Abstract
To remove high oxygen content is important to make high quality oil and valuable products. In this paper, the research on homogeneous catalytic deoxygenation reactions, including decarboxylation (DCX)/decarbonylation (DCN), hydrodeoxygenation (HDO) is reviewed. Based on DCX/DCN, the classic radical reactions such as the Barton decarboxylation, Henkel, Hunsdiecker and Kochi reactions were introduced, the practice and overall performance are also discussed. In addition, the different reaction pathways and mechanisms were demonstrated and the key chemical processes have been selected from the literature as examples to elaborate the critical emphasis on the mechanistic understanding. The applications of the catalytic deoxygenation reactions for high-value products have also been highlighted. Overall, this review provides insight discussions on the DO issues and progresses in homogeneous catalytic aspects.
Keywords: Chemical engineering, Bio-oil, Upgrading, Deoxygenation (DO), Homogeneous catalysis, Catalyst, Bioconversion, Energy sustainability, Fuel technology, Natural product chemistry
Chemical engineering; Bio-oil; Upgrading; Deoxygenation (DO); Homogeneous catalysis; Catalyst; Bioconversion; Energy sustainability; Fuel technology; Natural product chemistry
1. Introduction
Biomass derived products have been considered to be renewable and environmental friendly (Saidur et al., 2011). In recent decades, there has been increased interest and demand for bio-mass products from bio-crude. The bio-crude quality depends not only on the characteristics of raw materials but also the technical processes such as biomass fast pyrolysis and hydrothermal liquefication (Liu et al., 2010; Savage et al., 2010; Yang et al., 2015). In general, bio-crude has high amounts of oxygen and acidotic groups, which is unsuitable for direct use (Mortensen et al., 2011; Saber et al., 2016). Therefore, it is necessary to include a catalytic refining stage to improve the quality for high-value bio-mass products.
A catalytic refinery based on thermochemistry is a typical technology for the deoxygenation (DO) and cracking processes. For conventional crudes, the cracking process is used to produce light hydrocarbon species. However, for those products derived from biomass (where the oxygen content may be over 10 wt.%), DO becomes the most important process in the upgrading of bio-crude (Bu et al., 2012; Kenar et al., 2017). Currently, decarboxylation (DCX), decarbonylation (DCN) and hydrodeoxygenation (HDO) reactions can be performed for the DO process.
In recent years, the processes of catalytic DO reactions have been intensively investigated (Robinson et al., 2016). They can include either homogeneous or heterogeneous complexes. As each of these has its advantages and disadvantages, the processes must be balanced accordingly. Compared with the heterogeneous catalytic process, homogeneous catalysis has the advantages of greater activity and greater selectivity, since the reactive sites among the ancillary ligands can achieve far better performances than heterogeneous catalytic processes, e.g., using enzymes (Trost, 2010). Additionally, the high water/liquid phase content of bio-crude could allow homogeneous catalysts to achieve rapid and selective DO reactions under the mild conditions (Dawes et al., 2015).
There are several general reviews relevant to bio-crude refining. Zacher et al. at Pacific Northwest National Laboratory reviewed the hydrodeoxygenation (HDO) of bio-oil, in which it focused on process integration as well as physical and chemical modification (Zacher et al., 2014). Several other reviews also giveintroductions on bio-crude refining (Peterson et al., 2008; Helwani et al., 2009; Demirbas, 2011; Mortensen et al., 2011; Chang et al., 2012; Elliott, 2015; Yuan et al., 2016; Pattanaik and Misra, 2017). However, there are few literature studies reporting about homogeneous catalytic DO. The purpose of this review is to fill this gap by providing an in depth insight into the homogeneous catalytic deoxygenation reactions for the production of high-value bio-mass products, including DCX/DCN and HDO.
2. Decarboxylation (DCX)/decarbonylation (DCN)
DCX/DCN is an effective way to remove -COOH and release CO2 or CO to produce n-alkanes with and without H2. In the HDO method, the oxygen atom is removed or partially removed by introducing hydrogen (H2) to form n-alkanes or synthesize products in the presence of a catalyst (Kubičková and Kubička, 2010). However, a DCX/DCN process using a catalyst to produce n-alkanes requires much less or even no H2, making the process simpler and more economical.
2.1. Classic radical reactions
Classic radical reactions of DCX/DCN are the Barton decarboxylation reaction, Henkel reaction, and Hunsdiecker, Kochi reaction and so on, which are all types of free radical oxidative reactions.
In the Barton decarboxylation, -COOH is removed to form a Barton ester (by heating with radical initiator) producing a small amount of H2, as shown in Figure 1 (Barton et al., 1979, 2015; Gutekunst and Baran, 2011). The Barton reaction provides a possible route for decarboxylation, however the derivatives can still be formed due to radical-substitution reactions (Barton and Beaton, 1960; Ishmuratov et al., 2005; Petrović and Čeković, 2010). For instance, when the thermal DCX/DCN of O-acetylpodocarpic acid and N-hydroxypyridine-2—thione, a high yield of alkene was obtained by heating (Cochrane et al., 1989). Although some reductive decarboxylation and decarboxylative oxygenation applications have been used for conversion of acids (Saraiva et al., 2009), Barton decarboxylation has mostly been applied in the organic synthesis (Sugimoto et al., 2009; Wim et al., 2011).
Figure 1.
The Barton decarboxylation.
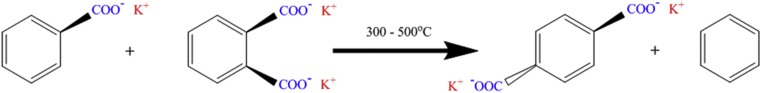
The Henkel process, also known as the Raecke process (Brolley et al., 1955), is widely used for converting the aromatic carboxylates into symmetrical aromatic dicarboxylates via thermal rearrangement with alkali metals. As shown in Figure 2, the process requires not only alkali salts as a catalyst but also a high temperature (e.g., 350–500 °C) and should be operated in inert conditions (Kucera and Jancar, 1998). Some results have shown that potassium salts are effective for the production of terephthalic acid from phthalic acid (Ogata et al., 1957, 1960). Radical oxidation plays important roles during the preparation of Terephthalic acid (Ogata et al., 1957; Jiang et al., 2008; Wang et al., 2013). Zuo et al. (2010) found that the inert pure nitrogen atmosphere can be replaced by CO2, which can enhance the radical oxidation and can lead to a high yield of toluene and p-toluic acid (over 90%) in the liquid-phase oxidation system (Xiao et al., 2010). Some studies have further applied supercritical CO2 for higher purity and a more active DCX/DCN process (Pérez et al., 2011).
Figure 2.
The classic process of Henkel reaction (Kucera and Jancar, 1998).
A similar Henkel route was also reported for producing furan and 2,5-furandicarboxylate (2,5-FDCA) (Pan et al., 2013), in which the conversion rate can reach up to 61% and the selectivity for 2,5-FDCA about 86% by ZnCl2 catalysis at 250 °C and a duration of 180 min. Other studies have also used this Henkel reaction to produce 2,5-furandicarboxylic acid with metal salts as the catalysts (e.g., potassium permanganate or gold nanoparticles), the results showed up to 99 mol% selectivity at 130 °C in water with air as an oxidant can be achieved. The Henkel route was also considered as an important process for bio-products such as furfural and 2,5-FDCA (Colonna et al., 2011).
