Abstract
The Chronic Kidney Disease in Children Study, a prospective cohort study with data collected from 2003 to 2018, provided the first opportunity to characterize the incidence of renal replacement therapy (RRT) initiation over the life course of pediatric kidney diseases. In the current analysis, parametric generalized gamma models were fitted and extrapolated for RRT overall and by specific treatment modality (dialysis or preemptive kidney transplant). Children were stratified by type of diagnosis: nonglomerular (mostly congenital; n = 650), glomerular–hemolytic uremic syndrome (HUS; n = 49), or glomerular–non-HUS (heterogeneous childhood onset; n = 216). Estimated durations of time to RRT after disease onset for 99% of the nonglomerular and glomerular–non-HUS groups were 42.5 years (95% confidence interval (CI): 31.0, 54.1) and 25.4 years (95% CI: 14.9, 36.0), respectively. Since onset for the great majority of children in the nonglomerular group was congenital, disease duration equated with age. A simulation-based estimate of age at RRT for 99% of the glomerular population was 37.9 years (95% CI: 33.6, 63.2). These models performed well in cross-validation. Children with glomerular disease received dialysis earlier and were less likely to have a preemptive kidney transplant, while the timing and proportions of dialysis and transplantation were similar for the nonglomerular group. These diagnosis-specific estimates provide insight into patient-centered prognostic information and can assist in RRT planning efforts for children with moderate-to-severe kidney disease who are receiving regular specialty care.
Keywords: dialysis, kidney disease, kidney transplantation, pediatrics, prospective studies, renal insufficiency, renal replacement therapy
Chronic kidney disease (CKD) in children is a progressive condition leading to end-stage renal disease (ESRD), which requires renal replacement therapy (RRT) in the form of dialysis or kidney transplantation (1–3). Pediatric kidney disease presents a unique public health challenge, since it is a relatively rare but severe disease with heterogeneous etiologies (4, 5). Parents, clinicians, and policy-makers need to understand when children with kidney disease will progress to RRT (6), with parents in particular placing a high value on understanding their child’s prognosis (7). To date, no longitudinal cohort studies have quantified timing and RRT outcomes for children with CKD using duration of disease as the time scale and translating it to ages at which given percentages of patients are expected to receive or need RRT.
There are heterogeneous causes of pediatric kidney disease, ranging from congenital anomalies of the kidney and urinary tract (CAKUT) to later childhood-onset glomerular diseases, each with its own trajectory (8–10). To characterize the progression from CKD to ESRD, the Chronic Kidney Disease in Children (CKiD) Study enrolled children with diverse CKD diagnoses who underwent standardized annual visits. Previous CKiD studies have demonstrated the particularly aggressive nature of glomerular diseases compared with CAKUT diagnoses, in terms of glomerular filtration rate (GFR) decline (9) and time to ESRD (11), and the critical roles of diagnosis, GFR, proteinuria (11), and other comorbid conditions (3) in estimating the onset of ESRD.
Our purpose in the present study was to describe the incidence of RRT over the course of kidney disease. In addition to overall nonglomerular diagnoses, we investigated patients with CAKUT versus those with other nonglomerular diagnoses; and for glomerular diagnoses, we separated patients with hemolytic uremic syndrome (HUS) from those with non-HUS glomerular diagnoses (12). While not every kidney disease diagnosis is represented in these groupings (e.g., minimal-change disease), nor are patients with very mild disease, CKiD participants comprise those with moderate-to-severe disease who require close monitoring. This analysis capitalized on the heterogeneous durations of CKD prior to entry into the CKiD Study, which enrolled only RRT-free participants and appropriately accounted for survivorship bias (i.e., peers of the enrolled cohort who were not enrolled in the study because they received RRT prior to study enrollment). Valid and accurate modeling of the incidence of disease provided us with an opportunity to extrapolate the data to the point at which nearly all (i.e., 99%) children in the study would be expected to experience the outcome, in terms of both time after disease onset and age. Lastly, we employed a competing-risks framework to decompose RRT into first dialysis or preemptive kidney transplant events to jointly describe the incidence of each specific modality. Children receiving a preemptive transplant have consistently better outcomes than those receiving dialysis first (13). Therefore, describing time to RRT by diagnosis will help inform clinical programs and facilitate the planning process for transplantation to optimize outcomes. We propose inferences that are generalizable to most children with moderate-to-severe CKD receiving regular specialty care, as well as a best-case scenario for physician and family discussions.
