Abstract
Rose (Rosa × hybrid L.) is one of the most important commercial ornamental crops cultivated worldwide for its beauty, fragrance and nutraceutical values. Characterization of rose germplasm provides precise information about the extent of diversity present among the cultivars. It also helps in cultivar identification, intellectual property right protection, variety improvement and genetic diversity conservation. In the present study, 109 Indian bred rose cultivars were characterized using 59 morphological and 48 SSR markers. Out of 48 SSRs used, 31 markers exhibited polymorphism and 96 alleles were identified with an average of 3.9 alleles per locus. Nei’s expected heterozygosity value of each locus ranged from 0.08 (with SSR ABRII/RPU32) to 0.78 (SSR Rh58). The similarity coefficient values ranged from 0.42 to 0.90 which indicated presence of moderated diversity among Indian cultivars. The neighbor-joining tree based on morphological data grouped the cultivars into two major clusters and several minor clusters based on their morphological resemblance. However, UPGMA dendrogram constructed using matching coefficient values grouped the cultivars into eight different clusters. Interpopulation analysis revealed higher genetic similarities between Hybrid Tea and Floribunda cultivars. An analysis for presence of population sub-structure grouped the Indian cultivars into eight different genetic groups. Analysis of molecular variance revealed apportioning of 97.59% of the variation to within subgroup diversity and 3.07% to between the cultivar groups. We have demonstrated here successful utilization of robust SSR to distinguish cultivars and assess genetic diversity among Indian bred rose cultivars. The information provided here is useful for cultivar identification and protection, cultivar improvement and genetic diversity conservation.
Electronic supplementary material
The online version of this article (10.1007/s12298-019-00735-8) contains supplementary material, which is available to authorized users.
Keywords: Rose, Indian cultivars, Genetic diversity, Morphological characterization, Molecular characterization, SSR, Population structure
Introduction
Rose (Rosa × hybrida L.) is among the top global ornamental crops cultivated for its flower, essential oil and other value-added products. The basic chromosome number of rose is x = 7 (Nybom et al. 2005) and the ploidy level of roses ranges from 2n = 2x = 14 to 2n = 10x = 70 in different wild species and cultivars (Bendahmane et al. 2013). The modern-day cultivated roses are complex hybrids derived mostly from diploid and tetraploid species of Asian and European origins (Zhang et al. 2006).
In India, scientific breeding work of roses started in recent times and was put to rigour in the last five decades. Along with institutionalised scientific efforts, few of the amateurs and private nurserymen also took up rose breeding leading to development of more than 500 cultivars belonging to different rose groups. Most of these cultivars were developed through conventional as well as mutation breeding approaches (Kaicker 1992). Majority of these cultivars have continuous flowering habit with a wide variety of flower forms, colours, scents and plant growth habits.
Development of various genomic tools in addition to the availability of genetic resources have lead to a revolution in crop improvement programs. Next generation sequencing (NGS) technologies are being used for sequencing of plant genomes and transcriptomes in a wide range of species. Sequencing data with the use of bioinformatic tools generate an array of genomic information useful for the development of molecular markers and construction of high-density genetic maps in addition to providing valuable information on genetic variations governing economically important traits. Genome sequence information is available for several rose species and cultivars (Nakamura et al. 2018; Lu et al. 2016; Foucher et al. 2015) which have been useful for identifying the molecular basis of different ornamental traits and to study diversity and evolution of roses.
Characterization and diversity analysis of available germplasm plays a major role in future improvement programmes in rose. Traditionally the classification was done based on morphological and physiological traits. Since these traits are highly influenced by environment as well as a wide range of overlaps in various characteristics, it’s recommended to avoid studying genetic diversity based on the morphological characters alone (Lewis 1957). Chemotaxonomic studies based on the presence of floral pigments, volatile compounds (Mikanagi et al. 2000; Flament et al. 1993) and isozyme markers were used for identification and characterization of roses (Grossi et al. 1998; Walker and Werner 1997; Kim and Byrne 1996; Kuhns and Fretz 1978). But the lower number of polymorphic isozyme markers and the inadequate number of biochemical assays to detect these biochemical markers limits the use of these marker systems in crop improvement programs.
DNA based marker systems are more efficient and informative for conducting genetic analysis and to understand the genetic relationships between the cultivars. Different molecular marker systems i.e. AFLP (amplified fragment length polymorphism), RFLP (restriction fragment length polymorphism), RAPD (randomly amplified polymorphic DNA), ISSR (inter-simple sequence repeats) have been developed and utilized for studying genetic diversity and relationship, DNA fingerprinting for genotype identity and for construction of genetic linkage maps for identification of potential genes in rose germplasm (Jiang and Zang 2018; Yang et al. 2016; Akond et al. 2012; Hubbard et al. 1992; Spillar et al. 2011). SSR markers are comparatively of recent origin and have several advantages over earlier marker systems due to its co-dominant inheritance, an abundant and relatively uniform distribution in the genome, high repeatability and polymorphism. Esselink et al. (2003) developed the first SSR markers in rose. In 2004, some of these microsatellite markers were used for the identification of allelic copies in polyploid roses (Esselink et al. 2004). Yan et al. (2005) identified 21 SSR and used for the construction of a draft genetic map of roses. Zhang et al. (2006) identified 30 SSR markers from R. wichuriana genomic DNA library and used for the development of tetraploid genetic map in roses. Oyant et al. (2008) identified 20 genomic SSRs and 44 EST-SSRs from bud expressed sequence tag database consisting of 2556 unigene sequences and genomic BAC library of rose with 384 clones. Later, Koning-Boucoiran et al. (2015) developed approximately 68,000 SNP markers from the rose EST database. Qi et al. (2018) developed organ-specific microsatellite markers (3245 genomic SSR primers and 33 EST-SSRs) in rose and tested them for diversity analysis in 48 rose genotypes. But very few studies were available for diversity analysis in Indian origin modern rose cultivars (Panwar et al. 2015; Prasad et al. 2006) and these were conducted in the limited number of Indian genotypes using RAPD and ISSRs markers. Considering the superiority of SSR markers in terms of robustness, amenability to comparison across laboratories etc. the present study was undertaken using morphological and molecular markers (SSRs) with an aim to characterize 109 Indian bred rose cultivars belonging to Hybrid Tea and Floribunda groups to ascertain the genetic diversity and presence of population sub-structure.
Materials and methods
Planting material
The rose cultivars listed in Table 1 were examined in this study (109 cultivars) and they belonged to the groups of Hybrid Teas (65) and Floribunda (44). These cultivars were obtained from the collection maintained at of Indian Agricultural Research Institute, New Delhi, India.
Table 1.
