Abstract
There have been 4 reported cases of squamous cell carcinoma (SCC) of the neovagina in transgender women. In this report, we present another case of neovaginal SCC in a transgender woman, which was HPV-related, with lung metastasis as the initial presentation, and which was also complicated by her previous history of metastatic renal cell carcinoma. This unique case highlights the diagnostic challenges in these unusual scenarios. Through this report, we hope to address the benefits of multidisciplinary tumor board rounds, provision of detailed clinical information, and familiarization of the transgender anatomy within the pelvis in this group of patients. We also propose that transgender women undergo a continuous annual follow-up after postoperative follow-up is completed.
Keywords: Transgender, Neovagina, Squamous cell carcinoma, HPV, Metastasis
Introduction
As genital reconstruction surgery for transgender women becomes more accessible and is more frequently performed, we are expecting to encounter more cases of neovaginal neoplasia, such as squamous cell carcinoma (SCC) [1, 2]. For male-to-female gender confirmation surgery, vaginoplasty is usually done by penile and scrotal skin inversion [3]. There have been 4 cases of SCC of the neovagina in transgender women reported, all presented as neovaginal discharge or bleeding [4, 5, 6, 7]. In this report, we present another case of neovaginal SCC in a transgender woman, with lung metastasis as the initial presentation. This case had also been further complicated by her previous history of metastatic renal cell carcinoma.
Case Presentation
We report the case of a 69-year-old transgender woman. The patient underwent genital reconstruction surgery at the age of 25, by combined penile and scrotal skin inversion, and has been on hormone therapy ever since.
Our patient was found having a left renal mass at the age of 65; therefore, she underwent left radical nephrectomy and retroperitoneal lymph node dissection. The pathology showed a left kidney renal cell carcinoma of clear cell type, grade 4. It invaded into the perinephric fat, and grossly extended into the renal vein. 0/4 lymph nodes were involved. The resection margins were negative for tumor. She developed an osseous metastasis in her left posterior rib 1 year after nephrectomy (at the age of 66), and received local radiation for the bone metastasis, as well as systemic targeted therapy by tyrosine kinase inhibitors.
In the fourth year after nephrectomy (at the age of 68), CT scans showed a new 1.5 × 1.5 cm nodule in the left lower lung of the patient. This lesion was presumed to be a new metastatic renal cell carcinoma. However, the nodule slowly grew to 3.3 cm in size during the next 12 months despite the systemic therapy for renal cell carcinoma.
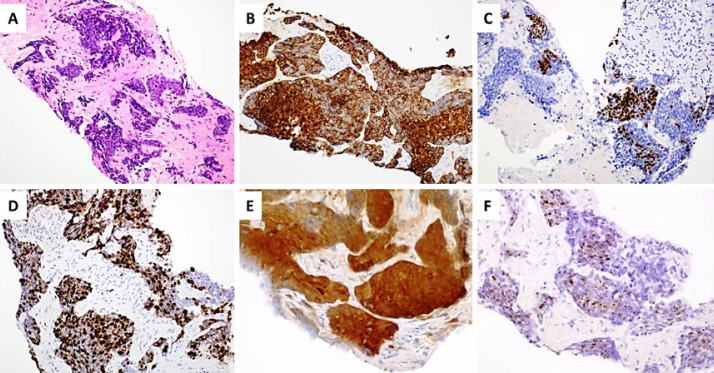
A transbronchial biopsy of the left lower lung lesion was performed, which showed a basaloid poorly differentiated carcinoma (Fig. 1A). Immunohistochemical stains showed that the tumor cells were diffusely positive for CK5/6 (Fig. 1B) and p40 (Fig. 1C), and negative for TTF1, PAX8, synaptophysin, and chromogranin. Ki67 stained approximately 80% of the tumor cell nuclei (Fig. 1D). These results supported a diagnosis of poorly differentiated SCC rather than a metastatic renal cell carcinoma or small cell carcinoma.
Fig. 1.