It was reported that the Hunsdiecker reaction could be carried out for DCX/DCN with silver salts and organic halides (Hunsdiecker and Hunsdiecker, 1942; Borodine, 2010). Figure 3 shows the Hunsdiecker reaction mechanism in the presence of silver salts and organic halides to generate the radical intermediates. In a typical process, the Ag+ will be replaced by Br, which can create the diradical intermediate 3. After the rapid DCX/DCN, n-alkanes and CO2 will be produced with some undesired organic halides (Johnson and Ingham, 1956).
Figure 3.
The typical Hunsdiecker reaction.
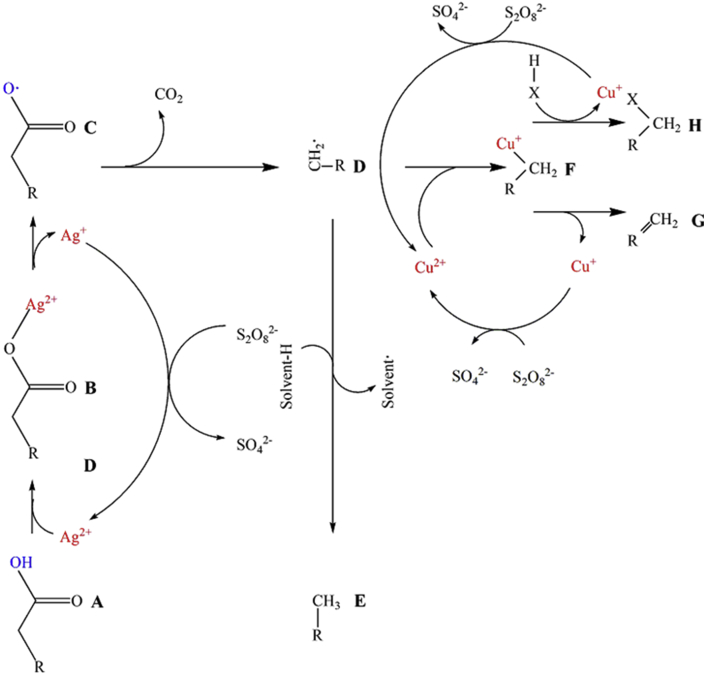
Kochi et al. (Kochi et al., 1967; Anderson and Kochi, 1970) further discovered that Ag(I)-catalyzed oxidative decarboxylation could remove COOH group(s) by using Cu2+ and sodium peroxydisulfate, which was widely applied to DCX/DCN of fatty acids. Fatty acids are the components derived from natural plant and algae oils, which are typically stearic acid (C18H36O2), palmitic acid (C16H32O2) and unsaturated fatty acids such as oleic acid (C18H34O2), linoleic acid (C18H32O2) and lignocellulosic, cellulosic and semicellulosic acid. Figure 4 shows the proposed mechanism in which Ag(I) complexes are converted to Ag(II) by persulfate oxidation and the Ag(II) subsequently oxidizes COOH into the acyloxy radical. The acyloxy radical is unstable, and breaks down to release CO2 and form an alkyl radical. By getting H from the solvent or chain, the alkyl radical is converted into alkane or alkene products. Additionally, Ag(II) accepts e− from H and is reduced to Ag(I). The catalytic copper (II) can produce alkenes by the oxidative elimination reaction (Fristad et al., 1984). The silver/copper decarboxylation/decarbonylation requires the stoichiometric peroxydisulfate or sulfate. To produce an alkane, a hydrogen donor solvent is needed.
Figure 4.
Kochi oxidative decarboxylation of fatty acids.
DCX/DCN by Ag(I)-catalyzed oxidation can be used in the decarboxylation of several saturated or unsaturated fatty acids. At 78 °C for 20 min, a 24% yield of 8(Z)-heptadecene with 79% purity was obtained from oleic acid. With the addition of Cu2+, (Z)-heptadeca-1,8-diene was found to be the major byproduct (Dawes et al., 2015). Additionally, silver/copper DCX/DCN was used for decarboxylating ortho-anisic acid to anisole (98 mol%), cinnamic acid to styrene (75%) and hydroxymethylfurfural to caprolactone (Buntara et al., 2011). Recently, Weng et al. (2017) reported the DCX/DCN of cross-link polyacrylic acid (PAA) and hydrophilic polymers, in which the cross-linking reaction was readily achieved with only 0.03 wt% AgNO3 and a small amount of persulfates in mild conditions (a room temperature in air). The results show promise in the use of oxidative decarboxylation to prepare hydrogels of copolymers and interpenetrating polymer network (IPN).
Unproductive reactions tend to occur as a result of the radical reactions, leading to disproportionation and rearrangement of the byproducts. Ag2O generation has been observed at temperatures above 200 °C, and can be further reduced to metallic silver. The results of two studies (Lee et al., 2002; Koen Binnemans et al., 2004) showed that intermediate silver carboxylates present as dimeric (RCO2)2Ag2 ring structures tended to generate Ag metal, CO2, and organic products at ~300 °C.
An insight view on the decarboxylation and simultaneous reduction was provided by Hatamura et al. (2015). The molecular structures of Ag(I) β-ketocarboxylates are shown in Figure 5. According to the DFT structures and the experimental results, Ag(I) β-ketocarboxylates can be classified into either type 1, type 2 or type 3. These indicate that the coordination of C=O to Ag is unstable, which leads to e− transfer during decomposition to produce (Ag2)+•. The intermediates 2 and 3a make the e− transfer. After e− transfer, the keto radical, CO2 and Ag can be produced from bond-cleavages. Thus, Ag(I) is the key to the radical oxidation for DCX/DCN of fatty acids.
Figure 5.
The Plausible structures of Ag(I) β-ketocarboxylates.
2.2. Organometallic precursor catalysts
The organometallic precursor catalysts, i.e., noble metal based catalysts (such as Rh-, Ir-, Pd-, Ru-) and transition metal based catalysts (such as FeCl2/KI, or FeI2/KI, Cu-), have been also applied for the catalytic decarboxylation, which could offer an alternative way to the DCX/DCN approaches. Table 1 summaries the results from the DCX/DCN of fatty acids by the organometallic precursor catalysts such as Rh-, Ir-, Pd- Ru-, Fe- and Cu-based catalysts. The results indicate that the use of Rh- and Ir-provides the best alkene selectivity in the reaction.
Table 1.
