Abstract
Background & Aims
The mucus layer in the human colon protects against commensal bacteria and pathogens, and defects in its unique bilayered structure contribute to intestinal disorders, such as ulcerative colitis. However, our understanding of colon physiology is limited by the lack of in vitro models that replicate human colonic mucus layer structure and function. Here, we investigated if combining organ-on-a-chip and organoid technologies can be leveraged to develop a human-relevant in vitro model of colon mucus physiology.
Methods
A human colon-on-a-chip (Colon Chip) microfluidic device lined by primary patient-derived colonic epithelial cells was used to recapitulate mucus bilayer formation, and to visualize mucus accumulation in living cultures noninvasively.
Results
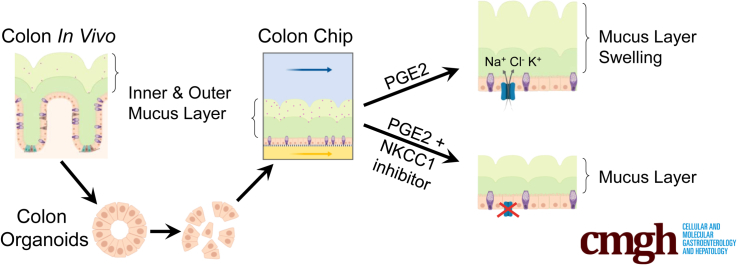
The Colon Chip supports spontaneous goblet cell differentiation and accumulation of a mucus bilayer with impenetrable and penetrable layers, and a thickness similar to that observed in the human colon, while maintaining a subpopulation of proliferative epithelial cells. Live imaging of the mucus layer formation on-chip showed that stimulation of the colonic epithelium with prostaglandin E2, which is increased during inflammation, causes rapid mucus volume expansion via an Na-K-Cl cotransporter 1 ion channel–dependent increase in its hydration state, but no increase in de novo mucus secretion.
Conclusions
This study shows the production of colonic mucus with a physiologically relevant bilayer structure in vitro, which can be analyzed in real time noninvasively. The Colon Chip may offer a new preclinical tool to analyze the role of mucus in human intestinal homeostasis as well as diseases, such as ulcerative colitis and cancer.
Keywords: Organ Chip, Intestine, Organoid, Microfluidic, Goblet Cell, Inflammatory Bowel Disease
Abbreviations used in this paper: BSA, bovine serum albumin; cAMP, adenosine 3′,5′-cyclic monophosphate; CFTR, cystic fibrosis transmembrane conductance regulator; Colon Chip, Colon-on-a-Chip; DPBS, Dulbecco’s phosphate-buffered saline; EdU, 5-Ethynyl-2′-deoxyuridine; HBSS, Hank’s balanced salt solution; MUC, mucin; NKCC1, Na-K-Cl cotransporter 1; Organ Chip, Organ-on-a-Chip; PBS, phosphate-buffered saline; PDMS, polydimethylsiloxane; PFA, paraformaldehyde; PGE2, prostaglandin E2; TFF3, Trefoil factor 3; TW, Transwell
Graphical abstract
Summary.
An in vitro method is described for studying colonic mucus physiology by integrating primary human colonic epithelial cells in a microfluidic organ-on-a-chip device. The Colon Chip produces a mucus layer with thickness and bilayered microstructure similar to the human colon.
The mucus layer in the human colon normally protects the intestinal epithelial cells against enormous numbers of luminal commensal bacteria and potential pathogens present in the gut lumen of healthy individuals.1, 2, 3, 4, 5 The human colonic mucus layer has a unique bilayer structure because it is composed of an inner layer that normally is impermeable to bacteria and a permeable outer layer.1,2,6 The integrity of the inner layer is most crucial in preventing direct contact of the bacteria with the colonic epithelium and associated chronic inflammation.1, 2, 3, 4 In addition, changes of mucus layer homeostasis can indirectly influence intestinal barrier function.6 Increased direct contact between these bacteria and the colonic epithelium can lead to gut barrier dysfunction and bacterial penetration through the epithelial tissue boundary. This can trigger injury and inflammation, for example, the mucus layer becomes penetrable to bacteria in dextran sodium sulfate–treated mice that develop colitis long before infiltration of immune cells is observed.4,5,7 Importantly, the inner colonic mucus layer in patients with ulcerative colitis, a common form of chronic inflammatory bowel disease affecting the colon,8 also has been shown to allow bacterial penetration.5,6 Prostaglandin E2 (PGE2) is increased during intestinal inflammation, as in patients with ulcerative colitis,9 in which it plays an essential role in wound healing.9, 10, 11 Recently, PGE2 also was shown to be a direct mediator of fluid secretion using intestinal epithelial organoids.12 This function is thought to be mediated mainly through activation of ion channels leading to ion flux–driven water flux into the lumen.12 In the past, short-term PGE2 treatment also has been reported to induce mucus secretion in an adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent manner mediated through activation of its receptor EP4 in murine intestinal loop studies and mouse proximal colon explants.13, 14, 15 However, the effect of PGE2 on the colonic mucus layer height and properties have been controversial and are not fully understood.13, 14, 15, 16
Unfortunately, it is impossible to study intestinal mucus physiology and changes in its behavior over time within the lumen of the living human colon to address these types of questions. Mucus physiology can be studied in vivo in animal models (eg, using intestinal loop studies)13,14; however, these methods are highly invasive, technically challenging, and often only low-resolution imaging is possible.17 More importantly, there are species-specific differences in mucus layer thickness and microstructure,1,2,6 and thus, there has been a search for new methods that could advance investigation in this area.6,17
Common challenges using in vitro cell culture models to investigate intestinal mucus physiology include the use of cancer-derived epithelial cells, such as Caco-2 and HT29-MTX cells, which results in secretion of the gastric mucin (MUC)5AC, but not typical intestinal MUC2.17,18 Primary human intestinal organoids can produce mucus, but because it is entrapped in the central lumen of the organoid, it is difficult to investigate its physiology.17 A few studies have shown accumulation of mucins on the surface of primary human ileal or rectal organoid fragments cultured on Transwell (TW) (Corning, Corning, NY) inserts, but these cultures only accumulate thin (<36 μm thick) mucus layers.19,20 Although a recent study reported obtaining a mucus layer of approximately 300-μm thickness by culturing human colonic epithelial cells on TWs under an air–liquid interface,21 this is still less than half of the thickness of the human colonic mucus layer (∼600 μm),6 and the inner mucus layer formed in vitro could be removed easily from the cell surface, which is not the case in vivo.22 Importantly, neither these cultures nor any other experimental in vitro method can reproduce the physiologically important bilayer structure that is seen in human colonic mucus.17 Thus, most studies of mucus biology rely on the use of short-lived ex vivo mouse or human tissue explants.22 Although most of our current understanding of the colon mucus layer originated from studies of tissue explants,4,6,15,22,23 they have significant limitations including the need for repeated access to clinical biopsy specimens. In addition, clinical samples can be used only once, they cannot be used for long-term (>1 day) cultures, and because the cells cannot be expanded in vitro, it is difficult to replicate results using the same donor.17
Human organ-on-a-chip (Organ Chip) microfluidic culture technology has been used to create a human Small Intestine Chip24,25 and Colon Chip,26 which are lined by primary human organoid-derived duodenal (Small Intestine Chip) or colonic intestinal epithelial cells (Colon Chip). These models present an alternative approach to confront this challenge. Organ Chips offer many advantages over the intestinal organoids from which they are derived, including the ability to continuously collect samples from both the luminal and abluminal compartments; the Small Intestine Chip also shows greater transcriptomic similarity to in vivo small intestine compared with organoids.25 In the present study, we set out to explore whether Organ Chip technology could be used to develop a method to recapitulate human colonic mucus layer formation in vitro.
Results
Reconstitution of a Polarized Colonic Epithelium
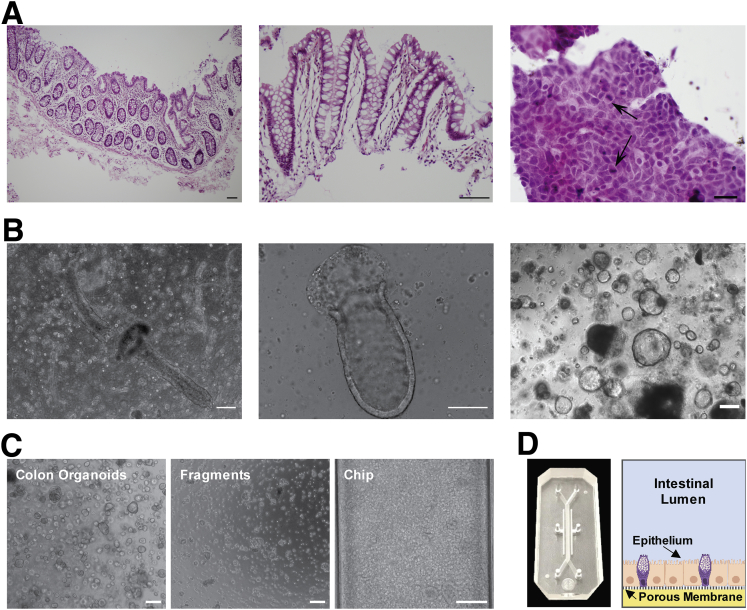
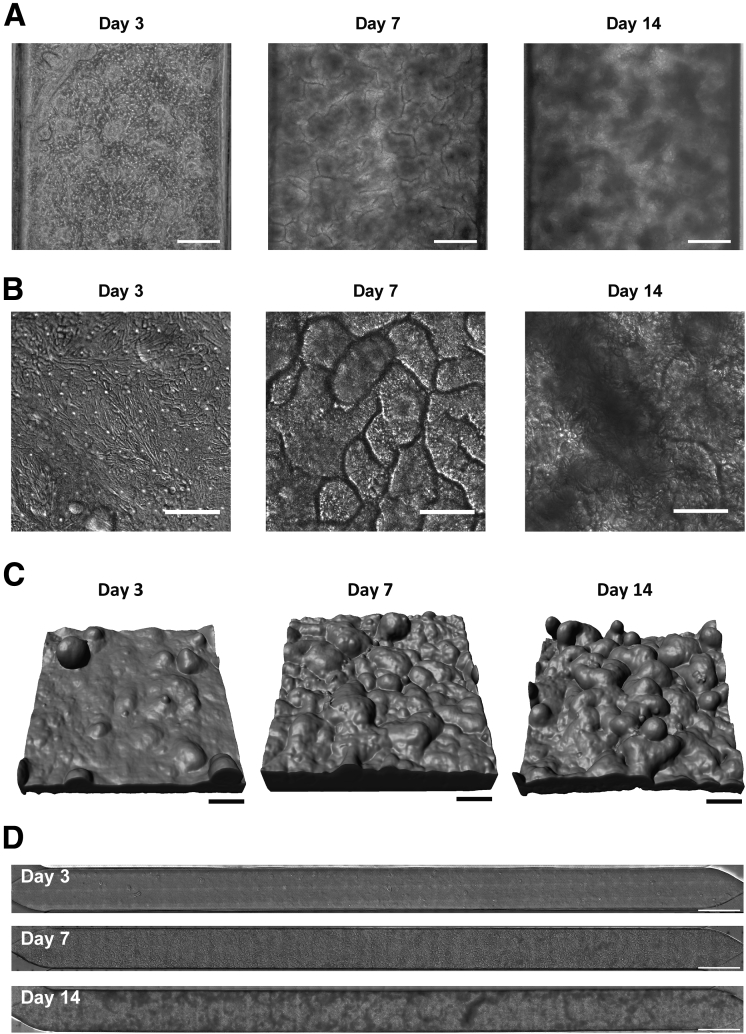
Primary colonic epithelial cells isolated from human colon resections and endoscopic biopsy specimens from the sigmoid and ascending colon (Figure 1A, Table 1) were first grown as colon organoids (Figure 1B), which then were fragmented and cultured on top of a porous extracellular matrix–coated membrane with 7-μm diameter pores in the top channel of a 2-channel microfluidic Organ Chip device composed of optically clear polydimethyl siloxane (PDMS), as previously described25,26 (Figure 1C and D). Hank’s balanced salt solution (HBSS) and stem cell expansion medium were perfused continuously (60 uL/h1) through the top (epithelial) and bottom channels, respectively. When cultured under these dynamic flow conditions, the human colonic epithelial cells formed a confluent flat monolayer by day 3 that developed into a dense undulating epithelial sheet lined by columnar colonic cells by day 7 (Figure 2A–C). The timeline of experiments is based on days after monolayer formation because there were small variations in the time course of differentiation (1–2 days) in different experiments and with different donors. The colonic epithelium retained this dense morphology for at least 2 weeks in culture (Figure 2A–C) and covered the entire channel uniformly (Figure 2D).
