Abstract
In the present study the potentials of aqueous extracts of the two plants, neem (Azadirachta indica) and Tulsi (Ocimum sanctum) were examined in alleviating arsenic toxicity in rice (Oryza sativa L.) plants grown in hydroponics. Seedlings of rice grown for 8 days in nutrient solution containing 50 μM sodium arsenite showed decline in growth, reduced biomass, altered membrane permeability and increased production of superoxide anion (O·−2), H2O2 and hydroxyl radicals (·OH). Increased lipid peroxidation marked by elevated TBARS (thiobarbituric acid reactive substances) level, increased protein carbonylation, alterated levels of ascorbate, glutathione and increased activities of enzymes SOD (superoxide dismutase), CAT (catalase), APX (ascorbate peroxidase) and GPX (glutathione peroxidase) were noted in the seedlings on As treatment. Exogenously added leaf aqueous extracts of Azadirachta indica (0.75 mg mL−1, w/v) and Ocimum sanctum (0.87 mg mL−1, w/v) in the growth medium considerably alleviated As toxicity effects in the seedlings, marked by reduced As uptake, restoration of membrane integrity, reduced production of ROS, lowering oxidative damage and restoring the levels of ascorbate, glutathione and activity levels of antioxidative enzymes. Arsenic uptake in the seedlings declined by 72.5% in roots and 72.8% in shoots, when A. indica extract was present in the As treatment medium whereas with O. sanctum extract, the uptake declined by 67.2% in roots and 70.01% in shoots. Results suggest that both A. indica and O. sanctum aqueous extracts have potentials to alleviate arsenic toxicity in rice plants and that A. indica can serve as better As toxicity alleviator compared to O. sanctum.
Keywords: Azadirachta indica, Ocimum sanctum, Antioxidants, Arsenic, Toxicity, Oxidative stress
Introduction
Increased industrial activities along with release of untreated effluents from the industries have resulted in accumulation of waste products, including various metals and metalloids, in the environment. Among different pollutants, the toxic metalloid arsenic (As) is a major pollutant and a potent carcinogen. In recent years, As has become an environmental pollutant of serious concern in many parts of the world (Sharma et al. 2012, 2014). It occurs naturally in different minerals and its availability in soil, water and air depends upon its release and mobilization from the sources (Roy and Saha 2002). Increased use of As in production of pigments, herbicides, wood preservatives along with various natural sources including volcanic eruptions lead to increased level of As in the environment (Tchounwou et al. 2012). Agricultural soils in many parts of the world like in India, China, Bangladesh, and USA are contaminated with up to three fold more As than basal level (Farooq et al. 2016). Drinking water and irrigation water in many countries around the world, more especially in Asia, South America are contaminated with high amounts of As. Though a non-essential element, As is readily taken up by plants along with the normal nutrient uptake and can accumulate inside the tissues to the toxic levels (Brackhage et al. 2014).
Excess As in the soil or in the growth medium causes retarded seed germination, decreases plant growth, ultimately leading to decline in crop productivity (Hettick et al. 2015). With high concentration of As in plant tissues, increased generation of various ROS (reactive oxygen species) such as O·−2 (superoxide), H2O2 and ·OH (hydroxyl radical) takes place leading to a state of oxidative stress in the cells (Mishra et al. 2011). Enhanced lipid peroxidation, DNA damage, protein oxidation and impairment of redox homeostasis in the cells are observed in As exposed plants due to oxidative stress (Mishra et al. 2011; Sharma et al. 2014). To cope up with the ROS induced damage, antioxidative defense system exists in the tissues which comprises of ascorbate and glutathione as non enzymic antioxidants as well as the antioxidative enzymes such as superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), guaiacol peroxidase (GPX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.7), monodehydroascorbatereductase (MDHAR, EC 1.6.5.4) and dehydroascorbatereductase (DHAR, EC 1.8.5.1). These antioxidants work in a specific and coordinated way to maintain a redox homeostasis inside the plants (Sharma et al. 2012).
Azadirachta indica or ‘Neem’ which is also called as Indian lilac belongs to family Meliacae and it is an evergreen or deciduous tree prevalent in tropical regions. Neem is being used in Ayurveda, Unani and homeopathy along with the modern medicine and it contains more than 100 bioactive ingredients which possess a wide range of medicinal, antibacterial, insecticidal and various other types of biological activities (Dohroo et al. 2016). The polyphenolic compounds inactivate the lipid free radicals and also prevent their formation by inhibiting hydroperoxides decomposition (Nahak and Sahu 2010). The polyphenols also chelate metal ions and hence reduce bioavailability of metals in plants (Harikrishnan et al. 2010). Ocimum sanctum or ‘Tulsi’ on the other hand has been used traditionally for many years due to its potent medicinal and antioxidative properties. The leaves of Tulsi possess a wide range of pharmacologically active volatile oils such as eugenol, carvacrol, euginal, linalool, caryophyllene, limatrol and many others (Pattanayak et al. 2010). In addition, a variety of triterpenoids, saponins, flavonoids and tannins are also present. Leaves also contain some water soluble flavanoids such as orientin and vicenin (Pattanayak et al. 2010).
Rice serves as important constituent in diets for the global population. Arsenic is taken up from the soil by the roots of rice plants in the form of either arsenite (AsIII) or arsenate (AsV) (Sharma et al. 2014). Both shoots and grains of rice can accumulate very high concentrations of As compared to other cereals and consuming As-contaminated rice grains may lead to severe health hazards like cancer of lung, skin, liver as well as respiratory and cardiovascular impairments (Guillod-Magnin et al. 2018). This necessitates a detailed study of As transport and the mechanisms of As induced toxicity in rice plants and to explore the methods for mitigating As uptake, its accumulation and toxicity effects in rice plants. To mitigate toxicity effects of the metals Mn, Ni, Cr, Al in plants, many chemicals like nitric oxide, salicylic acid, Mg, Ca and extracts of different parts of plants with high antioxidant potentials such as Terminalia arjuna, Phyllanthus emblica, etc. have been used with successful results (Srivastava and Dubey 2012; Pandey et al. 2013; Rajpoot et al. 2016; Pandey et al. 2019a). These chemicals or herbal extracts reduce the uptake of metals in the tissues and lower the degree of oxidative stress induced by the metals in the plants and thus help in mitigating metal toxicity effects in plants. As the plants A. indica and O. sanctum have proven antioxidant potential, in the present study we have used aqueous leaf extracts of these plants to examine possible alleviation of arsenic toxicity in rice plants. The studies were therefore conducted to investigate effects of As on growth parameters, ROS production, oxidative damage, level of antioxidants, ascorbate and glutathione and the activities of key antioxidative enzymes in rice seedlings grown in hydroponics. The aqueous extracts of A. indica and O. sanctum were examined for their role in alleviating As toxicity in the seedlings.
Materials and methods
Extracts from Azadirachta indica and Ocimum sanctum leaves
Fresh leaves from the tree of A. indica and from the shrub of O. sanctum were collected from within the campus of Banaras Hindu University, India. Leaves were dried in sun and crushed to powder. Five gram leaf powder of A. indica, mixed with 100 mL water was placed on a shaker for 72 h. After centrifugation at 12,000 ×g for 15 min, supernatant was collected and evaporated to dryness. The dried residue weighing 0.750 mg dissolved in 1 mL water served as stock solution of 0.75 mg mL−1 (w/v). Similarly O. sanctum extract was prepared by weighing 0.875 mg residue in 1 mL double distilled water and a stock solution of 0.875 mg mL−1 (w/v) was prepared. When 5 g dried leaf powder of A. indica was extracted in water, 0.750 mg dried residue was obtained, whereas with extraction of 5 g dried leaf powder of O. sanctum, 0.875 mg residue was obtained.
Rice plants and treatments
Rice (Oryza sativa L.) seeds of cultivar Malviya-36, obtained from agriculture farm of Banaras Hindu University were used. Seeds were surface sterilized with 0.1% sodium hypochlorite, soaked in water for 48 h and were then spread in Petri plates on moist filter paper and germinated for 5 days in an incubator (York Scientific Industries, New Delhi, India) maintained at 28 ± 1 °C with 80% relative humidity. Germinated seeds were then placed in 200 mL Yoshida nutrient solution in plastic pots (Yoshida et al. 1976) which served as control, whereas nutrient solutions containing either 3 mL A. indica aqueous extract or 3.5 mL O. sanctum aqueous extract also served as controls in order to examine any direct effects of extract in the absence of As . Nutrient solution containing 50 μM sodium arsenite (NaAsO2) served as As treatment solution, As treatment solution containing 3 mL A. indica (Ai) extract served as As + Ai treatment solution, and nutrient solution containing 50 μM NaAsO2 and 3.5 mL O. sanctum (Os) extract served as As + Os treatment solution. Preliminary experiments were performed and the doses 3 mL of 0.75 mg mL−1 stock of A. indica extract in 200 mL nutrient solution = 2.25 mg A. indica extract in 200 mL solution and 3.5 mL of 0.875 mg mL−1 stock of O. sanctum extract in 200 mL nutrient solution = 3.0625 mg O. sanctum extract in 200 mL nutrient solution were determined which elicited similar effects. In a greenhouse maintained at 28 ± 1 °C under 80% relative humidity and 12 h light/dark cycle with 190–200 μmol m−2 s−1 irradiance, pots were placed for growth of the seedlings. Seedlings were taken out at 4 and 8 days of growth for various analyses and biochemical determinations.