A The transbronchial biopsy of the left lower lung lesion showed a basaloid poorly differentiated carcinoma. B The tumor cells were diffusely positive for CK5/6. C The tumor cells were positive for p40. D Ki67 stained approximately 80% of the tumor cell nuclei. E The tumor cells were strongly positive for p16. F In situ hybridization showed that the tumor cells are strongly positive for high risk HPV RNA.
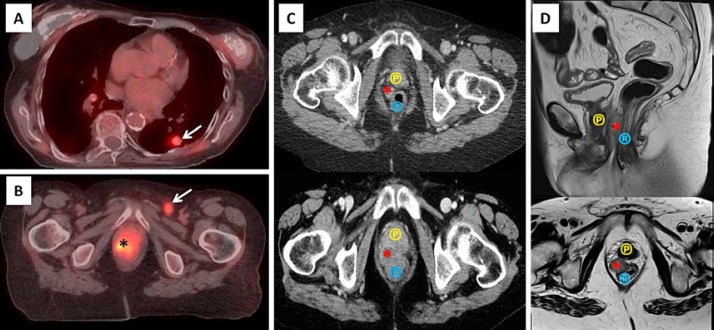
Since the CT scan did not demonstrate abnormalities other than the lung lesion and the bone metastases, a PET-CT was performed. Consistent with the previous CT scan, PET-CT showed an FDG-avid density within the left lower lung (Fig. 2A), with multiple additional nodular densities within both lungs. Additionally, an irregular region of marked increasing FDG activity that measures approximately 6 cm in the craniocaudal dimension was present within the pelvis with multiple moderate- to high-grade FDG-avid lymph nodes present within the left external iliac region and left inguinal region (Fig. 2B). Due to marked reduced sensitivity on the associated non-contrast CT component of the PET-CT, the female transgender anatomy within the pelvis was not clearly identified. The case was reviewed at the multidisciplinary tumor board rounds. On discussion, the transgender status of the patient was noted, and the FDG avidity within the pelvis was localized to the neovagina. Retrospective review of the CT imaging identified subtle changes within the soft tissues of the neovagina region over time (Fig. 2C). An MRI was also performed in the interim and identified a tumor within the neovagina (Fig. 2D).
Fig. 2.
A Axial-fused PET-CT demonstrates an FDG-avid left lower lobe pulmonary nodule (arrow) consistent with biopsy-proven squamous cell metastasis. B Axial-fused PET-CT using FDG demonstrates an FDG-avid mass within the neovagina (*) and an FDG-avid left inguinal lymph node (arrow). C Axial IV contrast-enhanced CT of the pelvis demonstrates the neovagina (*) 6 years before (top) and at the time of diagnosis (bottom). Note the increased soft tissue within the neovagina and loss of fat planes between neovagina, rectum (®), and prostate (℗) in lower image consistent with the tumor identified on PET-CT. D Sagittal T2 (top)/axial T2 (bottom) sequences of MRI of the pelvis demonstrates soft tissue thickening within the neovagina (*) located between the rectum (®) and the prostate (℗).
Given the above information, additional immunohistochemical stains were performed and showed that the tumor cells were strongly positive for p16 (Fig. 1E), suggesting a HPV-related SCC. In situ hybridization study further confirmed that the tumor cells are strongly positive for high-risk HPV RNA (Fig. 1F). The above study results were highly suggestive of a metastatic HPV-related SCC from the mucosa/skin of the oral cavity or anogenital region. A transvaginal biopsy was attempted but was not successful due to heavy bleeding. Nonetheless, a diagnosis of metastatic HPV-related SCC of the neovagina is established.
Due to the terminal stage of the disease, the patient was provided palliative radiotherapy and unfortunately passed away 4 months after the diagnosis.
Discussion
To our best knowledge, this is the fifth case of neovaginal SCC in transgender women after genital reconstruction surgery published to date [4, 5, 6, 7]. Within these 5 cases, including the current one, the age at reconstruction ranged from 21 to 33 years, with a median of 25 years. In all gender confirmation surgeries, skin grafts were used to create the cavity of the neovagina. In 4 cases, the skin was taken from the penis and scrotum. The age at diagnosis of the neovaginal tumor ranged from 42 to 78 years, with a median of 53 years. The latency period between the age at reconstruction and the age when diagnosed with cancer was between 18 and 45 years, with a median of 22 years.