List of rose cultivars used in the study
| Population | Genotypes |
|---|---|
|
Group-1 (Hybrid Teas) |
Abhisarika, Anurag, Arjun, Arka Parimala, Aruna, Ashwini, Bhim, Century Two seedling, Chambe di Kali, Chitra, Dil-Ki-Rani, Dr. B.P. Pal, Dr. Benjamin Pal, Dr. Bharat Ram, Dr. M.S. Randhawa, Dr. R.R. Pal, Dulhan, Eiffel tower X Queen Elizabeth, Ganga, Golden Afternoon, Haseena, Homage, Jawani, Lalima, Lal Makhmal, Madhosh, Maharani, Mother Teresa, Mridula, Mrinalini, Mrs. K. B. Sharma, Nayika, Nehru centenary, Nurjahan, Pink Montezuma, Preyasi, Priyadharshini, Pusa Ajay, Pusa Arun, Pusa Bahadur, Pusa Garima, Pusa Mansij, Pusa Mohit, Pusa Priya, Pusa Sonara, Raja Ram Mohan Roy, Raja S.S. Nalagarh, Rajkumari, Raktagandha, Raktima, Ranjana, Ratnaar, Sahasradhara, Shanti Pal, Shreyasi, Sir C. V. Raman, Soma, Sugandha, Surabhi, Surekha, Surkhab, Jawahar, Shiloz Mukherjee, Indian Princess, Pusa Gaurav |
|
Group-2 (Floribundas) |
Pusa Shatabdi, Akash Sundari, Delhi white Powder Puff, Delhi Pink Powder Puff, Anitha, Arunima, Banjaran, Chingari, Deepak, Delhi Brightness, Delhi Princess, Dr. S. S. Bhatnagar, Himangini, Jantar Mantar, Krishna, Lahar, Loree, Madhura, Manmatha, Manasi, Mohini, Navneet, Neelambari, Prema, Punchu, Pusa Abhishek, Pusa Barahmasi, Pusa Komal, Pusa Pitambar, Rupali, Sabnam, Sadabahar, Shola, Sindhoor, Suchitra, Surdas, Suryakiran, Suryodaya, Tarang, Pusa Virangana, Pusa Manhar, Pusa Urmil, Pusa Muskan, Rose Sherbet |
Morphological characterization
Morphological data was collected in the research farm of Division of Floricultural and Landscaping, ICAR-IARI, New Delhi, which is one of the DUS validation centres for Rose approved by PPV&FRA (Protection of Plant Varieties and Farmers Rights Act, 2001), India. A total of 59 morphological characters were selected from PPV&FRA drafted DUS guidelines developed for roses (Table 2). For each cultivar, 10 representative plants were selected for collecting data in two subsequent years.
Table 2.
List of morphological descriptors used for characterization
| S.NO | Characters | Characters | |
|---|---|---|---|
| 1 | Plant: Growth type (VG) | 31 | Flower: side view of lower part (VG) |
| 2 | Plant: Growth habit (VG) | 32 | Flower: Fragrance (MS) |
| 3 | Plant: Height (cm)(MS) | 33 | Sepal extensions |
| 4 | Young shoot: Anthocyanin colouration (VG) | 34 | Petal: Reflexing of petals one by one (VG) |
| 5 | Young shoot: Intensity of Anthocyanin colouration (VG) | 35 | Petal shape (VG) |
| 6 | Stem: Number of prickles (VG) | 36 | Petal incisions (VG) |
| 7 | Prickles: Predominant colour (VG) | 37 | Petal: Reflexing of margin (VG) |
| 8 | Prickle: Shape of lower side (VS) | 38 | Petal: Undulation of margin (VG) |
| 9 | Leaf: Size (MS) | 39 | Petal: length (MS) |
| 10 | Leaf: intensity of green colour on upper side (VG) | 40 | Petal: Width (MS) |
| 11 | Leaf: Anthocyanin colouration (VG) | 41 | Petal: Number of colours on inner side (VG) |
| 12 | Leaf: Glossiness of upper side (VG) | 42 | Varieties with one colour on inner side of petal: Intensity of colour excluding basal the spot (VG) |
| 13 | Leaflet: Undulations of margin (VG) | 43 | Petal: Colour of the majority portion of petal (VG) |
| 14 | Leaflet: Serration of margin (VG) | 44 | Varieties with two or more colours on inner side of petal: Secondary colour of petal (VG) |
| 15 | Terminal leaflet: Shape of blade (MS) | 45 | Varieties with two or more colours on inner side of petal: Tertiary colour of petal (VG) |
| 16 | Terminal leaflet: Shape of base of blade (MS) | 46 | Varieties with two or more colours on inner side of petal: Petal distribution of secondary colour on inner side (VG) |
| 17 | Terminal leaflet: Shape of apex of blade (VG) | 47 | Varieties with two or more colours on inner side of petal: Petal distribution of tertiary colour on inner side (VG) |
| 18 | Flowering shoot: Flowering laterals (VS) | 48 | Petal: Spot at base of inner side (VG) |
| 19 | Flowering shoot: Number of flowering laterals (MG) | 49 | Petal: Size of spot at base of inner side (VG) |
| 20 | Flowering shoot: Number of Flowers (MG) | 50 | Petal: Colour of spot at base of inner side (VG) |
| 21 | Flowering shoot: Number of flowers per lateral (MG) | 51 | Petal: Colour of spot at base of outer side (VG) |
| 22 | Flower bud: Shape of Longitudinal Section (VG) | 52 | Petal: Main colour on outer side (VG) |
| 23 | Flower: Type (VG) | 53 | Petal spot at base of outer side (VG) |
| 24 | Flower: Number of petals (MS) | 54 | Petal: Size of spot at the base of outer side (VG) |
| 25 | Flower: Colour group (VG) | 55 | Outer stamen: Predominant colour of the filament (VG) |
| 26 | Flower: Diameter (MS) | 56 | Seed vessel: Size (VG) |
| 27 | Flower: Colour of the centre (VG) | 57 | Hip: Shape of longitudinal section (VG) |
| 28 | Flower: Density of petals (MG) | 58 | Flower: Length of pedicel (MS) |
| 29 | Flower shape: View from above (VG) | 59 | Flower: Venation of Petals (VG) |
| 30 | Flower: Side view of upper part (VG) |
SSR marker amplification
DNA extraction was done using the CTAB method (Murray and Thompson 1980) with some modifications which included use of 2% CTAB in extraction buffer. Selected cultivars were analysed with 48 SSR markers obtained from the literature (Yan et al. 2005; Kimura et al. 2006; Jowkar et al. 2009). Out of these 48 SSR primers, 31 primers showed polymorphism and were used for further analysis. Primer sequence information, repeat motifs with amplified annealing temperatures and Nei’s heterozygosity values for each primer are presented in Table 3 (Nei 1973). DNA amplification was carried out in 25 µL reaction volume containing 2.50 μL Taq DNA buffer (10×), 2 µL of dNTPs (20 mM), 1 µL each of forward and reverse primers (1 µM), 0.33 µL of Taq DNA polymerase (5U/µL) (Genei, India), 2 µL of template DNA (20 ng/μL) and 16.17 µL autoclaved distilled water. The PCR amplification reaction was carried out in a thermal cycler programmed at 94 °C for 6 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 48–55 °C (depend upon primer annealing temperature) for one min and 72 °C for 2 min and a final extension step of 72 °C for 10 min before cooling to standby at 4 °C. PCR products were resolved in 3% Metaphor agarose gel using 1× TAE buffer. The sizes of amplified fragments were determined by using relative migration of DNA markers from the 100 bp DNA size standard. The SSR profiles were visualized and captured using Alpha Digi Doc Gel Documentation System and scoring was done using Alpha Ease FC 4.0 software (Yeh et al. 1999).