Growth of seedlings and uptake of arsenic
At 4th and 8th day of growth, length and fresh biomass of roots and shoots of the seedlings were determined in triplicate using 10 samples in each set. Dry weights were determined after drying in oven at 60 °C for 72 h. Relative water content (RWC) was determined following the method of Weatherley (1950). Concentration of arsenic in oven dried powdered root/shoot samples was determined according to Allen et al. (1986) employing ICP-OES (Inductive coupled plasma-optical emission spectrometer, Optima 7000 DV, Perkin Elmer, USA) and was expressed as µmoles g−1 tissue dry weight. The analytical standard used for ICP-OES analysis was TraceCERT®, 1 mg/L Arsenic in nitric acid (Merck). The ICP measurement data may be taken as estimates for the concentration of As in the samples, as we did not use standard reference material (SRM) processed at the same time as the experimental samples.
Measurement of superoxide anion production, H2O2 and their histochemical localizations
Production of O·−2 due to autoxidation of epinephrine was measured spectrophotometrically according to Mishra and Fridovich (1972) using freshly cut 3–5 mm pieces of root/shoot samples. Using 1.2 mM epinephrine (prepared in 0.1 N HCl) reaction was initiated and production of O·−2 was recorded at 480 nm using spectrophotometer (Spectronic 20D+, Thermo Scientific, USA). H2O2 was measured following Jana and Choudhuri (1981) using titanium sulfate. Absorption of colour developed was recorded spectrophotometrically at 410 nm and H2O2 level was calculated using extinction coefficient as 0.28 µM−1cm−1. Nitrobluetetrazolium (NBT) was used to detect histochemical localization of O2˙− in leaves following Frahry and Schopfer (2001). Dark blue insoluble formazon deposits were visible on leaf surface as a result of reaction of O2˙− with NBT, which were visualized using microscope. H2O2 was histochemically detected in leaves according to Thordal et al. (1997) employing DAB (3,3′-diaminobenzidine, Amresco, Solon, OH, USA). Reddish brown spots appeared on leaves due to reaction of DAB with H2O2 and peroxidase, which were visualized using microscope.
Determination of ·OH radicals and lipid peroxidation
Formation of ·OH in fresh root/shoot samples was quantified spectrophotometrically according to Halliwell and Gutteridge (1984) using a deoxyribose assay. The absorbance was recorded at 532 nm. The level of lipid peroxidation products was determined in root/shoot samples using 2-thiobarbituric acid (TBA) according to Heath and Packer (1968) and as modified according to Rajpoot et al. (2016) and expressed in terms of nmol TBARS (thiobarbituric acid reactive substances) g−1fresh weight using extinction coefficient of 155 mM−1cm−1. Histochemical localization of lipid peroxides was detected according to Pompella et al. (1987) using Schiff’s reagent. Pink coloured spots showing aldehyde formation were observed under microscope.
Alteration in integrity of plasma membrane
At various levels of treatments, alteration in integrity of root plasma membrane was assessed histochemically using Evan’s blue uptake according to Schützendübel et al. (2001). Dark blue stained roots, due to uptake of the dye Evan’s blue, were examined under microscope. Spectrophotometric assay was also used to quantify the extent of Evan’s blue dye taken up by the roots as described by Pandey et al. (2019a). The uptake of the dye was calculated in terms of its fold uptake by the roots.
Determination of non-enzymic antioxidants
The levels of reduced (ascorbate, AsA) and oxidized form of ascorbate (dehydroascorbate, DHA) as well as of total ascorbate (AsA + DHA) were determined following the method of Law et al. (1983). Initially total ascorbate and AsA were determined. The DHA level was determined by subtracting AsA from total ascorbate. The levels of different forms of glutathione (reduced glutathione GSH and oxidized glutathione GSSG) and of total glutathione (GSH + GSSG) were determined according to Griffith (1980) using sulphosalycilic acid, DTNB (5,5′-dithiobis-2-nitrobenzoic acid), glutathione reductase, NADPH and BSA (bovine serum albumin).
Activities of antioxidative enzymes
The activity of SOD was assayed following the method of Mishra and Fridovich (1972) using nitroblue tetrazolium (NBT) reduction method. Reduction of NBT was recorded by measuring absorbance at 560 nm. Catalase was assayed according to Beers and Sizer (1952). Decomposition of H2O2 was monitored at 240 nm using UV–Vis spectrophotometer. Catalase activity was expressed as µmol H2O2 oxidized min−1 mg−1 protein. APX activity was assayed following Nakano and Asada (1981). Oxidation of ascorbic acid was monitored at 290 nm using UV visible spectrophotometer. GPX activity was assayed following Egley et al. (1983) using guaiacol and H2O2. Enzyme activity was expressed in terms of mmol H2O2 reduced min−1 mg−1 protein. Assay of MDHAR was done using ascorbate oxidase and NADH. NADH oxidation was monitored at 340 nm using spectrophotometer and activity was expressed as nmol NADH oxidized min−1 mg−1 protein. Activity of DHAR was assayed following the method of Doulis et al. (1997). Reduction of DHA was monitored at 265 nm using UV–Vis spectrophotometer.
Non-protein thiol, protein-thiol, carbonylation of proteins and proteolysis
Total thiol content, which included protein thiol and non-protein thiol, was estimated following Dekok and Kuiper (1986) using DTNB. Total thiol content was calculated using extinction coefficient as 13,600 M−1 cm−1. The content of non-protein thiol was subtracted from total thiol to get the content of protein thiol. Contents were expressed in terms of nmol thiols g−1 tissue fresh weight. Method of Levine et al. (1994) as modified by Pandey et al. (2013) was followed to determine the extent of protein carbonylation in rice seedlings using DNPH reagent. Absorbance was recorded at 370 nm using UV–Vis spectrophotometer. Extinction coefficient of 22,000 M−1 cm−1was used to calculate the content of protein carbonyls and was expressed in terms of nmol mg−1 protein in the tissues.
Determination of protein
In all preparations, protein concentration was determined using Bradford (1976) reagent using BSA (bovine serum albumin, Sigma) as standard.
Statistical treatment of the data
Experiments for each analysis were conducted in triplicate. The values represent mean of three independent determinations ± standard deviation (SD). One way ANOVA and Tukey’s multiple range test were used to analyze the differences between control and treatment values. The typical mean with the highest absolute value and other means not statistically different from the highest value have been represented as letter ‘a’ and lesser mean values progressively have been represented as letters ‘b’ and ‘c’.
Results
Arsenic effects on growth of rice seedlings and role of A. indica and O. sanctum extracts
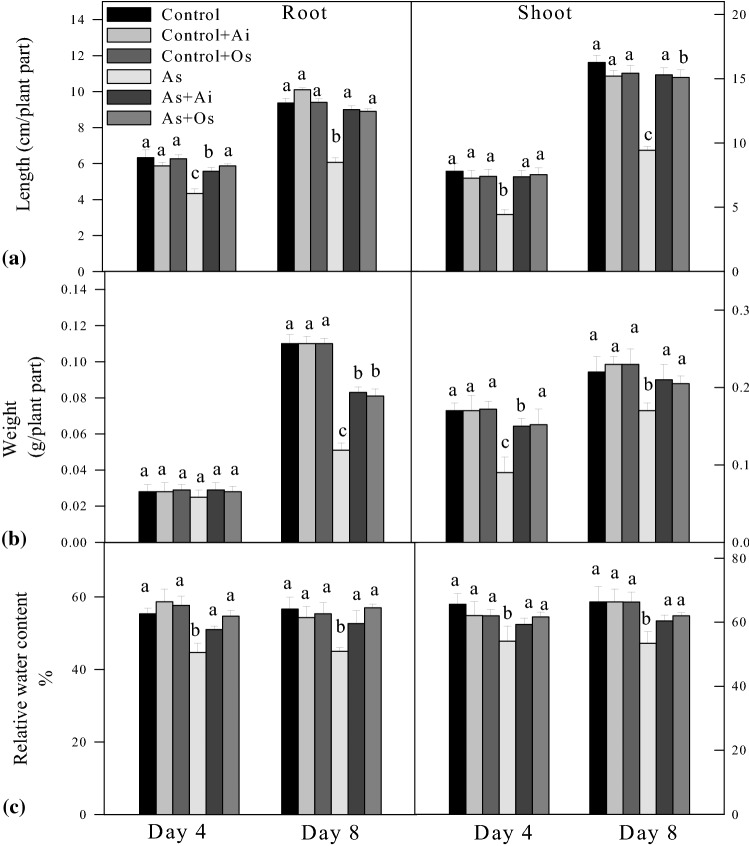
Rice seedlings were raised in hydroponics for 8 days under control and various treatments. Plant height including root/shoot length, fresh weight and relative water content (RWC) were recorded at day-4 and day-8 of treatment. A significant reduction in root/shoot length as well as fresh biomass and relative water content was apparent in 50 µM sodium arsenite treated seedlings compared to the seedlings raised in Yoshida nutrient solution (controls) (Fig. 1). Addition of either extract to the nutrient solution did not affect either root or shoot length. By day 8, As treatment caused a 35% reduction in root length and a 42% reduction in shoot length (Fig. 1a). Further, 50 µM As + O. sanctum treated seedlings showed 46.8% and 60.1% restoration in root and shoot growth respectively in comparison to As treated seedlings. Similarly, treatment with 50 µM As for 8 days caused 53.6% and 22.7% reductions in the fresh weights of roots and shoots respectively whereas when A. indica extract was present in 50 µM As containing growth medium, 62.7% restoration in root weight and 23.5% restoration in shoot weight were observed compared to As treated seedlings. Similarly, 50 µM As + O. sanctum treatment caused 58.8% and 20.5% restoration in root and shoot growth respectively in comparison to As treated seedlings (Fig. 1b). Relative water content declined by 20.5% and 11.5% in roots and shoots respectively due to 50 µM As treatment compared to controls and a restoration of 17.02% and 9.6% respectively in RWC was noted in As + A. indica treated seedlings compared to As treatment. Further 50 µM As + O. sanctum treatment caused 17.02% and 9.1% restoration in root and shoot RWC respectively in comparison to As treated seedlings (Fig. 1c). The ICP-OES analysis revealed that with 8 days of 50 µM As treatment, roots of the seedlings had 300.52 µmol As g−1dry wt., whereas shoots had As level of 251.78 µmol g−1 dry wt. (Table 1). Arsenic uptake by growing rice seedlings substantially declined with the addition of A. indica and O. sanctum extract in the growth medium. With A. indica extract in the medium, 72.5% decline in As uptake was noted in roots and 72.8% decline in shoots, whereas with the presence of O. sanctum extract, 67.2% decline in As uptake was recorded in roots and 70.01% decline in shoots as compared to As uptake level observed in 50 µM As treated seedlings. No As could be detected in control roots and shoots in ICP-OES analysis. The detection level of the instrument was 3.6 μg L−1 (ppb).
