Abstract
Improvements in life expectancy among people living with human immunodeficiency virus (PLWH) receiving antiretroviral treatment in the United States and Canada might differ among key populations. Given the difference in substance use among key populations and the current opioid epidemic, drug- and alcohol-related deaths might be contributing to the disparities in life expectancy. We sought to estimate life expectancy at age 20 years in key populations (and their comparison groups) in 3 time periods (2004–2007, 2008–2011, and 2012–2015) and the potential increase in expected life expectancy with a simulated 20% reduction in drug- and alcohol-related deaths using the novel Lives Saved Simulation model. Among 92,289 PLWH, life expectancy increased in all key populations and comparison groups from 2004–2007 to 2012–2015. Disparities in survival of approximately a decade persisted among black versus white men who have sex with men and people with (vs. without) a history of injection drug use. A 20% reduction in drug- and alcohol-related mortality would have the greatest life-expectancy benefit for black men who have sex with men, white women, and people with a history of injection drug use. Our findings suggest that preventing drug- and alcohol-related deaths among PLWH could narrow disparities in life expectancy among some key populations, but other causes of death must be addressed to further narrow the disparities.
Keywords: black women, drug- and alcohol-related deaths, health disparities, Hispanic adults, HIV, life expectancy, men who have sex with men, people who inject drugs
With advances over the past several decades in the efficacy and tolerability of antiretroviral therapy (ART), human immunodeficiency virus infection (HIV)-related morbidity and mortality have declined markedly and durably (1–3). Life expectancy, a key indicator encompassing mortality trends across the life span within a population, has shown a concomitant dramatic increase among people living with HIV (PLWH) accessing standard-of-care treatment (4, 5). A meta-analysis published in 2017 indicated that an additional 43 years of life at age 20 could be expected for PLWH on ART, a 10-year increase compared with findings from studies conducted in the late 1990s (6).
After steady increases in life expectancy over the several preceding decades among the general US population, a 0.1-year decrease occurred between 2014 and 2015, and again between 2015 and 2016 (7, 8). This consecutive-year decline in life expectancy in the US general population was unprecedented and translates into nearly a quarter of a million years of life lost each year in the United States (9). A major driver of this decrease is increasing drug- and alcohol-related deaths, including opioid-related deaths (10–12). PLWH have been shown to have higher rates of underlying substance use disorders (including opioids) and chronic pain (for which opioids could be prescribed) (13, 14).
Not all PLWH experience equivalent life expectancy; significant disparities exist among key populations. Previous estimates of life expectancy published from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) indicated that life expectancy at age 20 was 51.4 years overall in 2006–2007; life expectancy at age 20 was 48.4 years among nonwhite PLWH compared with 56.9 years among white individuals, 28.8 years among people who inject drugs, and 69.3 years among men who have sex with men (MSM) (4). These findings were consistent with other studies that have shown a 10-year difference in life expectancy among black compared with white PLWH, a 20-year shorter life expectancy among people who inject drugs and those with hepatitis C virus coinfection, and an equivalent or increased life expectancy among MSM compared with heterosexual PWLH (15–21). It is unknown whether the increase in drug- and alcohol-related deaths has affected disparities in life expectancy among key populations with HIV.
In this study, we estimated observed life expectancy estimates for several key populations of PLWH using mortality rates from the NA-ACCORD, highlighting disparities between key populations and their appropriate comparison groups. Next, we developed the Lives Saved Simulation (LISSO) model to estimate the potential narrowing of “expected” life-expectancy disparities that could occur between key populations and their appropriate comparison groups from a simulated 20% reduction in the number of drug- and alcohol-associated deaths among adults with HIV. A 20% reduction is the goal set forth in the Healing Communities Studies, which are funded by the National Institutes of Health’s Helping to End Addiction Long-Term initiative (22).
METHODS
Study population
The NA-ACCORD is a collaboration of >20 interval and clinical cohorts collecting longitudinal data from PLWH (≥18 years old) in Canada and the United States (http://www.naaccord.org/). This collaboration is the North American region of the National Institute of Health’s International Epidemiology Databases to Evaluate AIDS consortium. Details of the NA-ACCORD collaboration and its cohorts have been published elsewhere (23). Briefly, participants are eligible to be enrolled in the NA-ACCORD from contributing clinical cohorts if they have ≥2 clinical visits within 12 months. After enrollment, participants do not have to be retained in care to be retained in the NA-ACCORD. Individual-level data are pooled and harmonized and then undergo quality-control procedures for accuracy and completeness at the central Data Management Core (University of Washington, Seattle, Washington). The data then undergo additional quality-control procedures and assembly into analysis-ready summary files at the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, Maryland). The institutional review boards at each participating cohort and at the Johns Hopkins University School of Medicine have approved all human subject activities conducted within the NA-ACCORD.
Cohorts were included in the present study if they provided cause-of-death information (20 cohorts). Individuals were included in this analysis if they were prescribed ART between January 2004 and December 2015.
Deaths and alcohol- and drug-related deaths
Information regarding deaths was obtained through linkages with vital statistics registries (including the US National Death Index Plus), physician report, and/or outreach to the decedent’s survivors. Previously, the NA-ACCORD reported similar mortality rates among cohorts that do, and do not, link to death registries (4). Alcohol- and drug-related deaths were identified in cohorts submitting National Death Index Plus matching or death certificate information using International Classification of Diseases (revisions 9 and 10) codes identified by the National Center for Health Statistics (K70, X40–X44, X60–X64, X85, and Y10–Y14) (24). A free-text search in cause-of-death data was applied to the deaths that were submitted from physician report and/or outreach to the decedent (see Web Appendix 1, available at https://academic.oup.com/aje).
