Abstract
Background
Infections produced by extensively drug-resistant (XDR) gram-negative bacilli (GNB) in solid organ transplant (SOT) are an important cause of morbidity and mortality. Ceftazidime/avibactam (CAZ-AVI) is a novel β-lactam/β-lactamase combination antibiotic with anti-GNB activity, but experience in real clinical practice with CAZ-AVI in lung transplant (LT) recipients is limited.
Methods
We conducted a retrospective study of patients with XDR-GNB infection who received at least 3 days of CAZ-AVI in the Department of Lung Transplantation Between December 2017 and December 2018 at China-Japan friendship hospital (CJFH). The general information, clinical manifestations, laboratory examinations, treatment course, and outcomes were summarized.
Results
A total of 10 patients who underwent LT at our center were included. They were all males with a mean age 51 years. Infections after LT included pneumonia and/or tracheobronchitis [n=9; 90% (9/10)], cholecystitis and blood stream infection (BSI) (n=1, patient 8). In these 10 LT recipients, the incidence of various airway complications was 70% (7/10). Carbapenem-resistant Klebsialla pneumoniae (CRKP) was the predominant pathogen, being detected in 9 patients. Multilocus sequence typing (MLST) analysis showed that all 9 CRKP isolates belonged to ST11. Six patients (6/10, 60%) started CAZ-AVI as salvage therapy after a first-line treatment with other antimicrobials. CAZ-AVI was administered as monotherapy or in combination regimens in 20% (2/10) and 80% (8/10) of patients respectively. There were no difference in temperature before and after CAZ-AVI treatment (P>0.05). White blood cell (WBC) at 7 days, and procalcitonin (PCT) at 7 days and 14 days significantly dropped (P<0.05). After 7–14 days of CAZ-AVI treatment, the PaO2/FiO2ratio (P/F ratio) significantly improved (P<0.05). Nine patients (9/10, 90%) obtained negative microbiologic culture of CRKP/CRPA, with a median time to was 6.7 days (range, 1–15 days). However, 5 patients (5/10, 50%) had relapse of CRKP/CRPA infections in the respiratory tract regardless of whether negative microbiologic culture was obtained or not. The 30-day survival rate was 100%, and the 90-day survival rate was 90% (1/10). No severe adverse events related to CAZ-AVI occurred.
Conclusions
CAZ-AVI treatment of CRKP/ CRPA infection in LT recipients was associated with high rates of clinical success, survival, and safety, but recurrent CRKP/CRPA infections in the respiratory tract did occur.
Keywords: Extensively drug-resistant bacteria, gram-negative bacilli (GNB), carbapenem-resistant K. pneumoniae, ceftazidime-avibactam, lung transplant (LT), outcomes
Introduction
In recent years, the emergence of multidrug-resistant (MDR) organisms has generated worldwide concern (1,2). Extensively drug-resistant (XDR) bacteria tend to primarily be gram-negative bacilli (GNB), and the prevalence of XDR-GNB is on the rise in China and other countries (3).
There has been a worldwide increase in the number of infections produced by MDRGNB in solid organ transplantation (SOT) recipients. These infections are important cause of the related morbidity and mortality (4). GNB are the leading causative agents of blood stream infection (BSI) in SOT recipients within the first year after SOT (5). The SOT population seems particularly at risk to develop carbapenem-resistant Enterobacteriaceae (CRE) infections (6). Infections caused by these so-called superbugs are associated with high mortality because therapeutic options are limited (7-10).
Data concerning MDR bacterial infection in lung transplant (LT) recipients are sparse (11-14), it has been observed that the MDR bacterial infections were primarily caused by GNB such as P. aeruginosa and K. pneumoniae (15).
Ceftazidime-avibactam (CAZ-AVI) demonstrated potent activity against molecularly confirmed extended-spectrum β-lactamases (ESBL)-producing (MIC90 0.5 µg/mL; 99.9% susceptible), plasmid-mediated AmpC-producing (MIC90 0.5 µg/mL; 100% susceptible), and ESBL- and AmpC-producing (MIC90 1 µg/mL; 100% susceptible) isolates of E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis (16).
In 2015, the U.S. Food and Drug Administration (FDA) approved CAZ-AVI, but it was not available for routine clinical use in China during the study period.
CAZ-AVI in the treatment of infections due to carbapenem-resistant K. pneumoniae (CRKP) was only reported in renal transplantation and liver transplantation in China (17-19).
Experience in real clinical practice with CAZ-AVI in LT recipients is limited. Thus, we herein describe 10 cases from our center along with a comprehensive review on the efficacy and safety of CAZ-AVI in XDR-GNB infection in LT recipients.
