Abstract
The genetic transformation of plants is an important biotechnological tool used for crop improvement for many decades. The present study was focussed to investigate various factors affecting genetic transformation of potato cultivar ‘Kufri Chipsona 1’. It was observed that explants pre-cultured for 2 days on MS2 medium (MS medium containing 10 µM silver nitrate, 10 µM BA, 15 µM GA3), injured with a surgical blade and co-cultivated with Agrobacterium tumefaciens strain EHA105 [O.D600 (0.6)] for 2 days results in maximum transient β-glucuronidase (GUS) expression. The addition of 100 µM acetosyringone in MS2 medium also increased rate of transient GUS expression in both the explants. Clumps of putative transgenic shoots were regenerated using the optimised culture conditions from leaf and internodal explants. The stable integration of T-DNA was established using histochemical staining for GUS and amplification of DNA fragment specific to nptII and uidA genes. Within the clumps, around 67.85% of shoots showed uniform GUS expression in all the tissues and about 32.15% shoots show intermittent GUS expression establishing chimeric nature. Uniform GUS staining of the tissue was used as initial marker of non-chimeric transgenic shoots. Quantitative expression of nptII transgene was found to be directly proportional to uniformity of GUS staining in transgenic shoots. The present investigation indicated that manipulation of culture conditions and the medium composition may help to get transgenic shoots with uniform expression of transgene in all the tissues of potato cultivar ‘Kufri Chipsona 1’.
Keywords: Agrobacterium tumefaciens, Pre-culture period, Co-cultivation, Injury, Chimeric shoots
Introduction
One half of the global root and tuber crop productivity is contributed by potato (Solanum tuberosum); the third most important food crop of world (Millam 2004; FAOSTAT 2019). Potato production of about 388,190,674 tonnes was reported in 2017, of which 12% share was contributed by India, the second largest producer (FAOSTAT 2019; GOI 2018). Unfortunately, India witnesses about 30–50% of the crop loss each year due to various diseases to which blights and viral diseases contribute significantly (Bairwa et al. 2016; APEDA 2003). To effectively address this problem, there is a need to develop disease resistant cultivars (Millam 2004). Conventionally, plant breeding is considered as an effective and viable approach for crop improvement (Breseghello and Coelho 2013). However, a narrow genetic base coupled with a limited area of sexual reproduction, makes breeding a challenging task in potato (Barrell et al. 2013). Further, the tetraploid status of the crop, inbreeding depression and requirement to screen large progeny of populations complicates conventional breeding (Millam 2004; Barrell et al. 2013). Over decades, plant genetic transformation has been providing a solution to problems associated with conventional breeding of potato.
Potato genetic transformation using Agrobacterium tumefaciens was firstly reported in 1986 (An et al. 1986). In no time, attempts were made to genetically transform important potato cultivars throughout the world (Stiekema et al. 1988; Visser et al. 1989; Jongedijk et al. 1992; Snyder and Belknap 1993; Dale and Hampson 1995; Conner et al. 1994; Chakraborty et al. 2000; Sawahel 2002; Felcher et al. 2003; Khatun et al. 2012; Fatahillah et al. 2016; Farhanah et al. 2017). The efficiency of genetic transformation is reported to be influenced by many factors such as explant type, infection time, mode of injury, pre-culture period, co-cultivation time, etc. (Heeres et al. 2002; Aggarwal et al. 2011). Further, the genetic transformation in potato has been reported as genotype-dependent phenomena, which mandates the optimization of factors for every cultivar (De Block 1988; Trujillo et al. 2001; Chakravarty et al. 2007; Han et al. 2015). There are about 52 potato varieties available in India (Kumar et al. 2014). Among these ‘Kufri Chipsona 1’ is the most important processing grade cultivar having highest seed tuber germination, dry matter content and yield (31.15 tonnes per hectare) (Rana et al. 2009; Sadawarti et al. 2018). Despite of its importance, till date there is no report on optimisation of transformation protocol for this important processing grade potato cultivar. Therefore, in the present study, Agrobacterium-mediated genetic transformation protocol was optimized for effective delivery and integration of T-DNA in potato cultivar ‘Kufri Chipsona 1’ and to sort the transgenic shoots on the basis of pattern and extent of expression of transgenes.
Materials and methods
Plant material and chemicals
Cultures of potato cultivar ‘Kufri Chipsona 1’ (CS-1) available at TIFAC-CORE, Thapar Institute of Engineering and Technology, Patiala were maintained on Murashige and Skoog medium (1962) containing 87 mM sucrose and 0.7% (w/v) agar (basal MS medium) in 300 ml glass culture bottles (Kasablanka Ltd, Mumbai). The cultures were multiplied on MS1 medium (basal MS medium containing 10 µM silver nitrate) to get a good quality leaf and internodal explants (Kaur et al. 2017). Unless otherwise mentioned all the chemicals and plant growth regulators were purchased from HiMedia Laboratories Ltd. (Mumbai, India). Other fine chemicals including enzymes were purchased from Thermo Scientific Laboratories Ltd. (Mumbai, India).
Antibiotic sensitivity of explants
Kanamycin tolerance was determined by culturing leaf explants excised from 3 to 4 weeks old microshoots of cultivar CS-1 on shoot regeneration medium (Kaur et al. 2017) (MS1 medium supplemented with BA; 10 µM and GA3; 15 µM, referred to as MS2 medium) containing kanamycin (0, 10, 30, 50, 70, 100 mg/l). The kanamycin was added to the autoclaved medium after it has cooled down to 40–45 °C. Observations were made after approximately 4 weeks of culture.
Agrobacterium-mediated genetic transformation
For optimization of genetic transformation protocol for potato cultivar CS-1, initially Agrobacterium tumefaciens strains LBA4404 and EHA105 were used following the previously optimized genetic transformation conditions for Eucalyptus tereticornis (Aggarwal et al. 2011). Leaf and internodal explants injured with a surgical blade were infected with A. tumefaciens strains LBA4404 and EHA105 harboring binary vector pBI121 (Chen et al. 2003). T-DNA region of pBI121 contains nos promoter driven nptII (neomycin phosphotransferase) gene (kanamycin resistance) as the selectable marker and CaMV35S promoter driven uidA gene as a reporter (Jefferson et al. 1987). Based on transient assay scores, A. tumefaciens strain EHA105 was used for further all experiments.
A. tumefaciens cultures were grown overnight on incubator shaker (250 rpm/28 °C) in 50 ml YEP medium containing selection antibiotics rifampicin (15 mg/l) and kanamycin (50 mg/l). The fresh culture was raised in the same medium and grown till the O.D600 reaches about 0.6. One hour prior to infection, acetosyringone (100 µM) was added to the bacterial culture. Leaf and internodal explants were excised from actively growing microshoots of cultivar CS-1 and pre-cultured on MS2 medium for 1–5 days. These were differently injured with a surgical blade, hypodermic needle, glass beads or sandpaper. Injured explants were infected with overnight growing suspensions of A. tumefaciens strain EHA105 for different durations of time (5–30 min) and co-cultivated on MS2 medium containing different concentrations of acetosyringone (0–100 µM) for varying periods (24–120 h). Incubation of explants was carried out under 16/8 h light/dark cycle or under complete dark conditions at 25 ± 1 °C.