Fig. 1.
Effect of arsenic (As), As + Azadirachta indica extract (Ai) and As + Ocimum sanctum (Os) extract treatments in the growth medium on a length, b fresh weight and c relative water content of roots and shoots of rice seedlings at 4 and 8 days of treatment. Seedlings were grown in hydroponics for 8 days under control, control + A. indica extract (control + Ai), control + O. sanctum (control + Os), 50 µM As (As) and 50 µM As + A. indica extract (As + Ai) and 50 µM As + O. sanctum extract (As + Os). Values are mean ± SD based on three independent determinations and bars indicate standard deviations. The typical mean with the highest absolute value and other means not statistically different from the highest value have been represented as letter ‘a’ and lesser mean values progressively have been represented as letters ‘b’ and ‘c’
Table 1.
Arsenic content in roots and shoots of rice seedlings at 8 days of growth under various treatments
| Samples | Treatments | As content (µmol g−1 dry weight) |
|---|---|---|
| Root | Control | ND |
| Control + Ai | ND | |
| Control + Os | ND | |
| As | 300.52 ± 2.229*** | |
| As + Ai | 80.27 ± 1.137*** | |
| As + Os | 96.21 ± 2.012*** | |
| Shoot | Control | ND |
| Control + Ai | ND | |
| Control + Os | ND | |
| As | 251.78 ± 3.009*** | |
| As + Ai | 68.4 ± 2.088*** | |
| As + Os | 75.5 ± 1.023*** |
Seedlings were raised in Yoshida nutrient solution (control), nutrient solution containing A. indica extract (Control + Ai), nutrient solution containing O. sanctum extract (Control + Os), nutrient solution containing 50 µM As2O3 (As), nutrient solution containing 50 µM As2O3 and A. indica extract (As + Ai) and nutrient solution containing 50 µM As2O3 and O. sanctum extract (As + Os). ND depicts not detected. Values are mean ± SD based on three independent determinations and asterisks (***) represent significant differences compared to control at p ≤ 0.001 according to Tukey’s multiple range test
Effect of A. indica and O. sanctum extracts on As induced generation of reactive oxygen species
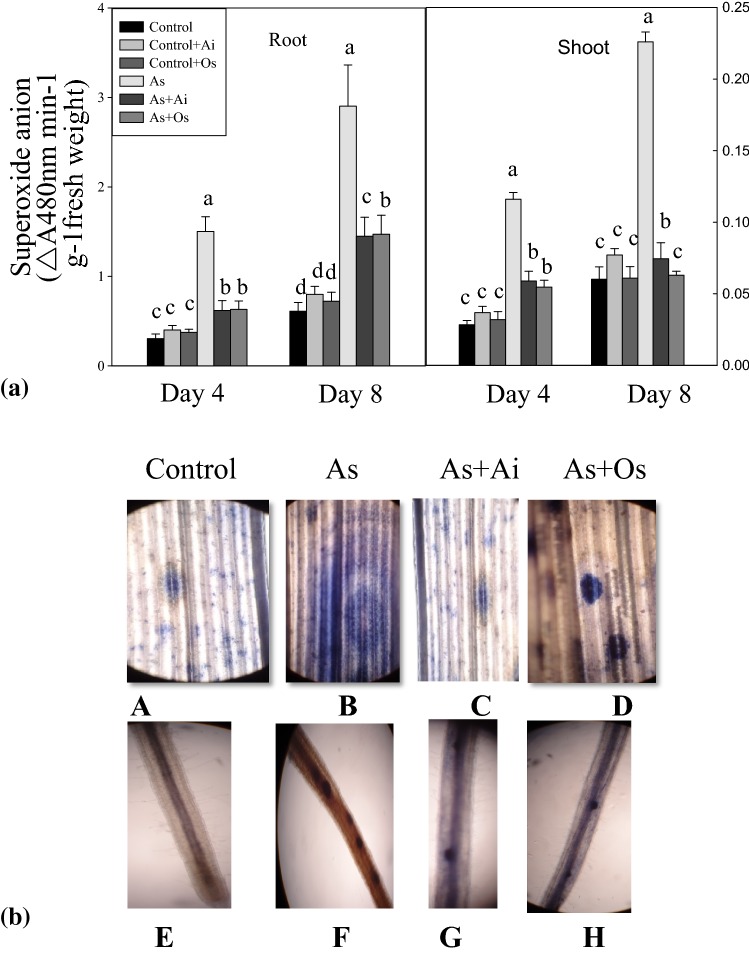
In rice seedlings grown in presence of As, increased formation of O·−2 was noted in comparison to controls (Fig. 2). With As treatment for 8 days, 66.6% increased O·−2 generation was noted in roots and 44.3% in shoots. A significant decline of As induced O.-2production could be noted with A. indica or O. sanctum extracts in the medium (Fig. 2a). Production of O·−2 was histochemically observed in leaves using the dye NBT. Blue colored formazon spots were clearly evident as a result of NBT reaction with O·−2. The intensity of dark blue stained spots increased in leaves due to As treatment, showing enhanced O·−2 production on As treatment. In leaves of rice seedlings treated with As + A. indica extract or As + O. sanctum extract, less intense patches were observed in comparison to As treated seedlings indicating suppression of As induced O·−2 generation in rice leaves due to the extracts of A. indica or O. sanctum (Fig. 2b).
Fig. 2.
a Effect of As, As + Ai and As + Os treatments in the growth medium on the levels of superoxide anion in roots and shoots of rice seedlings at 4 and 8 days of growth. b Microscopic view of localization of superoxide anion (O·−2) in situ in rice roots and leaves using NBT staining. Rice seedlings were raised in hydroponics under different treatments and all statistical analyses were done as described in Fig. 1
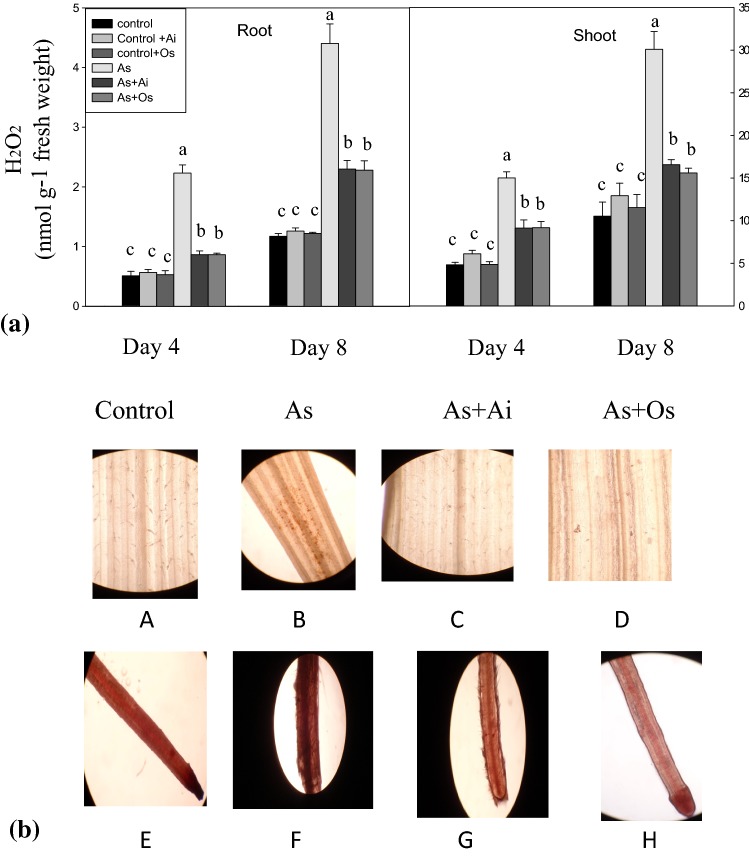
With As treatment, seedlings showed higher level of H2O2 production. In 8 days As treated seedlings, H2O2 level increased by 85.7% in roots and 91.5% in shoots, while the production of H2O2 declined by 41.02% in roots and 34.7% in shoots when A. indica extract was included in As containing medium (Fig. 3a). Similarly, with O. sanctum extract in As treatment medium, 41.5% decline in H2O2 content in roots and 38.3% decline in shoots were noted as against H2O2 content in As-stressed seedlings. When localization of H2O2 was examined histochemically with DAB staining, untreated control seedlings showed lesser accumulation of H2O2 marked by very few spots while more intense stained spots were seen in seedlings treated with As (Fig. 3b). When leaves from seedlings grown in As + A. indica or As + O. sanctum containing medium were examined after DAB staining, the intensity of spots were less intense in comparison with the intensity in As treated seedlings which suggests a protective role of A. indica and O. sanctum extracts by lowering H2O2 production under As treatment (Fig. 3b).