Figure 1.
Patient-derived human Colon Chip. (A) H&E-stained histologic sections (6 μm) of human colon in vivo. Colon tissue with intact epithelium, lamina propria, muscularis mucosa, and submucosa. Scale bar: 100 μm (left). Dissociated epithelium including lamina propria. Scale bar: 100 μm (middle). Smear of a 2-day colon organoid in Matrigel culture. Scale bar: 20 μm (right). Arrows indicate mitotically active epithelial cells. (B) Primary human colonic epithelium in Matrigel culture. Colonic crypts embedded in Matrigel after isolation from human colon resection (left). Shortening of crypt structure embedded in Matrigel 1 day after isolation (middle). Closed colonic crypts (colon organoids) in Matrigel culture 7 days after isolation (right). Scale bars: 200 μm. (C) Phase-contrast images of the preparation of primary human colonic epithelial cells for seeding on the Chip starting with colon organoids that are fragmented before seeding on the chip. Scale bars: 200 μm. (D) Photograph (left) and schematic cross-sectional view (right) of the primary human Colon Chip microfluidic device with the colonic epithelium cultured in the top channel on the upper surface of the porous membrane that separates it from the lower channel.
Table 1.
Donors Used in Experiments
| Organoid ID | Colon region | Goblet cells (number of experiments) | Mucus layer (number of experiments) |
|---|---|---|---|
| C07 | Sigmoid | Yes (2) | Yes (1) |
| C08 | Sigmoid | Yes (>5) | Yes (>5) |
| C09 | Sigmoid | Yes (1) | Yes (2) |
| H447 | Ascending | Yes (1) | Yes (1) |
| H480 | Ascending | Yes (1) | Yes (1) |
Figure 2.
Epithelium development in the Colon Chip after 3, 7, and 14 days of culture after monolayer formation. (A) Phase-contrast microscopic images of colonic epithelial cells cultured on the Colon Chip. Scale bars: 200 μm. (B) Differential interference contrast images of the colonic epithelium (viewed from above). Scale bars: 100 μm. (C) Three-dimensional confocal microscopic reconstruction of z-stack images of the epithelial cells based on F-actin staining. Images are representative of 2 independent experiments. Scale bars: 100 μm. (D) Differential interference contrast images of the full length of the channel of the Colon Chip. Images are representative of 2 independent experiments. Scale bars: 1000 μm.
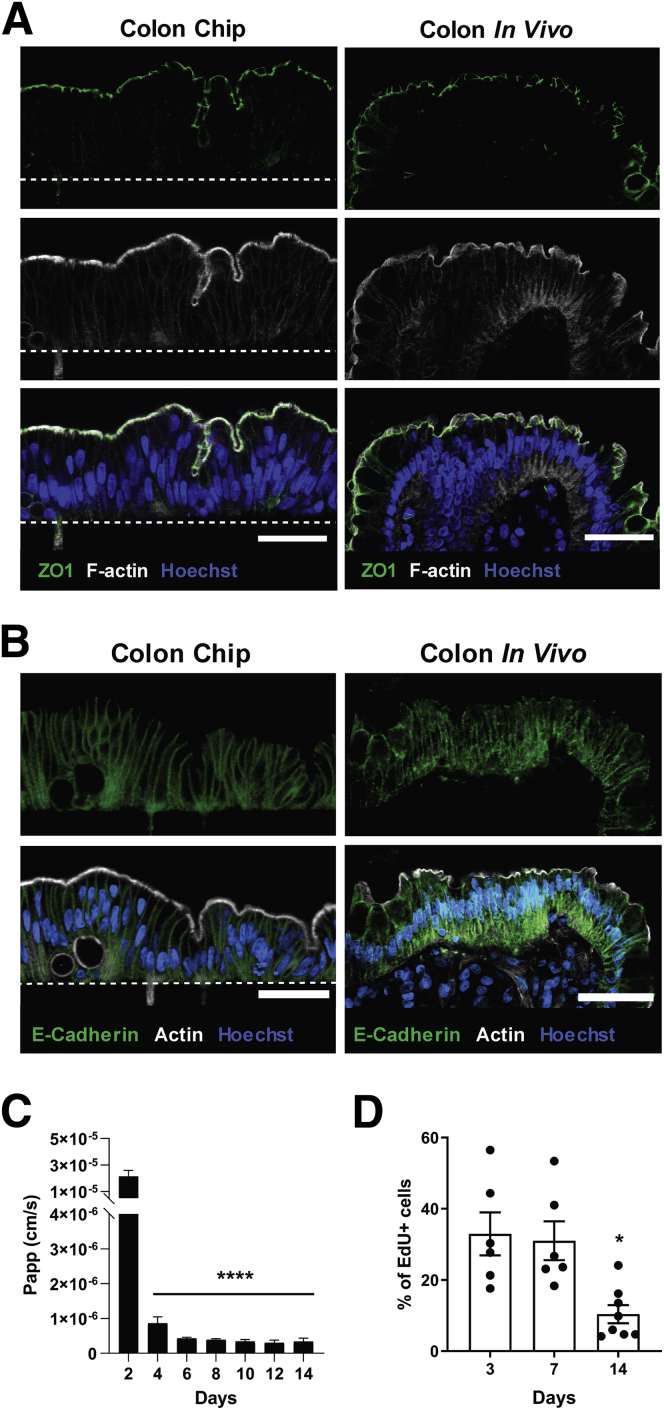
The polarized structure of the colonic epithelium is crucial for intestinal barrier function, as well as for uptake and transport of ions and water in the colon.27 Immunofluorescence confocal microscopy confirmed that the colonic cells formed a polarized columnar epithelium in the Colon Chip on day 7 that closely resembled that seen in human colon in vivo, as evidenced by restriction of nuclei to the basal cytoplasm and appearance of zonula occludens 1–containing tight junctions and an F-actin–rich brush-border along the apical surface of the epithelium (Figure 3A), as well as basolateral localization of E-cadherin–containing adherens junctions (Figure 3B). Analysis of barrier function measured with the small fluorescent tracer Cascade blue (550 daltons), confirmed that the colonic epithelium formed a tight intestinal barrier beginning at 4 days of culture on-chip, which was maintained over 2 weeks (Figure 3C), as previously observed in a human Small Intestine Chip.25 Importantly, under these culture conditions, the human colonic epithelium also retained a subpopulation of proliferating cells during the entire 2 weeks of culture (Figure 3D).
Figure 3.
Formation of a polarized colonic epithelium within the Colon Chip. (A) Immunofluorescence confocal microscopic images of histologic cross-sections of the Colon Chip (left) compared with human colon in vivo (right) showing a polarized epithelium with tight junctions labeled with zonula occludens 1 (ZO1) (green) and brush border stained for F-actin (gray) restricted to the apical regions, and Hoechst-stained nuclei (blue) localized at the cell base. Images are representative of 3 independent experiments. Scale bars: 50 μm. White dashed line indicates the top of the porous PDMS membrane in the Colon Chip. (B) Cross-sectional immunofluorescence confocal microscopic images of the Colon Chip (left) compared with human colon in vivo (right) showing a polarized epithelium with basolateral adherens junctions labeled with E-cadherin (green) and brush border stained for F-actin (gray) restricted to the apical regions, and Hoechst-stained nuclei (blue) localized at the cell base. Images are representative of 3 independent experiments. Scale bars: 50 μm. (C) Intestinal barrier function of the colonic epithelium measured over 14 days of culture on-chip by quantifying the apparent permeability (Papp) of Cascade blue (550 daltons) (n = 3–11 chips). ****P < .0001 compared with day 2. (D) Quantification of cell growth by measuring EdU incorporation over 18 hours using flow cytometry (n = 6–8 chips, 2 independent experiments compiled). *P < .05 compared with day 3 and day 7. All data represent means ± SEM.
Goblet Cell Differentiation
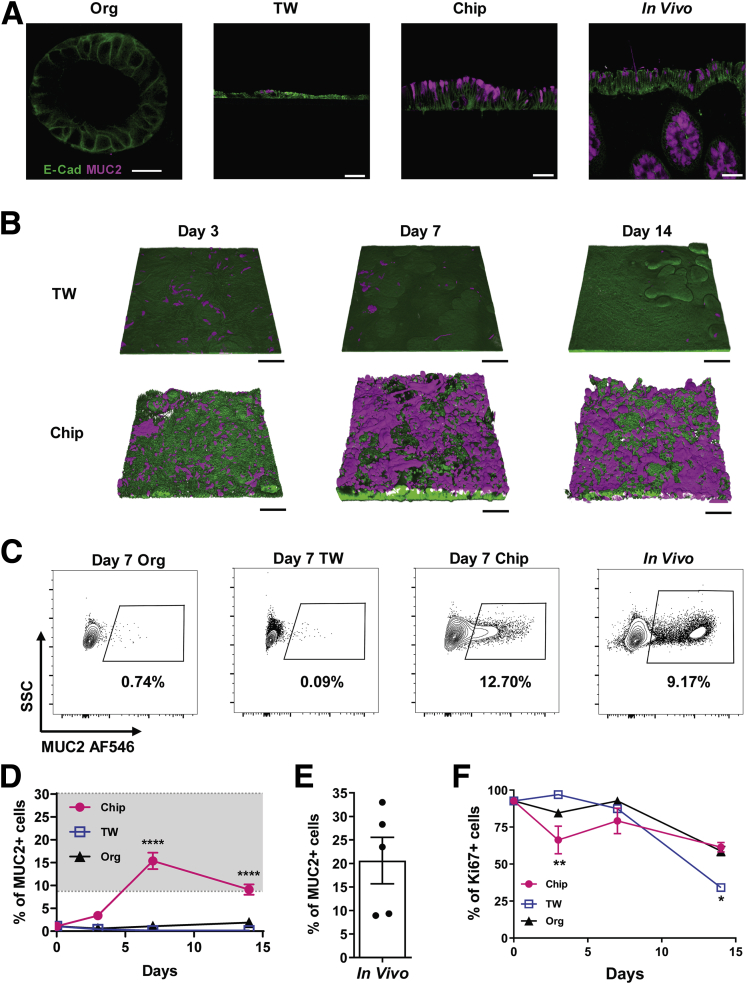
The presence of goblet cells in the colonic epithelium is a critical requirement for any study of mucus physiology because these are the specialized intestinal cells that produce and secrete MUC2, which is a major component of intestinal mucus.28 MUC2 polymers are densely packed in large secretory vesicles in goblet cells, which give the cells their typical goblet shape.28 As expected based on past work that showed stem cell expansion medium drives the proliferation of stem cells in organoid cultures,29 we found that our organoids, and TW cultures created using cells isolated from these organoids, formed few, if any, goblet cells at 1 week of culture when cultured in this medium (Figure 4A), and similar results were obtained even when TW cultures were maintained for 14 days (Figure 4B). Past work has shown that it is necessary to switch from stem cell expansion medium to a differentiation medium to induce goblet cell differentiation in primary human intestinal cells.19,20,29,30 Surprisingly, however, when the same organoid-derived colonic epithelial cells were cultured in the same stem cell expansion medium under microfluidic conditions in the Colon Chip, high levels of goblet cell differentiation were observed, as indicated by appearance of MUC2+ epithelial cells that were similar in morphology and number to those seen in histologic sections of human colon (Figure 4A). These MUC2+ cells could be detected as early as 3 days of culture and they expanded greatly in number by 1 to 2 weeks, covering larger areas of the epithelium (Figure 4B). Flow cytometric analysis of live epithelial cell populations harvested from colon organoids, TWs, Colon Chips, and human colonic tissue confirmed that there was little goblet cell differentiation in the TW and organoid cultures, whereas approximately 15% of the epithelial cells differentiated into goblet cells in the Colon Chip, which is similar to the percentage of goblet cells we measured in human colon tissue samples (∼10%–30% depending on the donor) (Figure 4C–E).