Comparison of DCX/DCN of fatty acids.
| Catalyst | Fatty acid | Catalyst loadings | Time (h) | Temp (°C) | Yield alkene | Reference |
|---|---|---|---|---|---|---|
| RhCl3 | stearic acid | 1% (mol/mol) | 1 | 280 | 90% | Foglia and Barr (1976) |
| IrCl(CO)(PPh3)2 | stearic acid | 2% (mol/mol) | 5 | 200 (KI, Ac2O) | 97% | Maetani et al. (2011) |
| PdCl2 | stearic acid | 2% (mol/mol) | 3 | 280 | 15% | Nôtre et al. (2010) |
| PdCl2(PPh3)2 | nonanoic acid | 0.01 mol% | 0.5 | 230 | 68% | Liu et al. (2014) |
| Ru3(CO)12 [Ru(CO)2(EtCO2)]n | 10-undecenoic acid | 0.87wt% | 4 | 250 | ~60% | Muench et al. (2016) |
| FeCl2 | aliphatic carboxylic acids | 10 mol% | 3 | 250 | 79% | Maetani et al. (2012) |
| Cu(OAc)2 | vinylic carboxylic acids | 2 mol% | 12 | 110 | 45% | Cui et al. (2012) |
RhCl3 serves as one of the best organometallic precursor catalysts. Rhodium trichloride (RhCl3) and triphenylphosphine can efficiently catalyze the DCX/DCN of stearic acid to alkanes, such as 2-heptadecene, 3-heptadecene and 1-heptadecene (Foglia and Barr, 1976), as shown in Table 1. The proposed mechanism is shown in Figure 6. There are four kinds of Rh complexes during the DCX/DCN of stearic anhydride, i.e., chlorocarbonyl [bis-triphenylphosphine] Rhodium(I) (type 2), six coordinate acyl-metal (type 3), five coordinate acyl (type 4), and six coordinate alkyl rhodium metal (type 5). After Type 2, Type 3 takes place, and then Type 4 is initiated by the elimination of triphenylphosphine or CO, which later converts into Type 5 generating an acid, 1-heptadecene and Type 2 for recycle. The RhCl3 can also be used in the Cativa™ Process for the manufacture of acetic acid (Kozub et al., 2000), and the reaction might also be useful for the production of benzoic acid derivatives (Jing et al., 2013).
Figure 6.
Decarbonylation of stearic acid by Rh- complexes.
IrCl(CO)(PPh3)2, i.e., Vaska's complex, can be used as a catalyst for DCX/DCN of fatty acids to alkenes in the presence of KI, the alkene yield can reach to 97% (Maetani et al., 2011). Iridium catalysis has also been used in the Cativa™ process (Sunley and Watson, 2000), Figure 7 shows the proposed mechanism. In a typical process, the acyl Ir (type A) could be produced by the condensation of the C(O)-O bond from Ac2O and carboxylic acids. Type A then generates the alkyl Ir by DCX/DCN, Type B. The Ir hydride complex C may be produced by the elimination of β-Hydride from Type B, which forms the Ir(I) complex. Additionally, Type C produces alkenes and the alkyl Ir, Type D. It should be noted that Type C might lead to different pathways, forming both n-C15H30 and n-C14H28.
Figure 7.
Decarbonylation reaction mechanism of Ir-complex.
Palladium-based catalysts, such as PdCl2, PdBr2, Pd(OAc)2, (dba)3Pd2, Pd(PPh3)4 and PdCl2(PPh3)2 also show high activity during decarboxylation. The DCX/DCN of aliphatic carboxylic acids into olefins can occur with palladium-based catalysts. The Pd-precursor at 110 °C can be used to effectively eliminate decarbonylation and β-hydride. PdBr2 has proved to be more active than PdCl2, since 60–75% yield can be achieved with Pd(PPh3)4 (0.01 mol%) for the DCX/DCN of carboxylic or dicarboxylic acids at about 190 °C (Nôtre et al., 2010). For myristic acid, a 91% yield was observed with PdCl2, Ph3P and Pivalic Anhydride, and 55% of 1,9-decadiene and 65% of 11-dodecadiene were obtained from the diacids. Nôtre et al. also reported that about 96% 1-heptadecene yield was achieved at 18 h and 110 °C with additive trialkylamines for the DCX/DCN of aliphatic carboxylic acids. Liu et al. (2014) reported that the decarbonylative dehydration using a low catalyst loading (PdCl2 (PPh3)2) under relatively mild and solvent-free conditions can achieve a 90% yield by the addition of isophthalic acid with 0.1% Pd catalyst under specific conditions (132 °C for 2 h in 1 atm of N2–CO). Additionally, direct DCX/DCN of benzoic acids was observed by Congyang et al. (2009). A Pd-catalyst was used in the Heck coupling system (Fu et al., 2016).
There are four main reactions in the catalyzed carbonylation mechanism, oxidative addition, coordination, insertion of carbon monoxide (transmetalation), and reductive elimination (Beller and Wu, 2013). A similar Pd-catalyzed decarboxylative cross-coupling mechanism for the decarbonylation of fatty acids was reported, in which the decarbonylation proceeds via the formation of an anhydride, coordinated to the palladium atom to produce an alkene, as shown in Figure 8.
Figure 8.
Pd-catalyzed carbonylation reactions.
Recently, [Ru(CO)2(EtCO2)]n, an isomerization-decarboxylation catalyst precursor complex, has also been studied. It isomerizes the substrate and then facilitates the decarboxylation without additional co-reagents (Muench et al., 2016; Yang et al., 2016). For 9-cis-octadecenoic acid, the conversion to alkene can be carried out without pre-catalysis at 250 °C and 24 h, while a 36% yield of alkene conversion with 0.10 wt % Ru3(CO)12 was found. A 60% yield of 10-undecenoic acid was achieved under the conditions of 0.89 wt % Ru3(CO)12 at 250 °C and 4 h duration (Muench et al., 2016). Moser et al. (2016) further reported that 77.6% heptadecene and 18.0% heptadecane was obtained by Ru-catalyzed DCX/DCN of fatty acids. There were also other reactions such as the decarboxylation, dehydrogenation, isomerization, hydrogenation, and cyclization/aromatization took place, which made a mixture of predominantly alkenes (Knothe et al., 2017). Doll et al. (2017) calculated and fitted the activation energy of the oleic acid decarboxylation reaction, which was 249 kJ mol−1 (Knothe et al., 2017).
Some transition metal based homogeneous catalysts were proved to be highly active. Iron-based catalysts have been found to be effective catalysts for the DCX/DCN processes. Iron-catalyzed DCX/DCN reactions of fatty acids to α-olefins (Maetani et al., 2012). The reaction was accelerated by the addition of KI and Ac2O, decarbonylation of stearic acid produced a 79% yield of heptadecenes. FeI2 serves a similar function to FeCl2/KI, and FeI(CO)2Cp, and can generate a 40% yield in the presence of KI. The proposed mechanism for this is shown in Figure 9. In the first step, acid anhydrides are produced from carboxylic acid and Ac2O by FeCl2, proceeding to iron-carbonyl B. KI or Phosphide reacts with CO and 1 to form the active catalytic species A. The DCX/DCN of B then produces the alkyl iron C, which eliminates β-hydride into α-olefins and iron hydride D. D then releases acyl iron A, and hence the cycle is completed. It was also found that D may generate by-products, isomerization olefins.
Figure 9.
Decarbonylation reaction by Fe-based homogeneous catalysts.
Additionally, copper-catalyzed decarboxylative alkenylation has also been reported (Cui et al., 2012). The pathways lead to the formation of alcohols, esters, and hydrocarbons to make (E)-alkenes. The results indicated Cu0 was more active than CuBr2, CuBr and Cu(OAc)2·H2O, and the 62% product yield was achieved with 1 mol% Cu. Copper-catalyzed protodecarboxylation of aromatic carboxylic acids was investigated using DFT calculations, as shown in Figure 10. The results indicate that the type [(phen)Cu]+ is an important precursor for a successful decarboxylation. The calculations showed the species [(phen)Cu]+ would be formed by coordinating anions or carboxylate ions to Cu(I) via a phenyl anion intermediate. The [(phen)Cu]+ would be rapidly transmitted, leading to the DCX/DCN or the reactivity of the benzoic acids. Thus, the ρ-system affects the DCX/DCN.
Figure 10.
Copper-catalyzed decarboxylative alkenylation.