METHODS
Study population
The CKiD Study is a longitudinal observational cohort study that enrolled children with kidney disease from 56 pediatric nephrology programs across the United States and Canada. Enrollment occurred in 3 waves targeting different stages and durations of disease. The first wave of enrollment took place between 2003 and 2010 (n = 586) for children aged 1–16 years with a GFR less than 90 mL/minute/1.73 m2 (14) with any diagnosis; the second wave occurred in 2011–2013 (n = 305) for children aged 1–16 years with a GFR of 45–90 mL/minute/1.73 m2 and equal proportions of glomerular and nonglomerular diagnoses; and the third wave began in 2016 and recruited children with nonglomerular diagnoses within 5 years of disease onset (n = 149 enrolled as of January 2019). At the time of analysis, 922 participants (89%) contributed follow-up time. At annual study visits, researchers collected data on biomarkers of CKD progression, general health, and several other clinical domains. For participants who were unable to complete full study visits, short-form interviews (Web-based, telephone, or in-person) provided data on CKD progression, general health, and RRT status (dates of dialysis initiation and/or kidney transplant).
All participants and families provided informed consent/assent, and the study protocols were approved by the institutional review boards of all participating sites. A complete description of the study design has been previously published (15).
Exposures
The primary exposure was diagnosis of kidney disease, defined broadly as nonglomerular and glomerular diseases (9). Nonglomerular diseases included CAKUT diagnoses (i.e., obstructive uropathy, dysplastic kidneys, reflux nephropathy, bilateral hydronephrosis, agenesis of abdominal musculature, and VACTERL [vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities] syndrome) and other (non-CAKUT) nonglomerular diagnoses (e.g., autosomal recessive polycystic kidney disease, renal infarct, cystinosis). Patients with glomerular diseases were stratified as those with HUS and those with other glomerular diagnoses (e.g., focal segmental glomerulosclerosis, systemic lupus erythematosus, chronic glomerulonephritis, familial nephritis). The HUS category was analyzed separately because it is known to differ from other glomerular diagnoses (12, 16, 17) and represented a small subgroup of patients (n = 49). A full description of kidney disease diagnoses is provided in Web Table 1 (available at https://academic.oup.com/aje). Within each diagnostic category, we explored whether sex and race (white vs. nonwhite) and whether CAKUT versus other nonglomerular diagnoses modified the estimated time by which 99% of children would receive RRT.
Time scale and outcome
The time scale was duration of time after disease onset, in years. Disease onset was estimated as the first manifestation of pathology as reported by the site clinician(s) treating each participant. The primary outcome was time to RRT, defined as the first occurrence of dialysis or transplantation. The date of first RRT was documented either by study personnel at each clinical site or by participant report confirmed through site review of medical records. Very few deaths occurred in this population (n = 2), and those participants were treated as censored at the time of death.
Statistical analysis
Nonparametric estimates of the cumulative incidence of RRT incorporated right-censoring and late-entry (i.e., left-truncated) observations using extended Kaplan-Meier methods. Since previous RRT was an exclusionary enrollment criterion, we employed methods for late entries to address survivorship bias for valid estimation of the cumulative incidence of RRT. These methods appropriately reweight the observed population to estimate an unobserved population of persons who experienced an event (i.e., RRT) before they could have enrolled in the study. In this application, all individuals entered late, since they were not enrolled at the time of disease onset, but the heterogeneous durations of disease at study entry allowed for characterization of the incidence of RRT over the course of up to 25 years.
Parametric survival models were fitted to data subjected to left-truncation (i.e., due to heterogeneous durations of CKD at study entry) and right-censoring (i.e., RRT-free at last time seen) using the generalized gamma (GG) family to model the incidence of RRT across the duration of CKD using maximum likelihood methods (18). The GG family of distributions is defined by 3 parameters—location β (linked to the median), scale σ (linked to the interquartile ratio), and shape κ (linked to the positions of the first and third quartiles relative to the median value (19))—and is denoted as GG(β, σ, κ), in which the Weibull (WE) distribution (WE(β, σ)) corresponds to a GG with a shape parameter of 1.