Figure 4.
Spontaneous goblet cell differentiation in the Colon Chip. (A) Cross-sectional confocal immunofluorescence microscopic images of epithelial cells in a colon organoid (Org), Transwell culture (TW), Colon Chip (Chip), and human colon tissue section (In Vivo) stained for E-cadherin (E-Cad) (green) and MUC2 (magenta). Cultures shown are 7 days after monolayer formation. Images are representative of 2 independent experiments. Scale bars: 20 μm (Org), and 50 μm (TW, Chip, In Vivo). (B) Three-dimensional reconstructions of confocal immunofluorescence microscopic z-stack images of epithelial cells in TW cultures and Colon Chips on days 3, 7, and 14 after monolayer formation showing epithelium stained for E-cadherin (green) and MUC2 (magenta). Images are representative of 2 independent experiments. Scale bars: 100 μm. (C) Contour plots of flow cytometric quantification of MUC2+ cells in colon Org, TW, Chip on day 7 of culture vs cells isolated from human colon tissue (In Vivo). (D) Graph showing the results of the full cytometric analysis on days 3, 7, and 14 (gray area indicates approximate range of in vivo values; n = 3–9 devices, 2 experiments compiled). ****P < .0001 compared with TW and Org. (E) Flow cytometric quantification of MUC2+ cells isolated from human colon tissue (n = 2 donors, 2–3 samples per donor). (F) Flow cytometric quantification of the percentage of Ki67-labeled proliferating cells in Chip, TW, and Org on days 3, 7, and 14 by flow cytometry (n = 3–8 devices, 2 independent experiments compiled). *P < .05, **P < .01. All data represent means ± SEM. SSC, Side Scatter.
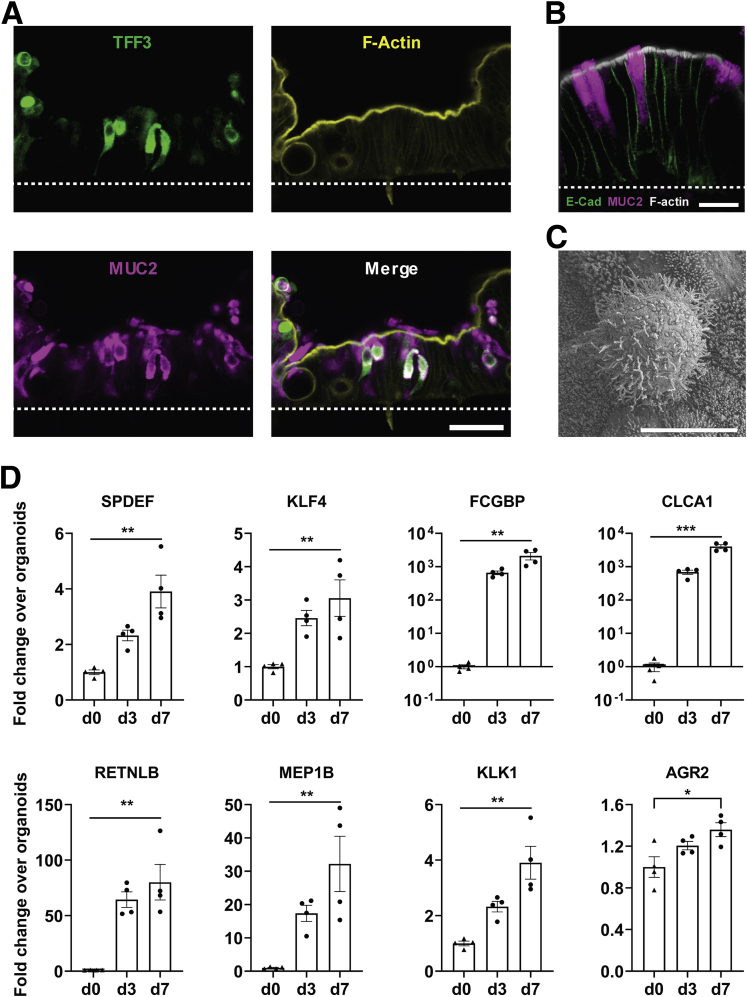
Importantly, despite supporting spontaneous goblet cell differentiation, the Colon Chip cultures were simultaneously able to maintain a proliferative cell subpopulation at levels similar to those present in the organoid and TW cultures (Figure 4F). Although past work has shown that some goblet cell differentiation can be induced in TW cultures and organoids by replacing the stem cell expansion medium with differentiation medium,19,29,31 these cultures cease proliferating and are short-lived. The MUC2+ cells in the Colon Chip also were positive for goblet cell marker Trefoil factor 3 (TFF3)28 (Figure 5A), accumulated MUC2-containing mucus granules apically (Figure 5B), and showed a surface morphology distinct from microvilli-covered enterocytes when analyzed by scanning electron microscopy (Figure 5C), which are similar to features observed in human goblet cells in vivo.28 Furthermore, over the course of the 7-day cultures, there were significant increases in expression of intestinal epithelial cell genes encoding the SAM pointed domain ETS factor and Kruppel-like factor 4 transcription factors that control goblet cell maturation in the human colon,32,33 as well as genes encoding multiple proteins present within mucus granules of human colonic goblet cells,28,34 including Fc-γ binding protein, chloride channel accessory 1, resistin-like molecule β, Meprin A subunit β, Kallikrein 1, and anterior gradient 2 protein disulfide isomerase family member, when compared with the cells when they were cultured as organoids (Figure 5D). Thus, the Colon Chip effectively provided an environment for goblet cell differentiation under conditions in which proliferative cells also remain present, as occurs in human colon in vivo, whereas other culture models do not.
Figure 5.
Differentiation of mature goblet cells in the Colon Chip. (A) Cross-sectional immunofluorescence confocal microscopic images of the Colon Chip stained for goblet cell markers TFF3 (green) and MUC2 (magenta), and for the brush-border marker F-actin (yellow). Images are representative of 2 independent experiments. White dashed line indicates the top of the porous membrane in the Colon Chip. Scale bar: 50 μm. (B) Higher magnification view of an immunofluorescence confocal micrograph showing cross-section of the epithelium within the Colon Chip containing goblet cells stained for MUC2 (magenta), E-cadherin (E-Cad) (green), and F-actin (gray). Image is representative of 2 independent experiments. Scale bar: 20 μm. (C) Scanning electron micrograph of the apical surface of a goblet cell within the epithelium cultured in the Colon Chip surrounded by enterocytes covered with apical microvilli. Scale bar: 5 μm. (D) Graphs showing the fold change in the levels of transcripts encoding SAM pointed domain ETS factor (SPDEF), Kruppel-like factor 4 (KLF4), Fc-g binding protein (FCGBP), chloride channel accessory 1 (CLCA1), resistin-like molecule β (RETNLB), Meprin A subunit β (MEP1B), Kallikrein 1 (KLK1), and anterior gradient 2, protein disulfide isomerase family member (AGR2) in colonic epithelial cells cultured in the Colon Chip on day 3 (d3) or day 7 (d7) relative to their levels in cells contained within colonic organoids before seeding on chip on day 0 (d0) (d0 = 1.0). *P < .05, **P < .01, and ***P < .001 compared with d0. All data represent means ± SEM.
Analysis of Intestinal Mucus Accumulation and Bilayer Structure in Living Cultures
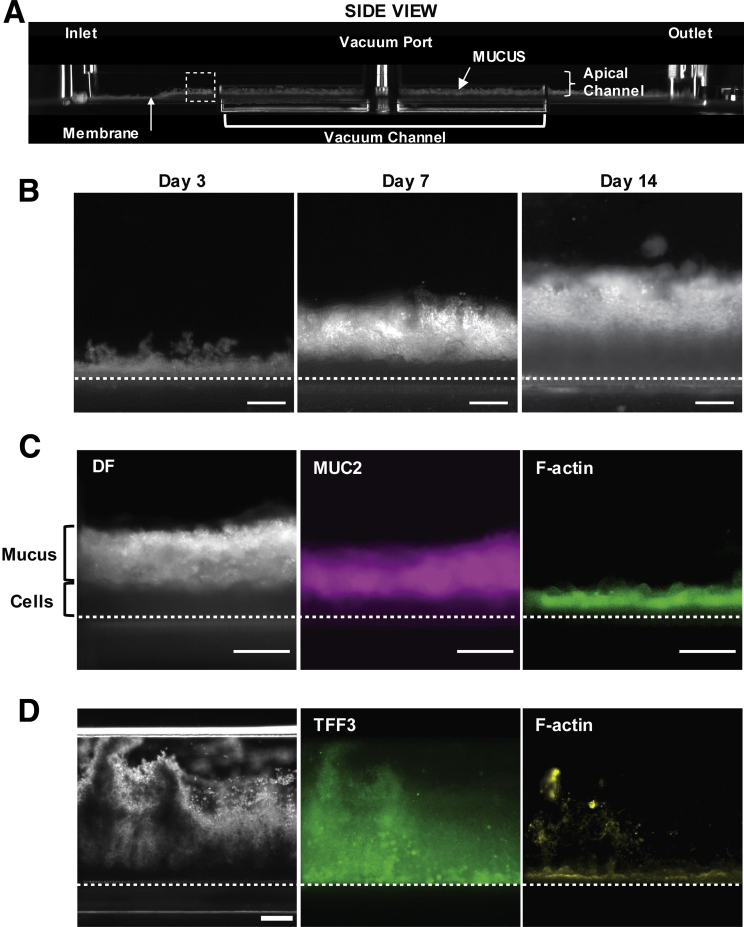
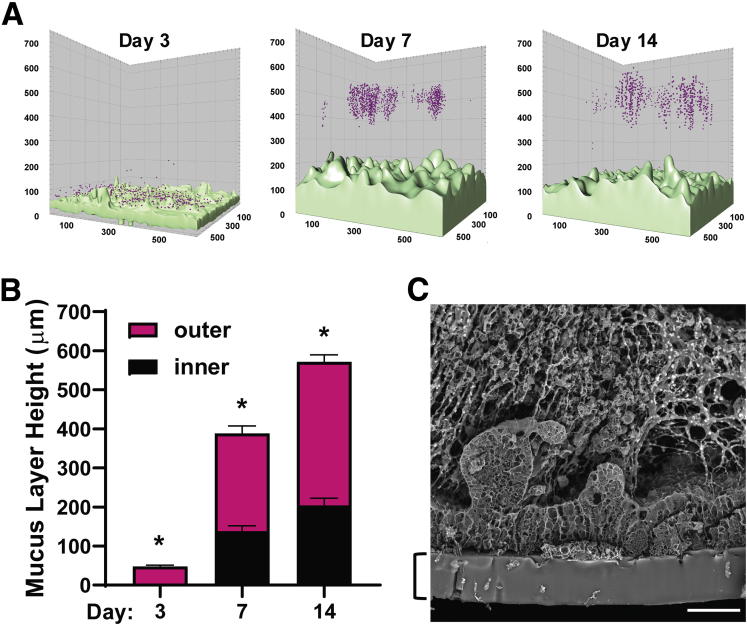
Given the spontaneous differentiation of large numbers of goblet cells in the Colon Chip that produce MUC2, which is the main mucin in colonic mucus, we next investigated if a physiologically relevant mucus bilayer forms on-chip. The existence of a mucus layer within the lumen of the apical epithelial channel was suggested by the appearance of increasing opacity of the Colon Chip over time when viewed from above by light microscopy (Figure 2A and D). Importantly, because the microfluidic Organ Chip is optically clear and has defined linear channel geometries (Figure 1D), we were able to develop a method to visualize live cultures in cross-sections across the entire channel. This was accomplished by slicing approximately 2 mm of the PDMS material away from the sides of the upper and lower channels, then turning the device 90° on its side, and analyzing it using dark-field microscopy (Figure 6A and B). By using this method, we detected the formation of a high light-scattering layer above the cells that was well developed by 1 week and when we analyzed the same chips over time, it continued to increase in height over the following week (Figure 7A and B ). This layer stained positively for MUC2 (Figure 7C) and TFF3 (Figure 7D), and resided on top of the apical F-actin–rich brush border of the epithelium (Figure 7C and D), confirming that this material that accumulated over time in the Colon Chip is indeed a thick mucus layer. Fixation of intestine tissue with paraformaldehyde has been shown to produce a compression artifact (thinning of the layer) and disrupt the inner layer17,35; thus, an important advantage of this method is that it enables the in situ study of mucus development in its native state over an extended period of time in living cultures.