3. Homogeneous Hydrodeoxygenation (HDO)
Hydrodeoxygenation (HDO) is a process to use hydrogen or hydrogen resources to remove O atoms from compounds. Organometallics are commonly considered as catalysts for the homogeneous catalysis process. However, according to recent reports, the homogeneous Lewis acids have higher activities for the HDO process (see Table 2).
Table 2.
The HDO by homogeneous catalysts.
| Catalyst | Feedstocks | Catalyst loadings | Time (h) | Temp (°C) | Yield Hydrocarbons | References |
|---|---|---|---|---|---|---|
| B(C6F5)3 | tristearin | 5 mol% | 6 | Room Temp | 95% | Li et al. (2015) |
| [Cp*Ru(CO)2]2 | glycol | 10% (mol/mol) | 8 | 170 | 70% | Sullivan et al. (2014) |
| [(4′-Ph-terpy)Ru(H2O)3](OTf)2 | 2,5-Hexanedione | 1 mmol/L = 0.1 mol % | 16 | 200 | 96% | Stanowski et al. (2012) |
| [Pd(tpy)Cl]Cl | Benzaldehyde | 5 % (mol/mol) | 4 | 100 | 90% | Delucia et al. (2018) |
| Ni(COD)2 | alkyl aryl ethers | 20 % (mol/mol) | 16 | 80 | 98% | Sergeev et al. (2011) |
| b-diketiminato magnesium | isocyanates | 10 mol% | 1.5 | 60 | 76% | Yang et al. (2017) |
Parks and Piers (1996) have reported firstly that the trispentafluorophenylborane (B(C6F5)3), a homogeneous Lewis acid, can be used for a HDO with a reductant such as silane. These results indicate that Lewis acid complexes such as B(C6F5)3, can be used for HDO of carbonyl by reaction with silane, as shown in Figure 11. B(C6F5)3 was applied to catalyze HDO of fatty acids and biomass derivatives for alkanes and alkenes (Li et al., 2015). The results showed that the reaction can take place even at room temperature with the reducing agent poly(methylhydrosiloxane) (PMHS). Dichloromethane and cyclohexane serve as effective solvents. The catalyst system can also be applied to HDO of ketones, aldehydes, ethers, esters, carboxylic acids (Gevorgyan et al., 2001; Chandrasekhar et al., 2002; Nimmagadda and Mcrae, 2006) and even lignin model compounds with Et3SiH as the reducing agent (Elias and Thibault, 2013). So far, this is the only metal-free method reported for catalyzing the HDO of fatty acids and biomass derivatives to alkanes and alkenes.
Figure 11.
Reduction reaction mechanism of homogeneous Lewis acid B(C6F5)3.
Organometallics are effective catalysts for HDO. Some Organometallics have been applied for the catalytic decarboxylation of levulinic acid. The HDO process requires that the homogeneous catalysts must be very stable in water- and acid-mediums.
The complex ruthenium(II) appears to be water and acid-stable. The complex [(4′-Ph-terpy)Ru-(H2O)3](OTf)2 (terpy = 2,2′:6′,2″-terpyridine), and its iridium analogue [(4′-Ph-terpy)Ir(OTf)3] are active for the HDO of 2,5-hexanedione and 2,5-dimethylfuran. Those complexes have higher oxidation states (+3) than that of the metal−ligand bonds. During the catalytic process, the isoelectronic relationship (RuII/d6 → IrIII/d6) can be altered slightly to complete the cycle (Sullivan et al., 2014). The complexes [Ru(2,2′-dipicolylamine)(OH2)3](OTf)2 and [Ru(6,6′-bis(aminomethyl)-2,2′-bipyridine)(OH2)2] (OTf)2 were also found to be active. For the HDO of 2,5-hexanedione, a 94% yield of 2,5-hexanediol or 55% yield of 2,5-dimethyltetrahydrofuran was obtained at 150 °C with H3PO4 in H2O. Also, about 55% 2,5-hexanediol and 23% 2,5-dimethyltetrahydrofuran were converted from 2,5-dimethylfuran at 175 °C.
[Cp*Ru(CO)2]2 (Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl, i.e., η5-C5Me5) has been developed as stable organometallic catalyst. At reaction conditions of 170 °C, 4 atm of H2, 70% hexane was obtained from 1,2-hexanediol (Stanowski et al., 2012). Similar to Decarboxylation/decarbonylation, [Cp*Ru(CO)2]2 serves as precatalyst in these reactions. In addition to the hydride intermediates Cp*Ru(CO)2H (Andrews and Klaeren, 1989) or [Cp*Ru(μ-H4)RuCp*] (Cheng and Bullock, 2002), some alternative redox olefin complexes (Saidi et al., 2010) and reduced-carbon aldehyde complexs (Andrews and Gould, 1991) have also been developed (Clarke et al., 2007). In the typical process, as shown in Figure 12, the hydrodehydroxylation, deoxygenation, and hydrocracking reactions are the main pathways.
Figure 12.
Typical Pathways of Decarboxylation/decarbonylation by Ru- complex.
The Ru- complex can also be used to upgrade the real bio-oil. Mahfud et al. reported the use of RuCl2(PPh3)3 to upgrade the water-soluble fraction of pyrolysis oil, and 1,2-ethanediol was found to be main product (Mahfud et al., 2007). Huang et al. (2010) also used the Ru- complex for HDO of bio-oil fraction, and observed a dramatic improvement of bio-oil quality. Use of the Ru-complex can significantly promote bio-oil quality, although specific fractions of bio-oil are improved more than others. Additionally, Busetto et al. (2011) achieved the reduction of bio-oil in pyrolysis of white poplar. Thus, there is potential for the application of ruthenium(II) complexes for upgrading the real bio-oil even in mild conditions.
Pd-based organometallics offer the highest selectivity. Wang et al. (2017) reported the HDO of phenols with an ArOSO2F intermediates Pd(OAc)2 complex. In the system, SO2F2 was observed to produce aryl fluorosulfates which then underwent Pd(cat.)/Et3N/HCO2H mediated hydrogenation to the arene products (Wei et al., 2004; Zhu et al., 2015; Chen et al., 2016). When DMSO was used under optimal conditions, a 99% yield was achieved. A possible mechanism (Peter et al., 2010) is shown in Figure 13. In the first step, phenol 1 reacts with SO2F2 to form intermediate 2, then intermediate 2 forms ArLnPdOSO2F II by Pd(0) catalysis. The intermediate III is subsequently generated from the reaction of FSO2OCH3 with HCOO(-), releasing CO2 to produce intermediate IV. Lastly, arene 3 can be produced from intermediate IV, releasing the Pd(0) complex. [Pd(tpy)Cl]Cl and [Ni(tpy)](PF6)2 has been also observed to be active in the hydrodeoxygenation of model lignin monomers (Delucia et al., 2018). The Pd-tpy catalyst exhibited excellent selectivity during hydrodeoxygenation towards benzyl alcohol, C6H5CHO and (C6H5)2CO. An almost complete conversion of benzyl alcohol to the desired toluene could be achieved at 100 °C and 20 bar H2.
Figure 13.
HDO mechanism of Pd-catalytic system.
