Abstract
Introduction:
Low plasma renin activity hypertension is prevalent in Afro-Caribbean persons. Reduced angiotensin converting enzyme 2 activity from the counter angiotensin converting enzyme 2 /angiotensin-(1-7)/Mas receptor axis of the renin angiotensin aldosterone system has been reported in people with pre-hypertension, type 2 diabetes mellitus and chronic renal disease. This study investigates whether an imbalance in the regulatory mechanisms between the pressor arm of the renin angiotensin aldosterone system (angiotensin converting enzyme/angiotensin II/AT1 receptor) and the depressor axis (angiotensin converting enzyme 2/angiotensin-(1-7)/Mas receptor) predisposes persons of African descent to hypertension.
Methods:
In total, 30 normotensives and 30 recently diagnosed hypertensives aged 18–55 of Afro-Caribbean origin who are naïve to antihypertensive treatment will be recruited from public sector polyclinics in Barbados. Demographic and anthropometric data, clinical blood pressure readings, 24-hour urine collections and venous blood samples will be collected. Biological samples will be analysed for renin angiotensin aldosterone system peptide markers using radioimmunoassay.
Conclusion:
We describe the design, methods and rationale for the characterization of renin angiotensin aldosterone system mechanisms that may contribute to hypertension predisposition in persons of African descent. Our findings will characterize any imbalance in the counter axes of the renin angiotensin aldosterone system in hypertensive Afro-Caribbeans with a potential view of identifying novel approaches with the use of renin angiotensin aldosterone system and mineralocorticoid blockers to manage the condition.
Keywords: Renin angiotensin aldosterone system, angiotensin II, blood pressure, Afro-Caribbean, hypertension, angiotensin-(1–7)
Introduction
Hypertension is a major risk factor for cardio-renal diseases and is highly prevalent in persons of African descent compared with other ethnic groups.1,2 Studies have reported that Afro-Caribbean populations in the UK have a higher incidence of end-stage renal failure and strokes compared to their European counterparts.3 Although there has been an increase in knowledge of blood pressure (BP) homeostasis, there is still a paucity of mechanistic studies to explain the increased susceptibility of populations of African descent to the development of essential hypertension.4 Low-renin hypertension has been reported to be the predominant form of the disease in African Americans5–7 comprising two main phenotypes, a primary aldosteronism (PA)/inappropriate aldosterone secretion phenotype characterized by low-renin and high aldosterone, and a Liddle phenotype characterized by low-renin and low aldosterone as a result of true Liddle syndrome, where the renal tubular epithelial sodium channel (ENaC) is overactive.8 A number of genetic variants contribute to the PA phenotype including CYP11B2 (encoding aldosterone synthase), KCNJ5, ATP1A1, ATP2B3, CACNAD1D, ARMC5 and possibly others.9,10 In contrast, the genetic variants contributing to the Liddle phenotype include SCNN1B, GRK4, NEDD4L, CYP4A11, NPPA, UMOD and possibly others.10 These genetic variants are common in African persons with uncontrolled hypertension.11
The renin angiotensin (Ang) aldosterone system (RAAS) regulates sodium (Na+), potassium (K) and volume balance, which directly influences vascular tone and sympathetic nervous system (SNS) activity, making these two systems key modulators of BP homeostasis.12 We have previously shown the mechanisms by which RAAS contributes to cardiac and vascular disease.13–19 The association of the biologically active axis composed of Ang converting enzyme 2 (ACE2), the ligand Ang-(1-7) and its Mas receptor, namely the ACE2/Ang-(1-7)/Mas receptor axis, in hypertensive vascular disease was first demonstrated in studies in which urinary Ang-(1-7) excretion rates were markedly reduced in untreated hypertensive patients16 and restored by 6 months of effective antihypertensive therapy with oral captopril.20 Figure 1 outlines the mechanisms of ACE and ACE2 from the counter axes and their roles in the formation of Ang II and Ang-(1-7). Augmented ACE 2 and neprilysin enzymatic activities are suggested to promote the protective axis of the RAAS21 whereas ACE activity leading to the formation of Ang II promotes the pro-inflammatory and hypertensive axis of the RAAS. Studies showed reduced ACE2 activity in pre-hypertensive subjects as well as reduced ACE2 activity in diabetic and renal disease patients.12,22,23 In contrast, the influence of ethnic characteristics in the predisposition of African descent hypertensive individuals remains incompletely characterized. Although there has been a clear demonstration of the importance of RAAS blockade in managing hypertension,24 persons of African descent have not benefited significantly from RAAS blockade monotherapy.5 Recent genetic mutations in aldosterone synthetase have been linked to higher levels of aldosterone synthesis and may protect persons of African descent from sodium depletion especially in hot and arid environments11,25 but may be related to a higher BP when exposed to a higher salt intake. This may be a contributing mechanism towards elevated BP in this population and supports the Grim and Wilson Hypothesis.26,27 Most treatment guidelines suggest other classes of drugs such as thiazides, thiazide-like diuretics and calcium channel blockers as first line monotherapy for persons of African ancestry.5 In contrast, the thorough landmark therapeutic guidelines put forth by the International Society on Hypertension in Blacks5 stressed the value of ACE inhibitors and Ang receptor blockers (ARBs) as important cardio-renal protective agents in the African American population.5 More so, physiologically individualized therapy based on renin/aldosterone phenotyping significantly improves BP control.8 Patients with a PA phenotype tend to respond best to aldosterone antagonists, whereas patients with a Liddle phenotype respond best to amiloride.8
Figure 1.