Figure 6.
Method for noninvasive visualization of mucus layer height on-chip using dark-field microscopy. (A) Schematic of how the chip is prepared for side-view imaging. (B) Whole-chip side-view images of live Colon Chips on days 3, 7, and 14 by dark-field microscopy showing cells and the overlying mucus layer over time.
Figure 7.
Development of MUC2+ mucus layer on Colon Chip over time. (A) Dark-field (DF) microscopic image of the side of an optically clear Colon Chip showing the entire length of the device with horizontal microfluidic upper and lower channels along its length, as well as an upper inlet, outlet, and vacuum port; a side vacuum channel also is apparent in this view. Note that mucus can be detected in the bottom half of the apical channel above the epithelium, which is cultured on the horizontal membrane that separates the upper and lower channels. The dashed square corresponds to regions shown in panels B–D. (B) DF images of the same living Colon Chip on days 3, 7, and 14 after monolayer formation showing a reflective mucus layer overlying the epithelium that progressively increases in thickness over time above the darker cell region The dashed line indicates a porous membrane. Images are representative of 3 independent experiments. Scale bars: 200 μm. (C) Higher-magnification images of a region similar to the area shown in the dashed square in panel A within a Colon Chip fixed on day 7, showing the presence of a thick mucus layer visualized by DF microscopy and MUC2 staining (MUC2) overlying the F-actin–rich brush border of the colonic epithelium (F-actin). The dashed line indicates a porous membrane. Images are representative of 2 independent experiments. Scale bars: 200 μm. (D) Side-view images of Colon Chip fixed on day 7, showing the presence of a thick mucus layer visualized by DF microscopy and TFF3 staining (TFF3) overlying the F-actin–rich brush border of the colonic epithelium (F-actin). The dashed line indicates a porous membrane. Images are representative of 2 independent experiments. Scale bar: 200 μm.
One of the unique properties of human colonic mucus is its bilayer structure, which is characterized by an inner layer that is impenetrable by bacteria and an outer penetrable mucus layer that normally is populated by commensal microbes. This feature of the mucus layer has been probed previously in tissue explants using fluorescent 1-μm diameter microbeads to mimic bacteria.22 When we flowed similar fluorescent microbeads through the lumen of the epithelial channel and allowed them to settle, we observed a bead-free region approximately 200 μm in height above the apical membrane of the epithelial cells on days 7 and 14 (Figure 8A and B), which is similar to the thickness of the dense impenetrable inner mucus layer observed in human colon tissue explants using this method.22 The spread of the beads trapped in the mucus above the impenetrable layer corresponded to the penetrable outer mucus layer (Figure 8A and B). When we quantified the total height of the mucus layer (both inner and outer layers) formed on-chip, we found that it reached approximately 570 μm by day 14 (Figure 8B). Scanning electron microscopic analysis of a cross-section of the Colon Chip also showed the presence of a fibrous network within the thick mucus layer in tight contact with the upper surface of the epithelium (Figure 8C), which again is similar to what has been observed in tissue explants analyzed using lectin staining.36 Thus, the microfluidic human Colon Chip is an in vitro method that supports spontaneous accumulation of a mucus layer in tight apposition to the apical surface of a cultured human colonic epithelium that replicates the thickness and unique bilayer properties shown by human colonic mucus in vivo.
Figure 8.
Formation of a mucus bilayer in the human Colon Chip. (A) A pseudocolor 3-dimensional reconstruction of representative z-stack confocal images of the Colon Chip perfused with 1-μm fluorescent beads (magenta) 3, 7, or 14 days after monolayer formation; the cells were live stained with calcein AM (light green). Images are representative of 2 independent experiments. (B) Quantification of the thickness of the inner (impenetrable) and outer (penetrable) mucus layers based on the distribution of the beads (n = 3 chips, 3 independent regions per chip; all values were significantly different between all days). *P < .05 for the inner and outer layers. Similar results were obtained in 2 independent experiments. (C) Scanning electron micrograph of a cross-section of the epithelium within a Colon Chip showing the presence of a filamentous network within the mucus layer in direct contact with the apical surface of the cells. The bracket indicates the PDMS membrane. Image is representative of 2 independent experiments. Scale bar: 50 μm. All data represent means ± SEM.
Modeling the Response of Colonic Epithelium to PGE2 on-Chip
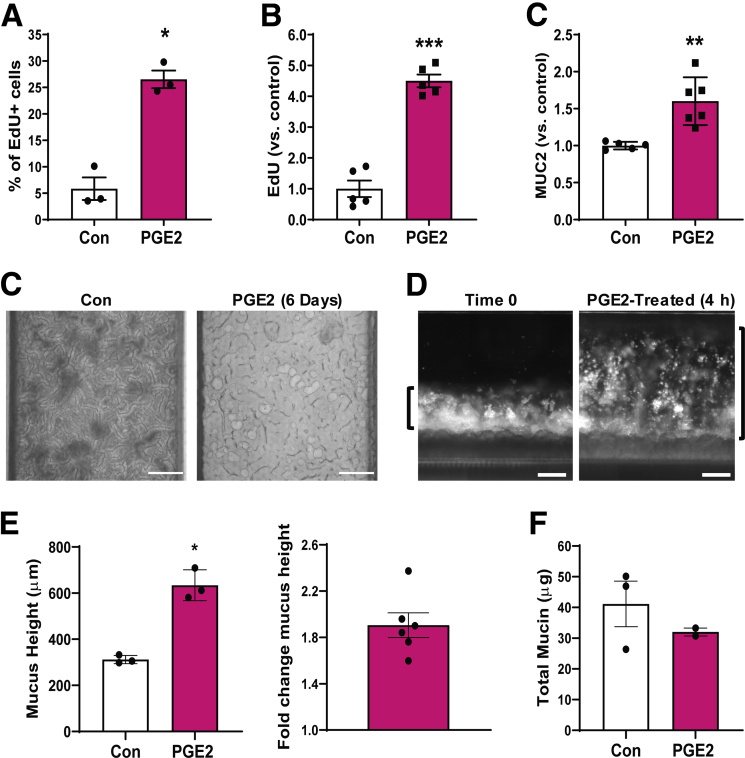
PGE2 has been reported to be increased in patients with ulcerative colitis, and it appears to contribute to healing of intestinal ulcers by increasing cell proliferation and altering mucus physiology.9, 10, 11,13, 14, 15 To explore if the human Colon Chip method can be used to study this response in vitro, we perfused PGE2 (1.4 nmol/L) through the basal channel from days 2 to 8. This 6-day exposure to PGE2 resulted in a 4.5-fold increase in proliferating cells compared with control chips, as measured by 5-Ethynyl-2′-deoxyuridine (EdU) incorporation (Figure 9A), as well as a 1.6-fold increase in MUC2+ cells (Figure 9B). The increase in EdU incorporation is consistent with reports that PGE2 promotes cell proliferation and is essential for wound healing.9, 10, 11
Figure 9.
PGE2-induced mucus layer swelling on-chip. (A) Graph shows quantification of EdU incorporation measured over 18 hours in epithelial cell monolayers within Colon Chips after 6 days of PGE2 treatment compared with untreated controls (Con) detected using flow cytometry (n = 3 chips per treatment). Graphs showing changes in (B) EdU incorporation measured over 18 hours and (C) MUC2+ cells in epithelial monolayers within Colon Chips after 6 days of PGE2 treatment relative to untreated controls (control value set to 1) detected using flow cytometry (n = 5 chips for panel A; n = 5–6 chips for panel B; 2 independent experiments compiled). (C) Bright-field images from above Colon Chips that were untreated or treated with PGE2 for 6 days. Scale bars: 200 μm. (D) Dark-field side-view images of a Colon Chip with epithelial cells and mucus layer before and after PGE2 treatment for 4 hours. Images are representative of 5 independent experiments. Scale bars: 200 μm. (E) Change in mucus height after 4 hours of PGE2 treatment over the mucus height before treatment in the same chips (n = 3–6, similar results were obtained in 5 independent experiments). (F) Total mucin amount per chip with and without 4-hour PGE2 treatment (n = 2–3) (not statistically significant by t test). *P < .05, **P < .01, and ***P < .001 compared to con. All data represent means ± SEM.
In these studies, we noticed that the PGE2-treated chips developed blockage of the apical channel, which prevented fluid flow, suggesting that there also might be increased mucus production. This was surprising because the PGE2-treated Colon Chips appeared to contain less light-obscuring material compared with control Chips when imaged from above by bright-field microscopy (Figure 9C), which stands in direct contrast to the increased opacity associated with the mucus layer development we observed in our earlier studies. Past animal and tissue explant studies exploring the effect of PGE2 on mucus production have produced contradictory results. Short-term PGE2 treatment has been reported to increase colonic mucus accumulation in murine intestinal loop studies and mouse proximal colon explants,13, 14, 15 but no increase in mucus secretion could be detected in response to PGE2 treatment in isolated human colonic crypts16 or distal colon mouse tissue explants.15
Thus, we set out to evaluate the short-term effect of PGE2 on mucus layer height using the human Colon Chip. Short-term (4 h) treatment of the Colon Chip with PGE2 resulted in an increase in mucus height of 321.8 ± 44.3 μm, which is equivalent to an approximately 2-fold increase compared with control chips (Figure 9D and E). Interestingly, however, there did not appear to be an associated increase in the total amount of mucins when quantified using an Alcian blue–binding assay (Figure 9F). This result suggests that the increase in height we detected was not the result of secretion of more mucin materials, but rather of swelling of the pre-existing mucus.
