Abstract
Background
Anemia of inflammation (AI) is the most common cause of anemia in the critically ill, but its diagnosis is a challenge. New therapies specific to AI are in development, and they require accurate detection of AI. This study explores the potential of parameters of iron metabolism for the diagnosis of AI during an ICU stay.
Methods
In a nested case-control study, 30 patients developing AI were matched to 60 controls. The iron parameters were determined in plasma samples during an ICU stay. Receiver operating characteristic curves were used to determine the iron parameter threshold with the highest sensitivity and specificity to predict AI. Likelihood ratios as well as positive and negative predictive values were calculated as well.
Results
The sensitivity of iron parameters for diagnosing AI ranges between 62 and 76%, and the specificity between 57 and 72%. Iron and transferrin show the greatest area under the curve. Iron shows the highest sensitivity, and transferrin and transferrin saturation display the highest specificity. Hepcidin and ferritin show the lowest specificity. At an actual anemia prevalence of 53%, the diagnostic accuracy of iron, transferrin, and transferrin saturation was fair, with a positive predictive value between 71 and 73%. Combining iron, transferrin, transferrin saturation, hepcidin, and/or ferritin levels did not increase the accuracy of the AI diagnosis.
Conclusions
In this explorative study on the use of different parameters of iron metabolism for diagnosing AI during an ICU stay, low levels of commonly measured markers such as plasma iron, transferrin, and transferrin saturation have the highest sensitivity and specificity and outperform ferritin and hepcidin.
Keywords: Anemia of inflammation, Diagnosis, Transferrin, Iron, Critically ill
Introduction
Anemia is very common in the critically ill. Consequently, 40% of these patients receive a red blood cell (RBC) transfusion during their ICU stay [1, 2]. Given the association between RBC transfusion and adverse outcome [3], other strategies for treating anemia in these patients are potentially highly valuable. Factors contributing to anemia in critically ill patients are hemolysis, blood loss, and iatrogenic factors, including frequent blood sampling and hemodilution. However, the most important contributor to anemia in the critically ill is thought to be inflammation [4].
Inflammation causes increased erythrophagocytosis, leading to a shortened RBC life span, and contributes to iron sequestration in macrophages [5]. Proinflammatory cytokines also stimulate hepcidin production, which causes degradation of ferroportin, resulting in inhibition of iron transport from enterocytes and macrophages into the circulation [6]. Further, during inflammation, transferrin levels are low [7], resulting in decreased iron transport to the bone marrow for erythropoiesis. Hereby, in anemia of inflammation (AI), there is no absolute iron deficiency, but rather a low iron availability.
The presence of anemia is associated with a longer ICU stay [8], calling for new interventions. Treatment options for AI that are gaining attention include increasing iron availability for erythropoiesis by decreasing inflammation using an anti-IL-6 receptor antibody [9, 10] or directly inhibiting hepcidin using a compound that inactivates hepcidin [11, 12]. However, prior to adoption of potential new therapies, it is crucial that AI be correctly diagnosed. The accuracy of hepcidin in diagnosing iron deficiency anemia has shown promising results in several patient populations [13, 14, 15] and may be useful for AI identification as well. However, hepcidin is currently not a bedside measurement, and the available measurement methods are expensive. In addition, the cutoff levels for hepcidin are unclear, and there is significant intertest variation [16, 17]. Therefore, we aim to explore the diagnostic test performance of commonly available iron parameters as well as of hepcidin for the development of AI during an ICU stay.
Subjects and Methods
Study Design
This study is a post hoc analysis of the Molecular Diagnosis and Risk Stratification of Sepsis (MARS) project, a prospective observational cohort study at the mixed surgical-medical ICUs of 2 tertiary hospitals (ClinicalTrials.gov NCT01905033). The Medical Ethics Committees of both centers approved an opt-out consent method. All patients admitted to the ICUs between January 2011 and July 2013 older than 18 years and with an expected stay longer than 24 h were eligible for inclusion [18].
Patient Selection
Patients with AI (n = 30) were selected from the MARS cohort as described previously [19]. No formal power calculation was performed. Group sizes were chosen based on previous studies [13]. AI was defined by the development of anemia (Hb level ≤9.7 g/dL or ≤6 mmol/L) during an ICU stay while complying with the diagnosis of sepsis [20]. Anemia was defined as Hb ≤6 mmol/L to select the clinically relevant patients, since these patients are likely to receive a blood transfusion if their hemoglobin level declines further to 4.3–5.0 mmol/L, which is the range at which transfusion is considered at our institution. Sepsis was used as a criterion in order to select patients with severe inflammation from the database.
