Abstract
Four right-handed patients who presented with an isolated impairment of speech or language had transactive response DNA-binding protein of 43 kDa (TDP-43) type B pathology. Comportment and pyramidal motor function were preserved at presentation. Three of the cases developed axial rigidity and oculomotor findings late in their course with no additional pyramidal or lower motor neuron impairments. However, in all 4 cases, postmortem examination disclosed some degree of upper and lower motor neuron disease (MND) pathology in motor cortex, brainstem, and spinal cord. Although TDP-43 type B pathology is commonly associated with MND and behavioral variant frontotemporal dementia, it is less recognized as a pathologic correlate of primary progressive aphasia and/or apraxia of speech as the presenting syndrome. These cases, taken together, contribute to the growing heterogeneity in clinical presentations associated with TDP pathology. Additionally, 2 cases demonstrated left anterior temporal lobe atrophy but without word comprehension impairments, shedding light on the relevance of the left temporal tip for single-word comprehension.
Keywords: Frontotemporal lobar degeneration, Motor neuron disease, Primary progressive aphasia, Speech apraxia, TAR DNA-binding protein
INTRODUCTION
Dementias can present in different forms and can be classified into amnestic, behavioral, aphasic and visuospatial types. The most common underlying neuropathologic entities include Alzheimer’s disease (AD), frontotemporal lobar degeneration (FTLD)-tau (corticobasal degeneration, progressive supranuclear palsy, Pick), and FTLD-transactive response DNA-binding protein (TDP) (types A–D). The relationships between clinical profile and underlying disease are probabilistic rather than deterministic such that each neuropathologic entity is associated with typical and atypical manifestations. This was most convincingly demonstrated for AD, which is most commonly associated with an amnestic dementia but which can also have primary aphasic or behavioral presentations (1, 2). The current report was occasioned by 4 patients who presented with progressive impairments in speech and language (primary progressive aphasia [PPA] and apraxia of speech [AOS]) and who were found at postmortem to have the pathology of FTLD-TDP, type B, a neuropathologic entity that has been associated with behavioral variant frontotemporal dementia (bvFTD) and motor neuron disease (MND) but not PPA or AOS.
According to harmonized pathological criteria, TDP 43 proteinopathies are subtyped on the basis of TDP inclusion morphology and localization: Types A to D (3). These TDP subtypes are also associated with certain mutations, patterns of cerebral atrophy, and clinical phenotypes on a probabilistic, not deterministic, basis.
FTLD-TDP type A is characterized by frequent phospho-TDP-positive cytoplasmic inclusions (NCI), frequent short dystrophic neurites (DNs), and few intranuclear inclusions (NIIs) concentrated in the superficial cortical laminae. The most common phenotypic manifestations are bvFTD and PPA of the agrammatic and logopenic types (1, 3). A series of patients with TDP type A and PPA with uniquely selective impairments in the auditory and oral modalities potentially adds to the heterogeneity associated with type A pathology (4). Genetic mutations that predict type A include those that encode progranulin (GRN) and, to a lesser extent, TANK-binding kinase 1 (TBK1) and C9orf72 (5–7).
Type B histopathology is marked by frequent NCIs, few DNs, and the absence of NIIs in all cortical layers. Corresponding clinical syndromes include bvFTD, MND, and bvFTD-MND (3). Carriers of C9orf72 hexanucleotide repeat expansions typically demonstrate pathology consistent with type B. Uncommon mutations in genes encoding T-cell restricted antigen-1 and TBK1 have also been described in association with type B neocortical change (8, 9).
The TDP immunohistochemical pattern of type C is one of numerous long DNs, few NCIs, and no NIIs. Patients with the semantic variant of PPA or semantic dementia, depending on the lateralization of temporal lobe atrophy, demonstrate relatively tight clinicopathologic associations with type C neuropathology (1). With exceedingly rare exception, type C appears to be sporadic.
A rare fourth subtype, type D, is associated with mutations in the gene encoding valosin-containing protein causing familial inclusion body myositis with early onset Paget’s disease of bone and FTD (10, 11). A proposed fifth subtype, Type E, is marked by p62-negative, ubiquitin-negative, phospho-TDP-positive aggregates. These aggregates take the form of granulofilamentous neuronal inclusions, grains, and oligodendroglial coiled bodies. Type E appears to be associated with a rapidly progressive bvFTD syndrome (12).
