Abstract
Background:
Magnetic resonance imaging (MRI) using nanostructures has been a proper method for tumor targeting purposes. Different MRI nanomaterials, targeting agents and anticancer drugs have been used for targeting of tumors.
Objectives:
This study aims to consider the MRI property of doxorubicin (DOX)-loaded gadolinium/13X zeolite/folic acid (Gd3+/13X/FA) nanocomposite.
Material and Methods:
In this in vitro study, Gd3+/13X/FA/DOX nanocomposite was prepared and the X-ray diffraction, scanning electron microscopy and MTT assay were conducted to evaluate the physicochemical properties of the nanocomposite. MRI was performed at 25°C using a 1.5 T clinical system to determine the T1 relaxation times and subsequently, the T1 relaxivity.
Results:
The size of the nanocomposite was in the range of 80-200 nm. The nanocomposite without DOX loading (Gd3+/13X/FA) showed compatibility for A549 cells for all concentrations while DOX-loaded nanocomposite was toxic for 62% of the cells at the concentration of 0.4 mg/ml. The T1 relaxivity of Gd3+/13X/FA/DOX nanocomposite was 4.0401 mM-1s-1.
Conclusion:
Gd3+/13X/FA/DOX nanocomposite shows a T1 relaxivity similar to the conventional gadolinium chelates, and a successful DOX loading.
Keywords: Magnetic Resonance Imaging, Relaxivity, Gadolinium, Doxorubicin, Folic Acid, 13X zeolite
Introduction
Magnetic resonance imaging (MRI) using nanostructures is an efficient method used for different biomedical applications such as cancer imaging [ 1 ], inflammation detection [ 2 ] and perfusion imaging [ 3 ]. MRI-guided targeted drug delivery represents another significant application of MRI, which has been important, recently [ 4 ]. A favorable procedure, which is useful for detection and treatment of cancers, includes combination of diagnostic and therapeutic features. The imaging methods are to detect tumor areas by improving the signal between the neoplasm and healthy tissues surrounding while the theraputic agents are released in the tumor site [ 5 ]. Magnetic nanoparticles are appropriate to be used in these procedures because of their ability to improve contrast in MRI as a medical diagnostic tool for therapeutic purposes such as hyperthermia and targeted drug delivery [ 6 ].
Among various contrast agents of MRI, gadolinium (Gd3+)-based materials have an optimal structure to produce positive signal in MRI due to a significant effect on the reduction of T1 relaxation time. They have been widely used in clinical MRI as chelate forms. However, gadolinium chelates have some disadvantages, including low detection sensitivity, toxicity attention and short blood circulation time [ 7 ]. On the other hand, Gd3+ nanoparticles have been used more recently due to their low toxicity, good solubility, excellent physicochemical properties and high relaxivity [ 8 ]. Recent advances in molecular imaging have produced an additional requirement for the use of targeted contrast materials and an increase in their sensitivity [ 9 ].
Folic acid (FA) is a vitamin with low molecular weight, which is used as a tumor-targeting agent for various types of tumor cells [ 10 ]. Because of high binding affinity of folate receptor for folic acid, FA can move into the cancer cells via receptor-mediated endocytosis [ 11 , 12 ]. Therefore, anticancer drug can be imported to the cells. FA has also other advantages such as non-immunogenic property, stability and low cost [ 11 ].
Doxorubicin (DOX) is an anticancer drug, which has been widely used for treatment of different types of cancer. Direct intravascular injection of DOX in chemotherapy patients has severe toxicity to normal cells and shows the side effects due to the low specificity of DOX to cancer cells [ 13 ]. The problem is solved by systems of tumor-targeted drug delivery [ 14 ]. One of these systems was DOX-loaded Gd-FA-Si nanoplatform, which was developed by Zhang et al. The nanosystem also showed capability as a T1 contrast agent for MRI [ 15 ]. In another study with ZnO@Gd2O3 nanoparticles, attachment of DOX and FA to the surface of the nanoparticles was performed through amino groups. The resultant multimodal nanostructures were reported as good T2 contrast agents for MRI [ 12 ].