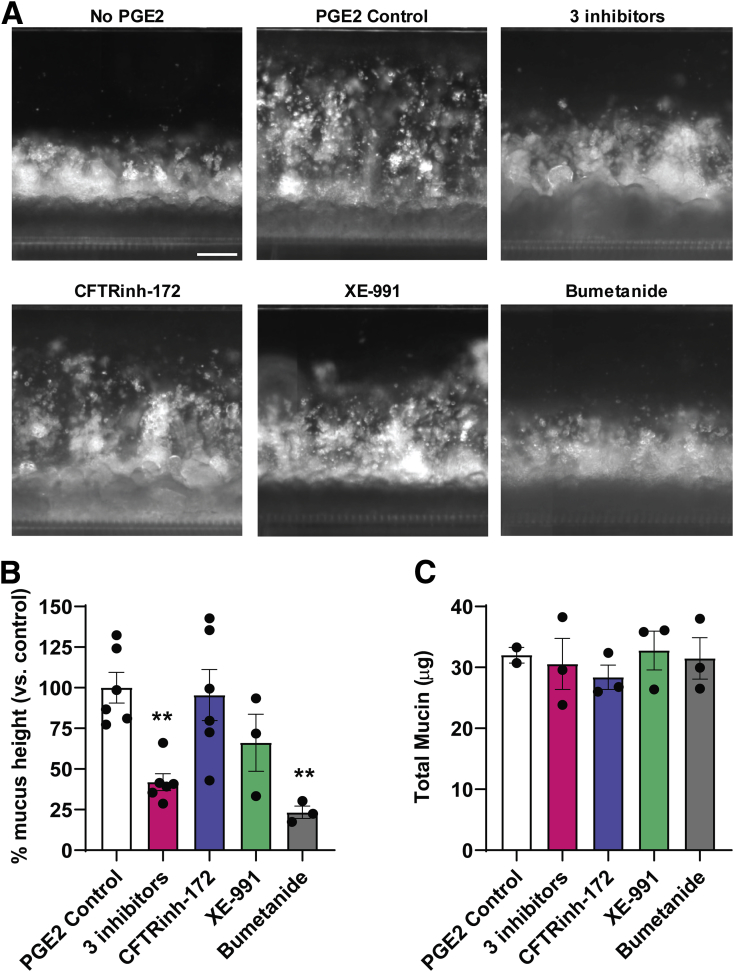
Although PGE2 has a multitude of functions in intestinal physiology, it plays an important role in the regulation of fluid secretion via ion transport.12 Because mucus production has been shown to depend on fluid secretion in mouse small intestine,37,38 we tested if this property of PGE2 was responsible for its ability to increase mucus height rapidly. PGE2-induced fluid secretion can be blocked by inhibiting ion channels,12 therefore we pretreated the human Colon Chip with a combination of 3 different ion channel inhibitors, CFTRinh-172, XE-991, and bumetanide, which are chemical inhibitors of the cystic fibrosis transmembrane conductance regulator (CFTR), KV7 voltage-gated potassium channel (KCNQ), and Na-K-Cl cotransporter 1 (NKCC1) respectively, before infusing PGE2. The combination of the 3 different ion channel inhibitors led to a significant reduction in the PGE2-induced increase in mucus height, confirming the importance of ion transport in this hydration response (Figure 10A and B).
Figure 10.
Modulation of PGE2-induced changes in mucus layer thickness by ion channel inhibition. (A) Dark-field side-view images of a Colon Chip with epithelial cells and mucus layer before (no PEG2) and after PGE2 treatment for 4 hours without (PGE2 control) or with CFTRinh-172 (50 μmol/L), XE-991 dihydrochloride (20 μmol/L), bumetanide (100 μmol/L), or a combination of all 3 ion channel inhibitors. Images are representative of 2 independent experiments. Scale bar: 200 μm. (B) Quantification of changes in mucus layer height based on the dark-field images shown in panel A and (C) the total mucin production measured using an Alcian blue assay induced by PGE2 in the absence (PGE2 control) or presence of CFTRinh-172 (50 μmol/L), XE-991 dihydrochloride (20 μmol/L), bumetanide (100 μmol/L), or a combination of all 3 ion channel inhibitors (data are presented relative to control chips treated with PGE2 alone; n = 2–6; 2 independent experiments compiled). **P < .01 vs control. All data represent means ± SEM.
To analyze the effects of each ion channel inhibitor individually, different Colon Chips were pretreated with each of these ion channel inhibitors before exposing them to PGE2. Suppression of the CFTR and Kv7 K+ channel activity had no significant effect, however, inhibition of the basolateral NKCC1 Na-K-Cl cotransporter with bumetanide significantly reduced PGE2-induced mucus layer height on-chip (Figure 10A and B). None of these channel inhibitors altered the total mucin content (Figure 10C). Thus, the increase in mucus height we observed on short-term exposure to PGE2 largely was owing to ion and fluid secretion–induced swelling of pre-existing mucus. The reduction of mucus swelling by inhibition of NKCC1, but not CFTR or Kv7 K+, could be owing to the fact that NKCC1 is a co-transporter of multiple different ions (Na+, 2 Cl-, K+), whereas both other ion channels transport only single ions (Cl- or K+, respectively). This could indicate that PGE2-induced swelling of the mucus layer involves multiple different ions rather than just one single ion type.
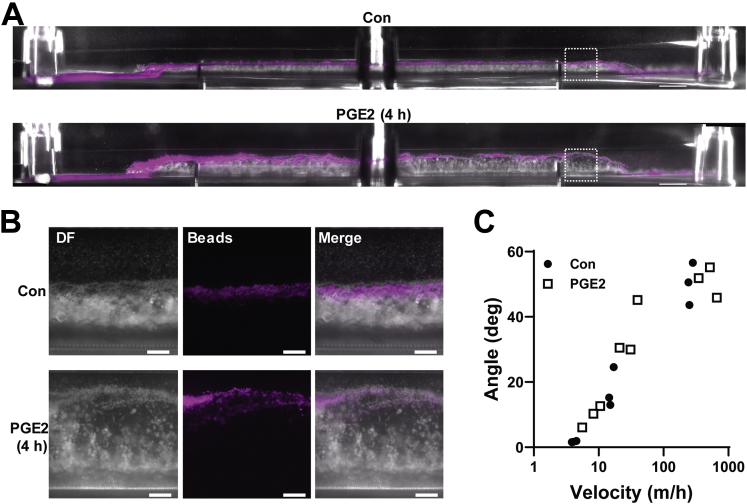
Because the structural integrity of the colonic mucus layer is an essential part of the intestinal barrier, local changes in mucin organization or concentration owing to swelling could affect structural properties of the mucus as well. Notably, the inner impenetrable mucus layer was preserved during PGE2-induced swelling of the mucus layer (Figure 11A and B). Furthermore, when we exposed the mucus layers of control and PGE2-treated Colon Chips to increasing flow velocities, we found that the mucus layers in both chips showed similar bending angles, and, hence, similar material responses to shear stress (Figure 11C, Supplementary Video 1). This suggests that despite changes in the hydration state of the mucus layer, its gross structural integrity was maintained.
Figure 11.
Inner mucus layer is not affected by PGE2. (A) Side view of combined dark-field and fluorescent image of a Colon Chip with a mucus layer labeled with 1-μm fluorescent beads (magenta) without (control [Con]) and with PGE2 treatment for 4 hours (PGE2 [4 h]). The dashed square indicates a region of the chips represented in the higher-magnification images in panel B. Images are representative of 2 independent experiments. Scale bars: 1000 μm. (B) Side-view images of Colon Chip with or without 4 hours of PGE2 treatment with 1-μm fluorescent beads (magenta) overlaid on dark-field (DF) images. Images are representative of 2 independent experiments. Scale bars: 200 μm. (C) Response of the mucus layer to shear forces measured in angle over increasing velocity, with a higher angle being equivalent to stronger bending of the mucus layer (each data point represents 1 chip, n = 3 per treatment). No significant difference by linear regression fits between PGE2 and Con.
Discussion
Although the structure and function of the human colonic mucus bilayer is highly relevant for intestinal pathophysiology, previous investigation of its properties could be performed only in short-term (<1 day) ex vivo tissue explants. Although a recent study using colonic epithelial cells cultured under an air–liquid interface in TWs showed mucus layer accumulation,21 the mucus layer was thinner and more readily removed than observed in vivo, and this required use of a differentiation medium that depleted stem cells,19,20,29,30 which limited its ability to perform long-term experiments or recovery studies. The key point, however, is that none of the past studies using human colonic epithelial cells cultured in TWs, organoids, or any other in vitro model17 resulted in the production of a thick mucus layer with a normal bilayer structure similar to that seen in vivo. In contrast, in the present study, we showed that a microfluidic 2-channel human Colon Chip enables long-term culture of primary human colonic epithelial cells under dynamic flow conditions. Moreover, this system supports the spontaneous differentiation of large numbers of highly differentiated, mucus-producing goblet cells at similar levels to those observed in human colon in vivo, while still maintaining a healthy subpopulation of proliferative cells. Importantly, under these culture conditions, the human colonic epithelial cells produced a mucus bilayer containing an impenetrable layer closely apposed to the apical surface of the epithelium, directly overlaid by a penetrable mucus layer, with a total thickness of 500–600 μm, which is similar to that seen in living human colon.22 Thus, this culture method recapitulates the development of a thick human colonic mucus layer with its unique bilayer structure.
Previous work has shown that germ-free mice do not have an inner mucus layer and weeks of bacterial colonization are required for an inner mucus layer to form.39 In contrast, in this human Colon Chip, the inner mucus layer is established without any bacteria being present on-chip. Because the epithelial cells cultured on the Colon Chip come from patients who had been in contact with a complex microbiome before their isolation as organoids, it is possible that they were influenced by this experience (eg, epigenetically) and no longer require their continued presence to form an inner mucus layer. However, because the inner mucus layer in germ-free mice is penetrable to microbeads,39 whereas the Colon Chip and the inner layer in human colon are not, this could be related to species-specific differences between human beings and mice.
Another novel feature of the human Colon Chip method is that the optical clarity of the microfluidic device allows live noninvasive visual analysis of mucus accumulation and physiology over time in culture. The dynamic changes in mucus layer thickness induced in vivo by the inflammatory mediator PGE2 could be replicated, quantified, and analyzed on-chip. This showed that rapid changes in mucus layer height after short-term exposure to PGE2 are mediated primarily by altering the hydration state of pre-existing mucus via ion secretion through NKCC1, and not as a result of additional mucus secretion. However, suppression of NKCC1 did not fully abolish the effect of PGE2, indicating that other ion channels may be involved in PGE2-induced mucus swelling. Although analysis of the pathways that mediate these processes is beyond the scope of this study, changes in cAMP-mediated HCO3- secretion38,40 or in PGE2-mediated glycosylation of mucins41 could be involved. Thus, to further dissect the mechanism of water-mediated mucus swelling in response to PGE2, future studies could be performed in which each type of ion is removed individually from the culture medium, as performed previously,12,38 and the role of cAMP induction could be explored as well.
