Abstract
Cadmium (Cd) is a heavy metal ion leading to morphological and physiological disorders in plants; a specific toxicity target is the membrane lipids. The total lipids were separated by thin-layer chromatography, and the fatty acid composition of the total (TLs), polar lipids (PLs) and triacylglycerol (TAG)—a neutral lipid—was analyzed in maize seedlings in hydroponics and treated by various Cd concentrations (0–200 µM Cd). The TLs and PLs significantly decreased in roots after Cd treatment, suggesting the onset of lipid peroxidation mediated by oxygen free radicals, that induce alterations of the membrane structure and function. There were also increases in the TAG from 28.2 to 36.9% of TLs, and the TAG/PLs ratio varied from 0.59 to 0.84, in control and after exposure to 200 µM Cd, respectively. The TAG plays potent roles in membrane turnover serving as energy and carbon resources for the biosynthesis of membrane lipids, to preserve membrane structure and function, and therefore cell homeostasis in response to Cd. In shoots, a significant increase in the levels of C16:0, C18:1, and C18:2, while a decrease in that of C18:3 was observed, suggesting inhibition of desaturases enzymes. These lead to impairment of the chloroplast membrane. The total lipid content did not change under Cd stress. The PLs, however, decreased from 22.4 to 13.6 mg g−1 DW; their percent to TLs varied from 86.6 to 52.5%, in control, and after Cd treatment, respectively. In conclusion, the accumulation of TAG may represent a defense strategy by which maize seedlings can withstand the effects of Cd toxicity, leading to reduced oxidative stress.
Keywords: Cadmium, Neutral lipids, Polar lipids, Thin layer chromatography, Triacylglycerol, Zea mays
Introduction
According to FAO, maize (Zea mays L.) is the main commonly cultivated crop in Latin America, Africa, and Asia. Previous studies have shown that heavy metals limit plant productivity worldwide. Nowadays, heavy metal pollution of the environment is a subject of profound concern. Essential mineral elements such as Zn, Fe, and Cu play crucial roles in plants as enzyme cofactors in many biological processes. However, non-essential minerals produced harmful effects. Heavy metal ions can spread in soils owing to high levels of industrial effluents and human waste. These include sewage treatment and intensive use of agrochemicals such as pesticides and fertilizers (Satarug et al. 2003).
Contamination of soils with cadmium (Cd)–a toxic heavy metal–as a result of the use of phosphate fertilizers, sewage, sludge, mine, and industrial wastes, induces primary toxicity symptoms such as inhibition of plant growth, alteration of photosynthesis and oxidative stress (Liu et al. 2008). Cd in the nutrient medium affect the uptake and the translocation of essential mineral nutrients such as Zn, Fe, and Mn, through Cd-competition at the root absorption-sites for the membrane transporters, leading to decreased nutrient uptake, and alteration of mineral homeostasis (Rodríguez-Serrano et al. 2009). It can also bind to proteins and substitute essential minerals such as Zn, Fe, and Ca in their prosthetic groups (Fagioni et al. 2009). Previous studies have shown alterations in the activity of enzymes, such as peroxidases and catalases, leading to increased production of reactive oxygen species (ROS) and peroxidation of chloroplast lipids (Sharma and Dietz 2009). A primary process in hyper-accumulating plants is the Cd translocation in the xylem in free form to the shoot which is influenced by the transpiration rate (Lu et al. 2009; Kranner and Colville 2011). Increasing Cd concentrations in shoots caused the inhibition of many processes: the photochemical reaction and the carbon fixation of the photosynthesis (Andresen and Küpper 2013; Küpper et al. 2007; Perreault et al. 2011). In addition, leaf chlorosis correlated with Fe deficiency.
Polar lipids (PLs) play a role as structural constituents of the membranes; the neutral lipids (NLs), however, serve as an energy and carbon storage compounds such as triacylglycerol (TAG). Cd induces great disturbance to PLs such as galactolipids, phospholipids, and sulfolipids. Increasing TAG accumulation has shown to play an essential role as energy resources for the membrane lipid biosynthesis and promoting root growth under stress (Grillitsch et al. 2011). Nutrient deficiencies, high salinity, excess light, and heat stress were efficient in inducing the accumulation of TAG in many algal species under stressful conditions (Hu et al. 2008; Khotimchenko and Yakovleva 2004; Légeret et al. 2016; Siaut et al. 2011). TAG accumulation has been shown to provide energy and carbon storage sources in microalgal cells under nitrogen-depletion conditions (Popko et al. 2016; Sato et al. 2014; Wang et al. 2009). The biosynthesis of membrane lipids from fatty acids resulting from TAG degradation is a crucial phenomenon in maintaining membrane homeostasis in actively growing cells of yeast, Saccharomyces cerevisiae (Kohlwein and Henry 2011).
Previous results have shown the crucial role of TAG in promoting growth in unicellular eukaryotic organisms under mineral deficiencies. In plants, however, its role in coping with heavy metal stress is still unknown. To provide further insight into the role of NLs in the tolerance mechanisms, we analyzed changes in the TAG and PLs compositions in root and shoot of maize seedlings grown under Cd stress.