The present harmonized scheme of TDP subtypes is regarded for the relative strength of association between phenotype and histopathology. Type B, commonly associated with MND and behavioral phenotypes, is infrequently affiliated with speech and language presentations. The following patients presented with isolated speech and language syndromes and unexpectedly demonstrated type B neuropathology at postmortem examination.
MATERIALS AND METHODS
Cases
All 4 patients described here presented for clinical evaluation at the Northwestern University Neurobehavior and Memory Clinic. Clinical scans were obtained by standard magnetic resonance imaging (MRI).
Pathologic Data
Brain autopsies were performed by the Neuropathology Core of the Northwestern University Alzheimer’s Disease Center (ADC) affiliated with the Mesulam Center for Cognitive Neurology and Alzheimer’s Disease. Autopsies were performed with written informed consent. Gross and microscopic evaluations were performed in a manner blinded to clinical and immunohistochemical diagnosis. Gross atrophy was assessed in frontal, temporal, and parietal lobes, motor cortex, and hippocampus. Microscopic neuronal loss and gliosis was semiquantitatively evaluated in frontal, temporal, and parietal cortex, hippocampus, subiculum, substantia nigra, and hypoglossal nucleus. White matter rarefaction of the corticospinal tracts was assessed.
Paraffin-embedded, 5-μm brain sections were immunohistochemically analyzed. The sections were incubated with the following primary antibodies: phosphorylated TDP of 43 kDa (TDP-43) (pS409/410-2, mouse monoclonal, 1:2500, CosmoBio, Tokyo, Japan), phosphorylated tau (AT8, S202/T205, mAb, 1:500, Thermo Fisher Scientific, Waltham, MA), alpha-synuclein (mAb, 1:40, Leica, Buffalo Grove, IL), and p62 (SQSTM1, mouse monoclonal, 1:750, Taipei, Taiwan).
Case Descriptions
Patient 1
Patient 1 first noticed word-finding problems at the age of 59. He identified difficulty retrieving nouns, such as household items and names of longtime colleagues. Reading, once his favored pastime, became progressively laborious. A chemistry professor, he found reading professional literature to be increasingly effortful and time-consuming. He withdrew from reading certain sections of the daily newspaper, namely the humor and editorial pages. Spelling mistakes became more frequent, and he became increasingly reliant on others to edit the content and organization of his written documents. These impairments led him to withdraw from work at the age of 61. His activities of daily living were otherwise preserved.
His initial examination, 3 years into his course, disclosed a focal impairment in language. Spontaneous speech was marked by frequent word-finding hesitations, circumlocutions, and phonemic paraphasias (convention/convocation and deck/dock). The Western Aphasia Battery (WAB) (13) disclosed mild impairment—an Aphasia Quotient of 90.4. According to the WAB and bedside testing, repetition and single-word comprehension were preserved, and there was no evidence of semantic loss. Confrontation naming disclosed a prominent anomia. His score on the Boston Naming Test (14) was 24/60. Comportment, autobiographical memory, visuospatial skills, and face recognition were preserved. His examination was consistent with an anomic PPA.
Reexamination 1 year later (4 years following symptom onset) detected additional impairments of grammar in speech. Comprehension of single words and syntactically complex sentences remained unimpaired; however, there was no evidence of speech apraxia or dysarthria. Autobiographical memory and comportment remained spared. The basic neurologic examination did not detect pyramidal signs or fasciculations. An MRI in the fourth year of his course disclosed marked left anterior temporal atrophy (Fig. 1A).