Zeolites are microporous crystalline aluminosilicate materials, which have the unique chemical structure, and consist of channels and holes [ 16 ]. They can act as a substitute for guest molecules like gadolinium ions [ 17 ]. Thus, different studies have been carried out using various types of zeolites in MRI. In one study, the chemical stability of Gd3+-loaded NaY zeolite was investigated at low pH and in the presence of some cations with the concentrations similar to gastrointestinal tract. Besides, the effect of Gd3+ loading on the relaxivity was studied [ 18 ]. In another study, Gd3+-loaded NaY or NaA zeolites were introduced as potential MRI contrast agents in the high field; in addition, the relationship between the structure of the zeolites and the relaxivities was investigated [ 19 ].
Although the different Gd3+-loaded zeolites have been investigated for MRI; in addition, DOX and FA have been used in the structure of the various targeted agents, to our knowledge, the MRI property of Gd3+/13X/FA/DOX nanocomposite has not been investigated. This study aims to consider Gd3+/13X/FA/DOX nanocomposite potential as an MRI contrast agent with the ability of DOX loading.
Material and Methods
Preparation of Gd3+/13X/FA/DOX nanocomposite
In this in vitro study, the 13X zeolite was prepared based on the study carried out by Mesgari-Shadi et al [ 20 ]. It was dispersed in 2 ml distilled water using an ultrasound device for 2 h. Then, gadolinium chloride solution with a ratio of 2.6 wt% zeolite was added to the suspension, stirred at room temperature for 24 h with a magnetic stirrer and centrifuged. FA was added to the suspension containing 5 g of the zeolite in 10 ml of distilled water. Then prepared Gd3+/13X/FA nanocomposite was dispersed in 5 ml of doxorubicin solution, stirred for 12 h at 25 °C, centrifuged and dried under vacuum for 24 h. The final product was Gd3+/13X/FA/DOX.
Characterization
Several characterization tools were employed to identify the physicochemical properties of Gd3+/13X/FA/DOX nanocomposite. To collect X-ray diffraction patterns (XRD) at room temperature and record scanning electron microscope (SEM) images, a Philips diffractometer and a Philips ES 30 kW device were used, respectively. MR imaging of the samples was carried out at 25 °C using a 1.5 T horizontal bore MRI system (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany).
In vitro MTT assay
MTT (3-(4, 5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide) assay is one of the routine methods to evaluate cytotoxicity or cell viability. In this study, A549 alveolar adenocarcinoma cells were incubated in 96-well plates (8×103 cells for each well). Then, the cells were cultured at 5% CO2 and 37 °C. Different concentrations, including 0.05, 0.1, 0.2 and 0.4 mg/ml of Gd3+/13X/FA or Gd3+/13X/FA/DOX nanocomposites were added to the culture media and the A549 cells were incubated for 24 h. Next steps were carried out as follow:
- Adding 50 μL of 2 mg/ml MTT solution and 150 μL culture medium to each well and incubating for 4 h at 37° C and 5% CO2.
- Washing wells with Phosphate-buffered solution and removing the residual media.
- Adding dimethyl sulfoxide and Sorenson buffer as solubilizer to each well.
- Measuring the absorbance at 570 nm using an ELISA plate reader (BioTeck, Bad Friedrichshall, Germany).
MRI and relaxivity measurement
Different concentrations of Gd3+/13X/FA/DOX nanocomposite were prepared in the glass tubes and dispersed by ultrasound device (SOLTEC, Italy) for 30 minutes. Addition of 10 ml agar to the nanocomposite samples was carried out to prevent from precipitation and aggregation of the samples. For the MRI study, the glass tubes were placed in a water-containing plastic phantom, which was adjusted in the center of a quadrature head coil.