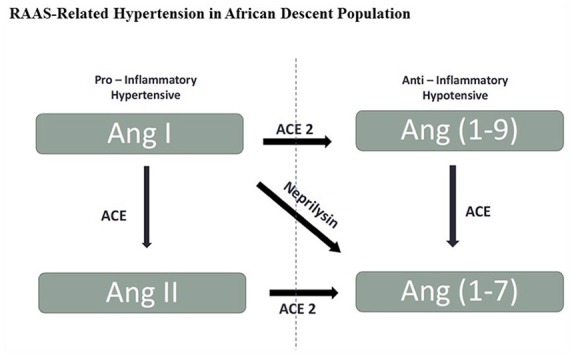
Potential mechanisms of angiotensin converting enzyme (ACE) and ACE2 in the formation of angiotensin (Ang) II and Ang-(1-7).
Baseline plasma renin activity in persons of African descent may not reflect a shift in the balance between the pressor and depressor arms of the RAAS, as a deficit in the activity of the ACE2/Ang-(1-7)/Mas axis allows even normal levels of tissue Ang II to exert vasoconstrictor and hypertrophic long-lasting responses. We hypothesize that a shift in the balance between Ang II and Ang-(1-7) and their respective pressor and depressor axes components may be previously unrecognized markers of hypertension development. This concept is supported by low Ang-(1-7) expression observed in untreated essential hypertensive non-black subjects16 and the increased Ang-(1-7) levels found in females of Afro-Caribbean descent in a genetically unmixed normotensive population of Afro-Caribbean in Barbados (Table 1).4,28
Table 1.
Summary of RAAS peptide analysis in the normotensive Afro-Caribbean population.
| Variable | All subjects | Females | Males | Sex differences p value |
|---|---|---|---|---|
| Plasma renin activity, ng/mL/h | 0.66 ± 0.53 | 0.67 ± 0.62 | 0.65 ± 0.43 | 0.874 |
| Plasma angiotensin II, pg/mL | 116.16 ± 148.33 | 146.20 ± 203.32 | 86.11 ± 43.85 | 0.204 |
| Plasma angiotensin-(1-7), pg/mL | 68.77 ± 55.40 | 75.60 ± 62.43 | 61.94 ± 47.91 | 0.431 |
| Urinary angiotensin II, pg/mL | 80.45 ± 62.28 | 79.77 ± 58.95 | 81.16 ± 66.77 | 0.938 |
| Urinary angiotensin-(1-7), pg/mL | 163.38 ± 50.58 | 179.82 ± 45.28 | 146.29 ± 50.98 | 0.016 |
| Urinary creatinine, mg/dL | 288.02 ± 72.26 | 312.14 ± 61.72 | 262.94 ± 74.99 | 0.013 |
| Urinary sodium, nmol/L | 174.08 ± 30.76 | 171.46 ± 21.44 | 176.80 ± 38.43 | 0.541 |
| Urinary potassium, nmol/L | 65.61 ± 25.06 | 63.00 ± 8.82 | 68.32 ± 34.82 | 0.454 |
RAAS: renin angiotensin aldosterone system.