Regeneration and multiplication of transgenic shoots
For selection of the transformants, co-cultivated explants were repeatedly washed with sterile distilled water containing broad-spectrum antibiotic; cefotaxime (200 mg/l). The explants were blotted dry to remove excess water before transfer to MS2 medium containing 200 mg/l cefotaxime and 100 mg/l kanamycin (selection medium). Explants were sub-cultured after every 2 weeks on the selection medium. Regenerated putative transformed shoot clumps were further multiplied on MS1 medium (Kaur et al. 2017) containing kanamycin (100 mg/l). The multiplied shoot clumps were used for confirmation of transgene integration and to study the extent of expression of reporter gene/selection marker gene using histochemical analysis and real-time PCR.
Histochemical assay and selection of transgenic lines
Transient GUS expression was assayed after 2 days of culture on selection medium. Unless otherwise mentioned, 100 explants from each treatment were randomly collected and assayed for transient GUS expression as described earlier (Aggarwal et al. 2011). The explants were then washed with 70% (v/v) ethanol to remove chlorophyll. Explants showing blue coloration were scored as GUS positive.
About 56 putatively transformed, kanamycin resistant single shoots (excised from shoot clumps) were assayed for GUS expression to establish chimeric or non-chimeric nature of the microshoots. The shoots showing uniform GUS expression (blue colour) in all the tissues were scored as putative non-chimeric, while others showing intermittent blue colouration in certain tissues were scored as chimeric. Chimeric and putatively non-chimeric shoots were multiplied on MS1 medium containing kanamycin (100 mg/l) and the lines were maintained separately. For further analysis of chimerism, four transgenic lines were selected on the basis of extent of GUS expression.
PCR screening of transgenic lines
DNA from transformed shoots and untransformed controls was isolated as described by Doyle and Doyle (1990) and integration of nptII and uidA genes was confirmed by PCR amplification using gene-specific primers as described earlier (Aggarwal et al. 2011). Further, PCR amplification of Agrobacterium specific 16S rRNA fragment from transformed and non-transformed shoots (Weisburg et al. 1991) was also carried out using similar amplification conditions taking bacterial genomic DNA as a positive control. Ethidium bromide stained, agarose gels (1% w/v) with amplified fragments were visualized under UV transilluminator (BioRad, USA).
Real time PCR for detection of chimeras
Total RNA was isolated using 100 mg of each transgenic lines and non-transformed tissues according to the method described by Chang et al. (1993). Tissue samples were homogenised in buffer containing CTAB (2% w/v), Polyvinyl pyrollidone (2%), Tris hydrochloride (100 mM), Ethylenetetraacetic acid (25 mM), Sodium chloride (2 M), β-mercaptaethanol (0.2% v/v) and incubated for 20 min at 65 °C. Equal amount of chloroform isoamyl alcohol (24:1 v/v) was added to the slurry and the supernatant was retained after centrifugation at 5000 rpm for 20 min at 4 °C. RNA was precipitated using lithium chloride (10 M) and pelleted by centrifugation at 12000 rpm/30 min at 4 °C. The pellet was dissolved in RNase free water. The cDNA was synthesized from total RNA by reverse transcription using The RevertAid First Strand cDNA Synthesis Kit (Fermentas Life Sciences, USA) as per manufactures instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of nptII gene was conducted using the Real Master Mix SYBR ROX Master Mix (5 prime, GmbH, Hamburg) using 100 times diluted cDNA, 2x SYBR green and 5 µM each of forward/reverse primers on Realplex 2.2 real-time PCR system (Eppendorf AG, Hamburg) to determine critical thresholds (Ct), using primers (Forward 5′-GAATGAACTGCAGGACGAG-3′ and Reverse 5′-ATACTTTCTCGGCAGGAGCA-3′). For assessment of relative expression of nptII gene in transgenic lines; the housekeeping genes; β-actin (Forward 5′-AGGAGCATCCTGTCCTCCTAA-3′ and reverse 5′-CACCATCACCAGAGTCCAACA-3′) and elongation factor (EF)1-α (forward 5′-GATGGTCAGACCCGTGAACA-3′ and reverse 5′-CCTTGGAGTACTTCGGGGTG-3′) were used as an endogenous controls (Volkov et al. 2003, Tang et al. 2017). Conditions for the qRT-PCR reactions were as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min, and dissociation 68 °C for 20 s. Change in expression was quantified by calculating the difference between threshold cycle value of target gene and internal controls. Data was analysed using Realplex 2.2 real time PCR inbuilt software and change in gene expression was calculated using ∆∆Ct.
Statistical analysis
Unless otherwise mentioned, for optimization of genetic transformation protocol, each experiment was performed three times using 100 explants each treatment. However to test the chimerism, total number of transgenic shoots tested was limited to 56 only. The data were analyzed by one way ANOVA and means were compared with Duncan’s Multiple Range Test (DMRT). Real time analysis was performed using three experimental replicates of three biological replicates. All analyses and graphical representations were carried out with GraphPad Prism version 5.0.
Results
Antibiotic sensitivity
The tolerance limit of leaf explants of potato cultivar CS-1 to kanamycin was tested on MS2 medium containing different concentrations of the antibiotic. In the absence of antibiotic, shoot organogenesis was observed from 35.66% explants, whereas in the presence of kanamycin, organogenesis was not observed in any of the explant. Further, the survival rate of explants drastically decreases with increase in kanamycin concentration and all the explants on the medium containing 100 mg/l kanamycin died (Fig. 1). Therefore, pBI121 harboring nptII gene (imparting resistance to kanamycin) was used as a binary vector and 100 mg/l of kanamycin was added in the selection medium.
Fig. 1.
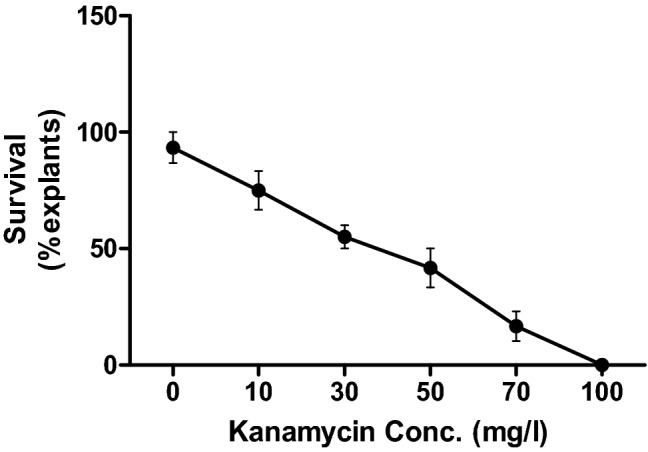
The effect of kanamycin on the survival of leaf explants excised from microshoots of potato cultivar ‘Kufri Chipsona 1'. Data were recorded after 4 weeks of culture
Factors affecting Agrobacterium-mediated genetic transformation
Factors affecting the genetic transformation of potato such as bacterial strain, infection time, mode of injury, culture density, etc. were investigated. Leaf and internodal explants were infected with two strains of A. tumefaciens, namely, EHA105 and LBA4404 (carrying binary vector pBI121). A significantly higher transient GUS expression was observed from both the explants when infected with strain EHA105 (Fig. 2). Thus, for the optimization of genetic transformation protocol, subsequent factors were standardized using A. tumefaciens strain EHA105. The present investigation revealed that internodal explants pre-cultured for 2 days on MS2 medium before infection, showed maximum transient GUS expression (Table 1), but in case of leaf explants, no significant difference between transient GUS expressions was observed after pre-culture period of 1–4 days. It was noteworthy that transient GUS expression significantly decreased in leaf explants pre-cultured for more than 4 days, whereas, in the case of internodal segments, a significant decrease was observed after 3 days of pre-culture.