Table 3.
List of polymorphic SSR markers (31) and their details
| SSR | Primer sequences | Source | Band size (bp) | NA | Ta (°C) | h |
|---|---|---|---|---|---|---|
| Rh79 |
F: TTCTTCTTGCTCGCCATTTTGATT R:GAACGTCCACCACCACCCACTCTG |
Yan et al. (2005) | 135, 142, 149, 160, 174 | 5 | 50 | 0.76 |
| RhAB9 |
F:GTCAATTTGTGCATAAGCTC R:GTGAGAACAGATGAGAAATG |
Yan et al. (2005) | 101, 116, 124 | 3 | 50 | 0.6 |
| Rh48 |
F:GATAGTTTCTCTGTACCCCACCTA R:TTGACCAGCTGCAACAAAATTAGA |
Yan et al. (2005) | 99, 107, 127, 136, 144 | 5 | 50 | 0.39 |
| Rh80 |
F:CATGCCAAACGAAATGAGTTA R:TTATCTAAAGGGCTGCTGTAAGTT |
Yan et al. (2005) | 134, 148 | 2 | 50 | 0.47 |
| Rh96 |
F:GCCGATGGATGCCCTGCTC R: AGATTCCCTGCGACATTCACATTC |
Yan et al. (2005) | 276, 282, 306 | 3 | 50 | 0.44 |
| Rh50 |
F: TGATGAAATCATCCGAGTGTCAG R: TCACTTTCATTGGAATGCCAGAAT |
Yan et al. (2005) | 240, 303, 340 | 3 | 50 | 0.49 |
| Rh58 |
F: ACAATTTAGTGCGGATAGAACAAC R: GGAAAGCCCGAAAGCGTAAGC |
Yan et al. (2005) | 238, 258, 274, 286, 291, 300 | 6 | 50 | 0.78 |
| RhABT12 |
F: CAAGTTTGTCTCCTTGGACC R: CATAGATGATTATCCTAGAGCC |
Yan et al. (2005) | 156,174,200,225 | 4 | 50 | 0.44 |
| Rh65 |
F: AGTACGCCGACGCAGATCCAGTGA R: ACGGCGTTGTAGGTCGTCATTCTC |
Yan et al. (2005) | 128,140,160 | 3 | 50 | 0.54 |
| Rh78 |
F: AAAGAAACGCGAAATCTATGATGC R: TCTGGATGGGATTTAAAAGACAGG |
Yan et al. (2005) | 216,296,336 | 3 | 50 | 0.52 |
| Rh77 |
F: CAACTGAAAGGAACAAATGGATGT R: GGAATGGCTTGTAAATTTGTGATT |
Yan et al. (2005) | 246,262,280,309 | 4 | 50 | 0.69 |
| Rh93 |
F: GCTTTGCTGCATGGTTAGGTTG R: TTCTTTTTGTCGTTCTGGGATGTG |
Yan et al. (2005) | 244,275 | 2 | 50 | 0.45 |
| RhAB38 |
F: GAGGTGGTCGATTCCATGTC R: TTACCGTTCTACCTAAGTGACTAAC |
Yan et al. (2005) | 120,139,173 | 3 | 50 | 0.55 |
| Rh60 |
F: TCTCTTTTCACGGCCACCACT R: TGAATCCAAGGCCGTATAGTTAGA |
Yan et al. (2005) | 244, 272, 300 | 3 | 50 | 0.51 |
| Rh85 |
F: ACTTTTGGGCGTTCATCGCATTACAC R:GGCTATATGGGCTCAAGTCTAGACAA |
Yan et al. (2005) | 203, 221 | 2 | 50 | 0.48 |
| Rh98 |
F: GGCCTCTAGAGTTTGGGATAGCAG R: ACGACGTCAATAACTCCATCAGTC |
Yan et al. (2005) | 160, 221 | 2 | 50 | 0.28 |
| Rh72 |
F: CCAAAAGACGCAACCCTACCATAA R: TCAAAACGCATGATGCTTCCACTG |
Yan et al. (2005) | 254, 276, 282, 310 | 4 | 50 | 0.58 |
| Rh73 |
F: GGTTAGACGGGTGGAAGAAG R: ACTGCCGATAGAAGTATTTCATCA |
Yan et al. (2005) | 150, 175, 205 | 3 | 55 | 0.62 |
| RhAB28 |
F: GCAGATGTTATTCATGTTAA R: CCAAGTATTTTAGTTTCTTC |
Yan et al. (2005) | 164, 180, 190 | 3 | 55 | 0.64 |
| ABR II/Rpu4 |
F: CTTGTTCAAAGGGTTCTCTG R: CACCTAAATGATGCTTTTCC |
Jowkar et al. (2009) | 274, 312 | 2 | 50 | 0.49 |
| ABRII/Rpu7 |
F: GAAACCCAGTTATTGATGCCTGGA R: CCAGATTATTGATGCCTGAT |
Jowkar et al. (2009) | 213, 220 | 2 | 50 | 0.49 |
| ABRII/Rpu10 |
F: AAGATTGGTGTTGGGTGTTA R: CTCGTTCTGGTTCTGTCTTC |
Jowkar et al. (2009) | 115, 118, 122 | 3 | 50 | 0.15 |
| ABR II/RPU11 |
F: AGTTGGACCTGTTTTCTTCA R: AGCACGACGACGAGTTTC |
Jowkar et al. (2009) | 205, 236, 248, 275 | 4 | 57 | 0.69 |
| ABRII/RPU12 |
F: GAAGAAGAACGACTGAGAGC R: GAGCAGAGAATTTCCATTTG |
Jowkar et al. (2009) | 103,109,117,131 | 4 | 50 | 0.5 |
| ABRII/RPU14 |
F: AGCACTTACAGGCTCATCAT R: CCTCCAAGTCAAGTTCTACG |
Jowkar et al. (2009) | 206, 216, 222, 230 | 4 | 50 | 0.52 |
| ABRII/RPU32 |
F: TAGAGCTATTTTCGATTCGG R: GGGTGACACAGAGAGAGAGA |
Jowkar et al. (2009) | 238, 265 | 2 | 50 | 0.08 |
| ABRII/RPU33 |
F: TCTCTCTCTGTGTCACCCTC R: CGTCTCCCTCTTCTTCTTCT |
Jowkar et al. (2009) | 150, 180 | 2 | 50 | 0.29 |
| ABRII/Rpu36 |
F: TAGTTGAGAGCTCGGAGAAG R: GAAGTTACAAGACGAAACCG |
Jowkar et al. (2009) | 235, 255 | 2 | 50 | 0.29 |
| RA013a |
F: GAGGGAAAGAGATACACAAA R: GTAAGACCTTGCGTGTTCATA |
Kimura et al. (2006) | 145, 155 | 2 | 55 | 0.37 |
| RA023b |
F: CATCCTCGGTGTTGCGTTGA R: TGTCTCCAGCAACCTTTTTTTCCC |
Kimura et al. (2006) | 174, 194 | 2 | 50 | 0.49 |
| RA043a |
F: GCAACGTACTTCAATTTCCAC R: CAAGCTCAGAACTGAGACAC |
Kimura et al. (2006) | 123, 136, 149, 179 | 4 | 50 | 0.63 |