Furthermore, our data indicate that the colonic mucus may be able to undergo significant expansion without losing barrier function or structural stability, highlighting the remarkable characteristics of this physiologically important structure. These findings show the usefulness of the Colon Chip as an in vitro tool for evaluation of mucus structure and function, which could advance our understanding of mucus physiology in disease contexts. Considering recent advances in bacterial co-cultures in intestinal microfluidic models,26,42, 43, 44 this microfluidic Colon Chip lined by patient-derived colonic epithelial cells also may facilitate the development of new therapeutics or probiotics that modulate the mucus barrier, as well as provide a novel testbed for personalized medicine.
Materials and Methods
Isolation of Human Colon Epithelial Cells
Human colon epithelium was isolated from colon resections or endoscopic tissue biopsy specimens (Table 1). Full-thickness pieces of the human colon were obtained anonymously from healthy regions of colonic resection specimens processed in the Department of Pathology at Massachusetts General Hospital under an existing Institutional Review Board–approved protocol (#2015P001859). Specimens were restricted to those with (nonneoplastic) disorders, and regions collected for isolation were determined to be healthy based on gross examination. Endoscopic biopsy specimens were collected from macroscopically grossly unaffected regions of the colon of de-identified pediatric and young adult patients undergoing endoscopy for abdominal complaints. Informed consent and developmentally appropriate assent were obtained at Boston Children’s Hospital from the donors’ guardian and the donor, respectively. All methods were performed in accordance with the Institutional Review Board of Boston Children’s Hospital approval (protocol number IRB-P00000529).
For the isolation of colonic crypts, colon resections were processed by removing the colon epithelium with lamina propria, and then the epithelial layer or the entire biopsy specimen was digested with 2 mg/mL collagenase I (17100-017; Thermo Fisher Scientific, Waltham, MA) supplemented with 10 μmol/L Y-27632 (Y0503; Sigma-Aldrich, St. Louis, MO) for 40 minutes at 37°C with intermittent agitation, as described.25,45 Colon organoids were grown embedded in growth factor–reduced Matrigel (354230, lot 7317015; Corning, Corning, NY) and stem cell expansion medium was supplemented with 10 μmol/L Y-27632.19,25 Stem cell expansion medium was composed of advanced Dulbecco’s modified Eagle medium F12 (12634-010; Thermo Fisher Scientific) supplemented with the following: L-Wnt3a, R-spondin, noggin–conditioned medium (65% vol/vol) (produced by the CRL-3276 cell line; American Type Culture Collection, Manassas, VA), 1× GlutaMAX (35050-061; Thermo Fisher Scientific), 10 mmol/L HEPES (15630-106; Thermo Fisher Scientific), recombinant murine epidermal growth factor (50 ng/mL) (315-09; Peprotech, Rocky Hill, NJ), 1× N2 supplement (17502-048; Thermo Fisher Scientific), 1× B27 supplement (17504-044; Thermo Fisher Scientific), 10 nmol/L human (Leu15)-gastrin I (G9145; Sigma-Aldrich), 1 mmol/L N-acetyl cysteine (A5099; Sigma-Aldrich), 10 mmol/L nicotinamide (N0636; Sigma-Aldrich), 10 μmol/L SB202190 (S7067; Sigma-Aldrich), 500 nmol/L A83-01 (2939; Tocris, Bristol, UK), and primocin (100 μg/mL) (ant-pm-1; InvivoGen, San Diego, CA).
Colon Chip Cultures
The Colon Chip uses the exact same chip device design as our previously described Small Intestine Chip,24, 25, 26 but the origin of the organoids is intestinal region–specific. The Organ Chip devices are composed of PDMS and contain 2 parallel microchannels (width × height: apical channel, 1000 × 1000 μm; basal channel, 1000 × 200 μm), separated by a 50-μm thick PDMS porous membrane (7-μm pore diameter, 40-μm spacing), purchased from Emulate, Inc (CHIP-S1 Stretchable Chip, RE00001024 Basic Research Kit; Emulate, Inc, Boston, MA). After activation of the channel surfaces with 0.5 mg/mL sulfo-SANPAH solution (A35395; Thermo Fisher Scientific), the inner surfaces of both channels and the porous PDMS membrane were coated with 200 μg/mL rat tail collagen type I (354236; Corning) and 1% Matrigel (354230, lot 7317015; Corning) in Dulbecco’s phosphate-buffered saline (DPBS), as previously described.25,26 Colon organoids then were isolated from Matrigel by incubating in cell recovery solution (354253; Corning) for 40 minutes on ice and then spun down at 400g for 5 minutes at 4°C. The colonic organoids were fragmented by incubating them in TrypLE Express Enzyme (12605010; Thermo Fisher Scientific), diluted in DPBS 1:1 (vol:vol), and supplemented with 10 μmol/L Y-27632 (2 mL/well of a 24-well plate) for 1 minute 45 seconds in a 37°C water bath. After adding the same volume of stem cell expansion medium with 10 μmol/L Y-27632, the organoid fragments were spun down at 400g for 5 minutes at 4°C and then resuspended at 6 × 106 cells/mL. The colonic organoid fragments were seeded on the extracellular matrix (ECM)-coated membrane in the apical channel of the Colon Chip (6 × 105 cells/cm2) in stem cell expansion medium supplemented with 10 μmol/L Y-27632 while filling the basal channel with the same medium, and the chips were incubated overnight at 37°C under 5% CO2 to promote cell adhesion. The following day, both channels were washed once with stem cell expansion medium, and then the chips were inserted into Pod portable modules (RE00001024 Basic Research Kit; Emulate, Inc) and placed within a ZOË culture instrument (Emulate, Inc), where they were perfused (60 μL/h) with stem cell expansion medium in the basal channel and HBSS with calcium and magnesium (21-023-cv; Corning) supplemented with 100 μg/mL primocin (ant-pm-1; InvivoGen) in the apical channel. The timeline of experiments is stated as days after monolayer formation because there was a small amount of variability (1–2 days) in terms of the time required for monolayer formation between different experiments and donors. Table 1 lists all donors used in these studies.
TW Culture Inserts
TW culture inserts (6.5 mm) with a 0.4-μm pore polyester membrane (3470; Corning) were coated as described earlier for the chip, and seeded with colon organoid fragments at the same density (6 × 105 cells/cm2) on the top side of the TWs in stem cell expansion medium supplemented with 10 μmol/L Y-27632, and the same medium was added to the bottom chamber. Similar to the chips, the TWs were incubated overnight at 37°C under 5% CO2, and the following day, the TWs were washed once with stem cell expansion medium before adding 1 mL stem cell expansion medium on the basal side and 250 μL of HBSS with calcium and magnesium supplemented with 100 μg/mL primocin to the apical side; medium was changed every 2 days thereafter.
Organoid Cultures
Colon organoid fragments were resuspended in growth factor–reduced Matrigel at 1 × 106 cells/mL and plated in 24- or 48-well plates (50 or 10 μL drops/well, respectively), and covered with stem cell expansion medium supplemented with 10 μmol/L Y-27632 (500 μL or 200 μL/well, respectively). Stem cell expansion medium was changed every 2 days thereafter.
Immunofluorescent Microscopy
Colon Chips were fixed with 200 μL of 2% paraformaldehyde (PFA) (15730; Electron Microscopy Sciences, Hatfield, PA), 25 mmol/L HEPES (15630-080; Thermo Fisher Scientific) in DPBS (14190-144; Gibco, Waltham, MA), with 200-μL filter tips at 4°C on a rocker overnight. TWs and organoids were fixed at room temperature for 15 minutes. Chips were either stained directly or sectioned at 250 μm with a vibratome (VT1000S; Leica, Wetzlar, Germany). Fresh-frozen, 7-μm, in vivo, tissue sections were fixed with 2% PFA for 12 minutes at 4°C. All samples were blocked and permeabilized using 0.1% Triton X-100 (Sigma) and 5% bovine serum albumin (BSA) (A2153; Sigma) in DPBS for 1 hour at room temperature. Samples then were stained overnight at 4°C in 2% BSA in DPBS with the following primary antibodies: anti-MUC2 (H-9, sc-515106, 1:100; Santa Cruz, Dallas, TX), anti–E-cadherin (HECD-1, ab1416, 1:100; Abcam, Cambridge, UK), anti–zonula occludens 1 (ZO1-1A12, 33-9100, 1:200; Abcam), anti-TFF3 (EPR3974, ab108599, 1:200; Abcam). The next day, after 3 washes of phosphate-buffered saline (PBS), samples then were stained overnight at 4°C in 2% BSA in DPBS containing secondary antibodies and phalloidin: goat anti-mouse IgG1 Alexa Fluor 647 (A-21240, 1:100; Invitrogen, Carlsbad, CA), goat anti-mouse IgG2b Alexa Fluor 555 (A-21147, 1:500; Invitrogen), phalloidin Alexa Fluor 488 (A12379, 1:200; Invitrogen), and phalloidin Alexa Fluor 647 (A22287, 1:200; Invitrogen). The next day, after 3 DPBS washes, staining with Hoechst 33342 (H3570, 1:2000; Life Technologies, Carlsbad, CA) for 30 minutes was performed.
Images were taken using a Leica SP5 laser scanning confocal immunofluorescence microscope with a 680- to 1080-nm, multiphoton, pulsed IR laser Chameleon Vision 2 with precompensation and Non-Descanned Detectors; a 470- to 670-nm white-light laser; and a 488-nm argon laser coupled to HyD detectors. Acquired images were analyzed using IMARIS software (Bitplane, Zurich, Switzerland).
Flow Cytometry
For assessment of proliferating cells, culture medium (stem cell expansion medium basally, HBSS apically) containing 10 μmol/L EdU (Click-iT Plus EdU Alexa Fluor 350 Flow Cytometry Assay Kit, C10645; Invitrogen) was perfused through the apical and basal channels of the chip for 18 hours before harvesting cells. Cells were isolated from the Colon Chips and TWs by incubation in 1 mg/mL collagenase IV in TrypLE Express Enzyme supplemented with 10 μmol/L Y-27632 (100 μL/channel in the chips; 100 μL above and 500 μL below the membrane in TWs) for 1 hour at 37°C. Detached cell fragments were incubated for an additional 45 minutes at 37°C for up to 1 hour until a single-cell suspension was obtained. Epithelial cells were isolated from colonic organoids as described earlier except that the organoids, after extraction from Matrigel, were incubated in 200 μL enzyme solution for 45 minutes (up to 1 hour) at 37°C. Cells also were isolated from human colon resections by dissecting the tissue as described earlier. Colonic crypt isolation and digestion into single cells was performed as previously described.46 In short, minced tissue was incubated in 8 mmol/L EDTA in DPBS (14190-144; Gibco) while slowly rotated for 75 minutes at 4°C, followed by vigorous shaking of the sample to enrich for dissociated colonic crypts. To obtain a single-cell suspension, colonic crypts were incubated in Disaggregation Medium (advanced Dulbecco’s modified Eagle medium/F12, 1× GlutaMAX, 10 mmol/L HEPES, 1× N-2, 1× B-27, 10 mmol/L nicotinamide, 1 mmol/L N-acetyl-L-cysteine, 10 μmol/L Y-27632, 2 U/mL dispase (17105041; Gibco), 200 KU DNAse I/mL (D5025; Sigma), and incubated for 30 minutes at 37°C with occasional agitation. All harvested cells were centrifuged, resuspended in flow staining buffer comprising 1% fetal bovine serum (10082-147; Gibco), 25 mmol/L HEPES (15630-080; Thermo Fisher Scientific), 1 mmol/L EDTA (15575-020; Thermo Fisher Scientific), and 0.05% sodium azide (BDH7465-2; VWR, Radnor, PA) in DPBS (14190-144; Gibco). Surface staining was performed in 100 uL staining buffer for 30 minutes, followed by fixation in 2% PFA (Staining panels 1 and 3) for 15 minutes or overnight fixation with eBioscience Foxp3/Transcription Factor Staining Buffer Set (00-5523-00; Invitrogen) (panel 2). After fixation, EdU staining was performed following the manufacturer’s instructions (Click-iT Plus EdU Alexa Fluor 350 Flow Cytometry Assay Kit, C10645; Invitrogen), followed by intracellular staining in 1× saponin (Click-iT Plus EdU Alexa Fluor 350 Flow Cytometry Assay Kit, C10645; Invitrogen).
