Abstract
Sudden unexplained death in childhood (SUDC) affects children >1-year-old whose cause of death remains unexplained following comprehensive case investigation and is often associated with hippocampal abnormalities. We prospectively performed systematic neuropathologic investigation in 20 SUDC cases, including (i) autopsy data and comprehensive ancillary testing, including molecular studies, (ii) ex vivo 3T MRI and extensive histologic brain samples, and (iii) blinded neuropathology review by 2 board-certified neuropathologists. There were 12 girls and 8 boys; median age at death was 33.3 months. Twelve had a history of febrile seizures, 85% died during apparent sleep and 80% in prone position. Molecular testing possibly explained 3 deaths and identified genetic mutations in TNNI3, RYR2, and multiple chromosomal aberrations. Hippocampal abnormalities most often affected the dentate gyrus (altered thickness, irregular configuration, and focal lack of granule cells), and had highest concordance between reviewers. Findings were identified with similar frequencies in cases with and without molecular findings. Number of seizures did not correlate with hippocampal findings. Hippocampal alterations were the most common finding on histological review but were also found in possibly explained deaths. The significance and specificity of hippocampal findings is unclear as they may result from seizures, contribute to seizure pathogenesis, or be an unrelated phenomenon.
Keywords: Dentate gyrus, Hippocampal abnormalities, Neuropathology, Pediatric, Seizure, Sudden unexplained death in childhood
INTRODUCTION
Sudden unexplained death in childhood (SUDC) is the unexpected death of a child over age 1 year that remains unexplained despite thorough review of clinical history, circumstances of death, and a complete autopsy with ancillary testing (1). SUDC occurs in 1.0–1.4 of 100 000 live births (2). It shares features with sudden infant death syndrome and sudden unexpected death in epilepsy (SUDEP), including death often during a sleep period, found in the prone position, male gender, and a history of individual or familial febrile seizures (3–6). SUDC likely results from varying etiologies (7). Comprehensive investigation is required to exclude an underlying explanation for the death. Although forensic agencies aim to perform complete autopsies, scene investigation, and ancillary studies, available funding is often limited and regional protocols for death investigation and autopsy vary considerably across institutions and jurisdictions. Autopsy practice audits for SUDC cases by the San Diego SUDC Research Project (151 cases) and by Irish pathologists (45 cases) identified marked variation between cases from different locations and different pathologists (4, 7, 8).
Genetic etiology may increase SUDC risk and could cause or contribute to some deaths through cardiac or epilepsy mechanisms (9, 10). Postzygotic de novo mutations can cause somatic mosaicism, which can impact brain development and neurologic functions and contribute to causing SUDC and SUDEP. These include abnormal neuronal migration, disorders associated with brain overgrowth, epileptic encephalopathies, intellectual disability, autism, and other neuropsychiatric diseases (11, 12). The most common neuropathologic findings in SUDC are hippocampal abnormalities (13, 14). The San Diego SUDC Research Project identified hippocampal abnormalities in ∼50% of cases, including multiple structural abnormalities such as single ectopic cells in the molecular layer, dispersion of the dentate gyrus (DG), ectopic granule cells in the hilus, irregular DG configuration, focal lack of DG granule cells without gliosis and without blood vessels penetrating the DG, clusters of ectopic granule cells in the molecular layer, DG hyperconvolution, variation in DG thickness, granule cell heterotopias, and hilar gliosis (8). Deaths associated with hippocampal maldevelopment may potentially result from unwitnessed seizure during sleep (15, 16). Among SUDC cases without hippocampal abnormalities, cause of death remained unexplained and raises the possibilities of undetected neuropathology, cardiac channelopathies, and metabolic disorders. Another SUDC neuropathologic study found hippocampal alterations in many cases and focal cortical dysplasia was the next most common abnormality (14). Other nonhippocampal lesions were not clearly associated with SUDC.