Two groups of 30 control patients were selected from a pool of 130 available controls from the MARS cohort, who either had sepsis and high Hb (Hb level ≥11.3 g/dL or ≥7 mmol/L) or no sepsis and high Hb. The Hb levels of the AI patients and control patients were chosen to create a clear distinction between patients with anemia and patients with a high Hb. The patients in the AI group were matched to the controls for age (±5 years) and sex (exact) by the Optimal Matching method from the MatchIt package of R statistics [21].
Excluded from all groups were postoperative patients (to avoid including patients who became anemic due to blood loss); patients who received RBC transfusions prior to or during the blood sampling period (which ranged from 1 to 31 days after ICU admission); patients who received iron or erythropoietin therapy; and patients in conditions which may induce or alter chronic anemia, including chronic renal failure, hematological disease, chemotherapy, or AIDS.
Sample Selection and Analysis
Three blood samples were taken from the patients throughout the course of their ICU stay, of which the first blood sample was taken on ICU admission [19]. The samples were centrifuged at room temperature at 1,500 g for 15 min, and the plasma was stored at −80°C. The measurements were done on heparin anticoagulated plasma, which is adequate for testing of parameters of iron metabolism [22, 23]. Plasma iron, transferrin, and ferritin was measured by immunoturbidimetric methods (Roche Cobas c702). Transferrin saturation was calculated using the formula plasma iron/(25.2 × transferrin). The hepcidin level was measured with an enzyme-linked immunosorbent assay kit (R&D) and analyzed on a SpectraMax M2 (Molecular Devices).
Statistical Analysis
The levels of iron, transferrin, transferrin saturation, ferritin, and hepcidin did not change over time (data not shown). Therefore, the data from all the sample moments were taken together for evaluation of the diagnostic ability of iron parameters for AI [24]. Patients that developed anemia while having sepsis were defined as cases and used as the reference standard for the diagnosis of AI. Receiver operating characteristic curves were used to determine the iron parameter threshold with the highest sensitivity and specificity (Youden index) to predict AI. The traditional academic point system was used to classify the accuracy of the test [25].
Also, the combined predictive value of the iron parameters for AI development was calculated (see online suppl. Additional file 1; see www.karger.com/doi/10.1159/000497123 for all online suppl. material). Using the Youden index, the test characteristics of the iron regulators were evaluated. Sensitivity, specificity, and the positive and negative likelihood ratios were calculated. The positive predictive value (PPV) and the negative predictive value (NPV) were calculated for different prevalence rates of AI. To calculate the PPV and NPV in this nested case-control study, the group of control patients was adjusted by the sample fraction of 2.17. This sample fraction was calculated by the total of patients classified as potential controls from the cohort (n = 130) divided by the number of matched controls (n = 60) [26].
Data that were not normally distributed are expressed as median and interquartile range. Categorical variables are expressed as n (%). To test for differences between more than two groups, the Kruskal-Wallis test was used. Categorical variables were compared with the χ2 test or Fisher's exact test. A p value <0.05 was considered statistically significant. Statistical uncertainty was expressed with 95% confidence intervals if possible. All analyses were performed using R statistics.
Results
Subjects
The baseline characteristics of the patients are presented in Table 1. Age was similar and the number of females and males was the same in all patient groups due to matching. Although not statistically significant, the patients with AI were possibly more severely ill than the control patients, which is reflected in a higher APACHE (Acute Physiology and Chronic Health Evaluation) IV score (p = 0.08) and a statistically significantly longer ICU stay (p < 0.01). The median time for AI patients to become anemic was 8 days (interquartile range 4–11). The patients categorized as having AI all had low iron or low transferrin saturation, in combination with a high ferritin level [19, 27].
Table 1.