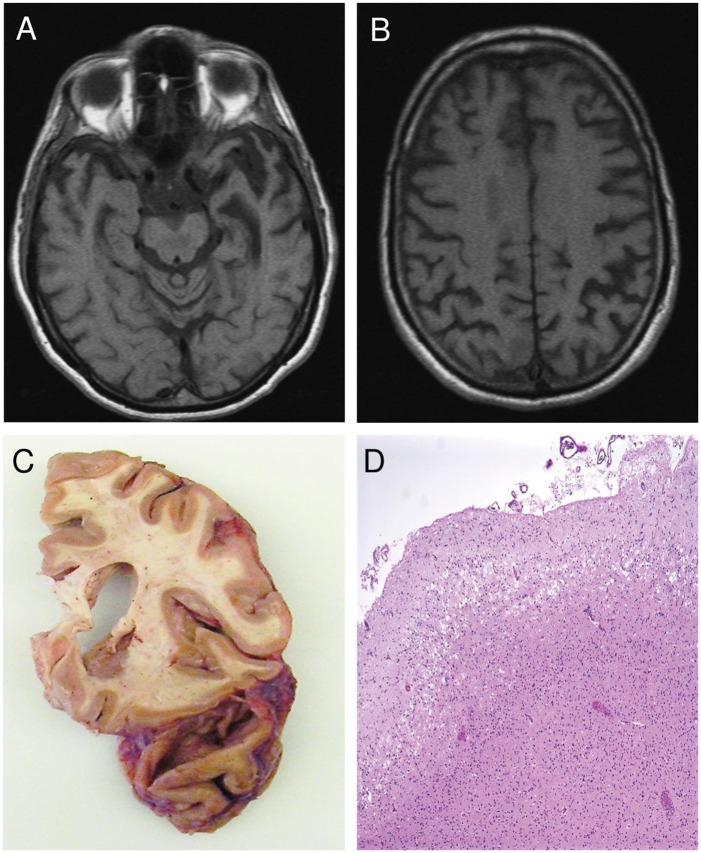
FIGURE 1.
Patient 1 imaging and pathological results. T1-weighted magnetic resonance imaging of Patient 1 shows marked left anterior temporal lobe (ATL) atrophy (A) and left-sided dorsal parietal atrophy (B). Autopsy of Patient 1 shows severe left temporopolar atrophy (C). Hematoxylin and eosin stain of left ATL reveals severe microvacuolation but no infarct (D).
The autopsy showed prominent left temporopolar atrophy (Fig. 1C). Histologic examination of the left anterior temporal lesion revealed severe superficial microvacuolation but no infarct (Fig. 1D). Neuronophagia was mild in the motor cortex. There was mild loss of Betz cells and gliosis in motor cortex. Neuronal loss and gliosis in the anterior horns was moderate at all 3 levels. Neuronophagia was mild in the cervical and thoracic anterior horns and moderate in the lumbosacral anterior horns. There was patchy and mild rarefaction of pontine corticospinal tracts as well as in ventral and lateral corticospinal tracts throughout the spinal cord. The cerebral peduncles and medullary pyramids were relatively spared. Skein-like inclusions and Lewy-like bodies were rare in the hypoglossal nucleus and anterior horns. Sparse Bunina bodies were visualized in hypoglossal nucleus and anterior horn cells at all 3 levels. Phosphorylated TDP-positive cytoplasmic inclusions were more frequent in the left superior temporal gyrus (Fig. 2A), as compared with right. There were no phospho-TDP-positive NIIs. There were sparse neurofibrillary tangles in the entorhinal cortex, hippocampus, and locus coeruleus but there was otherwise no Alzheimer type or tau pathology by Gallyas stain or AT8 immunostain. No Lewy body pathology was visualized. In sum, the postmortem examination disclosed TDP type B and concomitant amyotrophic lateral sclerosis (ALS) pathology.
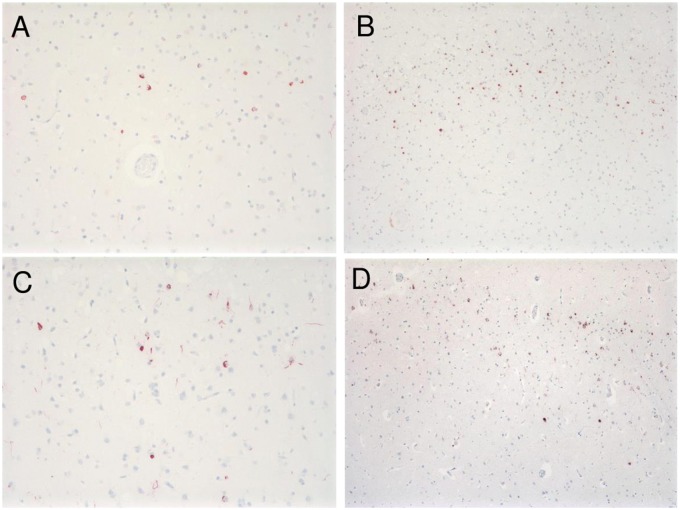
FIGURE 2.