Primer sequence motifs (F: forward and R: reverse); Ta, Annealing temperatures; band sizes of each amplified marker; NA, number of the amplified fragments generated by SSR primers; h, Nei’s expected heterozygosity values for markers
Data analysis
Morphological data was analysed using NTSYS software version 2.2 (Rohlf 2005). The dendrogram was generated following the neighbour-joining (NJ) method using pair-wise Euclidean distance values. For molecular data, the bands at each polymorphic marker were noted as either present (1) or absent (0) across the sample lanes. These scores were used to designate genotypic status for each SSR marker. The resultant data matrix was used to compute the genetic diversity statistics using POPGENE 32, version 1.31 (Yeh et al. 1997). The similarity matrix was prepared using dice similarity coefficient values and a dendrogram was generated by the unweighted pair group method with arithmetic mean (UPGMA). A model-based cluster analysis was done to determine the optimal number of genetic clusters found among rose cultivars using STRUCTURE analysis which group’s individuals into a number of genetic clusters based on the multi-locus genotypic data (Pritchard et al. 2000). The admixture model and correlated allele frequencies were applied for each run with 50,000 burn-in periods (iteration) and 75,000 Markov Chain Monte Carlo (MCMC) replication and the absolute value of the second order rate of change of the likelihood distribution determined as eight using Structure Harvester online package (Evanno et al. 2005; Earl and VonHoldt 2012). Data was also subjected to the analysis of molecular variation (AMOVA) using Arlequin 3.1 software (Excoffier et al. 1992). Fixation indices and population pairwise FST values were also calculated (Beaumont and Nichols 1996).
Results
Out of the 48 SSR markers tested, amplicons obtained from 31 SSRs showed polymorphism. A total of 96 alleles were scored in 109 rose cultivars analysed and the number of alleles ranged from 2 to 6 with an average of 3.09 alleles per locus (Table 3). The most robust polymorphic SSR was Rh58 which amplified the highest number of alleles (n = 6). The molecular weight of the allelic bands varies from 99 bp (Rh48) to 340 bp (Rh50) with different SSRs. Nei’s expected heterozygosity (h) or gene diversity computed following Nei (1973) ranged from 0.08 (ABRII/RPU32) to 0.78 (Rh58) with a mean value of 0.49 for locus (Table 3). SSRs Rh58, Rh79, Rh77, ABRII/RPU11, RhAB28, RA043A, and Rh73 were found to have high Nei’s heterozygosity (h) values (0.78, 0.76, 0.69, 0.69, 0.64, 0.63 and 0.62 respectively) could be used for efficient diversity analysis and characterization of rose cultivars.
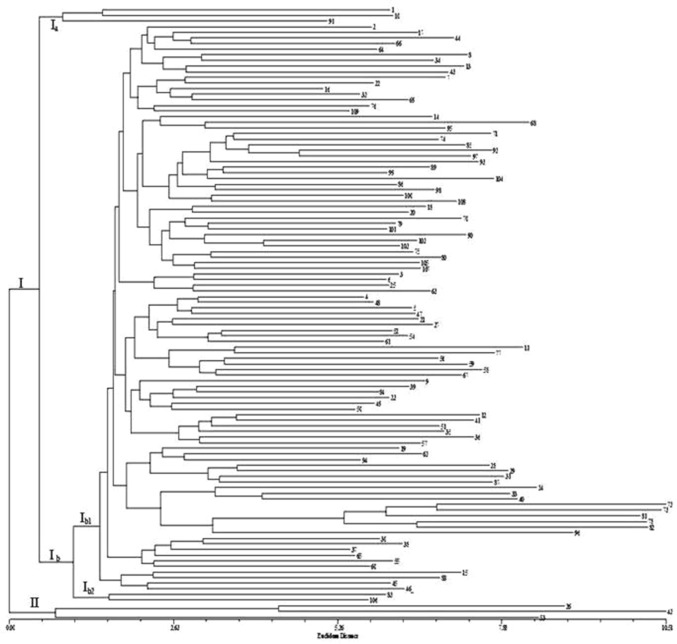
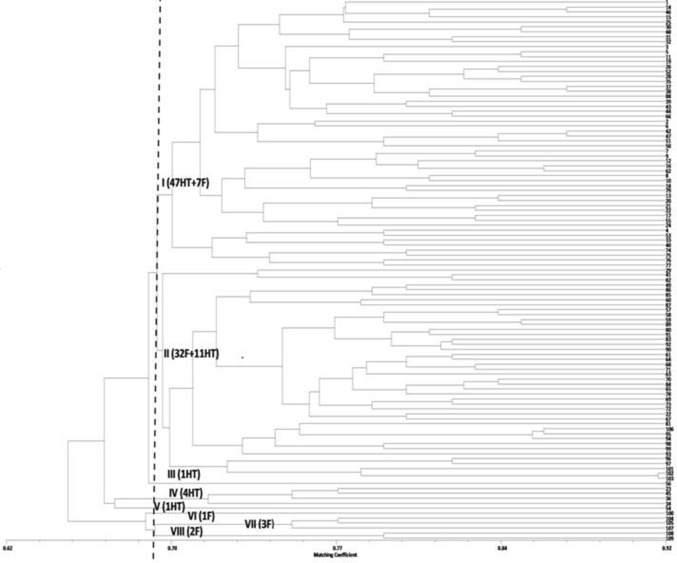
Neighbor-joining (NJ) dendrogram (Fig. 1) based on morphological characters separated the cultivars primarily on the basis of floral traits such as petal colour, flower type and flowering behaviour. Cultivars with bi and tricoloured petals were separated from the remaining group of cultivars due to a higher priority for flower colour trait in DUS test guidelines. Classification based on above horticultural traits is more helpful for selection of cultivars for landscape use rather than genetic relatedness. The UPGMA dendrogram generated using matching coefficient values on the basis SSR data revealed genetic relationships among 109 Indian bred roses. At an average matching coefficient value of 0.69, a total of 109 cultivars were grouped into 8 different clusters. Cluster I and II contain a maximum number of cultivars 54 and 43 followed by clusters IV, VII and VIII with 4, 3 and 2 cultivars each and remaining clusters III, V and VI included only one cultivar each (Fig. 2).
