Abstract
Background:
Increased carotid-femoral pulse wave velocity (cf-PWV), a surrogate of increased aortic stiffness, is a risk factor for cardiovascular events and all-cause mortality in end-stage renal disease (ESRD). To minimize the deleterious effects of an increased aortic stiffness in ESRD patients, several interventions have been developed and cf-PWV has been used to monitor responses.
Objective:
The aim of this study was to determine the effects of pharmacologic interventions that target aortic stiffness on cf-PWV and systolic blood pressure (SBP) in adults with ESRD.
Study design:
This study implements a systematic review and meta-analysis.
Data sources:
MEDLINE, EMBASE, Cochrane Central, Health Technology Assessment, and EBM databases were searched.
Study eligibility, participants, and interventions:
Randomized and non-randomized studies involving adults (>18 years) with ESRD of any duration, receiving or not renal replacement therapy (hemodialysis, peritoneal dialysis) and exposed to a pharmacologic intervention whose effects were assessed by cf-PWV.
Methods:
Study screening, selection, data extraction, and quality assessments were performed by 2 independent reviewers. Narrative synthesis and quantitative data analysis summarized the review.
Results:
We included 1027 ESRD participants from 13 randomized and 5 non-randomized studies. Most pharmacologic interventions targeted bone mineral metabolism disorder or hypertension. Treatment with vitamin D analogues or cinacalcet did not decrease cf-PWV or SBP over placebo or matched controls (P > .05). Calcium-channel blockers (CCB) decreased cf-PWV and SBP compared with placebo or standard care (P < .05). Renin-angiotensin system inhibitors did not show any advantage over placebo in decreasing cf-PWV (P > .05).
Limitations:
Quality of evidence ranged from very low to moderate. Overall evidence was limited by the low number of studies, small sample sizes, and methodological inconsistencies.
Conclusions:
Pharmacologic interventions targeting aortic stiffness in ESRD have mixed effects on reducing cf-PWV, with some strategies suggesting potential benefit. The quality of evidence, however, is insufficient to draw definitive conclusions on their use to slow progression of aortic stiffness in ESRD. Further well-designed studies are needed to confirm these associations and their impact on cardiovascular outcomes in ESRD.
Registered in PROSPERO (CRD42016033463)
Keywords: vascular stiffness, aortic stiffness, pulse wave velocity, end-stage renal disease, dialysis
Abrégé
Contexte:
L’accroissement de la vitesse de l’onde de pouls carotido-fémorale (VOPcf), un substitut à l’accroissement de la rigidité aortique, constitue un facteur de risque d’événements cardiovasculaires et de mortalité toutes causes confondues en contexte d’insuffisance rénale terminale (IRT). Plusieurs interventions pharmacologiques ont été développées pour minimiser les effets délétères de l’accroissement de la rigidité aortique chez les patients atteints d’IRT, et la VOPcf a été employée pour en mesurer la réponse.
Objectif:
Mesurer les effets d’interventions pharmacologiques ciblant la rigidité aortique sur la VOPcf et la pression systolique (PS) d’adultes atteints d’IRT.
Type d’étude:
Revue systématique et méta-analyse.
Sources:
Les bases de données MEDLINE, EMBASE, Cochrane Central, EMB et du Service d’évaluation des technologies de la santé ont été consultées.
Admissibilité, participants et interventions:
Ont été sélectionnées les études réparties aléatoirement ou non, peu importe leur durée, qui portaient sur des adultes atteints d’IRT, recevant ou non une thérapie de remplacement rénal (hémodialyse, dialyse péritonéale), qui avaient été exposés à une intervention pharmacologique dont les effets avaient été mesurés avec la VOPcf.
Méthodologie:
Deux réviseurs indépendants ont procédé à la recherche et à la sélection des études, à l’extraction des données et à l’évaluation de leur qualité. Une synthèse narrative et une analyse quantitative des données ont synthétisé les résultats de la revue.
Résultats:
L’étude porte sur un total de 1 027 sujets atteints d’IRT issus de 13 études à répartition aléatoire et de 5 études non réparties aléatoirement. La plupart des interventions pharmacologiques ciblaient l’hypertension ou un trouble du métabolisme de la densité osseuse. Lorsque comparés à un placebo ou à un témoin, les traitements impliquant un analogue de la vitamine D ou le cinacalcet n’ont eu aucun effet réducteur sur la VOPcf ou la PS (p>0,05). Les bloqueurs des canaux calciques ont montré un effet réducteur sur la VOPcf et la PS en comparaison du placebo ou du traitement standard (p<0,05). Les inhibiteurs du système rénine-angiotensine n’ont présenté aucun avantage pour réduire la VOPcf par rapport au placebo (p>0,05).
Limites:
La qualité des données recueillies variait de très pauvre à modérée. L’ensemble des données recueillies est limité par le faible nombre d’études, la petite taille des échantillons et par des divergences méthodologiques.
Conclusion:
Les interventions pharmacologiques ciblant la rigidité aortique en contexte d’IRT ont eu des résultats mitigés sur la réduction de la VOPcf, quoique certaines stratégies suggèrent de potentiels avantages. La qualité des données recueillies est toutefois insuffisante pour conclure de façon définitive que ces interventions ralentissent la progression de la rigidité aortique chez les patients atteints d’IRT. Des études bien conçues sont nécessaires pour confirmer ces associations et leur incidence sur les issues cardiovasculaires en contexte d’IRT.
What was known before
Increased aortic stiffness as measured by carotid-femoral pulse wave velocity (cf-PWV) is a risk factor for cardiovascular events and all-cause mortality in end-stage renal disease (ESRD). Several pharmacologic interventions have been developed to slow the progression of aortic stiffness in ESRD, and cf-PWV has been proposed to monitor responses.
What this adds
Most pharmacologic interventions that target aortic stiffness in ESRD are associated with mixed effects on reducing cf-PWV, with some strategies such as control of blood pressure with calcium-channel blockers suggesting potential benefit. Evidence, however, is insufficient to recommend their routine use for this purpose and further well-designed studies are necessary to determine the clinical relevance of these associations.
In ESRD, increased aortic stiffness is recognized as a risk factor for cardiovascular events and all-cause mortality.1,2 Increased arterial stiffness leads to an earlier return of peripherally reflected pressure waves, resulting in increased cardiac workload due to augmentation of the central pulse pressure.3,4 Although several non-invasive methods exist to evaluate aortic stiffness,5 cf-PWV has been established as an independent marker associated with non-fatal cardiovascular events and all-cause mortality in ESRD.2 As atherosclerosis, increased blood pressure, alterations in bone mineral metabolism and vascular calcification are prominent during the transition from chronic kidney disease to ESRD, these mechanisms have been associated with the accelerated progression of aortic stiffness that occurs in chronic dialysis patients.6 To minimize the deleterious effects of these risk factors, several interventions have been proposed and cf-PWV has been used to monitor responses. In a previous systematic review and meta-analysis, we reported the effects of non-pharmacologic-based interventions on reducing cf-PWV and systolic blood pressure (SBP).7 In this investigation, we systematically analyzed existing randomized and non-randomized studies that assessed pharmacologic interventions aimed at control of hypertension (renin-angiotensin system inhibitors, calcium-channel blockers), bone mineral imbalance (vitamin D analogues, calcimimetics, and phosphate binders), serum homocysteine levels, and anemia correction (recombinant human erythropoietin) and without any restriction on the type of comparator, in patients (≥18 years old) with ESRD of any duration and receiving or not any renal replacement therapy (hemodialysis and peritoneal dialysis) with the purpose of evaluating their effects on cf-PWV. Second, we evaluated the impact on SBP.