Fig. 2.
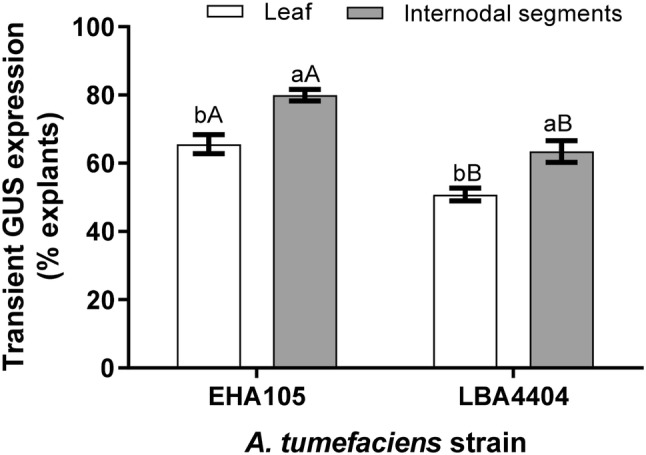
The efficiency of Agrobacterium tumefaciens strains (EHA105 and LBA4404 carrying binary vector pBI121) for the transfer of T-DNA to leaf and internodal segments of ‘Kufri Chipsona 1’. The transient GUS activity was assayed after two days of culture on the selection medium. Data were analyzed using ANOVA and means were compared with Duncan’s Multiple Range Test (DMRT) at P < 0.05. The bars with the same lowercase letter (between explants) and uppercase letter (within explant) are not significant at P < 0.05
Table 1.
Factors effecting transient GUS expression in leaf and internodal explants of potato cultivar ‘Kufri Chipsona 1’ after infection with Agrobacterium tumefaciens strain EHA105 harbouring pBI121 as binary vector
| Factors | Transient GUS expression | |
|---|---|---|
| Leaf | Internode | |
| Preculture period (days) | ||
| 0 | 61.38a | 71.94ab |
| 1 | 64.44a* | 73.89a* |
| 2 | 65.19a | 80.28a |
| 3 | 65.55a* | 74.83a* |
| 4 | 62.33a* | 66.24b* |
| 5 | 53.50b* | 51.49c* |
| 6 | 44.49c* | 51.39c* |
| 7 | 42.49c* | 45.22 cd* |
| Co-cultivation time (days) | ||
| 1 | 51.04b* | 56.91b* |
| 2 | 61.22a | 75.58a |
| 3 | 48.11b | 74.83a |
| 4 | 51.05b | 74.64a |
| 5 | 47.25b | 69.72a |
| Mode of injury | ||
| Sandpaper | 44.99a* | 47.78b* |
| Hypodermic needle | 41.39a | 19.22c |
| Glass Beads | 42.36a* | 50.00b* |
| Surgical blade | 50.42a | 73.83a |
| Infection time (min) | ||
| 5 | 13.75c | 28.88d |
| 10 | 34.03b* | 39.58c* |
| 15 | 48.33a | 63.88a |
| 20 | 38.54b | 50.97b |
| 30 | 34.50b* | 32.41c* |
| Bacterial density (O.D) | ||
| 0.2 | 49.86b* | 55.55d* |
| 0.4 | 54.16b* | 63.33c* |
| 0.6 | 73.89a | 89.29a |
| 0.8 | 57.72b | 77.22b |
| 1.0 | 51.59b | 65.28c |
| AcetosyringoneConc (µM) | ||
| 0 | 59.72b* | 68.05b* |
| 50 | 63.88b* | 65.64b* |
| 100 | 77.77a* | 81.94a* |
| Photoperiod (light/dark hrs) | ||
| 24/0 | 56.94b | 77.78a |
| 0/24 | 70.83a | 83.61a |
Filter sterilised Acetosyringone solution was added to the autoclaved co-cultivation medium for evaluating its effect. Each experiment was repeated three times with hundered explants each experiment. Data were analysed for each factor separately using ANOVA and means were compared by Duncan’s Multiple Range Test (DMRT) at P < 0.05. Mean values followed by same lowercase letter (within explant) and ‘*’ (between explants) are not significant at P < 0.05
Leaf and internodal segments pre-cultured for 2 days and injured using sandpaper, surgical blade, glass beads or hypodermic needle before infection also showed varied response (Table 1). Wounding the internodes with a scalpel and hypodermic needle before infection resulted in highest (73.83%) and lowest (19.22%) transient GUS expression respectively. No significant effect of mode of injury was observed in the case of leaf explants.
In order to determine the optimal density of bacterial cultures for genetic transformation, both explants were infected with A. tumefaciens strain EHA105 suspensions of varied cell densities (O.D.600 0.2–1.0). In both the explants, maximum transient GUS expression was observed in explants infected with A. tumefaciens growing at O.D.600 of 0.6. Internodal explants showed a significant increase in transient GUS expression with increasing bacterial density from 0.2 to 0.6. But in case of the leaf explants, bacterial culture densities (0.2, 0.4, 0.8 and 1.0) were not found to have a significant effect on transient GUS expression (Table 1).
The impact of infection time on transient GUS expression in both types of explants (leaf and internodal) was also observed. It was noted that both type of explants infected for 15 min with A. tumefaciens strain EHA105 resulted in maximum transient GUS expression (Table 1). It was noteworthy that deviation from infection time of 15 min adversely impacted the transient GUS expression.
A significant effect of co-cultivation periods and conditions on transient GUS expression in leaf and internodal explants of potato cultivar CS-1 was observed. Co-cultivation of explants with Agrobacterium for 2 days induced maximum transient GUS expression in leaf (61.22%) and internodal (75.58%) explants. Further increase in co-cultivation duration decreased percent explants showing transient GUS expression. However, this decrease was significant in the case of leaf explants only (Table 1). Incorporation of acetosyringone, was also found to significantly influence transient GUS expression of explants. It was observed that the addition of 100 µM acetosyringone increased transient GUS expression in both explants (59–77.8% in leaf explants and from 68 to 82% in internodal explants). Furthermore, explants co-cultivated in complete dark conditions showed higher transient GUS expression (Table 1).
