Abstract
Influenza continues to cause severe illness in millions and deaths in hundreds of thousands annually. Vaccines are used to prevent influenza outbreaks, however, the influenza virus mutates and annual vaccination is required for optimal protection. Vaccine effectiveness is also affected by other potential factors such as the human immune system, a mismatch with the chosen candidate virus, and egg adaptation associated with egg-based vaccine production. This article reviews the influenza vaccine development process and describes the implications of the changes to the cell-culture process and vaccine strain recommendations by the World Health Organization since the 2017 season. The traditional manufacturing process for influenza vaccines relies on fertilized chicken eggs that are used for vaccine production. Vaccines must be produced in large volumes and the complete process requires approximately 6 months for the egg-based process. In addition, egg adaptation of seed viruses occurs when viruses adapt to avian receptors found within eggs to allow for growth in eggs. These changes to key viral antigens may result in antigenic mismatch and thereby reduce vaccine effectiveness. By contrast, cell-derived seed viruses do not require fertilized eggs and eliminate the potential for egg-adapted changes. As a result, cell-culture technology improves the match between the vaccine virus strain and the vaccine selected strain, and has been associated with increased vaccine effectiveness during a predominantly H3N2 season. During the 2017–2018 influenza season, a small number of studies conducted in the United States compared the effectiveness of egg-based and cell-culture vaccines and are described here. These observational and retrospective studies demonstrate that inactivated cell-culture vaccines were more effective than egg-based vaccines. Adoption of cell-culture technology for influenza vaccine manufacturing has been reported to improve manufacturing efficiency and the additional benefit of improving vaccine effectiveness is a key factor for future policy making considerations.
Keywords: Influenza, vaccine, cell-culture technology
Introduction
Influenza causes up to 5 million cases of severe illness worldwide and between 290,000 and 650,000 respiratory deaths each year.1 Its seasonal appearance is associated with increased visits to emergency rooms and primary care physicians, increased absenteeism at work and school, and increased hospitalizations—particularly in the elderly and persons with chronic conditions.2 Influenza vaccines are widely used to prevent outbreaks. However, antigenic drift (due to mutations in key viral antigens) in the virus and a lack of long-lasting antibody titers even with vaccination means that optimal protection can only be achieved with annual vaccinations developed by careful surveillance and prediction of emerging strains. Despite a well-established system of surveillance and vaccine production, current influenza vaccines, even when given annually, do not provide complete protection. There are a number of reasons for this including characteristics of the human immune system, mismatch between the circulating strain and the vaccine strain, and egg adaptation of viral seeds.3 New and emerging manufacturing technologies such as cell-culture isolation of the seed virus may improve the closeness of vaccine virus match to circulating strains and thereby improve effectiveness and, thus, further reduce the burden of disease from influenza. In the case of a pandemic, it also offers an opportunity to provide for a more rapid response, which obviates the reliance on eggs for vaccine bulk production.
Background
Influenza is an infectious respiratory disease that has three different genera or types, A, B, and C, which are antigenically distinct.4 Influenza type A is further classified into subtypes, such as A/H1N1 and A/H3N2, by surface glycoproteins hemagglutinin (HA) and neuraminidase. Influenza B types are differentiated by lineages such as B/Yamagata and B/Victoria (Figure 1).2,4
Figure 1.
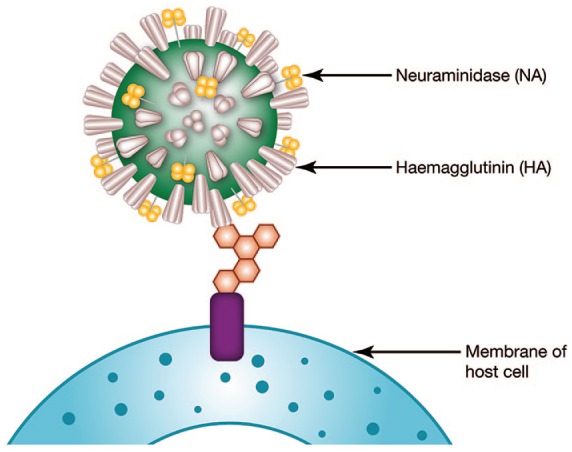
Anatomy of an influenza virus.