Although sexual dimorphism in RAAS peptides has been found in Afro-Caribbean normotensives,4 the activities of the counter axes of RAAS in Afro-Caribbean normotensive and naïve hypertensive Afro-Caribbean persons remain unknown. We intend to resolve this lack of knowledge through the following: (a) identifying a representative sample of normotensive and hypertensive Afro-Caribbean participants over a 2-year period in Barbados; (b) collecting demographic data, biological samples and other relevant study data from the participants; (c) analysing biological samples by radioimmunoassay (RIA) to assess the expression of peptides from both pressor and depressor axis of the RAAS; and (d) making inferences from the analysed data on the relative balance shift of the peptides from both axes of the RAAS and disseminating findings to community interest groups.
Our previous study remains the only attempt to identify RAAS sex differences in the expression of RAAS peptides in an Afro-Caribbean population.4 The analyses in that study were carried out at Wake Forest University and the analysis of the RAAS peptides from the current study will be done in the same laboratory. Past studies from Dr. Ferrario have demonstrated: (a) reduced urinary Ang-(1-7) expression and content in untreated essential hypertensive subjects;16 (b) chronic normalization of arterial pressure in essential hypertensive subjects treated with either captopril20 or the dual inhibitor of ACE and neprilysin (Omapatrilat)29 was also associated with increases in Ang-(1-7); (c) prehypertension, chronic kidney disease and diabetes are associated with reduced ACE2 activity; and (d) increased circulating Ang II and serum aldosterone in untreated hypertensive women.30 These prior findings justify the clinical relevance of the proposed study.
Here we describe the protocol we will use to determine whether a relative over expression of the pressor arm of the RAAS (ACE/Ang II/AT1 receptor) over the depressor axis (ACE2/Ang-(1-7)/Mas receptor) may be one of the contributing factors that predispose persons of African descent to hypertension and its sequelae.
Methods
Setting
Barbados, an island in the Eastern Caribbean, has a population of approximately 280,000 people of which 92% are of African origin.31 The Afro-Caribbean participants will be recruited from the following government polyclinics: Sir Winston Scott Polyclinic, Bradford Taitt Polyclinic and Edgar Cochrane Polyclinic. These three of nine polyclinics strategically located across the island provide comprehensive public sector primary care on the island. Along with the Queen Elizabeth Hospital (Bridgetown, Barbados), these polyclinics are well-resourced public care facilities that are a part of the universal healthcare system established by the 1969 Health Services Act of Barbados and the Drug Services Act in 1980.32–34
Participants
The proposed study is observational and evaluates protein markers of RAAS in Afro-Caribbean persons. It will study 30 normotensives and 30 hypertensives, as defined by the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) Guidelines for the management of arterial hypertension,35 who are naïve to antihypertensive treatment and are of Afro-Caribbean origin and within the age group of 18–55 years. Participants will be selected from three polyclinics: Winston Scott Polyclinic, Bradford Taitt Polyclinic and Edgar Cochrane Polyclinic. The age group of 18–55 years for participants is stipulated as having the highest incidence of hypertension in the Afro-Caribbean population.36 The chosen polyclinics are affiliated with the Faculty of Medical Sciences’ Family Medicine programme at the University of the West Indies, Cave Hill Campus, Barbados.
Inclusion/exclusion criteria for patient recruitment
Participants (aged 18–55 years) who are normotensive or being evaluated for primary hypertension will be included in the study. Participants with at least one of the following criteria will be excluded from the study: high alcohol consumption as defined by the National Health Service in the UK (>14 units for females and males per week; 1 unit = 8 g alcohol) and recreational drug use; smokers as defined by the World Health Organization37 > 1 year; diabetes (diagnosed at HBA1c of 48 mmol/mol (6.5%) or more); diagnosed with hypertension (self-reported history of diagnosis or by a chart record) at ⩾ 140/90 mmHg) and on antihypertensive medication; diagnosed with any condition that can contribute to secondary hypertension; persons with secondary forms of high BP; clinically evident vascular disease inclusive of atherosclerosis, peripheral artery disease, aneurysm, peripheral venous disease and vein thromboembolisms, or a body mass index (BMI) <18 Kg/m2 or >36 Kg/m2; pregnant women or those declaring to be seeking to become pregnant.