We previously reported a paucity of data within the first 2 years of CKD onset among participants with nonglomerular diagnoses (20), and there were also relatively few children (19%) with glomerular diagnoses enrolled within 1 year of disease onset. To address this when fitting a parametric model, we administratively truncated person-time at 1 year (see the Web Appendix for a full description) (18). This process omitted children whose observed follow-up fell entirely within the first year after diagnosis (6 nonglomerular patients and 1 glomerular patient), which resulted in an analytical population of 915 (i.e., 922 – 7) participants. This process ignores observed time within the first year after diagnosis for other children, only considering them to “enter” follow-up at 1 year after onset (12 nonglomerular patients and 47 glomerular patients). To assess the fit of the model to the data, we used the estimated cumulative incidence at 1 year (i.e., 1 – S(1)) to offset the nonparametric estimates of incidence and complete unobserved incidence of RRT during the first year due to administrative truncation. We then extrapolated the model to estimate the time by which 99% of children would receive RRT, with standard errors derived using the delta method. Overlaying the nonparametric and parametric cumulative incidence functions provided a visual display of correct model specification when data were available.
Influential data were assessed using the Wasserstein distance metric (21) and the extreme studentized deviate statistic (22). To evaluate the validity of each model, we used cross-validation methods (23, 24) with the data divided into 10 random samples of 10% each. Methods for influential data points and cross-validation methods are fully described in the Web Appendix, with results shown in Web Table 2.
For children with nonglomerular (nearly all congenital) disease, the time by which RRT occurs after disease onset closely corresponds to age. However, the vast majority of CKiD participants with a glomerular diagnosis had disease onset at heterogeneous ages. We sought to estimate the age by which 50% (i.e., median) and 99% of children with glomerular–non-HUS diagnoses would be expected to have RRT, accounting for survival bias. Specifically, we estimated the distribution of ages at RRT if all children with a glomerular diagnosis had been enrolled at the time of disease onset, including those who were observed and unobserved (i.e., unseen or not enrolled due to fast progression).
This distribution was based on 3 groups: persons who had an observed event; those who were right-censored (i.e., would have an event in the future); and those who were unseen and had an RRT event prior to enrollment (i.e., fast progressors). For the second group, we imputed an RRT event time that was conditional on the last contributed event-free person-time for each individual based on the parametric model. For the third group, the assumptions for late entries allowed calculation of the proportion who were ineligible for enrollment due to previously having had an RRT event and enabled reweighting of the population to appropriately account for these children. Specifically, for each participant who entered the CKiD Study outcome-free at  years after disease onset, the number of unobserved individuals corresponds to
years after disease onset, the number of unobserved individuals corresponds to . RRT times for these unobserved individuals were similarly drawn within the eligible window for this weighted pseudopopulation, thus completing the data for those unseen. Bootstrap methods were used to fit the GG model and repeat the data-completion process for the second and third groups to account for sampling and imputation variabilities. Within each bootstrap sample, we estimated the 50th and 99th percentiles of ages at RRT. From 500 samples, we report the median values and 2.5th and 97.5th percentiles of these quantiles as the expected ages (and 95% confidence intervals) at which half and nearly all children with glomerular–non-HUS diagnoses are expected to receive RRT. Importantly, we were not able to estimate times for children with very mild disease who were unlikely to require regular care at clinics similar to CKiD sites, or for diagnoses not represented in the CKiD Study.
. RRT times for these unobserved individuals were similarly drawn within the eligible window for this weighted pseudopopulation, thus completing the data for those unseen. Bootstrap methods were used to fit the GG model and repeat the data-completion process for the second and third groups to account for sampling and imputation variabilities. Within each bootstrap sample, we estimated the 50th and 99th percentiles of ages at RRT. From 500 samples, we report the median values and 2.5th and 97.5th percentiles of these quantiles as the expected ages (and 95% confidence intervals) at which half and nearly all children with glomerular–non-HUS diagnoses are expected to receive RRT. Importantly, we were not able to estimate times for children with very mild disease who were unlikely to require regular care at clinics similar to CKiD sites, or for diagnoses not represented in the CKiD Study.
Saturated GG models (18) were fitted separately with sex and race (white vs. nonwhite) as putative modifiers of timing of RRT for both diagnostic groups, and we additionally compared CAKUT with other nonglomerular diagnoses, as well as age at disease onset (age <10 years vs. ≥10 years) for glomerular diagnoses. We determined whether a 1-sample model was as appropriate as saturated models using a likelihood ratio test with 3 degrees of freedom (corresponding to the covariate modifying the β, σ, and κ parameters).