Influenza A is the most frequent cause of influenza in humans.2 The A/H3N2 strain has the highest rate of morbidity and mortality. One analysis of 31 influenza seasons in the United States showed that in 22 seasons in which influenza A/H3N2 was the dominant strain mortality rates were 2.7 times higher than in the non-H3N2-dominant seasons.5 Similar results were seen in other studies in other parts of the globe.6,7 In the winter of 2014–2015 in England, 28,484 deaths were due to influenza with 93% occurring in persons 65 years or older.8 In the 2017 influenza season in Australia when H3N2 was estimated to make up 55% of cases, there were 745 deaths compared with the 5-year average of 176. Most of the deaths were in the elderly.9 In the 2017–2018 season in the United States, H3N2 viruses predominated and there were 172 pediatric deaths due to influenza, which was the highest number of influenza-related deaths in children reported in a single nonpandemic season.10
The more genetically stable influenza B viruses, B/Yamagata and B/Victoria, have been circulating for 40 years and yet it is still challenging to determine which one will be dominant during a season.2 Influenza C is less common and has only caused mild respiratory infections.2
Influenza-related complications are myriad and include pneumonia, bronchitis, sinus infections, ear infections, and exacerbation of many chronic conditions such as asthma, congestive heart failure, and chronic obstructive pulmonary disease.11 Most who are infected with influenza experience mild illness that does not require medical intervention and resolves within 2 weeks; however, for others influenza complications can require hospitalization and can lead to death.11 In the United States, an analysis of 31 consecutive influenza seasons (1976–2007) showed that the estimated average number of annual influenza deaths due to pneumonia and influenza causes was 6309 with a low of 961 in 1986–1987 and a high of 14,715 in 2003–2004.5 The estimated average number of influenza-associated deaths with underlying respiratory and circulatory causes was 23,607 with a low of 3349 in 1986–1987 and a high of 48,614 in 2003–2004. Most deaths (89.4%) were seen in persons aged 65 years or older.5 When excess mortality is observed, an outbreak is considered an epidemic. When a new influenza virus emerges due to antigenic shift, a pandemic can occur and mortality rates can soar.12 In the 1918 Spanish Influenza pandemic up to up to 50 million people died worldwide; pandemics have also occurred in 1957, 1968, 1977, and 2009.2,3
Although the severity of the outbreak and the groups most affected vary in each influenza epidemic, certain high-risk groups have been identified who should receive vaccination and treatment.2 In the United States, the recommendation is for annual influenza vaccination of everyone 6 months and older with any licensed, age-appropriate influenza vaccine.13 The World Health Organization (WHO) recommends influenza vaccination for all high-risk groups (e.g. pregnant women at any stage of pregnancy, children aged between 6 months and 5 years, older adults aged >65 years, individuals with chronic medical conditions, and health-care workers).1
Technology for influenza vaccine production
Influenza vaccines are available in different forms: inactivated influenza vaccines (IIV) and live attenuated influenza vaccines (LAIV). IIVs are produced to protect against three (trivalent) or four (quadrivalent) influenza viruses; whereas LAIVs are produced as quadrivalent only. IIVs can be manufactured in different ways. IIVs can be made from a whole virus that is chemically inactivated, concentrated, and then purified. Further processing can create split virus vaccines, which are treated with detergent to separate the viral envelope and expose the interior viral proteins and subviral elements.14 Further purification can create subunit vaccines in which the HA protein is enriched. Since the 1970s, most vaccines are split virus or subunit because they have comparable effectiveness with fewer adverse reactions. The split virus and subunit vaccines are less immunogenic in populations that have not been exposed to influenza vaccines; in a seasonal setting, two doses of these vaccines are needed for young children.14 To improve the immunogenicity in certain populations such as the elderly, a high-dose version of the trivalent vaccine was approved for use in 2010 that has four times the standard HA dose.14 Adjuvants—substances that increase immune responses to an antigen—can be added to influenza antigens to improve vaccine immunogenicity, particularly for vulnerable populations. An influenza vaccine with MF59 adjuvant, which is a squalene oil-in-water emulsion system, is licensed in the United States, Europe, and some countries in the Asia pacific region including Australia for persons aged 65 years and older who do not have a history of severe allergic reaction to the vaccine or its components.15 A number of adjuvants are used in pandemic influenza vaccines including aluminum salt, MF59, and AS03.16 Other adjuvants are under investigation including immunostimulatory DNA sequences and bacterium-derived components.14
A key characteristic of influenza virus is continuous antigenic drift which requires that the vaccine be reformulated each year because of ongoing viral evolution through antigenic drift (Figure 2). This need for annual reformulation is unique to the influenza vaccines.17
Figure 2.