Patient characteristics
| Anemia of inflammation (n = 30) | Septic controls, high Hb (n = 30) | Nonseptic controls, high Hb (n = 30) | p value | |
|---|---|---|---|---|
| Male, n (%) | 19 (63) | 19 (63) | 19 (63) | 1.00 |
| Median age (range), years | 65 (20–85) | 66 (22–81) | 64 (21–79) | 0.99 |
| Median APACHE IV score (range) | 83 (45–155) | 72 (45–128) | 71 (17–104) | 0.08 |
| Admission type, n (%) | ||||
| Surgical | 7 (23) | 4 (13) | 7 (23) | 0.66 |
| Medical | 17 (57) | 16 (53) | 13 (43) | 0.75 |
| Neurological | 6 (20) | 10 (33) | 10 (33) | 0.72 |
| Median Hb level (range), g/dL | ||||
| ICU admission | 11.4 (9.9–15.0) | 13.9 (11.7–16.2) | 13.3 (11.2–15.4) | <0.01 |
| Second sample | 9.0 (8.2–9.4) | 12.6 (11.2–16.2) | 13.0 (11.4–15.7) | <0.01 |
| Third sample | 8.4 (7.4–9.3) | 12.8 (11.4–16.8) | 12.8 (11.2–14.7) | <0.01 |
| ICU mortality, n (%) | 4 (13) | 2 (7) | 3 (10) | 0.69 |
| Median length of ICU stay (range), days | 18 (7–84) | 11 (5–101) | 9 (5–49) | <0.01 |
Test Characteristics of Iron Parameters for Diagnosing AI
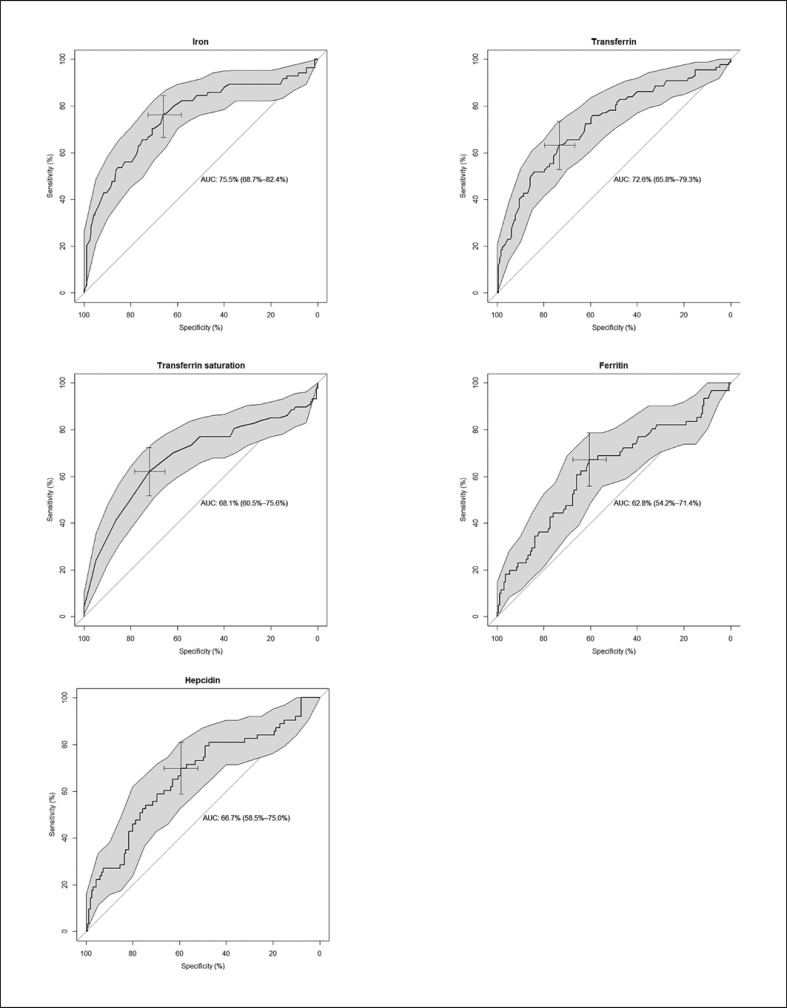
Iron and transferrin showed the greatest areas under the curve (AUCs) (76% [95% CI: 69–82] and 73% [95% CI: 66–79], respectively) (Table 2). The optimal cutoff values of the iron parameters for AI diagnosis as determined by the receiver operating characteristic curve analysis (Fig. 1), as well as their sensitivity and specificity, are presented in Table 2. The probability for AI increases when the iron and transferrin (saturation) levels are below the presented cutoff values. Regarding hepcidin and ferritin, the probability for AI increases when levels are higher than the presented cutoff values. Iron has the highest sensitivity for AI diagnosis, followed by ferritin. The specificity for AI diagnosis is highest for transferrin and transferrin saturation, followed by iron. Hepcidin and ferritin show the lowest specificity.
Table 2.