The T1 weighted images were prepared using a spin echo pulse sequence with different TRs (250, 450, 1000, 1800 and 2500 ms) and a constant TE of 11 ms, a matrix size of 384 × 384 , a field of view (FOV) of 189 × 189 mm2 and a slice thickness (ST) of 3 mm. The signal intensity values of the samples were measured using DicomWorks software. Plotting the curves of the T1 relaxation time and subsequently calculating of the 1/T1 relaxation rates were carried out using MATLAB software (r2018b). The 1/T1 values were used to plot the T1 relaxivity graph, which showed the relationship between the amounts of 1/T1 and the different Gd3+ concentrations of Gd3+/13X/FA/DOX nanocomposite.
Results
Preparation and characterization of the nanocomposite
Figure 1 shows the schematic illustration of the prepared Gd3+/13X/FA/DOX nanocomposite.
Figure1.
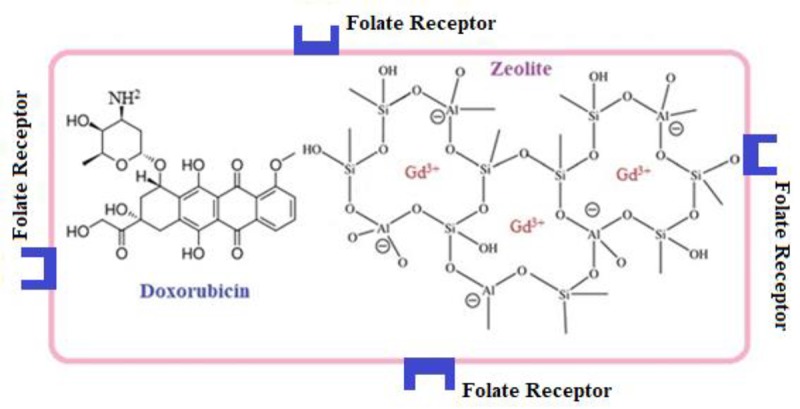
Schematic illustration of Gd3+/13X/FA/DOX nanocomposite.
XRD spectrum showed the peaks related to the 13X zeolite (Figure 2a). According to Figure 2b, the size of the nanocomposite was in the range of 80-200 nm.
Figure2.
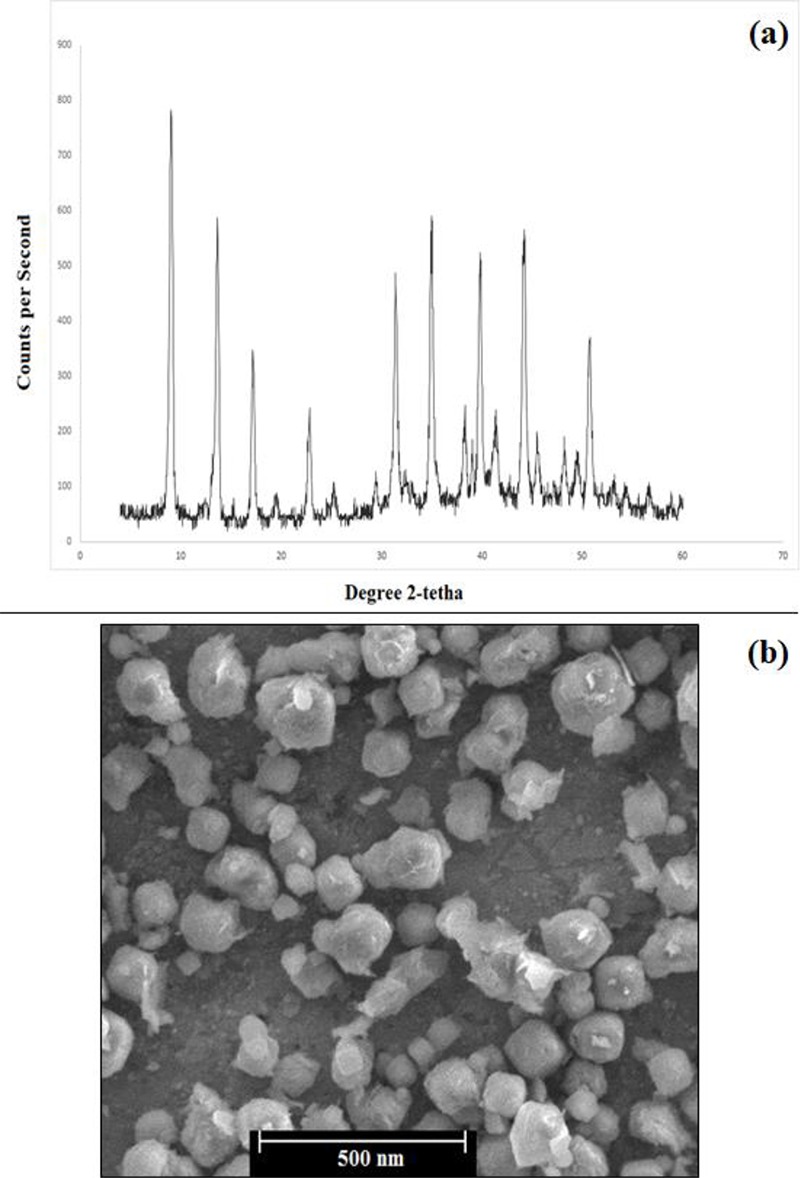
a) XRD spectrum and b) SEM image of Gd3+/13X/FA/DOX nanocomposite.