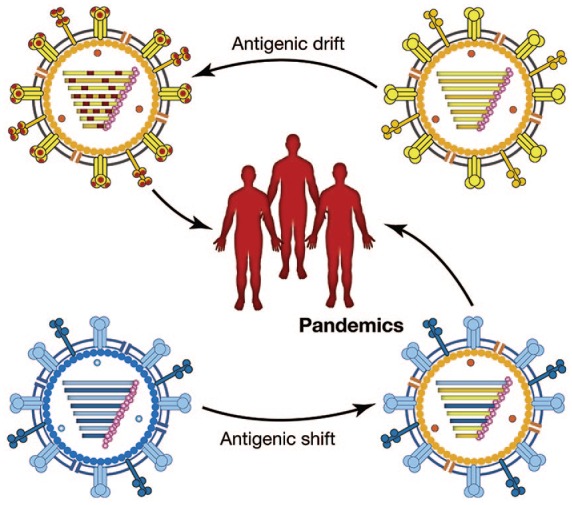
Antigenic drift and antigenic shift in influenza vaccines.
Antigenic drift occurs in all influenza types (A, B, and C) and is caused by small mutations in the antibody binding sites of hemagglutinin, neuraminidase, or both. Antigenic shift occurs only in influenza type A and is caused by exchanges of whole gene segments (often from birds or pigs) in hemagglutinin or neuraminidase that leads to the development of a new subtype of the virus. Antigenic shift is associated with pandemics.2,17,18
The candidate vaccine viruses (CVVs) included each year in the vaccines are chosen by the WHO based on information gathered by the WHO Global Influenza Surveillance and Response System (GISRS).18 This system works with 141 National Influenza Centers in 111 countries who supply specimens to WHO Collaborating Centers to identify the next year’s influenza virus. A network of associated laboratories tests approximately 1 million samples annually and provide thousands of samples of influenza viruses to WHO for further evaluation. Twice each year, vaccine consultation meetings are held to recommend vaccine strains; for the Northern hemisphere in February and for the Southern hemisphere in August.18,19 Production of the vaccines in such large volumes and the final release assay for quality assurance requires approximately 6 months, therefore vaccine composition choice must be made expeditiously each year.18
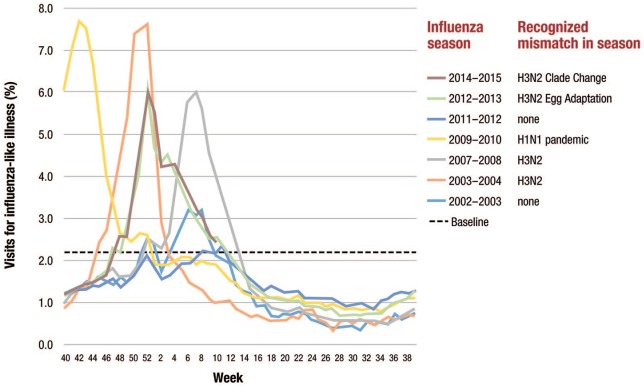
Influenza vaccines have a good safety record, but their effectiveness varies widely. One important issue is the potential for mismatch between the vaccine-selected strain and circulating strain, which is not uncommon. Between 2002 and 2015, five virus mismatches to the influenza vaccine had occurred.20 The potential effect of these mismatches can be seen in the increase in outpatient visits seen in those years (Figure 3).
Figure 3.
Visits for influenza-like illness during selected influenza seasons.
Influenza manufacturing processes
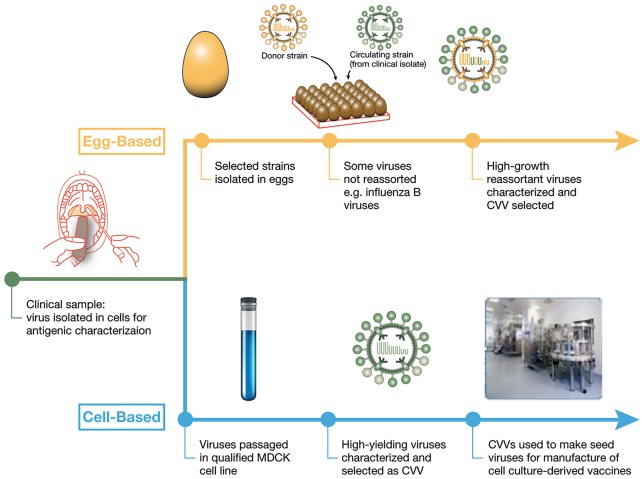
The seed virus isolation and manufacturing process for influenza vaccines can also directly affect the match of the vaccine virus to circulating strains and thereby the effectiveness of influenza vaccines (Figure 4).
