Abstract
Background:
Glucose transporter (Glut), a cellular transmembrane receptor, has a key role in the metabolism of cell glucose and is also associated with various human carcinomas.
Objective:
In this study, we evaluated a magnetic resonance (MR) imaging contrast agent for tumor detection based on paramagnetic gadolinium oxide (Gd2O3) coated polycyclodextrin (PCD) and modified with glucose (Gd2O3@PCD-Glu) for the targeting of overexpressed glucose receptors.
Material and Methods:
In this experimental study, 3T magnetic resonance imaging (MRI) scanner was used to assess the specific interactions between Glut1-overexpressing tumor cells (MDA-MB-231) and Gd2O3@PCD-Glu NPs. Furthermore, the capacity of transporting Gd2O3@PCD-Glu NPs to tumor cells was evaluated.
Results:
It was found that the acquired MRI T1 signal intensity of MDA-MB-231 cells that were treated with the Gd2O3@PCD-Glu NPs increased significantly. Based on the results obtained, Gd2O3@PCD-Glu NPs can be applied in targeting Glut1-overexpressing tumor cells in vivo, as well as an MRI-targeted tumor agent to enhance tumor diagnosis.
Conclusion:
Results have shown that glucose-shell of magnetic nanoparticles has a key role in diagnosing cancer cells of high metabolic activity.
Keywords: Magnetic Resonance Imaging, Gadolinium, Contrast media
Introduction
Carcinoma cells have a higher uptake of glucose compared to normal cells. This is due to their properties of hyper metabolism and relatively rapid proliferation [ 1 ]. This is also observed in tumor cell membranes, with higher glucose transporter (Glut) expression such as Glut-1. Glut-1, is found in most tumor cell lines and tumor tissues. Analogs, which have structural similarities with D-glucose, can also be transported by Glut-l to Glut-4. The high metabolic needs of tumor cells lead to increased uptake of these analogs. The ability of hexose phosphate isomerase to transform D-glucose-6-phosphate into 1, 6-diphosphate is hampered by these analogs. Therefore, this hinders further metabolism. Positron emission tomographic (PET) studies with fluorodeoxyglucose have been able to utilize Glut-1 for imaging tumor tissues [ 2 , 3 ]. Several diagnostic substances which are in tandem with the aforementioned targeting vectors have been developed and applied in different imaging modalities such as PET, single photon emission computed tomography (SPECT) [ 4 , 5 ], optical imaging [ 6 , 7 ], as well as magnetic resonance imaging [ 8 , 9 ].
A useful imaging modality for distinguishing cancerous masses from non-cancerous masses is PET. It can also be used in the evaluation and staging of recurrent diseases (for instance cancer). Furthermore, PET scans are used to assess the effectiveness of a treatment method by assessing death of tumor cells or in terms of their sugar consumption [ 10 ]. A commonly used glucose analog in PET imaging is 2-fluoro-2-deoxy-D-glucose molecule (18FDG). It has been shown to be efficient in tumor metabolic imaging; however, its clinical usage has been hindered as a result of high cost, inadequacy as well as difficulty in accessibility. Moreover, owing to the short half-life of positron emitting isotopes, PET imaging must be done within a very short period of time. The synthesis and chemical analysis of 18FDG are time-consuming and difficult.
MRI is an effective molecular imaging (MI) modality due to its high spatial resolution as well as tremendous soft tissue contrast. MI techniques facilitate the quantification of molecular changes related to the development of pathologic states, leading to early diagnosis of diseases like cancer [ 11 ]. However, as a result of MRI low sensitivity, acquired images could suffer from insufficient contrast, hence limitation of clinical applications. To enhance contrast as well as diagnostic accuracy, several exogenous contrast agents (CAs) have been developed and applied in MRI [ 12 ]. Compared to conventional contrast agents, nanoparticles offer several merits that one of them is loadability, in which the concentration of the imaging agent can be adjusted to suit the particular nanoparticle in the synthesis process. Tunability is also another merit, in which the circulation time of the agent in the blood stream or a target organ can be elongated. Lastly, nanoparticles have abilities to act as multifunctional MI agents because they have several features which can be utilized simultaneously in different imaging modalities such as MRI [ 13 ]. Amongst the different nanoparticles, gadolinium oxide (Gd2O3) nanoparticle is mostly used as a result of its large specific surface area and good paramagnetic property (high spin magnetic moment, s=7/2), hence making it suitable for magnetic resonance imaging (MRI) [ 14 ]. A study by Bridot et al. [ 15 ] have shown the suitability of organic dye-functionalized Gd2O3 nanoparticles for both MRI and fluorescence imaging. Conversely, the introduction of a suitable polymer on the surface of MNPs is a way of overcoming some shortfalls of Gd2O3 MNPs, including the strong dipole-dipole attraction between particles, safety and aggregation [ 16 ]. Moreover, efficient surface modification of MNPs offers protection to the magnetic core from the surrounding environment as well as inducing special physico-chemical properties. Hence, by attaching target groups, biological activities can be improved. Variations of MNPs with cyclodextrin (CD), a type of macrocyclic oligosaccharide, have been utilized in several ways due to favorable properties such as truncated cone chemical structure with an internal hydrophobic cavity, outstanding biocompatibility, non-toxicity, and biodegradability [ 17 ].
Earlier studies have been conducted towards improving the water solubility of Gd contrast agent by introducing various sugar groups into their structures [ 18 , 19 ]. Further development of structures causing the use of glucose and galactose/mannose moieties have also been supported, by the latter used to improve targeting in vivo and can also serve as substrate for native enzyme [ 20 ].
Therefore, in present experimental study, CD and glucosamine on the surface of magnetic nanoparticles were spontaneously used and provided significant features for designing nano-contrast agents and consequently enhanced their solubility and stabilization. They also facilitate a magnetically conductible system to the target site as an alternative to 18FDG that has no radioactive half-life, widely available, cost effective, efficient anticancer effects, with very little side effects on normal human breast cells as well as highly precise in MRI oncology and cancer diagnosis at the initial stages. We also showed that these particles subsequently accumulate in the cytoplasm of MDA-MB-231 (Glut1 overexpressing tumor cells) examined by in vitro magnetic resonance imaging.
Material and Methods
In this experimental study, breast cancer cell line MDA-MB-231 and mouse mammary cancer cell lines (4T1), MCF-10A (non-tumorigenic epithelial breast cell line) ), Dulbecco’s modified Eagle’s medium (DMEM) and Roswell Park Memorial Institute medium (RPMI-1640) cell culture media and DTPA, bare Gd2O3 NPs (<100 nm), β-cyclodextrine (CD) 1-ethyl-3-[3-(dimethylamino)-propyl] carbodiimide (EDC), N-hydroxysuccinimide (NHS), phosphate-buffered saline (PBS; 10 mM, pH=7.4) were purchased from Pasteur Institute (Tehran, Iran), Gibcol (USA), fetal bovine serum HyClone (USA) and Sigma Aldrich (St. Louis, MO), respectively. We procured Gd-DOTA (Dotarem®) from Bayer Health Care Pharmaceuticals Inc. (Montville, NJ, USA), while additional materials were from Merck KGaA (Darmstadt, Germany).
