Abstract
Background
Acute liver failure (ALF) is a life-threatening event associated with high mortality. Currently, only estimates are available for its incidence. The aim of the study was to assess the incidence of ALF in Germany, using the accounting data of the largest statutory health insurance company, which represents 26.5 million people.
Methods
The analysis included patients insured from 1 January 2014 to 31 December 2018. Coding with the International Statistical Classification of Diseases and Related Health Problems and the German operation and procedure codes were used to identify the patients, whose age, sex, liver transplantations (LT), and fatal outcomes were then recorded and extrapolated to the total population. As a validity check, the extrapolated LT results were compared with the LT that were actually performed.
Results
The calculated incidence of ALF was 1.13/100 000 person-years, representing 4652 cases. Women were more often affected (52% versus 48%, p < 0.001). The overall rate of mortality within 3 months was 47%. A total of 203 LT were recorded in 176 patients. Men received 41% of the LT, women 59% (p < 0.137). The 1-year overall mortality rate after LT was 20%. The 203 calculated transplantations corresponded to 228 actually performed transplantations.
Conclusion
The incidence of ALF was higher than previously estimated for Germany, with only a very low rate of LT despite high mortality. When extrapolating to the general population of Germany, it must be borne in mind that those insured by the company concerned do not represent a valid sample.
Acute liver failure (ALF) is defined as a potentially reversible, acute impairment of liver function characterized by jaundice, coagulopathy with international normalized ratio (INR) >1.5, and hepatic encephalopathy in patients without pre-existing liver disease (1). ALF is considered infrequent, but is associated with high mortality without liver transplantation (LT) (2– 4). Therefore, it is important to identify ALF in good time to refer patients to a liver transplant center. Since there are no national registries, the true incidence is unknown. Currently, only estimates based on cohort studies are available, while population-based data are rare (5, 6).
ALF can be caused by toxic, viral, autoimmune, or, more rarely, hereditary liver diseases. In Europe, North America, and Japan, the most common reasons for ALF are drug-induced liver injury (DILI), acute viral hepatitis, and cryptogenic liver failure (2– 4). The impact of viral hepatitis has decreased in the past few decades, due partly to immunization programs (4). However, acute viral hepatitis is still the most common cause of ALF in developing countries (7). Worldwide, occasional cases of ALF are caused by autoimmune hepatitis and Wilson’s disease (8, 9).
The Federal Association of the AOK (Allgemeine Ortskrankenkasse) is the largest German health insurance organization with about 26.5 million insured individuals in 2018 (10). This amounts to almost one third of the German population (11).
The aim of the study was to assess the incidence of ALF in Germany, using the accounting data of the AOK Federal Association. We also investigated gender distribution, etiologies, drug prescriptions before ALF, death and the subsequent necessity of LT.
Methods
Study design
The German health care system is based on statutory and private health insurance (12). The vast majority of people have statutory health insurance provided by different organizations (13). We evaluated the incidence of ALF in Germany based on the accounting data of the AOK, which is the largest statutory health insurance company. The data were extrapolated to the total population. As a validity check, figures on LTs actually performed in Germany with the main diagnosis ALF were requested from Eurotransplant and compared with the study results.
The study was performed in accordance with the Declaration of Helsinki, and the approval of the local ethics committee was obtained beforehand.
Data analysis and epidemiology
Data were collected prospectively by the AOK and analyzed anonymously in cooperation with the AOK Research Institute (WIdO). The analysis included all patients insured without interruption from 1 January 2014 to 31 December 2018. This also included patients born or deceased in the investigated period. To identify patients with ALF we used the inpatient diagnostic codes based on the International Statistical Classification of Diseases and Related Health Problems, 10threvision, German Modification (ICD-10-GM) as reported to the AOK.
To exclude incorrectly coded patients with chronic or alcoholic liver disease and/or primary or secondary carcinoma of the liver, concomitantly assigned codes suggesting pre-existing liver disease were used to adjust the primary search. Data obtained from standardized procedures and surgery based on the German operation and procedure codes (OPS) were used to identify patients with evaluation and/or LT.