Some transition metals can also be used in the organometallic complexes. Ni-SIPr system (Sergeev and Hartwig, 2011) is an effective approach, in which the complexes from Ni(COD)2 (COD, 1,5-cyclooctadiene) and PCy3 (Cy, cyclohexyl) may have good selectivity on the HDO of Aryl Ethers. Additionally, the nickel carbene complex can convert aromatic C–O bonds into arenes and alcohols at 80–120 °C. Some forms of nickel complexes use N-heterocyclic carbenes (NHCs) as active sites, which can achieve 85% yields of toluene and o-cresol. The Ni-SIPr system (Dankwardt, 2004; Guan et al., 2008; Mamoru et al., 2008) provides a new approach for the HDO of aromatic C–O compounds.
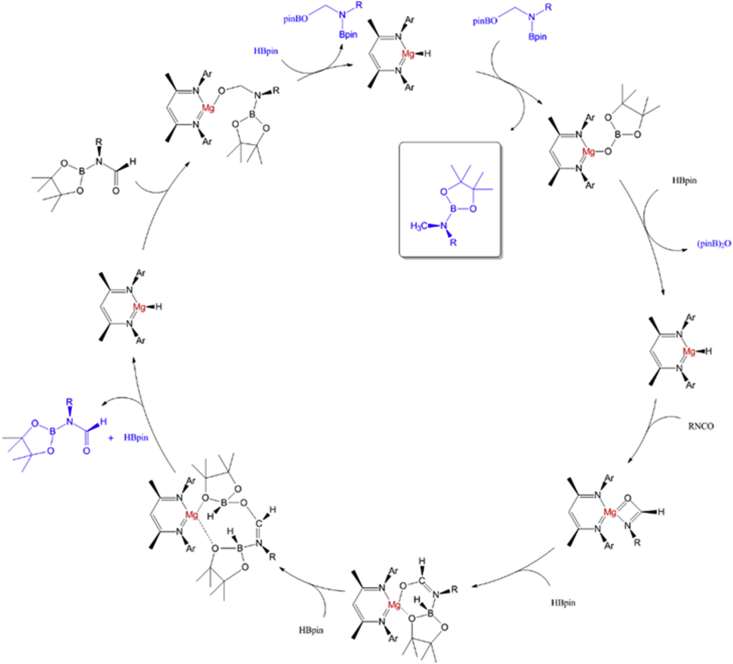
Recently, homogeneous magnesium catalysts have been studied for use in HDO reactions (Arrowsmith et al., 2011; Mukherjee et al., 2014; Lampland et al., 2015). Yang et al. (2017) used a b-diketiminato magnesium catalyst for the HDO of isocyanate at 60 °C over 1.5 h, and a 76% conversion rate was achieved. They postulated a generic mechanism for HDO with b-diketiminato magnesium catalysis (Figure 14). In this pathway, the magnesium hydride first generates formamidate and borate intermediates. The borate intermediate then reacts with the Lewis acid HBpin and the boron is transferred to the magnesium hydride. Additionally, other compounds will be consumed by the B–H/Mg–O complex, producing R(pinB)NCH2-OBpin, which releases species 6 and 4 for the catalytic cycle.
Figure 14.
Mechanism for the Mg- catalysed hydrodeoxygenation (Ar = 2,6-iPr2C6H3) (Yang et al., 2017).
All in all, some single-site homogeneous catalytic systems are able to provide selective HDO reactions, which shows promise for finding new ways of forming valuable products under mild conditions. It should, however, be noted that recovery of the homogeneous catalyst is still difficult. Some new recycling methods for environmental-friendly or cost-effective processing are encouraging, but need to be further explored to overcome these difficulties.
4. Conclusions and prospective
Significant progress has been made in recent years regarding the methods, materials, and mechanistic study of homogeneous catalytic deoxygenation reactions for high-value products. Due to the difference between bio-crude and conventional crudes, the homogeneous catalysis of DCX/DCN and HDO have provided a new direction. Classic radical reactions of DCX/DCN such as Barton decarboxylation, the Henkel reaction, and the Hunsdiecker and Kochi reaction proceed via free radicals, which have lower selectivities. Organometallic precursor catalysts and organometallic complexes such as trispentafluorophenylborane (B(C6F5)3), [Cp*Ru(CO)2]2, [Pd(tpy)Cl]Cl, Ni(COD)2 and B–H/Mg–O complexes can achieve high DO activity and selectivity at mild conditions, which can improve crude bio-oil quality significantly.
Currently, the most common experimental processes are still set at batch scale. The scale-up process is likely to drastically alter the kinetics and mass transfer, meaning that the efficiencies and selectivities of products observed at batch scales may no longer be relevant. To overcome this limitation, the use such as external stimuli should be investigated in specific processes for kinetic control. Additionally, the problem of catalyst recovery also needs further investigation before these processes can be practical.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the National Natural Science Foundation of China [21767015] and National Key R&D Program of China [2018YFC1902101], Collaborative Innovation Center of Western Typical Industry Environmental Pollution Control.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Qingqing Guan, Email: guanqq.qingqing@gmail.com.
Ping Ning, Email: ningping1958@163.com.
References
- Anderson J.M., Kochi J.K. Silver(II) complexes in oxidative decarboxylation of acids. J. Org. Chem. 1970;35:2450–2460. [Google Scholar]
- Andrews M.A., Gould G.L. Photoinduced net (2 + 2 + 2) cycloreversion of platinum(II) glycolate complexes: a new approach to the generation of reduced, coordinatively unsaturated metal species and the activation of carbohydrate carbon- carbon single bonds. Organometallics. 1991;22:387–389. [Google Scholar]
- Andrews M.A., Klaeren S.A. Selective hydrocracking of monosaccharide carbon-carbon single bonds under mild conditions. Ruthenium hydride-catalyzed formation of glycols. J. Am. Chem. Soc. 1989;20:4131–4133. [Google Scholar]
- Arrowsmith M., Hill M.S., Hadlington T., Kociok-Köhn G., Weetman C. Magnesium-catalyzed hydroboration of pyridines. Organomet. 2011;30:5556–5559. [Google Scholar]
- Barton D.H.R., Beaton J.M. A synthesis of aldosterone acetate. J. Am. Chem. Soc. 1960;83:74–75. [Google Scholar]
- Barton D.H.R., Hesse R.H., Pechet M.M., Smith L.C. The mechanism of the barton reaction. J. Chem. Soc. Perkin. Trans. 1979;86:1159–1165. [Google Scholar]
- Barton D.H.R., Beaton J.M., Geller L.E., Pechet M.M. A new photochemical Reaction1. J. Am. Chem. Soc. 2015;82:294–298. [Google Scholar]
- Beller M., Wu X.F. Transition Metal Catalyzed Carbonylation Reactions. Springer International Publishing; London, United Kingdom: 2013. A discussion between carbonylation, noncarbonylation and decarbonylation; pp. 215–221. [Google Scholar]
- Borodine A. Ueber Bromvaleriansäure und Brombuttersäure. Eur. J. Org Chem. 2010;119:121–123. [Google Scholar]
- Brolley J.E., Dickinson W.C., Henkel R.L. Angular dependence of the neutron-induced fission process. II. Phys. Rev. 1955;99:159–165. [Google Scholar]
- Bu Q., Lei H., Zacher A.H., Wang L., Ren S., Liang J., Wei Y., Liu Y., Tang J., Zhang Q. A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis. Bioresour. Technol. 2012;124:470–477. doi: 10.1016/j.biortech.2012.08.089. [DOI] [PubMed] [Google Scholar]
- Buntara T., Noel S., Phua P.H., Melian-Cabrera I., de Vries J.G., Heeres H.J. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. Engl. 2011;50:7083–7087. doi: 10.1002/anie.201102156. [DOI] [PubMed] [Google Scholar]