Materials and methods
Growth conditions
The maize seeds (Alistrong cv.) were purchased from Semences de Provence (France). The seed material was disinfected by immersion in 10% hydrogen peroxide, washed extensively with tap water, and then with distilled water. Seeds were soaked 12 h in aerated distilled water to increase the germination rate. Germination was carried on filter papers in Petri dishes at 25 °C in the dark for 4 days, then the seedlings were grown in plastic pots containing aerated Hoagland-modified nutrient solutions for 14 days. The seedlings were then transplanted to 6 l of fresh nutrient solutions with different concentrations of Cd(NO3)2: 0, 25, 50, 100 and 200 μM. The following is the composition of macronutrients (in mmol l−1): KNO3 (2), Ca(NO3)2 (2.5), KH2PO4 (1), MgSO4 (1), and micronutrients (in µmol l−1): Fe-K-EDTA (50), H3BO3 (30), MnSO4 (10), CuSO4 (1), ZnSO4 (1), and (NH4)6Mo7O24 (0.2), at pH 5.7 (Hoagland and Arnon 1950). The artificial growing conditions were: day/night temperature 25/22 ± 1 °C, 16 h-photoperiod and a photon flux density of 150 µmol m−2 s−1 photosynthetic active radiation, provided by mercury lamps.
Plant harvesting and chlorophyll determination
After 3 days of Cd treatment, maize seedlings were harvested, separated into roots and shoots, the roots rinsed many times with distilled water, wiped on filter paper, then fresh weight (FW) of the two plant parts determined. The dry weight (DW) of the plant samples was estimated after oven-drying for several days at 50 °C and until constant sample weight. The growth parameters shown in Fig. 2 were determined as described by Chaffai et al. (2009). The total chlorophyll extraction was done in acetone solution at 80% (Arnon 1949).
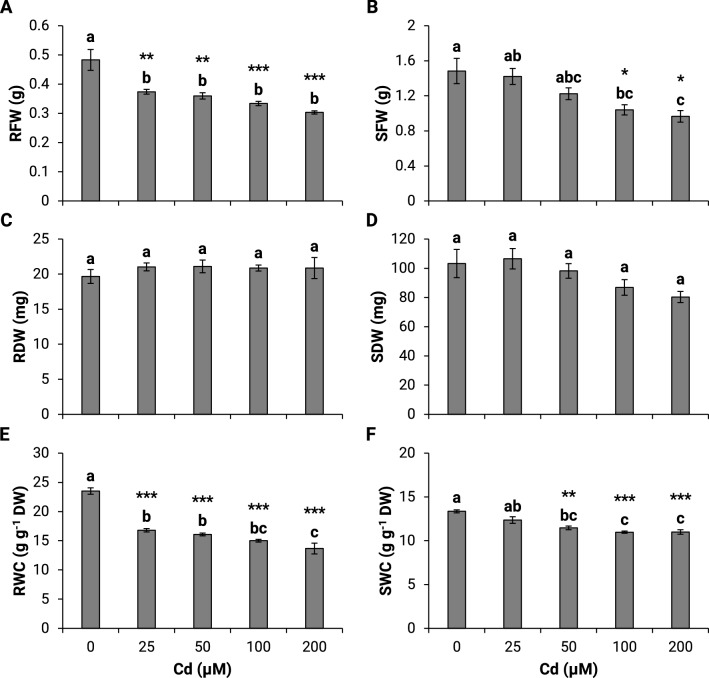
Fig. 2.
Effect of different cadmium concentrations on the fresh weight (a, b), dry weight (c, d) , and water content (e, f) in roots and shoots of maize seedlings in hydroponic culture. RFW, root fresh weight; SFW, shoot fresh weight; RDW, root dry weight; SDW, shoot dry weight; RWC, root water content; SWC, shoot water content. Statistical analysis was performed using Minitab version 18.1. One-way analysis of variance (ANOVA) followed by Tukey’s pairwise comparisons was performed to determine significant differences between means of five independent replicates (n = 5). The same lower-case letters are not significantly different means. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Fatty acid extraction and analysis
Lipids were extracted according to the method of Folch et al. (1957) and modified by Bligh and Dyer (1959). The neutral (nonpolar) lipids were separated using silica gel plates by thin-layer chromatography (TLC) into four classes: triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG) and Free fatty acids (FFA). The mobile phase mixture comprises petroleum ether, ethyl ether, and acetic acid at the proportions 70/30/0.4 (v/v/v) (Mangold 1961). Polar lipids (galactolipids and phospholipids) do not migrate into the recommended solvent mixture (Fig. 1). Neutral lipids migrated and were resolved upon development with the mobile phase, whereas phospholipids remained at the origin (Fried and Sherma 1999). The total polar (PL) and neutral (TAG) lipid spots were scraped off from the TLC plates. For gas chromatography analysis, the fatty acids in the plant samples were converted into fatty acid methyl esters (FAME). For lipid extraction, plant samples were placed in a glass tube and homogenized in 2 ml of hexane. The trans-methylation reaction of fatty acids was carried out by adding: sodium methylate 1% (0.5 ml), an internal standard of methyl heptadecanoic acid (C17:0), H2SO4 1 N (0.2 ml), and sodium chloride 5% (1.5 ml) (Carreau and Dubacq 1978). The superior phase containing the FAME was evaporated off under a stream of N2 to reduce its volume to a minimum. The analysis of FAME was performed on 6890 Agilent gas chromatography equipped with a flame ionization detector (GC-FID HP 6890 Series, Agilent Technologies, Palo Alto, CA, USA) equipped with an HP-Innowax type (HP 1909 1 N-133) capillary column (Polar stationary phase: polyethylene glycol, 30 m × 0.25 mm id, film thickness 0.25 μm). The maximum temperature of the column was maintained at 260 °C, allowing the separation of fatty acids according to the length of their chain and their unsaturation.