In vitro MTT assay
Figure 3 illustrates the MTT assay results for A549 cells following 24 h incubation with various concentrations of Gd3+/13X/FA and Gd3+/13X/FA/DOX nanocomposites. The relative cell viability of Gd3+/13X/FA/DOX was lower than Gd3+/13X/FA nanocomposite for all concentrations. Gd3+/13X/FA showed the cell viability greater than 80% even for the concentration of 0.4 mg/ml as the highest concentration. On the other hand, for Gd3+/13X/FA/DOX, the relative cell viability was less than 80% for all samples so that the cell killing at the concentration of 0.4 mg/ml was higher than 60%.
Figure3.
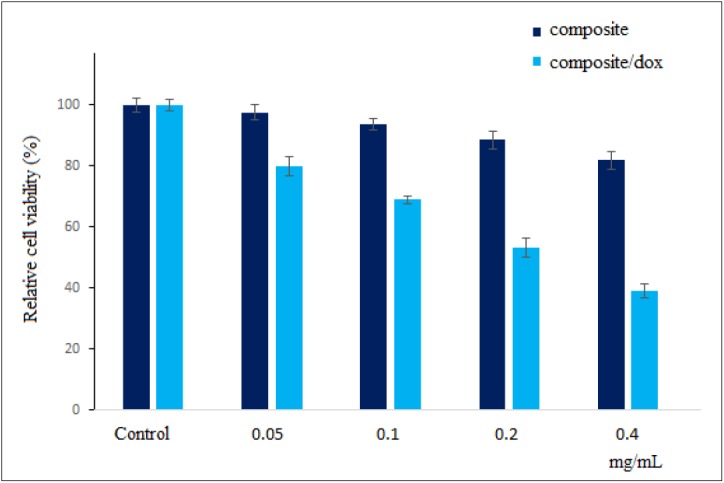
Relative A549 cell viability following 24 h incubation with various concentrations of Gd3+/13X/FA and Gd3+/13X/FA/DOX nanocomposites.
MRI Study
Figure 4a shows a sample of the T1 weighted image of Gd3+/13X/FA/DOX nanocomposite with various Gd3+ concentrations, which was prepared with TR = 450 ms and TE = 11 ms. Increasing of the samples’ brightness as a function of Gd3+ concentration is seen in the figure. Moreover, increasing of TR was led to the incremental signal intensity at the same concentrations.
Figure4.