To determine the specificity of hippocampal microscopic findings for SUDC pathogenesis, the availability of whole brains for systematic tissue sampling is essential and the subsequent analyses are lacking in the current literature. Limitations of previous studies include the retrospective case accrual, limited systematic evaluation outside the hippocampus, and unavailability of hippocampal sections for all cases (17). This reflects the rarity of SUDC cases as well as a lack of standardization between referring agencies and neuropathologists. Thus, systematic, comprehensive tissue sampling of hippocampi and other regions was often not possible retrospectively, making earlier neuropathological reports for SUDC limited and unable to clearly define the specificity of these findings in SUDC or their potential role as causes or results of seizures. The main goal of this study was to construct a systematic approach to neuropathologic investigations of SUDC as a multi-institutional joint effort at the Sudden Unexpected Death in Childhood Registry and Research Collaborative (SUDCRRC). Here, we introduce a standard brain tissue sampling protocol coupled with ancillary testing including molecular analyses, performed in a prospective blinded manner to evaluate etiologic specificity of these findings in SUDC and their potential role in possibly explained and unexplained causes of death.
MATERIALS AND METHODS
Case Enrollment and Data Collection
This study was approved by the New York University Institutional Review Board (IRB s14-01061). Autopsy cases enrolled in the SUDCRRC were included in the study. The SUDCRRC is a multidisciplinary collaborative including NYU Langone School of Medicine, Columbia University, The Mayo Clinic and 10 forensic pathologist investigators at Medical Examiner and Coroner Offices across the United States. Cases were referred to the SUDCRRC by the SUDC Foundation, treating physicians, medical examiners, coroners and family self-referrals. Twenty cases were prospectively enrolled over 5 years. Release of deceased child’s medical records, and whole exome sequencing from autopsy blood/tissue was obtained with decedent’s parent(s)/guardian(s) informed consent. Children aged 12 months to 18 years were enrolled if the initial gross postmortem investigation did not identify a clear cause of death (i.e. negative or nonspecific autopsy findings, inconclusive or unremarkable death scene investigation). Cause of death was determined by a consensus panel of board-certified forensic pathologists who reviewed all case materials for each case including circumstances of death, autopsy reports, histology slides, and ancillary test results including cardiac and neuropathology reports. Cases were excluded if the manner of death was unnatural (i.e. homicide, suicide or accident). All autopsies were full unrestricted autopsies that included a microscopic review of major organs, and performed at local governmental forensic agencies.
Ancillary Studies
Postmortem toxicologic testing was performed by the submitting forensic agency for every case, and microbiologic testing for 90% of cases. Whole exome sequencing was performed on patient genomic DNA from whole blood or fresh frozen liver samples. We sequenced the decedent and both biological parents using a Roche Nimblegen EZCap V3 or IDT xGen exome kit. We subjected input trios to relatedness quality controls to ensure that none violated expected interrelatedness patterns. Analyzed sequences were aligned and compared with reference sequences and variants were reported per the American College of Medical Genetic guidelines. The results of ancillary studies were used to assist in classification of deaths as either possibly explained or unexplained.
Neuropathologic Evaluation
Brains from 20 subjects were fixed in neutral buffered formalin for 2 weeks, screened using an ex vivo 3T MRI protocol, photographed, and examined in a standardized fashion by a board-certified neuropathologist (18, 19). For each case 15–17 macroscopic photographs of cerebrum, and 1 each of brainstem and cerebellum were obtained. Tissue sections from 23 brain regions were sampled using a modified NYU Alzheimer Disease Research Center protocol (Table 2). The sampling protocol incorporated hippocampus bilaterally at 3 levels to include lateral geniculate nucleus, pes, and pulvinar. Regions of hippocampal abnormality detected on postmortem 3T MRI imaging were additionally sampled. Tissue sections were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB) according to standard protocols. Whole slide imaging of 23 H&E- and 23 LFB-stained microscopic tissue sections was performed at 40× objective magnification using a Leica SCN400 Biosystem Aperio Slide Scanner (Leica, Buffalo Grove, IL). Digitized photographs of whole brain and digital slide scanned images were reviewed by 2 board-certified neuropathologists blinded to all clinical and autopsy data to generate a total of 40 independent reviews. Two neuropathologists were randomly assigned to independently review each case from a pool of 4 neuropathologists and were each provided an electronic list to record presence or absence of 62 observations across 14 anatomic brain regions (including 30 hippocampal observations, 15 on either side). Findings were classified as concordant if observed by both neuropathologists and discordant if only seen by 1 neuropathologist on blinded review. Hippocampal findings were scored as present or absent based on published descriptions of hippocampal microscopic findings in SUDC (14).
TABLE 2.