Fig. 1.
NJ-Clustering based on Euclidian distance values of 109 cultivars based on diversity for morphological traits. Samples labelled 1 to 65 are Hybrid Teas and 66–109 are Floribundas
Fig. 2.
UPGMA dendrogram depicting genetic relationships among the 109 selected rose cultivars based on SSR polymorphism. Samples labelled 1–65 are Hybrid Teas and 66–109 are Floribundas
Interpopulation analysis revealed minor variability among Hybrid Tea and Floribunda populations. The number of polymorphic loci observed were 31 with 100% polymorphism in both population groups. Observed and expected numbers of alleles (Na & Ne) in Hybrid Tea and Floribunda populations were 2.96 ± 1.04, 3 ± 1.09 and 2.18 ± 0.81, 2.2 ± 0.82 respectively. Shannon’s Information Index (I) was higher in the Floribunda group of cultivars (0.83 ± 0.32) as compared to Hybrid Tea population (0.81 ± 0.33). Variability observed for the heterozygosity parameters in Hybrid Tea (Ho = 0.48 ± 0.29, He = 0.53 ± 0.31and h = 0.49 ± 0.16) and Floribunda (Ho = 0.49 ± 0.16, He = 0.5 ± 0.16 and h = 0.5 ± 0.16) types (Table S1) were minor and not significant in nature.
Similarity coefficient values calculated for each pair of cultivars ranges from 0.42 to 0.90 with an average value of 0.69. Cultivars Suchitra and Surekha were found to be the most distinct with lowest similarity coefficient value 0.42 followed by cultivars Sherbet—Mansi (0.43); Mrinalini—Surekha (0.45); Maharani—Surekha (0.461) and Surekha—Pusa Virangana (0.465). Highest degree of similarity was observed between cultivars Suryakiran and Suryodaya with matching coefficient value 0.9 followed by the cultivar pairs Pusa Mansij and Raja Surendra Singh Nalagarh; Dr Bharat Ram and Raja Ram Mohan Roy; Priyadharshini and Pusa Ajay; Dr R. R. Pal and Jawahar respectively (0.88, 0.875, 0.875 and 0.87) (Figs. 1 and 2).
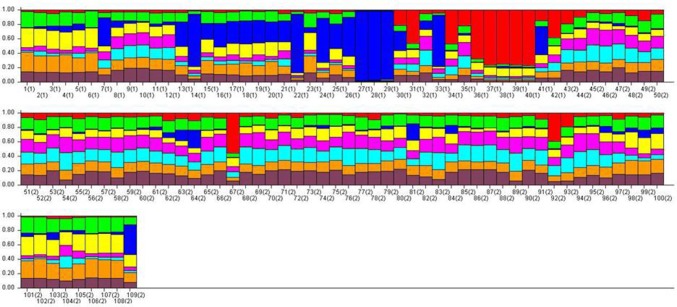
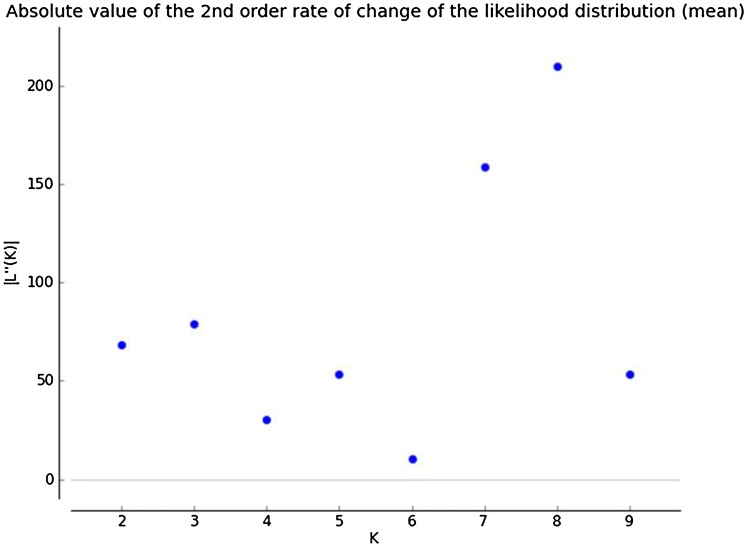
Population structure of the cultivars was analysed by the Bayesian approach. The estimated membership fractions of 109 cultivars for different values of K ranged from 2 to 9 (Fig. 3). Absolute value for the second order rate of change of the likelihood distribution (mean) was observed at K = 8 suggesting eight genetically distinct clusters (Fig. 4). Based on the membership fractions, the accessions with higher membership values were assigned to corresponding eight subgroups (Fig. 3). Analysis of molecular variance (AMOVA) revealed that maximum variation of 97.59% was observed within groups of individual genotypes while between the groups the variation reported was 3.07% and negligible variation was noticed within the group (Table S2).
Fig. 3.
Bar diagram for the 109 Indian rose cultivars at K = 8 indicating commonness of alleles among the cultivars belonging different cluster groups. Colour codes in bar diagram indicate the allelic affiliation with respect to the groups (colour figure online)
Fig. 4.
Biplot of absolute value of the second order rate of change of the likelihood distribution (mean), |L″(K)| against the inferred K values indicating the estimated memberships for K = 2 to 9
Discussion
SSRs or microsatellite markers are highly polymorphic, ubiquitous, reproducible, co-dominant markers and they were found to be the effective marker systems for studying genetic diversity. In this study, 31 polymorphic markers were used to characterize Hybrid Tea and Floribunda roses and 100% polymorphism was noticed in both the populations. This result is in agreement with earlier studies on rose genotypes that reported 98.5% polymorphism among the rose cultivars for RAPDs (Rai et al. 2015). Panwar et al. (2015) and Prasad et al. (2006) had also reported 94% and 87.5% polymorphism with ISSR and RAPD markers in Indian rose cultivars and fragrant roses. However, RAPDs and ISSRs being random markers are considered to have low repeatability and comparability across laboratories, hence, SSRs are considered more suitable due to their robust nature. In the present study, SSRs were found to be informative and exhibited a higher number of alleles (na) and Nei’s heterozygosity values (h) with relatively higher discriminating power and were identified as the most suitable markers for rose diversity analysis.