Materials and Methods
Data Sources and Search Strategy
The review was conducted in accordance with the Cochrane Collaboration methods, Systematic Reviews standards,8 and PRISMA guidelines.9 The study protocol has been published10 and registered in PROSPERO (www.crd.york.ac.uk/prospero; CRD42016033463). A comprehensive, systematic search strategy was implemented using MEDLINE, EMBASE, Cochrane Central databases, the Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment Database, CADTH’s “Grey Matters Light,” OVID, EBM reviews, and gray literature for studies published between January 1965 and May 2019. We accessed material and research produced by organizations outside of academic publishing journals including the “Grey Matters Light” of the Canadian Agency for Drugs and Technologies in Health. A detailed description of the reviewing methods including study screening, inclusion and exclusion criteria, and assessment of the quality of evidence has been published elsewhere.7 Two independent reviewers evaluated quality according to study design. The risk of bias in randomized studies was evaluated with the Cochrane Collaboration tool.8 For non-randomized studies, we used the “SIGN50” (cohort studies)11 and the NIH Quality Assessment Tool (cross-sectional studies and before-and-after single cohort designs).12 The original search strategy aimed to capture both pharmacologic and non-pharmacologic interventions (Appendix 1).
Inclusion and Exclusion Criteria
We included adults (≥18 years old) with end-stage renal disease (ESRD) defined as stage-5 chronic kidney disease (estimated glomerular filtration rate [e-GFR] < 15 mL/min/1.73 m2) of any duration and receiving or not renal replacement therapy. Studies involving pharmacologic interventions in adults with kidney transplantation as the current modality of renal replacement were excluded; in a separate study, we have reported the impact of non-pharmacologic interventions, including kidney transplantation, on cf-PWV in ESRD.7 This study included adults with ESRD participating in randomized control trials and non-randomized studies (cohort, case-control, cross-sectional, and single cohorts with before-and-after design) with at least 10 participants, involving a pharmacologic intervention whose effect was assessed by cf-PWV.
Study Screening, Review, and Abstraction
All abstracts and titles were screened by 2 independent reviewers using pre-specified criteria. Abstract selection was restricted to those published in English, French, Italian, or Spanish. Nonhuman, in vitro, modeling and pediatric studies or systematic/narrative reviews were excluded. Full-text eligible reports underwent a second screening of the “Materials and Methods” sections to confirm that adult patients with ESRD were included, that cf-PWV was incorporated, and that a pharmacologic intervention was tested. One of the reviewers screened all full-text copies while a second reviewer randomly verified 75% of all reports. Selected reports underwent full-text review by 2 reviewers for final inclusion decision using pre-specified criteria. Subsequently, eligible studies were abstracted by 2 independent reviewers using a piloted and standardized electronic form. All disagreements were resolved by consensus with a third independent reviewer. If data from selected studies were incomplete, we attempted to contact the principal study author.
Extracted Variables
Extracted data included the following: (1) study characteristics, design, and methods: title, authors, journal/source/year, language of publication, country, type of study design, study period, publication status, total number of patients, case ascertainment, inclusion/exclusion criteria, single or multicenter study, randomization, allocation, and concealment and blinding methods (where applicable); (2) sample characteristics: age, sex, type of renal replacement therapy, dialysis vintage, comorbidities, duration of follow-up, and type of arterial stiffness instrumentation; (3) interventions and co-interventions: type of pharmacologic therapy, dose, frequency and duration of treatment, type of comparator, and its dose; (4) outcomes: reduction in cf-PWV, decrease in systolic blood pressure (SBP), and side effects associated with the intervention.
Outcomes
The primary outcome was the reduction in cf-PWV associated with the pharmacologic intervention, and secondary outcomes included effects on SBP. Our decision for choosing SBP as a secondary outcome was based on the determinant nature of this parameter on aortic stiffness. We rationalized that measurements of SBP would provide information on its relationship with aortic stiffness. In addition, diastolic blood pressure is not as frequently reported as SBP in these interventional studies. Consequently, by choosing SBP, we expected to minimize the proportion of missing data.
Quality of Evidence
To assess the certainty in the evidence and strength of recommendations for all pharmacologic interventions in this review, 2 reviewers evaluated quality of evidence according to 5 domains of GRADE recommendations.13
Statistical Analyses
All studies were synthesized descriptively, and the outcomes were reported as mean differences and their 95% confidence intervals (CIs). For each study arm, we calculated the mean difference (95% CI) between the pre-treatment baseline and the end of treatment. Eligible studies were initially grouped according to the intervention strategy, study design, and completeness of information. After assessment of study quality and completion of descriptive data summary, we investigated the likelihood of combining data (i.e. pooling of individual effect estimates) from several independent studies that addressed the same intervention. Decision for pooling data was based on both clinical and statistical heterogeneity. Clinical heterogeneity included differences on inclusion and exclusion criteria, study design characteristics, methodological quality, patient characteristics at baseline, duration of exposure, doses, dialysis modality, and directionality of effect estimates. Analysis of statistical heterogeneity was accomplished by using forest plots and reported by the I2 test. An I2 value less than 30% with a non-significant chi-square statistic (P > .10) was suggestive of low statistical heterogeneity. In addition, sensitivity (i.e. study quality) and sub-group (i.e. treatment duration) analyses within each intervention were performed with the purpose of improving the strength of these associations. When appropriate, we estimated pooled mean differences and their 95% CI using the inverse variance method and the random effects model.14 To minimize the “double counting” error in cross-over studies, half of the total number of study participants were allocated to each study arm. Inter-group differences were analyzed using the Cochrane χ2 test with P ≤ .10. All analyses were performed using RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen).
Results
Characteristics of Studies
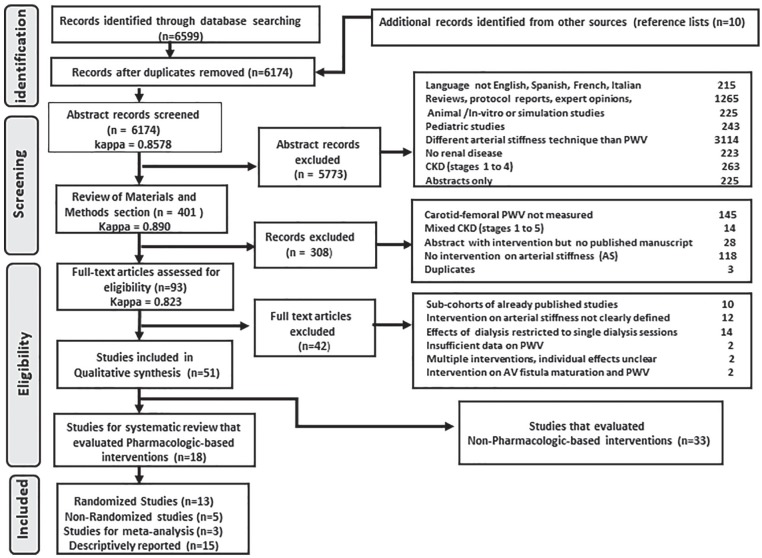
The literature search yielded 6607 citations (Figure 1). After completion of full-text review and abstraction, we included 18 studies that reported at least 1 pharmacologic intervention (13 randomized and 5 non-randomized studies). Ten studies were conducted in Europe (4 Denmark, 4 France, 1 Spain, 1 Hungary), 4 in Asia (2 China, 1 Japan, 1 Taiwan), 2 in Australasia (Australia/New Zealand), and 2 in North America (1 Canada, 1 United States). Thirteen publications were single-center studies and 5 involved multiple site participation. Most studies (14 of 18) were published after the year 2000, with 10 reports published after 2009. Table 1 summarizes the study characteristics, and Table 2 illustrates the cf-PWV recording devices and side effects reported for the intervention and comparator. Among the randomized studies, 3 were cross-over and 10 were parallel randomized trials. Non-randomized studies included 2 cohorts with matched controls and 3 single cohorts with before-and-after measurements. Studies were clustered according to the arterial stiffness mechanism targeted by the intervention into the following categories: (1) management of bone-mineral metabolism disorder (8 studies), (2) control of hypertension (8 studies), (3) correction of anemia (1 study), and (4) reduction of serum homocysteine levels (1 study). For strategies targeting bone mineral metabolism disorder, we identified interventions that involved supplementation with vitamin D analogues (4 studies), calcimimetics (3 studies), and phosphate binders (1 study). For control of hypertension, 3 classes of antihypertensive agents were studied, including renin-angiotensin system (RAS) inhibitors, calcium-channel blockers (CCB), and beta-blockers. Within this interventional category, 5 studies evaluated effects of single antihypertensive drugs compared with placebo or matched controls, while 3 reports involved “head-to-head” comparisons that evaluated 2 different active treatments.