Demographic characteristics
Age, sex, race/ethnicity, and HIV acquisition risk were classified at entry into the NA-ACCORD. Age was estimated from year of birth. Sex was classified as male or female; there was an insufficient sample size to estimate observed mortality rates by age group and calendar period among transgender and intersex individuals. Race was identified as black or white, regardless of Hispanic ethnicity. Hispanic ethnicity (yes/no) and HIV acquisition risk (MSM and having a history of injection drug use (IDU)) were collected. These characteristics were used to identify the following key populations of interest: black (vs. white) MSM, black (vs. white) women, Hispanic (vs. non-Hispanic) adults, and people with a history of IDU (vs. those without).
CD4 count and a history of an acquired immune deficiency syndrome (AIDS)-defining illness were measured to describe the population and to allow for comparison of our estimates with previous estimates of life expectancy in PLWH (3). CD4 count was measured as close to study entry as possible, within the window of before to 3 months after study entry. A clinical AIDS diagnosis at, or prior to, study entry was defined as diagnosis with an AIDS-defining opportunistic infection or cancer.
Statistical analysis: observed mortality rates and life expectancy, and simulated mortality rates and life expectancy using the LISSO model
Participants contributed deaths and person-years from age 20 years or January 1, 2004, (whichever came last) to the date of death, loss to follow-up (defined as a gap of >2 years without a CD4 or HIV RNA measurement), or administrative censoring at either the date through which the cohort ascertained cause of death information or December 31, 2015 (whichever came first).
Observed mortality rates per 1,000 person-years were calculated as the number of observed deaths divided by the number of observed person-years × 1,000. Observed mortality rates were estimated in 5-year age groups (20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, ≥80) for each calendar period (2004–2007, 2008–2011, and 2012–2015) within each key population and comparison group. Then mortality rates were standardized to the 2000 US Standard Population (25) to account for the increasing mean age in the age groups between calendar periods. In some of the key populations and comparison groups, mortality rates were unstable, particularly at older ages, due to smaller amounts of person-time and deaths observed. Age groups were then collapsed to 10-year age groups (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, ≥80) to increase the stability of the mortality rates.
Observed life expectancy was estimated using single-decrement, abridged period life tables (a standard approach) constructed from the observed mortality rates estimated for each decade of age in each calendar period for each key population and comparison group; the Chiang method was used to calculate the 95% confidence intervals (26). Life expectancy estimates can be interpreted as the average additional number of years that a 20-year-old with treated HIV will live, assuming constant age-specific mortality rates across that individual’s lifetime.
Anticipated (i.e. “expected”) life expectancy gained from a specified decrease in drug- and alcohol-related deaths was estimated by adapting the approach developed by Case and Deaton, which estimates the number of “lives saved” for each calendar year and age stratum after a reduction in a cause-specific mortality rate (11). In this approach, the population saved experiences the mortality rate of their peers as well as the opportunity for a proportion of the population to age into older age categories. For more details of the approach, please see Web Appendix 2 and the supplemental information to Case and Deaton’s paper (27). The Case and Deaton approach uses aggregated population counts and mortality rates in calendar time and age strata.
We built the LISSO agent-based simulation model using individual-level data from the NA-ACCORD, including the age at death for those who are saved. We randomly selected 20% of the individuals (i.e., agents) in each key population and comparison group who were observed to die from drug- and alcohol-related causes to be “saved” and subsequently experience the same overall mortality risk as their peers (i.e., the probability of death within the calendar period and age stratum) and/or the opportunity to age into the older age groups (Figure 1). The random selection of the 20% saved was simulated 100 times, and mortality rates were estimated for each simulation; the median mortality rates were used to estimate the expected life expectancy at age 20 in each calendar year period within each key population and comparison group. These life expectancy estimates can be interpreted as the average additional number of years that a 20-year-old with treated HIV would live if there was a 20% decrease in drug- and alcohol-related deaths, assuming constant age-specific mortality rates across that individual’s lifetime.
Figure 1.
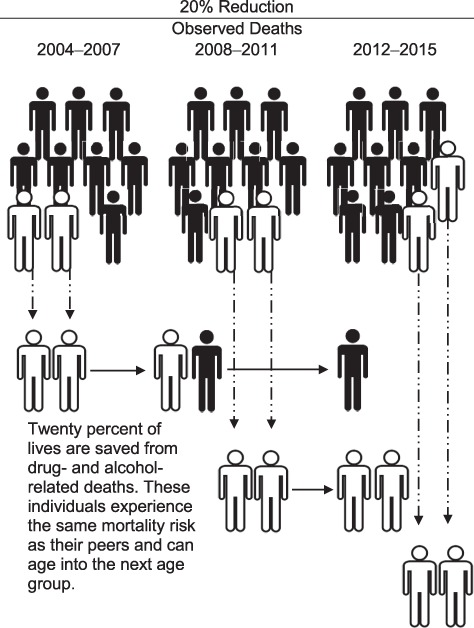
A schematic of the Lives Saved Simulation model, an agent-based simulation model that randomly selects 20% of those who died to be “saved” and experience the subsequent mortality risk of their peers, as well as the opportunity to age into older age groups.
Table 1.