Regeneration of transgenic shoots
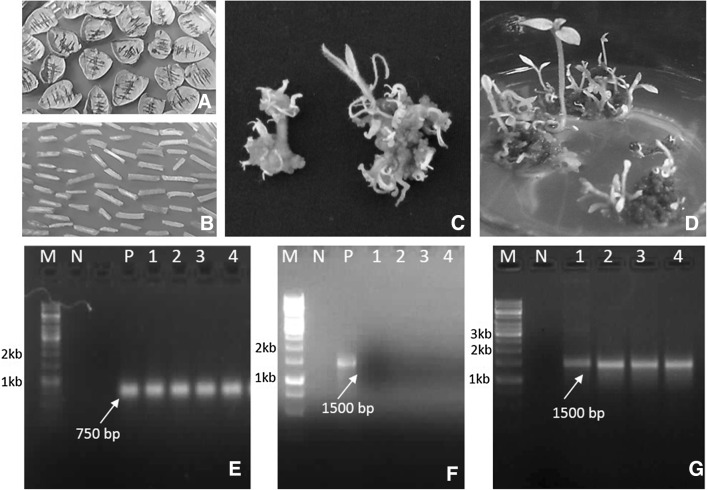
Callus initiation was observed from midrib region and cut ends of leaf and internodal explants of cultivar CS-1 cultured on MS2 medium containing kanamycin (100 mg/l). Although, callogenesis was observed from 46.64% of leaf and 43.33% of internodal explants, yet, regeneration of transformed shoots was observed from only 10.37% of leaf and 15.97% of internodal explants (Table 2). The shoots regenerated from the callus as clumps of about 2–4 shoots (Fig. 3c, d; Table 2).
Table 2.
Frequency of genetic transformation of potato cultivar ‘Kufri Chipsona 1’ genetically transformed with Agrobacterium tumefaciens strain EHA105 harbouring binary vector pBI121
| Explants | Mean explants callusing (%) | Mean number of shoots regenerated per callus | % regeneration of transformed shoots |
|---|---|---|---|
| Leaf | 46.64 ± 2.89a | 1.67 ± 0.33b | 10.37 ± 2.59b |
| Internode | 43.33 ± 3.85a | 4.00 ± 1.53a | 15.97 ± 4.86a |
The experiment was repeated three times using total 90 explants. Mean values were compared using Newman’s Keul test at P < 0.05. The values followed by same lower case letters are not significantly different
Fig. 3.
Shoot organogenesis following A. tumefaciens infection and testing of T-DNA integration; A, B leaf and internodal explants excised from 3 week old cultures of potato cultivar ‘Kufri Chipsona 1’; C, D shoot organogenesis from C internodal and D leaf explants on selection medium containing kanamycin (100 mg/l) and cefotaxime (200 mg/l); E Amplification of nptII gene (750 bp); F 16S rRNA (1500 bp); GuidA gene (1500 bp) from genomic DNA of transformed shoot (M 1 kb marker, N negative control, P positive control, 1-4 lines of shoots transformed with Agrobacterium strain EHA105 carrying binary vector pBI121)
Molecular analyses could not differentiate chimeras
Shoots clumps regenerated from the explants were subjected to GUS assay for confirmation of stable integration of T-DNA. For this purpose, a small part of single microshoot from the clump was excised for GUS assay and rest part of the same shoot was multiplied using nodal segments on MS1 medium. The microshoots were divided into two categories i.e. microshoots with uniform GUS expression and with intermittent GUS expression. Total 56 shoots were tested, out of which 67.85% showed uniform GUS expression and categorised as putative non-chimeric (Table 3). For the further analysis, four transgenic lines were selected which includes one chimeric (T1) and three putative non-chimeric transgenic (T2–T4) lines. Although, the regeneration of kanamycin resistant shoots (Fig. 3c, d) and appearance of blue coloration following assay with 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) (Fig. 4a) was an indicator of successful delivery of nptII (kanamycin resistance) and uidA (β-glucoronidase) gene, yet molecular analyses were carried to establish stable integration of T-DNA. Amplification of 750 bp (Fig. 3e) and 1500 bp (Fig. 3g) fragments with nptII and uidA gene-specific primers was observed in all the transgenic lines. Further, no amplification of DNA fragment specific to 16S rRNA from different tissue of regenerated shoots (Fig. 3f) indicates the complete elimination of bacteria from all the transgenic shoot cultures lines.
Table 3.
Chimerism of potato cultivar ‘Kufri Chipsona 1’ genetically transformed with Agrobacterium tumefaciens strain EHA105 harbouring binary vector pBI121
| Number of shoots tested from clumps (5th selection) | PCR positive shoots | non-chimeric shoots | Mean non-chimerism (%) |
|---|---|---|---|
| 56 | 56 | 38 | 67.85 |
The data were recorded after complete removal of chlorophyll from the shoots and shoots showing blue colour across the plant were scored as non-chimeric
Fig. 4.
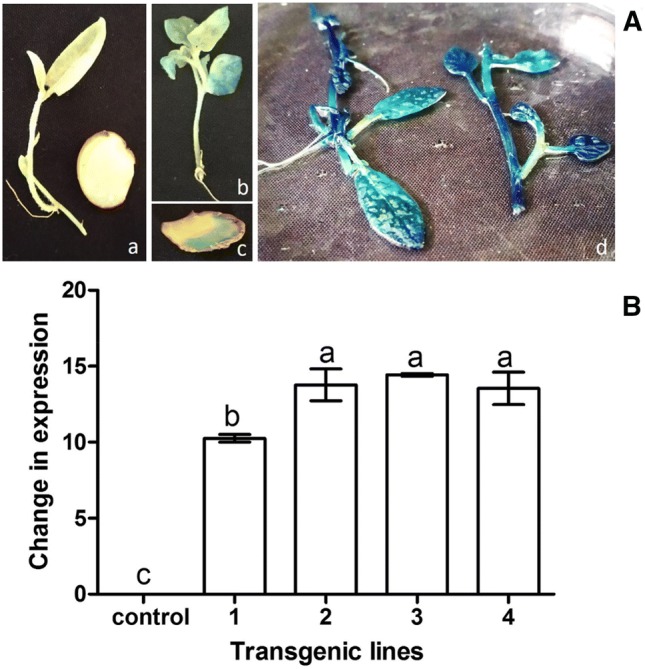
Detection of chimerism in four transgenic lines of potato cultivar ‘Kufri Chipsona 1’ transformed with binary vector pBI121 carrying both uidA and nptII genes; A Histochemical GUS staining of a untransformed shoots, b,c chimeric shoots and tuber, d non-chimeric shoots and tuber; B Determination of relative amount of the nptII transgenes, compared with non-transgenic control using RT-PCR
Real time PCR confirms non-chimeric shoots
Chimeric and putative non-chimeric transgenic lines were subjected to quantification of nptII gene expression using qRT-PCR. A positive correlation between extent of transformation and expression levels of nptII gene was observed. It was observed that in chimeric transgenic line (TI), nptII gene was expressed at lower level (Fig. 4b), whereas the expression was higher in case of putatively non-chimeric lines (T2–T4). It was noteworthy that more intense was blue colouration the higher was the nptII gene expression.