Fig. 3.
a Effect of As, As + Ai and As + Os treatments in the growth medium on the levels of hydrogen peroxide (H2O2) in roots and shoots of rice seedlings at 4 and 8 days of growth. Seedlings were raised and statistical analyses were done as in Fig. 1. b Histochemical detection of hydrogen peroxide in situ in rice roots and leaves by 3,3′-diaminobenzidine (DAB) uptake method. Views of DAB stained leaves as observed under microscope (a, b, c, d) and DAB stained roots (e, f, g, h). Dark brown spots represent localization of H2O2 (color figure online)
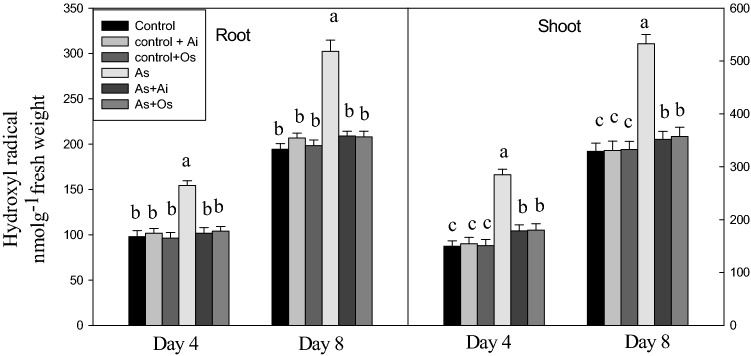
The production of another ROS, OH· was also higher in the seedlings on As treatment. The level of OH· was 55.5% higher in roots and 61.9% higher in shoots of As-treated seedlings as against the levels in untreated controls (Fig. 4). Arsenic induced production of OH. declined in both roots and shoots when A. indica or O. sanctum extracts were present in As treatment medium. This indicates a marked role of A. indica and O. sanctum extracts in lowering As induced OH· production in the seedlings (Fig. 4).
Fig. 4.
Effect of As, As + Ai and As + Os treatments in the growth medium on the levels of hydroxyl radical in roots and shoots of rice seedlings at 4 and 8 days of growth. Rice seedlings were raised in hydroponics under control, control + A. indica extract (Control + Ai), Control + O. sanctum extract (Control + Os), 50 µM As (As) and 50 µM As + A. indica extract (As + Ai) and 50 µM As + O. sanctum extract (As + Os). Analyses of the data were similar as described in Fig. 1
Arsenic induced lipid peroxidation and roles of the extracts
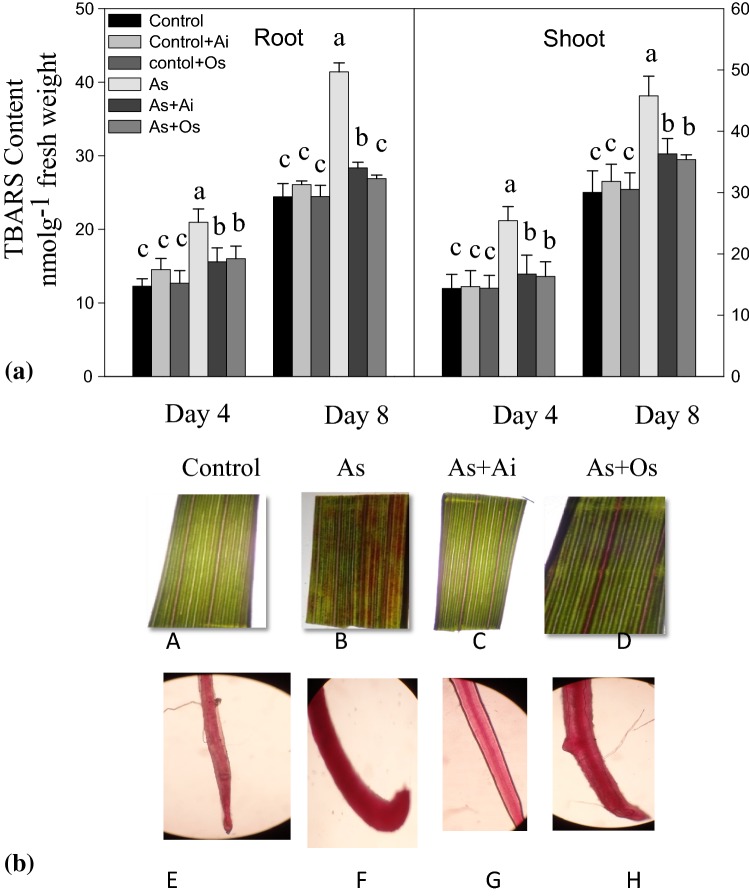
In As-treated seedlings, the content of lipid peroxides was higher than the content in controls (Fig. 5a). TBARS level, indicative of lipid peroxides, increased by 52.4% in shoots and 69.5% in roots in the seedlings due to 50 µM As treatment for 8 days. Decrease in the production of TBARS was observed by 31.5% in roots and 20.7% in shoots in seedlings treated with As + A. indica extract compared to As treated seedlings. Similarly, with O. sanctum treatment, lesser level of lipid peroxides was noted in roots and shoots than the level in As-treated seedlings (Fig. 5a). This is suggestive of protective roles of the extracts of A. indica or O. sanctum to the seedlings by lowering lipid peroxidation under As toxicity. With Schiff’s reagent, lipid peroxidation was also examined histochemically. Peroxidation of lipids causes aldehyde production which reacts with Schiff’s reagent and forms bright red products. Lipid peroxides formation increased marked by intense bright red colored spots in As treated rice seedlings in comparison with controls (Fig. 5b). Whereas, in seedlings grown with As + A. indica or As + O. sanctum extract in the medium, lesser lipid peroxidation was noted than As-grown seedlings, suggesting that the plant extracts lower lipid peroxidation in As-exposed seedlings.
Fig. 5.
a Effect of As, As + Ai and As + Os treatments in the growth medium on the level of thiobarbituric acid reactive substances (TBARS, a measure of lipid peroxidation) in roots and shoots of rice seedlings at 4 and 8 days of growth. b Histochemical detection of lipid peroxides in stained rice leaves. Pink-red stained portions represent extent of lipid peroxidation in the leaves (a, b, c, d) and roots (e, f, g, h) of stained rice seedlings (color figure online)
Arsenic induced membrane damage in root tips
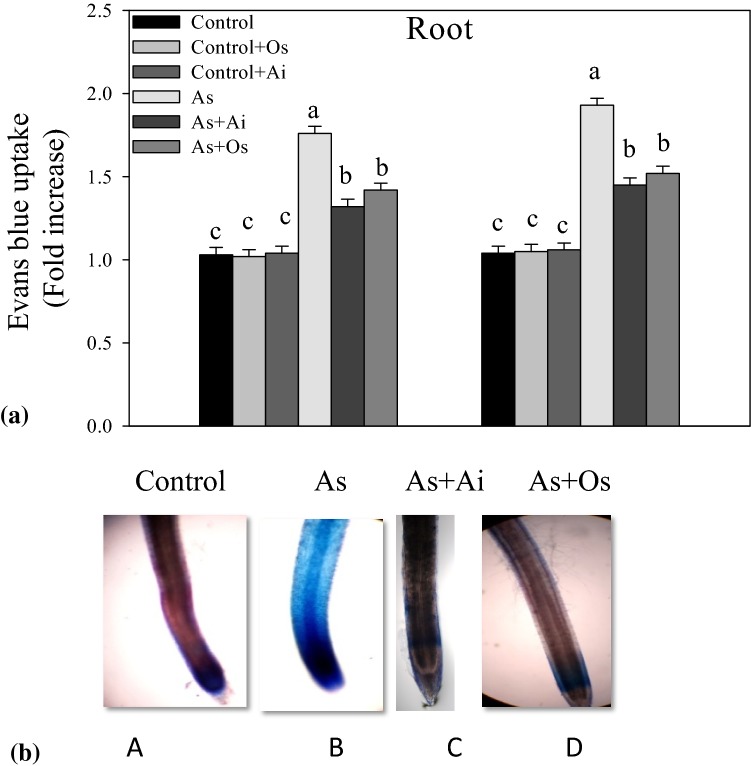
Evan’s blue dye uptake method was used to assess membrane damage in roots under various treatments. A higher uptake of the dye was observed in the roots of As-treated seedlings, in comparison to controls (Fig. 6). Whereas, with the presence of A. indica or O. sanctum extract in the growth medium, dye uptake by roots declined in comparison to As-treated seedlings (Fig. 6a). In histochemical observations, roots of As treated seedlings showed intense blue stain in comparison with controls, whereas roots of seedlings grown in As + A. indica or As + O. sanctum containing medium showed less intense blue stain suggesting the protective role of A. indica and O. sanctum extracts against As induced plasma membrane damage (Fig. 6b).