Methods
Patient selection and study design
We conducted a retrospective study of patients with XDR-GNB infection who received at least 3 days of CAZ-AVI treatment in the Department of Lung Transplantation between December 2017 and December 2018 at China-Japan friendship hospital (CJFH). The isolates of XDR-GNB were all resistant to any carbapenem, and only the first episode of XDR-GNB infection was included.
To summarize the characteristics (demographic and clinical) of the infections, their treatment course and outcomes (e.g., 90-day mortality).
A standard dosage of ceftazidime-avibactam was administered 2.5 g intravenously every 8 hours, with adjustments for renal impairment made according to manufacturer recommendations (20).
CAZ-AVI was not available for routine clinical use in China during the study period. No company had any type of involvement in the study.
Methodology
Clinical records
At the time of pulmonary sampling, clinical characteristics (purulent secretions, abundance of secretions, temperature), laboratory parameters, chest X-ray/chest computed tomography (CT) imaging and results of microbiologic cultures were collected.
Definitions
Pneumonia was defined according to the International Society for Heart and Lung Transplantation (ISHLT) consensus statement for the standardization of definitions of infections in cardiothoracic transplant recipients (21).
Tracheobronchitis was defined as positive culture of microbiologic samples, normal appearance or moderate interstitial infiltrates on chest X-ray, and at least one of the previously described clinical signs (21,22).
Bacteremia was classified as positive blood cultures and clinical signs of systemic inflammatory response syndrome.
Colonization was defined as positive culture of microbiologic samples with no clinical, laboratory, or radiological signs (21).
Infection onset was defined as the collection date of the index culture (i.e., the first culture that yielded the study isolate).
Salvage therapy was defined as antibiotic therapy administered after clinical and/or microbiological failure of a first-line treatment regimen or when it had not been possible to continue the previous therapy because of the onset of severe side effects.
Relapse was defined as the onset of a second microbiologically documented XDR-GNB infection in a patient whose original infection had been classified as a clinical cure (with or without microbiological confirmation).
Bronchoscopy procedures and microbiologic samples
Electronic bronchoscope was performed in most of the patients (except patient 7). During the postoperative period, bronchoscopy was performed according to clinical requirement with invasive respiratory tract samples collected by bronchial aspiration (BA) and by bronchoalveolar lavage (23). Tracheobronchial secretions were sent for bacteriological examination. Donor airway samples were obtained by BA during the surgical procedure before transplantation. Macroscopic aspects of the tracheobronchial tree and airway complications after LT were also recorded.
Isolate collection and microbiological investigation
The last isolates of XDR-GNB cultured before the usage of CAZ-AVI from 10 patients were collected for analysis. Antimicrobial susceptibility testing of CAZ-AVI was tested according to the Clinical and Laboratory Standards Institute (CLSI). Other antimicrobial susceptibility testing results were collected from the laboratory of clinical microbiology and infectious diseases. Nine CRKP isolates underwent multilocus sequence typing (MLST) and polymerase chain reaction (PCR) to detect capsular serotype and β-lactamase genes.
Statistical analysis
Statistical analysis was performed by GraphPad Prism 5. Continuous variables were analyzed using Student’s t-test (matched measure) or one-way ANOVA (repeated measure). All P values were two-sided, with statistical significance defined as a P<0.05.
Results
Clinical features
There were a total of 10 LT recipients who were all males (mean age, 51 years; range, 31–68 years). Native lung disease included idiopathic pulmonary fibrosis (IPF) (n=6), pulmonary hypertension (PH) (n=3), and bronchiectasis (n=1). Single LT (SLT) and bilateral LT (BLT) were performed in 4 patients and 6 patients respectively. Days from LT procedures to positive microbiologic findings of CRKP or carbapenem-resistant Pseudomonas aeruginosa (CRPA) ranged from 1 day to 321 days. Demographic data are shown in Table 1.
Table 1. Demographic data of the patients.