Genetic relationships among 109 Indian bred rose cultivars belonging to Hybrid Tea and Floribunda groups were studied using UPGMA dendrogram and the cultivars were grouped into 8 different clusters. Out of 54 cultivars placed in cluster-I, majority (47) were Hybrid Teas and only seven cultivars belonged to Floribunda group (Neelambari, Pusa Shatabdi, Jantar Mantar, Deepak, Delhi Brightness, Delhi Princess and Dr S. S. Bhatnagar). These Floribunds group cultivars also had genetic makeup similar to other HT cultivars, this conclusion was supported by the similarity of morphological features of these cultivars (Floribundas) with HT roses; the features included presence of medium to large size double flowers with dense petal arrangement (Pusa Shatabdi, Neelambari), long flowering stock (Jantar Mantar, Dr S.S. Bhatnagar, Pusa Shatabdi), absence of flowering shoot laterals (Delhi Princess, Dr S. S. Bhatnagar) and medium to tall plant height and semi-upright plant growth habit (Jantar Mantar, Pusa Shatabdi and Dr S. S. Bhatnagar).
The cluster-II comprised majority of the floribunda cultivars (32/43) and a few of the HT roses (11/43). The typical Floribunda cultivars possessed characteristic perpetual flowering habit, flowers in clusters on multiple flowering shoots, shorter plant height than average HT type and bed or stiff shrub type of plant growth. The Hybrid Tea cultivars (Mridula, Pusa Garima, Raktagandha, Surekha, Soma, Sugandha, Surabhi, Surkhab, Indian Princess, Pusa Gaurav, Jawani) present within cluster-II also shared some of the morphological characteristics with Floribundas including presence of multiple flowers on flower stocks (Mridula, Soma, Surabhi, Surekha, and Pusa Gaurav), stiff shrubby plant growth type with semi-upright growth (Indian Princess). Interestingly some of these cultivars were found to be bred from the crosses, Grandiflora × Hybrid Teas (Mridula, Surekha, Pusa Gaurav, Jawani) and Hybrid Tea × Floribunda (Surabhi). A higher share of floribunda genetic background in this HT cultivar could be a reason for separation these cultivars from a majority of Hybrid tea cultivars of cluster-I. Cultivars, C.V. Raman (HT), Shanti Pal (HT) and Suchitra (F) were separated into three different clusters (III, V and VI) and revealed distant genetic relationship with the remaining group of cultivars.
Cluster IV contained four Hybrid Tea types having comparable morphological features for plant growth habit, leaf and flower characteristics, and were also bred from crosses of Grandiflora and Hybrid Tea types. Cultivars, Tarang, Pusa Virangana, Pusa Urmil were present in cluster-VII had higher morphological similarity with each other except for some variation with prickle characteristics, flower colour and fragrance. The higher level of similarity among these floribunda cultivars was expected because cultivars Pusa Virangana and Pusa Urmil were derived from single parent ‘Jantar Mantar’ through open pollination and natural mutagenesis but the cultivar Tarang derived from Grandiflora parent ‘Queen Elizabeth’ also exhibited morphological and genetic similarity with them. The higher genetic resemblance among cultivars was observed for SSR markers among the Floribunda cultivars, Pusa Muskan and Rose Sherbet, but they deferred for the morphological characters such as growth habit, flowering laterals and flower colour and both the cultivars were placed in cluster-VIII (Fig. 1). Majority of the cultivars belonging to HTs and Floribundas groups were clustered together and some degree of separation was noticed at higher matching coefficient values which indicated presence of narrow variation among the majority of the genotypes. Similar results with a lower degree of variation between cultivated rose hybrids belonging to HT and Floribunda groups were noticed by Mohapatra and Rout (2005) using RAPD markers. Ben-Meir and Vainstein (1994) detected the highest level of genetic similarity between Hybrid Tea and Floribunda categories of roses using human-derived minisatellite probes. Vainstein et al. (1993) also reported higher genetic similarities among cultivated rose groups (Hybrid Tea, Floribunda, Polyantha and Miniature) by using 28 human-derived microsatellite probes. In the present investigation, interpopulation analysis also revealed minor genetic variability between both the cultivar groups.
Results from AMOVA indicated the narrow variability (3.07%) between Floribunda and Hybrid Tea group cultivars. The amount of variation (97.59%) noticed was due to genotypic variability of individuals within the groups. The between group variation was minimum (Table S2). Computation of Wright’s F statistic revealed that FIT, FIS values were 0.03 and zero indicating presence of greater homogeneity of individual cultivars as expected considering that rose cultivars are vegetative propagated. The estimate of FST was 0.03 which indicates the presence of lower genetic differentiation of cultivars belonging to both the groups. Similarly, pairwise FST estimate among the subpopulation groups has indicated higher similarity among the subpopulations. The lower variability observed among rose cultivars could be due to its free outcrossing nature, which results in free gene flow among populations ultimately contributing to greater genetic variability within groups (Akond et al. 2012; DeVries and Dubois 1996). All most all the cultivars used in the present study belong to modern rose classes (Hybrid Teas and Floribundas). Modern class (Hybrid Teas, Floribundas and Grandifloras and Miniatures) of rose cultivars were derived from 8 to 10 common wild ancestral species belongs to Asian and European origin (Zlesak 2006). A shift in genetic makeup from European to Asian background was also observed in cultivated modern types due to repeated crosses with Asiatic types while attempting transfer of continuous flowering habit as well as tea perfume in European types (Liorzou et al. 2016). Hybrid Tea cultivars were the first group of modern rose cultivars derived from crosses of Hybrid Perpetuals (derived from R.chinensis, R. gallica and R. centifolia) and Tea roses (originated from R. chinensis and R. gigantea) whereas other modern rose class ‘Floribundas’ were developed from the crosses of Hybrid Teas and Polyantha roses (latter developed from species R. multiflora and R. wichuriana) (Marriott 2003). Addition of genetic material from the Polyantha group of roses in Floribundas could be attributed to slight higher heterozygosity with this group as compared to the Hybrid Teas. Similarly, Azeem et al. (2012) and Rusanov et al. (2005) reported lower genetic variability of cultivated roses using RAPD and SSR markers. Akond et al. (2012) using SSRs also observed higher similarity and narrow genetic base of modern cultivars (Miniature roses and hybrid breeding lines) as compared to their wild relatives. Smulders et al. (2009) also characterized, 734 Hybrid Tea varieties using 11 microsatellite markers and reported relatively close genetic relationships among them.