Fig. 6.
a Loss of plasma membrane integrity in the roots of rice seedlings at various treatments, measured in terms of Evan’s blue uptake. Rice seedlings were raised in hydroponics for 8 days under different treatments as mentioned in Fig. 1. b Evan’s blue stained root portions are seen (color figure online)
Arsenic induced alterations in antioxidants in the seedlings and the role of leaf extracts
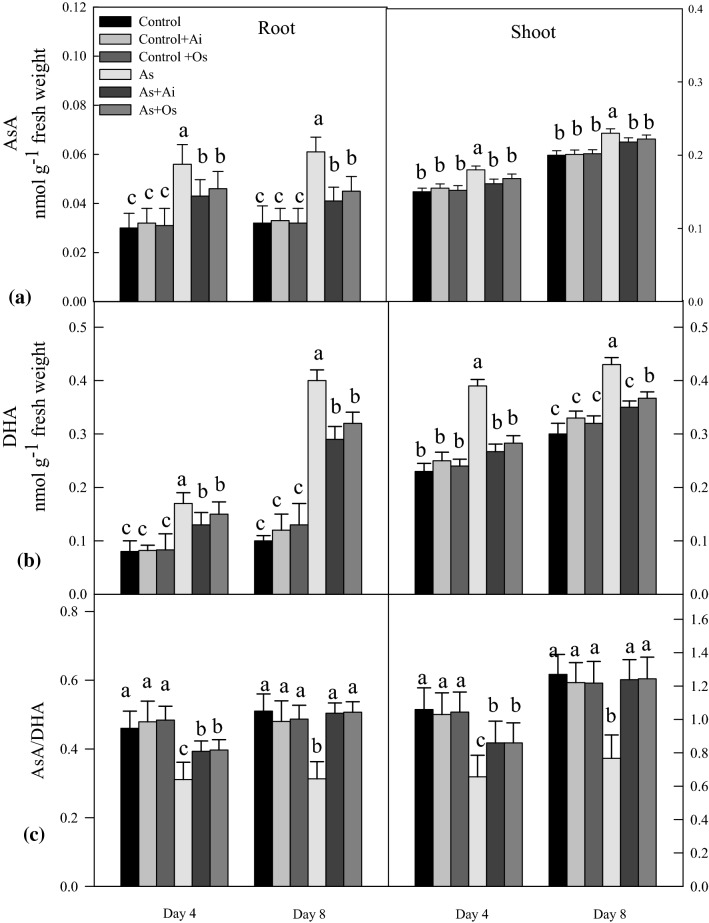
The potential antioxidants ascorbate and glutathione were examined for their level in the seedlings under different treatments. Reduced ascorbate (AsA) level as well the level of its oxidized form (DHA) was higher, however AsA/DHA ratio declined in the seedlings with As treatment (Fig. 7a). The level of AsA increased by 87.4% in roots and 20% in shoots due to 50 µM As treatment for 8 days, whereas the level of AsA declined by 32.7% in roots and 10.55% in shoots when extract of A. indica was included in the As medium. Similarly, with O. sanctum extract in As containing medium, decline in AsA level was noted in roots and shoots. Root DHA level increased to 112.5% and shoot level to 69.5% with 8 days of As treatment (Fig. 7b). Whereas with the addition of A. indica or O. sanctum extract in As treatment medium, decline in root and shoot DHA level was seen as against DHA levels As-treated seedlings. A 39.61% decline in AsA/DHA ratio in roots and 39.7% decline in ratio in shoots was evident due to 50 µM As treatment for 8 days (Fig. 7c), whereas with the addition of A. indica and O. sanctum extracts in As treatment medium, significant restoration in the ratio AsA/DHA was noted comparable to untreated controls.
Fig. 7.
Effect of As, As + Ai and As + Os treatments in the growth medium on the levels of a reduced ascorbate (AsA), b dehydroascorbate (DHA), and c ratio of AsA/DHA in roots and shoots of rice seedlings at 4 and 8 days of growth. Rice seedlings were raised in hydroponics as described in Fig. 1. Values are mean ± SD based on three independent determinations and bars indicate standard deviations
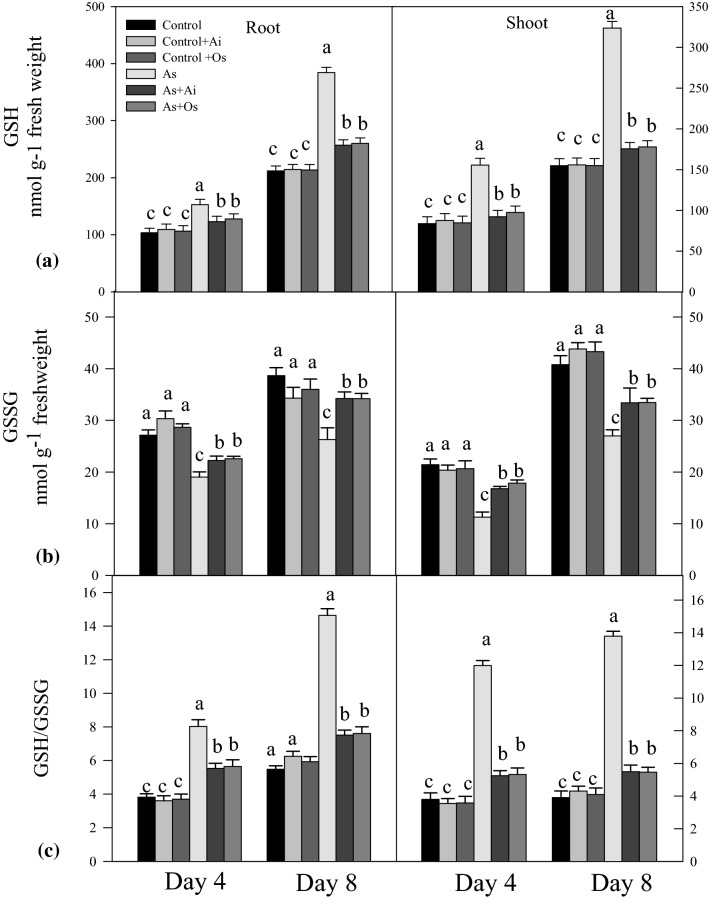
With As treatment, elevated level of GSH and a decline in GSSG level was noted in the seedlings. Arsenic treated seedlings for 8 days had 81.3% higher GSH level in roots and 109.1% higher level in shoots as against untreated controls (Fig. 8a). With extract of A. indica in As containing medium, 33.1% decline in GSH level in roots and 45.76% decline in shoots, and with O. sanctum extract 32.31% decline in GSH in roots and 45.0% decline in shoots was evident as against GSH levels in seedlings grown in presence of As. Oxidized glutathione (GSSG) level declined by 32.02% in roots and 33.8% in shoots due to As treatment, whereas the level was restored by 30.2% in roots and 23.7% in shoots with extract A. indica in As containing medium. Similarly, seedlings grown in As + O. sanctum containing medium, the level of GSSG was restored by 23.8% in roots and 30.1% in shoots as compared to As treated seedlings (Fig. 8b). An increased ratio of GSH/GSSG was noted in the seedlings after treatment with As (Fig. 8c).
Fig. 8.
Effect of As, As + Ai and As + Os treatments in the growth medium on the contents of a reduced glutathione (GSH), b oxidized glutathione (GSSG), and c ratio of GSH/GSSG in roots and shoots of rice seedlings at 4 and 8 days of growth. Treatment conditions of the seedlings and statistical analyses of the data were similar as described in Fig. 1
Antioxidative enzyme activities and role of leaf extracts
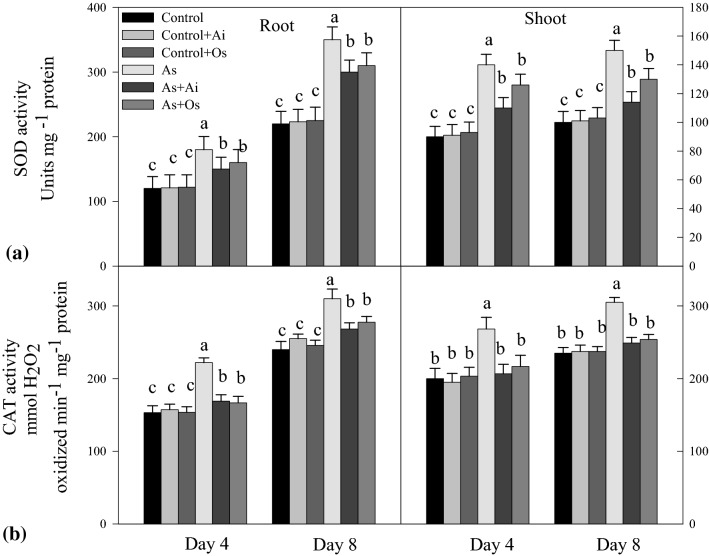
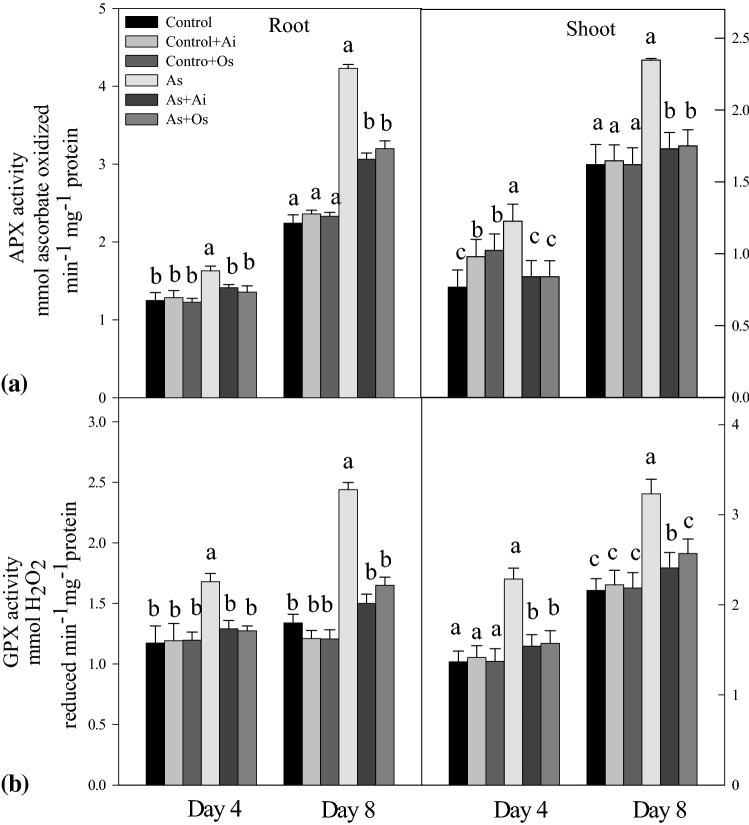
On exposure to As, the activities of SOD, CAT, APX and GPX increased in the seedlings. Arsenic treatment for 8d caused 59.1% increased activity of SOD in roots and 50% increased activity in shoots over controls. Whereas, with the addition of A. indica or O. sanctum extract in As treatment medium, SOD activity declined in roots and shoots in comparison to the activity in As treated seedlings (Fig. 9a). On As treatment, 29.06% higher CAT activity was noticed in roots and 29.7% higher in shoots, whereas the activity declined by 18.2% in roots and 18.4% in shoots with incorporation of A. indica extract in As containing medium. Similarly seedlings growing in 50 µM As + O. sanctum medium for 8 days showed 10.4% and 16.7% reduced CAT activities in roots and shoots respectively as against the activities in As-treated seedlings (Fig. 9b). This indicates restoration in activity levels of the enzymes SOD and CAT in the seedlings with the addition of A. indica and O. sanctum extracts in As treatment medium (Fig. 9). Similar to SOD and CAT, with As treatment, APX activity increased by 87.4% in roots and 44.8% in shoots, whereas with the addition of extracts of A. indica and O. sanctum in As treatment medium, APX activity was lowered in both roots and shoots as against the activity in As-treated seedlings (Fig. 10a). Similarly, As treatment resulted in 82.08% increased GPX activity in roots and 49.5% increased activity in shoots over controls but it declined in both roots and shoots with the addition of either of the extracts in As treatment medium (Fig. 10b).