Characteristics of Persons With Human Immunodeficiency Virus Using Antiretroviral Therapy, According to Key Population and Comparison Group, North American AIDS Cohort Collaboration on Research and Design, United States and Canada, 2004–2015
| Characteristic |
Black MSM
(n = 4,569) |
White MSM
(n = 17,433) |
Black Women
(n = 6,696) |
White Women
(n = 2,880) |
Hispanic Adults
(n = 10,707) |
Non-Hispanic Adults
(n = 81,582) |
IDU
(n = 17,798) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, yearsa | ||||||||||||||
| 20–39 | 2,366 | 52 | 5,708 | 33 | 2,843 | 42 | 1,141 | 40 | 4,418 | 41 | 23,443 | 29 | 2,973 | 17 |
| 40–49 | 1,497 | 33 | 7,029 | 40 | 2,432 | 36 | 1,100 | 38 | 3,511 | 33 | 29,284 | 36 | 7,234 | 41 |
| 50–59 | 576 | 13 | 3,690 | 21 | 1,179 | 18 | 502 | 17 | 2,094 | 20 | 21,133 | 26 | 6,515 | 37 |
| ≥60 | 130 | 3 | 1,006 | 6 | 242 | 4 | 137 | 5 | 684 | 6 | 7,722 | 9 | 1,076 | 6 |
| Male sexa | 4,569 | 100 | 17,433 | 100 | 0 | 0 | 0 | 0 | 8,975 | 84 | 70,279 | 86 | 15,105 | 85 |
| Racea,b | ||||||||||||||
| White | 0 | 0 | 17,433 | 100 | 0 | 0 | 2,880 | 100 | 0 | 0 | 38,979 | 48 | 5,630 | 32 |
| Black | 4,569 | 100 | 0 | 0 | 6,696 | 100 | 0 | 0 | 0 | 0 | 32,134 | 39 | 8,735 | 49 |
| Hispanic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10,707 | 100 | 0 | 0 | 1,525 | 9 |
| Other/unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10,469 | 13 | 1,908 | 11 |
| HIV acquisition risk groupa | ||||||||||||||
| MSM | 4,569 | 100 | 17,433 | 100 | 0 | 0 | 0 | 0 | 3,897 | 36 | 25,383 | 31 | 0 | 0 |
| Heterosexual contact | 0 | 0 | 0 | 0 | 3,586 | 54 | 1,534 | 53 | 1,961 | 18 | 9,856 | 12 | 0 | 0 |
| IDU | 0 | 0 | 0 | 0 | 1,067 | 16 | 827 | 29 | 1,525 | 14 | 16,273 | 20 | 17,798 | 100 |
| Other/unknown | 0 | 0 | 0 | 0 | 2,043 | 31 | 519 | 18 | 3,324 | 31 | 30,070 | 37 | 0 | 0 |
| CD4 cell count, cells/mm3c | ||||||||||||||
| 2004–2007 | ||||||||||||||
| Total | 2,440 | 10,315 | 4,149 | 1,916 | 6,334 | 50,705 | 12,944 | |||||||
| <200 | 779 | 32 | 2,014 | 20 | 1,150 | 28 | 439 | 23 | 1,586 | 25 | 12,952 | 26 | 3,820 | 30 |
| 200–349 | 563 | 23 | 2,501 | 24 | 1,037 | 25 | 447 | 23 | 1,463 | 23 | 12,264 | 24 | 3,211 | 25 |
| 350–500 | 438 | 18 | 2,103 | 20 | 752 | 18 | 378 | 20 | 1,071 | 17 | 9,615 | 19 | 2,260 | 17 |
| ≥500 | 591 | 24 | 3,348 | 32 | 1,083 | 26 | 597 | 31 | 1,513 | 24 | 13,832 | 27 | 2,932 | 23 |
| Missing | 69 | 3 | 349 | 3 | 127 | 3 | 55 | 3 | 701 | 11 | 2,042 | 4 | 721 | 6 |
| 2008–2011 | ||||||||||||||
| Total | 1,071 | 3,819 | 1,593 | 577 | 2,442 | 18,078 | 3,202 | |||||||
| <200 | 289 | 27 | 688 | 18 | 444 | 28 | 130 | 23 | 598 | 24 | 4,580 | 25 | 927 | 29 |
| 200–349 | 259 | 24 | 962 | 25 | 419 | 26 | 142 | 25 | 590 | 24 | 4,737 | 26 | 915 | 29 |
| 350–500 | 254 | 24 | 906 | 24 | 314 | 20 | 121 | 21 | 520 | 21 | 3,919 | 22 | 635 | 20 |
| ≥500 | 252 | 24 | 1,168 | 31 | 374 | 23 | 173 | 30 | 556 | 23 | 4,339 | 24 | 617 | 19 |
| Missing | 17 | 2 | 95 | 2 | 42 | 3 | 11 | 2 | 178 | 7 | 503 | 3 | 108 | 3 |
| 2012–2015 | ||||||||||||||
| Total | 1,058 | 3,299 | 954 | 387 | 1,931 | 12,799 | 1,652 | |||||||
| <200 | 174 | 16 | 408 | 12 | 167 | 18 | 80 | 21 | 284 | 15 | 2,252 | 18 | 309 | 19 |
| 200–349 | 164 | 16 | 495 | 15 | 185 | 19 | 67 | 17 | 311 | 16 | 2,176 | 17 | 329 | 20 |
| 350–500 | 244 | 23 | 631 | 19 | 200 | 21 | 63 | 16 | 385 | 20 | 2,547 | 20 | 327 | 20 |
| ≥500 | 445 | 42 | 1,664 | 50 | 384 | 40 | 168 | 43 | 769 | 40 | 5,411 | 42 | 614 | 37 |
| Missing | 31 | 3 | 101 | 3 | 18 | 2 | 9 | 2 | 182 | 9 | 413 | 3 | 73 | 4 |
| Clinical AIDS diagnosisd | 1,087 | 24 | 3,873 | 22 | 1,721 | 26 | 704 | 24 | 2,308 | 22 | 15,798 | 19 | 3,617 | 20 |
| Deaths while under follow-up | 390 | 9 | 1,267 | 7 | 772 | 12 | 331 | 11 | 1,148 | 11 | 12,201 | 15 | 4,672 | 26 |
| Loss to follow-upe | 1,173 | 26 | 4,669 | 27 | 1,305 | 19 | 630 | 22 | 2,717 | 25 | 13,493 | 17 | 1,877 | 11 |
| Years of follow-upf | 4.3 (2.2, 7.9) | 5.0 (2.5, 9.3) | 5.4 (2.7, 9.8) | 5.3 (2.6, 10.0) | 5.0 (2.5, 9.6) | 5.5 (2.7, 10.1) | 6.3 (2.9, 11.0) | |||||||
Abbreviations: AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency syndrome; IDU, injection drug use; MSM, men who have sex with men.
a Year of birth, sex, race, and HIV acquisition risk group were measured at entry into the North American AIDS Cohort Collaboration on Research and Design.
b Race was defined as black or white regardless Hispanic ethnicity.
c CD4 count was measured as close to study entry as possible, within the window from before to 3 months after entry.
d History of clinical AIDS diagnoses was defined by International Classification of Diseases, Ninth Revision, codes for AIDS-defining opportunistic infections and cancers.
e Loss to follow-up was defined as a gap of >2 years without a CD4 or HIV RNA measurement.
f Values are expressed as median (interquartile range).