Figure 4.
Traditional egg-based and emerging cell-based manufacturing processes for influenza vaccines compared.
CVV, candidate vaccine viruses; MDCK, Madin–Darby Canine Kidney.
For more than 70 years, influenza vaccines have been manufactured through an egg-dependent process.4 Viruses to be used in manufacturing are either obtained directly from clinical samples by the WHO GISRS and passaged directly in eggs to become the CVV or, if the virus does not grow well in eggs, they will undergo a process called reassorting in order to obtain a better growing virus. Reassorting is performed by co-infecting eggs with the chosen potential CVV and with a ‘donor’ strain, which is a well-characterized virus that grows well in eggs. As the viruses grow in the eggs, they may swap gene segments resulting in a virus that still contains the important antigenic elements of the proposed CVV but now grows better in eggs because the virus contains genes from the donor virus. On a larger scale, this new reassorted virus is chosen to be the CVV and many embryonated eggs are infected with the virus. After an incubation period, the allantoic fluid is harvested from the eggs, the virus is killed (or inactivated), and the key viral antigens are purified for use in influenza vaccine.21,22
The traditional egg-based process is a time-tested process but can be associated with some challenges. For inactivated vaccines, each dose of vaccine requires three to four eggs depending on the whether it is TIV or quadrivalent influenza vaccine (QIV), which means the coordination of the production of more than 100 million embryonated chicken eggs in flocks that must be pathogen free (clean). Ensuring clean flocks can be challenging as can maintaining sterility throughout the manufacturing process. Lapses in hygiene can lead to the need to reject large amounts of vaccine.23 In addition, embryonated hens’ eggs are not the ideal substrate for all virus strains; the H3N2 strains do not grow well.23
Importantly, the manufacturing process for inactivated influenza vaccines requires that the influenza virus infects the cells used in the process (e.g. avian cells for eggs). Seasonal viruses grow in humans and thus naturally grow in some mammalian cells. The influenza virus needs to bind to a cellular receptor in order to infect a cell. Avian cells have different receptors than are on the surface of mammalian cells. This means that for a human influenza virus to grow well in avian cells it needs to adapt to bind the avian receptor in a process known as egg adaptation. Unfortunately, the region in which the adaption occurs on the influenza virus is the same region that is dominant antigenically. This means that as the virus adapts to grow in eggs, it has the potential to differ antigenically to circulating viruses. This difference may drive egg-based vaccines to potentially be less effective at preventing influenza infection than their nonegg adapted mammalian cell grown counterparts.24
In 2012 a manufacturing process was approved by the United States Food and Drug Administration (FDA) that uses cell-culture technology for influenza vaccine production. In 2016 this process also extended to include both isolation and bulk production, which was also approved by the FDA for the development of WHO-isolated CVVs (versus egg-based).4 Starting in the 2017–2018 season, WHO has provided strain recommendations for seasonal influenza vaccines for both egg-derived and cell-derived CVVs.25
The cell-culture technology process isolates and grows viruses in Madin–Darby Canine Kidney (MDCK) cells rather than fertilized eggs. The production of cell-based influenza vaccines and the cell-grown CVVs allows for the elimination of the potential for egg-adapted changes and grows viruses that are closer to the circulating strain.25,26 In addition, cell-based production is not dependent on a supply of eggs; instead MDCK cells can be frozen in large quantities if needed for a pandemic.25 Cell-based and recombinant influenza vaccines are available for use in the United States and, recently, a cell-based QIV was also licensed in the EU. Efforts are underway for these vaccines to be made available in more geographies.27,28
Cell-derived viruses have been shown to better match circulating viruses than egg-derived viruses.29–31 The WHO Collaborating Centre data for influenza have been analyzed to determine the match of circulating viruses between the 2003 and the 2017–2018 season to selected MDCK-derived and egg-derived viruses, some of which were chosen for the vaccine in the respective season. A retrospective analysis showed that a substantially higher proportion of the MDCK propagated viruses matched the circulating influenza A/H3N2 viruses than the egg-propagated viruses.32
