functions in gynostemium and ovule development. Plant Cell Physiol. 2012;53:1053–1067. doi: 10.1093/pcp/pcs048. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Colombo M, Brambilla V, Marcheselli R, Caporali E, Kater MM, Colombo L. A new role for the SHATTERPROOF genes during Arabidopsis gynoecium development. Dev Biol. 2010;337:294–302. doi: 10.1016/j.ydbio.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Ehlers K, Bhide AS, Tekleyohans DG, Wittkop B, Snowdon RJ, Becker A. The MADS box genes ABS, SHP1, and SHP2 are essential for the coordination of cell divisions in ovule and seed coat development and for endosperm formation in Arabidopsis thaliana. PLoS ONE. 2016;11:e0165075. doi: 10.1371/journal.pone.0165075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell. 2003;15:2603–2611. doi: 10.1105/tpc.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C, Liljegren SJ, Yanofsky MF. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science. 2000;289:436–438. doi: 10.1126/science.289.5478.436. [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Hu Y, Ma H. Specific expression of the AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant J. 1996;10:343–353. doi: 10.1046/j.1365-313X.1996.10020343.x. [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. SnapShot: control of flowering in Arabidopsis. Cell. 2010;141(550):550.e1–550.e2. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- Hong JK, Kim SY, Kim KS, Kwon SJ, Kim JS, Kim JA, Lee SI, Lee YH. Overexpression of a Brassica rapa MADS-box gene, BrAGL20, induces early flowering time phenotypes in Brassica napus. Plant Biotechnol Rep. 2013;7:231–237. doi: 10.1007/s11816-012-0254-z. [DOI] [Google Scholar]
- Jack T, Sieburth L, Meyerowitz E. Targeted misexpression of AGAMOUS in whorl 2 of Arabidopsis flowers. Plant J. 1997;11:825–839. doi: 10.1046/j.1365-313X.1997.11040825.x. [DOI] [PubMed] [Google Scholar]
- Kater MM, Dreni L, Colombo L. Functional conservation of MADS-box factors controlling floral organ identity in rice and Arabidopsis. J Exp Bot. 2006;57:3433–3444. doi: 10.1093/jxb/erl097. [DOI] [PubMed] [Google Scholar]
- Kord H, Shakib AM, Daneshvar MH, Azadi P, Bayat V, Mashayekhi M, Zarea M, Seifi A, Ahmad-Raji M. RNAi-mediated down-regulation of SHATTERPROOF gene in transgenic oilseed rape. 3 Biotech. 2015;5:271–277. doi: 10.1007/s13205-014-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoda N, Wada M, Kusaba S, Kano-Murakami Y, Masuda T, Soejima J. Overexpression of MdMADS5, an APETALA1-like gene of apple, causes early flowering in transgenic Arabidopsis. Plant Sci. 2002;162:679–687. doi: 10.1016/S0168-9452(02)00024-9. [DOI] [Google Scholar]
- Kramer EM, Jaramillo MA, Di Stilio VS. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics. 2004;166:1011–1023. doi: 10.1534/genetics.166.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Lewis MW, Fletcher JC. RABBIT EARS is a second-whorl repressor of AGAMOUS that maintains spatial boundaries in Arabidopsis flowers. Plant J. 2006;45:369–383. doi: 10.1111/j.1365-313X.2005.02633.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature. 2000;404:766–770. doi: 10.1038/35008089. [DOI] [PubMed] [Google Scholar]
- Lü S, Du X, Lu W, Chong K, Meng Z. Two AGAMOUS-like MADS-box genes from Taihangia rupestris (Rosaceae) reveal independent trajectories in the evolution of class C and class D floral homeotic functions. Evol Dev. 2007;9:92–104. doi: 10.1111/j.1525-142X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Ma YQ, Li DZ, Zhang L, Li Q, Yao JW, Ma Z, Huang X, Xu ZQ. Ectopic expression of IiFUL isolated from Isatis indigotica could change the reproductive growth of Arabidopsis thaliana. Plant Physiol Biochem. 2017;121:140–152. doi: 10.1016/j.plaphy.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Ma H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell. 1992;71:119–131. doi: 10.1016/0092-8674(92)90271-D. [DOI] [PubMed] [Google Scholar]
- Mizzotti C, Mendes MA, Caporali E, Schnittger A, Kater MM, Battaglia R, Colombo L. The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J. 2012;70:409–420. doi: 10.1111/j.1365-313X.2011.04878.x. [DOI] [PubMed] [Google Scholar]
- Mühlhausen A, Lenser T, Mummenhoff K, Theißen G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013;73:824–835. doi: 10.1111/tpj.12079. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- Rijpkema AS, Vandenbussche M, Koes R, Heijmans K, Gerats T. Variations on a theme: changes in the floral ABCs in angiosperms. Semin Cell Dev Biol. 2010;21:100–107. doi: 10.1016/j.semcdb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cazorla E, Ortuño-Miquel S, Candela H, Bailey-Steinitz LJ, Yanofsky MF, Martínez-Laborda A, Ripoll JJ, Vera A. Ovule identity mediated by pre-mRNA processing in Arabidopsis. PLoS Genet. 2018;14:e1007182. doi: 10.1371/journal.pgen.1007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A reverse trend--MADS functions revealed. Trends Plant Sci. 2000;5:315–317. doi: 10.1016/S1360-1385(00)01690-3. [DOI] [PubMed] [Google Scholar]
- Theissen G. Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol. 2001;4:75–85. doi: 10.1016/S1369-5266(00)00139-4. [DOI] [PubMed] [Google Scholar]
- Thiruvengadam M, Chung IM, Yang CH. Overexpression of Oncidium MADS box (OMADS1) gene promotes early flowering in transgenic orchid (Oncidium Gower Ramsey) Acta Physiol Plant. 2012;34:1295–1302. doi: 10.1007/s11738-012-0926-x. [DOI] [Google Scholar]
- Wei B, Liu D, Guo J, Leseberg CH, Zhang X, Mao L. Functional divergence of two duplicated D-lineage MADS-box genes BdMADS2 and BdMADS4 from Brachypodium distachyon. J Plant Physiol. 2013;170:424–431. doi: 10.1016/j.jplph.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Wu W, Chen F, Jing D, Liu Z, Ma L. Isolation and characterization of an AGAMOUS-like gene from Magnolia wufengensis (Magnoliaceae) Plant Mol Biol Rep. 2012;30:690–698. doi: 10.1007/s11105-011-0385-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 The cDNA sequence of IiSHP2 and the amino acid sequence of the encoded product. (DOCX 16 kb)