To determine the joint distribution of incidence of first dialysis or transplant, we used parametric mixture models using the GG family to estimate the proportions of patients receiving dialysis (π) and transplantation (1 – π) and times to each competing event for nonglomerular and glomerular–non-HUS diagnoses separately (25). We excluded children with HUS, since they constituted a small diagnostic group. To incorporate the upper bound for the time by which all events were expected to occur from the parametric modeling of the composite event, participants who were free of the event were considered interval-censored (26) between the time of the last known event-free time and 45 years (for nonglomerular diagnoses) or 27.5 years (for glomerular–non-HUS diagnoses). We conducted sensitivity analyses with the upper limit of the interval censoring increasing incrementally to 60 years and 40 years, respectively.
All analyses were conducted in Stata 15 (StataCorp LLC, College Station, Texas) with parametric modeling using the “streg” and “stcrmix” commands and custom software for mixtures of GG and Weibull distributions. Graphs were constructed in R 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Table 1 presents the demographic and clinical characteristics of the 915 participants. A total of 650 (71.0%) children had a nonglomerular form of kidney disease, with 86.3% having disease onset at birth and an additional 7.4% having onset within 1 year of age, reflecting the congenital and very-early-onset nature of their disease. A total of 265 (29.0%) children had glomerular disease and were further stratified by HUS (n = 49) and non-HUS (n = 216) diagnoses. Of the patients with HUS, 77.5% had disease onset by 5 years of age. In contrast, among those with glomerular–non-HUS diagnoses, 25.9% had disease onset by 5 years of age. The group with glomerular–non-HUS diagnoses had higher proportions of children with nonwhite race, low income, and low maternal education, as well as a higher urinary protein: creatinine ratio than the other two groups. Differences in annual percent decline in GFR by diagnosis demonstrated the distinction between the 3 diagnostic groups, as previously documented (27).
Table 1 .
Sociodemographic, Clinical, and Longitudinal Characteristics of Participants in the Chronic Kidney Disease in Children (CKiD) Study, by Type of Kidney Disease Diagnosis, 2003–2018
| Variable | Type of Kidney Disease Diagnosis | |||||
|---|---|---|---|---|---|---|
|
Nonglomerular
(n = 650) |
Glomerular–HUS
(n = 49) |
Glomerular–Non-HUS
(n = 216) |
||||
| No. | % | No. | % | No. | % | |
| Demographic factors | ||||||
| Age at study entry, yearsa | 9.4 (5.3–13.3) | 10.5 (6.8–14.2) | 14.6 (11.9–16.0) | |||
| Male sex | 430 | 66.2 | 24 | 49.0 | 116 | 53.7 |
| Nonwhite race | 196 | 30.2 | 12 | 24.5 | 112 | 51.9 |
| Hispanic ethnicityb | 88 | 13.6 | 4 | 8.2 | 38 | 17.7 |
| Canadian resident | 74 | 11.4 | 7 | 14.3 | 22 | 10.2 |
| Socioeconomic status at study entry | ||||||
| Annual household income, US$ or Can$ | ||||||
| ≤36,000 | 249 | 39.3 | 13 | 27.1 | 93 | 44.7 |
| 36,001–75,000 | 187 | 29.5 | 16 | 33.3 | 57 | 27.4 |
| >75,000 | 197 | 31.1 | 19 | 39.6 | 58 | 27.9 |
| Maternal education less than collegec | 233 | 36.5 | 13 | 27.1 | 100 | 47.8 |
| Kidney disease factors | ||||||
| GFR at study entry, mL/minute/1.73 m2a | 50 (37–63) | 59 (44–74) | 61 (44–78) | |||
| Annual change in GFR, %a | −3.2 (−8.5 to −0.3) | −1.8 (−6.4 to 0.2) | −6.0 (−15.9 to −1.4) | |||
| Urinary protein:creatinine ratio at study entry, mg/mg Cra | 0.31 (0.12–0.80) | 0.33 (0.17–1.18) | 0.75 (0.23–2.64) | |||
| Urinary protein:creatinine ratio, mg/mg Crd | ||||||
| ≤0.20 | 257 | 40.5 | 17 | 35.4 | 48 | 22.4 |
| 0.21–1.99 | 324 | 51.0 | 26 | 54.2 | 106 | 49.5 |
| ≥2.00 | 54 | 8.5 | 5 | 10.4 | 60 | 28.0 |
| Age at disease onset, years | ||||||
| 0 (disease present at birth) | 561 | 86.3 | 1 | 2.0 | 23 | 10.6 |
| 0.1–1.0 | 48 | 7.4 | 4 | 8.2 | 4 | 1.9 |
| 1.1–5.0 | 11 | 1.7 | 33 | 67.3 | 29 | 13.4 |
| 5.1–10.0 | 14 | 2.2 | 9 | 18.4 | 58 | 26.9 |
| >10.0 | 16 | 2.5 | 2 | 4.1 | 102 | 47.2 |
| Duration of follow-up per participant, yearsa | 5.92 (3.17–8.24) | 5.11 (3.12–7.57) | 3.97 (2.37–5.30) | |||
| Total duration of follow-up, years | 3,756 | 281 | 894 | |||
| Renal replacement therapy | 192 | 29.5 | 10 | 20.4 | 76 | 35.2 |
| Dialysis | 98 | 15.1 | 8 | 16.3 | 60 | 27.8 |
| Kidney transplant | 94 | 14.5 | 2 | 4.1 | 16 | 7.4 |
Abbreviations: GFR, glomerular filtration rate; HUS, hemolytic uremic syndrome.