TDP immunohistochemistry. TDP-positive granular cytoplasmic inclusions are seen in left superior temporal gyrus in Patient 1, 200× (A), in left temporal pole in Patient 2, 100× (B), in right superior temporal gyrus in Patient 3, 200× (C), and in left superior temporal gyrus in Patient 4, 100× (D). Intranuclear inclusions in all 4 patients were absent.
Patient 2
At the age of 64, a right-handed man noticed difficulty finding words. There was difficulty retrieving names of those well known to him. He increasingly resorted to circumlocutory speech to convey his thoughts. His ability to read became uncharacteristically slowed. Because of these communication difficulties he withdrew from his position as a realtor. At home, he continued to carry out his customary duties and remained an avid golfer. Neuropsychological evaluation 2 years after symptomatic onset disclosed selective impairments in confrontation naming and spelling. In specific, his education-adjusted performance on the Multilingual Aphasia Examination (MAE) (15) Visual Naming fell in the fifth percentile and, on the MAE Oral Spelling, he scored in the second percentile.
Examination 2 years after onset revealed dysarthria, particularly for lingual consonants. There were frequent word-finding pauses, simplifications, and anomia. Repetition and comprehension remained intact. Word comprehension was intact. The other cognitive domains, including insight, were preserved. The basic neurological examination demonstrated right-sided posturing with stressed gait but did not disclose evidence of MND. An MRI in the second year of his course showed left anterior temporal lobe (ATL) atrophy (Fig. 3A). The history and examination were suggestive of an anomic PPA and subsequent emergence of dysarthria.
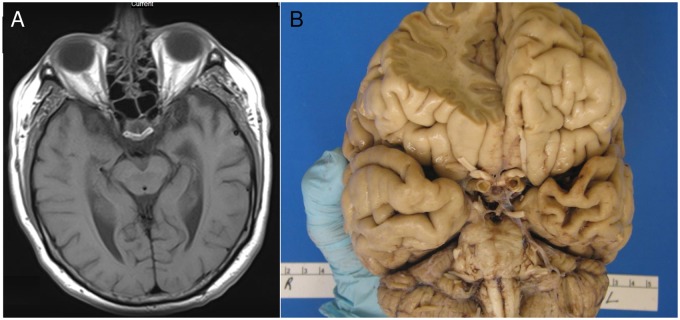
FIGURE 3.
Patient 2 imaging and pathological results. T1-weighted magnetic resonance imaging of Patient 2 shows left anterior temporal lobe (ATL) atrophy (A). Gross postmortem examination of Patient 2 redemonstrates severe left ATL atrophy (B).
On reexamination 4 years after onset he was anarthric, precluding assessment of grammar. Writing did not disclose clear agrammatism. Comprehension remained preserved for single words and for noncanonical syntactic structures. His basic neurological examination showed axial rigidity and new eye movement abnormalities in a supranuclear pattern. Palate and tongue weakness was noted.
Autopsy of Patient 2 (7 years following symptom onset) showed marked ATL atrophy on the left (Fig. 3B). Neuronal loss and gliosis were severe in the hypoglossal nucleus and moderate in the cervical spinal cord anterior horn neurons. Sparse Bunina bodies were observed in the hypoglossal nucleus and cervical anterior horns. Immunohistochemistry revealed frequent phosphorylated TDP-43-positive cytoplasmic inclusions in the bilateral inferior frontal gyri and bilateral temporal poles (Fig. 2B). Overall, TDP pathologic burden was more severe in the left hemisphere. There were no cortical NIIs. TDP pathology was also mild in the motor cortex, frequent in the striatum and hypoglossal nucleus, and sparse in the cervical anterior horns. AD neuropathologic change (ADNC) (16) was low: A1 + B1 + C0; AT8 immunostain reflected this minimal tangle pathology only. Alpha-synuclein immunostaining was negative for Lewy bodies. The postmortem findings were consistent with FTLD-TDP type B with ALS pathology.