The observed mortality rates, the LISSO model, and the expected mortality rates were estimated using SAS (SAS Institute, Inc., Cary, North Carolina). The single-decrement, abridged period life table approach to life expectancy was estimated using Microsoft Excel (Microsoft Corp., Redmond, Washington).
RESULTS
Among 132,664 participants in 20 cohorts with cause-of-death data available, 92,289 were prescribed ART and under observation in our study, of whom 13,349 (14%) died; median follow-up was 5.4 (interquartile range, 2.6–10.0) years. Most participants were male (86%), 42% were white, 35% were black, 12% were Hispanic, 34% were aged ≥50 years, and 19% had IDU for their HIV acquisition risk. Although 20% of adults had an AIDS-defining illness, 29% of adults had a CD4 count ≥500 cells/mm3 at study entry. CD4 count at study entry increased most dramatically from 2008–2011 to 2012–2015 in all key populations and comparison groups, which is reflective of the 2012 “treat all” guidelines change that recommended all receive prompt treatment, regardless of CD4 count. Characteristics stratified by key population and comparison group can be found in Table 1.
Overall mortality rates decreased over time in all key populations and comparison groups. Correspondingly, observed life expectancy increased from 2004–2007 to 2012–2015 in both the key populations and comparison groups (Figure 2, Web Table 1). Observed life expectancy among white MSM in 2012–2015 (60.3 years at age 20) exceeded life expectancy of white men in the general US population (57.1 years at age 20 in 2015); however, observed life expectancy estimates for other key populations and comparison groups of PLWH were less than life expectancy in the general US population (8).
Figure 2.
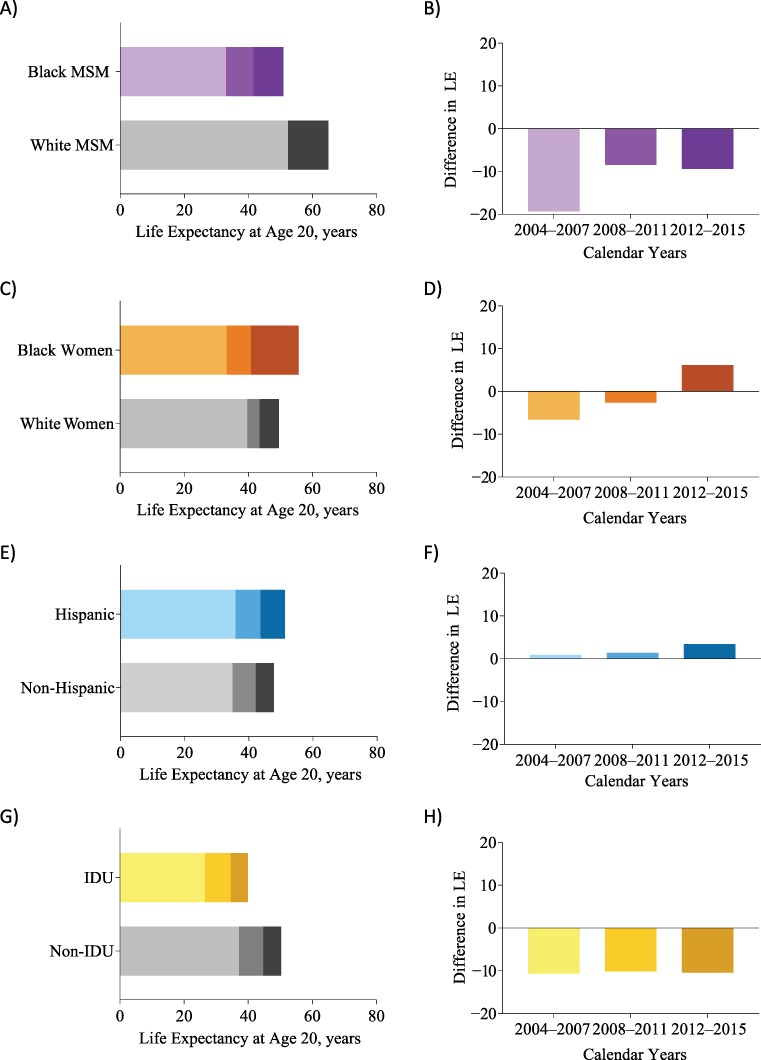
Observed increases in life expectancy (LE) at age 20 years among people with human immunodeficiency virus (left column, panels A, C, E, and G), and corresponding changes in disparities in key populations and their comparison groups (right column, panels B, D, F, and H) in the North American AIDS Cohort Collaboration on Research and Design, United States and Canada, 2004–2015. IDU, injection drug use; MSM, men who have sex with men.
Disparities in observed life expectancy at age 20 narrowed for black versus white MSM from 2004–2007 (33.0 vs. 52.3 years, respectively) to 2012–2015 (50.9 vs. 60.3 years, respectively), and for IDU versus non-IDU history (26.5 in 2004–2007 and 39.9 in 2012–2015 vs. 37.1 in 2004–2007 and 50.3 in 2012–15, respectively, Figure 2, Web Table 1). Reflective of the general population, Hispanic adults with HIV had greater observed life expectancy than non-Hispanic adults with HIV, and the disparity grew (for 2004–2007, 35.9 vs. 34.9 years; for 2012–2015, 51.2 vs. 47.8 years, respectively) (28). Among black versus white women, observed life expectancy was higher among white women in 2004–2007 (33.1 vs. 39.7 years) and 2008–2011 (40.8 vs. 43.4 years), but black women had longer observed life expectancy in 2012–2015 (55.7 vs. 49.5 years).