Discussion
The era of early 1980s was marked with successful reports on Agrobacterium-mediated gene delivery in plants including potato (An et al. 1986; Stiekema et al. 1988; Visser et al. 1989; De Block 1988). Since then numerous studies on the development of genetic transformation protocols of potato has been reported in other cultivars (Feher et al. 1991; Sidorov et al. 1999; Valkov et al. 2011; Wendt et al. 2011, 2012). Among various methods, Agrobacterium-mediated gene delivery is considered highly efficient and successful (Conner et al. 1997; Si et al. 2003). In potato, Agrobacterium-mediated genetic transformation protocol has been developed using various explants such as tuber discs (Si et al. 2003; Stiekema et al. 1988), stem segments (Beaujean et al. 1998; Han et al. 2015) and leaves (Banerjee et al. 2006; Trujillo et al. 2001), but all these protocols are highly genotype dependent (Chakravarty et al. 2007; Han et al. 2015). Thus, there is a need to optimise genetic transformation protocol for every cultivar of potato. Although genetic transformation has been optimised for many potato cultivars, but no definitive protocol is available for one of the most important processing grade Indian potato cultivar CS-1. Therefore, the present study was focussed to optimize various factors involved in genetic transformation of potato cultivar CS-1 and to establish the nature of regenerated transgenic lines with respect to the extent of transformation.
As an initial step of genetic transformation studies, antibiotic tolerance levels in potato cultivar CS-1 was investigated. The toxicity level of kanamycin in leaf explants of this cultivar was found to be much higher (100 mg/l). Earlier studies reported the toxicity of kanamycin to the potato and other plants at lower levels (Aggarwal et al. 2011; Trujillo et al. 2001; Si et al. 2003; Banerjee et al. 2006; Alvarez and Ordas 2007; Jube and Borthakur 2009). In many other plants, kanamycin toxicity levels were found to be less than 50 mg/l (Molla et al. 2011; Aggarwal et al. 2011; Veale et al. 2012). However in this study, about 55% of leaf explants were able to survive on the medium containing 50 mg/l kanamycin and necrosis of 100% explants was induced only on medium containing 100 mg/l kanamycin (Fig. 1). These results are in line with previous reports indicating the requirement of higher kanamycin levels in explants of potato cultivars ‘chubaek’, ‘Namjak’, ‘Jasim’, ‘Jopoong’, ‘Desiree’ and ‘Agria’ (Shin et al. 2011; Kamrani et al. 2015). Toxicity of kanamycin, (an antibiotic of aminoglycosides group) is due to inhibition of translational events in chloroplastic/mitochondrial ribosomes (Keeling et al. 2001; Kurtz 1974) and changes in the cytoplasmic membrane (Padilla and Burgos 2010). Expression of neomycin phosphotransferase (nptII) can detoxify aminoglycosides (Padilla and Burgos 2010), thus, allowing the selection of transgenic plants on medium containing kanamycin.
To improve genetic transformation efficiency, two Agrobacterium tumefaciens strains, namely, LBA4404 and EHA105 were tested for their efficacy for T-DNA delivery to potato cultivar CS-1. Higher rate of transient GUS expression was observed from the leaf (65.58%) and internodal (79.99%) explants infected with A. tumefaciens strain EHA105 (Fig. 2). The superiority of strain EHA105 over other strains has been previously reported in many studies (Hood et al. 1986; Terakami et al. 2007; Aggarwal et al. 2011). Although, it has also been reported that strain EHA105 is difficult to eliminate from the cultures and require higher levels (about 400 mg/l) of cefotaxime as compared to strain LBA4404 (Terakami et al. 2007; Maheswaran et al. 1992), yet in the present study cefotaxime at a concentration of 200 mg/l was sufficient to eliminate the bacteria from cultures.
The genetic transformation is also reported to be influenced by many other factors such as pre-culture period, co-cultivation period, mode of infection, bacterial density, etc., which play an important role in the delivery of T-DNA (Aggarwal et al. 2011; Tournier et al. 2003; Yasmeen 2009; Yevtushenko and Misra 2010). It has been reported that pre-culture of explants on medium containing auxins or cytokinins increase T-DNA delivery and its stable integration into the genome (Gohlke and Deeken 2014; Chateau et al. 2000). In the present study, it was observed that both the types of explants pre-cultured on MS2 medium for 2 days before infection resulted maximum transient GUS expression (Table 1).
The mode of injury was also observed to have an important role and use of a surgical blade (soft cuts) for injuring the explant induced maximum transient GUS expression. It was important to note that the role of mode of injury was important in case of internodal explants only where the transient GUS expression increased from 19.22% (pricking with a hypodermic needle) to 73.33% (injury with a scalpel) (Table 1). These results are in line with previous observations, where variation in response of leaf and internodal explants resulting from the mode of injury has been reported in potato (Visser et al. 1989; Beaujean et al. 1998).
The density of bacterial suspension used for infecting explants was also found to affect the transient GUS expression in potato cultivar CS-1 (Table 1). Higher densities of A. tumefaciens cultures were reported to increase the chances of tissue damage, thus reducing efficiency of transformation (Zhong 2007). The genetic transformation was further reported to be dependent upon infection time and duration of the co-cultivation period (Aggarwal et al. 2011; Niu et al. 2000). In this study, the highest efficiency was observed from leaf and internodal explants infected for 15 min with A. tumefaciens strain EHA105 at an O.D of 0.6 followed by co-cultivation for 2 days. These results are in line with earlier findings in other plants (Aggarwal et al. 2011; Niu et al. 2000). It has also been observed that medium composition and incubation conditions during co-cultivation period influence T-DNA transfer (Zambre et al. 2003; Zuker et al. 1999; Godwin et al. 1991; Tournier et al. 2003). In the present study, the explants co-cultivated under continuous dark were found to increase transient GUS expression.
Phenolic compounds are important for activating vir gene cascade during Agrobacterium-mediated T-DNA transfer (Hess et al. 1991). Therefore, the addition of acetosyringone (a phenol) during co-cultivation is reported to improve T-DNA delivery (Subramoni et al. 2014). In the present study, incorporation of 100 µM of acetosyringone was found to result in maximum transient GUS expression (Table 1), which is in line with earlier findings in Eucalyptus, Camellia sinenis and Glycine max (Lopez et al. 2014; Li et al. 2017; Tournier et al. 2003).