Anatomic Locations of Brain Tissue Sampled for Microscopic Analysis
| Block | Anatomic Location | Laterality |
|---|---|---|
| A | Superior frontal gyrus | Unilateral |
| B | Pre- and postcentral gyri, to include Brodmann area 1, 2, 3, 4 | Unilateral |
| C | Superior and middle temporal gyri | Unilateral |
| D | Inferior frontal (orbital) area | Unilateral |
| E | Hippocampus and inferior temporal gyrus, at level of and to include lateral geniculate nucleus | Left |
| F | Hippocampus and inferior temporal gyrus, at level of and to include lateral geniculate nucleus | Right |
| G | Anterior hippocampus at the level of the pes | Left |
| H | Anterior hippocampus at the level of the pes | Right |
| I | Posterior hippocampus at the level of the pulvinar | Left |
| J | Posterior hippocampus at the level of the pulvinar | Right |
| K | Angular gyrus | Unilateral |
| L | Occipital lobe (Calcarine sulcus) to include area 17, 18, and 19 | Unilateral |
| M | Basal forebrain with striatum, level of anterior commissure | Unilateral |
| N | Amygdala with adjacent hypothalamus | Unilateral |
| O | Thalamus, at level through subthalamic nucleus | Unilateral |
| P | Midbrain, through brachium conjunctivum | Entirely |
| Q | Pons, through locus ceruleus | Entirely |
| R | Lower Pons, at inferior border V cranial nerve | Entirely |
| S | Medulla | Entirely |
| T | Medulla | Entirely |
| U | Cerebellar hemisphere with cortex and dentate nucleus | Unilateral |
| V | Basal ganglia | Unilateral |
| W | Insular cortex | Unilateral |
| X | Sections of focal abnormalities detected by neuroimaging or macroscopic examination | Based on imaging findings |
Data Analysis
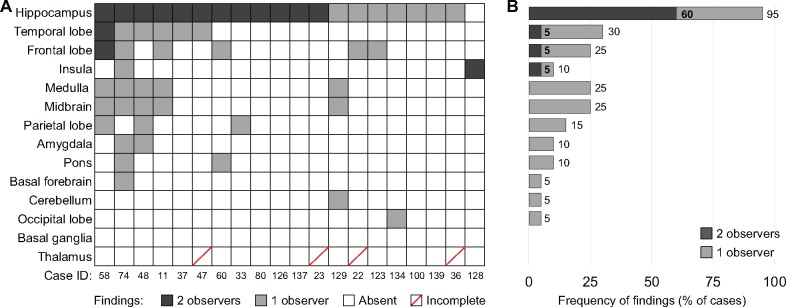
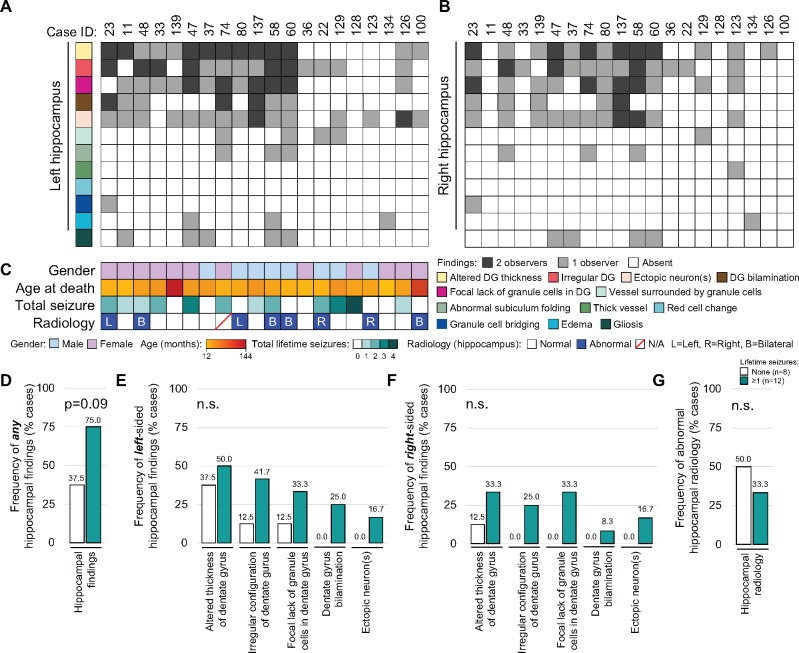
A data matrix containing anatomic region-specific observations (62 variables and 14 anatomic regions) was used for analysis. For each variable, a finding was defined as (i) absent (white box/bar), (ii) present and observed by 1 neuropathologist (light gray box/bar), (iii) present and observed by both neuropathologists (dark gray box/bar), or (iv) data incomplete (white box with red diagonal line). An overview analysis of 14 broad anatomic regions was initially performed to survey the landscape of brain pathology (Fig. 1B, C). Subsequent characterization of hippocampal microscopic findings was performed (12 variables, Fig. 2A, B). Heatmaps and bar graphs were generated in R, Excel and Illustrator. Fisher exact test and analysis of variance (ANOVA) was performed in Excel.