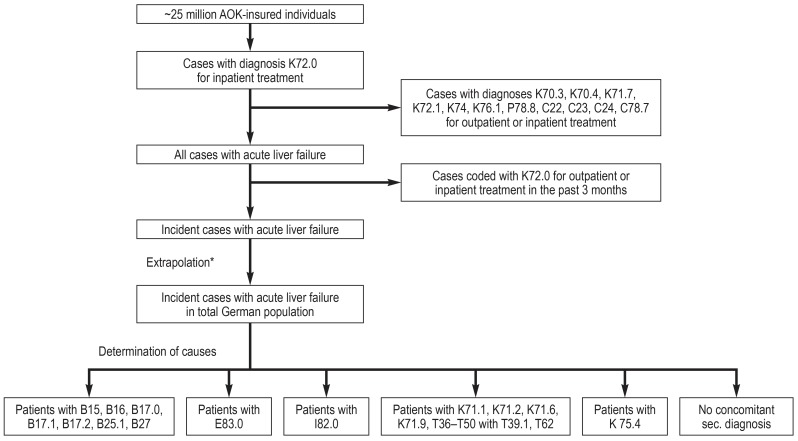
Specifically, the primary search included all patients assigned the principal discharge ICD code K72.0 ‘acute and subacute liver failure’ for an inpatient treatment. Data sets were eliminated in the event of simultaneous assignment of the following codes for in- or out-patient treatment: K72.1 ‘chronic liver failure’; K74 ‘liver cirrhosis’; K70.3 ‘alcoholic cirrhosis’; K70.4 ‘alcoholic liver failure’; P78.8 ‘congenital cirrhosis’; K76.1 ‘cirrhose cardiaque’; K71.7 ‘toxic liver disease with cirrhosis’; C22 ‘malignant neoplasm of liver and intrahepatic bile ducts’; C23 ‘malignant neoplasm of gallbladder’; C24 ‘malignant neoplasm of other and unspecified parts of biliary tract’; C78.7 ‘secondary malignant neoplasm of liver and intrahepatic bile duct’. To ensure that only incident cases were included, patients coded two or more times with K72.0 within a 3-month period were counted only once. Incident cases identified were extrapolated to the German general population with adjustment for sex and age (Figure 1). This procedure is routinely used by governmental institutions for analyses of the German health care system (11).
Figure 1.
Determination of incidence of acute liver failure in Germany, 2014–2018
AOK – Allgemeine Ortskrankenkasse (the largest German health insurance company); sec., secondary
*Extrapolation was performed using a statistic of the total population of Germany. Adjustments were made for sex and age.
Etiology and outcome
The ALF patient data sets were used to record sex, age at time of diagnosis, concomitant diagnoses indicating etiology, and secondary diagnoses (causes of ALF and chronic disorders). Prescriptions given within 3 months before coding of K72.0 were scrutinized for potentially hepatotoxic drugs published as possible causes of ALF (2, 14– 18) and were compared with prescriptions in all inhabitants. The defined daily doses were not considered; patients with at least one prescription of potentially hepatotoxic drugs in a given 3-month period were counted once.
All ALF patients were screened for OPS codes 1–920.24 ‘complete evaluation for liver transplantation with listing’, 1–920.34 ‘re-evaluation for liver transplantation with listing or remaining on waiting list’, 1–920.04 ‘complete evaluation for liver transplantation without listing’, 1–920.14 ‘partial evaluation for liver transplantation without listing’, 1–920.44 ‘re-evaluation for liver transplantation with removal from waiting list’, and 5–504 ‘liver transplantation’. Possibly related fatal outcomes were defined as death within 3 months after coding of diagnosis K72.0 or within 12 months after coding of OPS 5–504. The data search at Eurotransplant was performed consistently with the search at the AOK. Patients with simultaneously coded chronic liver diseases were excluded.
Statistical analysis
Incidence, gender, age, concomitant secondary diagnoses, death, and LT were reported as descriptive statistics. Sex distribution, mortality, and prescriptions were compared by means of a two-sided binominal test (19). Exact numbers were used for calculation, but rounded numbers are presented here.