Ethical considerations
The study was approved by the University of the West Indies, Ministry of Health Institutional Review Board and the Ministry of Health, Barbados. Written informed consent will be obtained from all participants.
Sample size calculation
The sample of participants was determined using data gathered in a previous study in which a significant difference in Ang-(1-7) was found between normotensive male and female counterparts of an Afro-Caribbean population4 (Table 1). A statistically significant difference in the levels of an Ang peptide level was found in normotensive participants so it is anticipated that a greater difference may be found between normotensive and hypertensive Afro-Caribbean persons visiting the polyclinics. Therefore, the sample size at 95% confidence level and 80% power with standardised effect size of 0.663 will be 26–36 participants per group.
Recruitment and enrolment
Regular communication between the principal investigators (PIs) and staff at the three polyclinics will be maintained to monitor patient recruitment. Additionally, on a weekly basis for the first 3 to 6 months, the PIs will collect the following recruitment patient information: age, BMI, sex and BP status as per ESC/ESH Guidelines (normotensive (less than 140/90 mm Hg) and hypertensive (greater than 140/90 mm Hg)) from all three polyclinic sites. The data collected will also be communicated to all three polyclinics so that matching of age, BMI and sex between hypertensive and normotensive subjects can be accomplished. To execute this, hypertensive participants will be recruited first and the normotensive participants will be matched from pre-existing patient databases at the polyclinics.
Clinical protocol
There will be two scheduled site visits for each normotensive and hypertensive participant. Participants will be asked to complete an informed consent form and complete a pre-screening survey. The survey will remain anonymous to protect the identity of the participants and no personal identifying information will be collected. All participants and their respective samples will be coded. On the first visit, consented participants will be assessed for hypertension using an established and standardized protocol for diagnosing hypertension. Each participant will undergo a BP measurement that is standardized across the polyclinics. They will be in a seated position and the OMRON 705IT (HEM-759-E) (Omron Corporation, Kyoto, Japan) will be used to evaluate their BP. Participants will be instructed to sit in quiet room for no less than 5 minutes followed by three to five consecutive BP readings recorded at 1 minute intervals in the absence of the researcher.38 An average of the first three readings will be recorded with additional readings if any of the first three readings differ by 4 mmHg. Demographic and anthropometric data will be collected at the first visit. Participants will be provided a container with 20 mL of 6 N HCL (Sigma, St. Louis MO.) to collect a 24-hour urine sample. Participants will be given a tutorial on the safe and correct method of collecting the 24-hour urine sample as follows:
On the first morning when you awake, empty your bladder into the toilet. Please make a note on the collection container of the date and time you did this. After this, collect and store in the container all urine you pass during the day and the following night.
When you feel the need to have a bowel movement, first try to pass urine into the jug for transfer to the container (that is, try not to pass urine directly into the toilet with a bowel motion).
The following morning when you awake, at the same time as on the previous day (that is 24-hour after you started), complete the collection by emptying your bladder into the jug for transfer into the container. Make a note of the time you complete the collection and if you missed any collection. If the collection is not exactly 24 hours later do not worry.
Keep the top of the collection container closed and store the container in a dark and cool place. If you pass urine but forget to collect it, please tell us when you hand your collection in, and if possible, try to estimate how much was lost. You can do this by noting how much you collect in the jug on the next occasion. Some people find it useful to attach their pants to their outer clothes with a safety pin as a reminder to make a collection each time they go to the toilet.
During the second visit, anthropometric measurements inclusive of waist circumference, height, weight and clinical BP measurements will be noted. Average BP readings, recorded by the method described above, will be calculated and expressed in the form of mean ± standard deviation (SD). Then a 24-hour urine sample will be collected. Fasting samples of venous blood will be collected in vacutainer tubes (Becton Dickinson, Franklin, NJ) containing potassium ethylenediaminetetraacetic acid (as an anticoagulant) and a mixture of peptidase inhibitors including 0.44 mM 1,20 ortho phenanthroline monohydrate (Sigma, St. Louis MO.), 0.12 mM pepstatin (Peninsula Labs, Belmont CA) and 1 mM Na p-hydroxymercuribenzoate (Sigma, St. Louis MO).