| Patient No. | Gender | Age | Native lung disease | Surgical type | Airway complications | Days from LT to XDR-GNB acquired | Antibiotics before CAZ-AVI |
|---|---|---|---|---|---|---|---|
| 1 | Male | 68 | IPF | SLT (L) | Anastomotic fistulas with bronchial stenosis | 5 | Tigecycline + polymyxin B |
| 2 | Male | 33 | PH | BLT | Bronchial stenosis | 37 | Polymyxin B + imipenem/cilastatin |
| 3 | Male | 54 | IPF | BLT | N | 321 | Ceftazidime + polymyxin B, piperacillin/tazobactam + polymyxin B |
| 4 | Male | 35 | PH | BLT | Anastomotic fistulas | 9 | None |
| 5 | Male | 31 | Bronchiectasis | BLT | Anastomotic fistulas | 150 | Tigecycline + polymyxin B |
| 6 | Male | 67 | IPF | SLT (L) | Anastomotic fistulas with bronchial stenosis | 246 | None |
| 7 | Male | 58 | IPF | BLT | N | 188 | None |
| 8 | Male | 67 | IPF | SLT (R) | Anastomotic fistulas | 1 | Tigecycline + polymyxin B |
| 9 | Male | 67 | IPF | SLT (L) | N | 8 | None |
| 10 | Male | 31 | PH | BLT | Bronchial stenosis | 8 | Tigecycline + polymyxin B |
IPF, idiopathic pulmonary fibrosis; PH, pulmonary hypertension; SLT, single-lung transplant; BLT, bilateral-lung transplant; CAZ-AVI, ceftazidime-avibactam; XDR-GNB, extensively drug-resistant Gram-negative bacteria.
Airway complications
In these 10 LT recipients, the incidence of various airway complications was 70% (7/10). Airway complications included 5 cases of anastomotic fistula and 2 cases of bronchial stenosis. Among these patients, two of them combined anastomotic fistula with bronchial stenosis. XDR-GNB isolated from the respiratory tract in these 7 patients with airway complications were all CRKP. Four cases and 3 cases of CRKP isolated respectively before and after airway complications.
Clinical manifestations
The main symptoms included excessive phlegm and/or purulent secretions (9/10, 90%), dyspnea (6/10, 60%), fever (6/10, 60%), PaO2/FiO2 ratio decreased (4/10, 40%), and somnolence (1/10, 10%). One patient manifested as high fever, chills, and upper abdominal pain.
Laboratory examination
At the onset of infection, white blood cell (WBC) elevated in all patients except patient 7 who had leukocytopenia and neutropenia. His WBC level elevated to 6.1×109/L after subcutaneous injection granulocyte cell stimulating factor (GCSF). Procalcitonin (PCT) ranged from 0.23 to 5.39. Six patients with pneumonia showed mild to severe lung infiltration in chest X-ray or chest CT.
Antimicrobial susceptibility testing and microbiological characteristics
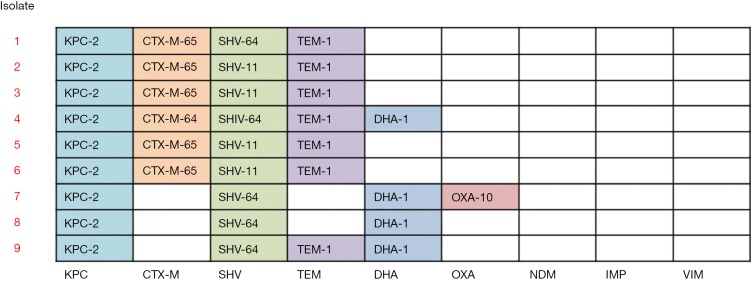
Antimicrobial susceptibility results for 10 XDR-GNB isolates are listed in Table 2. MLST analysis showed that all 9 CRKP isolates belonged to ST11. The capsular serotype of isolates 1, 5, 8, 9, and 10 is KL47, and the capsular serotype of isolates 2, 4, 6, and 7 is KL64. The β-lactamase genes detected in CRKP isolates are shown in Figure 1.
Table 2. Characteristics of isolates recovered from lung transplant recipients.
| Isolate | Organism | MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C/A | CAZ | IMP | MEM | ATM | FEP | TZP | TGC | CST | ||
| 1 | K. pneumoniae | 2 | ≥64 | 4 | ≥16 | ≥64 | ≥64 | >64/4 | 0.5 | 8 |
| 2 | K. pneumoniae | 4 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | 2 | ≥16 |
| 3 | P. aeruginosa | 8 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | NA | ≤0.5 |
| 4 | K. pneumoniae | 8 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | 2 | ≥16 |
| 5 | K. pneumoniae | 8 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | 0.5 | 0.5 |
| 6 | K. pneumoniae | 0.5 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | 1 | 16 |
| 7 | K. pneumoniae | <0.125 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | ≤0.5 | ≤0.5 |
| 8 | K. pneumoniae | 4 | ≥64 | 8 | ≥16 | ≥64 | ≥64 | >64/4 | 0.5 | 2 |
| 9 | K. pneumoniae | 4 | ≥64 | 8 | ≥16 | ≥64 | ≥64 | >64/4 | 0.5 | 2 |
| 10 | K. pneumoniae | 2 | ≥64 | ≥16 | ≥16 | ≥64 | ≥64 | >64/4 | 2 | ≤0.5 |
NA, not available; C/A, ceftazidime-avibactam; CAZ, ceftazidime; IMP, imipenem; MEM, meropenem; ATM, aztreonam; FEP, cefepime; TZP, piperacillin-tazobactam; TGC, tigecycline; CST, colistin.