a Values are expressed as median (interquartile range).
b Data on Hispanic ethnicity were missing for 2 participants.
c Data on maternal education were missing for 19 participants.
d Data on urinary protein:creatinine ratio were missing for 18 participants (15 nonglomerular, 2 HUS, and 1 glomerular–non-HUS).
Figure 1 presents the cumulative incidence of first renal replacement therapy (dialysis or transplant) after disease onset over the course of 50 years, for the nonglomerular, glomerular–non-HUS, and HUS groups. Nonparametric estimates of RRT incidence, incorporating late entries, were possible for up to 19 years for glomerular–non-HUS diagnoses and 25 years for nonglomerular and HUS diagnoses. The nonparametric functions commenced at 1 year, due to administrative truncation, and were informed by the estimated survival at 1 year from the parametric functions (i.e., 1 – S(1)). Despite the low sample size of the HUS group, incidences of RRT were similar between glomerular–HUS and nonglomerular diagnoses. Parametric models for both nonglomerular and glomerular–non-HUS groups demonstrated excellent fit with the Kaplan-Meier curves. The median times to event were 18.9 years (95% confidence interval (CI): 17.7, 20.1) and 7.6 years (95% CI: 3.8, 11.4) after kidney disease onset for the nonglomerular and glomerular–non-HUS groups, respectively. For those with nonglomerular disease, the parametric model-estimated time from disease onset by which 99% of participants would experience RRT was 42.5 years (95% CI: 31.0, 54.1), and for those with glomerular–non-HUS disease, the time was estimated to be 25.4 years (95% CI: 14.9, 36.0).
Figure 1 .

Incidence of renal replacement therapy (RRT) after kidney disease onset among participants with nonglomerular (blue; n = 650), glomerular–hemolytic uremic syndrome (HUS) (green; n = 49), and glomerular–non-HUS (red; n = 216) diagnoses, Chronic Kidney Disease in Children Study, 2003–2018. Continuous step functions represent nonparametric estimates of the cumulative incidence of RRT. Dashed lines represent group-specific parametric survival models based on the generalized gamma (GG) family, with parameters listed as GG(β, σ, κ) in the figure key. 99th percentile times to RRT (in years) after kidney disease onset are presented for patients with glomerular–non-HUS (red dot; 25.4 years, 95% confidence interval: 14.9, 36.0) and nonglomerular (blue dot; 42.5 years, 95% confidence interval: 31.0, 54.1) disease.
Two participants in the nonglomerular group and 3 in the glomerular–non-HUS group were identified as influential data points, because of early RRT incidence (within 3.5 years and 1.25 years, respectively). However, very similar results for both models were obtained when administrative truncation was moved to 5 years and 1.5 years, respectively, providing evidence that these individuals contributed valid data and should not be excluded. More importantly, these models were validated in a cross-validation test set of data comparing the observed and expected survival functions when 10% were randomly excluded. There was no evidence of significant departures from the expected survival functions, thus demonstrating satisfactory model validation. A full description of the cross-validation results is presented in the Web Appendix.
To explore differences in time to RRT by sex and race, we fitted saturated GG models. On the basis of likelihood ratio tests, among participants with a nonglomerular diagnosis, there was no evidence of differing times to RRT by sex (P = 0.488) or race (P = 0.243), and this was similar in the glomerular group (sex: P = 0.506; race: P = 0.498). Furthermore, among patients with nonglomerular diagnoses, we also explored differences between CAKUT (n = 488) and other nonglomerular (n = 162) diagnoses. There were no statistical differences between these two etiological subgroups when comparing the saturated GG model with the null GG model (P = 0.200), and the cumulative incidence functions were similar. The same phenomenon was observed for age at disease onset (<10 years vs. ≥10 years, with n = 114 and n = 102, respectively) among children with glomerular–non-HUS diseases (P = 0.121).