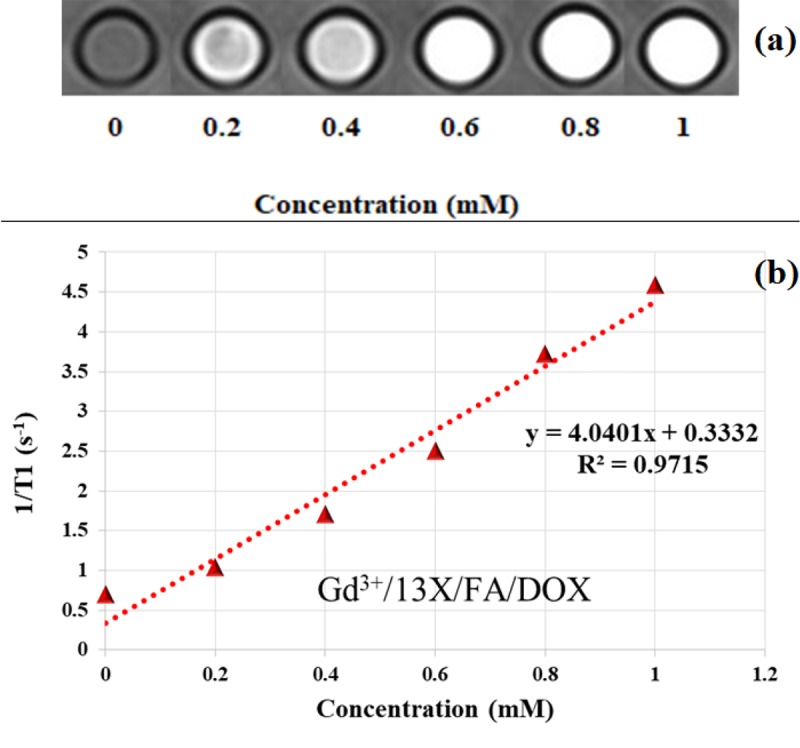
a) T1 weighted image of the various Gd3+ concentrations of Gd3+/13X/FA/DOX nanocomposite, b) T1 relaxivity graph of the nanocomposite
According to Figure 4b, which shows the T1 relaxivity graph, the linear relationship between 1/T1 relaxation rates with Gd3+/13X/FA/DOX nanocomposite concentrations is seen. The T1 relaxivity of Gd3+/13X/FA/DOX was calculated 4.0401 mM-1s-1.
Discussion
Characterization and in vitro MTT assay
In XRD spectrum, the peaks of 13X zeolite were seen; however, Gd3+ did not show any peaks because of its ionic form resulted from ion exchange synthesis method.
According to the MTT assay results (Figure 3), Gd3+/13X/FA indicated high compatibility with the cells for all concentrations while Gd3+/13X/FA/DOX showed cell-killing effect for all concentrations. This finding indicates the high ability of Gd3+/13X/FA/DOX to kill A549 alveolar adenocarcinoma cells confirming the successful loading of DOX to Gd3+/13X/FA nanocomposite.
MRI
As seen in Figure 4a, increasing the concentration of Gd3+/13X/FA/DOX nanocomposite results in a more pronounced effect on the T1 relaxation time shortening. Gd3+ is a paramagnetic ion, which can affect both T1 and T2 relaxation times. In the low concentrations of gadolinium ions, the T1 shortening effect is dominant leading to the signal intensity increasing, and consequently, the positive contrast enhancement of the T1 weighted MR images. The TR amount controls the T1 weighting of the image. Therefore, difference for TR has a major effect on the image contrast.
T1 relaxivity shows the relationship between 1/T1 relaxation rate and Gd3+ concentrations. Based on Figure 4b, this relationship is linear for Gd3+/13X/FA/DOX nanocomposite. The linear dependence of 1/T1 to gadolinium ion concentration is necessary for each MRI contrast agent. The relaxivity of the nanocomposite is affected by the quantity of the gadolinium ions and the number of the available water protons for the Gd3+ magnetic centers. 13X zeolite is an X type zeolite with a high capacity for ion exchange, and Si/Al ratio of 1-1.5 [ 21 ]. Due to the low ratio of Si to Al, it shows hydrophilic property. The gadolinium ions as magnetic centers are encapsulated in the supercages, pores and channels of the zeolite. Because of the large size of the supercage cavity, the number of the Gd3+ in the supercages is higher than those of the pores and channels. Therefore, it is expected that the gadolinium ions have the highest access to the water protons through the supercages, and enough access via the pores and channels of the zeolite. In Gd3+/13X/FA/DOX nanocomposite, FA as targeted agent and DOX as anticancer agent are present. Since FA can bind to the OH groups of the zeolite, it occupies some of the pores and channels. Thus, the access of the gadolinium ions to the water protons via the pores and channels is limited. Although DOX cannot occupy the pores and channels of the zeolite due to the large size, it can be placed in the mouth of the pores and channels, and prevent from the access of the Gd3+ to the bulk water. Both of these situations lead to the lowering of the T1 relaxivity for Gd3+/13X/FA/DOX nanocomposite.