The optimized genetic transformation conditions during the present study resulted in callus induction as well as regeneration of shoots clumps from both leaf and internodal explants (Table 2). The genetic transformation efficiency in the present study was found as 10.37% and 15.97% from leaf and internodal explants respectively (Table 2). It is noteworthy that in previous studies, genetic transformation efficiency of potato was reported in the range of 1–5% (De Vetten et al. 2003; Rakosy-Tican et al. 2007), which is about three times lower than the present efficiency. It has been previously reported that the occurrence of chimeras is a major concern, which also affect the pattern of expression in regenerants of subsequent subculture cycles (Faize et al. 2010). This reduce the transformation efficiencies significantly (Bhowmik et al. 2019). Thus, in the present study attempts were made to test the chimerism. To evaluate the occurrence of chimeras in present study, the individual shoots from the regenerated clumps were subjected to GUS assay and based on blue colouration, transgenic shoots were classified as chimeric and putative non-chimeric. One chimeric line (T1) and three putative non-chimeric lines (T2–T4) were multiplied and maintained on MS1 medium using nodal segments. Amplification of uidA and nptII fragments along with the absence of 16S rRNA fragment from T1 to T4 lines confirmed stable transformation and complete elimination of bacteria from the plant tissue but was unable to differentiate between chimeric and non-chimeric tissues (Fig. 3e–g). Earlier, it has been demonstrated that quantification of transgene expression can be used for identification of chimerism (Faize et al. 2010). In this report, RT-PCR was used to confirm stable integration of nptII gene in tobacco and apricot. Thus in the present study qRT-PCR was used to quantify nptII gene in chimeric and putative non-chimeric transgenic lines (Fig. 4b). It was noteworthy that in T1 (chimeric transgenic line), lower nptII gene expression was observed whereas in other transgenic lines (T2–T4) with uniform expression of GUS, nptII gene was expressed to higher levels. It was also interesting to note that intensity of blue colouration in GUS assay was directly proportional to levels of the gene expression.
In conclusion, the methodology described in the present study leads to improved genetic transformation efficiency and provide a basic study to detect chimerism in Indian potato cultivar ‘Kufri Chipsona 1’. The study can be used as a platform for carrying out genetic manipulations of the crop.
Funding
This work was supported by the Council of Scientific and Industrial Research, Extramural Research Division, New Delhi through the Project 38(1465)/18/EMR-II.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal D, Kumar A, Reddy MS. Agrobacterium tumefaciens mediated genetic transformation of selected elite clone(s) of Eucalyptus tereticornis. Acta Physiol Plant. 2011;33:1603–1611. doi: 10.1007/s11738-010-0695-3. [DOI] [Google Scholar]
- Alvarez R, Ordas RJ. Improved genetic transformation protocol for cork oak (Quercus suber L.) Plant Cell Tiss Organ Cult. 2007;91:45–52. doi: 10.1007/978-1-61779-818-4_28. [DOI] [Google Scholar]
- An G, Watson BD, Chiang CC. Transformation of tobacco, tomato, potato and Arabidopsis thaliana using binary Ti vector system. Plant Physiol. 1986;81:301–305. doi: 10.1104/pp.81.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APEDA (2003) Agricultural & processed food products export development authority post harvest manual for export of potatoes
- Bairwa A, Venkatasalam EP, Sudha R, Umamaheswari R, Sharma S, Singh BP, et al. Managementof late blight disease in Kharif potato at Karnataka. Potato J. 2016;43:173–181. [Google Scholar]
- Banerjee AK, Prat S, Hannapel DJ. Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens mediated transformation. Plant Sci. 2006;170:732–738. doi: 10.1016/j.plantsci.2005.11.007. [DOI] [Google Scholar]
- Barrell PJ, Meiyalaghan S, Jacobs JME, Conner AJ. Applications of biotechnology and genomics in potato improvement. Plant Biotechnol J. 2013;11:907–920. doi: 10.1111/pbi.12099. [DOI] [PubMed] [Google Scholar]
- Beaujean A, Sangwan RS, Lecardonnel A, Sangwan-Norreel BS. Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J Exp Bot. 1998;49:1589–1595. doi: 10.1093/jxb/49.326.1589. [DOI] [Google Scholar]
- Bhowmik SSD, Cheng AY, Long H, Tan GZH, Hoang TML, Karbaschi MR, et al. Robust genetic transformation system to obtain non-chimeric transgenic chickpea. Front Plant Sci. 2019;10:524. doi: 10.3389/fpls.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breseghello F, Coelho ASG. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.) J Agric Food Chem. 2013;61:8277–8286. doi: 10.1021/jf305531j. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Chakraborty N, Datta A. Increased nutritive value of transgenic potato by expressing a non-allergenic seed albumin gene from Amaranthus hypochondriacus. Proc Natl Acad Sci USA. 2000;97:3724–3729. doi: 10.1073/pnas.97.7.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty B, Pruski GW, Flinn B, Gustafson V, Sharon R. Genetic transformation in potato: approaches and strategies. Am Potato J. 2007;84:301–311. doi: 10.1007/bf02986242. [DOI] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. doi: 10.1007/BF02670468. [DOI] [Google Scholar]
- Chateau S, Sangwan RS, Sangwan-Norreel BS. Competance of Arabidopsis thaliana genotypes and mutants for Agrobacterium tumefaciens-mediated gene transfer: role of phytohormones. J Exp Bot. 2000;51:1961–1968. doi: 10.1093/jexbot/51.353.1961. [DOI] [PubMed] [Google Scholar]
- Chen P-Y, Wang C-K, Soong S-C, To K-Y. Complete sequence of the binary vector pBI121 and its applications in cloning T-DNA insertion from transgenic plants. Mol Breed. 2003;11:287–293. doi: 10.1023/A:1023475710642. [DOI] [Google Scholar]
- Conner AJ, Williams MK, Abernethy DJ, Fletcher PJ, Genet RA. Field performance of transgenic potatoes. N Z J Crop Hortic. 1994;22:361–371. doi: 10.1080/01140671.1994.9513847. [DOI] [Google Scholar]
- Conner AJ, Jacobs JME, Genet RA. Transgenic potatoes versus “traditional” potatoes: what’s the difference? In: McLean GD, Waterhouse PM, Evans G, Gibbs MJ, editors. Commercialization of transgenic crops: risk, benefit and trade considerations. Canberra: Cooperative Research Centre for Plant Science and Bureau of Resource Sciences; 1997. pp. 23–36. [Google Scholar]