FIGURE 1.
The frequency of microscopic findings in different brain regions. (A) Heatmap illustrates region-specific findings in individual cases. Cell color depicts presence or absence of a finding, and concordance between 2 independent neuropathologists (dark gray—finding present, reported by both neuropathologists; light gray—finding present, reported by one neuropathologist; white—no findings reported by both neuropathologists; white with red line—only 1 observation available, considered incomplete). (B) Bar graph shows the frequency of findings (percent of cases, x-axis) identified in different anatomic regions of the brain (y-axis), corresponding to (A). Dark and light gray indicates if findings were reported by 2 or 1 neuropathologists, respectively.
FIGURE 2.
Heterogeneity of hippocampal findings between patients. Heatmaps illustrate the presence or absence of specific hippocampal findings for left (A) and right hippocampus (B). Color-coded rows indicate specific hippocampal findings, and columns (Case ID) represent the individual patients (n = 20). Cell colors depict the presence or absence, and concordance between the 2 independent neuropathologists (dark gray—finding present, reported by both neuropathologists; light gray—finding present, reported by 1 neuropathologist; white—lesion absent, reported by both neuropathologists). (C) Clinical characteristics of patients, including gender, age at time of death (months), number of total lifetime seizures and presence of abnormal hippocampal radiology (blue, present; white, absent; white with red line, no data) and the laterality (R, right; L, left; B, bilateral) are shown. Cases were divided into those with (teal, n = 12) and without (white, n = 8) seizure history, and the percentage of cases with any hippocampal findings (D), and the most frequently identified left- (E) or right-sided (F) hippocampal findings were evaluated. (G) Frequency of hippocampal radiologic abnormalities with seizures was evaluated. The Fisher exact test was used for statistical analysis of categorical data (D–G).
RESULTS
Demographic and Clinical Characteristics
The demographic and clinical characteristics of the cohort are summarized in Table 1. The series consisted of 14 toddlers (12–36 months), and 6 children, 40–142 months, enrolled over a 5-year period. The median and average ages of cases in this series were 33.3 and 39.4 months, respectively. The age range of our cases overlapped with other larger series, suggesting that these cases are representative of the SUDC population (4, 8). The proportion of females to males was 3–2 (12 girls and 8 boys; Table 1). In total 9 children had a history of simple seizures and 3 had a history of complex febrile seizures (mean 2 seizures, median 2 seizures, range 1–4 seizures). Mean age at first seizure was 18.2 months (median age 16.5 months). Among the 20 cases, 85% died during apparent sleep, 80% were prone, 45% face down, and 30% to the side. Ten children had a recent minor viral-type illness, 1 child had autism and 4 had either speech or developmental delays. All deaths were sudden and unexpected. Autopsy examination revealed cardiomegaly in 2 cases but otherwise showed no significant natural disease. Postmortem 3T-MRI scanning of ex vivo brains revealed subtle hippocampal layer abnormalities for 8 cases (4 bilateral, 2 left hippocampus only, and 2 right hippocampus only). No correlation between imaging and microscopic hippocampal findings was seen (Fig. 2). The development, implementation and results of the ex vivo MRI screening protocol are beyond the scope of this initial report.
TABLE 1.