Preparation of Gd2O3@PCD-DG NPs
The synthesis of DTPA-dianhydride
The process of synthesizing DTPA-dianhydride has been previously described [ 21 ]. Briefly, 7.6 mmol of DTPA 3 g was dissolved in dimethyl sulfoxide 10 mL, acetic anhydride 20 mL, and pyridine 3 mL as a base under anhydrous conditions. In this step, the mixture was heated at 65 °C for 24 h. Afterwards, it was cooled, filtered, and washed twice in acetic anhydride and anhydrous diethyl ether. White powder was produced after drying the residue at constant weight under vacuum (52 kPa) at 40 °C. Application of DTPA-bis-Anhydride (DTPA-DA) as monomer in condensation polymerization endows the produced polyester with myriad acid and amide functional groups as selective gadolinium ions chelating agents preventing toxic gadolinium leakage.
Conjugation of D-glucosamine hydrochloride to Gd2O3/PCD (Gd2O3/PCD-Glu)
Gd2O3 (0.3 g) was initially dispersed in anhydrous DMSO (10 mL). CD (0.1 mmol, 0.12 g) was then added to the as-prepared dispersion followed by addition of 0.5 mL of dried triethylamine (TEA). The reaction mixture was stirred at room temperature for at least 12 h under room temperature to form stable complex between hydroxyl group of CD and Gd2O3. Afterwards, DTPA-DA (0.78 mmol, 0.28 g) was added to the mixture and stirred for another 12h. Gd2O3/PCD was centrifuged (12000 rpm) and washed three times with deionized water and ethanol. Finally, white powder was obtained and dried in a desiccator.
Conjugation of D-glucosamine hydrochloride to Gd2O3/PCD (Gd2O3/PCD-Glu) was carried out using the following procedure: Briefly, to 0.7 g Gd2O3/PCD NPs dissolve in 10mL dried DMSO, 0.4g l-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.3g N-hydroxysuccinimide (NHS) was added. The mixed solution was stirred under room temperature for at least 12 h. Afterwards, 0.45 g (2.5 mmoL) of DG was added to the solution and stirred for another 12 h. The reaction mixture was then centrifuged (9000 rpm) and the precipitate was washed three times with deionized water and ethanol, and then dried.
Characterization
The Fourier transform infrared (FTIR) spectra were obtained via a Nexus 670 model FTIR spectrophotometer (Thermo Nicolet, USA) at 400-4000 cm-1 at room temperature using KBr pellets. 10 mg samples were heated from room temperature to 800 °C, at a heating rate of 10 °C/min in nitrogen. Zeta potential measurements were conducted using a ZetaPALS instrument (Brooken Haven, USA). Nicomp 380 ZLS Zeta potential/Particle sizer (PSS Nicomp, USA) was used for DLS analysis. The concentration of Gd was measured using inductively coupled plasma atomic emission spectroscopy (ICP-OES 730-ES, Varian). FEI Magellan 400 microscope was used to obtain the field emission scanning electron microscopy (FESEM) images. X’Pert PRO MPDPANalytical (Netherlands) X-ray diffractometer with Cu target (40 kV, 40 mA) was used to obtain the X-ray diffraction (XRD) patterns while JEM-1400 transmission electron microscope (Japan) was used to obtain the transmission electron microscopy (TEM) images. The sample suspension was dropped on a 200 mesh copper grid deposited by carbon, and dried in air. VSM measurements were done by VSM 7400 model (Lakeshore Cryotronics Inc., OH, USA). A 3 T MRI scanner (Siemens Prisma MRI Scanner using head coil) was used for all phantoms, in vitro, and in vivo MR imaging.
Hemolysis assay
The procedures of a previously reported method were followed in evaluating the hemolytic activity of the prepared samples against human red blood cells (HRBCs) [ 22 ]. Briefly, centrifugation of fresh human blood stabilized with EDTA was carried out at 2000 rpm for 10 min to remove plasma as supernatant, while the resulting precipitate was rinsed in PBS (pH 7.4) and washed four times. Afterwards, PBS was used to dilute the RBC suspension 10 times. 200 µL of the diluted HRBCs suspension was added to 800 µL of each Gd2O3@PCD-Glu sample at different concentrations (1.95–1000 mg mL-1) as well as positive (800 µL Triton X100, 2% v/v) and negative (800 µL of PBS buffer, pH 7.4) controls. Incubation of these samples lasted for 2 h at room temperature with moderate shaking. Lastly, centrifugation of all samples at 10000 rpm for 2 min was done, in addition to measuring the absorbance of supernatant (hemoglobin) via UV-visible spectrophotometer at 541 nm. The following equation was used to calculate the hemolytic activity percentage of the various samples:
Cell culture
The Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% of fetal bovine serum (FBS), 100 U/ml of penicillin and 100 μg/mL of streptomycin at 37 °C in a humidified incubator with 5% of CO2, was used to incubate MCF-10A (epithelial normal breast) cells lines as well as Glut1-overexpressing MDA-MB-231 (breast carcinoma) [ 9 ]. For in-vivo experiments, mouse mammary cancer cell lines (4T1) were cultured in RPMI-1640 + FBS 10%.
Cytotoxicity assay
MTT method was used to assess the cytotoxicity of Gd2O3@PCD-Glu in MDA-MB-231 cells. In brief, 2 × 104 cells per well were seeded in 96-well plates for 24 h at 37 °C. A culture medium was then replaced with a fresh DMEM medium of varying concentrations of NPs (0, 1.5, 3.12, 6.25, 12.5, 25, 50,100 µgr/mL). 24 and 48 h after incubation, 10 μl of MTT in a concentration of 5 mg/mL was added to each well. This was followed by incubation of the cells for an additional 4 h at 37 °C. Lastly, ELISA reader Thermo Multiscan MK3 at 570 nm, was used to measure the reaction mixture on each of the 96-well culture plate. All experiments were conducted in triplicate. The rate of survival was calculated using the following equation:
OD(treated) represents the absorbance of cells incubated by nanoparticles while OD(control) signifies absorbance of cells without nanoparticles.