Fig. 9.
Effect of As, As + Ai and As + Os treatments in the growth medium on the activities of a superoxide dismutase (SOD) and b catalase (CAT) in roots and shoots of rice seedlings at 4 and 8 days of growth. Treatment conditions of the seedlings and statistical analyses of the data were similar as described in Fig. 1
Fig. 10.
Effect of As, As + Ai and As + Os treatments in the growth medium on the activities of a ascorbate peroxidase (APX) and b guaiacol peroxidase (GPX) in roots and shoots of rice seedlings at 4 and 8 days of growth. Rice seedlings were raised in hydroponics under different treatments and analyses of the data were done as described in Fig. 1
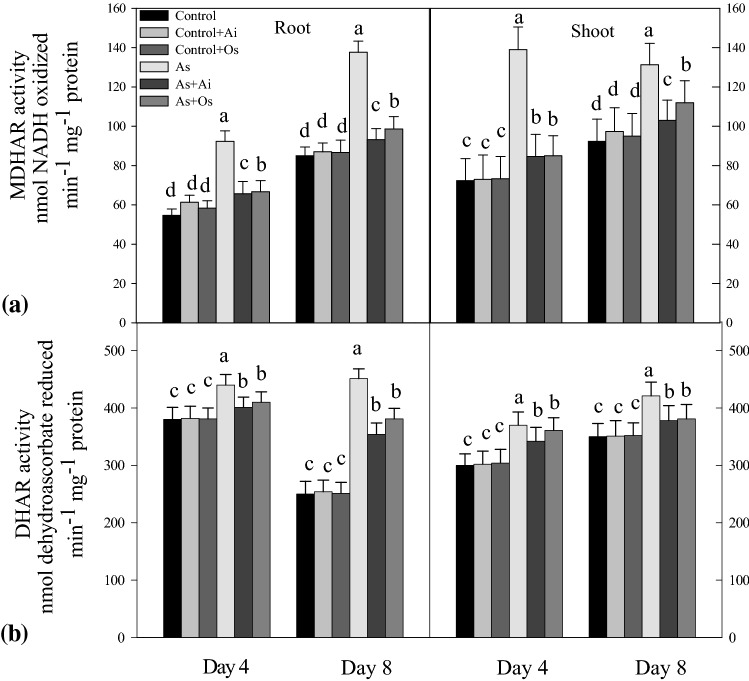
The enzymes MDHAR and DHAR also showed elevated activity levels in the seedlings with As treatment. MDHAR activity increased by 61.8% in roots and 42.7% in shoots with As treatment, but with the addition of A. indica extract in the medium, 31.9% decline in root enzyme activity and 21.5% decline in shoot enzyme activity was observed (Fig. 11a). Similarly, compared to As-treated seedlings, with O. sanctum treatment, decline in root and shoot MDHAR activities were observed. Like MDHAR, higher root and shoot DHAR activities were noted in As treated seedlings compared to controls (Fig. 11b), whereas with the addition of A. indica or O. sanctum extracts in As containing medium, root and shoot enzyme activities declined as compared to the activities in As treated seedlings (Fig. 11b).
Fig. 11.
Effect of As, As + Ai and As + Os treatments in the growth medium on the activities of a monodehydroasorbate reductase (MDHAR) and b dehydroascorbate reductase (DHAR) in roots and shoots of rice seedlings at 4 and 8 days of growth. Treatment conditions for the seedlings and analyses of the data were similar as described in Fig. 1
Levels of thiols, protein carbonylation and effects of extracts
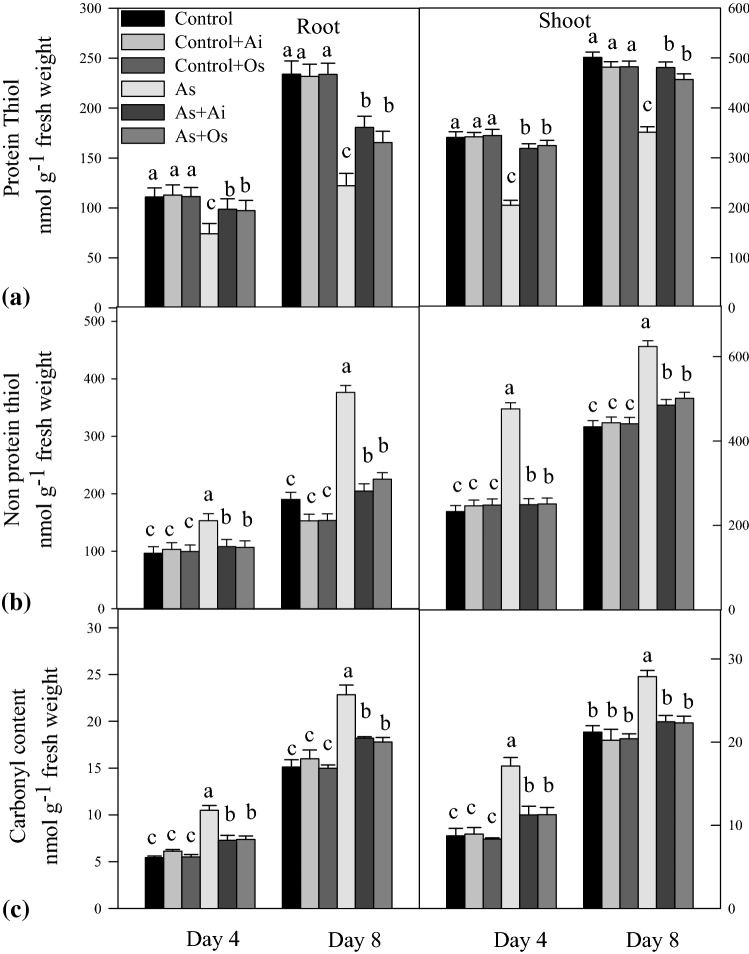
A marked reduction in the level of protein thiol and increase of non-protein thiol was evident in rice seedlings with As treatment (Fig. 12a). In As treated 8 days grown seedlings, protein thiol declined by 47.7% and 29.8% in roots and shoots respectively as against untreated controls, whereas this decline became less with A. indica or O. sanctum extract in the medium. This indicates a protective role of A. indica and O. sanctum extracts for protein thiols under As toxicity in rice seedlings (Fig. 12a). In As-treated seedlings, level of non-protein thiol increased by 105% and 37% in roots and shoots respectively as against controls, whereas with A. indica or O. sanctum extracts in the medium, the increased level of non-protein thiols remained only up to 10 to 23% in roots and 7–9% in shoots compared to controls (Fig. 12b). Arsenic treatment caused increase in protein carbonylation in the seedlings (Fig. 12c). With 50 µM As treatment in the medium, the level of protein carbonyls was higher by 53% and 27% in roots and shoots respectively in comparison to the level in untreated seedlings. Whereas, when rice seedlings were grown in presence of As + A. indica extract in the medium, the As induced elevated level of protein carbonyls was lower by 33% and 18% respectively in roots and shoots. Similarly, with O. sanctum extract, As induced elevated level of protein carbonyls declined by 30% in roots and 18% in shoots. This indicates protective roles of A. indica as well as O. sanctum extracts against As induced protein carbonylation in the seedlings (Fig. 12c).
Fig. 12.