Results
Epidemiology
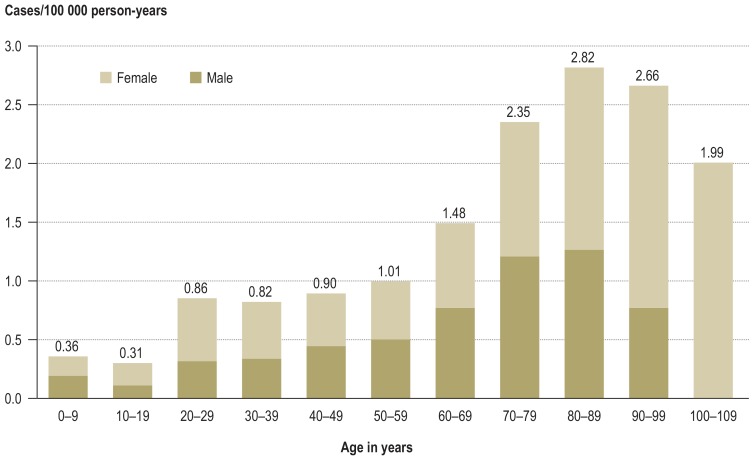
In total, 4652 cases of ALF were identified in the period from 1 January 2014 to 31 December 2018 (Table 1), resulting in a mean incidence of 1.13 per 100 000 person-years. These 4652 cases occurred in 4621 patients with a median age of 60 years (range 0–101). ALF occurred most frequently in the ninth decade (Figure 2), but 64% of patients were younger than 70 years (20).
Table 1. Incidence of acute liver failure in Germany, 2014–2018.
| Year | Female | Male | All cases | Female | Male | All inhabitants | Incidence/100 000 person-years |
| 2014 | 449 | 419 | 867 | 41 362 080 | 39 835 457 | 81 197 537 | 1.07 |
| 2015 | 499 | 394 | 893 | 41 661 561 | 40 514 123 | 82 175 684 | 1.09 |
| 2016 | 437 | 388 | 825 | 41 824 535 | 40 697 118 | 82 521 653 | 1.00 |
| 2017 | 522 | 534 | 1056 | 41 948 786 | 40 843 565 | 82 792 351 | 1.28 |
| 2018 | 532 | 479 | 1011 | 42 052 522 | 40 966 691 | 83 019 213 | 1.22 |
| 2014–2018 | 4652*1 | 1.13*2 | |||||
*1 All cases with acute liver failure 2014–2018
*2 Mean incidence 2014–2018
Figure 2.
Age distribution for incidence of acute liver failure in Germany, 2014–2018
Etiology and concomitant diagnoses
For the majority of patients with ALF (56%), no concomitant secondary diagnosis was identified that could be used to determine the etiology (eTable). The potentially hepatotoxic drugs prescribed for patients with ALF and for the general population are presented in Table 2.
eTable. Concomitant secondary diagnoses (ICD-10-GM codes) for outpatient or inpatient treatment in the investigated period.
| Secondary diagnoses*1 | ALF patients with diagnosis | ALF patients with diagnosis and LT | ||
| (n) | (% of all ALF patients)*2 | (n) | (% of ALF patients with diagnosis) | |
| Liver disease*3 | ||||
| B15 acute viral hepatitis A | 118 | 2.6 | 3 | 2.8 |
| B16 acute viral hepatitis B | 244 | 5.3 | 4 | 1.6 |
| B17.0 acute viral hepatitis D | 7 | 0.2 | 0 | – |
| B17.1 acute viral hepatitis C | 64 | 1.4 | 0 | – |
| B17.2 acute viral hepatitis E | 162 | 3.5 | 11 | 6.5 |
| B25.1 cytomegaloviral hepatitis | 23 | 0.5 | 4 | 17.3 |
| B27 infectious mononucleosis | 96 | 2.1 | 26 | 26.5 |
| E83.0 disorders of copper metabolism (Wilson’s disease) | 26 | 0.6 | 11 | 44.4 |
| I82.0 Budd-Chiari syndrome | 48 | 1.0 | 11 | 22.5 |
| K70 alcoholic liver disease (without K70.3. K70.4) | 196 | 4.2 | 0 | – |
| K71 toxic liver disease (without K71.7) | 946 | 20.5 | 56 | 6.0 |
| K75.4 autoimmune hepatitis | 352 | 7.6 | 27 | 7.6 |
| K76.0 fatty change of liver | 843 | 18.2 | 23 | 2.7 |
| T36-T50 poisoning by drugs, medications, and biologically active substances (without T39.1) | 277 | 6.0 | 11 | 4.0 |
| T39.1 poisoning by 4-aminophenol derivatives*4 | 176 | 3.8 | 4 | 2.0 |
| T62.0 toxic effect of ingested mushrooms | 96 | 2.1 | 7 | 7.5 |
| General condition | ||||
| C00-C99 malignant neoplasms (without C22, C23, C24, C78.7) | 1143 | 24.7 | 35 | 3.0 |
| E10-E14 diabetes mellitus | 1524 | 33.0 | 42 | 2.8 |
| F10 mental behavioral disorders due to use of alcohol | 668 | 14.5 | 15 | 2.3 |
| I25 chronic ischemic heart disease | 1212 | 26.2 | 7 | 0.6 |
| J44 chronic obstructive pulmonary disease | 970 | 21.0 | 16 | 1.6 |
*1 Multiple assessments possible.