For participants with nonglomerular diagnoses, time since kidney disease onset approximated age, but this was not the case for children with glomerular–non-HUS disease due to heterogeneous age at disease onset. To estimate the median and 99th percentile ages at RRT in the glomerular disease group, we used the model shown in Figure 1 and the bootstrap and imputation methods described above. The results from a single imputation are presented in Figure 2. The gray points represent observed RRT events; the solid black points represent the right-censored participants with an imputed age at RRT. The open points display estimates for the unseen persons who experienced RRT prior to study enrollment; bubble sizes are commensurate to weights in the pseudopopulation. Diagonal reference lines depict ages at RRT. This simulation recaptured the natural course described in Figure 1: The median age at kidney disease onset was 9.5 years, and the median time to RRT after disease onset was 7.3 years. The estimated median age at RRT was 15.2 years (95% CI: 11.5, 17.7), and the 99th percentile was 37.9 years (95% CI: 33.6, 63.2).
Figure 2 .
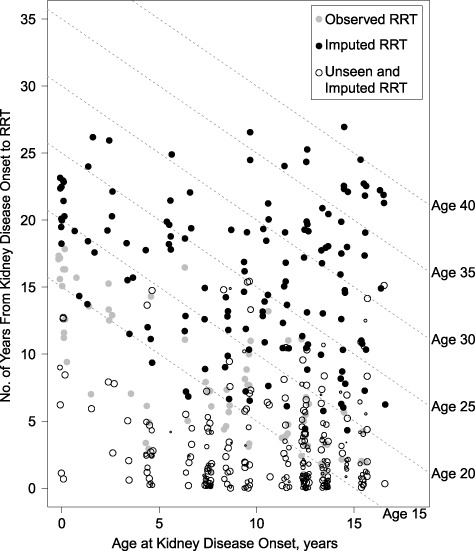
Characterization of age at renal replacement therapy (RRT) among patients with glomerular (non–hemolytic uremic syndrome) chronic kidney disease, by age at disease onset (x-axis) and years from onset to RRT (y-axis), Chronic Kidney Disease in Children Study, 2003–2018. The graph shows observed (gray) and imputed (black) data from a single imputation. Solid points denote observed study participants, and open points denote unseen fast progressors. The data and model were based on those presented in Figure 1. Dashed reference lines are provided for different ages at RRT.
To explore differences in RRT modality as competing events and compare the distributions of times to preemptive transplantation and first dialysis, we fitted parametric mixture models with interval censoring that incorporated the upper limit identified by the models using the composite outcome of RRT (45 years and 27.5 years for nonglomerular and glomerular–non-HUS diagnoses, respectively). Web Figure 1 displays the nonparametric and parametric cumulative incidences of transplantation and dialysis, by nonglomerular (Web Figure 1A) and glomerular–non-HUS (Web Figure 1B) diagnoses, and demonstrates outstanding model fit. The mixture parameter represents the proportion of patients who are expected to receive dialysis first (49%, 95% CI: 38, 60), with the complement being the proportion receiving a kidney transplant (51%) by 45 years after disease onset. The timing of dialysis and transplantation were similar for patients with nonglomerular diagnoses, indicating that approximately half would receive a preemptive transplant and the other half would receive dialysis first, and at very similar times. In contrast, persons with glomerular–non-HUS diseases were much more likely to receive dialysis (84%) than a transplant (16%) as the first RRT by 27.5 years after disease onset. The inferences were robust to increased upper censoring bounds (results shown in Web Table 3 and Web Figure 2).
DISCUSSION
The CKiD Study, as a prospective longitudinal cohort study, provided us with the opportunity to describe the course of kidney disease among study participants from onset and to extrapolate models for estimates of times when children similar to CKiD participants will progress to ESRD, including fast progressors. The estimates of 42.5 years for children with nonglomerular diseases and about 25 years after disease onset for children with glomerular–non-HUS diseases are measures of the expected progression of disease in children with mild-to-moderate forms of CKD. Further, recognizing the variable age of onset among patients with glomerular–non-HUS diagnoses, the model was used to indicate that nearly all those with childhood-onset glomerular disease and with a nadir GFR below 90 mL/minute/1.73 m2 will require RRT by age 38 years (with half experiencing RRT by age 15 years).