Eight different genetic groups were identified within the rose cultivars analysed using the Beysian model-based approach. The model-based approach uses genetic information to determine the population membership of each cultivar without assuming predefined populations. They also allocate either members or some portion of their genome to a number of groups based on multilocus genotypes (Chen et al. 2007). Population structure of 109 accessions arranged based on inferred ancestry (K = 8) are shown in Fig. 3. Majority of the small and large flowered cultivars were separated into different subgroups except for a few. Population groups (K = 8) obtained via structure analysis showed presence of genetic admixtures in larger proportions than expected from morphological analyses. Presence of common patterns of allelic variations in cultivars across the population groups irrespective of the morphological groups are indicative of the sharing of allelic variations between the Floribunda and Hybrid Tea group cultivars. The results are comparable to the earlier reports that analysed different rose populations in order to understand the genetic relationship between different ancestral populations to study the genetic shift between the populations of different geographical regions (Riek et al. 2013; Liorzou et al. 2016; Jiang and Zang 2018). The findings have implications in rose cultivar improvement programs since results suggest that useful genetic variations present in one group can be transferred beneficially to other cultivars from a diverse breeding group.
Conclusion
Microsatellite markers were found to be highly informative for detecting polymorphism and to study the diversity among rose cultivars. Narrow variability was noticed among modern cultivated roses because of the use of a limited gene pool for breeding these cultivars. The dendrograms constructed using molecular data clearly separates the cultivars based on their genetic similarities and dissimilarities. The SSRs used in the present study is helpful for cultivar identification, protection of cultivars against unauthorised commercialization and also for documentation of genetic diversity for conservation of Indian rose germplasm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Indian Council of Agricultural Research for the fellowship to the first author (AV) and for the facilities provided for conducting this work. The views expressed in this article are authors own and do not reflect the official position of the Indian Council of Agricultural Research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akond M, Jin S, Wang X. Molecular characterization of selected wild species and miniature roses based on SSR markers. Sci Hortic. 2012;147:89–97. [Google Scholar]
- Azeem S, Khan AI, Awan FS, Riaz A, Bahadur S. Genetic diversity of rose germplasm in Pakistan characterized by random amplified polymorphic DNA (RAPD) markers. Afr J Biotechnol. 2012;11(47):10650–10654. [Google Scholar]
- Beaumont MA, Nichols RA. Evaluating loci for use in the genetic analysis of population structure. Proc R Soc Lond Biol Sci. 1996;263:1619–1626. [Google Scholar]
- Bendahmane M, Dubois A, Raymond O, Bris ML. Genetics and genomics of flower initiation and development in roses. J Exp Bot. 2013;64:847–857. doi: 10.1093/jxb/ers387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Meir H, Vainstein A. Assessment of genetic relatedness in roses by DNA finger print analysis. Sci Hortic. 1994;58:158–164. [Google Scholar]
- Chen C, Durand E, Forbes F, Francois O. Bayesian clustering algorithms ascertaining spatial population structure: a new computer program and a comparison study. Mol Ecol Resour. 2007;7(5):747–756. [Google Scholar]
- DeVries DP, Dubois LAM. Rose breeding: past, present, prospects. Acta Hortic. 1996;424:241–248. [Google Scholar]
- Earl DA, VonHoldt BM. Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour. 2012;4(2):359–361. [Google Scholar]
- Esselink GD, Smulders MJM, Vosman B. Identification of cut rose (Rosa hybrida) and rootstock varieties using robust sequence tagged microsatellite site markers. Theor Appl Genet. 2003;106:277–286. doi: 10.1007/s00122-002-1122-y. [DOI] [PubMed] [Google Scholar]
- Esselink GD, Nybom H, Vosman B. Assignment of allelic configuration in polyploids using the MAC-PR (microsatellite DNA allele counting-peak ratios) method. Theor Appl Genet. 2004;109:402–408. doi: 10.1007/s00122-004-1645-5. [DOI] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distance among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament I, Debonneville C, Furrer A. Volatile constituents of roses: characterization of cultivars based on the headspace analysis of living flower emissions. In: Teranishi R, Buttery RG, Sugisawa H, editors. Bioactive Volatile Compounds from Plants. Washington DC: American Chemical Society; 1993. pp. 269–281. [Google Scholar]
- Foucher F, Oyant HSL, Hamama L. Towards the rose genome sequence and its use in research and breeding. Acta Hortic. 2015;1064:167–175. [Google Scholar]
- Grossi C, Raymond O, Boisson S, Jay M. Rosa taxonomy and hierarchy of markers defined by ACT STATIS. J Chem Sci. 1998;54:25–34. [Google Scholar]
- Hubbard M, Kelly J, Rajapakse S, Abbott A, Ballard R. Restriction fragment length polymorphisms in Rose and their use for cultivar identification. HortScience. 1992;27(2):172–173. [Google Scholar]
- Jiang L, Zang D. Analysis of genetic relationship in Rosa rugosa using conserved DNA-derived polymorphism markers. Biotechnol Biotechnol Equip. 2018;32(1):88–94. [Google Scholar]
- Jowkar A, Mardi M, Kermani JKM, Kafi M, Pirseyedi SM, Mahmoodi P, Ghaffari MR, Fatahi R. Isolation and characterization of microsatellite loci from Rosa pulverulenta. Mol Ecol Resour. 2009 doi: 10.1111/j.1755-0998.2010.02898. [DOI] [Google Scholar]
- Kaicker US. Rose breeding in India and cytology of induced mutants of H.T.cv Folklore. Acta Hortic. 1992;320:105–112. [Google Scholar]
- Kim Y, Byrne DH. Inter-specific hybrid verification of Rosa with isozymes. HortScience. 1996;31:1207–1209. [Google Scholar]
- Kimura T, Nishitani C, Iketani H, Ban Y, Yanamato Y. Development of microsatellite markers in rose. Mol Ecol Recour. 2006;6(3):210–212. [Google Scholar]