Patient Demographics and Clinical Characteristics
| Gender | Age (months) | Race | GA (weeks) | Development | Any Sz | Type of Sz | Number of Sz | Age at onset (months) | Longest Seizure (minutes) | Cardiorespiratory History | Terminal Activity | Prone | Face Down | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | F | 17.4 | W | 39.2 | WNL | Yes | Simple | 1 | 14 | 0.5 | Sleep | Yes | Yes | |
| 22 | M | 21.5 | W | 38 | Motor/speech delay, autism spectrum | Yes | Simple | 2 | 9 | 2.5 | Sleep | Yes | ||
| 23 | F | 22 | W | 39.3 | WNL | Yes | Simple | 2 | 16 | 1 | Sleep | Yes | Unknown | |
| 33 | F | 41.8 | W | 41 | WNL | Yes | Simple | 2 | 23 | 1 | Sleep | Yes | Yes | |
| 36 | F | 17.7 | W | 39.3 | WNL | Sleep | Yes | Yes | ||||||
| 37 | M | 39.9 | H/W | 40.2 | Speech delay | Exercise | ||||||||
| 47 | F | 39.9 | W | 36.4 | Speech delay | Yes | Simple and complex | 3 | 24 | 90 | Sleep | Yes | Yes | |
| 48 | F | 34.8 | W | 35 | WNL | Yes | Simple | 1 | 29 | 2 | Frequent URI, Pneumonia | Sleep | Yes | |
| 58 | M | 32.8 | W | 39.4 | WNL | Yes | Simple | 2 | 14 | 3 | Sleep | Yes | Yes | |
| 60 | M | 22.2 | W | 37.5 | WNL | Sleep | Yes | Yes | ||||||
| 74 | F | 24.9 | EA/H/W | 36.1 | Speech delay | Yes | Complex | 2 | 11 | 2 | VSD | Sleep | Yes | |
| 80 | M | 28.7 | W | 40.5 | WNL | Sleep | Yes | Yes | ||||||
| 100 | F | 102 | W | 39.6 | WNL | UNK | Yes | |||||||
| 123 | M | 30.9 | W | 39 | Motor and speech delay, poor visual function | Reactive airway disease, frequent URIs | Sleep | Unknown | ||||||
| 126 | F | 34.5 | W | 40.4 | Motor and speech delay, hypotonic | Yes | Simple | 1 | 17 | 1 | Sleep | Yes | Yes | |
| 128 | F | 33.8 | W | 39.6 | WNL | Yes | Simple and complex | 4 | 13 | 8 | obstructive sleep apnea, reactive airway disease | Rest/unknown | Yes | |
| 129 | M | 44.6 | H/W | 39.3 | WNL | Yes | Simple | 3 | 18 | 2 | Reactive airway disease | Sleep | ||
| 134 | F | 14.2 | W | 38.2 | WNL | Sleep | ||||||||
| 137 | M | 43 | H/W | 39.3 | WNL | Yes | Simple | 1 | 30 | 4 | Sleep | Yes | Yes | |
| 139 | F | 142 | W | 37.2 | WNL | Reactive airway disease, wheezing | Sleep |
F, female; M, male; W, white; H, Hispanic; EA, East Asian; GA, gestational age; WNL, within normal limits; Sz, Seizure; VSD, ventricular septal defect; URI, upper respiratory infection.
Microscopic Changes Were Most Frequently Identified in the Hippocampus
Ten (50%) brains were normal on macroscopic examination, with both pathologists in agreement. For remaining cases neuropathologists did not agree on presence of edema (10 cases); hippocampal asymmetry (5 cases); congestion (4 cases), dusky discoloration of gray matter (2 cases), asymmetry of the cerebellar hemispheres (2 cases); dilation of the fourth ventricle (2 cases), and dysplasia of the corpus callosum, white matter discoloration, punctate hemorrhage, a remote small infarct, white matter cyst, dysplasia of the corpus callosum, and misfolding of the subiculum in 10% of cases. Because prior reports indicated hippocampal pathology in SUDC (7, 14), we sampled both hippocampi in every case (Table 2). Other brain regions were sampled extensively from either the left or the right side in each case. Brain microscopic evaluation revealed the hippocampus had the most frequent microscopic findings, identified in 95% of cases (19/20) by at least 1 neuropathologist (Fig. 1A, B and Table 3). Hippocampal pathology at lateral geniculate nucleus level (see “Materials and Methods”) was concordant in 63% of cases (12/19). When the analysis included anterior and posterior hippocampal sections, all cases had at least 1 hippocampal microscopic finding (Table 3). Other regions with concordant microscopic findings included the temporal and frontal lobe in 1 case, and insula in 1 case, suggesting that the lesions were incidental observations. Beyond this, there was no consistent anatomic distribution of microscopic findings, and concordance between neuropathologists was low (Fig. 1A, B and Table 3).
TABLE 3.