Fig. 1.
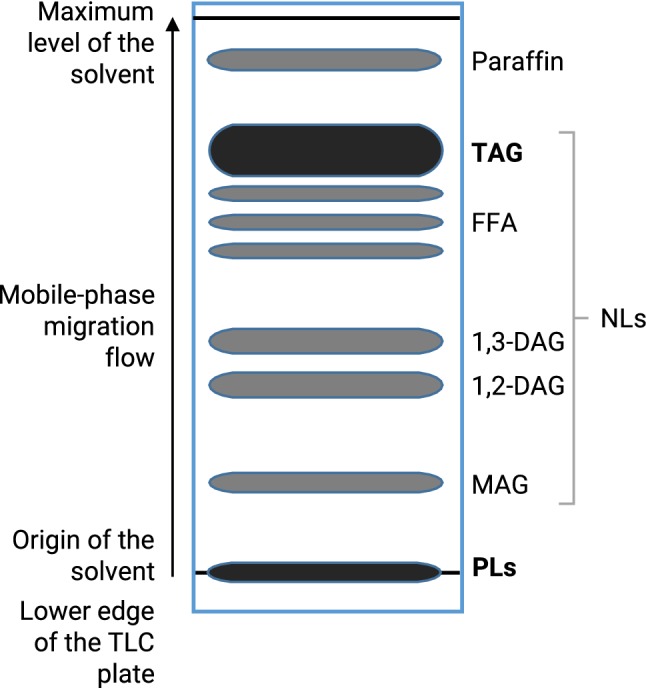
A typical schematic thin layer chromatography (TLC) separation of major neutral lipid classes on a silica-gel plate developed with a solvent mixture of petroleum ether/ethyl ether/acetic acid (70:30:0.4, v/v/v) (Mangold 1961). PLs, polar lipids; MAG, monoacylglycerol; NLs, neutral lipids; 1,2-DAG, 1,2 monoacylglycerol; 1,3-DAG, 1,3 diacylglycerol; FFA, free fatty acids; TAG, triacylglycerol. The bands of PLs and TAG showed in black were scraped off the TLC plate and extracted
Data acquisition and processing was performed by the Hewlett-Packard ChemStation Rev. A.0401 under the following conditions: flow rate of 1.5 ml min−1, mode split, a split, the ratio of 60:1, the temperature of the injector 250 °C, the temperature of the detector 275 °C. The oven temperatures: isotherm at 150 °C for 1 min, from 150 to 200 °C at a rate of 15 °C min−1, from 200 to 225 °C at a rate of 2 °C min−1, then isotherm at 225 °C for 2 min. The fatty acids identification was done by comparing their retention times with those of standard fatty acid methyl esters under the same conditions, and their amounts calculated using the internal standard (C17:0) as a reference.
Statistical analysis
One-way ANOVA was performed using Minitab version 18.1 (Minitab Inc., State College, PA, USA). Differences between pairs of means were compared for statistical significance using the Tukey test at the 95% confidence level, showing the significant difference at P ≤ 0.05. In tables and figures, results represent means and standard error (± SE); those labeled with the same letter are not significantly different.
Results
Effect of cadmium on growth and total chlorophylls
This study examined the effect of varying Cd concentrations on maize growth. The Cd exposure induced a decrease in the fresh weight of the roots by 23 and 37%, at 25 µM and 200 µM Cd, respectively (Fig. 2a). A less pronounced effect was observed in the shoots, with a 32.5% decrease at 100 and 200 µM Cd (Fig. 2b). There were no significant changes in the root and shoot dry weight (Fig. 2c, d). The roots’ water content decreased markedly from 29 to 42% at 25 µM and 200 µM Cd, respectively. However, the shoot’s water content showed a lesser decrease by about 16.7% after exposure to 50, 100, and 200 µM Cd (Fig. 2e, f). Exposure to Cd had decreased the total chlorophyll content by 27.3 and 55.7%, at 50 and 200 µM, respectively (Fig. 3).
Fig. 3.