Using the models presented here, we aimed to describe the life-course epidemiology of RRT for this population of children with kidney disease. These estimates provide important general prognostic information to help inform the RRT planning process in pediatric and adult nephrology programs. Providing an upper bound of ESRD incidence is epidemiologically relevant, since identifying high-risk individuals remains a challenge because of heterogeneous disease progression. Most importantly, qualitative research suggests that families often feel disempowered by their perceived knowledge imbalance as compared with physicians and are concerned about receiving false hope regarding their child’s prognosis (7). The results from this epidemiologic study are distinct from clinical prediction tools used to estimate or discriminate the timing of ESRD or RRT based on a patient’s current disease severity (3, 11, 28).
This study further documents the differences between etiologies of kidney disease and the higher risk and progression related to glomerular–non-HUS diagnoses (29), where earlier RRT planning and clinical vigilance are needed. In this study, diagnostic groups with more rapid progression to RRT were more likely to receive dialysis than preemptive transplantation. We therefore recommend early discussions in pediatric nephrology clinics for the high-risk glomerular diagnosis as a means to increase the likelihood of avoiding or shortening time spent on dialysis.
In a European study of registry data comprising persons with nonglomerular diagnoses, Wühl et al. (30) described the ages at which RRT occurred, which corresponded to disease duration, since this type of kidney disease is congenital. The authors estimated that the median age (i.e., disease duration) at RRT, among persons who received RRT, was 40 years (30). A major difference between that study and ours was that only patients who initiated RRT were included in the European study. In the CKiD cohort, the study population was not selected by the outcome; rather, participants were followed prospectively, which was a major strength. The differing estimates are probably due to the selection of CKiD participants at particularly high risk for CKD progression. However, it is important to note that a substantial number of children in CKiD experienced minimal or no change in kidney function during the course of the study. Specifically, Pierce et al. (9) demonstrated that 20% of children with kidney disease, of any etiology, had no change in kidney function or improving kidney function over a limited number of follow-up years. This heterogeneity of disease progression gives confidence that our results pertain to the broad spectrum of children with similar diagnoses and disease severity, who represent a challenge for pediatric nephrologists.
This study provides an epidemiologic forecast of RRT events that have not occurred. It is not certain that these events will occur, and we cannot identify or impute from these models when events will occur for particular individuals. However, the extremely good fit from the parametric models suggests that the extrapolation is valid if participants who are currently event-free have similar trajectories as those who were observed to have RRT. We note that there are people with kidney abnormalities who experience a very mild disease course and may require RRT very late in life or not at all. This subgroup is not represented here, because they did not meet eligibility criteria for the CKiD Study and these results probably are not generalizable to them. The fact that HUS, as a glomerular diagnosis, demonstrated slower progression than other glomerular diseases suggests that specific diagnoses may have distinct trajectories that warrant further study. In addition, limited representation of children with a particular diagnosis, including other diagnoses not represented in CKiD, meant that we could not fully characterize the diagnosis-specific incidences of RRT. Given the rare nature of pediatric kidney disease and the diversity of diagnoses, it remains an epidemiologic challenge to further stratify study participants by these rare diagnoses to describe diagnosis-specific disease progression. We also note that initiation of dialysis does not preclude future transplants from occurring, and previous literature has shown that transplantation frequently occurs within 1 year after dialysis initiation among persons with pediatric CKD (31). Lastly, while the cross-validation assessment yielded confidence in these models, validation in a truly external population would be ideal.
Knowledge of what the future holds for a child newly diagnosed with CKD is highly important to families (7) and clinicians (3, 11), but the information needs to be communicated in a meaningful way. Extrapolating from prospective data from the largest observational cohort of children with CKD compiled to date, we estimated that children receiving regular specialty care with moderate-to-severe nonglomerular CKD will require RRT by approximately 42.5 years after disease onset, and that those with glomerular–non-HUS CKD will require RRT by approximately 25.4 years after disease onset (around 38 years of age). These diagnosis-specific estimates provide insight into patient-centered prognostic information and can assist in RRT planning efforts for this population of patients.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (grants U01-DK-66143, U01-DK-66174, U24-DK-082194, and U24-DK-66116).
We acknowledge Megan Carroll for assistance in identifying outlying data points and Dr. Meredith Atkinson for helpful comments in the preparation of this work.