Relaxivity Measurements
The diagnostic ability of Gd2O3@PCD-Glu NPs and Dotarem (clinical MRI contrast agent as a control), was evaluated according to the protocol of phantom imaging [ 23 , 24 ]. Phantom agar gels of Gd2O3@PCD-Glu NPs and Dotarem were obtained from 2.5% w/v agar solutions in PBS (0.1 M, pH 7.4) with varying concentrations (0–0.64 mM (Gd), determined by ICP-MS). The imaging parameters used for measurements of T1 and T2 relaxation times include external field (H) = 3 T, temperature = 22 Cº, NEX = 3, slice thickness = 5 mm, flip angle of 90°, the number of signal averages of 3, field of view 128 × 128 mm2, and metric sizes 256 × 256, spacing (gap) = 1 cm, and bandwidth = 15.63. T1 relaxation time for each sample was obtained by varying repetition times (TR = 50, 200, 400, 600, 800, 1100, 1300, 1500, 1800, 2000 ms) with fixed echo time at TE = 11 ms. Similarly, T2 relaxation times were measured by varying echo times (TE = 10, 30, 60, 90, 130, 170, 210, 240, 270, 350 ms) and fixed TR = 3,000 ms. The signal intensities of each sample were obtained using the manual approach of drawing their regions of interest. Monoexponential curve fitting of signal intensity against time (repetition or echo times) was applied in calculating the relaxation rates (R1 = 1/T1 and R2 = 1/T2). The values of specific relaxivity (r1 and r2) were obtained by fitting the curve of 1/T1 and 1/T2 (s-1) against the concentration of Gd (mM) in agar gels, respectively.
MDA-MB-231 cells magnetic resonance imaging
In order to evaluate the targeting ability of Gd2O3@PCD-Glu NPs in in-vitro, MDA-MB-231 cells (of density = 1 × 106 cells/well) were seeded into 6-well plates with 2 ml fresh medium. It was incubated afterwards at 37°C and 5% CO2 overnight to achieve cell confluence. This was later substituted with a fresh non-glucose medium (2 mL) with PBS (control), Gd2O3@PCD-Glu with Gd+3 concentrations of 0, 12.5, and 50 μg/mL. Cells were incubated at 37°C and 5% CO2 for a further 6 h. The choice of Gd+3 concentrations was according to the acceptable cytotoxicity of the MTT assay. Then, the cells were washed with PBS 5 times, trypsinized, centrifuged, and resuspended in 1 ml PBS (containing 0.5% agarose) in 2 ml Eppendorf tubes for MR imaging. A 3 T Siemens Prisma system was used for all MR imaging. Conventional spin-echo sequence with the following inputs: TR/TE =500/12 ms, 220 × 320 matrices, 82× 120 mm field of view, 140 Hz/Px of bandwidth, and slice thickness of 3 mm was used for the acquisition of T1-weighted images [ 11 ].
In vivo MRI tumor imaging studies
The conduct of all animal studies was in accordance with relevant national and international guidelines of Tehran University of Medical Sciences (Approval number: IR.TUMS.REC.1394.1461). Ten female BALB/c mice (6–8 weeks old, 20-25 g) were purchased from Iran Pasteur Institute. They were housed under constant 12-h dark and light cycles, in addition to standard diet and water ad libitum. All animals were randomly assigned to two groups (5 in each): the experimental and control groups. Each animal was xenografted in the right foreleg muscle with 2 × l06 (4T1). Two weeks after grafting, the tumors had reached a size of about 1-2 cm in diameter.
The T1-contrast ability of Gd2O3@PCD-Glu NPs for targeted imaging of an in vivo tumor model was verified using mouse mammary cancer cell lines (4T1), collected via incubation with 0.05% trypsin-EDTA. They were centrifuged and resuspended in 0.2 mL PBS. Afterwards, 2 ×106 cells were subcutaneously implanted into the right flank of each mouse. Two weeks later, the diameter of the xenografted tumor ranged between 1.0-2.0 cm. A 3 T Siemens Prisma system was also used for In vivo MR imaging. A 250 µL of the Gd2O3@PCD-Glu NPs suspension was administered to mice through tail vein injection at a dose of 0.1 mg/kg Gd of body weight [ 25 ]. Dotarem was used in the control group. The T1-weighted MR images were acquired before and 30 min, 1 h and 6 h after administration using a conventional spin-echo sequence with the following parameters: TR: 600 ms; TE: 8.6 ms; FA: 150°; slice thickness: 2 mm; FOV: 110 mm; matrix size: 192 × 154 and NEX: 6.
For quantitative data analysis of T1-weighted images, the serial post-injection MRI images were processed into DICOM (digital imaging and communication in medicine) images using Dicom Works Software (v 1.3.5) [ 26 , 27 ]. The contrast-to-noise ratio (CNR) at different time points was obtained by manually placing the region of interest (ROI) around the tumor.
Statistical analysis
All data were presented as mean ± standard error of the mean (SEM), while star mark was used to denote statistical significance (p<0.05) between groups as determined by two-tailed T-test assuming unequal variances when comparing two groups, unless particularly outlined. Significant differences (p<0.05) between multiple groups were determined using ANOVA.
Results
FTIR analysis
The FTIR spectra of DTPA-DA, Gd2O3, Gd2O3@PCD, and Gd2O3@PCD-Glu are shown in Figure 1. The FTIR spectrum of Gd2O3 core (Figure 1) shows peaks around 1492 cm-1, 1393 cm-1 and 540 cm-1 which are related to the Gd=O, Gd-O-Gd and Gd-O vibrations, respectively. The FTIR spectrum of DTPA-DA shows the bands at around at 1822, 1776 and 1113 cm-1 which are attributed to the asymmetric and symmetric stretching vibrations of C=O and C-N stretching in anhydride [ 28 ] (Figure 1a). The bands of C=O stretching vibrations (1739 cm-1 and 1634 cm-1), in FTIR spectrum of Gd2O3 @PCD and attributed to ester and carboxylate groups, confirmed the presence of PCD moieties on the surface of the core Gd2O3 [ 29 ] (Figure 1b).
Figure 1.