Figure 1.
PRISMA flow chart.
Note. AV = arteriovenous; CKD = chronic kidney disease; PWV = pulse wave velocity.
Table 1.
Characteristics of Studies With Pharmacologic Interventions Included in This Systematic Review.
| Author (reference) | Study design | Qualitya | Intervention (n) | Comparator (n) | Age (years) intervention, comparator | Exposure (months) | Effect size mean difference (95% CI) (m/s) |
|---|---|---|---|---|---|---|---|
| Vitamin D supplementation | |||||||
| Hewitt et al15 | Parallel RCT | Low risk of biasb | Cholecalciferol 50 000 IU/week × 8 weeks + monthly × 4
months HD (n = 21) |
Placebo HD (n = 24) |
61 ± 14 64 ± 14 |
6 | −1.20 (–4.2, 1.8) P = .43 |
| Mose et al16 | Parallel RCT | Low risk of biasb | Cholecalciferol 3000 IU/day × 6
months HD/PD (n = 22) |
Placebo HD/PD (n = 19) |
68 ± 9 67 ± 13 |
6 | +0.70 (–1.7, 3.1) P = .56 |
| Marckmann et al17 | Parallel RCT | High risk of biasb | Cholecalciferol 40 000 IU/week × 8 weeks HD only (n = 12) |
Placebo HD only (n = 13) |
70 ± 13 68 ± 14 |
2 | +0.0 (–2.9, 2.9) P = .99 |
| Hansen18 | Randomized cross-over | High risk of biasb | Paricalcitol HD (n = 7) |
Alfacalcidol HD (n = 3) |
64 ±16 64 ± 14 |
4 | Paricalcitol: –7.1% (–15.9%, 1.7%) Alfacalcidol: 17.6% (13.7%, 21.5%) Difference: P = .04 |
| Cinacalcet | |||||||
| Bonet et al19 | Before/after | Fairc | Cinacalcet 35 mg (30-60 mg)/day HD (n = 21) |
Before treatment | 51.3 ± 18 | 12 | −0.7 (–5.8, 4.4) P = .79 |
| Chow et al20 | Cohort | Acceptable(+)d | Cinacalcet 25-100 mg daily PD (n = 33) |
Matched group PD (n = 37) |
57 ± 10 60 ± 13 |
12 | −0.04 (–0.86, 0.78) P = .92 |
| Poulin et al21 | Randomized DB cross-over | Unclear risk of biasb | Cinacalcet 30 mg daily × 7 days HD (n = 21) |
Placebo (for 7 days) Cross-over |
68 ± 4 | 1-week × treatment (1-week washout) | −0.00 (–3.5, 3.5) P = .99 |
| Phosphate binders | |||||||
| Othmane et al22 | Cohort | Acceptable (+)d |
Sevelamer 2400 mg daily, increased to 4800 mg daily at
discretion × 10 months HD (n = 13) |
Matched controls (by age, sex, dialysis duration,
diabetes) HD (n = 13) |
54.7 ± 8.7 54.0 ± 9.3 | 10.8 ± 2.3 | −1.8 (–3.4, –0.15) P = .042 |
| Pharmacologic control of hypertension | |||||||
| RAS inhibitor monotherapy | |||||||
| Pannier et al23 | Randomized cross-over | Low risk of biasb | Quinapril 20 mg single dose HD (n = 12) |
Placebo Cross-over |
53 ± 12 | 172 hours | At nadir: –1.40 (–4.6, 1.8) P = .39 |
| Yu et al24 | Parallel RCT | Unclear risk of biasb | Ramipril 1.25 mg, 3 times/week × 2 weeks, 2.5 mg 3
times/week, after HD (n = 24) |
Placebo HD (n = 22) |
45 ± 13 48 ± 14 |
12 | −0.40 (–2.31, 1.51) P = .68 |
| Peters et al25 | Parallel RCT | Low risk of biasb | Irbesartan 150 mg/day × 2 weeks, 300 mg
thereafter HD (n = 26) |
Placebo HD (n = 30) |
61 ± 16 62 ±14 |
12 | 0.40 (–0.7, 1.5) P = .49 |
| Calcium-channel blocker monotherapy | |||||||
| London et al26 | Parallel RCT | Low risk of biasb | Nitrendipine 20 mg/day × 7 weeks, then 20 mg twice ×
day) HD (n = 18) |
Placebo HD (n = 19) |
57 ± 11 57 ± 12 |
4 | −1.87 (–3.7, –0.06) P = .04 |
| Saito et al27 | Cohort | Unacceptable (0)d | Nifedipine 10-20 mg daily HD (n = 47) |
Age-matched HD (n = 46) |
52 ± 11 51 ± 4 |
24 | Nifedipine: –2% controls: +10% P < .01 |
| Head-to-head trials (antihypertensive classes) | |||||||
| London et al28 | Parallel RCT | Low risk of biasb | Perindopril 2 mg/day, 3 times/weeke
HD (n = 14) |
Nitrendipine 20 mg/day 3 times/weekd
HD (n = 10) |
55 ± 3 52 ± 5 |
12 | −0.33 (–1.25, 0.59) P = .48 |
| Sun et al29 | Parallel RCT | High risk of biasb | Losartan 50 mg/day (n = 33) |
Bisoprolol 5 mg/day (n = 32) |
57 ± 10 59 ± 10 |
12 | −0.5 (–0.70, –0.31) P = .021 |
| Georgianos et al30 | Parallel RCT | High risk of biasb | Atenolol 100 mg maximum)/3 times × week HD (n = 60) |
Lisinopril 40 mg maximum)/3 times/week HD (n = 49) |
52 ± 12 53 ± 13 |
6 | −14.8% (1.5%, 28.5%) P = .03 |
| Serum homocysteine | |||||||
| Zoungas et al31 | Parallel RCT | Unclear risk of biasb | Folic acid 15 mg/day PD, HD, or awaiting dialysis (n = 156) |
Placebo PD, HD, or awaiting dialysis (n = 159) |
56 ± 13 56 ± 14 |
12 | −0.31 (–1.20, 0.57) P = .49 |
| Correction of anemia | |||||||
| London et al32 | Before/after | Poorc | rec-Human erythropoietin 100 IU/kg IV (repeated 10-35
weeks) (n = 11) |
Before treatment HD |
29 (17-49) All patients |
8.75 | 1.0 (–1.1, 3.2) P = .36 |
Note. CI = confidence interval; HD = hemodialysis; PD = peritoneal dialysis; rec = recombinant; RCT = randomized controlled trial.
Although tools for observational studies are specific to the methodological design, they are equivalent to the rating level of grading.8,11,12
The Cochrane Collaboration’s tool for assessing risk of bias in randomized controlled trials—Interpretation: low risk of bias: plausible bias unlikely to seriously alter the results; unclear risk of bias: plausible bias that raises some doubt about the results; high risk of bias: plausible bias that seriously weakens confidence in the results.
NIH Quality Assessment Tool for cross-sectional studies and single cohorts before-and-after (called pre-and-post) studies with no control group—Interpretation: good quality: minimal risk of bias with a low risk of measurement errors or other confounding factors that would results from “flaws” in the design or conduct of the study (this is equivalent to low risk of bias); fair quality: presence of some risk of bias in confounding, selection, information, and measurement derived from several “flaws” in the design or conduct of the study. Also, there is some doubt about the ability of the study to accurately assess an association between the intervention or exposure and outcome; poor quality: poor internal validity and high risk for “flaws” in the design and/or execution of the study. There is high doubt about the results reported in the study or the ability of the study to accurately assess an association between the intervention or exposure and the outcome (this is equivalent to high risk of bias).