- Koning-Boucoiran CFS, Esselink GD, Vukosavljev M, Westende WPC, Gitonga VW, Krens F, Voprrips RE, et al. Using RNA-Seq to assemble a rose transcriptome with more than 13,000 full-length expressed genes and to develop the WagRhSNP 68 k Axiom SNP array for rose (Rosa L.) Front Plant Sci. 2015;6:249. doi: 10.3389/fpls.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns LJ, Fretz TA. Distinguishing rose cultivars by polyacrylamide gel electrophoresis isozyme variation among cultivars. J Am Soc Hortic Sci. 1978;103:509–516. [Google Scholar]
- Lewis WH. Revision of the genus Rosa in Eastern North America: a review. Am Rose Annu. 1957;42:116–126. [Google Scholar]
- Liorzou M, Pernet A, Li S, Chastellier A, Thouroude T, Michel G, Malecot V, et al. Nineteenth-century French rose (Rose sp.) germplasm shows a shift over time from a European to an Asian genetic background. J Exp Bot. 2016;67(15):4711–4725. doi: 10.1093/jxb/erw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, An H, Li L, Jain M. Genome survey sequencing for the characterization of the genetic background of Rosa roxburghii tratt and leaf ascorbate metabolism genes. PLoS ONE. 2016;11:147530. doi: 10.1371/journal.pone.0147530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott M. Modern Roses (Post-1800) In: Roberts A, Debener T, Gudin S, editors. Encyclopedia of Rose Science. Oxford: Elsevier Science; 2003. pp. 402–409. [Google Scholar]
- Mikanagi Y, Saito N, Yokoi M, Tatsuzawa F. Anthocyanins in flowers of genus Rosa sections Cinnamomeae (Rosa) Chinensis, Gallicanae and some modern garden roses. Biochem Syst Ecol. 2000;28:887–902. doi: 10.1016/s0305-1978(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Mohapatra A, Rout GR. Identification and analysis of genetic variation among rose cultivars using random amplified polymorphic DNA. Z Naturforsch. 2005;60:611–617. doi: 10.1515/znc-2005-7-817. [DOI] [PubMed] [Google Scholar]
- Murray M, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Hirakawa H, Sato S, Otagaki S, Matsumoto S, Tabata S, Tanaka Y. Genome structure of Rosa multiflora, a wild ancestor of cultivated roses. DNA Res. 2018;25(2):113–121. doi: 10.1093/dnares/dsx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. PNAS. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybom H, Werlemark G, Esselink GD, Vosman B. Sexual preferences linked to rose taxonomy and cytology. Acta Hortic. 2005;690:21–28. [Google Scholar]
- Oyant HSL, Crespel L, Rajapakse S, Zhang L, Foucher F. Genetic linkage maps of rose constructed with new microsatellite markers and locating QTL controlling flowering traits. Tree Genet Genomes. 2008;4:11–23. [Google Scholar]
- Panwar S, Singh KP, Sonah H, Deshmukh PK, Namita B, Prasad KV, Sharma TR. Molecular fingerprinting and assessment of genetic diversity in rose. Indian J Biotechnol. 2015;14:518–524. [Google Scholar]
- Prasad KV, Kumar S, Choudhary ML. Molecular characterization of fragrant rose cultivars. Indian J Hortic. 2006;63:229–234. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Chen X, Fang P, Shi S, Li J, Liu X, Cao X, Zhao N, Hao H, Li Y, Han Y, Zhang Z. Genomic and transcriptomic sequencing of Rosa hybrida provides microsatellite markers for breeding, flower trait improvement and taxonomy studies. BMC Plant Biol. 2018;18:119. doi: 10.1186/s12870-018-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai H, Raju DVS, Kumar AMB, Janakiram T, Namita S, Krishnan G, Rana JC. Characterization and analysis of genetic diversity among different species of rose using morphological and molecular markers. Indian J Agric Sci. 2015;85(2):240–245. [Google Scholar]
- Riek JD, Cock KD, Smulders MJM, Nybom H. AFLP-based population structure analysis as a means to validate the complex taxonomy of dog roses (Rosa section Caninae) Mol Phylogenet Evol. 2013;67(3):547–559. doi: 10.1016/j.ympev.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ (2005) NTSYSpc (Numerical Taxonomy & Multivariate Analysis System). Version 2.2, Exeter Software, Applied Biostatistics Inc., New York
- Rusanov K, Kovacheva N, Vosman B, Zhang L, Rajapakse S, Atanassov A, Atanassov I. Microsatellite analysis of Rosa damascena Mill. Accessions reveals genetic similarity between genotypes used for rose oil production and old damask rose varieties. Theor Appl Genet. 2005;111:804–809. doi: 10.1007/s00122-005-2066-9. [DOI] [PubMed] [Google Scholar]
- Smulders MJM, Esselink D, Voorrips RE, Vosman B. Analysis of a database of DNA profiles of 734 hybrid tea rose varieties. Acta Hortic. 2009;836:169–174. [Google Scholar]
- Spillar M, Linde M, Oyant HSL, Tasi CJ, Byrne DH, Smulders MJM, Foucher F, Debener T. Towards unweighted genetic map for diploid roses. Theor Appl Genet. 2011;122:489–500. doi: 10.1007/s00122-010-1463-x. [DOI] [PubMed] [Google Scholar]
- Vainstein A, Ben-Meir H, Zucker A (1993) DNA fingerprinting as a reliable tool for the identification and genetic analysis of ornamentals. In: Proceedings of XVIIth eucarpia symposium on “creating genetic variation in ornamentals”, San Remo, pp 63–68
- Walker CA, Werner DJ. Isozyme and randomly amplified polymorphic DNA (RAPD) analyses of Cherokeerose and its putative hybrids ‘Silver Moon’ and ‘Anemone’. J Am Soc Hortic Sci. 1997;122:659–664. [Google Scholar]
- Yan Z, Denneboom C, Hattendorf A, Dolstra O, Debener T, Stam P, Visser PB. Construction of an integrated map of rose with AFLP, SSR, PK, RGA, RFLP, SCAR and morphological markers. Theor Appl Genet. 2005;110:766–777. doi: 10.1007/s00122-004-1903-6. [DOI] [PubMed] [Google Scholar]
- Yang S, Guo N, Hong GE. Morphological and AFLP based genetic diversity in Rosa platycantha population in Eastern Tianshan Mountains of northwestern China. Hortic Plant J. 2016;2(1):55–60. [Google Scholar]
- Yeh FC, Yang RC, Boyle TBJ, Ye ZH, Mao JX. POPGENE, the user-friendly shareware for population genetic analysis. Canada: Molecular Biology and Biotechnology Centre, University of Alberta; 1997. [Google Scholar]
- Yeh FC, Yang RC, Boyle T (1999) Power Marker 3.23, Alpha Ease FC 4: Microsoft Window-based free Software for Population Genetic Analysis. ftp.microsoft.com/Softlib/HPGL.EXE
- Zhang LH, Byrne DH, Ballard RE, Rajapakse S. Microsatellite marker development in rose and its application in tetraploid mapping. J Am Soc Hortic Sci. 2006;131(3):380–387. [Google Scholar]
- Zlesak DC. Rosa x hybrida L. In: Anderson NO, editor. Flower Breeding and genetics: issues, challenges and opportunities for the 21st century. Dordrecht: Springer; 2006. pp. 695–738. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.