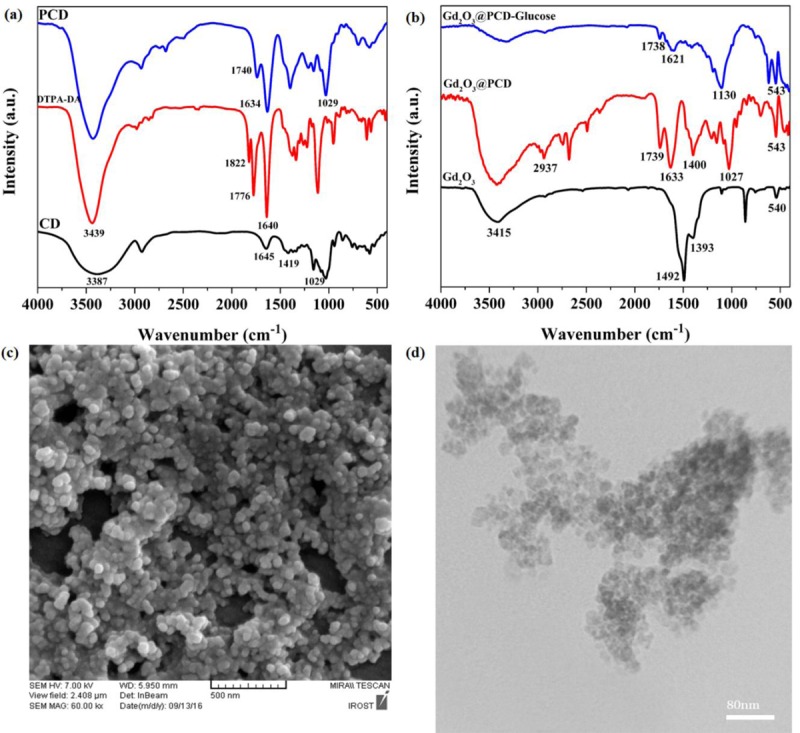
FTIR spectra of (a) CD, DTPA-DA and PCD. (b) Gd2O3, Gd2O3@PCD and Gd2O3@PCD-Glu NPs. (c) FE-SEM and (d) TEM image of Gd2O3@PCD-Glu NPs.
For Gd2O3@PCD-Glu (Figure 1b), the FTIR spectrum showed that the characteristic peaks of Gd2O3 and the polymer layer have been preserved. The appeared band at around 1130 cm-1 indicated an attachment of Glucoseamine to the surface of Gd2O3 @PCD.
Morphological analysis of NPs
Morphology and size assessment of the prepared samples were evaluated using FE-SEM and TEM analysis. As shown in Figures 1(a) and (b), Gd2O3@PCD-Glu NPs are spherical in shape and seemed to be uniform, which is in good agreement with the results of DLS analysis. Slight differences between data from FE-SEM and DLS could be as a result of the various processes involved in preparing the samples [ 30 ].
Dynamic light scattering (DLS) and Zeta potential analysis
DLS analysis was used to obtain the hydrodynamic size as well as size distribution of the prepared samples. Mean diameter of Gd2O3 and Gd2O3@PCD-Glu NPs was 91±5.3 nm (PDI= 0.321) and 118±6.2 nm (PDI= 0.277), respectively (Table 1). The particle size of the prepared polymer was useful in passive tumor target delivery of drugs loaded in NPs as a result of enhanced permeability and retention effect (EPR) [ 31 ], in addition to decreasing reticuloendothelial system (RES)-mediated clearance and avoiding renal filtration [ 32 ]. Polydispersity index (PDI) < 0.5 gave an indication of the suitable size distribution of NPs. Evaluation of the surface charge of the prepared NPs was carried out by measuring the surface zeta potential. Zeta potential values of Gd2O3 and Gd2O3@PCD-Glu shifted from +23.1mV to -5.06mv due to surface modification of Gd2O3 with cyclodextrin moieties as well as hydroxyl and carboxyl groups of the polymer coating layer.
Table 1.
DLS size, PDI and zeta potential of the Gd2O3 and Gd2O3@PCD-FA-DOX NPs.
| Nanoparticle | Hydrodynamic diameter(nm) | PDI | Zeta potential(mv) |
|---|---|---|---|
| Gd2O3 | 91± 5.3 | 0.321 | +23.1 |
| Gd2O3@PCD-Glu | 118±6.2 | 0.277 | -5.06 |
VSM analysis
The magnetic properties of Gd2O3 and Gd2O3NPs were evaluated using a vibrating sample magnetometer (VSM) at RT (300 K) (Figure 2). The amounts of magnetic saturation values (σs) of Gd2O3 and Gd2O3@PCD-Glu were 1 and 0.56 emu/g, respectively, hence indicating the paramagnetic property of the synthesized nanoparticles (linear relationship between magnetization (M) and applied field (H) with positive slope) [ 33 ]. The reasonable decrease in magnetization values of modified NPs can be attributed to the application of non-magnetic organic moieties (PCD) on the surface of the Gd2O3 [ 34 ].
Figure 2.
Magnetization curves of (a) Gd2O3 (b) Gd2O3@PCD-Glu. Magnetization (emu/g) plotted as a function of the applied field. (c): Hemolytic activity of Gd2O3@PCD-Glu NPs at concentration range of 1.95–1000 mg/mL (right to left). (d) XRD pattern of the Gd2O3@PCD-Glu NPs.
Hemolysis assay
Strict hemolysis could result in serious problems. Hence, hemolysis assay was done for Gd2O3 and Gd2O3@PCD-Glu NPs in order to evaluate their biosafety and toxic effects on erythrocytes [ 22 ]. Figure 2(c) shows the hemocompatibility of Gd2O3@PCD-Glu NPs similar to the negative control (PBS), which resulted in sedimentation. Whereas, hemolysis activities with no sedimentation were observed for the HRBCs treated with Triton X100 (2% v/v) (positive control). In addition, hemolytic activity of Gd2O3@PCD-Glu in the studied concentration range (1.95–1000 mg/mL) was less than the standard acceptance limit of 5%.
XRD analysis
Figure 2(d) shows the XRD patterns of Gd2O3 and Gd2O3@PCD-Glu NPs. The observed characteristic peaks of Gd2O3 was in consonance with that of a previous study [ 35 ]. It also showed that the crystalline structure of Gd2O3 was maintained after polymerization and glucoseamine conjugation processes.