Effect of As, As + Ai and As + Os treatments in the growth medium on a protein thiol, b non-protein thiol and c carbonyl content in roots and shoots of rice seedlings at 4 and 8 days of growth. Rice seedlings were raised in hydroponics under different treatments and analyses of the data were done as described in Fig. 1
Discussion
Our results show that As treatment to rice seedlings causes marked decline in growth with increase in production of ROS, increased As uptake by roots, increased oxidative stress in the tissues marked by enhanced peroxidation of lipids, carbonylation of proteins, increased membrane permeability, decreased protein thiols, altered AsA/DHA and GSH/GSSG ratios and increased activities of the enzymes SOD, CAT, APX, GPX, MDHAR and DHAR. Arsenic toxicity in crop plants including rice has been well established (Mishra et al. 2011; Hettick et al. 2015). Arsenic reduces growth of plants by interfering with key metabolic processes responsible for proper plant growth. Arsenic toxicity in plants is associated with overproduction of ROS, which in turn causes oxidative stress in the tissues (Sharma et al. 2012). When aqueous extracts of either A. indica or O. sanctum were added in As containing growth medium, in our studies, a marked reduction in As uptake, comparatively lesser production of ROS and substantial restoration in growth of seedlings was observed compared to As level, ROS production and growth parameters in As-treated seedlings. With A. indica extract, As uptake was more reduced compared to O. sanctum extract, suggesting that among these two herbal extracts A. indica better alleviates As toxicity in rice plants by reducing bioavailability of As. A. indica extract contains a large number of glycosides, tannins, phenolic compounds, saponins, proteins, amino acids, triterpenoids and steroids (Prasanth and Krishnaih 2014), therefore it is possible that the phenolic compounds might form complexes with As leading to its decreased uptake by the roots. This could be one of the reasons for the reduced uptake of As by the roots thereby leading to its reduced bioavailability in the tissues due to the presence of A. indica aqueous extract in the medium. The phenolics present in extract might be involved in scavenging free radicals generated under As toxicity and could provide antioxidative defense against As induced oxidative stress. Similarly, a wide range of natural products like euginal, eugenol, carvacrol, limatrol, saponins, flavanoids, triterpenoids, tannins, rosameric acid, propanoic acid, apigenin, and some water soluble flavanoids such as orientin and vicenin are present in leaves of O. sanctum (Hakkim et al. 2007). Due to the presence of these compounds, O. sanctum aqueous extract has strong chelating as well as free radical scavenging potential (Hakkim et al. 2007). These properties of O. sanctum appear to be responsible for reducing As uptake and providing antioxidative defense in the seedlings thereby lowering As bioavailability and its toxicity in the seedlings. A wide range of natural compounds present in A. indica and O. sanctum extract may form complexes with As leading to its decreased absorption by roots. The antioxidative properties of the phenolic compounds and flavanoids present in A. indica and O. sanctum extract could be responsible for its direct protective and antioxidative action within the tissues. Phenolic compounds are associated with the antioxidant capacity of plants and can directly inactivate free radicals and prevent decomposition of hydroperoxides into free radicals (Maisuthisakul et al. 2007).
In our studies, ROS production increased in the seedlings with As treatment, whereas when extracts of A. indica and O. sanctum were present in As containing medium, reduced formation of ROS was evident than the ROS level in As alone treated seedlings. Increased ROS production has been reported in a wide range of crops grown under excess levels of different metals (Verma and Dubey 2003; Caverzan et al. 2016; Pandey and Dubey 2019; Pandey et al. 2019a). Arsenic is a potent toxic metalloid and is responsible for inducing increased generation of ROS in plants (Mishra et al. 2011; Pandey et al. 2019b). The two commonly available toxic species of arsenic present in soil are arsenate (V) and arsenite (III), which are easily taken up by growing plants and thereafter cause increased ROS production in the tissues (Sharma et al. 2014; Pandey et al. 2019b). Electron transport activities of mitochondria, chloroplasts and plasma membranes appear to be responsible for leakage of electrons and increased formation of O·−2 in plant cells under arsenic toxicity (Sharma et al. 2014). Moreover arsenic induces anatomical and morphological changes in mitochondria and a rapid decline of mitochondrial membrane potential causes uncontrolled formation of O·−2 (Jomova et al. 2010). The electron transport chain activities of chloroplast, mitochondria, NADPH oxidase of plasma membrane, cell wall associated peroxidases are mainly involved in enhanced H2O2 production under stressful conditions in plants (Sharma et al. 2012). Rice and sunflower plants exposed to toxic levels of As show increased production of H2O2 (Mishra et al. 2011; Issam et al. 2015). In our studies, all the three ROS (O·−2, H2O2 and OH·) examined, showed increased levels in rice plants on As exposure and the level declined in the presence of A. indica or O. sanctum extracts in the treatment medium. This suggests that both A. indica and O. sanctum extracts alleviate As toxicity effects in rice seedlings by reducing As bioavailability and lowering ROS production. Peroxidation of membrane lipids is regarded as a biochemical marker of oxidative stress in plants. Increased lipid peroxidation in membranes causes alteration in membrane permeability. In our study, TBARS level, the byproducts of lipid peroxidation increased in As-treated seedlings, which indicate that lipid peroxidation increases in the seedlings on As treatment which further causes alteration in permeability of plasma membrane. A marked decline in TBARS level in As-treated seedlings was observed in our studies when A. indica or O. sanctum extracts were added in the medium. This suggests that A. indica and O. sanctum extracts reduce oxidative stress in the seedlings caused due to As and provide protection to the cellular biomolecules against oxidative stress. Alteration in membrane permeability in As exposed rice seedlings was also histochemically confirmed in our studies using Evan’s blue uptake assay. In presence of A. indica and O. sanctum extracts in As containing medium, a reduction in the internalization of Evan’s blue dye occurred inside the root cells compared to the dye internalized in As stressed stressed seedlings, suggesting that A. indica and O. sanctum extracts protect membrane integrity under As toxicity conditions.
It has been observed that under stressful conditions such as metal toxicity, plants restrict lipid peroxidation and membrane damage by increasing the level of non-enzymic antioxidants like reduced ascorbate and glutathione (Sharma et al. 2014). Reduced ascorbate helps in reduction of lipid peroxide radicals by formation of α-tocopherol (Fryer 1992) and in this way plays important role in protection of cellular components against oxidative damage caused due to ROS (Gill and Tuteja 2010). In our studies, with As treatment, decline in AsA/DHA ratio was noticed in the seedlings, while the ratio increased when A. indica or O. sanctum were present in As treatment medium. This suggests that the extracts of A. indica and O. sanctum appear to have roles in maintaining needed reduced ascorbate level in As treated seedlings. Arabidopsis plants expressing high levels of ascorbate show increased oxidative stress tolerance (Wang et al. 2010). GSH helps in maintaining thiol groups of proteins in reduced state and it serves as an important redox buffer in the cells (Dalton 1995). Due to its importance in plants, high concentration of GSH is maintained in the cells to serve as antioxidant to combat oxidative stress under stressful conditions (Sharma et al. 2012). Arsenic treatment to the seedlings, in our studies, caused elevated GSH level and decline in the level of GSSG. At 8th day of As treatment of seedlings, the GSH/GSSG ratio increased while the ratio declined when seedlings were grown in As + A. indica or As + O. sanctum containing medium. The increased ratios suggest increased production of GSH to modulate antioxidant potential to better combat As induced oxidative damage in the seedlings.
All the antioxidative enzymes examined in our studies, such as SOD, APX, CAT, GPX, MDHAR and DHAR showed their increased activities in the seedlings with As treatment, whereas with the presence of A. indica or O. sanctum extracts in As treatment medium, a significant reduction in all the enzyme activities was noticed. In order to withstand the oxidative damage that occurs under stressful conditions, increase in activities of antioxidative enzymes has been observed in plant tissues (Verma and Dubey 2003; Pandey and Dubey 2019). The enzymes CAT, APX and GPX help in detoxification of H2O2 produced in plant tissues on exposure to stress. Arsenic toxicity caused increase in CAT activity in Pteris vitata (Tartar et al. 2009) and Pteris ensiformis (He et al. 2017), increased APX activity in black gram (Srivastava and Sharma 2013). Due to the presence of A. indica and O. sanctum in As containing growth medium, the reduction in activities of antioxidative enzymes in comparison to the activity in As treated rice seedlings, suggests that A. indica and O. sanctum extracts contribute in maintaining the requisite antioxidative activity status and in alleviating As toxicity in rice seedlings. Ascorbic acid plays crucial role in the cells by maintaining the redox balance due to formation of its reduced form through enzymes MDHAR and DHAR (Arrigoni 1994). Our study reveals increase in the activity of both the enzymes MDHAR as well as DHAR in rice seedlings on As treatment and a lowering in activity due to the presence of A. indica and O. sanctum in As treatment medium. Increased MDHAR activity has been reported in As (Mishra et al. 2011), Mn (Fecht-Christoffers et al. 2003), Ni (Baccouch et al. 2001) and Cu (Cuypers et al. 2000) treated plants whereas increased activity of DHAR has been observed As treated plants (Mishra et al. 2011; Rahman et al. 2015).
Attempts have been made earlier to look for inorganic chemicals, hormones, herbal extracts which can be used to alleviate metal toxicity in plants and encouraging results have been obtained (Srivastava and Dubey 2012; Pandey et al. 2013; Rajpoot et al. 2016; Pandey et al. 2019a). These alleviators when added in the growth medium of plants, reduce uptake of metals and modulate antioxidative status of the plants so that plants can survive better under metal toxicity conditions. In excised rice leaves, it has been shown that Mn toxicity can be alleviated with application of nitric oxide by reducing the level of oxidative stress (Srivastava and Dubey 2012). Similarly, exogenously added Mg, Ca or salicylic acid in the hydroponics growth medium of rice alleviates Al toxicity by reducing uptake of Al and elevating antioxidative defense potential in plants (Pandey et al. 2013). Among herbs, Terminalia arjuna bark aqueous extract alleviates Ni toxicity, whereas aqueous extract from dried fruits of Phyllanthus emblica alleviates Cr toxicity in growing rice seedlings (Rajpoot et al. 2016; Pandey et al. 2019a). Herbal extracts due to the presence of phenolics, alkaloids, terpenoids and many antioxidant compounds serve as metal chelators and free radical scavengers and reduce metal uptake by the plants (Hakkim et al. 2007; Rajpoot et al. 2016; Pandey et al. 2019a). In areas where water is contaminated with As species, toxicity due to As is a major constraint in rice cultivation and studies have shown that leaf aqueous extracts of A. indica and O. sanctum contain compounds with high antioxidant capacity and radical scavenging activity, this prompted us to look into the alleviating activity of these two extracts towards As toxicity in growing rice seedlings. Addition of both of these extracts retarded uptake of As by roots of rice plants and modulated the levels of AsA, GSH as well as activities of antioxidative enzymes, in order to provide better antioxidant potential to As-stressed rice seedlings. This shows potential alleviating role of these two herbal extracts in mitigating As toxicity in rice plants.