Patient 3
Patient 3, a right-handed man, began to experience progressive speech and language changes at the age of 60. He remained fully employed and carried out his usual daily living activities. He reported a lifelong difficulty with spelling. His only son also had early difficulty with spelling and reading. Three paternal aunts suffered dementia with onset in their 60s, though none came to autopsy. On examination, his speech was labored but not dysarthric. He had difficulty sequencing speech sounds. Word-finding hesitations and circumlocutions were evident. His naming and repetition were preserved. His performance on the Boston Naming Test (14) was 57 out of 60. On the Northwestern Anagram Test, a speech independent measure of grammatical abilities, he scored 23/30, indicating impaired syntactic processing (17). The remaining cognitive domains were spared. The basic neurological examination was unrevealing, except for mild left-sided facial weakness.
Reexaminations 1 and 2 years later demonstrated gradual decline. He became severely dysarthric and dysphagic. Written output became progressively agrammatic. He developed supranuclear gaze palsy, bradykinesia, and axial rigidity. The history and examination were consistent with an admixture of AOS and agrammatism with late emergence of a progressive supranuclear palsy-like phenotype.
At autopsy, 5 years following symptomatic onset, there was left hemisphere atrophy in frontal, parietal, and temporal cortex. Additionally, there was neuronal loss and gliosis in left motor cortex. White matter rarefaction was severe in the internal capsule and moderate elsewhere in the descending corticospinal tracts. Phosphorylated TDP-43 immunostaining revealed frequent cytoplasmic inclusions in all layers of bilateral middle/inferior frontal gyrus, inferior parietal regions, and superior temporal gyrus (Fig. 2C). In most regions, TDP pathology was left hemisphere predominant; in the superior temporal gyrus; however, TDP pathology was more marked on the right. Additionally, TDP-positive cytoplasmic inclusions were moderate in the hypoglossal nucleus and sparse in the cervical anterior horns. Tau immunostaining did not disclose PSP pathology. ADNC was low: A1 + B1 + C2 (16). Alpha-synuclein immunostains in limbic areas were negative. The neuropathologic examination was consistent with both TDP type B and MND.
Patient 4
At the age of 51, a right-handed man noticed speech changes. Examination 1 year later revealed labored output without additional evidence of dysarthria. There were phonemic sequencing errors consistent with speech apraxia as well as mild naming difficulties. Comprehension, repetition, and grammar were spared. Elemental examination revealed slowed vertical optokinetic responses. Tone, bulk, and power were preserved, and there were no fasciculations. The clinical picture was consistent with AOS with a component of anomic aphasia.
Reexamination 3 years after symptom onset revealed anarthria and agrammatic writing. There was clear limitation of voluntary eye movements in the vertical plane with preservation of the oculocephalic reflex. No additional motor findings were elicited.
At autopsy, 4 years after symptom onset, there was mild-moderate asymmetric atrophy in left frontal, temporal, and parietal cortex. Histologic examination revealed superficial microvacuolation in left inferior frontal gyrus, left temporal pole, and left motor cortex. Neuronal loss and gliosis were moderate in primary motor cortex. Phospho-TDP-positive cytoplasmic inclusions were present in all cortical layers with predominance in bilateral inferior frontal gyri, left temporal pole, and left superior temporal gyrus (Fig. 2D). NCIs were frequent in the left motor cortex and absent on the right. There were no NIIs. Skein-like inclusions and Lewy-like bodies were frequent in substantia nigra in lateralized fashion. There were sparse skein-like inclusions and Bunina bodies in the hypoglossal nucleus, as well. Tau immunostaining was negative. Alpha-synuclein immunostains of amygdala and cingulate gyrus were unrevealing. The pathologic examination was consistent with TDP type B pathology with concomitant ALS pathology.