Relaxivity measurement
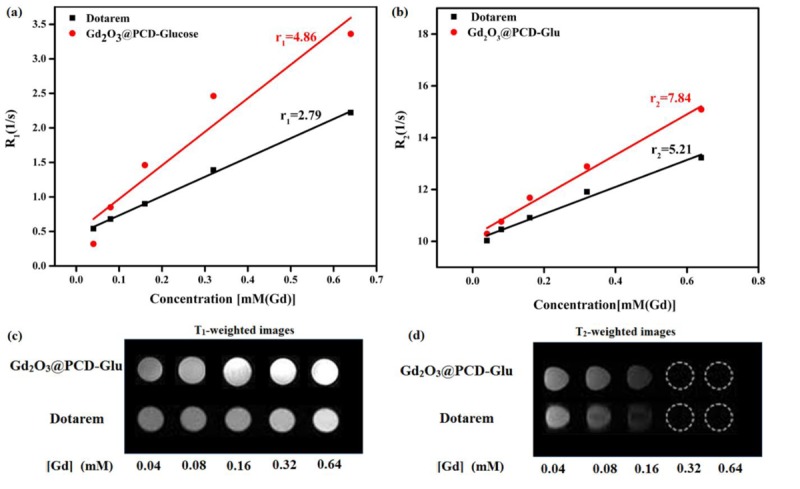
To evaluate the potential application of Gd2O3@PCD-Glu NPs as a new contrast agent, T1-T2-weighted MRI images were performed in aqueous suspensions of the NPs at certain concentrations. In Figure 3, Gd2O3@PCD-Glu showed more efficient contrast enhancement than that of Dotarem at the same concentration. The molecular structure of PCD as a coating agent is a reason for this rise in magnetic resonance sensitivity of Gd2O3@PCD-Glu. PCD is composed of several hydroxyl groups. As a result, it attracts water molecules, increases local water density, and also improves the rate of water exchange of Gd2O3@PCD-Glu [ 36 , 37 ]. These in vitro MR imaging results implied that Gd2O3@PCD-Glu would produce same imaging effects with commercial contrast agent, Dotarem, by decreasing its dose. Gd-based complexes always are limited by adverse effects such as nephrogenic systemic fibrosis. However, a decrease in the agent’s dose improves its clinical safety [ 38 ]. Quantitative analysis was done by measuring the values of the longitudinal relaxation time (T1) for different concentrations of Gd complex in aqueous. Figure 3 shows that the increased concentration of Gd2O3@PCD-Glu and Dotarem led to a decrease in T1 and an increase in longitudinal relaxation rate R1 (R1 = 1/T1). At the same time, Gd2O3@PCD-Glu demonstrated a shorter T1 values than Dotarem at the same concentration. longitudinal (r1) and teransverse (r1) relaxivities of the particles were calculated by measuring the relaxation rate as a function of Gd ions concentration. Calculated r1 value for Gd2O3@PCD-Glu was 4.86 mM−1 • s−1 which was more than that of Dotarem (2.79 mM−1 • s−1).
Figure3.

(a) T1 and (b) T2 relaxivity plot of aqueous suspension of Gd2O3@PCD-Glu NPs and Dotarem. (c) T1-weighted and (d) T2-weighted MR images of Gd2O3@PCD-Glu at 3.0 T MR system.
In vitro cytotoxicity assay
Cytotoxicity of the target sample against MCF-10A epithelial normal breast and cancerous breast carcinoma MDA-MB-231 cells at treatment times of 24 and 48 h was carried out using MTT assay. As illustrated in Figure 4(a), cytotoxicity of Gd2O3@PCD-Glu against MCF-10A cells, as compared to control was about 7% after 24 h (p > 0.05) and 13% after 48 h (p < 0.05) at concentration of 100 μg/mL, revealing their biocompatible property. It could be proposed that Gd2O3 coating with PCD led to a reduction in Gd leakage. For MDA-MB-231 cells (Figure 4(b)), cytotoxicity of Gd2O3@PCD-Glu NPs, in comparison with control, was about 10% after 24 h (p < 0.05) and 21% after 48 h (p < 0.05) at concentration of 100 μg/mL.
Figure4.
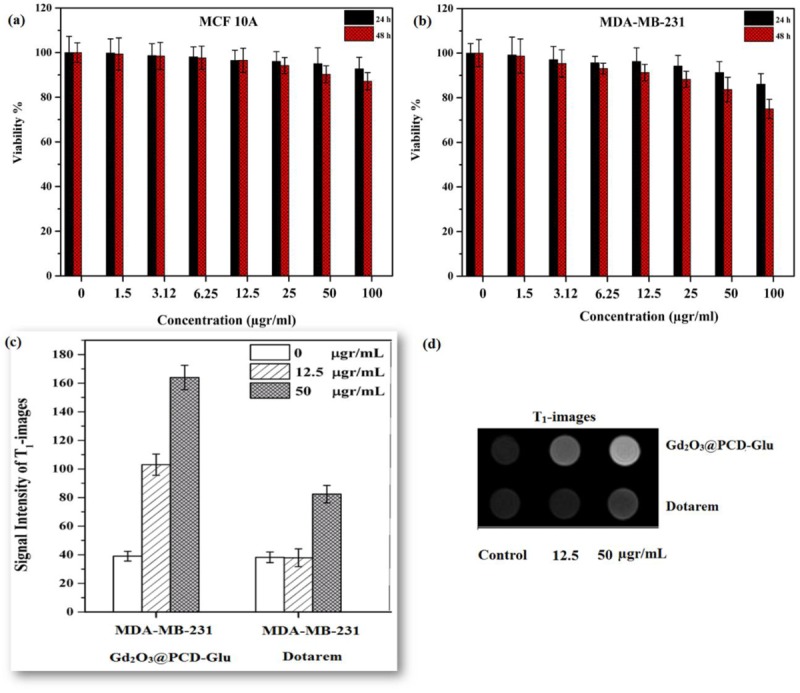
Cytotoxicity of Gd2O3@PCD-Glu nanoparticles measured by MTT assay in MCF 10A (a) and MDA-MB-231 (b) cells after 24 and 48 h incubation. Data is expressed as mean ± S.E.M. (n = 3). (c) Signal intensity analysis for T1-weighted MR images. (d) T1- weighted MR images of Gd2O3@PCD-Glu and Dotarem in MDA-MB-231 cells at different concentration of NPs after incubation for 6 h on 3T MR system.
In vitro MR imaging
The use of Gd2O3@PCD-Glu as the probe for the in vitro MR imaging of MDA-MB-231 cells was evaluated. Dotarem was also investigated similarly for comparison. Figure 4(c) and (d) shows T1-weighted MR images of MDA-MB-231 cells before and after treatment with Gd2O3@PCD-Glu or Dotarem for 6 h. For the MDA-MB-231 cells treated with Gd2O3@PCD-Glu, the in vitro MR signal intensities (Figure 4c) were found to have a significant increase when compared to untreated control cells or the cells treated with Dotarem. In contrast, Dotarem had no effects in the MR signal intensity.
In vivo MR imaging
Further investigation was conducted using Gd2O3@PCD-Glu as the probe for in vivo MR imaging of 4T1 tumors in comparison to Dotarem for similar objective. Significant visualization of tumors was observed for both Gd2O3@PCD-Glu and Dotarem, following intravenous injection of an aqueous suspension of Gd2O3@PCD-Glu or Dotarem in PBS into tumor-bearing mice. Axial T1-weighted images of the tumor enhanced by the Gd2O3@PCD-Glu or Dotarem are shown in Figure 5. As illustrated in Figure 5, the CNR of tumor for Gd2O3@PCD-Glu increased from 0.89±0.23 to 4.85±1.01 within 1 h after injection and gradually reduced to 1.99±0.36 within 6 h. There was a steady rise in the CNR of tumor with time, up to a peak value after 1 h. Afterwards, a steady decline in the CNR was observed, and attained baseline after 6 h (p = 0.64). In contrast, in the control group, CNR for the mice under the same treatment does not increase obviously over time post-injection and achieved 1.22±0.23, 1 h after injection and reduced to 0.95±0.22 after 6 h (Figure 5c, P-value <0.05).