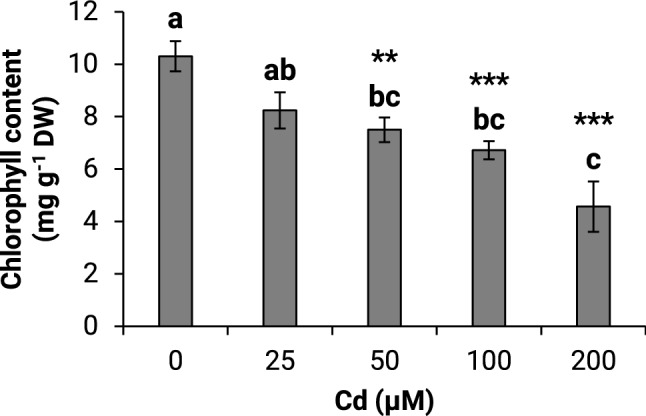
Effect of different cadmium concentrations on total chlorophyll content of maize seedlings in hydroponic culture. The same lower-case letters are not significantly different means. *P ≤0.05, **P ≤ 0.01, ***P ≤ 0.001
Effect of Cd on the fatty acid composition of total lipids, neutral and polar lipids
The total fatty acid profile from roots included palmitic (C16:0), oleic (C18:1), linoleic (C18:2), linolenic (C18:3), and lignoceric (C24:0) acids (Table 1). The C18:2 was the most abundant fatty acid, accounting for about 36.5% of total fatty acids (TFA) in control roots, while C18:1 was the minor component. Cd exposure reduced the root total fatty acid amount by about 37.8%, and increased the C18:1 level by about 60.5%, while the other fatty acids remained unchanged.
Table 1.
Fatty acid composition of total lipids analyzed in roots of maize treated with different Cd concentrations for 3 days in hydroponics
| Cd (µM) | Fatty acid (mol%) | Total fatty acids (mg g−1 DW) | ||||
|---|---|---|---|---|---|---|
| C16:0 | C18:1 | C18:2 | C18:3 | C24:0 | ||
| 0 | 29.68 ± 1.76a | 4.09 ± 0.32b | 36.50 ± 2.05ab | 4.23 ± 0.53a | 32.29 ± 4.79a | 8.10 ± 0.63a |
| 25 | 29.21 ± 0.67a | 7.37 ± 0.10a | 44.33 ± 0.36a | 5.71 ± 0.03a | 13.08 ± 0.22a | 5.16 ± 0.43b |
| 50 | 24.28 ± 1.81a | 6.39 ± 0.43a | 43.08 ± 3.52ab | 5.12 ± 0.39a | 17.61 ± 6.31a | 5.24 ± 0.33b |
| 100 | 23.89 ± 1.92a | 5.92 ± 0.35a | 32.98 ± 2.96b | 5.54 ± 0.24a | 32.74 ± 5.63a | 5.08 ± 0.45b |
| 200 | 25.64 ± 1.39a | 6.56 ± 0.59a | 36.28 ± 1.59ab | 5.70 ± 0.35a | 19.54 ± 3.74a | 4.70 ± 0.67b |
The results are mean (± SE) of five independent experiments (n = 5) and expressed as mol%. Means with the same lower-case letters in the same column are not significantly different at P ≤ 0.05 using one-way ANOVA and Tukey’s multiple comparisons with Minitab version 18.1
In shoots, the predominant fatty acid was α-linolenic (C18:3), accounting for 53.3% of TFA in control, followed by C18:2, C22:1, C16:0, and C24:0, while C18:1 was the minor component (Table 2). Cd exposure decreased the α-C18:3 levels by about 12.5% significant from 50 µM of Cd exposure. Those of C18:2, however, increased significantly by 19% from 25 µM Cd and C18:1 by 71% from 100 µM Cd.
Table 2.
Fatty acid composition of total lipids analyzed in shoots of maize treated with different Cd concentrations for 3 days in hydroponics
| Cd (µM) | Fatty acid (mol%) | Total fatty acids (mg g−1 DW) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:1 | C18:2 | C18:3 | C22:1 | C24:0 | ||
| 0 | 13.81 ± 0.38b | 2.38 ± 0.08c | 15.83 ± 0.26b | 53.26 ± 0.61a | 14.00 ± 0.32a | 4.21 ± 1.22a | 25.84 ± 1.03a |
| 25 | 15.69 ± 0.16a | 2.55 ± 0.08c | 17.93 ± 0.26a | 50.02 ± 1.05ab | 12.87 ± 0.42ab | 1.04 ± 0.17b | 20.59 ± 1.20a |
| 50 | 15.76 ± 0.48a | 2.93 ± 0.12bc | 18.74 ± 0.86a | 46.99 ± 1.98b | 11.32 ± 0.49c | 1.65 ± 0.14ab | 21.78 ± 1.27a |
| 100 | 15.93 ± 0.36a | 4.39 ± 0.42a | 19.50 ± 0.32a | 45.64 ± 0.69b | 12.75 ± 0.41abc | 1.61 ± 0.10ab | 21.98 ± 1.32a |
| 200 | 14.80 ± 0.18ab | 3.75 ± 0.06ab | 19.17 ± 0.20a | 47.21 ± 0.16b | 12.46 ± 0.04bc | 2.44 ± 0.52ab | 23.73 ± 1.40a |
The results are mean (± SE) of five independent experiments (n = 5) and expressed as mol%. Means with the same lower-case letters in the same column are not significantly different at P ≤ 0.05 using one-way ANOVA and Tukey’s multiple comparisons with Minitab version 18.1
The total lipids (TLs) were separated through thin layer chromatography using the system of Mangold; while the PLs remained at the origin, the NLs revealing four different classes-monoacylglycerol (MAG), diacylglycerol (DAG), free fatty acids (FFA) and triacylglycerol (TAG). In roots, the TAG made up about 28.2% of TLs in control; the C16:0 predominated, making up 61.9% of TFA. The fatty acid profile and content of TAG showed no significant change after Cd exposure; the TAG percent, however, increased markedly to 36.9% of TLs (Table 3). The fatty acid profile of PLs of roots contained C16:0 as the predominant fatty acid representing 52.3% of the control (Table 3). While this profile did not change following Cd treatment, the amount of PLs, however, decreased by 46.6%, and the percent from 47.8 to 25.5% of TLs (Table 3).