Staining Panel 1 (2% PFA fixation) comprised the following: anti-MUC2 (H-9, sc-515106, 1:100; Santa Cruz), anti-mouse–IgG2b–546 (A-21143, dilution 1:100; Invitrogen), and Click-iT Plus EdU Alexa Fluor 350 Flow Cytometry Assay Kit (C10645; Invitrogen). Staining Panel 2 (Foxp3) comprised the following: anti-Ki67–APC (Ki-67, 350514, dilution 1:20; BioLegend, San Diego, CA), and Click-iT Plus EdU Alexa Fluor 350 Flow Cytometry Assay Kit (C10645; Invitrogen). Staining Panel 3 (fresh in vivo tissue) comprised the following: anti-CD45–Brilliant Violet 570 (HI30 clone, 304034, dilution 1:50; BioLegend), anti-CD235a–Pacific Blue (HI264 clone, 349108, dilution 1:40; BioLegend), anti-CD11b–Brilliant Violet 570 (ICRF44, 301325, dilution 1:40; BioLegend), anti-CD31-421 (WM59, 303124, dilution 1:20; BioLegend), EpCAM-PE/Cy7 (CO17-1A, 369815, dilution 1:20; BioLegend), anti-MUC2 (H-9, sc-515106, 1:100; Santa Cruz), and anti-mouse–IgG2b Alexa Fluor 546 (A-21143, dilution 1:100; Invitrogen). All panels included 20 nmol/L Syto16 (S7578, dilution 1:500; Thermo Fisher Scientific), zombie NIR dye (423106, dilution 1:500; BioLegend), Fc block (422302, dilution 1:20; BioLegend). Stained cells were analyzed using the LSRFortessa (BD Biosciences, San Jose, CA). Results were analyzed using FlowJo V10 software (FlowJo, LLC, Ashland, OR).
RNA Isolation, Reverse Transcription, and Quantitative Reverse-Transcription Polymerase Chain Reaction
The cells of colonic organoids, day 3 chips, and day 7 Colon Chips were harvested with RLT buffer from the RNeasy Mini Kit (74106; Qiagen, Hilden, Germany). RNA was extracted using the RNeasy Mini Kit (74106; Qiagen) followed by complementary DNA synthesis with the SuperScript VILO complementary DNA Synthesis Kit (1754250; Invitrogen). Reverse-transcription polymerase chain reaction was performed using TaqMan Fast Advanced Master Mix (4444965; Applied Biosystems, Foster City, CA), TaqMan Gene Expression Assays (Hs02786624_g1 for glyceraldehyde-3-phosphate dehydrogenase, Hs00358836_m1 for Kruppel-like factor 4, Hs00171942_m1 for SAM pointed domain ETS factor, Hs00356521_m1 for anterior gradient 2, protein disulfide isomerase family member, Hs00395669_m1 for resistin-like molecule β, Hs00175398_m1 for Fc-γ binding protein, Hs01086545_m1 for Kallikrein 1, Hs00983260_m1 for Meprin A subunit β, and Hs00976287_m1 for chloride channel accessory 1; Thermo Fisher Scientific), and run on a QuantStudio 7 Flex Real-Time polymerase chain reaction System (Applied Biosystems). All results were normalized relative to glyceraldehyde-3-phosphate dehydrogenase expression and day 0 organoid respective gene expression.
Permeability
Cascade Blue hydrazide Trilithium Salt (550 daltons) (C3239; Invitrogen) at 50 μg/mL in HBSS was added to the top epithelial channel of the Colon Chip to assess barrier permeability. The concentration of dye that diffused through the membrane into the basal channel was measured in the effluent, and apparent paracellular permeability was calculated as previously described.25
Light Microscopy
The top view Colon Chip images were acquired using a differential interference contrast or phase-contrast microscopy (Axio Observer Z1; Zeiss, Oberkochen, Germany). Frozen sections were obtained during different stages of the isolation of colon epithelial cells, stained with H&E, and imaged.
Side View Imaging of Mucus Accumulation on-Chip
To image mucus accumulation in living cultures on-chip, approximately 2 mm PDMS were cut away from each side of the chip parallel to the channels using a razor blade secured in a press. The chips were rotated onto one side on a glass slide coated with glycerine solution (11513872; Leica), and the top side was covered with glycerine and a cover glass. The images were acquired with an inverted microscope (Axio Observer Z1; Zeiss) using a 2.5× objective (0.06 numerical aperture (NA), 441010-9901; Zeiss) and condenser (0.35 NA, 424241-0000-000; Zeiss) with phase ring 2 used for dark-field imaging. Fluorescent images were acquired using an X-cite LED light source (Excelitas Technologies, Waltham, MA). Mucus height and area were analyzed in side view images of the Colon Chip using Fiji software (https://imagej.net/Fiji).
PGE2 Treatment
For long-term PGE2 studies, the basal channel of the Colon Chips was perfused with stem cell expansion medium 1.4 nmol/L PGE2 (P5640; Sigma) for 6 days, starting on day 2. To quantify cell proliferation, 10 μmol/L EdU (C10645; Invitrogen) was perfused through both channels of the chip for 18 hours before enzymatically detaching cells on day 8 and performing flow cytometric analysis. Short-term treatment with PGE2 and ion channel inhibitors was performed 7 days after monolayer formation. In short, Colon Chips were perfused with medium (stem cell expansion medium basally, HBSS apically) containing 50 μmol/L CFTRinh-172 (S7139; Selleckchem, Houston, TX), 20 μmol/L XE-991 dihydrochloride (20010; Tocris), 100 μmol/L bumetanide (S1287; Selleckchem), or a combination of the 3 inhibitors at 60 μL/h for 4 hours, followed by side view imaging to determine the baseline height of the mucus layer before PGE2 treatment. After baseline side view imaging, the chips then were switched to cotreatment with 1.4 nmol/L PGE2 (basal channel) and the respective ion channel inhibitors (apically and basally). After 4 hours of cotreatment with inhibitors and PGE2, side view imaging was performed to determine the swelling of the mucus layer.
Scanning Electron Microscopy
To visualize the epithelium and mucus layer on-chip, SEM analysis was performed using Colon Chips that had a top channel that was not irreversibly bonded to the membrane, which allowed the device to be dismantled manually as described previously.47 Cells were fixed with 4% PFA (157–4; Electron Microscopy Sciences) and 2.5% glutaraldehyde (G7776; Sigma) in DPBS and incubated in 0.5% osmium tetroxide (19152; Electron Microscopy Sciences) in 0.1 mol/L sodium cacodylate buffer (pH 7.4) before serial dehydration in ethanol. Samples then were dried using a critical point drier and imaged using field emission SEM (S-4700; Hitachi, Schaumburg, IL).
For cross-sectional SEM images, Colon Chips were fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer (P5244; Sigma) overnight and washed with water before being flash-frozen in liquid nitrogen. Frozen chips were sectioned into 5-mm cross-sections on dry ice, lyophilized, mounted on aluminum pin mounts with conductive carbon tape, sputter-coated with gold, and examined with a Tescan Vega3 GMU scanning electron microscope (Brno, Czechia).
Analysis of Inner and Outer Mucus Layers
Cells in the Colon Chips were stained for live cells by perfusing both channels for 30 minutes with medium (stem cell expansion medium basally, HBSS apically) containing 10 μmol/L calcein AM (C3100MP; Invitrogen) (200 μL/h); the medium perfused through the apical channel also contained 1 μm FluoSpheres Carboxylate-Modified Microspheres (70 μL/mL, F13083; Invitrogen). Colon Chips were incubated under static conditions for 40 minutes to allow the fluorescent beads to settle, and then z-stack images of 2–3 areas of each chip were collected using a Leica SP5 confocal microscope. Calcein AM was imaged with a 488-nm argon laser, and beads were visualized using the multiphoton laser at 1000 nm. Confocal z-stacks were reconstructed and analyzed using IMARIS software (Bitplane). To determine the thickness of the inner and outer mucus layers, a Gaussian distribution was fit to the data using Matlab (Natick, MA) and the height of the outer layer was determined using the middle 90% of the Gaussian distribution of the beads. The inner layer was set as the distance between the apical cell surface and the lower bound of the outer layer.
Shear Stress Deformation Assay
Increasing flow rates were applied to the Colon Chips using a Fusion Touch Syringe Pump (Chemyx, Stafford, TX). Side view images were acquired by transmitted light microscopy and movies were generated during flow and stop cycles of the pump at 1.6 mL/h, 6 mL/h, and 10 mL/h. Images then were analyzed using Fiji software by tracing the movements of mucus strains while flow was applied compared with the final position after flow was stopped. The angle between flow and no flow was calculated (great angle equals great deformation). Linear regression model was fit to data sets. Uncertainties of the fits were used to evaluate differences between PGE2 and Control.
Drawings
All drawings were created with BioRender (Toronto, ON).
Alcian Blue Mucin Assay
Mucus was loosened from the apical surfaces of the colon chips by reducing disulfide bonds with 250 mmol/L Tris (2-carboxyethyl) phosphine (C4706; Sigma-Aldrich). After a 1-hour incubation, the mucus layer was separated mechanically by washing the apical channel with PBS. Samples were frozen, lyophilized overnight, and reconstituted in PBS. The total mucus amount was determined using an Alcian blue colorimetric assay adapted from Hall et al.48 Briefly, a standard curve was created using serial dilutions of the submaxillary gland mucin (Sigma), ranging from 0 to 500 μg/mL. Samples were diluted into the linear range of the curve using PBS. Samples and standards were equilibrated with filtered Richard Allan Scientific Alcian blue (88043; Thermo Fisher Scientific) for 2 hours. The resulting precipitant was separated by centrifugation at 1870g for 30 minutes. This was followed by a series of wash/spin cycles at 1870g in a resuspension buffer composed of 40% ethanol, 0.1 mol/L acetic acid, and 25 mmol/L magnesium chloride. The mucin pellets then were dissociated with a 10% sodium dodecyl sulfate solution (71736; Sigma) and absorbance was measured with a microplate reader (Synergy HT, BioTek, Winooski, VT) at 620 nm. Mucin concentration values for samples were interpolated from a linear fit of the standard curve.
Statistical Analysis
All graphs are shown as means ± SEM and significant differences between 2 groups were determined using a 2-tailed unpaired Student t test. To determine significant differences between 3 groups or more, 1-way analysis of variance with the Tukey multiple comparisons test was used. With 3 groups or more and 2 independent variables, 2-way analysis of variance with the Tukey multiple comparisons test was used to determine statistical significance. Prism 7 (GraphPad Software, San Diego, CA) was used for statistical analysis.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
All authors had access to the study data and reviewed and approved the final manuscript.