The SIGN50 tool for assessing methodological quality in cohort studies—Interpretation: high quality (++): majority of criteria met, little or no risk of bias, and results unlikely to be changed by further research; acceptable (+): most criteria met, some flaws in the study with an associated risk of bias, and conclusions may change in the light of further studies; low quality (0) or unacceptable: either most criteria not met or significant flaws relating to key aspects of study design, and conclusions likely to change in the light of further studies.
Double dose if DBP > 95 mm Hg.
Table 2.
Aortic Stiffness Recording Devices, Effects on Heart Rate, and Side Effects Associated With Pharmacologic Interventions.
| Author (reference) | Effects on heart rate | Recording device cf-PWV | Intervention-reported side effects | Comparator-reported side effects |
|---|---|---|---|---|
| Cholecalciferol | ||||
| Hewitt et al15 | Not reported | SphygmoCor PVx (AtCor Medical, Sydney, Australia) |
Bone fractures = 1 Serum calcium >10.4 mg/dL on ≥1 occasion = 3 Serum phosphate >5.0 mg/dL on ≥1 occasion = 17 Gastrointestinal events =3 Withdrawal due to death =1 |
Bone fractures = 0 Serum calcium >10.4 mg/dL on ≥1 occasion = 2 Serum phosphate >5.0 mg/dL on ≥1 occasion = 20 Gastrointestinal events = 3 Withdrawal due to death = 1 |
| Mose et al16 | Not reported | SphygmoCor CPV, (AtCor Medical, Sydney Australia) |
Hypercalcemia = 1 Cerebrovascular disease = 1 |
Hypercalcemia = 0 Cerebrovascular disease = 2 |
| Marckmann et al17 | Not reported | Applanation tonometer (Millar, SPT-301B, Houston, Texas) |
Small but significant rise in FGF-23 Mild to moderate hypercalcemia = 5 |
None reported |
| Hansen18 | Not reported | SphygmoCor (AtCor Medical, Sydney Australia) |
Alfacalcidol: Gastrointestinal = 3 Dermatologic = 1 Death = 1 Respiratory = 1 Cardiovascular/stroke = 10 Musculoskeletal = 1 Infectious = 12 |
Paricalcidol: Gastrointestinal = 5 Dermatologic = 0 Death = 0 Respiratory = 1 Cardiovascular/stroke = 6 Musculoskeletal = 2 Infectious = 8 |
| Cinacalcet | ||||
| Bonet et al19 | No effect | Complior system (Artech Medical, Paris, France) |
Not reported | Not reported |
| Chow et al20 | Not reported | Complior system (Artech Medical, Paris, France) |
Severe hypocalcemia = 1 Death = 2 |
None reported |
| Poulin et al21 | No between-group differences | Complior SP (Artech Medical, Paris, France) |
Not reported | Not reported |
| Phosphate binders (sevelamer) | ||||
| Othmane et al22 | No differences | PulsePen device (DiaTecne, Milan, Italy) |
Not reported | Not reported |
| RAS inhibitors monotherapy | ||||
| Pannier et al23 | Not reported | Not specified | Not reported | Not reported |
| Yu et al24 | No effect with ramipril | Not specified | Severe cough = 2 Hypertension = 1 |
Traumatic intracranial hemorrhage = 1 |
| Peters et al25 | No effect with irbesartan | SphygmocCor (AtCor Medical, Sydney Australia) |
Diarrhea = 2 Hypotension = 1 Myocardial infarction = 1 Poor general condition = 1 |
Death = 3 |
| Calcium-channel blocker monotherapy | ||||
| London et al26 | No effect on heart rate | Transcutaneous Doppler flow | Post-dialysis ionized calcium: 1.43 ± 0.07 mmol/L | Post-dialysis ionized calcium: 1.41 ± 0.06 mmol/L |
| Saito et al27 | Not reported | PWV 200 (Fukuda Elect Co, Tokyo) |
Not reported | Not reported |
| Head-to-head comparisons | ||||
| London et al28 | No effect by perindopril or nitrendipine | Transcutaneous Doppler flow/applanation tonometry | Not reported | Not reported |
| Sun et al29 | Decreased with bisoprolol. No changes with losartan |
Complior (Colson, DuPont Medical, France) |
Losartan: Fasting insulin levels were reduced |
Bisoprolol: Fasting insulin levels were not reduced |
| Georgianos et al30 | No between-group differences | Continuous Doppler flow | Not reported | Not reported |
| Other pharmacologic treatments | ||||
| Zoungas et al31 | No differences in heart rate | Applanation tonometry (transducers: Millar Mikro-Tip, SPT-301; Houston, TX, USA) |
Treatment discontinuation due to adverse events:
6 Cobalamin deficiency (<160 pmol/L): 6 |
Treatment discontinuation due to adverse effects:
2 Cobalamin deficiency (<160 pmol/L) 6 |
| London et al32 | Lower heart rate at end of treatment | Not specified | Not reported | Not reported |
Note. cf-PWV = carotid-femoral pulse wave velocity; PWV = pulse wave velocity; RAS = renin-angiotensin system.
Bone Mineral Metabolism Disorder
Vitamin D analogues
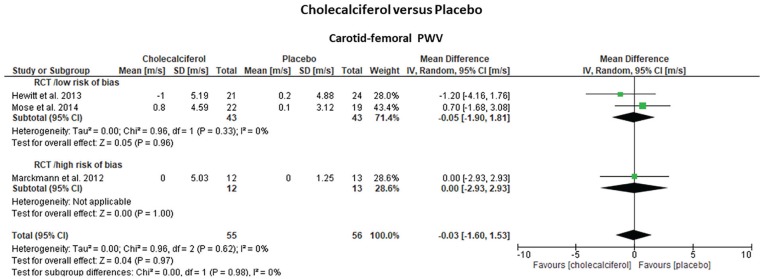
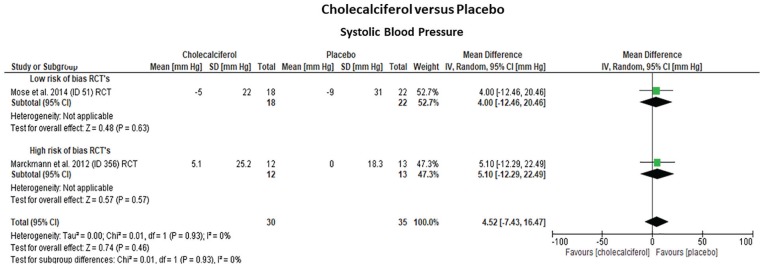
Three randomized trials15-17 (2 low risk; 1 high risk of bias) that included 111 hemodialysis patients assessed the effects of cholecalciferol on cf-PWV and SBP over placebo (Table 1; Figures 2 and 3). One additional trial (high-risk bias) compared the effects of paricalcitol (n = 7) versus alfacalcidol (n = 3).18 Treatment duration was 2 to 6 months for cholecalciferol and 4 months for alfacalcidol. The pooled effect estimates indicated that cholecalciferol did not reduce cf-PWV (–0.03 m/s, 95% CI: [–1.60; 1.53], P = .97) or SBP (+4.5 mm Hg, 95% CI: [–7.43; 16.47], P = .46) compared with placebo. Statistical heterogeneity was low (I2 = 0%) and study quality (cf-PWV: P = .97; SBP: P = .93) or treatment duration (cf-PWV: P = .82) did not modify the overall effect estimates. In contrast, paricalcitol slightly decreased cf-PWV (–7.1%, 95% CI: [–15.9%; +1.72%]), while alfacalcidol resulted in an increase (+17.6%, 95% CI: [+13.7%; +21.5%]), with the differences between the groups being marginally significant (P = .04). There were no significant effects on SBP (paricalcitol: +1.1%, 95% CI: [–2.0; 13] vs alfacalcidol: –5.1%, 95% CI: [–16.3; 6.1]; P > .05). This study, however, documented a high rate of attrition and was stopped prematurely.