Table 3.
Fatty acid composition of triacylglycerol (TAG) and polar lipids (PL) analyzed in roots of maize treated for 3 days with 200 µM Cd in hydroponics
| Lipid class | Fatty acids (mol%) | Total lipids (mg g−1 DW) | Percent of total lipids | ||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:1 | C18:2 | C18:3 | C24:0 | |||
| TAG | |||||||
| Control | 61.86 ± 6.38a | 8.47 ± 0.53a | 20.97 ± 2.26a | 18.16 ± 0.00a | 4.76 ± 0.00a | 2.28 ± 0.33a | 28.2 |
| Cd | 62.84 ± 8.60a | 8.92 ± 0.79a | 16.85 ± 2.79a | 10.87 ± 0.00b | 3.21 ± 0.74a | 1.73 ± 0.28a | 36.9 |
| PL | |||||||
| Control | 52.29 ± 2.93a | 6.49 ± 0.61a | 37.76 ± 1.93a | 8.19 ± 3.14a | 2.08 ± 0.00b | 3.88 ± 0.25a | 47.8 |
| Cd | 40.98 ± 4.92a | 8.88 ± 2.43a | 38.16 ± 0.15a | 10.35 ± 5.59a | 3.24 ± 0.00a | 2.07 ± 0.03b | 25.5 |
The results are mean (± SE) of three independent experiments (n = 3) and expressed as mol%. Means with the same lower-case letters in the same column are not significantly different at P ≤ 0.05 using one-way ANOVA and Tukey’s multiple comparisons with Minitab version 18.1
The fatty acid composition of the shoots revealed several changes, mostly in the polar lipid fraction (Table 4). These alterations were through the increase of C16:0 and C18:2 levels in PLs (19.4 and 14.7% increases, respectively) and the decrease of that of C18:3 (9.4% decrease) following 200 µM of Cd exposure. The fatty acid analysis in shoots showed that the most significant changes were in PLs following the Cd treatment, such as the increase in the C18:2 level by 14.7%, and the decrease in that of the major fatty acid C18:3 by 9.4% (Table 4). The percent of PLs decreased markedly from 86.6 to 52.5% of TLs; their amounts were reduced by 39.4% upon the addition of Cd (Table 4).
Table 4.
Fatty acid composition of triacylglycerol (TAG) and polar lipids (PL) analyzed in shoots of maize treated for 3 days with 200 µM Cd in hydroponics
| Lipid class | Fatty acids (mol%) | Total lipids (mg g−1 DW) | Percent of total lipids | ||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:1 | C18:2 | C18:3 | C24:0 | |||
| TAG | |||||||
| Control | 21.46 ± 4.19a | 66.47 ± 5.09a | 3.49 ± 0.31b | 2.28 ± 0.33a | 2.20 ± 0.54a | 5.00 ± 1.08a | 19.4 |
| Cd | 20.08 ± 0.98a | 69.48 ± 1.19a | 4.84 ± 0.24a | 1.79 ± 0.18a | 2.32 ± 0.17a | 3.89 ± 0.55a | 16.4 |
| PL | |||||||
| Control | 17.60 ± 0.58b | 2.74 ± 0.42a | 12.52 ± 0.26b | 61.13 ± 0.82a | 6.02 ± 0.42a | 22.38 ± 0.38a | 86.6 |
| Cd | 21.02 ± 0.68a | 3.41 ± 0.10a | 14.36 ± 0.54a | 55.38 ± 1.73b | 5.83 ± 1.38a | 13.57 ± 1.30b | 52.5 |
The results are mean (± SE) of three independent experiments (n = 3) and expressed as mol%. Means with the same lower-case letters in the same column are not significantly different at P≤0.05 using one-way ANOVA and Tukey’s multiple comparisons with Minitab version 18.1
Discussion
In the present study, Cd exposure induced a significant inhibition of maize growth, principally in roots; the leaves showed outward chlorosis. Heavy metal contamination of the environment has been increasing worldwide. Cd is a heavy metal causing reduced plant growth. Previous studies have shown a reduction in root elongation and browning symptoms following a mineral nutrient disordering induced by Cd (Lux et al. 2011). The cell walls constitute the primary site for Cd accumulation in plants due to the negatively charged groups, inhibiting the uptake of essential nutrients. There is evidence that root deterioration by Cd leads to a disturbance in soil water gaining. It was likely that the toxicity effect of Cd involved reduced water uptake and content (Perfus-Barbeoch et al. 2002).