Laboratory analysis
For RAAS peptide analysis, all volumes of urine and blood samples will be recorded and processed after collection and stored at -80oC until they are sent for analysis at the laboratories. Plasma obtained from blood samples and urine samples will be submitted to the Biomarker Analytical Core/Hypertension and Vascular Disease Center, Wake Forest University Health Sciences for analysis of the RAAS peptides by RIA. The following variables will be measured in the 24-hour urine samples: volume, creatinine, sodium, potassium, Ang II and Ang-(1-7) and from plasma samples, serum aldosterone, plasma ACE and ACE2 activities, Ang II and Ang-(1-7). In addition, plasma samples will be processed for the measurement of Ang-(1-12), a novel blood and tissue non-renin dependent Ang II-forming substrate.39,40
The RAAS peptides, Ang-(1-12), Ang II and Ang-(1-7) will be analysed in plasma and also in the urine samples with the exception of Ang-(1-12) after preparation. These samples will be extracted using SepPak columns (Waters Associates, Watford, Hertfordshire, England) and activated with 5 mL sequential washes of a mixture of ethanol: water, 4% acetic acid (83:13:4), methanol, ultrapure water and 4% acetic acid. The samples will be applied to the column, washed with ultrapure water and acetone and eluted with two 1 mL and one 1.5 mL washes of a mixture of ethanol:water:4% acetic acid. The weight of the eluate will be recorded and two 2 ml aliquots from the total eluate will be transferred into conical bottom polystyrene tubes and dried. The eluted sample will be reconstituted into a Tris buffer with 0.1% bovine serum albumin. Ang peptides will be measured using RIA. All samples will also be corrected for recoveries. The assay for measurements of the human sequence of Ang-(1-12) are documented.41
Data collection and analysis
Patient information and respective samples will be coded and only the PIs will have a master key of codes and patients’ names. Coded samples will be dispatched for analysis. No personal identifying information will be collected and cross-referenced with the names of the participants. All patient files will be stored in the polyclinic document/records department, which is secure and accessible only by the attending physician or nurse. Research data will be kept between the PIs in a secure, locked filing cabinet.
Data will be retrieved and summarized from the respective laboratories and from the clinical sites. Outliers will be removed from the analysis. Outliers are data points that would fall outside a threshold of two SD away from the mean value. Analytes that do not demonstrate a normal distribution will be transformed. Statistical analysis will be done at the 95% confidence level with statistical significance established at p ⩽ 0.05. The Student’s t-test will be used to identify statistical differences of the mean values of the peptides at the 95% confidence level. Laboratory data will be collected and summarized from the analysis of samples to help predict or detect trends, which could determine if there is a gradient effect as the disease stage develops. Peptide analysis data will be summarised as mean ± SD.
Expected outcome and impact
We describe in detail the design and methods of a novel study approach to investigate the role of the pressor and depressor axes of RAAS and, in particular, whether overexpression of the pressor arm of the RAAS (ACE/Ang II/AT1 receptor) over the depressor axis (ACE2/Ang-(1-7)/Mas receptor) may be one of the contributing factors that predisposes persons of African descent to hypertension. Moreover, characterization of the association of aldosterone with elevated BP in persons of African descent is critical to the understanding of the therapeutic benefits of RAAS blockade among other classes of hypotensive dugs in persons of African ancestry. We exclude measurements of plasma renin activity as baseline values of this variable neither define nor augment the discriminatory sensitivity of the measured biomarkers that define the activity of terminal products of the RAAS. In keeping with this conclusion, comprehensive studies by Spence and colleagues indicated that stimulated not unstimulated plasma renin activity was helpful in detecting secondary but not primary causes of hypertension.8
Findings from this study will have a major impact in understanding and characterizing the neurohormonal factors accounting for excess cardiovascular morbidity and mortality in the African diaspora. Understanding the RAAS mechanisms that lead to increased susceptibility in the development of essential hypertension among Afro-Caribbean natives is of utmost importance in discovering modes of prevention, treatment and management. It has been shown that there is reduced urinary Ang-(1-7) expression in untreated non-black essential hypertensive subjects.16 In addition, prehypertension, chronic kidney disease and diabetes are associated with reduced ACE2 activity. This study attempts to ascertain similar findings in an Afro-Caribbean population where the use of ACE inhibitors and ARBs may be associated with increases in Ang-(1-7). Such approaches have been seen as useful in the chronic normalization of arterial pressure in essential hypertensive subjects treated with either captopril20 or the dual inhibitor of ACE and neprilysin (Omapatrilat).29 The data obtained from this study may potentially affect knowledge, attitudes and practices of physicians who prescribe RAAS inhibiting drugs to their patients. Moreover, there is a strong possibility that the use of RAAS inhibitors including mineralocorticoid inhibitors or ENaC blockers to manage hypertension in persons of African descent may be considered in the future for therapy.