The proportion of alcohol- and drug-related deaths was higher in all comparison groups than in key populations, except for those with IDU versus non-IDU risk; only being a white woman in 2008–2011 and 2012–2015 and having a history of IDU in 2012–2015 were associated with >20% drug- and alcohol-related deaths (Figure 3). Although the proportion of drug- and alcohol-related deaths increased among white women over time, the proportion of white women who had IDU risk was constant over time (52% in 2004–2007, 53% in 2008–2011, and 54% in 2012–2015).
Figure 3.
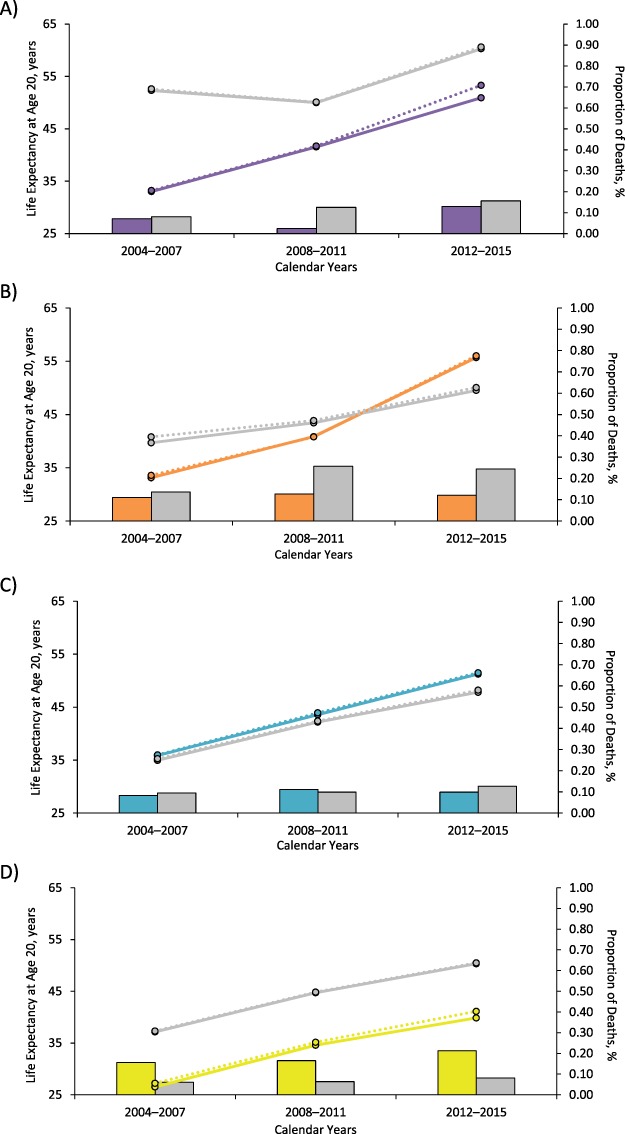
Observed (solid lines) life expectancy from the North American AIDS Cohort Collaboration on Research and Design (United States and Canada, 2004–2015) and expected (dashed lines) life expectancy from the Lives Saved Simulation model, after a 20% reduction in drug- and alcohol-related deaths, as well as the proportion of drug- and alcohol-related deaths (bars) among key populations and their comparison groups, including black (purple) compared with white (gray) men who have sex with men (A), black (orange) compared with white (gray) women (B), Hispanic (teal) compared with non-Hispanic (gray) adults (C), and people who have a history of injection drug use (yellow) compared with those who do not (gray) (D). Expected life expectancy after a 20% reduction in drug- and alcohol-related deaths was estimated as the median estimate from 100 simulations using the Lives Saved Simulation model.
After applying the LISSO model to reducing the number drug- and alcohol-related deaths by 20%, increases in life expectancy of ≥1 year were seen among black MSM (in 2012–2015), white women (in 2004–2007), and those with a history of IDU (in 2012–2015, Table 2). The observed and expected life-expectancy estimates were higher in the black (vs. white) women and Hispanic (vs. non-Hispanic) adults in 2012–2015. Although reducing the number of drug- and alcohol-related deaths by 20% had differential impact on the expected life expectancy among key populations and comparison groups, the percentage change in the life-expectancy disparity showed a narrowing for black (vs. white) MSM and those with a history of IDU (vs. non-IDU) in 2012–2015.
Table 2.
Changes in Life-Expectancy Disparities Comparing Observed Life Expectancy Estimates With Expected Life Expectancy After a 20% Reduction in Drug- and Alcohol-Related Deaths Among Key Populations of People With Human Immunodeficiency Virus and Comparison Groups, North American AIDS Cohort Collaboration on Research and Design, United States and Canada, 2004–2015
| Key Population | Years | Observed LE | Expected LE After a 20% Reduction in Drug- and Alcohol-Related Deaths | % Change in LE Disparity a | Expected LE Increase After Reducing Drug- and Alcohol-Related Deaths by 20% b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Key Population | Comparison | LE Disparity c | Key Population | Comparison | LE Disparity c | Key Population | Comparison | |||
| Black (vs. white) MSM | 2004–2007 | 33.0 | 52.3 | −19.3 | 33.2 | 52.6 | −19.4 | 0% | 0.2 | 0.3 |
| 2008–2011 | 41.5 | 50.0 | −8.4 | 41.7 | 50.1 | −8.4 | −1% | 0.2 | 0.1 | |
| 2012–2015 | 50.9 | 60.3 | −9.4 | 53.3 | 60.6 | −7.3 | −22% | 2.4 | 0.3 | |
| Black (vs. white) women | 2004–2007 | 33.1 | 39.7 | −6.6 | 33.6 | 40.8 | −7.2 | 10% | 0.4 | 1.1 |
| 2008–2011 | 40.8 | 43.4 | −2.6 | 40.8 | 43.9 | −3.1 | 19% | −0.1 | 0.4 | |
| 2012–2015 | 55.7 | 49.5 | 6.2 | 56.0 | 50.0 | 6.0 | −3% | 0.3 | 0.5 | |
| Hispanic (vs. non-Hispanic) | 2004–2007 | 35.9 | 34.9 | 0.9 | 36.0 | 35.2 | 0.8 | −19% | 0.1 | 0.3 |
| 2008–2011 | 43.6 | 42.2 | 1.4 | 43.9 | 42.4 | 1.6 | 11% | 0.4 | 0.2 | |
| 2012–2015 | 51.2 | 47.8 | 3.4 | 51.5 | 48.2 | 3.3 | −4% | 0.2 | 0.4 | |
| IDU (vs. non-IDU) | 2004–2007 | 26.5 | 37.1 | −10.6 | 27.2 | 37.3 | −10.1 | −5% | 0.7 | 0.2 |
| 2008–2011 | 34.6 | 44.7 | −10.1 | 35.2 | 44.8 | −9.7 | −5% | 0.6 | 0.1 | |
| 2012–2015 | 39.9 | 50.3 | −10.4 | 41.1 | 50.5 | −9.4 | −10% | 1.3 | 0.2 | |
Abbreviations: IDU, injection drug use; LE, life expectancy; MSM, men who have sex with men.