Figure 2.
Forrest plots of the effect estimates on carotid-femoral pulse wave velocity (PWV) from studies focused on vitamin D analogue treatment (cholecalciferol) to improve aortic stiffness compared with placebo in 3 randomized parallel clinical trials. Studies were grouped according to their risk of bias (low vs high).
Note. All cf-PWV values were non-adjusted for mean blood pressure. CI = confidence interval.
Figure 3.
Forrest plots of the effect estimates on systolic blood pressure from studies focused on vitamin D analogue treatment (cholecalciferol) to improve aortic stiffness compared with placebo in 3 randomized parallel clinical trials. Studies were grouped according to their risk of bias (low vs high).
Note. All cf-PWV values were non-adjusted for mean blood pressure. CI = confidence interval.
Calcimimetics
Three studies19-21 evaluated the effects of cinacalcet on cf-PWV over placebo or standard care, but only 2 studies reported data on SBP.19,21 The studies had different methodological designs (2 cohorts; 1 cross-over), study quality (1 fair; 1 acceptable; 1 unclear risk of bias), treatment duration (1 week, 12 months, and 12 months), time on dialysis, and dialysis modality (peritoneal dialysis: 1 study; hemodialysis: 2 studies). Although cinacalcet decreased serum parathyroid hormone levels, its effects on cf-PWV (–0.05 m/s, 95% CI: [–0.85; 0.74]) and SBP (–1.5 mm Hg, 95% CI: [–22.10, 19.18]; P = .89) were not different from placebo or standard care. The mean differences in cf-PWV for 2 of the studies20,21 were small and less variable (–0.04 m/s, 95% CI: [–0.86; 0.78], P = .92; –0.00 m/s, 95% CI: [–3.53; 3.53], P = .96) but larger and more variable (–0.7 m/s, 95% CI: [–5.80; 4.42], P = .79) for the other.19
Phosphate binders
In a single study,22 incremental doses of sevelamer for 11 months in hemodialysis patients (n = 13) with serum phosphorus levels above 1.86 mmol/L decreased cf-PWV (–0.83 m/s, 95% CI: [–2.1; 0.4], P = .21) compared with 13 controls matched for age, sex, diabetes, and dialysis duration (+0.93 m/s, 95% CI: [–0.15; 2.01], P = .09; sevelamer vs controls: P = .042) without any effects on SBP (sevelamer: +1.5 mm Hg, 95% CI: [–9.5; 12.5], P = .79; controls: +1.8 mm Hg, 95% CI: [–9.8; 13.4], P = .77; sevelamer vs controls: P = .98). Large differences in baseline calcium and phosphorus parameters might have decreased the strength of these associations.
Control of Hypertension
Eight studies assessed the effects of antihypertensive agents on cf-PWV and SBP in chronic hemodialysis patients. Three randomized trials evaluated monotherapy with RAS inhibitors (quinapril, ramipril, and irbesartan) compared with placebo. Two additional studies (1 randomized) evaluated effects of the CCB nitrendipine and nifedipine versus placebo or age-matched controls. Also, 3 “head-to-head” trials compared the RAS inhibitors losartan, perindopril, or lisinopril versus the CCB nitrendipine, or the beta-blockers atenolol or bisoprolol. These studies had large differences in treatment doses, inclusion criteria, duration of exposure, study design, and quality.
RAS inhibitors
Pannier et al23 evaluated the acute effects of a single dose of quinapril in 12 hypertensive hemodialysis patients with SBP greater than 160 mm Hg. Both SBP and cf-PWV decreased within the first 4 hours after quinapril administration compared with placebo. The cf-PWV decreased to a maximum of 17% from baseline values (13.2 ± 2.8 m/s; vs 13.6 ± 3.3 m/s) and was maintained for 52 hours after quinapril administration. Yu et al24 randomized escalating doses of ramipril against placebo in 46 normotensive hemodialysis patients with left ventricular hypertrophy. Ramipril decreased cf-PWV (–0.7 m/s, 95% CI: [–2.4; 0.9], P < .05) and SBP (–10 mm Hg, 95% CI: [–28; 7.9], P < .05) after 12 months of treatment, but the effect of ramipril on cf-PWV was not significantly different to the placebo group (mean difference: –0.40 m/s, 95% CI: [–2.3; 1.5] P = .68). Peters et al25 randomized treatment with irbesartan or placebo as add-on to standard hypertensive therapy (target SBP: 140 mm Hg) for 1 year in 56 hemodialysis patients and showed a decrease in SBP (–10.0 mm Hg, 95% CI: [–18.4; –1.7], P = .02) and cf-PWV (–0.8 cm/s, 95% CI: [–1.5; 0.0], P = .05) with irbesartan, but these effects were not significantly different to the placebo group (irbesartan vs placebo: SBP: P = .76; cf-PWV: P = .49). The effects on heart rate were also comparable.
Calcium-channel blockers
London et al26 randomized 40 hypertensive hemodialysis patients to a 4-month treatment with nitrendipine alone or placebo. Nitrendipine significantly reduced cf-PWV (–2.15 m/s, 95% CI: [–3.6; –0.71], P < .01) and pre-dialysis supine SBP (–30 mm Hg, 95% CI: [–45; –15], P < .01) from baseline, but no effects were observed in the placebo group (cf-PWV: –0.28 m/s, 95% CI: [–1.58; 1.02], P = .66; SBP: –10 mm Hg, 95% CI: [–27.3; 7.3], P > .05). The effect of nitrendipine on cf-PWV was significantly superior to placebo (nitrendipine vs placebo: –1.87 m/s, 95% CI: [–3.7; –0.06], P = .04). Saito et al27 compared the change in cf-PWV in response to nifedipine administered for 2 years in 47 hypertensive hemodialysis patients against an age-matched control group. At 2 years, cf-PWV decreased by 2% from baseline in the nifedipine group, but it increased 10% in the control group (nifedipine vs controls; P < .01). SBP did not change significantly between the groups. The 2 studies, however, showed large differences in effect estimates, and these were related to different baseline cf-PWV values (13.2 ± 2.3 m/s vs 9.4 ± 2.2 m/s), times of exposure (4 months vs 24 months), risk of bias (low risk vs unacceptable), and study design (randomized vs non-randomized).
Head-to-head trials
London et al28 randomized hemodialysis patients with left ventricular hypertrophy to 12 months perindopril (n = 14) or nitrendipine (n = 10). Perindopril and nitrendipine induced similar reductions in SBP (–27 mm Hg, 95% CI: [–32; –22], P < .001 vs −20 mm Hg, 95% CI: [–26; –14], P < .001) and cf-PWV (–2.0 m/s, 95% CI: [–2.5; –1.5], P < .001 vs −1.6 m/s, 95% CI: [–2.6; –0.7], P < .001) with a decrease in left ventricular hypertrophy only in patients receiving perindopril. Sun et al29 evaluated the effects of losartan (n = 33) against the beta-blocker bisoprolol (n = 32) on cf-PWV and insulin resistance for 12 months. SBP decreased similarly with either losartan or bisoprolol (P > 0.05; –11 mm Hg, 95% CI: [–16.4; –5.6] vs −13 mm Hg, 95% CI: [–18.6; –7.4]), but reductions in cf-PWV in the losartan group (–0.9 m/s, 95% CI: [–1.0; –0.8]) were significantly greater (P = .021) than with bisoprolol (–0.4 m/s, 95% CI: [–0.6; –0.22]). This difference persisted even after adjustment for age (P = .03). In an open-label trial of 109 hemodialysis patients, Georgianos et al30 studied the effects of incremental doses of lisinopril and atenolol, 3 times per week for 6 months, on cf-PWV and SBP (target SBP: <140 mm Hg). Atenolol induced greater reduction in cf-PWV relative to lisinopril (mean difference: –14.8%, 95% CI: [1.5; 28.5], P = .03) that persisted after adjustment for age, sex, race, and baseline SBP, but the effect on SBP was not different between the 2 groups (mean difference: –5.7 mm Hg, 95% CI: [–26.2; 14.8], P = .59).