The exposure of Phragmites australis to Cd resulted in a sharp decrease in the total chlorophyll content, and chlorosis, which is considered as being common toxicity symptoms (Andresen and Küpper 2013; Schützendübel et al. 2001). Fe deficiency (Krupa and Legge 1999), impairment of PSII photochemical and non-photochemical processes of photosynthesis due to Mg substitution by Cd in chlorophylls (Pietrini et al. 2003), the Ca replacement in the photosynthetic water splitting complex, the oxidative damage to the photochemical apparatus, are mechanisms underlying the Cd-induced chlorosis in plants (Faller et al. 2005). In cereals, the oxidative stress induced by Cd altered the photochemical and non-photochemical phases of photosynthesis (Dias et al. 2019).
Studies have previously reported that alteration in the relative proportions of lipid classes plays a crucial role in stress adaptation. Our results showed that excess Cd reduced polar lipids in roots, accompanied by an increase in the fraction of neutral lipids (TAG), suggesting an alteration of the lipid biosynthesis pathway towards the accumulation of TAG (Guschina and Harwood 2006). Any increase in fatty acyl chains is compensated for by the corresponding increase in the biosynthesis of neutral lipids, the most common form of energy under stressful conditions (Formighieri 2015; Harwood and Gurr 2013; Osanai et al. 2017); this may serve for nutrient uptake, the detoxification of ROS and the plant homeostasis (Choudhury et al. 2017; Parvaiz and Prasad 2011).
A profound alteration in energy metabolism plays a crucial role in stress acclimation and alleviation (Heazlewood et al. 2016); the nonpolar storage lipids are the main pools for chemical energy and supply building blocks for membrane lipid synthesis in microorganisms and eukaryotic cells (Rajakumari et al. 2008). An increase in the TAG levels, alongside lower polyunsaturated fatty acids, has been shown in a microalga, Chlorella vulgaris subjected to Cd stress (Chia et al. 2013). TAG is a principal storage glycerolipid accumulated in plants subjected to growth-limiting conditions (Tjellström et al. 2015; Meï et al. 2017) such as in the halotolerant green microalga Dunaliella tertiolecta under nitrogen starvation (Razeghifard 2013; Pick and Avidan 2017); it may also serve for the biosynthesis of osmolytes (sorbitol, proline, glycine betaine) to maintain the cell water flux and turgor under stress (Chakraborty and Chakraborty 2015). This storage lipid can play a crucial role in sustaining plant growth under stress. It is involved in the biosynthesis of other lipid compounds, such as sulfolipids and phospholipids, depleted as the consequences of stress-induced sulfur and phosphorus deficiencies.
In shoots, the impairment of the polar lipid fraction concomitant with changes in the levels of C18:1, C18:2, and C18:3, suggesting the Cd-induced either direct lipid peroxidation of chloroplast membranes or through alterations of desaturases activity. Those ensuring the conversion of C18:1 into C18:2—especially the oleoyl-desaturase—might be enhanced, while there was inhibition of the linoleoyl desaturase-Δ-15-desaturase—that converts C18:2 into C18:3. The increase in C18:2 level of the extra-plastidial membranes has been shown in tomato leaves, suggesting inhibition of desaturase activity upon the addition of Cd (Nouairi et al. 2006; Verdoni et al. 2001); those of polyunsaturated fatty acids had decreased in metal-stressed microalgae (Guschina and Harwood 2006), and the MGDG and PG—the abundant polar lipids of the plastids—decreased markedly in maize seedlings subjected to Cd stress (Chaffai et al. 2007, 2009).
C18:3 is a specific target for heavy metal stress, the main component of polar lipids in membranes, and has an essential role in the grana adjoining that allows light absorption during photosynthesis. Cd exposure, therefore, exerted alteration of the plasma membrane, inducing impairment of the photosynthesis in maize. Oxidative stress is the principal mechanism associated with the disruption of polyunsaturated fatty acids, occurring via the generation of ROS and enhanced lipoxygenase activity (Guo et al. 2007); these (C18:3 and C16:4) released from chloroplast galactoglycerolipids are utilized for TAG formation under N deprivation (Goncalves et al. 2013; Davidi et al. 2014; Pick and Avidan 2017). Lipid peroxidation has been shown to increase under Cd stress and associated with a decrease in the activity of Cu–Zn superoxide dismutase (Sandalio et al. 2001). The Cd mainly affected the membrane lipids in flax leaves, especially the relative proportions of polyunsaturated fatty acids, C18:2 and C18:3; a decrease in C18:3 with a corresponding increase in C18:2, C18:1, and C18:0 have been shown in lettuce and tomato seedlings grown in contaminated soil (Djebali et al. 2005; Verdoni et al. 2001). Exposure to 200 µM Cd has shown to result in sharp decreases in the total and polar lipid contents in leaves of Mesembryanthemum crystallinum and Sesuvium portulacastrum (Nouairi et al. 2006).
Conclusion
In conclusion, Cd treatment induced severe damage to roots—the primary targets for heavy metal stress—but to a lesser extent in shoots. Cd stress affected lipid metabolism and turnover. The increase in TAG with the concomitant decrease in PLs may reflect a regulation in the lipid pathway; the changes are necessary to fulfill energy supply to maintain membrane fluidity and homeostasis and to cope with the stress leading to reduced oxidative damage due to the onset of lipid peroxidation mediated by oxygen free radicals.