Other novel manufacturing processes are being developed to produce effective vaccines more quickly. In one process, recombinant technology is used to isolate the HA gene from a “wild type” influenza virus and then insert the HA gene in a baculovirus based expression system. This recombinant baculovirus infects insect cells and expresses both baculovirus proteins and the HA protein encoded by the inserted genes. The expressed influenza HA protein is harvested and purified.33 The resulting HA component is genetically identical to the HA of the selected strain. This process can produce some vaccine quantities within 6–8 weeks.33
Another type of manufacturing process is in development that uses plants to produce vaccines. This process, like the insect cell process above, also uses a virus that encodes for the DNA sequence of a chosen HA. The virus infects the host plant and again both the noninfluenza viral proteins and the selected HA protein are expressed.34 Very similar to the previous process, the HA is purified from the host cells and this purified HA acts as the relevant influenza component of the vaccine.35 The production process can produce a vaccine in 5–6 weeks.34,36
Historical review of the effectiveness of egg-derived vaccines
Although influenza vaccines are widely recommended as influenza prophylaxis, actual effectiveness can be suboptimal. There are several reasons for this: natural drift, population level immunogenicity, study methodology, seasonal variation, and strain selection issues (including egg adaptation). A meta-analysis of 10 randomized control trials found that the pooled effectiveness for conventional egg-based trivalent inactivated vaccines against all influenza strains over 12 seasons (between 1967 and 2011) was 59% [95% confidence interval (CI): 51–67] in adults aged 18–65 years.37
A more recent meta-analysis of test-negative design studies of egg-based influenza vaccine effectiveness included 56 studies from 2004 to 2015. Test-negative studies enroll patients who seek medical care for acute respiratory illness and whose clinical samples are then tested for influenza with RT-PCR. Those who test negative are used as controls in the analysis.38 The meta-analysis showed that the vaccine effectiveness for H1N1 was 67% (95% CI: 29–85), for H1N1pdm09 was 61% (95% CI: 57–65) and for type B was 54% (95% CI: 46–61). For H3N2, the overall pooled effectiveness was much lower at 33% (95% CI: 26–39)38 with even lower effectiveness from seasons with mismatch (23%). This lower effectiveness with H3N2 vaccine effectiveness was seen across all age groups; pediatric (43%), working-age adults (35%), and older adults (24%).38
Comparative effectiveness of egg-derived and cell derived influenza vaccines from the 2017–2018 season
Cell-based inactivated influenza vaccines have been shown to be modestly more effective than egg-based inactivated influenza vaccines. A 2017–2018 real-world observational study evaluated the relative effectiveness of inactivated influenza vaccines prepared in embryonated chicken eggs compared with those prepared in mammalian cells.39 The study included more than 13 million Medicare beneficiaries age 65 years or older—nearly all the vaccine recipients in the United States in this age group—who had received inactivated influenza vaccines in a cell-based quadrivalent form (n = 659,249) or four types of egg-based vaccines; quadrivalent (n = 1,863,654), high-dose trivalent (n = 8,489,159), adjuvanted trivalent (n = 1,473,536), or standard dose trivalent (n = 1,018,494). The primary data source was Medicare administrative files with patient details on enrollment, inpatient and outpatient care, physician office visits, and prescription drugs from 6 August 2017 to 4 August 2018. The primary outcome was influenza-related hospital encounters (i.e. inpatient hospitalizations and emergency department visits as defined by the International Classification of Disease, Tenth Revision, Clinical Modification codes for influenza). Other outcomes included only inpatient stays, influenza-related office visits, and hospital outpatient visits. The results were adjusted for imbalances between covariates using inverse probability of treatment weighting (IPTW). A Poisson regression was used to evaluate the prevention of influenza-related hospital encounters.