a % change in LE disparity = (expected LE disparity after a 20% reduction in drug- and alcohol-related deaths − observed LE disparity)/observed LE disparity. A negative value indicates a narrowing of the disparity with a 20% reduction in drug- and alcohol-related deaths.
b Expected LE increase after reducing drug- and alcohol-related deaths by 20% = (expected LE after a 20% reduction in drug- and alcohol-related deaths − observed LE) for the key population or comparison groups.
c LE disparity = LE in the key population − LE in the comparison group. A negative value indicates a lower life expectancy in the key population.
DISCUSSION
There was an increase in observed life expectancy in all key populations and comparison groups using ART from 2004–2007 to 2012–2015; however, disparities persisted in both observed life expectancies and expected life expectancies after simulating a 20% reduction in drug- and alcohol-related deaths. Disparities in observed life expectancy narrowed over time for black (vs. white MSM) and those with a history of IDU (vs. non-IDU). Observed life expectancy was lower among black (vs. white) women in 2004–2007 and 2008–2011, but it was greater among black versus white women in 2012–2015. After simulating a 20% reduction in drug- and alcohol-related deaths, expected life expectancy increased differentially in key populations and over time, with black MSM (in 2012–2015), white women (in 2004–2007), and those with IDU history (in 2012–2015) having the greatest increase in expected life expectancy. The observed and expected life-expectancy disparities and the percent change in life-expectancy disparity narrowed in all key populations (vs. comparison groups) in the most recent period (2012–2015), except for black (vs. white) women. Nevertheless, our results suggest that observed disparities in life expectancy among key populations persist, and that the specified 20% reduction in drug- and alcohol-related deaths among adults with HIV might slightly narrow disparities in some of the key populations of PLWH.
Our findings update observed life-expectancy estimates among PLWH and add nuance by demonstrating persistent disparities in observed life expectancy in key populations of PLWH. Although prior studies have reported trends in observed life expectancy among PLWH in the United States and Canada according to sex, race, ethnicity, HIV acquisition risk, and CD4 count (4, 15–18), our study examines disparities in observed life expectancy in key populations versus their comparison groups among PLWH. Compared with sex- and race-stratified observed life-expectancy estimates in similar years in the general population, our observed life expectancy at age 20 years among PLWH was lower in all key populations and comparison groups, with the exception of white MSM from 2012–2015, who had an observed life expectancy 3.2 years greater than white men at age 20 in the general population in 2015 (8). This difference could be due to an increased frequency of health-care encounters among white MSM with HIV and on treatment compared with white men in the general population. It is notable that the observed life-expectancy disparity in black versus white MSM with HIV narrowed but persisted, with the greatest decrease in disparity from 2004–2007 to 2008–2011, followed by a slight widening of the disparity from 2008–2011 to 2012–2015, resulting in an observed life expectancy gap of 9.4 years in black versus white MSM. This pattern was also present for IDU versus non-IDU history with HIV, resulting in an observed gap of 10.4 years in the most recent time period. In the context of HIV, where guidelines changed in 2012 to “treat everyone,” persistent racial disparities among MSM and disparities among IDU histories suggest the potential roles of structural racism and stigma, which can reduce access to and retention in health care and result in disparities in observed life expectancy (29–31).
Our finding of a greater observed life expectancy in black (vs. white) women in 2012–2015 was unexpected; this was a reversal of the difference in observed life expectancy prior to the most recent time period. Black women had the greatest increase in observed life expectancy in any group from one period to the next (14.9 years) from 2008–2011 to 2012–2015, as well as an 18-percentage-point increase in the proportion with a CD4 count ≥500 cells/mm3 at study entry. These increases might be attributed in part to timelier HIV testing, linkage to care, and treatment initiation efforts focused on women of color. Conversely, observed life expectancy among white women increased modestly, by 6.1 years, to 49.5 years at age 20 from 2008–2011 to 2012–2015. White women had nearly double the proportion of drug- and alcohol-related deaths as black women in 2008–2011 and 2012–2015, suggesting differential influence of the opioid epidemic on white and black women with HIV.
The application of the LISSO model is intended for public health planning; it estimates the corresponding increase in life expectancy from a specified decrease in drug- and alcohol-related mortality. Approximately 63% of 52,404 overdose deaths were attributed to opioids in 2015 in the United States (12). The number of overdose deaths continued to increase beyond the end of our study period (2015) until a plateau in 2018; thus, the impact of a 20% reduction in drug- and alcohol-deaths might result in greater increases in life expectancy after 2015 (32). There are numerous evidence-informed strategies to reduce opioid deaths, including broadening naloxone availability, expanding methadone-assisted treatment, monitoring opioid prescription information, and intensifying the punishments for the illicit supplying of opioids (33–36). However, preventing nonopioid drug- and alcohol-related deaths as well as other major causes of death (particularly the causes of death among younger adults, because those deaths are more influential on the life-expectancy estimate than deaths at older ages) for key PLWH populations must not be overlooked in order to further reduce disparities. The effectiveness of interventions to reduce drug- and alcohol-related deaths (ideally estimated from study populations of the key populations and comparison groups) must be incorporated by program decision-makers.