Frequency of Hippocampal Findings
| Hippocampal findings (n = 20) | Concordant | One observation |
|---|---|---|
| Lateral geniculate nucleus (any findings) | 65% | 95% |
| Alternating thickness of dentate gyrus (DG) | 45% | 80% |
| Irregular configuration of DG | 30% | 75% |
| Focal lack of granule cells in DG | 30% | 75% |
| Single or ectopic clusters of neurons in molecular layer or hilus | 15% | 75% |
| Dentate bilamination | 15% | 45% |
| Gliosis | 0% | 25% |
| Abnormal folding/architecture of subiculum | 0% | 20% |
| Vessels surrounded by granule cells in hilus | 0% | 20% |
| Edema of dentate granule cells | 0% | 15% |
| Granule cell bridging between DG convolution | 0% | 5% |
| Thick-walled vessels in hilus/DG | 0% | 5% |
| Red cell change | 0% | 0% |
| Pulvinar (any findings) | 25% | 65% |
| Pes (any findings) | 15% | 60% |
Concordant, observed by 2 neuropathologists; One observation, observed by at least 1 neuropathologist.
Altered Thickness of the DG Was the Most Common Hippocampal Lesion
To identify pathologies potentially associated with SUDC, detailed microscopic examination of the hippocampus was performed (Fig. 2A, B). The most frequent concordant hippocampal lesions at lateral geniculate nucleus level were (i) altered DG thickness (45%, 9/20), (ii) irregular DG configuration (30%, 6/20), (iii) focal loss of DG granule cells (30%, 6/20), and (iv) ectopic neurons (15%, 3/20; Figs. 2A, B, 3, and Table 1). Gliosis was observed in 25% of cases by one of the neuropathologists, although concordance on this finding was low. DG bilamination (i.e. double layering of DG granule cells), was previously proposed as a SUDC biomarker (13). However, concordance for DG bilamination was seen in only 25% of cases with a febrile seizure history (3/12), and in no cases without a febrile seizure history (Fig. 2A–C). Abnormal blood vessels and red neurons, suggestive of acute hypoxic-ischemic injury, were not seen in any case, and no case had hippocampal sclerosis. Hippocampal findings were identified bilaterally in many cases, although the frequency was slightly higher on the left side. We found no significant associations between specific hippocampal abnormalities and patient demographics, including gender and age (Fig. 2C andTable 3). There was a trend for higher incidence of hippocampal pathology in cases with a seizure history (Fig. 2D, 75% vs 37.5%, p = 0.09). However, specific hippocampal pathology, laterality of hippocampal lesions and radiologic abnormalities was not associated with lifetime seizure incidence (Fig. 2E–G).
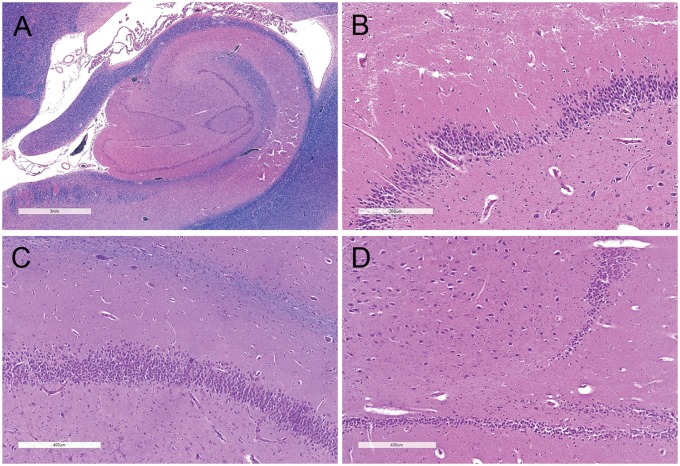
FIGURE 3.
Hippocampus at the level of the lateral geniculate nucleus. Luxol fast blue and hematoxylin and eosin-stained hippocampal sections showing (A) irregular DG configuration with looping (scale bar = 3 mm), (B) altered DG thickness with focal thinning (scale bar = 300 µm), (C) DG dispersion with ectopic neurons, (scale bar = 400 µm), and (D) focal DG granule cell loss (scale bar = 400 µm).