Although presence of FA and DOX reduces the T1 relaxivity value of Gd3+/13X/FA/DOX nanocomposite, its relaxivity is similar to the conventional gadolinium chelates. Additionally, the nanocomposite shows good ability for DOX loading. Comparing to other targeted porous nanocomposites, the T1 relaxivity of Gd3+/13X/FA/DOX is slightly lower than Gd-FA-Si nanoplatform [ 15 ]. It should be noted that the magnetic field strength was different in the studies.
Conclusion
In the present study, Gd3+/13X/FA/DOX nanocomposite was successfully prepared, and the MRI property of the nanocomposite was investigated. The in vitro MTT assay results confirmed the cytocompatibility of the Gd3+/13X/FA nanocomposite and the appropriate cell-killing effect of Gd3+/13X/FA/DOX, showing the successful loading of DOX. The MRI study revealed the capability of the nanocomposite to produce positive contrast in the T1 weighted images. Gd3+/13X/FA/DOX nanocomposite shows potential for using as T1 contrast material with successful loading of the anticancer drug. The investigation of the nanocomposite potential for tumor targeting in animal model can be done as a future work.
Acknowledgement
This work was adapted from the MSc thesis and supported by Medical Radiation Sciences Research Team, Tabriz University of Medical Sciences (Grant No: 1397.317).
Conflict of Interest: None
References
- 1.Oghabian M A, Guiti M, Haddad P, Gharehaghaji N, Saber R, Alam N R, et al. Detection sensitivity of MRI using ultra-small super paramagnetic iron oxide nano-particles (USPIO) in biological tissues. Conf Proc IEEE Eng Med Biol Soc. 2006:5625–6. doi: 10.1109/IEMBS.2006.260131. [DOI] [PubMed] [Google Scholar]
- 2.Lutz A M, Weishaupt D, Persohn E, Goepfert K, Froehlich J, Sasse B, et al. Imaging of macrophages in soft-tissue infection in rats: relationship between ultrasmall superparamagnetic iron oxide dose and MR signal characteristics. Radiology. 2005;234(3):765–75. doi: 10.1148/radiol.2343031172. [DOI] [PubMed] [Google Scholar]
- 3.Saharkhiz H, Gharehaghaji N, Nazarpoor M, Mesbahi A, Pourissa M. The Effect of Inversion Time on the Relationship Between Iron Oxide Nanoparticles Concentration and Signal Intensity in T1-Weighted MR Images. Iran J Radiol. 2014;11(2):e12667. doi: 10.5812/iranjradiol.12667. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren L, Chen S, Li H, Zhang Z, Zhong J, Liu M, et al. MRI-guided liposomes for targeted tandem chemotherapy and therapeutic response prediction. Acta Biomater. 2016;35:260–8. doi: 10.1016/j.actbio.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Ma Z, Wan H, Wang W, Zhang X, Uno T, Yang Q, et al. A theranostic agent for cancer therapy and imaging in the second near-infrared window. Nano Research. 2019;12(2):273–9. doi: 10.1007/s12274-018-2210-x. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inozemtseva O A, German S V, Navolokin N A, Bucharskaya A B, Maslyakova G N, Gorin D A. Encapsulated magnetite nanoparticles: preparation and application as multifunctional tool for drug delivery systems. Nanotechnology and Biosensors. 2018: 175–192. doi: 10.1016/B978-0-12-813855-7.00006-4. [DOI] [Google Scholar]
- 7.Stephen Z R, Kievit F M, Zhang M. Magnetite nanoparticles for medical MR imaging. Mater Today (Kidlington) 2011;14:330–8. doi: 10.1016/S1369-7021(11)70163-8. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C T, Padmanabhan P, Gulyás B Z. Gadolinium (iii) based nanoparticles for T 1-weighted magnetic resonance imaging probes. RSC Advances. 2016;6(65):60945–66. doi: 10.1039/C6RA07782J. [DOI] [Google Scholar]