Our agent-based simulation model is a novel adaptation of the Case and Deaton “lives saved” approach—it allows application of the approach to individuals by randomly saving 20% of those who died from drug- and alcohol-related causes and then applying subsequent mortality risk of their peers and the opportunity to age into older age groups to those saved. Creating new conditional probabilities of dying in each age interval after being “saved” employs the same assumptions as hazard independence (independence from competing causes of death) and force of mortality (uniform distribution of deaths across an age interval) as a cause-deleted life table, which is a more traditional approach.
Limitations include cause-specific mortality as determined by death certificate information and matching to the National Death Index Plus, which has unavoidable misclassification: There might be an underascertainment of drug- and alcohol-related deaths. Second, there are sequelae to long-term moderate to severe use of drugs and alcohol (37, 38). Preventing mortality from these causes is not a solution in and of itself; addressing significant health consequences related to substance use, such as liver disease, will be important. Third, we did not account for hepatitis C virus infection and its newer treatments (the first of which was approved in October of 2014), which could affect life expectancy, particularly among those with a history of IDU. Fourth, we were unable to account for socioeconomic-status differences between our key populations and their comparison groups; certainly, these differences affect life expectancy, but they probably do not wholly account for the racial and HIV acquisition risk–based disparities in our key populations and comparison groups. Fifth, the interpretation of life expectancy relies upon constant age-specific mortality trends across the life span. By restricting to the calendar period of January 2004 to December 2015, we eliminated the impact of the dramatically declining mortality rates that immediately followed the advent of effective HIV treatment and diminishing thresholds to initiate treatment that occurred prior to 2004. Sixth, our findings are most applicable to the target population of PLWH in the United States who have successfully linked into care and initiated ART (target population); the NA-ACCORD is the largest (albeit nonrandom) sample of this population with demographic characteristics similar to that of the Medical Monitoring Project (which was sampled to be representative of persons in HIV care in the United States; details of this comparison available at http://www.naaccord.org/). Finally, as life expectancy among PLWH continues to increase (even in the setting of persistent disparities), efforts must focus on improving not only the duration but also the quality of life among PLWH. Interventions to improve the quality of life among PLWH through prevention, screening, and treatment of non-HIV related comorbidities are essential to successful aging with HIV (39).
Life expectancy among PLWH continues to increase, with the good news of narrowing disparities in black and white MSM, and substantial increase in life expectancy among black women. However, life-expectancy disparities persist among black versus white MSM and persons with a history of IDU (vs. no IDU history), and gains in life expectancy over time among white women (for whom 20% of deaths were drug- and alcohol-related) did not keep pace with the other groups. The increases in expected life expectancy with a simulated 20% decrease in drug- and alcohol-related deaths narrowed life-expectancy disparities for most groups, however other causes of death must be addressed to further narrow life-expectancy disparities.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R01AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214, and Z01CP010176), the Centers for Disease Control and Prevention (contracts CDC-200-2006-18797 and CDC-200-2015-63931), the Agency for Healthcare Research and Quality (contract 90047713), the Health Resources and Services Administration (contract 90051652), the Canadian Institutes of Health Research (grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118), the Ontario Ministry of Health and Long Term Care, and the Government of Alberta (Canada). Additional support was provided by the National Cancer Institute, National Institute of Mental Health, and National Institute on Drug Abuse, as well as the Johns Hopkins Center for AIDS Research (grant P30AI094189).
We thank Dr. Stéphane Helleringer, Associate Professor, Johns Hopkins Bloomberg School of Public Health, for his insights into the application of the observed and simulated mortality rates into the life tables approach for calculating life expectancy.
NA-ACCORD Collaborating Cohorts and Representatives: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch; AIDS Link to the IntraVenous Experience: Gregory D. Kirk; Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso; Highly Active Antiretroviral Therapy (HAART) Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio SG Montaner, Benita Yip, Julia Zhu, Kate Salters, and Karyn Gabler; HIV Outpatient Study: Kate Buchacz and Jun Li; HIV Research Network: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States: Michael A. Horberg; Kaiser Permanente Northern California: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; Multicenter Hemophilia Cohort Study II: Charles Rabkin; Multicenter AIDS Cohort Study: Joseph B. Margolick, Lisa P. Jacobson, and Gypsyamber D’Souza; Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein; Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay; Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort: M. John Gill; Study of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin; Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig; University of California at San Diego: William C. Mathews; University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata, Heidi M. Crane, and Daniel R. Drozd; Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner; Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin; and Women’s Interagency HIV Study: Stephen J. Gange and Kathryn Anastos. NA-ACCORD Study Administration: Executive Committee: Richard D. Moore, Keri N. Althoff, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman; Administrative Core: Richard D. Moore, Keri N Althoff, and Aimee M. Freeman; Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Daniel R. Drozd, Liz Morton, Justin McReynolds, and William B. Lober; and Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Bin You, Brenna Hogan, Jinbing Zhang, Jerry Jing, Elizabeth Humes, Lucas Gerace, and Sally Coburn.
Preliminary findings were reported at the Conference on Retroviruses and Opportunistic Infections, March 4–7, 2018, Boston, Massachusetts; and the 23rd International Workshop on HIV and Hepatitis Observational Databases, March 28–30, 2019, Athens, Greece.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
K.N.A. serves on the Scientific Advisory Board for TrioHealth (not directly related to this study). M.J.S. reports research grants to his institution from Merck and Gilead (not directly related to this study). J.E.T. is a consultant for Gilead (not directly related to this study).
Conflict of interest: none declared.