Other Pharmacologic Treatments
A single study31 randomized 315 hemodialysis patients to 15 mg folic acid daily or placebo to lower serum homocysteine levels. After 12 months of treatment, plasma total homocysteine was reduced by 19%, but there was no significant reduction in cf-PWV compared with placebo (–0.31 m/s, 95% CI: [–1.2; 0.6], P = .49). London et al32 evaluated the impact of recombinant human erythropoietin on cf-PWV in 11 anemic hemodialysis patients before and 10 to 35 weeks after initiation of therapy, SBP and cf-PWV did not change significantly between the 2 times of measurement (before: 8.04 ± 2.6 m/s; after: 9.1 ± 2.52 m/s; P = .08).
Quality of evidence
Quality of evidence for both cf-PWV and SBP across all interventional categories ranged from very low to low except for cholecalciferol trials, where quality was considered moderate. Table 3 summarizes these results.
Table 3.
Quality of Evidence (GRADE Method)a for cf-PWV and SBP Outcomes in ESRD Patients Among Different Interventions.
| Intervention | Study designb | Risk of biasc | Inconsistencyc | Indirectnessc | Imprecisionc | Publication biasc | Upgrading factorsd | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| Carotid-femoral pulse wave velocity (cf-PWV) | ||||||||
| Cholecalciferol | High quality | Not an issue | Not an issue | Not an issue | Serious (–1) |
Undetected | No upgrade | Moderate quality (+++) |
| Cinacalcet | High quality | Serious risk (–1) |
Not an issue | Not an issue | Serious (–1) |
Undetected | No upgrade | Low quality (++) |
| RAS inhibitors | High quality | Serious risk (–1) |
Not an issue | Serious (–1) |
Serious (–1) |
Undetected | No upgrades | Very low quality(+) |
| Calcium-channel blockers | High quality | Serious risk (–1) |
Serious (–1) |
Not an issue | Serious (–1) |
Undetected | No upgrades | Very low quality (++) |
| Systolic blood pressure (SBP) | ||||||||
| Cholecalciferol | High quality | Not an issue | Not an issue | Not an issue | Serious (–1) |
Undetected | No upgrade | Moderate quality (+++) |
| Cinacalcet | High quality | Serious risk (–1) |
Not an issue | Not an issue | Serious (–1) |
Undetected | No upgrade | Low quality (++) |
| RAS inhibitors | High quality | Serious risk (–1) |
Not an issue | Serious (–1) |
Serious (–1) |
Undetected | No upgrades | Very Low quality(+) |
| Calcium-channel blockers | High quality | Serious risk (–1) |
Serious (–1) |
Not an issue | Serious (–1) |
Undetected | No upgrades | Very low quality (+) |
RAS = renin-angiotensin system.
GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) provides a structured and transparent evaluation of the importance of outcomes regarding interventions or management strategies according to comprehensive criteria for downgrading and upgrading certainty in evidence. GRADE classifies the quality of evidence into one of four levels as follows: high (very confident that the true effect lies close to that of the effect estimate); moderate (moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); low (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect); very low (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect).
GRADE starts with a baseline rating of HIGH for randomized trials and LOW for non-randomized studies. This baseline rating can then be adjusted (downgraded or upgraded) after considering 8 assessment criteria and making a judgment about quality based on these criteria.33
Reasons to downgrade the evidence: risk of bias, inconsistency, indirectness, imprecision, and publication bias. For these 5 criteria: if no serious concern exists, the quality is not downgraded from the baseline quality (e.g. high for RCTs); if serious concern exists, the evidence is downgraded one level, for example, from high to moderate (–1); if very serious concern exists, the evidence is downgraded 2 levels, for example, from high to low (–2).
Reasons to upgrade the evidence: large magnitude of effect, dose response, or effect of all plausible confounding factors would be to reduce the effect (where an effect is observed) or suggest a spurious effect (when no effect is observed).
Discussion
We analyzed data from 1027 ESRD patients included in 18 studies that evaluated effects of 4 different pharmacologic interventions on cf-PWV. Most studies (89%) focused on 1 of 2 major pharmacologic strategies: improvement in bone mineral metabolism disorder or management of hypertension. Based on very low-to-moderate quality evidence, our findings suggest that treatment of bone mineral metabolism disorder with active vitamin D analogues or cinacalcet does not cause significant reductions in cf-PWV or SBP over placebo or standard care. Sevelamer caused a discrete reduction in cf-PWV over controls, but this effect was confounded by differences in baseline serum parameters. The use of CCB may show an advantage over RAS inhibitors in decreasing cf-PWV, but results were affected by the small number of studies and differences in inclusion criteria, study quality, and deficient control for confounders. Single studies on folic acid and erythropoietin were underpowered and limited by study design and quality.
Bone Mineral Metabolism Disorder
Hypovitaminosis D is highly prevalent in patients with ESRD and may contribute to cardiovascular risk.34 Vitamin D analogue supplementation in chronic dialysis patients has been associated with improved parameters of bone mineral metabolism,17 reduction in parathyroid hormone levels, and increased survival.35 Because altered bone mineral metabolism increases the risk for vascular calcification and arterial stiffness, cholecalciferol supplementation has been proposed as an intervention to improve cf-PWV. Based on moderate quality evidence, our findings indicate that treatment of chronic dialysis patients with cholecalciferol for 2 to 6 months does not decrease cf-PWV and SBP over placebo. Paricalcitol may have a slight advantage over alfacalcidol, but the results are based on small numbers of patients. Because serum levels of 25(OH)D are expected to achieve optimal levels between 8 and 12 weeks15 after vitamin D supplementation, treatment for 6 months or less with cholecalciferol may not have been sufficiently long to induce reliable changes in cf-PWV. Furthermore, a previous meta-analysis36 showed a benefit of vitamin D supplementation (1-6 months) on cf-PWV over placebo (–0.93 m/s, 95% CI: [–1.71; –0.15], P = .02) in patients with chronic kidney disease stages 1 to 4, suggesting that once ESRD is reached, arterial stiffness becomes refractory to this intervention.
In pre-clinical studies, calcimimetics (e.g. cinacalcet) decrease parathyroid hormone levels, vascular calcification, and vascular stiffness.37,38 Based on very low quality of evidence, our findings suggest that despite a reduction in parathyroid hormone levels, the effects of cinacalcet on cf-PWV and SBP in ESRD participants were not superior to standard care or placebo. We speculate that the small number of studies, differences in design and quality, dialysis modality, and short follow-up periods may explain the lack of effect of cinacalcet on cf-PWV. An additional consideration is that cinacalcet has minimal impact on the regulation of Klotho levels,39 which may have greater effects on aortic stiffness compared with parathyroid hormone. The effect of cinacalcet may also have been offset by concomitant use of medications with opposite effects on vascular calcification (i.e. calcium-based phosphate binders).20
Treatment of hyperphosphatemia with sevelamer attenuates progression of aortic calcification in hemodialysis patients.40,41 Our review identified a single small cohort study suggesting that sevelamer modestly decreased cf-PWV in hemodialysis patients without affecting SBP. A major limitation, however, was the lack of control for baseline calcium and phosphorus parameters that made these differences less relevant.
Control of Hypertension
In ESRD, arteriosclerosis and vascular calcification are prominent and associated with increased SBP and aortic stiffness.3 Thus, lowering blood pressure with antihypertensive agents reduces cardiovascular morbidity and mortality in chronic dialysis patients.42 RAS inhibitors have been recommended as initial pharmacologic therapy for control of hypertension in ESRD.43 We identified 2 studies24,25 that evaluated the chronic effects of RAS inhibitors on aortic stiffness. While administration of RAS inhibitors decreased cf-PWV and SBP, these effects were not superior to placebo. However, the small sample sizes and the inconsistency of inclusion criteria, dosage, study quality, volume overload assessment, and medication compliance in these studies strongly support the need for additional well-designed trials to address the impact of RAS blockade on arterial stiffness in ESRD.