During this H3N2-dominated influenza season, the IPTW-adjusted results show that for influenza-related hospital encounters cell-based quadrivalent vaccine was modestly but significantly more effective than egg-based quadrivalent vaccine [relative vaccine efficacy (RVE) 11.0%, 95% CI: 7.9–14.0, p ⩽ 0.05], egg-based standard-dose trivalent (RVE 10.8, 95% CI 7.4–14.1, p ⩽ 0.05), and egg-based adjuvanted trivalent (RVE 7.5, 95% CI 4.1–10.7, p ⩽ 0.05), but not egg-based high-dose trivalent (RVE 2.3 95% CI: −0.8, to 5.3, p ⩽ 0.05). For influenza-related office visits only, the cell-cultured quadrivalent vaccine was significantly more effective than the egg-based quadrivalent vaccine (RVE 5.7%, 95% CI, 1.9–9.4, p ⩽ 0.05), the egg-based adjuvanted trivalent (RVE 11.5%, 95% CI, 7.9–15.0, p ⩽ 0.05), and the egg-based high-dose trivalent (RVE 5.1, 95% CI 1.6–8.4, p ⩽ 0.05), but not compared with the egg-based standard-dose trivalent (RVE 1.0%, 95% −3.5 to 5.3).39
In the 2017–2018 influenza season, half of US service members who were immunized received cell-based vaccines. A study analyzed the data from the Defense Medical Surveillance System who had received either a cell-based influenza vaccine or an egg-based influenza vaccine between 1 August 2017 and 28 April 2018. The analysis used two study designs. For the case test-negative design, which included laboratory-confirmed influenza cases, the adjusted RVE of cell-based influenza vaccines (n = 2467) compared with egg-based influenza vaccines (n = 3239) was 5% (95% CI: –10, 17). The second study design was based on cohorts as defined by outcomes for influenza-like illness (ILI) or diagnosed influenza. For all cohorts, 50% received the cell-based influenza vaccine. Compared with egg-based influenza vaccines, the adjusted RVE of cell-based influenza vaccines for ILI medical encounters was 2% (95% CI: 0, 4), for ILI hospitalization 16% (95% CI: –9, 35), for influenza-specific medical encounter 16% (95% CI: 11, 20), and for influenza hospitalization 46% (95% CI: –18, 76). These results showed that the RVE of cell-based influenza vaccines was similar to or greater than that of egg-based influenza vaccines but only statistically significant for ILI and influenza-specific medical encounters.40 Other outcomes are not statistically significant and therefore should be interpreted with caution.
A Kaiser Permanente Northern California study included all members (n = 3,015,891) from the ages of 4 years of age to 64 years of age over the 2017–2018 influenza season.31 The study evaluated persons who received an inactivated influenza vaccine that was either cell-based quadrivalent (n = 84,440) or egg-based (n = 932,874), which was mostly trivalent (86.5%). Influenza cases were confirmed with polymerase chain reaction. The vaccine effectiveness against influenza A compared with unvaccinated persons was 30.2% (95% CI: 17.1, 41.3, p < 0.0001) for the cell-based vaccine and 17.9% (95% CI: 12.1, 23.3) for the egg-based vaccine. When compared with each other the adjusted RVE of the cell-based versus the egg-based vaccine was 6.8% (95% CI: 17.1, 41.3). When compared with unvaccinated subjects, the adjusted absolute vaccine effectiveness (VE) for cell-based vaccines was 30.2% (95% CI: 17.1, 41.3; p < 0.0001) and for egg-based vaccines 17.9% (95% CI: 12.1, 23.3; p < 0.0001).31 Similar to the previous study the outcomes are not statistically significant and the authors concluded that increased power may have allowed for a definitive assessment on whether there was indeed a modest advantage with cell based vaccines over egg-based vaccines.
A large United States electronic medical record dataset of the 2017–2018 influenza season was retrospectively evaluated to investigate the effectiveness of cell-based vaccines compared with egg-based vaccines for persons presenting to primary care practices.41 Patients who were 4 years of age to 65 years of age and older were enrolled from 1 August 2017, to 31 March 2018, and received quadrivalent influenza vaccines that were either cell-based (n = 92,192) or egg-based (n = 1,255,983). The overall estimate of RVE as defined by the prevention of ILIs was significantly higher for the cell-based quadrivalent inactivated influenza vaccine compared with the egg-based vaccine 36.2% (95% CI: 26.1, 44.9, p < 0.001).41
A complicating factor for these studies is that cell-based manufacturing systems still use both egg-based CVVs as well as cell-based CVVs; the 2017–2018 cell-based vaccine included three cell-based CVVs and one egg-based CVV (H1N1). As a result, the egg-CVV virus may retain egg-characteristics and the improvements shown in the cell-based vaccine were most likely due to the cell-based components, particularly for H3N2. All four components for the 2019–2020 influenza vaccine are cell-based CVV for the first time and this offers an opportunity to monitor the outcomes for all these strains in the future.42
Conclusions
Seasonal influenza outbreaks and epidemics are a regular part of our modern world. Influenza vaccines are important in minimizing the effects of what can be a devastating disease. Although vaccines have been available for many years, the traditional manufacturing process, which relies on fertilized hens’ eggs is logistically complicated and egg-derived manufacturing may encounter extended delays due to the need for a continuous and large supply of eggs. In addition, egg-based vaccines are often affected by egg adaptation (particularly for H3N2) in seed strains, which may lead to a decrease in vaccine effectiveness.