- Dale PJ, Hampson KK. An assessment of morphogenic and transformation efficiency in a range of varieties of potato (Solanum tuberosum) Euphytica. 1995;85:101–108. doi: 10.1007/BF00023936. [DOI] [Google Scholar]
- De Block M. Genotype-independent leaf disk transformation of potato (Solanum tuberosum) using Agrobacterium tumefaciens. Theor Appl Genet. 1988;76:767–774. doi: 10.1007/BF00303524. [DOI] [PubMed] [Google Scholar]
- De Vetten N, Wolters AM, Raemakers K, van der Meer I, ter Stege R, Heeres E, Heeres P, Visser R. A transformation method for obtaining marker-free plants of a cross-pollinating and vegetatively propagated crop. Nat Biotechnol. 2003;21:439–442. doi: 10.1038/nbt801. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Faize M, Faize L, Burgos L. Using quantitative real-time PCR to detect chimeras in transgenic tobacco and apricot and to monitor their dissociation. BMC Biotechnol. 2010;10:53. doi: 10.1186/1472-6750-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2019) Food and Agriculture organisation of the United Nations (www.fao.org/faostat/en/#data/QC/;2019). Accessed 4 Oct 2019
- Farhanah A, Suharsono S, Wattimena GA, Widyastuti U. Genetic engineering of potato plant (Solanum tuberosum L.) cv. JalaIpam with MmPMA gene encoding plasma membrane H+-ATPase. Pak J Biotechnol. 2017;14:37–42. [Google Scholar]
- Fatahillah, Suharsono S, Astuti UW. Genetic transformation of potato (Solanum tuberosum L.) cv. Nooksack with FBPase/ClRan1 genes mediated by Agrobacterium tumefaciens. Pak J Biotechnol. 2016;13:187–192. [Google Scholar]
- Feher A, Felfoldi K, Preiszner J. PEG- mediated transformation of leaf protoplast of Solanum tuberosum L. cultivar. Plant Cell Tiss Organ Cult. 1991;27:105–114. doi: 10.1007/BF00048214. [DOI] [Google Scholar]
- Felcher KJ, Douches DS, Kirk WW, Hammerschmidt R. Expression of a fungal glucose oxidase gene in three potato cultivars with different susceptibility to late blight (Phytophthora infestans Mont. deBary) J Am Soc Hortic Sci. 2003;128:238–245. doi: 10.21273/jashs.128.2.0238. [DOI] [Google Scholar]
- Godwin I, Todd G, Ford-Lloyd B, Newbury HJ. The effect of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep. 1991;9:671–675. doi: 10.1007/BF00235354. [DOI] [PubMed] [Google Scholar]
- Gohlke J, Deeken R. Plant responses to Agrobacterium tumefaciens and crown gall development. Front Plant Sci. 2014;5:155. doi: 10.3389/fpls.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOI (2018) Monthly report on potato. Horticulture statistic division. Department of Agriculture, Cooperation and farmers welfare
- Han EH, Goo YM, Lee MK, Lee SW. An efficient transformation method for a potato (Solanum tuberosum L. var. Atlantic) J Plant Biotechnol. 2015;42:77–82. doi: 10.5010/JPB.2015.42.2.77. [DOI] [Google Scholar]
- Heeres P, Schippers-Rozenboom M, Jacobsen E, Visser RGF. Transformation of a large number of potato varieties: genotype dependent variation in efficiency and somaclonal variability. Euphytica. 2002;124:13–22. doi: 10.1023/A:1015689112703. [DOI] [Google Scholar]
- Hess KM, Dudley MW, Lynn DG, Joerger RD, Binns AN. Mechanism of phenolic activation of Agrobacterium virulence genes: development of a specific inhibitor of bacterial sensor/response systems. PNAS. 1991;88:7854–7858. doi: 10.1073/pnas.88.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood EE, Helmer GL, Fraley RT, Chilton MD. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol. 1986;168:1291–1301. doi: 10.1128/jb.168.3.1291-1301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongedijk E, De Schutter AAJM, Stolte T, Van den Elzen PJM, Cornelissen BJC. Increased resistance to potato virus-x and preservation of cultivar properties in transgenic potato under field conditions. Bio/Technology. 1992;10:422–429. doi: 10.1038/nbt0492-422. [DOI] [PubMed] [Google Scholar]
- Jube S, Borthakur D. Development of an Agrobacterium mediated transformation protocol for the tree-legume Leucaena leucocephala using immature zygotic embryos. Plant Cell Tiss Organ Cult. 2009;96:325–333. doi: 10.1007/s11240-008-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrani M, Ebadi A, Shiri M. Effect of explant, genotype and plant growth regulators on regeneration and Agrobacterium-mediated transformation of potato. J Agron. 2015;14:227–233. doi: 10.3923/ja.2015.227.233. [DOI] [Google Scholar]
- Kaur A, Reddy MS, Kumar A. Efficient, one step and cultivar independent shoot organogenesis of potato. Physiol Mol Biol-Plant. 2017;23:461–469. doi: 10.1007/s12298-017-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling KM, Brooks DA, Hopwood JJ, Li P, Thompson JN, Bedwell DM. Gentamicin-mediated suppression of Hurler syndrome stop mutations restores a low level of alpha-Liduronidase activity and reduces lysosomal glycosaminoglycan accumulation. Hum Mol Genet. 2001;10:291–299. doi: 10.1093/hmg/10.3.291. [DOI] [PubMed] [Google Scholar]
- Khatun A, Hasan MM, Bachchu MAA, Moniruzzaman M, Nasiruddin KM. Agrobacterium-mediated Genetic transformation of Potato (Solanum tuberosum L.) var. Cardinal and Heera. The Agriculturists. 2012;10:81–86. doi: 10.3329/agric.v10i1.11068. [DOI] [Google Scholar]
- Kumar V, Luthra SK, Bhardwaj V, Singh BP (2014) Indian potato varieties and their salient features. Technical bulletin no. 78. ICAR-Central Potato Research Institute, Shimla, India
- Kurtz DI. Fidelity of protein synthesis with chicken embryo mitochondrial and cytoplasmic ribosomes. Biochemistry. 1974;13:572–577. doi: 10.1021/bi00700a026. [DOI] [PubMed] [Google Scholar]
- Li S, Zhen C, Xu W, Wang C, Cheng Y. Simple, rapid and efficient transformation of genotype Nisqually-1: a basic tool for the first sequenced model tree. Sci Rep. 2017;7:2638. doi: 10.1038/s41598-017-02651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez SJ, Kumar RR, Pius PK, Muraleedharan N. Agrobacterium tumefaciens-mediated genetic transformation in Tea (Camellia sinensis [L.] O. Kuntze) Plant Mol Biol Rep. 2014;22:201a–201j. doi: 10.1007/BF02772730. [DOI] [Google Scholar]
- Maheswaran GM, Welander M, Hutchinson JF, Graham MW, Richards D. Transformation of apple rootstock M26 with Agrobacterium tumefaciens. J Plant Physiol. 1992;139:560–568. doi: 10.1016/S0176-1617(11)80370-6. [DOI] [Google Scholar]
- Millam S. Agrobacterium-mediated transformation of potato. In: Curtis I, editor. Transgenic crops of the world. Dordrecht: Springer; 2004. pp. 257–269. [Google Scholar]
- Molla MM, Nasiruddin KM, Al-Amin M, et al. Effect of growth regulators on direct regeneration of potato. Int Proc Chem Biol Environ Eng. 2011;12:205–210. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Niu X, Li X, Veronese P, Bressan RA, Hasegawa SC, Weller PM. Factors affecting Agrobacterium tumefaciens-mediated transformation of pepper mint. Plant Cell Rep. 2000;19:304–310. doi: 10.1007/s002990050017. [DOI] [PubMed] [Google Scholar]