Test characteristics (95% confidence intervals) of the iron parameters for AI diagnosis
| AUC, % | Cutoff for AI diagnosis | Sensitivity, % | Specificity, % | PLR | NLR | |
|---|---|---|---|---|---|---|
| Iron | 76 (69–82) | 4.4 µmol/L | 76 (66–85) | 66 (58–73) | 2.3 (1.9–2.7) | 0.4 (0.2–0.5) |
| Transferrin | 73 (66–79) | 1.46 g/L | 63 (52–73) | 72 (65–79) | 2.4 (1.9–3) | 0.5 (0.4–0.7) |
| Transferrin saturation | 68 (61–76) | 34% | 62 (56–80) | 72 (65–79) | 2.2 (1.7–2) | 0.5 (0.4–0.7) |
| Hepcidin | 67 (59–75) | 14.3 ng/mL | 68 (55–79) | 59 (51–67) | 1.7 (1.4–2.1) | 0.5 (0.3–0.8) |
| Ferritin | 63 (54–71) | 318 µg/L | 69 (56–80) | 57 (52–62) | 1.6 (1.3–2) | 0.6 (0.4–0.8) |
AI, anemia of inflammation; AUC, area under the curve; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Fig. 1.
Receiver operating characteristic curves of iron, transferrin, transferrin saturation, ferritin, and hepcidin values as diagnostic tests for anemia of inflammation in ICU patients.
Also, the negative and positive likelihood ratios are most favorable for iron, transferrin, and transferrin saturation, and most unfavorable for ferritin and hepcidin (Table 2). Combining iron, transferrin, and transferrin saturation with hepcidin or ferritin did not increase the AUC for AI, nor did combining iron with all parameters together increase the AUC (0.753 vs. 0.749) (online suppl. Additional file 1). Combining iron parameters with the APACHE IV score did not change the diagnostic performance for AI either (data not shown).
The PPV and NPV of a test are affected by the prevalence of the disease. We calculated the prevalence of anemia according to the WHO definition [28] in all patients acutely admitted to our ICU in 2016. From a total of 1,716 acutely admitted patients, 53% developed anemia while in the ICU. At an anemia prevalence of 53%, the NPV of iron parameters for AI ranges between 62 and 71%, and the PPV ranges between 64 and 73% (Table 3). When the AI prevalence is 70%, the NPVs of parameters of iron metabolism for AI are low, while the probability that AI is present when the test is positive is high (PPV). However, since the prevalence will vary between populations, the PPV and NPV were calculated for a lower prevalence as well (Table 3).
Table 3.
PPVs and NPVs (95% confidence intervals) of various prevalence values
| Pretest probability of disease (prevalence) |
|||
|---|---|---|---|
| 30% | 53% | 70% | |
| Iron1 | |||
| PPV | 49% (44–54) | 72% (68–75) | 84% (81–87) |
| NPV | 87% (83–89) | 71% (66–75) | 54% (49–59) |
| Transferrin | |||
| PPV | 50% (45–56) | 73% (68–77) | 85% (81–88) |
| NPV | 83% (79–85) | 64% (60–68) | 46% (42–51) |
| Transferrin saturation | |||
| PPV | 49% (44–54) | 71% (67–75) | 84% (80–87) |
| NPV | 82% (78–85) | 63% (58–67) | 45% (40–49) |
| Hepcidin | |||
| PPV | 43% (38–47) | 66% (62–70) | 80% (77–83) |
| NPV | 83% (80–87) | 66% (61–70) | 48% (43–53) |
| Ferritin | |||
| PPV | 41% (36–45) | 64% (60–68) | 79% (75–82) |
| NPV | 81% (77–84) | 62% (57–67) | 44% (39–49) |
PPV, positive predictive value; NPV, negative predictive value.
See Table 2 for the sensitivity and specificity values that apply to these calculations.
Discussion
In this study, the diagnostic accuracy of iron, transferrin, transferrin saturation, hepcidin, and ferritin for AI development during an ICU stay were explored. The relevance of such diagnostic criteria is underlined by our finding that critically ill patients with AI have a longer ICU stay than patients without AI. We showed that of the tested parameters, low plasma iron, transferrin, and transferrin saturation levels have the highest sensitivity and specificity for AI. At an estimated prevalence of AI of 53% in our critically ill patient cohort, the diagnostic accuracy of iron, transferrin, and transferrin saturation was fair. Thus, in our study, the use of iron, transferrin, and transferrin saturation to diagnose AI in the critically ill was better than using plasma hepcidin. This might be unexpected, since hepcidin is induced by inflammation. However, the regulation of hepcidin is complex and its level is influenced by many factors, which could be the reason for the low diagnostic performance of hepcidin in diagnosing AI. This study explored whether parameters of iron metabolism may aid in the diagnosis of AI by a simple analysis, investigating the single diagnostic accuracy of the respective iron regulators without any complementary variables such as patient characteristics and disease severity. Our results suggest that parameters of iron metabolism have sufficient diagnostic potential for further investigation.