Figure5.
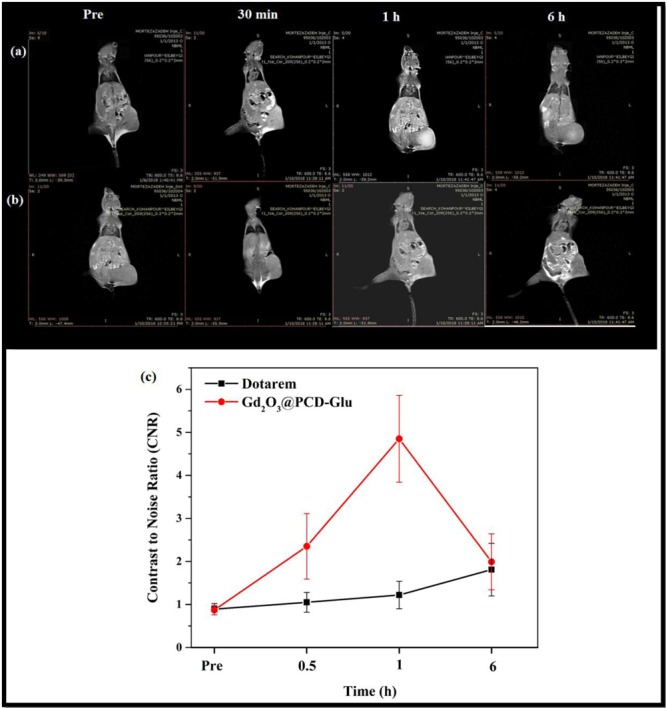
T1-weighted images of Mice tumor injected with (a) Gd2O3@PCD-Glu NPs and (b) Dotarem. (c) CNR analysis for T1-weighted images in tumor. Data represent mean ± SEM (n = 5), P-value < 0.05.
Discussion
In present study, the choice of DTPA as monomer for preparing cyclodextrin-based polymer as a coating agent was due to its availibiltiy, low cost, simple polymerization method as well as proper affinity towards Gd+3 ions. Polymerization was accomplished between β-CD and DTPA-DA in the presence of naked Gd2O3 NPs resulting in CD-based polyester containing appropriate functional groups for chelating of Gd2O3 core as well as further functionalization. Glucoseamine was then conjugated to the appeared acidic groups on the surface of Gd2O3 NPs to enhance the contrast agent’s targeting ability. In addition, the inductively-coupled plasma atomic emission spectroscopy (ICP-AES) results showed that the Gd concentration on the final NPs was approximately 60%. Generally, it is believed that owing to the strong chelating ability of DTPA towards Gd+3 ions [ 39 ], DTPA was selected as monomer to prepare cyclodextrin-based polymer as a coating agent.
It has been shown by studies that negatively charged NPs have poor propensities to absorb plasma proteins. Furthermore, their stability in blood circulation is better compared to positively charged NPs. This is because adsorption of protein leads to rapid clearance of NPs from blood circulation by microphages [ 40 , 41 ]. Also, negatively charged surfaces are considerably less hemolytic compared to positively charged surfaces [ 42 ].
Cytotoxicity results showed that Gd2O3@PCD-Glu NPs had less cytotoxicity against MCF-10A normal breast cells with respect to cancerous MDA-MB-231 cells due to higher metabolic rate and reproduction of cancerous cells compared to normal cells. In other words, attachment of Glucoseamine to Gd2O3@PCD increased cellular uptake of Gd2O3@PCD-Glu through phospholipid cell membrane. This could probably be due to the overexpression of Glut-1 receptors over the MDA-MB-231 cell surface, leading to more accumulation in the cytoplasm [ 9 ]. Increased uptake of these analogs was a result of the increased metabolic needs of the tumor cells.
Relaxivity measuements exhibited that introduction of PCD could be led to an increase in the molecular weight of contrast agent, extension of rotation time and consequently increased the relaxivity. According to literature, polar C=O groups in the polymer coating layer endow the contrast agent with acceptable water accessibility [ 43 ]. In some cases, poor water accessibility to the intraparticular Gd+3 ions inside the polymer can compromise the performance of T1-weighted MRI [ 44 ]. This challenge can be tackled using porous materials through which a water molecule can travel from the bulk water into the interior space [ 45 , 46 ]. Proper relaxivity of Gd2O3@PCD-Glu in our study after coating with PCD as a hydrophilic polymer layer could be due to the presence of polar carbonyl groups on the surface of Gd2O3 core. Water molecules easily diffused across this porous membrane.
Based on in vitro MRI results, our design facilitates significant targeting uptake of the particles in MDA-MB-231 cells overexpressing Glut1. In vivo MR imaging results indicate that Gd2O3@PCD-Glu could clearly and selectively enhance the contrast at the tumor area in T1-weighted MR images which could be due to targeting ability of NPs because of over-expression of Glut-1 receptors over the cell surface. Cancer cells require more glucose compared to normal cells due to their hyper metabolism and high rate of proliferation [ 47 ]. Gd2O3@PCD-Glu, having structural similarities with D-glucose, can be transported by Glut-l to Glut-4. An increase in the uptake of these analogs due to increased metabolic needs of tumor cells is observed during this period. Ethylenedicysteine-deoxyglucose (EC-DG), a glucose derivative, was developed by Yang et al. [ 48 ]. Their findings showed that it is involved in growth as well as proliferation of cells. Due to the similarities in the pathway(s) of glucosamine and glucose, it facilitates post-treatment follow-ups.
Conclusion
In summary, a new gadolinium (III)-based complex Gd2O3@PCD-Glu as a novel nano-contrast agent was successfully synthesized for MR molecular imaging. They have proper paramagnetic property, cyto-compatibility, blood-compatibility and high magnetic relaxivity for MR imaging and were suitable for intravenous injection. In terms of r1 and T1-weighted in vitro imaging, Gd2O3@PCD-Glu gave a better relaxation performance compared to commercial Dotarem. Hence, Gd2O3@PCD-Glu could serve as an efficient probe for targeted MR imaging in vitro as well as xenografted animal model tumors in vivo. The use of Gd2O3@PCD-Glu could allow for early detection of primary tumors as well as tumor metastasis. It also shows great promise in monitoring the reappearance of tumors.
Acknowledgement
This work was supported in part by the Research Chancellor of Tehran University of Medical Sciences (Grant no. 94-03-30-30035) Tehran, Iran.