Figure 1.
β-lactamase genes detected in 9 CRKP isolates. CRKP, carbapenem-resistant Klebsialla pneumoniae.
Treatment course
Infections after LT included pneumonia and/or tracheobronchitis [n=9; 90% (9/10)], cholecystitis, and BSI (n=1, patient 7). Patient 7 who had an acute episode of abdominal pain, high fever, and jaundice 4 months after LT was diagnosed as acute cholangitis. He recovered from it after undergoing endoscopic retrograde cholangiopancreatography (ERCP) and use of imipenem/cilastatin. One month later, he had chills, high fever, and abdomen pain with leukocytopenia. Abdominal CT and MRI indicated acute purulent cholecystitis. The bacteremia of three consecutive blood cultures of CRKP was definitely diagnosed. He obtained negative blood culture in the second day after emergent cholecystectomy and application of CAZ-AVI combined with tigecycline. Finally clinical success achieved quickly.
Six patients (6/10, 60%) started CAZ-AVI as salvage therapy after a first-line treatment with other antimicrobials. The first-line antibiotic regimens were all based on polymyxin B, in combination with tigecycline (n=4), with imipenem/cilastatin (n=1), and with ceftazidime and piperacillin/tazobactam successively (n=1). Five patients had clinical failure after first-line antibiotic regimens. One patient could not tolerate the neurotoxicity associated with systemic polymyxin therapy.
CAZ-AVI was administered as monotherapy or in combination regimens in 20% (2/10) and 80% (8/10) of patients respectively. All combination agents were started concomitantly with CAZ-AVI and administered for 72 hours or longer. Combinations included intravenous tigecycline (n=7) and polymyxin B (n=1). Median treatment duration was 15.7 days (range, 9–22 days). The dosage of CAZ-AVI in 6 patients was 2.5 g intravenously every 8 hours. Renal adjustment for ceftazidime-avibactam occurred in 4 (40%) patients.
Outcomes
At the onset of infection, the median simplified acute physiology score II (SAPS II) was 9.2 (range, 1–20), and the mean sequential organ failure assessment (SOFA) score was 5.8 (range, 1–13).
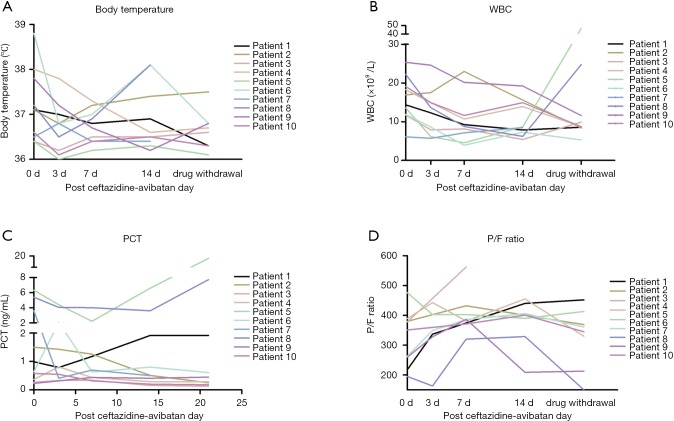
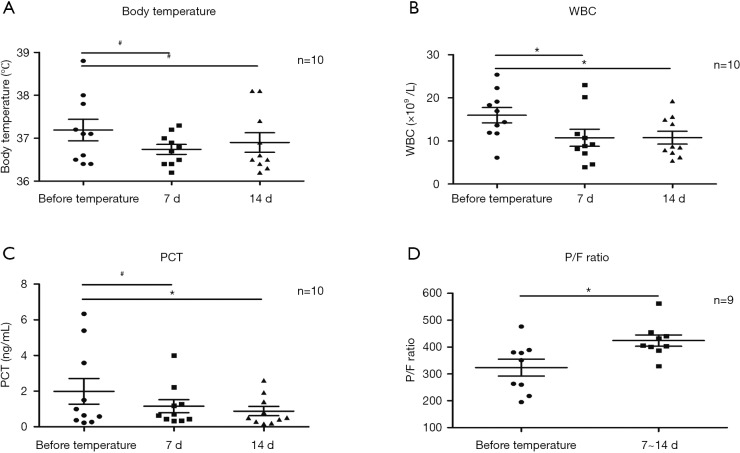
The dynamic curve of temperature, WBC, PCT, and P/F ratio are shown in Figure 2A,B,C,D. In patient 8, after the 2nd to 3rd day of CAZ-AVI, excessive phlegm was reduced dramatically, and his temperature dropped down to normal. However, on the 4th day, he had anastomotic fistulas and thoracic bleeding. He was reintubated in emergency. He had concurrent infection with carbapenem-resistant Acinetobacter baumannii (CRAB), and thus his WBC, PCT and body temperature elevated again. There was no difference in temperature before and after CAZ-AVI treatment (P>0.05) (Figure 3A). WBC at 7 days, and PCT at 7 days and 14 days significantly dropped (P<0.05) (Figure 3B,C). After 7–14 days of CAZ-AVI treatment, the PaO2/FiO2 ratio (P/F ratio) significantly improved (P<0.05) (Figure 3D).