Acknowledgements
I dedicate this work to the memory of my supervisor at the Master’s Level Prof Ali Tekitek who advanced my knowledge on thin-layer chromatography techniques through his full encouragement and insightful support. Also, I am grateful to Prof Brahim Marzouk, a retired professor of plant biochemistry and a pioneering expert of plant lipids, for proposing the research theme and plan of this work and his competent help and assistance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andresen E, Küpper H. Cadmium toxicity in plants. Met Ions Life Sci. 2013;11:395–413. doi: 10.1007/978-94-007-5179-8_13. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Carreau JP, Dubacq JP. Adaptation of a macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J Chromatogr A. 1978;151:384–390. doi: 10.1016/S0021-9673(00)88356-9. [DOI] [Google Scholar]
- Chaffai R, Seybou TN, Marzouk B, El Ferjani E. Effects of cadmium on polar lipid composition and unsaturation in maize (Zea mays) in hydroponic culture. J Integr Plant Biol. 2007;49:1693–1702. doi: 10.1111/j.1744-7909.2007.00585.x. [DOI] [Google Scholar]
- Chaffai R, Seybou TN, Marzouk B, El-Ferjani E. A comparative analysis of fatty acid composition of root and shoot lipids in Zea mays under copper and cadmium stress. Acta Biol Hung. 2009;60:109–125. doi: 10.1556/ABiol.60.2009.1.10. [DOI] [PubMed] [Google Scholar]
- Chakraborty U, Chakraborty B. Abiotic stresses in crop plants. New Delhi: CABI; 2015. [Google Scholar]
- Chia MA, Lombardi AT, MdaG Melão, Parrish CC. Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquat Toxicol. 2013;128–129:171–182. doi: 10.1016/j.aquatox.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- Davidi L, Shimoni E, Khozin-Goldberg I, Zamir A, Pick U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014;164:2139–2156. doi: 10.1104/pp.113.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MC, Santos C, Pinto G, Silva AMS, Silva S. Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat. Protoplasma. 2019;256:69. doi: 10.1007/s00709-018-1281-6. [DOI] [PubMed] [Google Scholar]
- Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel MH, Chaïbi W. Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol. 2005;7:358–368. doi: 10.1055/s-2005-837696. [DOI] [PubMed] [Google Scholar]
- Fagioni M, D’Amici GM, Timperio AM, Zolla L. Proteomic analysis of multiprotein complexes in the thylakoid membrane upon cadmium treatment. J Proteome Res. 2009;8:310–326. doi: 10.1021/pr800507x. [DOI] [PubMed] [Google Scholar]
- Faller P, Kienzler K, Krieger-Liszkay A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim Biophys Acta. 2005;1706:158–164. doi: 10.1016/j.bbabio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Folch JM, Lee GH, Stanley S. A simple method for the isolation of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Formighieri C. Solar-to-fuel conversion in algae and cyanobacteria. Cham: Springer; 2015. [Google Scholar]
- Fried B, Sherma J. Thin-layer chromatography, revised and expanded. 4. New York: CRC Press; 1999. [Google Scholar]
- Goncalves EC, Johnson JV, Rathinasabapathi B. Conversion of membrane lipid acyl groups to triacylglycerol and formation of lipid bodies upon nitrogen starvation in biofuel green algae Chlorella UTEX29. Planta. 2013;238:895–906. doi: 10.1007/s00425-013-1946-5. [DOI] [PubMed] [Google Scholar]
- Grillitsch K, Connerth M, Köfeler H, Arrey TN, Rietschel B, Wagner B, Karas M, Daum G. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: Lipidome meets Proteome. Biochim Biophys Acta Mol Cell Biol Lipids. 2011;1811:1165–1176. doi: 10.1016/j.bbalip.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Zhou H, Zhang X, Li X, Meng Q. Overexpression of CaHSP26 in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J Plant Physiol. 2007;164:126–136. doi: 10.1016/j.jplph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Guschina IA, Harwood JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45:160–186. doi: 10.1016/j.plipres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Harwood JL, Gurr MI. Lipid biochemistry: an introduction. Boston: Springer; 2013. [Google Scholar]
- Heazlewood JL, Jorrín-Novo JV, Agrawal GK, Mazzuca S, Lüthje S. Editorial: international plant proteomics organization (INPPO) world congress 2014. Front Plant Sci. 2016;7:1190. doi: 10.3389/fpls.2016.01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D, Arnon DI. The water culture method for growing plants without soil. Berkeley: College of Agriculture, University of California; 1950. [Google Scholar]
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- Khotimchenko SV, Yakovleva IM. Effect of solar irradiance on lipids of the green alga Ulva fenestrata Postels et Ruprecht. Bot Mar. 2004;47:395–401. doi: 10.1515/BOT.2004.050. [DOI] [Google Scholar]
- Kohlwein SD, Henry SA. Coordination of storage lipid synthesis and membrane biogenesis: evidence for cross-talk between triacylglycerol metabolism and phosphatidylinositol synthesis. J Biol Chem. 2011;286:1696–1708. doi: 10.1074/jbc.M110.172296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Colville L. Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environ Exp Bot. 2011;72:93–105. doi: 10.1016/j.envexpbot.2010.05.005. [DOI] [Google Scholar]