- Padilla IM, Burgos L. Aminoglycoside antibiotics: structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep. 2010;29:1203–1213. doi: 10.1007/s00299-010-0900-2. [DOI] [PubMed] [Google Scholar]
- Rakosy-Tican E, Aurori CM, Dijkstra C, Thieme R, Aurori A, Davey MR. The usefulness of the gfp reporter gene for monitoring Agrobacterium-mediated transformation of potato dihaploid and tetraploid genotypes. Plant Cell Rep. 2007;26:661–671. doi: 10.1007/s00299-006-0273-8. [DOI] [PubMed] [Google Scholar]
- Rana RK, Pandit A, Pandey SK. Profitability analysis of Kufri Chipsona-1 cultivation in Uttar Pradesh of India. Potato J. 2009;36:166–172. [Google Scholar]
- Sadawarti M, Patel K, Samadhiya RK, Gupta PK, Singh SP, Gupta VK, Roy S, Chakrabarti SK, Verma D. Evaluation of table and processing varieties of potato (Solanum tuberosum L.) FOR North-Central India. Int J Chem Stud. 2018;6:823–833. [Google Scholar]
- Sawahel WA. The production of transgenic potato plants expressing human alpha interferon using lipofectin-mediated transformation. Cell Mol Biol Lett. 2002;7:19–29. [PubMed] [Google Scholar]
- Shin DY, Seong ES, Na JK, Yoo JH, Kang WH, Ghimire BK, et al. Conditions for regeneration and transformation of Solanum tuberosum cultivars using the cotton glutathione S-transferase (Gh-5) gene. Afr J Biotechnol. 2011;10:15135–15141. doi: 10.5897/ajb11.1895. [DOI] [Google Scholar]
- Si HJ, Xie CH, Liu J. An efficient protocol for Agrobacterium mediated transformation with microtuber and the introduction of an antisense class I gene into potato. Acta Agron Sin. 2003;29:801–805. [Google Scholar]
- Sidorov VA, Kasten D, Pang S, Hajdukiewicz PTJ, Staub JM, Nehra NS. Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J. 1999;19:209–216. doi: 10.1046/j.1365-313X.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- Snyder GW, Belknap WR. A modified method for routine Agrobacterium mediated transformation of in vitro grown potato microtubers. Plant Cell Rep. 1993;12:324–327. doi: 10.1007/BF00237428. [DOI] [PubMed] [Google Scholar]
- Stiekema WJ, Heidekamp F, Louwerse JD, Verhoeven HA, Dijkhuis P. Introduction of foreign genes into potato cultivars Bintje and Desiree using an Agrobacterium tumefaciens binary vector. Plant Cell Rep. 1988;7:47–50. doi: 10.1007/bf00272976. [DOI] [PubMed] [Google Scholar]
- Subramoni S, Nathoo N, Klimov E, Yuan ZC. Agrobacterium tumefaciens responses to plant-derived signaling molecules. Front Plant Sci. 2014;5:322. doi: 10.3389/fpls.2014.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhang N, Si H, Calderon-Urrea A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods. 2017;13:85–93. doi: 10.1186/s13007-017-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakami S, Matsuta N, Yamamoto T, Sugaya S, Gemma H, Soejima J. Agrobacterium-mediated transformation of the dwarf pomegranate (Punica granatum L.) Plant Cell Rep. 2007;26:1243–1251. doi: 10.1007/s00299-007-0347-2. [DOI] [PubMed] [Google Scholar]
- Tournier V, Grat S, Marque C, El Kayal W, Penchel R, de Andrade G, et al. An efficient procedure to stably introduce genes into an economically important pulp tree (Eucalyptus grandis X Eucalyptus urophylla) Transgenic Res. 2003;12:403–411. doi: 10.1023/A:1024217910354. [DOI] [PubMed] [Google Scholar]
- Trujillo C, Rodriguez-Arango E, Jaramillo S, Hoyos R, Orduz S, Arango R. One-step transformation of two Andean potato cultivars (Solanum tuberosum L. subsp. andigena) Plant Cell Rep. 2001;20:637–641. doi: 10.1007/s002990100381. [DOI] [Google Scholar]
- Valkov VT, Gargano D, Manna C, Formisano G, Dix PJ, Gray JC, et al. High efficiency plastid transformation in potato and regulation of transgene expression in leaves and tubers by alternative 5′ and 3′ regulatory sequences. Transgenic Res. 2011;20:137–151. doi: 10.1007/s11248-010-9402-9. [DOI] [PubMed] [Google Scholar]
- Veale MA, Slabbert MM, Emmenes LV. Agrobacterium-mediated transformation of potato cv. Mnandi for resistance to the potato tuber moth (Phthorimaea operculella) S Afr J Bot. 2012;80:67–74. doi: 10.1016/j.sajb.2012.02.007. [DOI] [Google Scholar]
- Visser RGF, Jacobsen E, Hesseling-Meinders A, Schans MJ, Witholt B, Feenstra WJ. Transformation of homozygous diploid potato with an Agrobacterium tumefaciens binary vector system by adventitious shoot regeneration on leaf and stem segments. Plant Mol Biol. 1989;12:329–337. doi: 10.1007/BF00043210. [DOI] [PubMed] [Google Scholar]
- Volkov RA, Komarova NY, Panchuk II, Hemleben V. Molecular evolution of rDNA external transcribed spacer and phylogeny of sect. Petota (genus Solanum) Mol Phylogenet Evol. 2003;29:187–202. doi: 10.1016/S1055-7903(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Weisburg WA, Barns SM, Pelletier DA, Lane DJ. 16S rDNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Doohan F, Winckelmann D, Mullins E. Gene transfer into Solanum tuberosum via Rhizobium spp. Transgenic Res. 2011;20:377–386. doi: 10.1007/s11248-010-9423-4. [DOI] [PubMed] [Google Scholar]
- Wendt T, Doohan F, Mullins E. Production of Phytophthora infestans-resistant potato (Solanum tuberosum) utilising Ensifer adhaerens OV14. Transgenic Res. 2012;21:567–578. doi: 10.1007/s11248-011-9553-3. [DOI] [PubMed] [Google Scholar]
- Yasmeen A. An improved protocol for the regeneration and transformation of tomato (cv. Rio Grande) Acta Physiol Plant. 2009;31:1271–1277. doi: 10.1007/s11738-009-0364-6. [DOI] [Google Scholar]
- Yevtushenko DP, Misra S. Efficient Agrobacterium-mediated transformation of commercial hybrid poplar Populus nigra L. X P. maximowiczii A. Henry. Plant Cell Rep. 2010;29:211–229. doi: 10.1007/s00299-009-0806-z. [DOI] [PubMed] [Google Scholar]
- Zambre M, Terryn N, Clercq JD, De Buck S, Dillen W, Van Montagu M, et al. Light strongly promotes gene transfer from Agrobacterium tumefaciens to plant cells. Planta. 2003;216:580–586. doi: 10.1007/s00425-002-0914-2. [DOI] [PubMed] [Google Scholar]
- Zhong G (2007) Establishment of high efficient regenration system of Lilium and Agrobacterium-mediated genetic transformation. Ph.D. thesis, Southwest University Press
- Zuker A, Ahroni A, Tzfira T, Ben-Meir H, Vainstein A. Wounding by bombardment yields highly yields highly efficient Agrobacterium-mediated transformation of carnation (Dianthus caryophyllus L.) Mol Breed. 1999;5:367–375. doi: 10.1023/A:1009671131200. [DOI] [Google Scholar]