Conflict of Interest: None
References
- 1.Farhood B, Raei B, Ameri H, Shirvani M, Alizadeh A, Najafi M, et al. A review of incidence and mortality of colorectal, lung, liver, thyroid and bladder cancers in Iran and compared to other countries. Contemp Oncol. 2019;23(1):7. doi: 10.5114/wo.2019.84112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavo A C, Brown R S, Wahl R L. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–32. [PubMed] [Google Scholar]
- 3.Haberkorn U, Ziegler S I, Oberdorfer F, Trojan H, Haag D, Peschke P, et al. FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models. Nucl Med Biol. 1994;21:827–34. doi: 10.1016/0969-8051(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 4.Yang D J, Kim C G, Schechter N R, Azhdarinia A, Yu D F, Oh C S, et al. Imaging with 99mTc ECDG targeted at the multifunctional glucose transport system: feasibility study with rodents. Radiology. 2003;226:465–73. doi: 10.1148/radiol.2262011811. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Huang Z W, He L, Zheng S L, Li J L, Qin D L. Synthesis and evaluation of a technetium-99m-labeled diethylenetriaminepentaacetate-deoxyglucose complex ([99mTc]-DTPA-DG) as a potential imaging modality for tumors. Appl Radiat Isot. 2006;64:342–7. doi: 10.1016/j.apradiso.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Chen Y, Guo D J, Huang Z W, Cai L, He L. The synthesis of a D-glucosamine contrast agent, Gd-DTPA-DG, and its application in cancer molecular imaging with MRI. Eur J Radiol. 2011;79:369–74. doi: 10.1016/j.ejrad.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Levi J, Xiong Z, Gheysens O, Keren S, Chen X, et al. Near-infrared fluorescent deoxyglucose analogue for tumor optical imaging in cell culture and living mice. Bioconjug Chem. 2006;17:662–9. doi: 10.1021/bc050345c. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovar J L, Volcheck W, Sevick-Muraca E, Simpson M A, Olive D M. Characterization and performance of a near-infrared 2-deoxyglucose optical imaging agent for mouse cancer models. Anal Biochem. 2009;384:254–62. doi: 10.1016/j.ab.2008.09.050. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan X H, Hu H, Xiong F, Gu N, Geng X D, Zhu W, et al. Targeting Glut1-overexpressing MDA-MB-231 cells with 2-deoxy-d-g1ucose modified SPIOs. Eur J Radiol. 2012;81:95–9. doi: 10.1016/j.ejrad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Cheze-Le Rest C, Metges J P, Teyton P, Jestin-Le Tallec V, Lozac’h P, Volant A, et al. Prognostic value of initial fluorodeoxyglucose-PET in esophageal cancer: a prospective study. Nucl Med Commun. 2008;29:628–35. doi: 10.1097/MNM.0b013e3282f81423. [DOI] [PubMed] [Google Scholar]
- 11.Mortezazadeh T, Gholibegloo E, Alam N R, Dehghani S, Haghgoo S, Ghanaati H, et al. Gadolinium (III) oxide nanoparticles coated with folic acid-functionalized poly (β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: in vitro and in vivo studies. MAGMA. 2019;32(4):487–500. doi: 10.1007/s10334-019-00738-2. [DOI] [PubMed] [Google Scholar]
- 12.Dehghani S, Alam N R, Shahriarian S, Mortezazadeh T, Haghgoo S, Golmohamadpour A, et al. The effect of size and aspect ratio of Fe-MIL-88B-NH 2 metal-organic frameworks on their relaxivity and contrast enhancement properties in MRI: in vitro and in vivo studies. J Nanoparticle Res. 2018;20(10):278. doi: 10.1007/s11051-018-4376-2. [DOI] [Google Scholar]
- 13.Gholibegloo E, Mortezazadeh T, Salehian F, Forootanfar H, Firoozpour L, Foroumadi A, et al. Folic acid decorated magnetic nanosponge: An efficient nanosystem for targeted curcumin delivery and magnetic resonance imaging. Journal of Colloid and Interface Science. 2019;556:128–39. doi: 10.1016/j.jcis.2019.08.046. [DOI] [PubMed] [Google Scholar]
- 14.Narmani A, Farhood B, Haghi-Aminjan H, Mortezazadeh T, Aliasgharzadeh A, Mohseni M, et al. Gadolinium nanoparticles as diagnostic and therapeutic agents: Their delivery systems in magnetic resonance imaging and neutron capture therapy. J Drug Deliv Sci Technol. 2018;44:457–66. doi: 10.1016/j.jddst.2018.01.011. [DOI] [Google Scholar]
- 15.Bridot J L, Faure A C, Laurent S, Riviere C, Billotey C, Hiba B, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J Am Chem Soc. 2007;129:5076–84. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Zhu J, Chen Z, Xu C, Wang Y, Yao C. A novel bienzyme glucose biosensor based on three-layer Au–Fe3O4@ SiO2 magnetic nanocomposite. Sensors and Actuators B: Chemical. 2011;159:220–8. doi: 10.1016/j.snb.2011.06.076. [DOI] [Google Scholar]
- 17.Gholibegloo E, Mortezazadeh T, Salehian F, Ramazani A, Amanlou M, Khoobi M. Improved curcumin loading, release, solubility and toxicity by tuning the molar ratio of cross-linker to β-cyclodextrin. Carbohydr Polym. 2019;213:70–8. doi: 10.1016/j.jddst.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D A, Elemento E M, Aime S, Chaabane L, Botta M, Parker D. Glycoconjugates of gadolinium complexes for MRI applications. Chem Commun (Camb) 2006:1064–6. doi: 10.1039/b517997a. [DOI] [PubMed] [Google Scholar]
- 19.Parker D, Dickins R S, Puschmann H, Crossland C, Howard J A. Being excited by lanthanide coordination complexes: aqua species, chirality, excited-state chemistry, and exchange dynamics. Chem Rev. 2002;102:1977–2010. doi: 10.1021/cr010452+. [DOI] [PubMed] [Google Scholar]
- 20.Alauddin M M, Louie A Y, Shahinian A, Meade T J, Conti P S. Receptor mediated uptake of a radiolabeled contrast agent sensitive to beta-galactosidase activity. Nucl Med Biol. 2003;30:261–5. doi: 10.1016/s0969-8051(02)00392-x. [DOI] [PubMed] [Google Scholar]
- 21.Duarte M G, Gil M H, Peters J A, Colet J M, Elst L V, Muller R N, et al. Synthesis, characterization, and relaxivity of two linear Gd(DTPA)-polymer conjugates. Bioconjug Chem. 2001;12:170–7. doi: 10.1021/bc000065r. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Zheng L, Cai H, Sun W, Shen M, Zhang G, et al. Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging. Biomaterials. 2013;34:8382–92. doi: 10.1016/j.biomaterials.2013.07.070. [DOI] [PubMed] [Google Scholar]