Unlike the 4 previously reported cases, in which the patients presented with vaginal discharge as first symptom, the current patient did not have pelvic symptoms and presented with lung metastasis on routine follow-up imaging 1 year before the identification of the primary tumor within the neovagina. Given the patient's clinical history, it was presumed that the lung lesion was a metastasis from the previous renal cell carcinoma. This highlights the need for histological correlation as all new lesions may not be metastases from the same origin.
This diagnostic dilemma was also confounded by the reduced sensitivity of CT imaging within the pelvis. In retrospect, upon review of the CT study prior to PET-CT at the tumor board rounds, subtle changes on CT imaging were apparent with slightly increased prominence of the soft tissue within the neovagina. However, the conspicuity of tumor was reduced by the adjacent unopacified bowel and other adjacent soft tissues. It is for these reasons that MRI has been identified as the modality of choice for assessment of the pelvis in this patient cohort [8].
It is also apparent that the patient was not always identified as a transgender woman either on the clinical indication provided or on the interpreted images. This highlights the importance of familiarization with transgender pelvic anatomy on cross-sectional imaging to optimize interpretation and also the gathering of all clinical information to aid diagnosis.
After the identification of the neovaginal tumor, a transvaginal biopsy was attempted but was unsuccessful due to the heavy bleeding. Although approximately 10% of all pulmonary SCC show p16 positivity, high-risk HPV is extremely rare in a carcinoma of the lung [9]. Given the positive p16 and high-risk HPV in situ hybridization of the lung lesion and the radiological finding within the neovaginal, a diagnosis of metastatic neovaginal SCC to the lung was established.
Four of the 5 cases, all of which with penile and scrotal skin inversion, had been confirmed positive for HPV. Three of them (including the current one) were positive for high-risk HPV type, with another case having a history of low-risk HPV infection. The fifth case, with skin graft, was not tested for HPV; however, the immunostain for p16 turned out to be negative [4, 5, 6, 7]. Transgender women have a high prevalence of sexually transmitted infections, with a HPV infection rate of 52.4%, all of which have at least one high-risk genotype [10, 11, 12, 13, 14]. This could be explained by several contributory factors. First, the prevalence of HPV is generally high in the male genital region (up to 76%) [15], while most of the transgender women's neovagina are from the penile and scrotal skin grafts. Additionally, as unexpected pregnancy is not a concern, sexual protection may be used to a lesser extent than in nontransgender women, making HPV and other sexually transmitted diseases more easily transmitted.
Although they have a higher prevalence of HPV infection than nontransgender women, only 4% of all transgender women ever had a gynecological exam after follow-up of vaginoplasty was completed [2]. Therefore, it has been recommended that regular gynecological examinations including speculum and digital neovaginal examination should be applied for transgender women, even after postoperative follow-up is completed [2, 6]. Additionally, as for nontransgender women, cytologic smear testing every 3 years, starting at the age of 21 until the age of 70 is recommended for transgender women, especially for those with a history of genital warts [6].
Two of the previous reported transgender women succumbed to their diseases. The other two, however, showed a disease-free interval of at least 2 years prior to the publication of their cases. The patient in our report died of the disease 4 months after the diagnosis.
Conclusion
We report the fifth case of neovaginal SCC 44 years after male-to-female gender confirmation surgery. The diagnosis of this case was complicated by the patient's history of metastatic renal cell carcinoma and the unusual presentation of the disease. This highlights the benefits of multidisciplinary tumor board rounds in these unusual scenarios. Transgender anatomy offers specific diagnostic challenges within the pelvis, and familiarization with this is required to aid in the assessment and interpretation of images. This can also be aided by the provision of detailed clinical information on image requests. We advise gynecologists to encourage transgender women to undergo a continuous annual follow-up after postoperative follow-up is completed.
Statement of Ethics
This case study was approved by the Institutional Review Boards of University of British Columbia. The patient passed away, so consent could not be obtained. For the purpose of protection of the patient, any identifiable attributes were anonymized in the manuscript and its supplementary files.