Acknowledgment
The authors thank Anja Nordstrom (Harvard Medical School Electron Microscopy Facility) for help with scanning electron microscopy; Gregory Phelps for assistance with mucus layer analysis; Patrick Wallisch, Carlos Ng, and Elizabeth Calmari for technical assistance; and Angeliki Chalkiadaki and Alex Monreal for their guidance and excellent advice.
Footnotes
Author contributions Alexandra Sontheimer-Phelps designed, executed and analyzed all experiments, with input and supervision from Donald E. Ingber, David B. Chou, Rachelle Prantil-Baun, and Oren Levy; David B. Chou helped with flow cytometry; David B. Chou, Alessio Tovaglieri, and Viktoras Frismantas assisted with data interpretation; Camilla A. Richmond and David T. Breault provided advice on the establishment of organoid cultures and data interpretation; Thomas C. Ferrante assisted with fluorescence and transmitted light microscopy; Taylor Duckworth helped with chip experiment performance and bead data analysis; Cicely Fadel performed the Alcian blue assay; Viktoras Frismantas sectioned the Transwells; Arlene D. Sutherland helped with chip experiments and organoid cultures, and performed the quantitative reverse-transcription polymerase chain reaction; Sasan Jalili-Firoozinezhad and James C. Weaver helped with scanning electron microscopy; Magdalena Kasendra and Alessio Tovaglieri helped with developing the Colon Chip method; David B. Chou, Alessio Tovaglieri, and Magdalena Kasendra helped with generation of human organoid cultures from resections; Eric Stas generated the human organoid cultures from biopsy specimens; and Alexandra Sontheimer-Phelps and Donald E. Ingber wrote the manuscript, with input from all co-authors.
Conflicts of interest Donald E. Ingber is a founder and holds equity in Emulate, Inc, and chairs its scientific advisory board; and Alexandra Sontheimer-Phelps, Alessio Tovaglieri, Magdalena Kasendra, and Donald E. Ingber are co-inventors on related patent applications. The remaining authors disclose no conflicts.
Funding This research was supported by a Cancer Research UK STORMing grant (C25640/A29057), a Defense Advanced Research Projects Agency (DARPA) grant (W911NF1920023), a US Food and Drug Administration grant (HHSF223201310079C), a Bayer foundation fellowship, and the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Supplementary Material
Shear force assay. Side-view images compiled to movies at 6 mL/h-1 and 10 mL/h-1 flow rate without (no PGE2) or with 4 hours of PGE2 treatment (4 h PGE2).
References
- 1.Schütte A., Ermund A., Becker-Pauly C., Johansson M.E., Rodriguez-Pineiro A.M., Bäckhed F., Müller S., Lottaz D., Bond J.S., Hansson G.C. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc Natl Acad Sci U S A. 2014;111:12396–12401. doi: 10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo K., Ota H., Akamatsu T., Sugiyama A., Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson M.E., Gustafsson J.K., Sjöberg K.E., Petersson J., Holm L., Sjövall H., Hansson G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swidsinski A., Loening-Baucke V., Theissig F., Engelhardt H., Bengmark S., Koch S., Lochs H., Dörffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson M.E., Gustafsson J.K., Holmén-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjövall H., Hansson G.C. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Sluis M., De Koning B.A.E., De Bruijn A.C.J.M., Velcich A., Meijerink J.P.P., Van Goudoever J.B., Büller H.A., Dekker J., Van Seuningen I., Renes I.B., Einerhand A.W.C. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Loftus E.V. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Dey I., Lejeune M., Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi M., Rosenberg D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessner T.G., Cohn S.M., Schloemann S., Stenson W.F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology. 1998;115:874–882. doi: 10.1016/s0016-5085(98)70259-8. [DOI] [PubMed] [Google Scholar]
- 12.Fujii S., Suzuki K., Kawamoto A., Ishibashi F., Nakata T., Murano T., Ito G., Shimizu H., Mizutani T., Oshima S., Tsuchiya K., Nakamura T., Araki A., Ohtsuka K., Okamoto R., Watanabe M. PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci Rep. 2016;6:36795. doi: 10.1038/srep36795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belley A., Chadee K. Prostaglandin E(2) stimulates rat and human colonic mucin exocytosis via the EP(4) receptor. Gastroenterology. 1999;117:1352–1362. doi: 10.1016/s0016-5085(99)70285-4. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T., Miyawaki Y., Nishikawa A., Horiyama S., Yamauchi K., Kuwano S. Prostaglandin E2-mediated stimulation of mucus synthesis and secretion by rhein anthrone, the active metabolite of sennosides A and B, in the mouse colon. J Pharm Pharmacol. 1990;42:542–545. doi: 10.1111/j.2042-7158.1990.tb07055.x. [DOI] [PubMed] [Google Scholar]
- 15.Ermund A., Schütte A., Johansson M.E., Gustafsson J.K., Hansson G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halm D.R., Halm S.T. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol. 2000;278:C212–C233. doi: 10.1152/ajpcell.2000.278.1.C212. [DOI] [PubMed] [Google Scholar]
- 17.Lock J.Y., Carlson T.L., Carrier R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv Drug Deliv Rev. 2018;124:34–49. doi: 10.1016/j.addr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y., Lin Y., Davis K.M., Wang Q., Rnjak-Kovacina J., Li C., Isberg R.R., Kumamoto C.A., Mecsas J., Kaplan D.L. Robust bioengineered 3D functional human intestinal epithelium. Sci Rep. 2015;5:13708. doi: 10.1038/srep13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanDussen K.L., Marinshaw J.M., Shaikh N., Miyoshi H., Moon C., Tarr P.I., Ciorba M.A., Stappenbeck T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In J., Foulke-Abel J., Zachos N.C., Hansen A.-M., Kaper J.B., Bernstein H.D., Halushka M., Blutt S., Estes M.K., Donowitz M., Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduces mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol. 2016;2:48–62.e3. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Kim R., Sims C.E., Allbritton N.L. Building a thick mucus hydrogel layer to improve the physiological relevance of in vitro primary colonic epithelial models. Cell Mol Gastroenterol Hepatol. 2019;8:653–655.e5. doi: 10.1016/j.jcmgh.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafsson J.K., Ermund A., Johansson M.E., Schutte A., Hansson G.C., Sjovall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2012;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergström J.H., Birchenough G.M.H., Katona G., Schroeder B.O., Schütte A., Ermund A., Johansson M.E., Hansson G.C. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc Natl Acad Sci U S A. 2016;113:13833–13838. doi: 10.1073/pnas.1611400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalili-Firoozinezhad S., Gazzaniga F.S., Calamari E.L., Camacho D.M., Fadel C.W., Bein A., Swenor B., Nestor B., Cronce M.J., Tovaglieri A., Levy O., Gregory K.E., Breault D.T., Cabral J.M.S., Kasper D.L., Novak R., Ingber D.E. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3 doi: 10.1038/s41551-019-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A., Scholl W., Zhang C., Rickner H., Richmond C.A., Li H., Breault D.T., Ingber D.E. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep. 2018;8:2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tovaglieri A., Sontheimer-Phelps A., Geirnaert A., Prantil-Baun R., Camacho D.M., Chou D.B., Jalili-Firoozinezhad S., de Wouters T., Kasendra M., Super M., Cartwright M.J., Richmond C.A., Breault D.T., Lacroix C., Ingber D.E. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome. 2019;7:43. doi: 10.1186/s40168-019-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunzelmann K., Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 28.Birchenough G.M.H., Johansson M.E., Gustafsson J.K., Bergström J.H., Hansson G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T., Stange D.E., Ferrante M., Vries R.G.J., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Jung P., Sato T., Merlos-Suárez A., Barriga F.M., Iglesias M., Rossell D., Auer H., Gallardo M., Blasco M.A., Sancho E., Clevers H., Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 31.Kozuka K., He Y., Koo-McCoy S., Kumaraswamy P., Nie B., Shaw K., Chan P., Leadbetter M., He L., Lewis J.G., Zhong Z., Charmot D., Balaa M., King A.J., Caldwell J.S., Siegel M. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 2017;9:1976–1990. doi: 10.1016/j.stemcr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz J.P., Perreault N., Goldstein B.G., Lee C.S., Labosky P.A., Yang V.W., Kaestner K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noah T.K., Kazanjian A., Whitsett J., Shroyer N.F. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson M.E., Hansson G.C. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. In: McGuckin M., Thornton D., editors. Mucins. Methods in molecular biology (methods and protocols) Humana Press; 2012. p. 842. [DOI] [PubMed] [Google Scholar]
- 36.Nyström E.E.L., Birchenough G.M.H., van der Post S., Arike L., Gruber A.D., Hansson G.C., Johansson M.E. Calcium-activated chloride channel regulator 1 (CLCA1) controls mucus expansion in colon by proteolytic activity. EBioMedicine. 2018;33:134–143. doi: 10.1016/j.ebiom.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia M.A.S., Yang N., Quinton P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator–dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafsson J.K., Ermund A., Ambort D., Johansson M.E., Nilsson H.E., Thorell K., Hebert H., Sjövall H., Hansson G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson M.E., Jakobsson H.E., Holmén-Larsson J., Schütte A., Ermund A., Rodríguez-Piñeiro A.M., Arike L., Wising C., Svensson F., Bäckhed F., Hansson G.C. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18:582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang N., Garcia M.A.S., Quinton P.M. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. J Physiol. 2013;591:4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enss M.-L., Schmidt-Wittig U., Heim H.-K., Sewing K.-F. Prostaglandin E2 alters terminal glycosylation of high molecular weight glycoproteins, released by pig gastric mucous cells in vitro. Prostaglandins Leukot Essent Fatty Acids. 1995;52:333–340. doi: 10.1016/0952-3278(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim H.J., Li H., Collins J.J., Ingber D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah P., Fritz J., Desai M., Glaab E., Estes M., Zenhausern F., Wilmes P. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface (in revision) Nat Commun. 2016 doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jalili-Firoozinezhad S., Gazzaniga F.S., Calamari E.L., Camacho D.M., Fadel C.W., Nestor B., Cronce M.J., Tovaglieri A., Levy O., Gregory K.E., Breault D.T., Cabral J.M.S., Kasper D.L., Novak R., Ingber D.E. Complex human gut microbiome cultured in anaerobic human intestine chips. Nat Biomed Eng. 2019;3:520–531. doi: 10.1038/s41551-019-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 46.Jung P., Sommer C., Barriga F.M., Buczacki S.J., Hernando-Momblona X., Sevillano M., Duran-Frigola M., Aloy P., Selbach M., Winton D.J., Batlle E. Isolation of human colon stem cells using surface expression of PTK7. Stem Cell Rep. 2015;5:979–987. doi: 10.1016/j.stemcr.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jalili-Firoozinezhad S., Prantil-Baun R., Jiang A., Potla R., Mammoto T., Weaver J.C., Ferrante T.C., Kim H.J., Cabral J.M.S., Levy O., Ingber D.E. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018;9:223. doi: 10.1038/s41419-018-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall R.L., Miller R.J., Peatfield A.C., Richardson P.S., Williams I., Lampert I. A colorimetric assay for mucous glycoproteins using Alcian Blue [proceedings] Biochem Soc Trans. 1980;8:72. doi: 10.1042/bst0080072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shear force assay. Side-view images compiled to movies at 6 mL/h-1 and 10 mL/h-1 flow rate without (no PGE2) or with 4 hours of PGE2 treatment (4 h PGE2).
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
All authors had access to the study data and reviewed and approved the final manuscript.