In previous studies, hepcidin showed a high specificity for diagnosing iron deficiency in anemic ICU patients and anemic rheumatoid arthritis patients [13, 14], and it was a good discriminator between AI and iron deficiency in anemic patients due to different causes [15]. In these studies, the diagnostic accuracy of iron parameters other than hepcidin were not tested. However, a low iron level does not discriminate between iron deficiency and AI. Future research validating the use of parameters of iron metabolism to diagnose AI is needed, ideally using a prospective study in a larger patient population.
A limitation of this study is that the blood sampling period ranged from 1 to 31 days after ICU admission. Therefore, the iron parameters were not measured at similar time points across groups. Another limitation is that the nested case-control design in this diagnostic study could have led to overestimation of diagnostic accuracy [29]. However, to be able to calculate the PPV and NPV, we weighted the controls with the case-control sampling fraction [26], which is a strength of this study.
Conclusions
This explorative study assessing the possibility of using parameters of iron metabolism in the diagnosis of AI in critically ill patients shows that among the four biomarkers that were tested, plasma iron, transferrin, and transferrin saturation levels had the highest discriminative ability. Adding hepcidin did not increase the diagnostic accuracy for AI.
Statement of Ethics
The Medical Ethics Committees of both study centers (Academic Medical Center Medical Ethics Committee and Medical Ethics Committee University Medical Center Utrecht) approved this study. An opt-out consent method was used.
Disclosure Statement
The authors declare that they have no competing interests.
Funding Sources
This work was supported by Sanquin (grant No. PPOP-14-31).
Availability of Data and Materials
Data can be provided by the corresponding author in case of a reasonable request.
Author Contributions
Study design: M.B., R.v.B., and N.P.J. Contributed to the data: M.B., B.N., J.H., M.J.S., K.v.d.G., and O.L.C. Analyzed and interpreted the data: M.B., J.M.B., R.v.B., and N.P.J. Wrote the manuscript: M.B., R.v.B., and N.P.J. Reviewed the manuscript and approved the final submitted version: M.B., B.N., J.M.B., J.H., M.J.S., K.v.d.G., O.L.C., R.v.B., and N.P.J.
Supplementary Material
Supplementary data
Acknowledgements
The authors thank all members of the MARS Consortium for their participation in data collection and especially acknowledge: Friso M. de Beer, MD, Lieuwe D.J. Bos, MD, PhD, Gerie J. Glas, MD, Roosmarijn T.M. van Hooijdonk, MD, PhD, Laura R.A. Schouten, MD, Marleen Straat, MD, Esther Witteveen, MD, and Luuk Wieske, MD, PhD (Department of Intensive Care, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Arie J. Hoogendijk, PhD, Mischa A. Huson, MD, Brendon P. Scicluna, Tom van der Pol, MD, PhD, Lonneke A. van Vught, MD, PhD, and Maryse A. Wiewel, MD, PhD (Center for Experimental and Molecular Medicine, Academic Medical Center, University of Amsterdam); and Marc J.M. Bonten, MD, PhD, Jos F. Frencken, MD, Peter M.C. Klein Klouwenberg, MD, PharmD, PhD, Maria E. Koster-Brouwer, MSc, David S.Y. Ong, MD, PharmD, and Diana M. Verboom, MD (Department of Intensive Care Medicine, Julius Center for Health Sciences and Primary Care and Department of Medical Microbiology, University Medical Center Utrecht, Utrecht, The Netherlands).