Figure 2.
The dynamic curve of clinical features in 10 patients. (A) Temperature curve of 10 patients; (B) WBC curve in 10 patients; (C) PCT curve in 10 patients; (D) P/F ratio curve in 10 patients. WBC, white blood cell; PCT, procalcitonin; P/F ratio, PaO2/FiO2 ratio.
Figure 3.
Statistical figures of clinical features. (A,B,C,D) Statistical figures of temperature, WBC, PCT, and P/F ratio analyzed using Student’s t-test (matched measure) or one-way ANOVA (repeated measure). *P<0.05, #NS (by one-way ANOVA, repeated measure, Dunnett test, or Student’s t-test, matched measure). WBC, white blood cell; PCT, procalcitonin; P/F ratio, PaO2/FiO2 ratio.
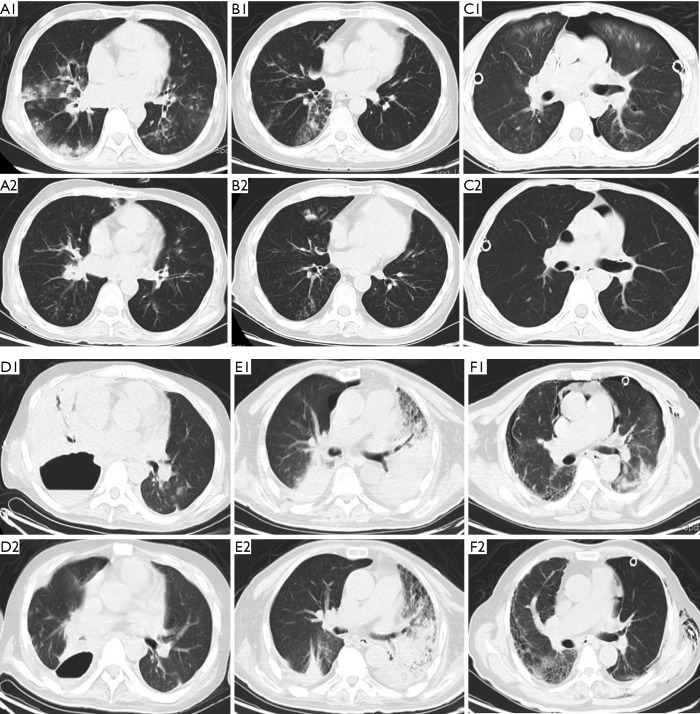
The lung infiltration of 6 patients with pneumonia was absorbed dramatically after administration of CAZ-AVI (Figure 4A,B,C,D,E,F).
Figure 4.
Chest CTs of 6 patients with pneumonia. (A1, B1, C1, D1, E1, and F1) Chest CT before the administration of CAZ-AVI in patient 2, 3, 4, 5, 8, and 9; (A2, B2, C2, D2, E2, and F2) chest CT after the administration of CAZ-AVI in patient 2, 3, 4, 5, 8, and 9. CT, computed tomography; CAZ-AVI, ceftazidime/avibactam.
Nine patients (9/10, 90%) obtained negative microbiologic culture of CRKP/CRPA; the median time was 6.7 days (range, 1–15 days). However, 5 patients (5/10, 50%) had relapse of CRKP/ CRPA infections in the respiratory tract regardless of whether negative microbiologic culture was obtained or not.
The 30-day survival rate was 100% and the 90-day survival rate was 90% (1/10). Treatment failures occurred in patient 8. He did have temporary clinical and radiological improvement after application of CAZ-AVI, but he died due to anastomotic fistulas and pneumonia 35 days after CRKP infection. The treatment course and clinical outcome are shown in Table 3.