DISCUSSION
All 4 patients presented with focal and isolated impairments of speech and language (Table). Patients 1 and 2 presented with an isolated anomic aphasia, and Patients 3 and 4 demonstrated speech apraxia with components of agrammatism and anomia, respectively. In contrast to the typical presentations of type B pathology, motor and behavioral impairments were not present. Late in the course of the disease, motor findings in the form of axial rigidity and ocular dysmotility were noted. Despite the absence of pyramidal signs in life, postmortem examination revealed neuropathological changes of MND consistent with type B. None of our cases demonstrated p62-positive stellate inclusions in cerebellum and hippocampus characteristic of C9orf72 mutation (18). Despite the PSP-like phenotypes seen in 3 of our cases, none demonstrated PSP-type FTLD-tau pathology at autopsy.
TABLE.
Comparison of Patients With Speech and Language Impairment and FTLD-TDP Type B Pathology
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Sex | Male | Male | Male | Male |
| Handedness | Right | Right | Right | Right |
| Age at onset (years) | 59 | 64 | 60 | 51 |
| Duration of isolated aphasia/AoS (years) | 4 | 2 | 2 | 3 |
| Presenting clinical features | ||||
| Language | Anomia | Anomia | Syntactic incomprehension | Anomia |
| Speech | None | None | AoS | AoS |
| Motor | None | None | None | None |
| Late clinical features (years after presentation) | ||||
| Language | Agrammatism (2) | No additional features | Agrammatism (1) | Agrammatism (3) |
| Speech | None | Dysarthria (2) | Dysarthria (1) | Anarthria (3) |
| Motor | None | Axial rigidity, SNO (4) | Axial rigidity, SNO (2) | SNO (3) |
| Atrophy | Severe left ATL | Severe left ATL | Left hemisphere | Left hemisphere |
| TDP pathology (granular CI) | L>R STG | IFG, ATL | L>R MFG, IPL | L>R parietal |
| R>L STG | ||||
| ALS pathology | ||||
| UMN | Sparse | Moderate | Frequent | Frequent |
| LMN | Moderate | Sparse | Frequent | Moderate |
AoS, apraxia of speech; ATL, anterior temporal lobe; STG, superior temporal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; CI, cytoplasmic inclusions; UMN, upper motor neuron; LMN, lower motor neuron.
These cases represent 4 of 22 cases (18%) of FTLD-TDP type B examined by the Neuropathology Core of the Northwestern University ADC. The remainder (82%) presented with behavioral and/or fronto-executive deficits, of which 11 demonstrated clinical signs of MND. Given the clinical focus of the Northwestern Neurobehavior and Memory Clinic, the proportion of type B cases with speech and language presentations is likely affected by referral bias. Taken together with those cases of TDP type B referred by the Northwestern Lois Insolia ALS Clinic—an additional 34 cases with the clinical phenotype of MND—our cases represent 4 out of a sum total of 56 cases of type B neuropathology (7%).
Patients 1 and 2 demonstrated marked atrophy at the tip of the left ATL (Figs. 1A, C and 3A, B). Although left ATL atrophy is the characteristic correlate of semantic PPA with severe single-word comprehension impairment, neither patient demonstrated single-word comprehension deficits. Consistent with this observation, recent evidence has emerged to suggest that loss of single-word comprehension may require not only ATL atrophy but also the caudal spread of this atrophy into the middle parts of the lateral temporal lobe (19).
Caselli et al (20) described a series of 7 patients with severe aphasia, articulatory impediments, and MND. Four progressed to death within 2 years, a rate of progression similar to those in our series. While their cases predate ubiquitin immunohistochemistry and the identification of FTLD-TDP, they link speech and language phenotypes with MND. Our patients were all male and onset was in their 50s and 60s. In the Caselli et al cases, mean age was 67 years with a range of 54–77; 3 of the 7 were male.