*2 Total of 4621 patients.
*3 2574 patients without diagnoses B15, B16, B17.0, B17.1, B17.2, B25.1, B27, E83.0, I82.0, K71, K75.4, T36-T50 including T39.1, T62.0; diagnoses K70 and K76.0 are not considered causes of ALF in a narrower sense.
*4 Including acetaminophen, phenacetin is no longer approved in Germany
ALF, Acute liver failure; ICD-10-GM, International Statistical Classification of Diseases and Related Health Problems, 10th revision, German Modification; LT, liver transplantation
Table 2. Prescriptions of hepatotoxic drugs in patients with acute liver failure (n= 4621 patients) compared with the general population*1.
| Hepatotoxic drug | Patients with ALF *3 | General population*4 | Comparison with general population | |||
| (n) | (% of all) | (n) | (% of all) | (%) | (p) | |
| Painkillers | ||||||
| Ibuprofen*2 | 310 | 6.7 | 7 145 928 | 8.7 | -1.97 | <0.001 |
| Diclofenac | 77 | 1.7 | 2 177 394 | 2.6 | -0.98 | <0.001 |
| Acetaminophen*2 | 34 | 0.7 | 991 534 | 1.2 | -0.47 | 0.017 |
| Antibiotics | ||||||
| Amoxicillin | 122 | 2.6 | 2 035 862 | 2.5 | 0.17 | 0.481 |
| Ciprofloxacin | 139 | 3.0 | 1 031 971 | 1.3 | 1.76 | <0.001 |
| Trimethoprim/sulfamethoxazole | 71 | 1.5 | 405 784 | 0.5 | 1.04 | <0.001 |
| Levofloxacin | 39 | 0.8 | 289 716 | 0.4 | 0.48 | <0.001 |
| Nitrofurantoin | 16 | 0.3 | 114 144 | 0.1 | 0.21 | 0.002 |
| Others | ||||||
| Phenprocoumon | 67 | 1.5 | 829 865 | 1.0 | 0.45 | 0.006 |
| Carbamazepine | 7 | 0.1 | 207 871 | 0.3 | -0.11 | 0.221 |
| Cytostatic preparations | 87 | 1.9 | 101 846 | 0.1 | 1.77 | <0.001 |
| All | 4621 | 82 341 288 | ||||
ALF – acute liver failure
*1 Comparison performed using a two-sided binominal test.
*2 Prescriptions only. Over-the-counter-medications are not included.
*3 Number of ALF patients with at least one prescription in the 3 months before coding of ALF
*4 The numbers of patients with at least one prescription were recorded for every quarter during the study period. The average numbers of patients per quarter are presented.
Of the 4621 ALF patients, 33% were coded with diabetes mellitus, 26% with chronic ischemic heart disease, and 25% with malignant neoplasms for inpatient or outpatient treatment (eTable).
Outcome
In total, 2166 of 4621 patients died within 3 months (47%): 1071 men (49% of the men) and 1095 women (45% of the women; p= 0.055). OPS codes for evaluation were assigned in 188 of 4621 patients (4.1%). A total of 209 evaluations with and without listing were encoded in these 188 patients.
Furthermore, 203 LT were identified in 176 patients (176/4621; 3.8%). Of these 176 patients, 73 were male (41% versus 59%; p=0.137). The median age of the LT patients was 33 years (range 0–66). Within the first year after LT, 34 out of 176 patients died (19.5%): 7 men (10% of the men) and 27 women (26% of the women; p= 0.035). The median age of the deceased patients was 38 years (range 1–57).