- 9.Lin Y S, Hung Y, Su J K, Lee R, Chang C, Lin M L, et al. Gadolinium (III)-incorporated nanosized mesoporous silica as potential magnetic resonance imaging contrast agents. The Journal of Physical Chemistry B. 2004;108(40):15608–11. doi: 10.1021/jp047829a. [DOI] [Google Scholar]
- 10.Sun C, Sze R, Zhang M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res A. 2006;78(3):550–7. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 11.Stella B, Arpicco S, Peracchia M T, Desmaële D, Hoebeke J, Renoir M, et al. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89(11):1452–64. doi: 10.1002/1520-6017(200011)89:11<1452::aid-jps8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Babayevska N, Florczak P, Woźniak-Budych M, Jarek M, Nowaczyk G, Zalewski T, et al. Functionalized multimodal ZnO@Gd2O3 nanosystems to use as perspective contrast agent for MRI. Applied Surface Science. 2017;404:129–37. doi: 10.1016/j.apsusc.2017.01.274. [DOI] [Google Scholar]
- 13.Shen X, Li T, Chen Z, Geng Y, Xie X, Li S, et al. Luminescent/magnetic PLGA-based hybrid nanocomposites: a smart nanocarrier system for targeted codelivery and dual-modality imaging in cancer theranostics. Int J Nanomedicine. 2017;12:4299. doi: 10.2147/IJN.S136766. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández M, Javaid F, Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci. 2018;9(4):790–810. doi: 10.1039/C7SC04004K. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Gao J, Qian J, Zhang L, Zheng K, Zhong K, et al. Hydroxylated mesoporous nanosilica coated by polyethylenimine coupled with gadolinium and folic acid: a tumor-targeted T1 magnetic resonance contrast agent and drug delivery system. ACS Appl Mater Interfaces. 2015;7(26):14192–200. doi: 10.1021/acsami.5b04294. [DOI] [PubMed] [Google Scholar]
- 16.Shin J, Jo D, Hong S B. Rediscovery of the importance of inorganic synthesis parameters in the search for new zeolites. AAcc Chem Res. 2019;52(5):1419–1427. doi: 10.1021/acs.accounts.9b00073. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Li L, Yu J. Applications of zeolites in sustainable chemistry. Chem. 2017;3(6):928–49. doi: 10.1016/j.chempr.2017.10.009. [DOI] [Google Scholar]
- 18.Bresinska I, Balkus K J. Studies of Gd (III)-exchanged Y-type zeolites relevant to magnetic resonance imaging. J Phys Chem. 1994;98(49):12989–94. doi: 10.1021/j100100a029. [DOI] [Google Scholar]
- 19.Csajbók É, Bányai I, Vander Elst L, Muller R N, Zhou W, Peters J A. Gadolinium (III)-loaded nanoparticulate zeolites as potential high-field MRI contrast agents: Relationship between structure and relaxivity. Chemistry. 2005;11(16):4799–807. doi: 10.1002/chem.200500039. [DOI] [PubMed] [Google Scholar]
- 20.Mesgari-Shadi A, Sarrafzadeh M H, Divband B, Barar J, Omidi Y. Batch adsorption/desorption for purification of scfv antibodies using nanozeolite microspheres. Microporous and Mesoporous Materials. 2018;264:167–75. doi: 10.1016/j.micromeso.2018.01.028. [DOI] [Google Scholar]
- 21.Anbia M, Aghaei M. Study of the effect of organic binders on 13X zeolite agglomeration and their CO2 adsorption properties. Scientia Iranica. 2019;26(3):1497–504. doi: 10.24200/sci.2018.21198. [DOI] [Google Scholar]