The data used in this analysis were collected by investigators in the Chronic Kidney Disease in Children (CKiD) Prospective Cohort Study (http://statepi.jhsph.edu/ckid). CKiD clinical coordinating centers: Children’s Mercy Hospital and University of Missouri–Kansas City (Kansas City, Missouri) (Principal Investigator, Dr. Bradley Warady) and Children’s Hospital of Philadelphia (Philadelphia, Pennsylvania) (Principal Investigator, Dr. Susan Furth); central biochemistry laboratory: University of Rochester Medical Center (Rochester, New York) (Principal Investigator, Dr. George Schwartz); data coordinating center: Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland) (Principal Investigators, Drs. Alvaro Muñoz and Derek K. Ng).
Conflict of interest: none declared.
Abbreviations
- CAKUT
congenital anomalies of the kidney and urinary tract
- CI
confidence interval
- CKD
chronic kidney disease
- CKiD
Chronic Kidney Disease in Children
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- GG
generalized gamma
- HUS
hemolytic uremic syndrome
- RRT
renal replacement therapy
References
- 1.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22(12):1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckardt K-U, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. [DOI] [PubMed] [Google Scholar]
- 3.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis. 2015;65(6):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CS, Pierce CB, Cole SR, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the Chronic Kidney Disease in Children Study. Clin J Am Soc Nephrol. 2009;4(4):812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol. 2015;10(4):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploos van Amstel S, Noordzij M, Warady BA, et al. Renal replacement therapy for children throughout the world: the need for a global registry. Pediatr Nephrol. 2018;33(5):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutman T, Hanson CS, Bernays S, et al. Child and parental perspectives on communication and decision making in pediatric CKD: a focus group study. Am J Kidney Dis. 2018;72(4):547–559. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JT, Mitsnefes M, Pierce C, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Hypertension. 2008;52(4):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce CB, Cox C, Saland JM, et al. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol. 2011;174(5):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harambat J, Stralen KJ, Kim JJ, et al. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furth SL, Pierce C, Hui WF, et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018;71(6):783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repetto HA. Long-term course and mechanisms of progression of renal disease in hemolytic uremic syndrome. Kidney Int Suppl. 2005;68(suppl 97):S102–S106. [DOI] [PubMed] [Google Scholar]
- 13.Amaral S, Sayed BA, Kutner N, et al. Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int. 2016;90(5):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) Prospective Cohort Study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spizzirri FD, Rahman RC, Bibiloni N, et al. Childhood hemolytic uremic syndrome in Argentina: long-term follow-up and prognostic features. Pediatr Nephrol. 1997;11(2):156–160. [DOI] [PubMed] [Google Scholar]
- 17.Caletti MG, Lejarraga H, Kelmansky D, et al. Two different therapeutic regimes in patients with sequelae of hemolytic-uremic syndrome. Pediatr Nephrol. 2004;19(10):1148–1152. [DOI] [PubMed] [Google Scholar]
- 18.Cox C, Chu H, Schneider MF, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. [DOI] [PubMed] [Google Scholar]
- 19.Matheson M, Muñoz A, Cox C. Describing the flexibility of the generalized gamma and related distributions. J Stat Distrib Appl. 2017;4(1):Article 15. [Google Scholar]
- 20.Ng DK, Moxey-Mims M, Warady BA, et al. Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease. Ann Epidemiol. 2016;26(11):780–787.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panaretos VM, Zemel Y. Statistical aspects of Wasserstein distances. Annu Rev Stat Appl. 2019;6(1):12.1–12.27. [Google Scholar]
- 22.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Dent Tech. 1983;25(2):165–172. [Google Scholar]
- 23.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453–473. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Harrell FE, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. [DOI] [PubMed] [Google Scholar]
- 25.Checkley W, Brower RG, Muñoz A, et al. Inference for mutually exclusive competing events through a mixture of generalized gamma distributions. Epidemiology. 2010;21(4):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(9):2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winnicki E, McCulloch CE, Mitsnefes MM, et al. Use of the kidney failure risk equation to determine the risk of progression to end-stage renal disease in children with chronic kidney disease. JAMA Pediatr. 2018;172(2):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staples AO, Greenbaum LA, Smith JM, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5(12):2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wühl E, Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reese PP, Hwang H, Potluri V, et al. Geographic determinants of access to pediatric deceased donor kidney transplantation. J Am Soc Nephrol. 2014;25(4):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]