To check the validity, data on the LT actually performed in Germany between 1 January 2014 and 31 December 2018 were requested from Eurotransplant (Arjan van Enckevort, Eurotransplant International Foundation [request@eurotransplant.org], personal communication via e-mail, 19 September 2019); 357 patients were listed in Germany with the diagnosis code K72.0, including patients listed for re-transplantation (Table 3). In those patients, 228 transplantations were performed, including re-transplantations.
Table 3. Patients from Germany with diagnosis K72.0 listed at Eurotransplant in the years 2014–2018, including re-listed patients*.
| Outcome | Patients |
| Deceased | 82 |
| Removed | 38 |
| Still on waiting list | 9 |
| Transplanted | 228 |
| Total | 357 |
* Personal communication via e-mail from Arjan van Enckevort, Eurotransplant International Foundation (request@eurotransplant.org), 19 September 2019
Discussion
Acute liver failure is a severe impairment of liver function, but its incidence has always been more or less unknown (4). In the current study, we report the incidence of ALF in the German population, extrapolating health care data from approximately 25 million people to the total population of 83 million (10). To our knowledge, this is the largest population-based study on ALF to date (5, 21, 22).
The primary conclusion of our analysis is that the incidence of ALF seems to be higher than previously estimated for Germany (23), comparable with that of the United States (24), but lower than recently reported for Taiwan and Thailand (5, 22). However, the Taiwanese incidence included ALF in acute alcoholic hepatitis, which should, strictly speaking, be classified separately (25). A synopsis of the published data is given in Table 4.
Table 4. Synopsis of published epidemiological data for acute liver failure (ALF) in the past decade.
| Author | Country or region | Cohort or population | Study design | Number of people | Incidence (in 100.000 person-years) |
| All causes of ALF | |||||
| Bretherick et al. 2011 (21) | Scotland | Residents of a geographically defined area including Edinburgh | Retrospective cohort study, data from the Scottish Liver Transplant Unit at the Royal Infirmary of Edinburgh | ˜5 064 200 | 0.62 |
| Ho et al. 2014 (5) | Taiwan | Taiwanese population | Population-based study, data randomly selected from the Longitudinal Health Insurance Database | 1 000 000 | 8.02 |
| Thanapirom et al. 2019 (22) | Thailand | Thai population | Population-based study, data from the statutory Universal Coverage Scheme (76% of all admissions to primary, secondary, and tertiary hospitals across 77 provinces) | ˜65 400 000 | 6.29 |
| Drug-induced liver injury | |||||
| Goldberg et al. 2015 (26) | California, USA | Northern California residents | Retrospective cohort study, data from the Kaiser Permanente Northern California Health Care health insurance service | ˜2 400 000 | 0.19 |
| Shen et al. 2019 (29) | China | Patients of 66 centers in mainland China | Retrospective cohort study with extrapolation to the population of mainland China | ˜1 361 000 000* | 23.8 |
* Extrapolation to this number of people
Furthermore, LT was rarely carried out despite the extremely high mortality associated with untreated disease. Previously published data about LT in ALF are scarce and often reflect specific cohorts (26); the rate ranges from 0% to 23% (15, 26– 29). The median age of the patients was higher than reported in cohort analyses (17, 18), but similar to that of patients from Japan (28) and patients with DILI (30, 31), as well as to that in the Taiwanese population-based analysis (5). Assuming an age limit of 70 years for LT (20), most patients were eligible for organ replacement. However, only 4.1% of patients were evaluated and 3.8% underwent LT. The low number of recorded assessments may arise from peculiarities of the German billing system: LT is coded as principal procedure, and the code for evaluation may not have been recorded as it is of subsidiary importance for billing purposes.
Given the high mortality it is more difficult to understand why the rate of LT is so low. Contraindications such as malignancies and cardiovascular comorbidity may have contributed to the low number of transplantations.
Patients with primary and secondary neoplasms of the liver were excluded by the study design. However, the concomitant secondary diagnoses reveal a high rate of other malignancies and also chronic ischemic heart disease in patients reported with ALF. We excluded patients coded with acute alcoholic liver failure. Nevertheless, diagnosis F10 ‘mental and behavioral disorders due to use of alcohol’ was coded in 14.4% and alcoholic liver disease (without alcoholic liver cirrhosis and alcoholic liver failure) in 4.2% of patients. Moreover, rapid progression to terminal liver failure and death may also be a factor in the low rate of LT. In the investigated period, 82 of 357 patients registered at Eurotransplant died on the waiting list. The impact of—structural or regional—access to transplantation is not known. With regard to the management and survival of patients with hepatocellular carcinoma, regional differences in access to treatment have recently been reported (32).