References
- 1.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14707558. [DOI] [PubMed] [Google Scholar]
- 2.Napolitano LM, Kurek S, Luchette Fa, Anderson GL, Bard MR, Bromberg W, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–1442. doi: 10.1097/TA.0b013e3181ba7074. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20009700. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008 Sep;36((9)):2667–74. doi: 10.1097/CCM.0b013e3181844677. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003246-200809000-00026%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/18679112. [DOI] [PubMed] [Google Scholar]
- 4.Prakash D. Anemia in the ICU: anemia of chronic disease versus anemia of acute illness. Crit Care Clin. 2012;28:333–343. doi: 10.1016/j.ccc.2012.04.012. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22713609. [DOI] [PubMed] [Google Scholar]
- 5.Sihler KC, Napolitano LM. Anemia of inflammation in critically ill patients. J Intensive Care Med. 2008;23:295–302. doi: 10.1177/0885066608320836. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18701529. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003 Aug;102((3)):783–8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13((6)):273–9. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. ABC (Anemia and Blood Transfusion in Critical Care) Investigators Anemia and blood transfusion in critically ill patients. JAMA. 2002 Sep;288((12)):1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15((6)):R204. doi: 10.1186/ar4397. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3978585&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-a inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15((5)):1. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013 Mar;121((12)):2311–5. doi: 10.1182/blood-2012-09-456756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eijk LT, John AS, Schwoebel F, Summo L, Vauléon S, Zöllner S, et al. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014 Oct;124((17)):2643–6. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasocki S, Baron G, Driss F, Westerman M, Puy H, Boutron I, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010;36:1044–1048. doi: 10.1007/s00134-010-1794-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20213069. [DOI] [PubMed] [Google Scholar]
- 14.van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CM, van Riel PL, van Ede AE, et al. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011 Dec;63((12)):3672–80. doi: 10.1002/art.30623. [DOI] [PubMed] [Google Scholar]
- 15.Thomas C, Kobold U, Balan S, Roeddiger R, Thomas L. Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol. 2011 Apr;33((2)):187–93. doi: 10.1111/j.1751-553X.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- 16.Uijterschout L, Swinkels DW, Domellöf M, Lagerqvist C, Hudig C, Tjalsma H, et al. Serum hepcidin measured by immunochemical and mass-spectrometric methods and their correlation with iron status indicators in healthy children aged 0.5-3 y. Pediatr Res. 2014 Oct;76((4)):409–14. doi: 10.1038/pr.2014.109. [DOI] [PubMed] [Google Scholar]
- 17.Delaby C, Vialaret JÔ, Bros P, Gabelle A, Lefebvre T, Puy H, et al. Clinical measurement of Hepcidin-25 in human serum: is quantitative mass spectrometry up to the job? EuPA Open Proteomics. European Proteomics Association. 2014;3:60–7. [Google Scholar]
- 18.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. MARS Consortium Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016 Apr;315((14)):1469–79. doi: 10.1001/jama.2016.2691. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 19.Boshuizen M, Binnekade JM, Nota B, van de Groep K, Cremer OL, Tuinman PR, et al. Molecular Diagnosis and Risk Stratification of Sepsis (MARS) Consortium Iron metabolism in critically ill patients developing anemia of inflammation: a case control study. Ann Intensive Care. 2018 May;8((1)):56. doi: 10.1186/s13613-018-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 21.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42((8)):1–28. [Google Scholar]
- 22.A B, X F, M L, NS Y, JP M Effect of anticoagulants on multiplexed measurement of cytokine/chemokines. Cytokine. 2012;60:438–46. doi: 10.1016/j.cyto.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.H K M M. Effects of heparin, citrate, and EDTA on plasma biochemistry of sheep: comparison with serum. Rev Med Vet. 2015;166:275–9. doi: 10.1016/j.rvsc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Steiner SH, Danila O, Cotton CA, Severn D, Mackay RJ. Comparing two binary diagnostic tests with repeated measurements. J R Stat Soc Ser C Appl Stat. 2016;65((2)):315–29. [Google Scholar]
- 25.Tape Thomas G. The Area Under an AUC Curve. University of Nebraska Medical Center. :p. http://gim.unmc.edu/dxtests/Default.htm. [Google Scholar]
- 26.Biesheuvel CJ, Vergouwe Y, Oudega R, Hoes AW, Grobbee DE, Moons KG. Advantages of the nested case-control design in diagnostic research. BMC Med Res Methodol. 2008 Jul;8((1)):48. doi: 10.1186/1471-2288-8-48. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2500041&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/18644127%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2500041%5Cnhttp://bmcmedresmethodol.biomedc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth E, Ganz T. Hematol Oncol Clin North Am. Vol. 28. Elsevier Inc: 2014. Anemia of inflammation. pp. 671–681. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. VMNIS. 2011:1–6. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Haemoglobin+concentrations+for+the+diagnosis+of+anaemia+and+assessment+of+severity#1. [Google Scholar]
- 29.Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005 Aug;51((8)):1335–41. doi: 10.1373/clinchem.2005.048595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Data can be provided by the corresponding author in case of a reasonable request.