- Krupa SV, Legge AH. Foliar injury symptoms of Saskatoon serviceberry (Amelanchier alnifolia Nutt.) as a biological indicator of ambient sulfur dioxide exposures. Environ Pollut. 1999;106:449–454. doi: 10.1016/S0269-7491(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- Légeret B, Schulz-Raffelt M, Nguyen HM, Auroy P, Beisson F, Peltier G, Blanc G, Li-Beisson Y. Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids. Plant Cell Environ. 2016;39:834–847. doi: 10.1111/pce.12656. [DOI] [PubMed] [Google Scholar]
- Liu K, Shen L, Sheng J. Improvement in cadmium tolerance of tomato seedlings with an antisense DNA for 1-aminocyclopropane-1-carboxylate synthase. J Plant Nutr. 2008;31:809–827. doi: 10.1080/01904160802043080. [DOI] [Google Scholar]
- Lu LL, Tian SK, Yang XE, Li TQ, He ZL. Cadmium uptake and xylem loading are active processes in the hyperaccumulator Sedum alfredii. J Plant Physiol. 2009;166:579–587. doi: 10.1016/j.jplph.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculik M, White PJ. Root responses to cadmium in the rhizosphere: a review. J Exp Bot. 2011;62:21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- Mangold HK. Thin-layer chromatography of lipids. J Am Oil Chem Soc. 1961;38:708–727. doi: 10.1007/BF02633061. [DOI] [Google Scholar]
- Meï CE, Cussac M, Haslam RP, Beaudoin F, Wong YS, Maréchal E, Rébeillé F. C1 metabolism inhibition and nitrogen deprivation trigger triacylglycerol accumulation in Arabidopsis thaliana cell cultures and highlight a role of NPC in phosphatidylcholine-to-triacylglycerol pathway. Front Plant Sci. 2017;7:2014. doi: 10.3389/fpls.2016.02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouairi I, Ghnaya T, Youssef NB, Zarrouk M, Ghorbel MH. Changes in content and fatty acid profiles of total lipids of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum under cadmium stress. J Plant Physiol. 2006;163:1198–1202. doi: 10.1016/j.jplph.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Osanai T, Park Y-I, Nakamura Y. Editorial: biotechnology of microalgae, based on molecular biology and biochemistry of eukaryotic algae and cyanobacteria. Front Microbiol. 2017;8:118. doi: 10.3389/fmicb.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaiz A, Prasad MNV. Environmental adaptations and stress tolerance of plants in the era of climate change. Berlin: Springer; 2011. [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313X.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Perreault F, Dionne J, Didur O, Juneau P, Popovic R. Effect of cadmium on photosystem II activity in Chlamydomonas reinhardtii: alteration of O-J-I-P fluorescence transients indicating the change of apparent activation energies within photosystem II. Photosynth Res. 2011;107:151–157. doi: 10.1007/s11120-010-9609-x. [DOI] [PubMed] [Google Scholar]
- Pick U, Avidan O. Triacylglycerol is produced from starch and polar lipids in the green alga Dunaliella tertiolecta. J Exp Bot. 2017;68:4939–4950. doi: 10.1093/jxb/erx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F, Iannelli MA, Pasqualini S, Massacci A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol. 2003;133:829–837. doi: 10.1104/pp.103.026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko J, Herrfurth C, Feussner K, Ischebeck T, Iven T, Haslam R, Hamilton M, Sayanova O, Napier J, Khozin-Goldberg I, Feussner I. Metabolome analysis reveals betaine lipids as major source for triglyceride formation, and the accumulation of sedoheptulose during nitrogen-starvation of Phaeodactylum tricornutum. PLoS ONE. 2016;11:e0164673. doi: 10.1371/journal.pone.0164673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Grillitsch K, Daum G. Synthesis and turnover of non-polar lipids in yeast. Prog Lipid Res. 2008;47:157–171. doi: 10.1016/j.plipres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Razeghifard R. Algal biofuels. Photosynth Res. 2013;117:207–219. doi: 10.1007/s11120-013-9828-z. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137:65–83. doi: 10.1016/S0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Sato A, Matsumura R, Hoshino N, Tsuzuki M, Sato N. Responsibility of regulatory gene expression and repressed protein synthesis for triacylglycerol accumulation on sulfur-starvation in Chlamydomonas reinhardtii. Front Plant Sci. 2014;5:444. doi: 10.3389/fpls.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001;127:887–898. doi: 10.1104/pp.010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14:43–50. doi: 10.1016/j.tplants.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G. Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011;11:7. doi: 10.1186/1472-6750-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB. Tracking synthesis and turnover of triacylglycerol in leaves. J Exp Bot. 2015;66:1453–1461. doi: 10.1093/jxb/eru500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni N, Mench M, Cassagne C, Bessoule J. Fatty acid composition of tomato leaves as biomarkers of metal-contaminated soils. Environ Toxicol Chem. 2001;20:382–388. doi: 10.1002/etc.5620200220. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U. Algal lipid bodies: stress induction, purification, and biochemical characterization in wild type and starchless Chlamydomonas reinhardtii, Eukaryot. Cell. 2009;8:1856–1868. doi: 10.1128/EC.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]