A secondary goal of the study was to determine the causes of ALF. In the majority of cases no additional diagnosis useful for determination of ALF was encoded, so the reported rate of cryptogenic ALF was higher than reported previously (18, 33, 34). The reasons for this are not clear. The fact that billing for health care costs essentially depends on the primary inpatient diagnosis and the principal procedure has certainly played a part. In order to investigate underlying reasons, medication prescriptions were recorded and screened for drugs implicated in idiosyncratic liver failure (2, 14– 18). Our study might confirm the hepatotoxic potential of drugs, but analysis is hampered by the complexity of influencing factors. The study design meant that intake of over-the-counter medications, not to mention illicit drugs, was not documented (35, 36). This must be remembered when interpreting the data.
To verify data acquisition and calculation, we compared the extrapolated number of LT with the number registered for Germany at Eurotransplant. The former was lower than the latter. The difference may reflect distinctions between AOK-insured individuals and the German general population in terms of comorbidities or socio-economic status (12). Adjustments in coding from one year to the next may also have resulted in discrepancies. Six patients underwent LT in a different year than listed: five had their operation in the subsequent year, while one was listed in 2015 and transplanted in 2018. Furthermore, 19 datasets from patients listed with the diagnosis K72.0 were deleted because chronic liver disease was coded simultaneously. This leads to the assumption that at the Eurotransplant registry the code K72.0 does not always imply ALF in the more restricted sense.
Interpretation of the results is limited by the study design. Despite the efforts to exclude differential diagnoses for ALF, inaccurate assignment of the code K72.0 for an inpatient treatment or of other codes for inpatient or outpatient treatments used for selection of our ALF patients is conceivable—and indeed suggested by the high rate of comorbidities or fatty livers, as well as ALF of unknown origin. Coding in Germany is done not only by physicians but also by medical controllers. However, ALF in the narrow sense is difficult to diagnose, and even experts do not succeed in identifying the cause of ALF in all patients (34). It is not known whether the diagnosis of K72.0 entails any financial benefit in individual cases. Furthermore, as only minimal data sets are reported to health insurance companies along with the ICD and OPS codes, laboratory data and clinical information are not available to confirm the diagnoses (37). Nevertheless, the use of billing data has specific advantages: because all inpatient treatments are included in screening (38), there is no systematic bias as found in cohort analyses from specialized liver centers.
Extrapolation of the results to the total population was adjusted for age and sex concordantly with health care analyses carried out by governmental institutions (11). In interpreting the findings, it should be borne in mind that sociodemographic factors other than age and sex may influence health in general and the incidence of certain diseases in particular (5). As AOK-insured individuals may differ from the general population in terms of sociodemographic characteristics (39), over- or underestimation is conceivable despite standardization of the extrapolation to the total population (40). It must also be kept in mind that health care companies’ populations are continuously changing because insurees are free to change from one company to another.
The main inpatient diagnosis and the procedure code suffice for billing the costs of in-hospital treatment. The analysis of the causes of ALF should therefore be interpreted with caution. Despite these limitations, however, this is the largest population-based study to date on ALF, its incidence, and subsequent LT.
In conclusion, our analysis of the health insurance data of 25 million individuals indicates, extrapolated to the total population of approximately 83 million people in Germany, an incidence of 1.13 per 100 000 person-years for ALF. Despite the high mortality, potentially lifesaving LT is seldom performed. Moreover, the ICD code does not distinguish between acute and subacute liver failure. The incidence cited here therefore includes both forms, which, however, do not differ clinically with regard to prognosis and treatment (1). The treatment of choice is LT.
THE CLINICAL PERSPECTIVE.
Acute liver failure
-
Definition
Acute liver failure (ALF) is a potentially reversible but life-threatening liver dysfunction defined by jaundice, coagulopathy, and hepatic encephalopathy without pre-existing liver disease.
-
Causes
The most common causes of ALF in western countries are intake of hepatotoxic drugs and acute viral hepatitis. Hepatotoxic drugs described as possible causes of ALF include acetaminophen, phenprocoumon, ibuprofen, diclofenac, amoxicillin, ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, nitrofurantoin, and carbamazepine. Herbal medicines, dietary supplements, illegal drugs such as ecstasy, or the death cap fungus can also cause ALF. In a high proportion of patients, the cause of ALF remains unclear (cryptogenic ALF).