Table 3. Treatment course and clinical outcome.
| Patient No. | Type of initial infection | Initial treatment regimen duration (d) | Combined antibiotics | Time to negative microbiologic culture (d) | Infection relapse | Clinical outcome at 90 days |
|---|---|---|---|---|---|---|
| 1 | Tracheobronchitis | 15 | Tigecycline | 6 | Recurrent tracheobronchitis | Alive |
| 2 | Pneumonia tracheobronchitis | 18 | Polymyxin B | 11 | Recurrent pneumonia | Alive |
| 3 | Pneumonia | 19 | None | 15 | Recurrent pneumonia | Alive |
| 4 | Pneumonia tracheobronchitis | 22 | Tigecycline | Respiratory colonization | Recurrent tracheobronchitis | Alive |
| 5 | Pneumonia tracheobronchitis | 16 | Tigecycline | 11 | 0 | Alive |
| 6 | Tracheobronchitis | 19 | None | 3 | 0 | Alive |
| 7 | Cholecystitis sepsis | 14 | Tigecycline | 1 | 0 | Alive |
| 8 | Pneumonia tracheobronchitis | 13 | Tigecycline | 2 | 0 | Dead |
| 9 | Pneumonia tracheobronchitis | 14 | Tigecycline | 9 | Recurrent tracheobronchitis | Alive |
| 10 | Tracheobronchitis | 14 | Tigecycline | 7 | 0 | Alive |
Adverse events
During the CAZ-AVI treatment period, 3 patients had blood urea and creatinine increased. One of these patients had combined polymyxin B treatment, which primarily contributed to renal dysfunction. Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) increased in 2 patients, concomitant with tigecycline in one patient, and voriconazole in the other. Three patients had elevation of alkaline phosphatase (ALP), γ-glutamyltranspeptadase (GGT) and total bilirubin (TBIL), two of them combined with tigecycline. In the blood and lymphatic system disorders, one patient had thrombocytosis.
No adverse events happened in the skin and subcutaneous tissue, the gastrointestinal system, and nervous system (Table 4).
Table 4. Adverse effects of ceftazidime-avibactam.
| System organ class | Adverse effects related with ceftazidime-avibactam | Incidence |
|---|---|---|
| Renal and urinary system | Blood urea and creatinine increased | 3/10 |
| Hepatobiliary system disorders | Alanine aminotransferase and aspartate aminotransferase increased | 2/10 |
| Alkaline phosphatase, γ-glutamyl transpeptadase and total bilirubin increased | 3/10 | |
| Gastrointestinal disorders | Nausea, vomiting, stomach ache, diarrhea | 0/10 |
| Skin and subcutaneous tissue disorders | Rash maculopapular, urticaria, pruritus | 0/10 |
| Blood and lymphatic system disorders | Eosinophilia | 0/10 |
| Thrombocytosis | 1/10 | |
| Nervous system disorders | Headache and dizziness | 0/10 |
No severe adverse events happened in our study.
Discussion
We designed an observational study of a prospectively collected cohort of adult LT recipients receiving CAZ-AVI in our center. The efficacy and safety of the CAZ-AVI in our cohort of LT recipients were approved. CAZ-AVI shows promising results, even in monotherapy, for the treatment of patients with severe infections due to CRKP/CRPA, especially in CRKP infection.
Avibactam is a member of a novel class of non-β-lactam/β-lactamase inhibitors, the diazabicyclooctanes (DBOs). Compared to currently available inhibitors for clinical use, DBOs are more potent and have a broader spectrum and a different mechanism of action (24).
In our study, 9 patients had good outcomes, especially the CRKP infection patients. All K. pneumoniae isolates produced KPC-2 carbapenemase. CAZ-AVI represents a potentially powerful tool for managing these infections in light of its demonstrated in vitro activity against CRE isolates that produce KPC enzymes (as well as extended-spectrum β-lactamases, AmpC β-lactamases, and oxacillinases) (25). Since the majority of CRE infections in the United States are caused by KPC-producing K. pneumoniae, CAZ-AVI may offer a significant improvement over previous treatment regimens (26). ST11 is predominant in China. Most K. pneumoniae ST11 isolates (96.2%) produced KPC-2 carbapenemase. KPC-2 is the most common carbapenemase type in K. pneumoniae (919/1,201, 76.5%) (27).
In our study, 5 patients had clinical failure after the first-line antibiotic regimens based on polymyxin B. Another patient could not tolerate the neurotoxicity associated with systemic polymyxin therapy. CRE treatment options include polymyxins such as colistin and polymyxin B (6). Concerns about polymyxins include toxicity, limited efficacy, dosing uncertainties, and resistance, including worrisome mcr-1 mediated resistance (28,29). Several articles have reported evidence for the superiority of CAZ-AVI over other active agents including colistin in the initial treatment of infections caused by carbapenemase-producing CRE (26,30-32). The use of CAZ-AVI was associated with improved clinical outcomes (33), especially decreased all-cause hospital mortality rate and improved benefit-risk outcomes (32).