The new process of cell-based isolation and manufacturing offers some advantages including an improved production process that may allow for faster development and a minimization of egg-adaptation. Studies indicate that viruses produced in a cell-based process more closely match the circulating virus than those produced in an egg-based process, and observational data from the 2017 to 2018 season provides some supportive evidence of potential improved effectiveness. As a result, both cell-based and other nonegg technologies used for the production of influenza vaccines will likely further reduce the burden of disease as investigators continue to search for the holy grail of a universal influenza vaccine.
Acknowledgments
The authors wish to thank C. Gordon Beck (Princeton Biomedical Communications, LLC, Princeton, NJ), for editorial assistance in the preparation of the manuscript.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Seqirus, Inc.
Conflict of interest statement: SR, CB, DKG, and AG are employees of Seqirus, Inc.
ORCID iD: Ashesh Gandhi  https://orcid.org/0000-0002-3829-3940
https://orcid.org/0000-0002-3829-3940
Contributor Information
Sankarasubramanian Rajaram, Seqirus, Quebec, Canada.
Constantina Boikos, Seqirus, Quebec, Canada.
Daniele K. Gelone, Seqirus Inc, Cambridge, MA, USA
Ashesh Gandhi, Medical Affairs, Americas, Seqirus Inc., Cambridge MA, USA.
References
- 1. World Health Organization. Influenza (seasonal), http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (2018, accessed 19 October 2018).
- 2. Wright PF. Influenza. In: Jameson JL, Fauci AS, Kasper DL, et al. (eds) Harrison’s principles of internal medicine. 20th ed. New York, NY: McGraw-Hill Education, 2018. [Google Scholar]
- 3. Settembre EC, Dormitzer PR, Rappuoli R. Bringing influenza vaccines into the 21st century. Hum Vaccin Immunother 2013; 10: 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouvier NM. The future of influenza vaccines: a historical and clinical perspective. Vaccines (Basel). 2018; 6: pii: E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza — United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59: 1057–1062. [PubMed] [Google Scholar]
- 6. Pebody R, Warburton F, Andrews N, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill 2015; 20: pii: 30013. [DOI] [PubMed] [Google Scholar]
- 7. Sullivan SG, Chilver MB, Carville KS, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro Surveill 2017; 22: pii: 17-00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pebody RG, Green HK, Warburton F, et al. Significant spike in excess mortality in England in winter 2014/15 – influenza the likely culprit. Epidemiol Infect 2018; 146: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Australian Government Department of Health. 2017. Influenza Season in Australia: a summary from the National Influenza Surveillance Committee. Canberra: Department of Health, 2017. [Google Scholar]
- 10. Centers for Disease Control and Prevention. CDC reported flu deaths in children exceeds seasonal high, https://www.cdc.gov/flu/spotlights/reported-flu-children-deaths.htm. 2018. (accessed 14 September 2018).
- 11. Centers for Disease Control and Prevention. People at high risk of developing serious flu–related complications, https://www.cdc.gov/flu/about/disease/high_risk.htm. (2018, accessed 14 September 2018).
- 12. Monto AS. Influenza: quantifying morbidity and mortality. Am J Med 1987; 82: 20–25. [DOI] [PubMed] [Google Scholar]
- 13. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm Rep 2018; 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev 2013; 26: 476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Flu vaccine with adjuvant: flu vaccine with adjuvant, brand name FLUAD, https://www.cdc.gov/flu/protect/vaccine/adjuvant.htm. (2018, accessed 12 December 2018).
- 16. Weir JP, Gruber MF. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses 2016; 10: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Treanor J. Influenza vaccine–outmaneuvering antigenic shift and drift. N Engl J Med 2004; 350: 218–220. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Types of seasonal influenza vaccine, http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/types-of-seasonal-influenza-vaccine. (2018, accessed 14 September 2018).
- 19. Ziegler T, Mamahit A, Cox NJ. 65 years of influenza surveillance by a world health organization-coordinated global network. Influenza Other Respir Viruses 2018; 12: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berlanda Scorza F, Tsvetnitsky V, Donnelly JJ. Universal influenza vaccines: shifting to better vaccines. Vaccine 2016; 34: 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. How influenza (flu) vaccines are made, https://www.cdc.gov/flu/protect/vaccine/how-fluvaccine-made.htm. (2018, accessed 12 September 2018).