Disclosure Statement
The authors report no conflicts of interest.
Funding Sources
The authors report no funding sources.
Author Contributions
Gang Wang and David Ferguson conceived and designed the study, collected the data, wrote, edited, and reviewed the manuscript. David Ferguson, Diana Ionescu, Lien Hoang, Sarah Barrett, Dirk van Niekerk, James Neil Rose, and Christian Kollmannsberger collected the data, edited and reviewed the manuscript. All authors gave final approval for publication. Gang Wang takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
References
- 1.Schober JM. Cancer of the neovagina. J Pediatr Urol. 2007;3((3)):167–70. doi: 10.1016/j.jpurol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Weyers S, De Sutter P, Hoebeke S, Monstrey G, T Sjoen G, Verstraelen H, et al. Gynaecological aspects of the treatment and follow-up of transsexual men and women. Facts Views Vis Obgyn. 2010;2((1)):35–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Colebunders B, Brondeel S, D'Arpa S, Hoebeke P, Monstrey S. An update on the surgical treatment for transgender patients. Sex Med Rev. 2017;5((1)):103–9. doi: 10.1016/j.sxmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Bollo J, Balla A, Rodriguez Luppi C, Martinez C, Quaresima S, Targarona EM. HPV-related squamous cell carcinoma in a neovagina after male-to-female gender confirmation surgery. Int J STD AIDS. 2018;29((3)):306–8. doi: 10.1177/0956462417728856. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes HM, Manolitsas TP, Jobling TW. Carcinoma of the neovagina after male-to-female reassignment. J Low Genit Tract Dis. 2014;18((2)):E43–5. doi: 10.1097/LGT.0b013e3182976219. [DOI] [PubMed] [Google Scholar]
- 6.Fierz R, Ghisu GP, Fink D. Squamous carcinoma of the neovagina after male-to-female reconstruction surgery: a case report and review of the literature. Case Rep Obstet Gynecol. 2019;2019:4820396. doi: 10.1155/2019/4820396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harder Y, Erni D, Banic A. Squamous cell carcinoma of the penile skin in a neovagina 20 years after male-to-female reassignment. Br J Plast Surg. 2002;55((5)):449–51. doi: 10.1054/bjps.2002.3868. [DOI] [PubMed] [Google Scholar]
- 8.Bertolotto M, Liguori G, Bucci S, Iannelli M, Vedovo F, Pavan N, et al. MR imaging in patients with male-to-female sex reassignment surgery: postoperative anatomy and complications. Br J Radiol. 2017;90((1072)):20170062. doi: 10.1259/bjr.20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatta LB, Balzarini P, Tironi A, Berenzi A, Benetti A, Angiero F, et al. Human papillomavirus DNA and p16 gene in squamous cell lung carcinoma. Anticancer Res. 2012;32((8)):3085–9. [PubMed] [Google Scholar]
- 10.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13((3)):214–22. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 11.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7((7)):453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 12.Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12((1)):1–17. doi: 10.1007/s10461-007-9299-3. [DOI] [PubMed] [Google Scholar]
- 13.Loverro G, Di Naro E, Caringella AM, De Robertis AL, Loconsole D, Chironna M. Prevalence of human papillomavirus infection in a clinic sample of transsexuals in Italy. Sex Transm Infect. 2016;92((1)):67–9. doi: 10.1136/sextrans-2014-051987. [DOI] [PubMed] [Google Scholar]
- 14.van der Sluis WB, Buncamper ME, Bouman MB, Elfering L, Ozer M, Bogaarts M, et al. Prevalence of neovaginal high-risk human papillomavirus among transgender women in The Netherlands. Sex Transm Dis. 2016;43((8)):503–5. doi: 10.1097/OLQ.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 15.Weyers S, Lambein K, Sturtewagen Y, Verstraelen H, Gerris J, Praet M. Cytology of the ‘penile’ neovagina in transsexual women. Cytopathology. 2010;21((2)):111–5. doi: 10.1111/j.1365-2303.2009.00663.x. [DOI] [PubMed] [Google Scholar]