Abbreviations
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- IDU
injection drug use
- LISSO
Lives Saved Simulation
- MSM
men who have sex with men
- NA-ACCORD
North American AIDS Cohort Collaboration on Research and Design
- PLWH
people living with human immunodeficiency virus
References
- 1. Anderegg N, Johnson LF, Zaniewski E, et al. All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS. 2017;31(suppl 1):S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teeraananchai S, Kerr SJ, Amin J, et al. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–266. [DOI] [PubMed] [Google Scholar]
- 7. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2016: NCHS Data Brief, No. 293. Hyattsville, MD: National Center for Health Statistics; 2017. https://www.cdc.gov/nchs/data/databriefs/db293.pdf. Accessed September 24, 2019. [Google Scholar]
- 8. Murphy SL, Xu J, Kochanek KD, et al. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 9. National Center for Health Statistics Health, United States, 2017: With a special feature on mortality. Hyattsville, MD: 2018. https://www.cdc.gov/nchs/data/hus/hus17.pdf. Accessed September 24, 2019. [PubMed] [Google Scholar]
- 10. Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder—and its treatment. JAMA. 2014;311(14):1393–1394. [DOI] [PubMed] [Google Scholar]
- 11. Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112(49):15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016: NCHS data brief, No. 294 . Hyattsville, MD: National Center for Health Statistics; 2017. https://www.cdc.gov/nchs/data/databriefs/db294.pdf. Accessed September 24, 2019. [Google Scholar]
- 13. Cunningham CO. Opioids and HIV infection: from pain management to addiction treatment. Top Antivir Med. 2018;25(4):143–146. [PMC free article] [PubMed] [Google Scholar]
- 14. Tsao JC, Plankey MW, Young MA. Pain, psychological symptoms and prescription drug misuse in HIV: a literature review. J Pain Manag. 2012;5(2):111–118. [PMC free article] [PubMed] [Google Scholar]
- 15. Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr. 2016;73(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53(1):124–130. [DOI] [PubMed] [Google Scholar]
- 17. Siddiqi AE, Hall HI, Hu X, et al. Population-based estimates of life expectancy after HIV diagnosis: United States 2008–2011. J Acquir Immune Defic Syndr. 2016;72(2):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49(10):1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lloyd-Smith E, Brodkin E, Wood E, et al. Impact of HAART and injection drug use on life expectancy of two HIV-positive cohorts in British Columbia. AIDS. 2006;20(3):445–450. [DOI] [PubMed] [Google Scholar]
- 20. Leszczyszyn-Pynka M, Ciejak P, Maciejewska K, et al. Hepatitis C coinfection adversely affects the life expectancy of people living with HIV in northwestern Poland. Arch Med Sci. 2018;14(3):554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters L, Mocroft A, Lundgren J, et al. HIV and hepatitis C co-infection in Europe, Israel and Argentina: a EuroSIDA perspective. BMC Infect Dis. 2014;14(suppl 6):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute on Drug Abuse HEALing Communities Study: developing and testing an integrated approach to address the opioid crisis (research sites). Rockville, MD: National Institutes of Health, 2018. https://grants.nih.gov/grants/guide/rfa-files/rfa-da-19-016.html. Accessed September 24, 2019. [Google Scholar]
- 23. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research And Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland: World Health Organization, 2004. https://apps.who.int/iris/bitstream/handle/10665/42980/9241546530_eng.pdf. Accessed September 24, 2019. [Google Scholar]
- 25. Klein RJ, Schoenborn CA Age adjustment using the 2000 projected U.S. population. Healthy People Statistical Notes, no. 20. National Center for Health Statistics; 2001. https://www.cdc.gov/nchs/data/statnt/statnt20.pdf. Accessed September 24, 2019. [PubMed] [Google Scholar]
- 26. Chiang CL. The Life Table and Its Applications. Malabar, FL: Robert E. Krieger Publishing Company; 1984. [Google Scholar]
- 27. Case A, Deaton A. Supporting information: Case and Deaton 10.1073/pnas.1518393112. Proc Natl Acad Sci U S A. 2015. https://www.pnas.org/content/pnas/suppl/2015/10/29/1518393112.DCSupplemental/pnas.201518393SI.pdf. Accessed September 24, 2019. [Google Scholar]
- 28. Smith DP, Bradshaw BS. Rethinking the Hispanic paradox: death rates and life expectancy for US non-Hispanic white and Hispanic populations. Am J Public Health. 2006;96(9):1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eaton LA, Driffin DD, Kegler C, et al. The role of stigma and medical mistrust in the routine health care engagement of black men who have sex with men. Am J Public Health. 2015;105(2):e75–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis. 2014;58(8):1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rebeiro PF, Abraham AG, Horberg MA, et al. Sex, race, and HIV risk disparities in discontinuity of HIV care after antiretroviral therapy initiation in the United States and Canada. AIDS Patient Care STDS. 2017;31(3):129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmad FB, Escobedo LA, Rossen LM, et al. Provisional drug overdose death counts. National Center for Health Statistics. 2019. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed September 24, 2019.
- 33. Hall WD, Farrell M. Reducing the opioid overdose death toll in North America. PLoS Med. 2018;15(7):e1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulozzi LJ, Weisler RH, Patkar AA. A national epidemic of unintentional prescription opioid overdose deaths: how physicians can help control it. J Clin Psychiatry. 2011;72(5):589–592. [DOI] [PubMed] [Google Scholar]
- 35. Russolillo A, Moniruzzaman A, Somers JM. Methadone maintenance treatment and mortality in people with criminal convictions: a population-based retrospective cohort study from Canada. PLoS Med. 2018;15(7):e1002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Center on Addiction Ending the Opioid Crisis: a Practical Guide for State Policymakers. New York, NY: National Center on Addiction and Substance Abuse, 2017. https://www.centeronaddiction.org/addiction-research/reports/ending-opioid-crisis-practical-guide-state-policymakers. Accessed September 24, 2019. [Google Scholar]
- 37. Macleod J, Oakes R, Copello A, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363(9421):1579–1588. [DOI] [PubMed] [Google Scholar]
- 38. Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. [DOI] [PubMed] [Google Scholar]
- 39. Althoff KN, Smit M, Reiss P, et al. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS. 2016;11(5):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]