Two previous studies have compared the outcomes of first-line CAZ-AVI treatment with those of other antimicrobial regimens in patients with CRE bacteremia (26,32). Survival might reasonably be expected to be worse than those recorded in settings where CAZ-AVI could be started promptly after infection onset. Instead, the 30-day mortality rate we observed (34.1%) is identical to that reported in patients whose CRE infections were treated with CAZ-AVI as the first-line antibiotic regimen (34).
CAZ-AVI was very active against P. aeruginosa (PA). Alatoom et al. reported 29 (94%) PA isolates were susceptible to CAZ-AVI (MIC50 1.5 mµg/mL). Ceftolozane-tazobactam and CAZ-AVI showed comparable activity against ESBL and PA (35). In a teaching hospital, 8 patients with infections due to MDR- or XDR-PA were treated with CAZ-AVI, including four strains resistant to ceftolozane/tazobactam (36).
In our study, 5 patients (5/10, 50%) had recurrent CRKP/CRPA infections in the respiratory tract which was much higher than the experience of Shields’ and Tumbarello’s study where infection relapses occurred in 13.5% and 8.7% of patients respectively. Meanwhile, the recurrence rate at 90 days was 10% in Sousa’s study (37). It is important to acknowledge that these differences may reflect the use of CAZ-AVI predominantly in different types of infections (bacteremia in Tumbarello’s study and pneumonia in that of Shields et al. (34,38). Pneumonia has been recently recognized as risk factor for CAZ-AVI resistance among patients with CRE infections (39). In our study, the incidence of airway complications was high, and it was unique complications in LT patients. Perhaps airway complications, especially bronchial stenosis and immunosuppressed status, were related with infection relapses. Furthermore, 3/37 (8.1%) and 3/138 (2.2%) of cases of KPC-Kp isolates reported by Shields et al. (34) and Tumbarello were associated with acquired in vitro resistance to CAZ-AVI (MICs >8 µg/mL) (38). We will report isolate resistance development in a following study.
This study is limited by its retrospective, single-center design, and the small patient population. Due to the study size, we are unable to make definitive conclusions about the effectiveness of CAZ-AVI in CRKP/CRPA infection in LT recipients. There is thus an urgent need to expand the sample size of the study and conduct a case-control study comparing patients with CRKP infection CAZ-AVI and non-CAZ-AVI in the future. Lastly, this was a retrospective study and prospective evaluation of our practice could be a useful approach in future studies.
Acknowledgments
Funding: Supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences: 2019PT320020.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the China-Japan Friendship Hospital, Beijing, China, and written informed consent was obtained from the patients or their next of kind.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008;8:159-66. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 2.Karaiskos I, Giamarellou H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother 2014;15:1351-70. 10.1517/14656566.2014.914172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan X, He L, Hu B, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clinical Microbiology and Infection 2016;22:S15-25. 10.1016/j.cmi.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Silva JT, Fernandez-Ruiz M, Aguado JM. Multidrug-resistant Gram-negative infection in solid organ transplant recipients: implications for outcome and treatment. Curr Opin Infect Dis 2018;31:499-505. 10.1097/QCO.0000000000000488 [DOI] [PubMed] [Google Scholar]
- 5.Oriol I, Sabe N, Simonetti AF, et al. Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: a single-centre prospective cohort study. Transpl Int 2017;30:903-13. 10.1111/tri.12984 [DOI] [PubMed] [Google Scholar]
- 6.Bartoletti M, Giannella M, Tedeschi S, et al. Multidrug-Resistant Bacterial Infections in Solid Organ Transplant Candidates and Recipients. Infect Dis Clin North Am 2018;32:551-80. 10.1016/j.idc.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 2009;9:228-36. 10.1016/S1473-3099(09)70054-4 [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Limbago BM, Patel JB, et al. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011;53:60-7. 10.1093/cid/cir202 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob Agents Chemother 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis 2016;35:1679-89. 10.1007/s10096-016-2710-0 [DOI] [PubMed] [Google Scholar]
- 11.Tebano G, Geneve C, Tanaka S, et al. Epidemiology and risk factors of multidrug-resistant bacteria in respiratory samples after lung transplantation. Transpl Infect Dis 2016;18:22-30. 10.1111/tid.12471 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigo-Troyano A, Sibila O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology 2017;22:1288-99. 10.1111/resp.13115 [DOI] [PubMed] [Google Scholar]
- 13.Husain S, Chan KM, Palmer SM, et al. Bacteremia in Lung Transplant Recipients in the Current Era. Am J Transplant 2006;6:3000-7. 10.1111/j.1600-6143.2006.01565.x [DOI] [PubMed] [Google Scholar]