- Busetto L., Camiletti C., Zanotti V., Albano V.G., Sabatino P. New metallapyrrole complexes from [M2(μ-CNMe2)(μ-CO)(CO)2(Cp)2]SO3CF3 (M=Fe, Ru) and acetonitrile anions; structure of [(Cp)(CO)FeC(NMe2)N(H)C(Me)C(CN)] J. Organometal. Chem. 2010;593–594:335–341. [Google Scholar]
- Chandrasekhar S.,., Ch Raji R., B Nagendra B. Rapid defunctionalization of carbonyl group to methylene with polymethylhydrosiloxane-B(C(6)F(5))(3) J. Org. Chem. 2002;67:9080. doi: 10.1021/jo0204045. [DOI] [PubMed] [Google Scholar]
- Chang H.K., Park S.H., Jeon J.K., Dong J.S., Jeong K.E., Park Y.K. Upgrading of biofuel by the catalytic deoxygenation of biomass. Kor. J. Chem. Eng. 2012;29:1657–1665. [Google Scholar]
- Chen W., Dong J., Li S., Liu Y., Wang Y., Yoon L., Wu P., Sharpless K.B., Kelly J.W. Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. Engl. 2016;55:1835–1838. doi: 10.1002/anie.201509016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.Y., Bullock R.M. Hydride transfer from (η5 -C5Me5 )(CO)2 MH (M = Fe, Ru, Os) to trityl cation: different products from different metals and the kinetics of hydride transfer. Organomet. 2002;21:2325–2331. [Google Scholar]
- Clarke M.L., Díaz-Valenzuela M.B., Slawin A.M.Z. Hydrogenation of aldehydes, esters, imines, and ketones catalyzed by a ruthenium complex of a chiral tridentate ligand. Organomet. 2007;26:16–19. [Google Scholar]
- Cochrane E.J., Lazer S.W., Pinhey J.T., Whitby J.D. Stereoid cd-ring synthons from podocarpic acid by use of the barton radical decarboxylation reaction. Tetrahedron Lett. 1989;30:7111–7114. [Google Scholar]
- Colonna M., Berti C., Fiorini M., Binassi E., Mazzacurati M., Vannini M., Karanam S. Synthesis and radiocarbon evidence of terephthalate polyesters completely prepared from renewable resources. Green Chem. 2011;13:2543–2548. [Google Scholar]
- Congyang W., Isabel P., Frank G. Palladium-catalyzed intramolecular direct arylation of benzoic acids by tandem decarboxylation/C-H activation. J. Am. Chem. Soc. 2009;131:4194–4195. doi: 10.1021/ja8100598. [DOI] [PubMed] [Google Scholar]
- Cui Z., Shang X., Shao X.F., Liu Z.Q. Copper-catalyzed decarboxylative alkenylation of sp3 C–H bonds with cinnamic acids via a radical process. Chem. Sci. 2012;3:2853–2858. [Google Scholar]
- Dankwardt J.W. Nickel-catalyzed cross-coupling of aryl grignard reagents with aromatic alkyl ethers: an efficient synthesis of unsymmetrical biaryls. Angew. Chem. 2004;43:2428–2432. doi: 10.1002/anie.200453765. [DOI] [PubMed] [Google Scholar]
- Dawes G.J.S., Scott E.L., Nôtre J.L., Sanders J.P.M., Bitter J.H. Deoxygenation of biobased molecules by decarboxylation and decarbonylation – a review on the role of heterogeneous, homogeneous and bio-catalysis. Green Chem. 2015;17:3231–3250. [Google Scholar]
- Delucia N.A., Das N., Overa S., Paul A., Vannucci A.K. Low temperature selective hydrodeoxygenation of model lignin monomers from a homogeneous palladium catalyst. Catal. Today. 2018;302:146–150. [Google Scholar]
- Demirbas M.F. Biorefineries for biofuel upgrading: a critical review. Appl. Energy. 2011;86:151–161. [Google Scholar]
- Doll K.M., Bantchev G.B., Walter E.L., Murray R.E., Appell M., Lansing J.C., Moser B.R. Parameters governing ruthenium sawhorse-based decarboxylation of oleic acid. Ind. Eng. Chem. Res. 2017;56:864–871. [Google Scholar]
- Elias F., Thibault C. Unprecedented organocatalytic reduction of lignin model compounds to phenols and primary alcohols using hydrosilanes. Chem. Commun. 2013;50:862–865. doi: 10.1039/c3cc47655c. [DOI] [PubMed] [Google Scholar]
- Elliott D.C. Biofuel from fast pyrolysis and catalytic hydrodeoxygenation. Curr. Opin. Chem. Eng. 2015;9:59–65. [Google Scholar]
- Foglia T.A., Barr P.A. Decarbonylation dehydration of fatty acids to alkenes in the presence of transition metal complexes. J. Am. Oil Chem. Soc. 1976;53:737–741. [Google Scholar]
- Fristad W.E., Fry M.A., Klang J.A. Persulfate/silver ion decarboxylation of carboxylic acids. Preparation of alkanes, alkenes, and alcohols. J. Org. Chem. 1984;15:3575–3577. [Google Scholar]
- Fu Z., Li Z., Song Y., Yang R., Liu Y., Cai H. Decarboxylative halogenation and cyanation of electron-deficient aryl carboxylic acids via Cu mediator as well as electron-rich ones through Pd catalyst under aerobic conditions. J. Org. Chem. 2016;81:2794–2803. doi: 10.1021/acs.joc.5b02873. [DOI] [PubMed] [Google Scholar]
- Gevorgyan V.,., Rubin M.,., Liu J.X., Yamamoto Y. A direct reduction of aliphatic aldehyde, acyl chloride, ester, and carboxylic functions into a methyl group. J. Org. Chem. 2001;32:1672–1675. doi: 10.1021/jo001258a. [DOI] [PubMed] [Google Scholar]
- Guan B.T., Xiang S.K., Wu T., Sun Z.P., Wang B.Q., Zhao K.Q., Shi Z.J. Methylation of arenes via Ni-catalyzed aryl C-O/F activation. Chem. Commun. 2008;12:1437–1439. doi: 10.1039/b718998b. [DOI] [PubMed] [Google Scholar]
- Gutekunst W.R., Baran P.S. C-H functionalization logic in total synthesis. Chem. Soc. Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- Hatamura M., Yamaguchi S., Takane S.Y., Yu C., Suganuma K. Decarboxylation and simultaneous reduction of silver(I) ß-ketocarboxylates with three types of coordination. Dalton Trans. 2015;44:8993–9003. doi: 10.1039/c5dt00773a. [DOI] [PubMed] [Google Scholar]
- Helwani Z., Othman M.R., Aziz N., Fernando W.J.N., Kim J. Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process. Technol. 2009;90:1502–1514. [Google Scholar]
- Huang F., Li W., Lu Q., Zhu X. Homogeneous catalytic hydrogenation of bio-oil and related model aldehydes with RuCl2(PPh3)3. Chem. Eng. Technol. 2010;33:2082–2088. [Google Scholar]
- Hunsdiecker H., Hunsdiecker C. Über den Abbau der Salze aliphatischer Säuren durch Brom. Eur. J. Inorg. Chem. 1942;3:23–24. [Google Scholar]
- Ishmuratov G.Y., Kharisov R.Y., Shayakhmetova A.K., Botsman L.P., Shitikova O.V., Tolstikov G.A. Ozonolysis of ricinolic acid derivatives and transformations of the ozonolysis products under barton reaction conditions. Chem. Nat. Compd. 2005;41:643–649. [Google Scholar]