-
Clinical presentation and course of disease
Patients may present with jaundice, increasing somnolence, disorientation, flapping tremor, or in extreme cases hepatic coma. Laboratory tests reveal elevated transaminases, bilirubin, and ammonia, as well as international normalized ratio (INR) > 1.5. ALF can be complicated by other organ failure, cerebral edema, or bleeding.
-
Treatment
Patients with ALF should be admitted to the hospital and, if possible, treated causatively, e.g., with acetylcysteine for acetaminophen intoxication. Intensive care is necessary in most cases. The King’s College criteria define the indications for liver transplantation, which in this situation is a life-saving procedure. Therefore, patients should be transferred in good time to a liver transplant center for assessment. Extracorporeal liver support therapy (“liver dialysis”) does not improve survival.
Key Messages.
In the largest population-based study on acute liver failure (ALF) to date, the billing data of approximately 26.5 million people were evaluated and the findings extrapolation to the total German population of 83 million.
The incidence was higher than recently estimated for Germany, at 1.13/100 000 person-years.
The median age of patients with ALF was 60 years (range: 0 to 101 years).
Despite the high mortality of 47%, liver transplantation was performed in only 3.8% of cases.
The diagnostic code used to define ALF, K72.0, does not distinguish between acute and subacute liver failure.
Footnotes
Conflict of interest statement Dr. Weiler has received consultancy payments and lecture fees from Astellas and Novartis, and reimbursement of travel costs from AbbVie, Astellas, Biotest, and Novartis.
Prof. Schnitzbauer has received consultancy payments from Astellas, Novartis, Integra Life Sciences, TEVA, and Chiesi, and reimbursement of travel costs from Astellas.
Prof. Zeuzem has received consultancy payments from AbbVie, Gilead, Intercept, and Janssen, and lecture fees from AbbVie, Gilead, and Merck/MSD.
PD Welker has received lecture fees and consultancy payments from AbbVie, Akcea, Amgen, Bayer, BMS, Gilead, Hexal, Novartis, and Roche, and reimbursement of travel costs from AbbVie, Astellas, Bayer, BMS, Novartis, Janssen, and Roche.
Dr Schlotmann declares that no conflict of interest exists.
References
- 1.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. 34 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a model to predict transplant-free survival of patients with acute liver failure. Clin Gastroenterol Hepatol. 2016;14:1199–1206 e2. doi: 10.1016/j.cgh.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 5.Ho CM, Lee CH, Wang JY, Lee PH, Lai HS, Hu RH. Nationwide longitudinal analysis of acute liver failure in taiwan. Medicine (Baltimore) 2014;93 doi: 10.1097/MD.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in adults with acute liver failure between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724–732. doi: 10.7326/M15-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manka P, Verheyen J, Gerken G, Canbay A. Liver failure due to acute viral hepatitis (A-E) Visc Med. 2016;32:80–85. doi: 10.1159/000444915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto K, Miyake Y, Ohira H, et al. Prognosis of autoimmune hepatitis showing acute presentation. Hepatol Res. 2013;43:630–638. doi: 10.1111/j.1872-034X.2012.01109.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Wang D, Ding N, Zheng C. Hepatic manifestations in Wilson‘s disease: Report of 110 Cases. Hepatogastroenterology. 2015;62:657–660. [PubMed] [Google Scholar]
- 10.AOK - Die Gesundheitskasse. Jahresbericht. https://aok-bv-jahresbericht2018.de/data/jahresbericht_2018_pdf/#44 (last accessed on 23 December 2019) 2018 [Google Scholar]
- 11.Statistisches Bundesamt. www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/Tabellen/zensus-geschlecht-staatsangehoerigkeit-2019.html;jsessionid=734C83DBFD878C31C051471D5D27C98D.internet741 (last accessed on 23 December 2019) [Google Scholar]
- 12.Busse R, Blumel M. Germany: Health system review. Health Syst Transit. 2014;16:1–296. [PubMed] [Google Scholar]
- 13.Gesundheitsberichterstattung des Bundes. www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=17317387&p_sprache=D&p_knoten=TR700. (last accessed on 23 December 2019) [Google Scholar]