CCB are non-dialyzable and thereby allow for improved control of hypertension in ESRD without dose adjustment.44 In hypertensive patients without renal disease, CCB reduce carotid intima-media thickness45,46 and aortic stiffness,47 but their effects in ESRD patients are unclear. In our review, we identified 2 studies that evaluated CCB in chronic dialysis patients. These studies indicate that nitrendipine and nifedipine significantly decrease cf-PWV and SBP compared with placebo or age-matched hemodialysis controls (Supplementary Table S-1). Interestingly, nitrendipine was associated with a moderate effect on aortic stiffness (–1.87 m/s), which if effective, may potentially decrease mortality in ESRD patients by approximately 28%.2 Another trial28 that compared perindopril against nitrendipine reported comparable effects on cf-PWV, but the effect sizes and variability were larger compared with other trials (see Supplementary Table S-1).
Overall, clinical studies have postulated several physiologic mechanisms associated with the effects of the different antihypertensive medications on aortic stiffness that range from lowering central aortic blood pressure to an enhancement of endothelial function and vasodilatation and/or reductions in oxidative stress and inflammation that affect arterial compliance.48 Differences among these mechanisms may account for the different effects of these medications on cf-PWV. Both angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers have a differential effect on central and peripheral blood pressure that may account for the observed amplification of the pulse pressure and potentially lead to a lesser effect on arterial stiffness.49,50 ACE inhibitors, in particular, have been shown to have an additional effect on reducing oxidative stress, inflammation, and improving vasodilation through reduction in angiotensin II, causing smooth muscle relaxation and remodeling of the vessel wall.51 In contrast, dihydropyridine-type CCB not only antagonize the L-type calcium channel but in animal models have been shown to have antioxidant effects.52 Evidence regarding beta-blockers, however, suggests that these agents may be inferior to other antihypertensive drugs53 in reducing aortic stiffness due to their dominant effect on peripheral versus central blood pressure.54
Limitations
Our rigorous methodology provided an extensive and comprehensive systematic review on existing pharmacologic interventions that target cf-PWV in ESRD patients. We recognize, however, that quality of evidence ranges from very low to moderate, and that despite our efforts to establish associations between changes in cf-PWV and these interventions, the results should be considered hypothesis-generating given the high methodological heterogeneity, low number of trials, small sample sizes, and lack of control for confounders.
In summary, pharmacologic interventions in ESRD are associated with mixed effects on cf-PWV and conclusions are limited by the paucity of studies, small sample sizes, and methodological inconsistencies. Medications that target bone mineral metabolism disorder do not appear to decrease aortic stiffness in ESRD. In contrast, management of hypertension with CCB may reduce aortic stiffness, but the quality of evidence is very low. The effect of RAS inhibitors on cf-PWV is not greater than placebo. Most importantly, in contrast to decreases in aortic stiffness associated with non-pharmacologic interventions in ESRD (kidney transplant, control of extracellular fluid volume, low calcium dialysate, and intradialytic exercise),7 evidence for pharmacologic therapies is insufficient to recommend their routine use for this purpose. Further well-designed studies are needed to confirm these associations and evaluate their impact on cardiovascular outcomes in ESRD.
Supplemental Material
Supplemental material, CJKHD_supplementary_APPENDIX_S-2_PRISMA_CHECKLIST for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease
Supplemental material, CLEAN_CJKHD_supplementary_Appendix_S-1 for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease
Supplemental material, CLEAN_Rodriguez_CJKHD_SupplementaryTABLE_S-1 for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease
Acknowledgments
We acknowledge Dr Beverley Shea, Becky Skidmore, Risa Shorr, and Raymond Daniel for their help and support to this review.
Footnotes
Ethics approval and consent to participate: Not applicable for this type of study.
Consent for publication: We have the authors’ consent for publication.
Availability of Data and Materials: All data are available on request.
Author Contributions: RAR and KDB conceived the study and its design; RAR created the analytical plan and drafting of the manuscript; RAR, MS, RH, and KDB participated in study screening, selection, data extraction, and quality assessment; RAR, KDB, and MA contributed to study interpretation and manuscript revisions. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Kidney Research Centre, Ottawa Hospital Research Institute, University of Ottawa.
ORCID iD: Rosendo A. Rodriguez  https://orcid.org/0000-0002-6039-9274
https://orcid.org/0000-0002-6039-9274
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434-2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 2. Verbeke F, Van Biesen W, Honkanen E, et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6(1):153-159. doi: 10.2215/CJN.05120610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 4. Zanoli L, Lentini P, Briet M, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019;30(6):918-928. doi: 10.1681/ASN.2019020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boutouyrie P, Fliser D, Goldsmith D, et al. Assessment of arterial stiffness for clinical and epidemiological studies: methodological considerations for validation and entry into the European Renal and Cardiovascular Medicine registry. Nephrol Dial Transplant. 2014;29(2):232-239. doi: 10.1093/ndt/gft309. [DOI] [PubMed] [Google Scholar]
- 6. Georgianos PI, Pikilidou MI, Liakopoulos V, Balaskas EV, Zebekakis PE. Arterial stiffness in end-stage renal disease-pathogenesis, clinical epidemiology, and therapeutic potentials. Hypertens Res. 2018;41(5):309-319. doi: 10.1038/s41440-018-0025-5. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez RA, Hae R, Spence M, Shea B, Agharazii M, Burns KD. A systematic review and Meta-analysis of Non-pharmacologic-based interventions for aortic stiffness in End-Stage Renal Disease. Kidney Int Rep. 2019;4(8):1109-1121. doi: 10.1016/j.ekir.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. Cochrane Collaboration; 2011. http://handbook.cochrane.org/v5.0.0/. [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez RA, Shea B, Hae R, Burns KD. The impact of intervention strategies that target arterial stiffness in end-stage renal disease: a systematic review protocol. Syst Rev. 2016;5(1):118. doi: 10.1186/s13643-016-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Scottish Intercollegiate Guidelines Network (SIGN). A Guideline Developer’s Book. Edinburgh, Scotland: SIGN50; 2010, 2012 http://www.sign.ac.uk/sign50.html. Accessed January 2018. [Google Scholar]
- 12. National Institutes of Health, National Heart, Lung and Blood Institute. Quality Assessment Tool for Before-After (Pre-post) Studies with No Control Group. Health-Pro. Guidelines. Cohort. Bethesda, MD: National Institutes of Health; 2014. http://www.nhlbi.nih.gov/health-topics.study-quality-assessment-tools. Accessed July 2017. [Google Scholar]
- 13. Atkins D, Briss PA, Eccles M, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(7):1143-1149. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mose FH, Vase H, Larsen T, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients–a randomized controlled trial. BMC Nephrol. 2014;15:50. doi: 10.1186/1471-2369-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marckmann P, Agerskov H, Thineshkumar S, et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant. 2012;27(9):3523-3531. doi: 10.1093/ndt/gfs138. [DOI] [PubMed] [Google Scholar]
- 18. Hansen D. A randomised clinical study of alfacalcidol and paricalcitol. Dan Med J. 2012;59:B4400. [PubMed] [Google Scholar]