Possible limitations of our study include (a) the reliance of self-reported data by participants when completing the pre-screening questionnaire to determine eligibility to the study; (b) the collection of the 24-hour urine samples; if patients forget to collect all urine within the time period, final concentrations of the tested variables may be inaccurate and hence impact results; and (c) the lack of genotyping to identify genetic variants of the PA phenotype in the hypertensive participants.
Acknowledgments
We are grateful to the Yale-TCC Pilot Project Program for their support in this study. We thank Dr Saria Hassan for helpful discussions and reviewing the manuscript. We also thank Dr Clarence Grim for his extensive reviews and suggestions.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by a grant from the National Institute on Minority Health and Health Disparities (#1U54MD010711-01).
Compliance with Ethical Standards: Our research was approved by the University of the West Indies-Cave Hill/Barbados Ministry of Health Research Ethics Committee/Institutional Review Board and all procedures will be followed in accordance with the ethical standards of the IRB and the Helsinki Declaration of 1975, as revised in 2000. Informed consent will be obtained from patients being included in the study.
The study proposal was presented in part at the Consortium of Southeastern Hypertension Control (COSEHC) 2018 Scientific – Quality Impact Practice Transformation Network Meeting, New Orleans, Louisiana, USA (21–25 March 2018).
References
- 1. Lane D, Beevers DG, Lip GYH. Ethnic differences in blood pressure and the prevalence of hypertension in England. J Human Hypertension 2002; 16: 267–273. [DOI] [PubMed] [Google Scholar]
- 2. Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension 1996; 28: 854–858. [DOI] [PubMed] [Google Scholar]
- 3. Lane DA, Lip GYH. Ethnic differences in hypertension and blood pressure control in the UK. Qjm-Monthly J Assoc Physicians 2001; 94: 391–396. [DOI] [PubMed] [Google Scholar]
- 4. Cohall DH, Scantlebury-Manning T, James S, et al. Renin-angiotensin-aldosterone system gender differences in an Afro-Caribbean population. J Renin-Angiotensin-Aldosterone Sys 2015; 16: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans - Consensus statement of the hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Internal Med 2003; 163: 525–541. [DOI] [PubMed] [Google Scholar]
- 6. Ferrario CM, Flack JM. Pathologic consequences of increased angiotensin II activity. Cardiovasc Drug Therapy 1996; 10: 511–518. [DOI] [PubMed] [Google Scholar]
- 7. Flack JM, Mensah GA, Ferrario CM. Using angiotensin converting enzyme inhibitors in African-American hypertensives: A new approach to treating hypertension and preventing target-organ damage. Current Medical Res Opin 2000; 16: 66–79. [PubMed] [Google Scholar]
- 8. Spence JD, Rayner BL. Hypertension in blacks: Individualized therapy based on renin/aldosterone phenotyping. Hypertension 2018; 72: 263–269. [DOI] [PubMed] [Google Scholar]
- 9. Dutta RK, Soderkvist P, Gimm O. Genetics of primary hyperaldosteronism. Endocr Relat Cancer 2016; 23: R437–454. [DOI] [PubMed] [Google Scholar]
- 10. Spence JD. Hypertension in Africa. Eur J Prev Cardiol 2019; 26: 455–457. [DOI] [PubMed] [Google Scholar]
- 11. Jones ES, Spence JD, McIntyre AD, et al. High frequency of variants of candidate genes in black Africans with low renin-resistant hypertension. Am J Hypertens 2017; 30: 478–483. [DOI] [PubMed] [Google Scholar]
- 12. Duffy SJ, Biegelsen ES, Eberhardt RT, et al. Low-renin hypertension with relative aldosterone excess is associated with impaired NO-mediated vasodilation. Hypertension 2005; 46: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrario CM. Angiotensin-(1-7) and antihypertensive mechanisms. J Nephrol 1998; 11: 278–283. [PubMed] [Google Scholar]
- 14. Ferrario CM, Ahmad S, Joyner J, et al. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1-7). Adv Pharmacol 2010; 59: 197–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrario CM, Ahmad S, Nagata S, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clinical Sci 2014; 126: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrario CM, Martell N, Yunis C, et al. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertension 1998; 11: 137–146. [DOI] [PubMed] [Google Scholar]