Ancillary Postmortem Studies Identified Cases With Possible Explained Underlying Etiologies
Postmortem respiratory viral cultures were positive for adenovirus in 2 cases, both had a recent viral type infection (Table 1). Rare pneumococcus organisms were found on a Gram stain of cerebrospinal fluid for 1 case, although there was no associated neutrophilic inflammation or leptomeningitis. Toxicologic studies were negative in all cases. Whole exome sequencing of postmortem blood samples identified reportable findings in 3 cases. One patient (No. 37) had cardiomegaly at autopsy (heart weight: 103 g; expected weight: 59–73 g) and heterozygosity for TNNI3 c.574C>T (p.Arg192Cys). A second patient (No. 100) was heterozygous for a pathogenic c.6737C>T (p.Ser2246Leu) variant in the RYR2 gene. This patient had a history of recent streptococcal infection; however, no organisms were detected on postmortem bacterial cultures or viral testing. A third patient (No. 74) had no reportable findings on whole exome sequencing, although microarray analysis revealed de novo duplication on chromosome 16p13.3, de novo terminal deletion on chromosome 4p16.3, de novo deletion on chromosome 1q43, and a maternally inherited deletion on chromosome 13 of uncertain clinical significance. At autopsy this patient had acute viral pneumonitis and a positive respiratory PCR panel for adenovirus and rhinovirus/enterovirus. No genetic abnormalities potentially related to cause of death or positive ancillary test results were identified in the other 17 cases (i.e. unexplained deaths).
Unexplained Cases Are Not Associated With Specific Demographics, Radiology or Hippocampal Pathology
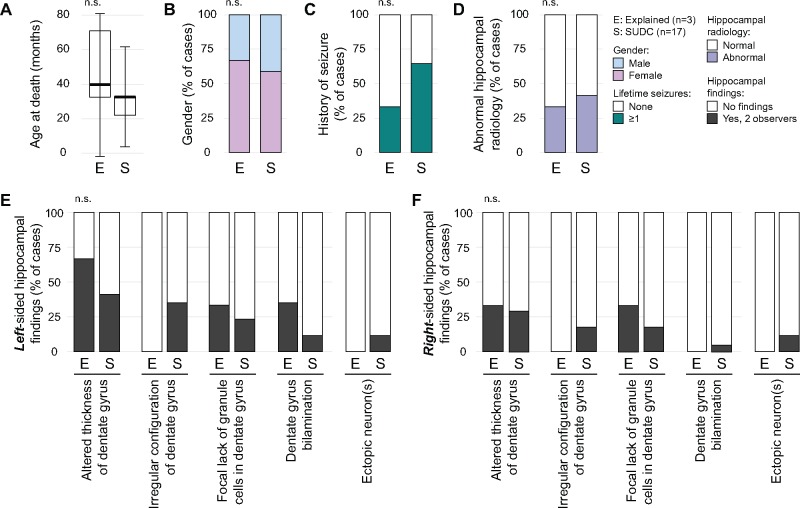
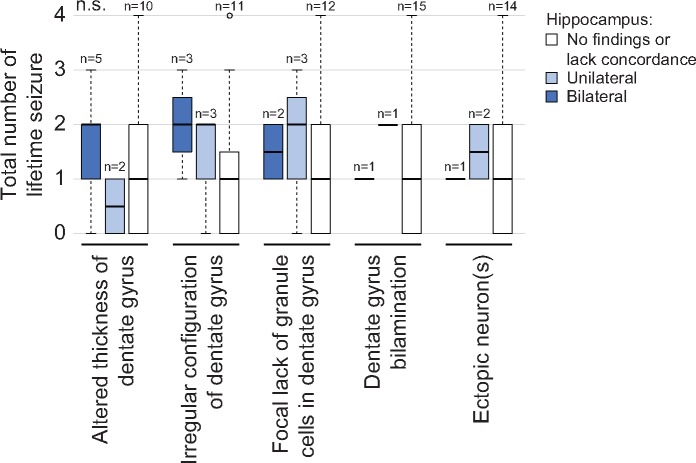
Autopsy findings and ancillary tests allowed cases to be segregated into 2 categories based on whether or not they were possibly explained based on results of molecular testing (n = 3) or remained unexplained (n = 17) deaths. The median age of possibly explained (39.9 ± 40.9 months) and unexplained (32.8 ± 28.8 months) cases was similar (p = 0.51), as was gender (p = 0.46; Fig. 4A, B). A slightly higher percentage of cases with a febrile seizure history was present in the unexplained group compared with the possibly explained group (Fig. 4C; 64.7%, 11/17 vs 33.3%, 1/3; p = 0.29). Hippocampal radiology was similar between groups (Fig. 4D, p = 0.46), as were specific hippocampal pathologies (Fig. 4E–G). Total lifetime seizure number in unexplained cases was not associated with either unilateral or bilateral hippocampal lesions (Fig. 5).