To conclude, it can be inferred that in growing rice plants As toxicity is associated with increase in uptake of As by the roots, increased formation of ROS (O2.−, OH· and H2O2), increased membrane permeability, increased oxidative stress in the tissues, altered levels of ascorbate, glutathione and elevated activities of the enzymes SOD, CAT, APX, GPX, MDHAR, DHAR so as to maintain sufficient antioxidant potential in rice seedlings in order to combat arsenic induced oxidative stress. Further, the aqueous extracts of A. indica and O. sanctum, when present in the medium appear to have potential in alleviating As toxicity in rice seedlings by reducing As bioavailability in rice roots and shoots and lowering oxidative stress in the tissues. Thus, both of these extracts can be used for As toxicity alleviation in rice. However, the details of molecules with antioxidant properties present in the extracts and the mechanism how they protect plants against the harmful effects of As need to be worked out in our future experiments with pure compounds.
Acknowledgements
The authors are grateful to the University Grants Commission, New Delhi for providing financial assistance to AG in form of research fellowship.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allen SE, Grimshaw HM, Rowland AP. Chemical analysis. In: Mooren PD, Chapman SB, editors. Methods in plant ecology. Oxford: Blackwell; 1986. pp. 285–344. [Google Scholar]
- Arrigoni O. Ascorbate system in plant development. J Bioenerg Biomembr. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- Baccouch S, Chaoui A, Ferjani EE. Nickel toxicity induces oxidative damage in Zea mays roots. J Plant Nutr. 2001;24:1085–1097. [Google Scholar]
- Beers RF, Sizer IW. Colorimetric method for estimation of catalase. J Biol Chem. 1952;195:133–139. [PubMed] [Google Scholar]
- Brackhage C, Huang JH, Schaller J, Elzinga EJ, Dudel EG. Readily available phosphorus and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.) Sci Rep. 2014;4:4944. doi: 10.1038/srep04944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caverzan A, Casassola A, Brammer SP. Antioxidative response of wheat plants under stress. Genet Mol Biol. 2016;39:1–6. doi: 10.1590/1678-4685-GMB-2015-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A, Vangronsveld J, Clijsters H. Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during the early stages of metal assimilation. Physiol Plant. 2000;110:512–517. [Google Scholar]
- Dalton DA. Antioxidant defenses of plants and fungi. In: Ahmad S, editor. Oxidative stress and antioxidant defenses in biology. New York: Chapman and Hall; 1995. pp. 298–355. [Google Scholar]
- De Kok LJ, Kuiper PJC. Effect of short-term dark incubation with sulfate, chloride and selenate on the glutathione content of spinach leaf discs. Physiol Plant. 1986;68:477–482. [Google Scholar]
- Dohroo A, Kanwal A, Ghai M. Recent developments in Neem (Azadirachta indica- A. Juss) derived antimicrobial constituents for control of human and plant diseases—a review. Ann Acad Med Siles. 2016;70:220–223. [Google Scholar]
- Doulis A, Debian N, Kingston-Smith A, Foyer CH. Characterization of chilling sensitivity in maize. I. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997;114:1031–1037. doi: 10.1104/pp.114.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egley GH, Paul RN, Vaughn KC, Duke SO. Role of peroxidase in the development of water impermeable seed coats in Sidaspinosa L. Planta. 1983;157:224–232. doi: 10.1007/BF00405186. [DOI] [PubMed] [Google Scholar]
- Farooq MA, Islam F, Ali B, Najeeb U, Mao B, Gill RA, Yan G, Siddique KH, Zhou W. Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot. 2016;132:42–52. [Google Scholar]
- Fecht-Christoffers MM, Maier P, Horst WJ. Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol Plant. 2003;117:237–244. [Google Scholar]
- Frahry G, Schopfer P. NADH-stimulated, cyanide-resistantsuperoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta. 2001;212:175–183. doi: 10.1007/s004250000376. [DOI] [PubMed] [Google Scholar]
- Fryer MJ. The antioxidant effects of thylakoid vitamin E (α-tocopherol) Plant Cell Environ. 1992;15:381–392. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;6:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Guillod-Magnin R, Brüschweiler BJ, Aubert R, Haldimann M. Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit Contam Part A. 2018;35:1164–1178. doi: 10.1080/19440049.2018.1440641. [DOI] [PubMed] [Google Scholar]
- Hakkim FL, Shankar CG, Girija S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J Agric Food Chem. 2007;55:9109–9117. doi: 10.1021/jf071509h. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. 1984;J219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikrishnan R, Kim M, Kim J, Balasundaram C, Jawahar S, Heo M. Identification and antimicrobial activity of combined extract from Azadirachta indica and Ocimum sanctum. Isr J Aquac. 2010;62:85–95. [Google Scholar]
- He S, Hu Y, Wang H, Li Q. Effects of indole 3 acetic acid on arsenic uptake and antioxidative enzymes in Pteriscretica var. nervosa and Pteris ensiformis. Int J Phytoremediation. 2017;19:231–238. doi: 10.1080/15226514.2016.1207609. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hettick BE, Carrell CJE, French AD, Klein DM. Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J Agric Food Chem. 2015;19:7097–7107. doi: 10.1021/acs.jafc.5b02487. [DOI] [PubMed] [Google Scholar]
- Issam S, Nawel N, Yassine C. Selenium alleviates the arsenic toxicity in sunflower seedling. J Adv Biol Biotechnol. 2015;3:101–109. [Google Scholar]
- Jana S, Choudhuri MA. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot. 1981;12:345–354. [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2010;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid and spinach (Spinacea oleracea) chloroplasts, the effect of hydrogen peroxide and paraquat. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assesment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–1418. [Google Scholar]
- Mishra HP, Fridovich I. The role of superoxide anion in autooxidation of the epinephrine and sample assay for SOD. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Mishra S, Jha AB, Dubey RS. Arsenite treatment induces oxidative stress, upregulates antioxidant system and causes phytochelatin synthesis in rice seedlings. Protoplasma. 2011;248:565–577. doi: 10.1007/s00709-010-0210-0. [DOI] [PubMed] [Google Scholar]
- Nahak G, Sahu RK. In vitro antioxidative activity of Azadirachtica indica and Melia azedarach leaves by DPPH scavenging assay. Nat Sci. 2010;8:22–28. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Pandey Poonam, Dubey Rama Shanker. Advances in Rice Research for Abiotic Stress Tolerance. 2019. Metal Toxicity in Rice and Strategies for Improving Stress Tolerance; pp. 313–339. [Google Scholar]
- Pandey P, Srivastava RK, Dubey RS. Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicol. 2013;22:656–670. doi: 10.1007/s10646-013-1058-9. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Gautam A, Pandey P, Dubey RS. Alleviation of chromium toxicity in rice seedling using Phyllanthus emblica aqueous extract in relation to metal uptake and modulation of antioxidative defense. S Afr J Bot. 2019;121:306–316. [Google Scholar]
- Pandey AK, Gautam A, Dubey RS. Transport and detoxification of metalloids in plants in relation to plant-metalloid tolerance. Plant Gene. 2019;17:100171. doi: 10.1016/j.plgene.2019.100171. [DOI] [Google Scholar]
- Pattanayak P, Behera P, Das D, Panda SK. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: an overview. Pharmacogn Rev. 2010;4:95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompella A, Maellaro E, Casini AF. Measurement of lipid peroxidation in vivo, a comparison of different procedures. Lipids. 1987;22:206–211. doi: 10.1007/BF02537304. [DOI] [PubMed] [Google Scholar]
- Prasanth GK, Krishnaih GM. Chemical composition of the leaves of Azadirachtaindica. Int J Adv Eng Technol Manag Appl Sci. 2014;1:31–40. [Google Scholar]
- Rahman A, Mostofa MG, Alam M, Nahar K, Hasanuzzaman M, Fujita M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int. 2015 doi: 10.1155/2015/340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpoot R, Rani A, Srivastava RK, Pandey P, Dubey RS. Terminalia arjuna bark extract alleviates nickel toxicity by suppressing its uptake and modulating antioxidative defense in rice seedlings. Protoplasma. 2016;253:1449–1462. doi: 10.1007/s00709-015-0899-x. [DOI] [PubMed] [Google Scholar]
- Roy P, Saha A. Metabolism and toxicity of arsenic: a human carcinogen. Curr Sci. 2002;82:38–45. [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld HR, Godbold DL, Polle A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001;127:887–898. doi: 10.1104/pp.010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Pallavi, Jha Ambuj Bhushan, Dubey Rama Shanker, Pessarakli Mohammad. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany. 2012;2012:1–26. [Google Scholar]
- Sharma Pallavi, Jha Ambuj, Dubey Rama. Books in Soils, Plants, and the Environment. 2014. Arsenic Toxicity and Tolerance Mechanisms in Crop Plants; pp. 733–782. [Google Scholar]
- Srivastava S, Dubey RS. Nitric oxide alleviates manganese toxicity by preventing oxidative stress in excised rice leaves. Acta Physiol Plant. 2012;34:819–825. [Google Scholar]
- Srivastava S, Sharma YK. Impact of arsenic toxicity on black gram and its amelioration using phosphate. ISRN Toxicol. 2013 doi: 10.1155/2013/340925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartar GMK, Rathinasabapathi B, Ma LQ. Characterization of glutathione reductase and catalase in the fronds of two Pteris Ferns upon arsenic exposure. Plant Physiol Biochem. 2009;47:960–965. doi: 10.1016/j.plaphy.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. PMC. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal CH, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Verma S, Dubey RS. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. [Google Scholar]
- Wang Z, Xiao Y, Chen W, Tang K, Zhang L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol. 2010;52:400–409. doi: 10.1111/j.1744-7909.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Weatherley PE. Studies in the water relation of cotton plant, I, the field measurement of water deficits in leaves. New Phytol. 1950;49:81–97. [Google Scholar]
- Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory manual for physiological studies of rice. 3. Philippines: International Rice Research Institute; 1976. p. 83. [Google Scholar]