- 19. Bonet J, Bayes B, Fernandez-Crespo P, Casals M, Lopez-Ayerbe J, Romero R. Cinacalcet may reduce arterial stiffness in patients with chronic renal disease and secondary hyperparathyroidism—results of a small-scale, prospective, observational study. Clin Nephrol. 2011;75(3):181-187. doi: 10.5414/cnp75181. [DOI] [PubMed] [Google Scholar]
- 20. Chow KM, Szeto CC, Kwan BC, Cheng PM, Pang WF, Leung CB, Li PK. Effect of cinacalcet treatment on vascular arterial stiffness among peritoneal dialysis patients with secondary hyperparathyroidism. Nephrology (Carlton). 2014;19(6):339-344. doi: 10.1111/nep.12223. [DOI] [PubMed] [Google Scholar]
- 21. Poulin A, Bellemare PL, Fortier C, et al. Acute effects of cinacalcet on arterial stiffness and ventricular function in hemodialysis patients. A randomized double-blinded crossover study. Medicine (Baltimore). 2017;96(21):e6912. doi: 10.1097/MD.0000000000006912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Othmane Tel H, Bakonyi G, Egresits J, et al. Effect of sevelamer on aortic pulse wave velocity in patients on hemodialysis: a prospective observational study. Hemodial Int. 2007;11(Suppl. 3):S13-21. doi: 10.1111/j.1542-4758.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 23. Pannier BM, Guerin AP, Marchais SJ, Cuche JL, Vicaut E, London GM. Pressure-independent improvement of aortic distensibility in hypertensive hemodialysed patients. Arch Mal Coeur Vaiss. 1994;87(8):1059-1061. [PubMed] [Google Scholar]
- 24. Yu WC, Lin YP, Lin IF, Chuang SY, Chen CH. Effect of ramipril on left ventricular mass in normotensive hemodialysis patients. Am J Kidney Dis. 2006;47(3):478-484. doi: 10.1053/j.ajkd.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 25. Peters CD, Kjaergaard KD, Jensen JD, et al. No significant effect of angiotensin II receptor blockade on intermediate cardiovascular end points in hemodialysis patients. Kidney Int. 2014;86(3):625-637. doi: 10.1038/ki.2014.69. [DOI] [PubMed] [Google Scholar]
- 26. London GM, Marchais SJ, Guerin AP, Metivier F, Safar ME, Fabiani F. Salt and water retention and calcium blockade in uremia. Circulation. 1990;82(1):105-113. doi: 10.1161/01.cir.82.1.105. [DOI] [PubMed] [Google Scholar]
- 27. Saito Y, Shirai K, Uchino J, Okazawa M, Hattori Y, Yoshida T, Yoshida S. Effect of nifedipine administration on pulse wave velocity (PWV) of chronic hemodialysis patients–2-year trial. Cardiovasc Drugs Ther. 1990;4(Suppl. 5):987-990. doi: 10.1007/bf02018306. [DOI] [PubMed] [Google Scholar]
- 28. London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90(6):2786-2796. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 29. Sun F, Song Y, Liu J, Ma LJ, Shen Y, Huang J, Zhou YL. Efficacy of losartan for improving insulin resistance and vascular remodeling in hemodialysis patients. Hemodial Int. 2016;20(1):22-30. doi: 10.1111/hdi.12327. [DOI] [PubMed] [Google Scholar]
- 30. Georgianos PI, Agarwal R. Effect of lisinopril and atenolol on aortic stiffness in patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10(4):639-645. doi: 10.2215/CJN.09981014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47(6):1108-1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 32. London GM, Zins B, Pannier B, et al. Vascular changes in hemodialysis patients in response to recombinant human erythropoietin. Kidney Int. 1989;36(5):878-882. doi: 10.1038/ki.1989.274. [DOI] [PubMed] [Google Scholar]
- 33. Ryan R, Hill S. How to GRADE the Quality of the Evidence (Version 3.0). Cochrane Consumers and Communication Group; December 2016. http://cccrg.cochrane.org/author-resources. [Google Scholar]
- 34. Lundquist AL, Nigwekar SU. Optimal management of bone mineral disorders in chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2016;25(2):120-126. doi: 10.1097/MNH.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19(1):179-184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 36. Dou D, Yang B, Gan H, Xie D, Lei H, Ye N. Vitamin D supplementation for the improvement of vascular function in patients with chronic kidney disease: a meta-analysis of randomized controlled trials. Int Urol Nephrol. 2019;51(5):851-858. doi: 10.1007/s11255-019-02088-3. [DOI] [PubMed] [Google Scholar]
- 37. Henley C, Colloton M, Cattley C, et al. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol Dial Transplan. 2005;20:1370-1377. [DOI] [PubMed] [Google Scholar]
- 38. Lopez I, Aguilera-Tejero E, Mendoza FJ, et al. Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J Am Soc Nephrol. 2006;17:795-804. [DOI] [PubMed] [Google Scholar]
- 39. Komaba H, Koizumi M, Tanaka H, Takahashi H, Sawada K, Kakuta T, Fukagawa M. Effects of cinacalcet treatment on serum soluble Klotho levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(5):1967-1969. doi: 10.1093/ndt/gfr645. [DOI] [PubMed] [Google Scholar]
- 40. Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245-252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 41. Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815-1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 42. Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomized controlled trials. Lancet. 2009;373:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Del Vecchio L, Teatini U, Locatelli F. Use of ACE inhibition and blood pressure management in deferring dialysis initiation. Panminerva Med. 2017;59(2):166-172. doi: 10.23736/S0031-0808.17.03293-1. [DOI] [PubMed] [Google Scholar]
- 44. Sica DA, Gehr TW. Calcium-channel blockers and end-stage renal disease: pharmacokinetic and pharmacodynamic considerations. Curr Opin Nephrol Hypertens. 2003;12(2):123-131. doi: 10.1097/00041552-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 45. Aslam S, Santha T, Leone A, Wilcox C. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70(12):2109-2115. doi: 10.1038/sj.ki.5001983. [DOI] [PubMed] [Google Scholar]
- 46. Yilmaz R, Altun B, Kahraman S, Ozer N, Akinci D, Turgan C. Impact of amlodipine or ramipril treatment on left ventricular mass and carotid intima-media thickness in non-diabetic hemodialysis patients. Ren Fail. 2010;32:903-912. [DOI] [PubMed] [Google Scholar]
- 47. Mahmud A, Feely J. Anti-hypertensive drugs and arterial stiffness. Expert Rev Cardiovasc Ther. 2003;1:65-78. [DOI] [PubMed] [Google Scholar]
- 48. Dudenbostel T, Glasser SP. Effects of antihypertensive drugs on arterial stiffness. Cardiol Rev. 2012;20:259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Protogerou AD, Stergiou GS, Vlachopoulos C, Blacher J, Achimastos A. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: Evidence for specific class-effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15(3):272-289. doi: 10.2174/138161209787354186. [DOI] [PubMed] [Google Scholar]
- 50. Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens. 2000;14(9):541-546. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 51. Hirata K, Vlachopoulos C, Adji A, O’Rourke MF. Benefits from angiotensin-converting enzyme inhibitor “beyond blood pressure lowering”: beyond blood pressure or beyond the brachial artery? J Hypertens. 2005;23(3):551-556. doi: 10.1097/01.hjh.0000160211.56103.48. [DOI] [PubMed] [Google Scholar]
- 52. Mason RP, Walter MF, Trumbore MW, Olmstead EG, Jr, Mason PE. Membrane antioxidant effects of the charged dihydropyridine calcium antagonist amlodipine. J Mol Cell Cardiol. 1999;31(1):275-281. doi: 10.1006/jmcc.1998.0867. [DOI] [PubMed] [Google Scholar]
- 53. Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113(5):657-663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 54. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366(9496):1545-1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CJKHD_supplementary_APPENDIX_S-2_PRISMA_CHECKLIST for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease
Supplemental material, CLEAN_CJKHD_supplementary_Appendix_S-1 for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease
Supplemental material, CLEAN_Rodriguez_CJKHD_SupplementaryTABLE_S-1 for Pharmacologic Therapies for Aortic Stiffness in End-Stage Renal Disease: A Systematic Review and Meta-Analysis by Rosendo A. Rodriguez, Matthew Spence, Richard Hae, Mohsen Agharazii and Kevin D. Burns in Canadian Journal of Kidney Health and Disease