- 22. National Institute for Allergies and Infectious Disease. Diagram of genetic reassortment in the production of vaccine, https://www.flickr.com/photos/niaid/5102830876. (2010, accessed 12 December 2018).
- 23. Hegde NR. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum Vaccin Immunother 2015; 11: 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13: e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Cell-based flu vaccines, https://www.cdc.gov/flu/protect/vaccine/cell-based.htm (2018, accessed 14 September 2018).
- 26. Rajaram S, Van Boxmeer J, Leav B, et al. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared to cell-based isolation and the possible implications for vaccine effectiveness. Presented at the IDWeek, 3–7 October 2018, San Francisco, CA. [Google Scholar]
- 27. Medicago. Medicago announces phase 3 study of VLP quadrivalent influenza vaccine, https://media.medicago.com/webfolder_download/951cb9f9114b2ed4e132b1c563ba870f/medicago-announces-phase-3-study-of-vlp-quadrivalent-influenza-vaccine/6936dd00ddfd77a354f293f8144269ffb22c029c/medicago-announces-phase-3-study-of-vlp-quadrivalent-influenza-vaccine.pdf (2017). (accessed 14 September 2018).
- 28. Novavax. Novavax NanoFlu™ vaccine demonstrates improved immune responses compared to egg-based, high-dose flu vaccine https://ir.novavax.com/news-releases/news-release-details/novavax-nanoflutm-vaccine-demonstrates-improved-immune-responses (2018). (accessed 14 September 2018).
- 29. Lu Y. Relative effectiveness of cell-cultured versus egg-based influenza vaccines, 2017-18. Presented at the Advisory Committee on Immunization Practices (ACIP) Meeting, 20 June 2018, Atlanta, GA. [Google Scholar]
- 30. Boikos C, Sylvester G, Sampalis J, et al. Effectiveness of the cell culture- and egg-derived, seasonal influenza vaccine during the 2017-2018 Northern Hemisphere Influenza Season. Presented at the Canadian Immunization Conference, 4–6 December 2018, Ottawa, Canada. [Google Scholar]
- 31. Klein N, Fireman B, Goddard K, et al. Vaccine effectiveness of flucelvax relative to inactivated influenza vaccine during the 2017-18 influenza season in Northern California. Presented at the IDWeek, 3–7 October 2018, San Francisco, CA. [Google Scholar]
- 32. Rajaram S. Cell-dervived viruses. Presented at the IDWeek, 3–7 October 2018, San Francisco, CA. [Google Scholar]
- 33. Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376: 2427–2436. [DOI] [PubMed] [Google Scholar]
- 34. Koberstein W. Innovative vaccine production using tobacco plants, https://www.bioprocessonline.com/doc/medicago-s-innovative-vaccine-production-using-tobacco-plants-0001 (2014). (accessed 14 September 2018).
- 35. Roldao A, Mellado MC, Castilho LR, et al. Virus-like particles in vaccine development. Expert Rev Vaccines 2010; 9: 1149–1176. [DOI] [PubMed] [Google Scholar]
- 36. Cone A. Tobacco being tested for flu vaccine production, https://www.upi.com/Tobacco-being-tested-for-flu-vaccine-production/8621521748240/ (2018, accessed 1 February 2019).
- 37. Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 36–44. [DOI] [PubMed] [Google Scholar]
- 38. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16: 942–951. [DOI] [PubMed] [Google Scholar]
- 39. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017-18. J Infect Dis 2018; 220: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 40. Cost E, Hu Z. Relative effectiveness of cell-based influenza vaccines compared with egg-based influenza vaccines, active component U.S. Service Members, 2017–18 Season [Poster LBB-22]. Presented at the International Conference on Emerging Infectious Diseases, 26–29 August 2018, Atlanta, GA. [Google Scholar]
- 41. Boikos C, Sylvester G, Sampalis J, et al. Effectiveness of the cell culture- and egg-derived, seasonal influenza vaccine during the 2017-2018 Northern Hemisphere influenza season. NFID Clinical Vaccinology Course, 9–10 November 2018, Bethesda, MD. [Google Scholar]
- 42. Kokosky G. Manufacturer to produce cell-based flu vaccine for next season, https://www.pharmacytimes.com/resource-centers/flu/vaccine-manufacturer-to-produce-cellbased-flu-shots-for-next-season (2019, accessed 25 July 2019).