- 17. Lieb W, Chen MH, Teumer A, et al. Genome-wide meta-analyses of plasma renin activity and concentration reveal association with the kininogen 1 and prekallikrein genes. Circ Cardiovasc Gen 2015; 8: 131–U278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santos RAS, Silva ACSE, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Nat Acad Sci USA 2003; 100: 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circulatory Physiol 2005; 289: H1560–H1566. [DOI] [PubMed] [Google Scholar]
- 20. Luque M, Martin P, Martell N, et al. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertension 1996; 14: 799–805. [DOI] [PubMed] [Google Scholar]
- 21. Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): An evolving story in cardiovascular regulation. Hypertension 2006; 47: 515–521. [DOI] [PubMed] [Google Scholar]
- 22. Keidar S, Strizevsky A, Raz A, et al. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects. Nephrol Dialysis Transplant 2007; 22: 597–601. [DOI] [PubMed] [Google Scholar]
- 23. Reich HN, Oudit GY, Penninger JM, et al. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 2008; 74: 1610–1616. [DOI] [PubMed] [Google Scholar]
- 24. Ferrario CM. Cardiac remodelling and RAS inhibition. Therapeutic Adv Cardiovasc Dis 2016; 10: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001; 104: 545–556. [DOI] [PubMed] [Google Scholar]
- 26. Brown AGM, Houser RF, Mattei J, et al. Hypertension among US-born and foreign-born non-Hispanic Blacks: National Health and Nutrition Examination Survey 2003–2014 data. J Hypertens. 2017; 35: 2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson TW, Grim CE. Biohistory of slavery and blood pressure differences in blacks today. A hypothesis. Hypertension 1991; 17: I122–128. [DOI] [PubMed] [Google Scholar]
- 28. Benn-Torres J, Bonilla C, Robbins CM, et al. Admixture and population stratification in African Caribbean populations. Ann Hum Genet 2008; 72: 90–98. [DOI] [PubMed] [Google Scholar]
- 29. Ferrario CM, Smith RD, Brosnihan B, et al. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertension 2002; 15: 557–564. [DOI] [PubMed] [Google Scholar]
- 30. Ferrario CM, Jessup JA, Smith RD. Hemodynamic and hormonal patterns of untreated essential hypertension in men and women. Ther Adv Cardiovasc Dis 2013; 7: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbados Statistical Service. Barbadian Population and Housing Census. Available at: http://www.barstats.gov.bb/files/documents/PHC_2010_Census_Volume_1.pdf (2013, accessed 28 November 2018).
- 32. Ministry of Health - Barbados. Drug Service. Available at: http://drugservice.health.gov.bb/index.php/main_pages (accessed 28 November 2018).
- 33. Hennis A, Hambleton I, Broome H, et al. Health Welfare and Aging in Barbados Sabe 2000. Washington, DC: Pan American Health Organization, 2005, pp. 9–63. [Google Scholar]
- 34. Rodney P. Caribbean State, health care and women: An analysis of Barbados and Grenada during the 1979-1983 period. Toronto: University of Toronto, Graduate Department of Education, 1998. [Google Scholar]
- 35. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 36. Unwin N, Rose AMC, George KS, et al. The Barbados Health of the Nation Survey: Core Findings 2015. St. Thomas, Barbados: Miller Publishing Company, 2015. [Google Scholar]
- 37. World Health Organization. Guidelines for controlling and monitoring the tobacco epidemic. Geneva: World Health Organization, 1998. [Google Scholar]
- 38. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 2019; 73: e35-e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrario CM, Ahmad S, Nagata S, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014; 126: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrario CM, Ahmad S, Varagic J, et al. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol 2016; 311: H404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrario CM, VonCannon J, Ahmad S, et al. Activation of the human angiotensin-(1-12)-chymase pathway in rats with human angiotensinogen gene transcripts. Front Cardiovasc Med 2019; 6: 163. doi: 10.3389/fcvm.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]