- 14.Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P, Wang C, Liu W, et al. Causes and outcomes of acute liver failure in China. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080991. e80991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadem J, Tacke F, Bruns T, et al. Etiologies and outcomes of acute liver failure in Germany. Clin Gastroenterol Hepatol. 2012;10:664–669 e2. doi: 10.1016/j.cgh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Wei G, Bergquist A, Broome U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 18.Escorsell A, Mas A, de la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13:1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 19.Sachs L. Angewandte Statistik. Berlin, Heidelberg: Springer-Verlag. 2004 889. [Google Scholar]
- 20.Sharpton SR, Feng S, Hameed B, Yao F, Lai JC. Combined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomes. Transplantation. 2014;98:557–562. doi: 10.1097/TP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretherick AD, Craig DG, Masterton G, et al. Acute liver failure in Scotland between 1992 and 2009; incidence, aetiology and outcome. QJM. 2011;104:945–956. doi: 10.1093/qjmed/hcr098. [DOI] [PubMed] [Google Scholar]
- 22.Thanapirom K, Treeprasertsuk S, Soonthornworasiri N, et al. The incidence, etiologies, outcomes, and predictors of mortality of acute liver failure in Thailand: a population-base study. BMC Gastroenterol. 2019;19 doi: 10.1186/s12876-019-0935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canbay A, Tacke F, Hadem J, Trautwein C, Gerken G, Manns MP. Acute liver failure: a life-threatening disease. Dtsch Arztebl Int. 2011;108:714–720. doi: 10.3238/arztebl.2011.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoofnagle JH, Carithers RL Jr., Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 25.Wendon J, Cordoba J, Dhawan A, et al. EASL Clinical Practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148:1353–1361 e3. doi: 10.1053/j.gastro.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrillow S, Bailey M, Pilcher D, et al. Characteristics and outcomes of patients with acute liver failure admitted to Australian and New Zealand intensive care units. Intern Med J. 2019;49:874–885. doi: 10.1111/imj.14167. [DOI] [PubMed] [Google Scholar]
- 28.Nakao M, Nakayama N, Uchida Y, et al. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol. 2018;53:752–769. doi: 10.1007/s00535-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 29.Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology. 2019;156:2230–2241 e11. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. doi: 10.1053/j.gastro.2013.02.006. 25 e1-3; quiz e19-20. [DOI] [PubMed] [Google Scholar]
- 31.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Goutte N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017;66:537–544. doi: 10.1016/j.jhep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288–296. doi: 10.1016/j.jhep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Ganger DR, Rule J, Rakela J, et al. Acute liver failure of indeterminate etiology: A comprehensive systematic approach by an expert committee to establish causality. Am J Gastroenterol. 2018 doi: 10.1038/s41395-018-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maharaj R, Pingitore A, Menon K, Kane P, Wendon J, Bernal W. Images of the Month: MDMA-induced acute liver failure and transient abdominal pneumatosis. Am J Gastroenterol. 2015;110 doi: 10.1038/ajg.2014.399. [DOI] [PubMed] [Google Scholar]
- 36.Kamijo Y, Soma K, Nishida M, Namera A, Ohwada T. Acute liver failure following intravenous methamphetamine. Vet Hum Toxicol. 2002;44:216–217. [PubMed] [Google Scholar]
- 37.Tan SS, Geissler A, Serden L, et al. DRG systems in Europe: variations in cost accounting systems among 12 countries. Eur J Public Health. 2014;24:1023–1028. doi: 10.1093/eurpub/cku025. [DOI] [PubMed] [Google Scholar]
- 38.Busse R, Nimptsch U, Mansky T. Measuring, monitoring, and managing quality in Germany‘s hospitals. Health Aff (Millwood) 2009;28:w294–w304. doi: 10.1377/hlthaff.28.2.w294. [DOI] [PubMed] [Google Scholar]
- 39.Böcken JB, Bernard Amhof Robert, editors. Gesundheitsmonitor 2008. Gesundheitsversorgung und Gestaltungsoptionen aus der Perspektive der Bevölkerung. Gütersloh: Bertelsmann Stiftung. 2008 [Google Scholar]
- 40.Hoffmann F, Icks A. [Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor] Gesundheitswesen. 2012;74:291–297. doi: 10.1055/s-0031-1275711. [DOI] [PubMed] [Google Scholar]