There has been a recent revival of interest in the association between MND and speech and language impairment. Among a series of neuropathologically confirmed FTLD-MND, Vinceti et al (21) reported 10 patients whose symptoms were heralded by language disturbance. At initial evaluation, however, 6 already had motor symptoms—including limb weakness, fasciculations, and cramping—and 5 had behavioral changes. In total 6 of the 10 were found to have type B neuropathologic change of which 2 demonstrated agrammatic PPA and 4 presented with semantic PPA (21). Long et al (22) characterized language profiles in 26 subjects with FTD-MND. Severity of language-based impairments on the Sydney Language Battery and Test for Reception of Grammar was comparable to those seen in PPA. Language phenotypes in patients with FTD-MND were notably heterogenous. Taken together with our series, the clinical heterogeneity of speech and language presentations associated with type B appears to span the full spectrum of PPA subtypes and AOS. The arguably unique feature of our cases was the initial presentation with isolated speech and language impairments.
These 4 patients expand the known clinicopathologic correlations associated with TDP pathology, type B. Although types A and C are of most relevance to isolated language impairments (1), type B appears to provide another neuropathologic correlate of dementias with speech and language presentations.
This study was supported by grants from the National Institute on Deafness and Other Communication Disorders (R01 008552) and the National Institute on Aging (AG13854), the Davee Foundation, and the Florane and Jerome Rosenstone Fellowship.
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 2014;137:1176–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendez MF, Joshi A, Tassniyom K, et al. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology 2013;80:561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackenzie IRA, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mesulam MM, Nelson MJ, Hyun JM, et al. Preferential disruption of auditory word representations in primary progressive aphasia with the neuropathology of FTLD-TDP type A. Cogn Behav Neurol 2019;32:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 2007;66:142–51 [DOI] [PubMed] [Google Scholar]
- 6. Koriath CAM, Bocchetta M, Brotherhood E, et al. The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: A longitudinal case report. Alzheimers Dement 2016;3:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bigio EH, Weintraub S, Rademakers R, et al. Frontotemporal lobar degeneration with TDP-43 proteinopathy and chromosome 9p repeat expansion in C9ORF72: Clinicopathologic correlation. Neuropathology 2013;33:122–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mackenzie IRA, Nicholson AM, Sarkar M, et al. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 2017;95:808–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Zee J, Gijselinck I, Van Mossevelde S, et al. TBK1 mutation spectrum in an extended European patient cohort with frontotemporal dementia and amyotrophic lateral sclerosis. Hum Mutat 2017;38:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 2004;36:377–81 [DOI] [PubMed] [Google Scholar]
- 11. Neumann M, Mackenzie IR, Cairns NJ, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP mutations. J Neuropathol Exp Neurol 2007;66:152–7 [DOI] [PubMed] [Google Scholar]
- 12. Lee EB, Porta S, Baer GM, et al. Expansion of the classification of FTLD-TDP: Distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 2017;134:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kertesz A. Western Aphasia Battery-Revised. San Antonio, TX: Harcourt Assessment, Inc; 2007 [Google Scholar]
- 14. Goodglass H, Kaplan E, Barresi B, Boston Diagnostic Aphasia Examination, 3rd ed Austin, TX: Pearson; 2000 [Google Scholar]
- 15. Benton AL, Hamsher KD, Sivan AB.. Multilingual Aphasia Examination, 2nd ed Iowa City, IA: AJA Associates; 1989 [Google Scholar]
- 16. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weintraub S, Mesulam MM, Wieneke C, et al. The Northwestern anagram test: Measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen 2009;24:408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Sarraj S, King A, Troakes C, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 2011;122:691. [DOI] [PubMed] [Google Scholar]
- 19. Mesulam MM, Rader BM, Sridhar J, et al. Word comprehension in temporal cortex and Wernicke area. Neurology 2019;92:e224–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol 1993;33:200–7 [DOI] [PubMed] [Google Scholar]
- 21. Vinceti G, Olney N, Mandelli ML, et al. Primary progressive aphasia and the FTD-MND spectrum disorders: Clinical, pathological, and neuroimaging correlates. Amyotroph Lateral Scler Frontotemporal Degener 2019;20:146–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Long Z, Irish M, Piguet O, et al. Clinical and neuroimaging investigations of language disturbance in frontotemporal dementia-motor neuron disease patients. J Neurol 2019;266:921–33 [DOI] [PubMed] [Google Scholar]