- 14.Riera J, Caralt B, López I, et al. Ventilator-associated respiratory infection following lung transplantation. Eur Respir J 2015;45:726-37. 10.1183/09031936.00095214 [DOI] [PubMed] [Google Scholar]
- 15.Whiddon AR, Dawson KL, Fuentes A, et al. Postoperative antimicrobials after lung transplantation and the development of multidrug-resistant bacterial and Clostridium difficile infections: an analysis of 500 non-cystic fibrosis lung transplant patients. Clin Transplant 2016;30:767-73. 10.1111/ctr.12746 [DOI] [PubMed] [Google Scholar]
- 16.Karlowsky JA, Biedenbach DJ, Kazmierczak KM, et al. Activity of Ceftazidime-Avibactam against Extended-Spectrum- and AmpC beta-Lactamase-Producing Enterobacteriaceae Collected in the INFORM Global Surveillance Study from 2012 to 2014. Antimicrob Agents Chemother 2016;60:2849-57. 10.1128/AAC.02286-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Li D, Wu H, et al. Medical experience in 13 cases of donor-derived drug-resistant Klebsiella pneumoniae infection. Practical Journal of Organ Transplantation (Electronic Version) 2018;6:9-12. [Google Scholar]
- 18.Li G, Li C, Xie J, et al. Experience in diagnosis and treatment of infection and bleeding caused by DCD-derived CRKP in kidney transplant recipients. Chin J Organ Transplant 2018;39:582-5. [Google Scholar]
- 19.Chen X, Han L, Qian Y, et al. The difference between liver and kidney transplantation—Donor derived infection of carbapenemresistant Klebsiella pneumonia. Prac J Organ Transplant (Electronic Version) 2018;6:45-8. [Google Scholar]
- 20.Acycaz (Ceftazidime-avibactam). Prescribing information. Cincinnati, Ohio: Forest Pharmaceuticals, Inc., 2016. [Google Scholar]
- 21.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011;30:361-74. 10.1016/j.healun.2011.01.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Loeches I, Povoa P, Rodriguez A, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med 2015;3:859-68. 10.1016/S2213-2600(15)00326-4 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Geneve C, Tebano G, et al. Morbidity and mortality related to pneumonia and TRACHEOBRONCHITIS in ICU after lung transplantation. BMC Pulm Med 2018;18:43. 10.1186/s12890-018-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sader HS, Castanheira M, Flamm RK. Antimicrobial Activity of Ceftazidime-Avibactam against Gram-Negative Bacteria Isolated from Patients Hospitalized with Pneumonia in U.S. Medical Centers, 2011 to 2015. Antimicrob Agents Chemother 2017;61:e02083-16. 10.1128/AAC.02083-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Duin D, Bonomo RA, Saravolatz LD. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin Infect Dis 2016;63:234-41. 10.1093/cid/ciw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother 2017. doi: . 10.1128/AAC.00883-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Wang X, Wang J, et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae: Data From a Longitudinal Large-scale CRE Study in China (2012-2016). Clin Infect Dis 2018;67:S196-205. 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- 28.Rojas LJ, Salim M, Cober E, et al. Colistin Resistance in Carbapenem-Resistant Klebsiella pneumoniae: Laboratory Detection and Impact on Mortality. Clin Infect Dis 2017;64:711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016;16:161-8. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 30.Zhong H, Zhao XY, Zhang ZL, et al. Evaluation of the efficacy and safety of ceftazidime/avibactam in the treatment of Gram-negative bacterial infections: a systematic review and meta-analysis. Int J Antimicrob Agents 2018;52:443-50. 10.1016/j.ijantimicag.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 31.Caston JJ, Lacort-Peralta I, Martin-Davila P, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 2017;59:118-23. 10.1016/j.ijid.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 32.van Duin D, Lok JJ, Earley M, et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clinical Infectious Diseases 2018;66:163-71. 10.1093/cid/cix783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King M, Heil E, Kuriakose S, et al. Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother 2017. doi: . 10.1128/AAC.00449-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shields RK, Potoski BA, Haidar G, et al. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin Infect Dis 2016;63:1615-8. 10.1093/cid/ciw636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alatoom A, Elsayed H, Lawlor K, et al. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis 2017;62:39-43. 10.1016/j.ijid.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Nunez O, Ripa M, Morata L, et al. Evaluation of ceftazidime/avibactam for serious infections due to multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist 2018;15:136-9. 10.1016/j.jgar.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 37.Sousa A, Perez-Rodriguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2018;73:3170-5. 10.1093/jac/dky295 [DOI] [PubMed] [Google Scholar]
- 38.Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin Infect Dis 2019;68:355-64. 10.1093/cid/ciy492 [DOI] [PubMed] [Google Scholar]
- 39.Shields RK, Nguyen MH, Chen L, et al. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother 2018. doi: . 10.1128/AAC.02497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]