- Jiang Q., Xiao Y., Tan Z., Li Q.H., Guo C.C. Aerobic oxidation of p- xylene over metalloporphyrin and cobalt acetate: their synergy and mechanism. J. Mol. Catal. Chem. 2008;285:162–168. [Google Scholar]
- Jing Z., Jun-Feng L., Angel U., Pillai A.F.X., Zhong-Ming S., Pinjing Z. Methoxy-directed aryl-to-aryl 1,3-rhodium migration. J. Am. Chem. Soc. 2013;135:17270–17273. doi: 10.1021/ja409049t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.G., Ingham R.K. The degradation of carboxylic acid salts by means of halogen - the hunsdiecker reaction. Chem. Rev. 1956;56:37–47. [Google Scholar]
- Kenar J.A., Moser B.R., List G.R. Chapter 2 – naturally occurring fatty acids : source, chemistry, and uses. Fat. Acids. 2017:23–82. [Google Scholar]
- Knothe G., Steidley K.R., Moser B.R., Doll K.M., Knothe G., Steidley K.R., Moser B.R., Doll K.M., Knothe G., Steidley K.R. Decarboxylation of fatty acids with triruthenium dodecacarbonyl: influence of the compound structure and analysis of the product mixtures. ACS Omega. 2017;2:6473–6480. doi: 10.1021/acsomega.7b01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi J.K., Bacha J.D., Bethea T.W. Free radicals in thermal and photochemical oxidative decarboxylations with lead(IV) J. Am. Chem. Soc. 1967;89:6538–6547. [Google Scholar]
- Koen Binnemans R.V.D., Ben Thijs, Iris Vanwelkenhuysen A., Geuens I. Structure and mesomorphism of silver alkanoates. Chem. Mater. 2004;16:2021–2027. [Google Scholar]
- Kozub P.A., Gryn G.I., Goncharov I.I. Investigations on platinum gauze surfaces used in the manufacture of nitric acid. Platin. Met. Rev. 2000;44:74–84. [Google Scholar]
- Kubičková I., Kubička D. Utilization of triglycerides and related feedstocks for production of clean hydrocarbon fuels and petrochemicals: a review. Waste. Biomass Valorization. 2010;1:293–308. [Google Scholar]
- Kucera F., Jancar J. Homogenous and heterogeneous sulfonation of polymers: a review. Polym. Eng. Sci. 1998;38:783–792. [Google Scholar]
- Lampland N.L., Hovey M., Mukherjee D., Sadow A.D. Magnesium-catalyzed mild reduction of tertiary and secondary amides to amines. ACS Catal. 2015;5:4219–4226. [Google Scholar]
- Lee S.J., Sang W.H., Choi H.J., Kim K. Structure and thermal behavior of a layered silver carboxylate. J. Phys. Chem. B. 2002;106:2892–2900. [Google Scholar]
- Li X.Y., Rui S., Fu M.C., Yao F. Conversion of biomass-derived fatty acids and derivatives into hydrocarbons using a metal-free hydrodeoxygenation process. Green Chem. 2015;17:2790–2793. [Google Scholar]
- Liu Z., Zhang F.S., Wu J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel. 2010;89:510–514. [Google Scholar]
- Liu Y., Kim K.E., Herbert M.B., Fedorov A., Grubbs R.H., Stoltz B.M. Palladium-catalyzed decarbonylative dehydration of fatty acids for the production of linear alpha olefins. Adv. Synth. Catal. 2014;356:130–136. doi: 10.1002/adsc.201301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetani S., Fukuyama T., Suzuki N., Ishihara D., Ryu I. Efficient iridium-catalyzed decarbonylation reaction of aliphatic carboxylic acids leading to internal or terminal alkenes. Organometallics. 2011;30:1389–1394. [Google Scholar]
- Maetani S., Fukuyama T., Suzuki N., Ishihara D., Ryu I. Iron-catalyzed decarbonylation reaction of aliphatic carboxylic acids leading to α-olefins. Chem. Commun. 2012;48:2552–2554. doi: 10.1039/c2cc18093f. [DOI] [PubMed] [Google Scholar]
- Mahfud F.H., Ghijsen F., Heeres H.J. Hydrogenation of fast pyrolyis oil and model compounds in a two-phase aqueous organic system using homogeneous ruthenium catalysts. J. Mol. Catal. Chem. 2007;264:227–236. [Google Scholar]
- Mamoru T., Toshiaki S., Naoto C. Nickel-catalyzed cross-coupling of aryl methyl ethers with aryl boronic esters. Angew. Chem. 2008;47:4866–4869. doi: 10.1002/anie.200801447. [DOI] [PubMed] [Google Scholar]
- Mortensen P.M., Grunwaldt J.D., Jensen P.A., Knudsen K.G., Jensen A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. Gen. 2011;407:1–19. [Google Scholar]
- Moser B.R., Knothe G., Walter E.L., Murray R.E., Doll K.M. Analysis and properties of the decarboxylation products of oleic acid by catalytic Triruthenium Dodecacarbonyl. Energ. Fuel. 2016;30:7443–7451. [Google Scholar]
- Muench S., Wild A., Friebe C., Häupler B., Janoschka T., Schubert U.S. Polymer-based organic batteries. Chem. Rev. 2016;116:9438–9484. doi: 10.1021/acs.chemrev.6b00070. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Ellern A., Sadow A.D. Magnesium-catalyzed hydroboration of esters: evidence for a new zwitterionic mechanism. Chem. Sci. 2014;5:959–964. [Google Scholar]
- Nimmagadda R.D., Mcrae C. A novel reduction reaction for the conversion of aldehydes, ketones and primary, secondary and tertiary alcohols into their corresponding alkanes. Tetrahedron Lett. 2006;47:5755–5758. [Google Scholar]
- Nôtre J.L., Scott E.L., Franssen M.C.R., Sanders J.P.M. Selective preparation of terminal alkenes from aliphatic carboxylic acids by a palladium-catalysed decarbonylation–elimination reaction. Tetrahedron Lett. 2010;51:3712–3715. [Google Scholar]
- Ogata Y., Tsuchida M., Muramoto A. The preparation of terephthalic acid from phthalic or benzoic acid. J. Am. Chem. Soc. 1957;79:6005–6008. [Google Scholar]
- Ogata Y., Hojo M., Morikawa M. Further studies on the preparation of terephthalic acid from phthalic or benzoic acid. J. Org. Chem. 1960;25:2082–2087. [Google Scholar]
- Pan T., Deng J., Xu Q., Zuo Y., Guo Q.X., Fu Y. Catalytic conversion of furfural into a 2,5-furandicarboxylic acid-based polyester with total carbon utilization. Chemsuschem. 2013;6:47–50. doi: 10.1002/cssc.201200652. [DOI] [PubMed] [Google Scholar]
- Parks D.J., Piers W.E. Tris(pentafluorophenyl)boron-Catalyzed hydrosilation of aromatic aldehydes, ketones, and esters. J. Am. Chem. Soc. 1996;118:9440–9441. [Google Scholar]
- Pattanaik B.P., Misra R.D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: a review. Renew. Sustain. Energy Rev. 2017;73:545–557. [Google Scholar]