- 23.Daryasari M P, Akhgar M R, Mamashli F, Bigdeli B, Khoobi M. Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. Rsc Advances. 2016;6:105578–88. doi: 10.1039/c6ra23182a. [DOI] [Google Scholar]
- 24.Zhang P, Hu L, Yin Q, Zhang Z, Feng L, Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release. 2012;159:429–34. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Azizian G, Riyahi-Alam N, Haghgoo S, Moghimi H R, Zohdiaghdam R, Rafiei B, et al. Synthesis route and three different core-shell impacts on magnetic characterization of gadolinium oxide-based nanoparticles as new contrast agents for molecular magnetic resonance imaging. Nanoscale Res Lett. 2012;7:549. doi: 10.1186/1556-276X-7-549. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad M W, Xu W, Kim S J, Baeck J S, Chang Y, Bae J E, et al. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci Rep. 2015;5:8549. doi: 10.1038/srep08549. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Vlerken L E, Amiji M M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv. 2006;3:205–16. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 28.Puech P A, Boussel L, Belfkih S, Lemaitre L, Douek P, Beuscart R. DicomWorks: software for reviewing DICOM studies and promoting low-cost teleradiology. J Digit Imaging. 2007;20:122–30. doi: 10.1007/s10278-007-9018-7. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalender W. Classic papers in modern diagnostic radiology. Springer Science & Business Media: 2004. [Google Scholar]
- 30.Ardestani M S, Arabzadeh A J, Heidari Z, Hosseinzadeh A, Ebrahimi H, Hashemi E, et al. Novel and facile methods for the synthesis of DTPA-mono-amide: a new completely revised strategy in radiopharmaceutical chemistry. Journal of radioanalytical and nuclear chemistry. 2010;283:447–55. [Google Scholar]
- 31.Heydarnezhadi S, Alam N R, Haghgoo S, Ghanaati H, Khoobi M, Gorji E, et al. Glycosylated Gadolinium as Potential Metabolic Contrast Agent vs Gd-DTPA for Metabolism of Tumor Tissue in Magnetic Resonance Imaging. Applied Magnetic Resonance. 2016;47:375–85. doi: 10.1007/s00723-015-0756-2. [DOI] [Google Scholar]
- 32.Gao Z, Lukyanov A N, Singhal A, Torchilin V P. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano letters. 2002;2:979–82. doi: 10.1021/nl025604a. [DOI] [Google Scholar]
- 33.Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9:1521–32. doi: 10.1002/smll.201201390. [DOI] [PubMed] [Google Scholar]
- 34.Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2) Tropical Journal of Pharmaceutical Research. 2013;12:265–73. doi: 10.4314/tjpr.v12i2.20. [DOI] [Google Scholar]
- 35.Sarmento B, Mazzaglia D, Bonferoni M C, Neto A P, Do Céu Monteiro M, Seabra V. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system. Carbohydrate polymers. 2011;84:919–25. doi: 10.1016/j.carbpol.2010.12.042. [DOI] [Google Scholar]
- 36.Akrami M, Khoobi M, Khalilvand-Sedagheh M, Haririan I, Bahador A, Faramarzi M A, et al. Evaluation of multilayer coated magnetic nanoparticles as biocompatible curcumin delivery platforms for breast cancer treatment. RSC Advances. 2015;5:88096–107. doi: 10.1039/c5ra13838h. [DOI] [Google Scholar]
- 37.Kumar S, Meena V K, Hazari P P, Sharma R K. FITC-Dextran entrapped and silica coated gadolinium oxide nanoparticles for synchronous optical and magnetic resonance imaging applications. Int J Pharm. 2016;506:242–52. doi: 10.1016/j.ijpharm.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Tarasi R, Khoobi M, Niknejad H, Ramazani A, Ma’mani L, Bahadorikhalili S, et al. β-cyclodextrin functionalized poly (5-amidoisophthalicacid) grafted Fe 3 O 4 magnetic nanoparticles: A novel biocompatible nanocomposite for targeted docetaxel delivery. Journal of Magnetism and Magnetic Materials. 2016;417:451–9. doi: 10.1016/j.jmmm.2016.05.080. [DOI] [Google Scholar]
- 39.Liu Y, Yang P, Wang W, Dong H, Lin J. Fabrication and photoluminescence properties of hollow Gd 2 O 3: Ln (Ln= Eu3+, Sm3+) spheres via a sacrificial template method. CrystEngComm. 2010;12:3717–23. doi: 10.1039/c0ce00145g. [DOI] [Google Scholar]
- 40.Dobrovolskaia M A, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–95. doi: 10.1021/mp800032f. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yim H, Yang S G, Jeon Y S, Park I S, Kim M, Lee D H, et al. The performance of gadolinium diethylene triamine pentaacetate-pullulan hepatocyte-specific T1 contrast agent for MRI. Biomaterials. 2011;32:5187–94. doi: 10.1016/j.biomaterials.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 42.Werner E J, Datta A, Jocher C J, Raymond K N. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angew Chem Int Ed Engl. 2008;47:8568–80. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 43.Lew S, Brooks M V. Nephrogenic systemic fibrosis: clinical review and education for nurse practitioners. The Journal for Nurse Practitioners. 2009;5:344–9. doi: 10.1016/j.nurpra.2008.10.011. [DOI] [Google Scholar]
- 44.Fang J, Chandrasekharan P, Liu X-L, Yang Y, Lv Y-B, Yang C-T, et al. Manipulating the surface coating of ultra-small Gd2O3 nanoparticles for improved T1-weighted MR imaging. Biomaterials. 2014;35:1636–42. doi: 10.1016/j.biomaterials.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Chen K J, Wolahan S M, Wang H, Hsu C H, Chang H W, Durazo A, et al. A small MRI contrast agent library of gadolinium(III)-encapsulated supramolecular nanoparticles for improved relaxivity and sensitivity. Biomaterials. 2011;32:2160–5. doi: 10.1016/j.biomaterials.2010.11.043. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Z, Thorek D L, Tsourkas A. Gadolinium-conjugated dendrimer nanoclusters as a tumor-targeted T1 magnetic resonance imaging contrast agent. Angew Chem Int Ed Engl. 2010;49:346–50. doi: 10.1002/anie.200905133. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Z, Thorek D L, Tsourkas A. Porous Polymersomes with Encapsulated Gd-labeled Dendrimers as Highly Efficient MRI Contrast Agents. Adv Funct Mater. 2009;19:3753–9. doi: 10.1002/adfm.200901253. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young C D, Lewis A S, Rudolph M C, Ruehle M D, Jackman M R, Yun U J, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]