- Pérez E., Fraga-Dubreuil J., García-Verdugo E., Hamley P.A., Thomas W.B., Housley D., Partenheimer W., Poliakoff M. Selective aerobic oxidation of para-xylene in sub- and supercritical water. Part 1. Comparison with ortho-xylene and the role of the catalyst. Green Chem. 2011;13:2389–2396. [Google Scholar]
- Peter H., Romilda B., Stefan W., Weigand J.J., Elsevier C.J. Mechanism of Pd(NHC)-catalyzed transfer hydrogenation of alkynes. J. Am. Chem. Soc. 2010;132:16900–16910. doi: 10.1021/ja1062407. [DOI] [PubMed] [Google Scholar]
- Peterson A.A., Vogel F., Lachance R.P., Fröling M., Jr M.J.A., Tester J.W. Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ. Sci. 2008;1:32–65. [Google Scholar]
- Petrović G., Čeković Ž. Alkylation of remote non-activated δ-carbon atoms: addition of δ-carbon radicals, generated by 1,5-hydrogen transfer in alkoxy radical intermediates, to activated olefins. Tetrahedron. 2010;30:1377–1390. [Google Scholar]
- Robinson A.M., Hensley J.E., Medlin J.W. Bifunctional catalysts for upgrading of biomass-derived oxygenates: a review. ACS Catal. 2016;6:5026–5043. [Google Scholar]
- Saber M., Nakhshiniev B., Yoshikawa K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016;58:918–930. [Google Scholar]
- Saidi V., Garry C., Irshad A., Nicholas K.M. Rhenium-catalyzed deoxydehydration of glycols by sulfite. Inorg. Chem. 2010;49:4744–4746. doi: 10.1021/ic100467p. [DOI] [PubMed] [Google Scholar]
- Saidur R., Abdelaziz E.A., Demirbas A., Hossain M.S., Mekhilef S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011;15:2262–2289. [Google Scholar]
- Saraiva M.F., Couri M.R.C., Hyaric M.L., Almeida M.V.D. The Barton ester free-radical reaction: a brief review of applications. Tetrahedron. 2009;65:3563–3572. [Google Scholar]
- Savage P.E., Levine R.B., Huelsman C.M. Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals. Royal Society of Chemistry; London: 2010. Hydrothermal processing of biomass; pp. 190–219. [Google Scholar]
- Sergeev A.G., Hartwig J.F. Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science. 2011;42:439–443. doi: 10.1126/science.1200437. [DOI] [PubMed] [Google Scholar]
- Stanowski S., Nicholas K.M., Srivastava R.S. [Cp*Ru(CO)2]2- catalyzed hydrodeoxygenation and hydrocracking of diols and epoxides. Organometallics. 2012;31:515–518. [Google Scholar]
- Sugimoto A., Fukuyama T., Sumino Y., Takagi M., Ryu I. Microflow photo-radical reaction using a compact light source: application to the Barton reaction leading to a key intermediate for myriceric acid A. Tetrahedron. 2009;65:1593–1598. [Google Scholar]
- Sullivan R.J., Latifi E., Chung B.K.M., Soldatov D.V., Schlaf M. Hydrodeoxygenation of 2,5-hexanedione and 2,5-dimethylfuran by water-, air-, and acid-stable homogeneous ruthenium and iridium catalysts. ACS Catal. 2014;4:4116–4128. [Google Scholar]
- Sunley G.J., Watson D.J. High productivity methanol carbonylation catalysis using iridium - the CativaTM process for the manufacture of acetic acid. Catal. Today. 2000;58:293–307. [Google Scholar]
- Trost B.M. Atom economy—a challenge for organic synthesis: homogeneous catalysis leads the way. Angew. Chem. Int. Ed. 2010;34:259–281. [Google Scholar]
- Wang Q., Cheng Y., Wang L., Xi L. Semicontinuous studies on the reaction mechanism and kinetics for the liquid-phase oxidation of p-xylene to terephthalic acid. Ind. Eng. Chem. Res. 2013;46:8980–8992. [Google Scholar]
- Wang X.Y., Leng J., Wang S.M., Asiri A.M., Marwani H.M., Qin H.L. A facile and mild Pd-catalyzed one-pot process for direct hydrodeoxygenation (HDO) phenols to arenes through a ArOSO2F intermediates transformation. Tetrahedron Lett. 2017;58:2340–2343. [Google Scholar]
- Wei Z., Christine Hiu-Tung C., Yimin L., Tadamichi N. A highly efficient microwave-assisted suzuki coupling reaction of aryl perfluorooctylsulfonates with boronic acids. Org. Lett. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng G., Huang Y., Thanneeru S., Li H., Alamri A., He J. Cross-linking of COOH-containing polymers using Ag(i)-catalyzed oxidative decarboxylation in aqueous solution. Soft Matter. 2017;13:5028–5037. doi: 10.1039/c7sm00825b. [DOI] [PubMed] [Google Scholar]
- Wim D., Mashentseva A.A., Seitembetov T.S. Allobetulin and its derivatives: synthesis and biological activity. Molecules. 2011;16:2443–2466. doi: 10.3390/molecules16032443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Luo W.P., Zhang X.Y., Guo C.C., Liu Q., Jiang G.F., Li Q.H. Aerobic oxidation of p-toluic acid to terephthalic acid over T(p-Cl)PPMnCl/Co(OAc)2 under moderate conditions. Catal. Lett. 2010;134:155–161. [Google Scholar]
- Yang G., Yeh T., Song W., Xu D., Wang S. A review of bio-oil production from hydrothermal liquefaction of algae. Renew. Sustain. Energy Rev. 2015;48:776–790. [Google Scholar]
- Yang J.D., Xian W., Song C.X., Zhang W.Q., Sun H.M. The thermolysis of Ru3(CO)12 with carboxylic acids revisited: stepwise assembly of Ru2 to Ru6 cluster frameworks. Chemistry Sel. 2016;1:5397–5403. [Google Scholar]
- Yang Y., Anker M.D., Fang J., Mahon M.F., Maron L., Weetman C., Hill M.S. Hydrodeoxygenation of isocyanates: snapshots of a magnesium-mediated C=O bond cleavage. Chem. Sci. 2017;8:3529–3537. doi: 10.1039/c7sc00117g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Chen H., Zhao W., Chao Y., Peng M., Sheng H. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016;40:865–877. [Google Scholar]
- Zacher A.H., Olarte M.V., Santosa D.M., Elliott D.C., Jones S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014;16:491–515. [Google Scholar]
- Zhu K., Hao J.H., Zhang C.P., Zhang J., Feng Y., Qin H.L. Diversified facile synthesis of benzimidazoles, quinazolin-4(3H)-ones and 1,4-benzodiazepine-2,5-diones via palladium-catalyzed transfer hydrogenation/condensation cascade of nitro arenes under microwave irradiation. RSC Adv. 2015;5:11132–11135. [Google Scholar]
- Zuo X., Niu F., Snavely K., Subramaniam B., Busch D.H. Liquid phase oxidation of p-xylene to terephthalic acid at medium-high temperatures: multiple benefits of CO2-expanded liquids. Green Chem. 2010;12:260–267. [Google Scholar]