FIGURE 4.
Comparison of patient characteristics and hippocampal pathology between SUDC and explained cases. A comparison of patient characteristics between cases with an explained cause of death (Explained [E], n = 3) and those whose death remained unexplained (SUDC [S], n = 17) is shown. (A) The median age (thick horizontal bar ± SD) at time of death and (B) gender distribution (blue, male; pink, female) are illustrated. (C) History of any seizures (white, 0; teal, ≥1) and (D) hippocampal radiology (white, normal; purple, abnormal) are also depicted. Specific left- (E) and right-sided (F) hippocampal lesions identified by microscopic evaluation were also compared (white, absent; dark gray, present; concordant observations only). Two-tailed t-test and Fisher exact test were used for statistical analysis of numerical (A) and categorical data (B–F), respectively.
FIGURE 5.
Among patients whose cause of death remains unexplained, the presence of hippocampal findings is not associated with frequency of lifetime seizure. Unexplained cases were divided based on absent, unilateral or bilateral observations of specific hippocampal findings most commonly identified in this series (x-axis). The number of lifetime seizures (y-axis) was compared between the groups. Thick horizontal bars represent the median number of seizures and error bars illustrate standard deviations. ANOVA was used for statistical analysis.
DISCUSSION
Our prospective neuropathologic data from 20 SUDC cases revealed that findings in the hippocampal DG are most common, but are not specific for this entity as they were also seen in 3 cases that were later possibly explained by genetic testing. Detailed neuropathologic examinations were performed in each case to identify potential brain lesion(s) that might have caused or contributed to the child’s death. Although studies have suggested a relationship between hippocampal changes of the DG and SUDC, these retrospective studies were limited by inconsistent methodologies, limited samples of DG and other brain regions, and lack of blinding (e.g. some were unblinded, whereas others may have had neuropathologists blindly review slides they had previously seen unblinded) (3, 7, 8, 13, 14). Ours is the first prospective series of unexpected pediatric deaths in which the entire brain was subjected to comprehensive and standardized neuropathologic examination, including postmortem neuroimaging, with bilateral hippocampus evaluated at multiple levels for every case, and independent reviewers who blindly assessed the gross and histologic data.
The lack of national guidelines for sampling brain tissue in forensic pediatric neuropathology contributes to marked differences in how brains are examined between centers. This limits aggregation or comparison of datasets to identify abnormalities and trends. Some medical examiner offices have protocols for pediatric neuropathology, but limited resources, including lack of uniform access to a board-certified neuropathologist, means most forensic pathology offices cannot consistently perform detailed brain examinations (20). We modified the NYU Alzheimer Disease Research Center protocol to sample both hippocampi at multiple levels to identify structural changes. Our comprehensive sampling strategy, familiar to most neuropathologists, captures key brain regions (Table 2). Consistent with previous reports, we identified a high frequency of hippocampal findings that were slightly more frequent in SUDC cases compared with possibly explained cases. Unlike other reports, we did not find DG bilamination significantly associated with SUDC. Notably, 1 patient with DG bilamination had no seizure history, although it is possible that seizures had occurred but were never identified. Our findings question the frequency of DG bilamination in SUDC cases, which is likely affected by both the number of hippocampal samples surveyed as well as specific methods and orientation of samples. Further, how subtle cases of bilamination are read and their specificity for SUDC or seizures remains unproven. There was no significant difference in the incidence of other hippocampal findings between possibly explained and unexplained cases, nor was there any significant left-right asymmetry (Fig. 4). Gliosis was observed in 25% of cases, although concordance between pathologists for this observation was low. Unlike previous series, acute red cell change, indicative of recent acute hypoxic-ischemic damage, was not found in any case (8). Although our sampling protocol was orientated toward detecting hippocampal alterations, we did not identify any specific histopathologic patterns that were associated with SUDC when compared with potentially explained cases.
Approximately one-third of SUDC cases had febrile seizures, which is 10-fold higher than age-based population rates. This history with frequent hippocampal findings has led some investigators to posit that a SUDEP-like mechanism contributes to some of these deaths (5, 13, 14, 17, 21). Kinney et al (13) suggested that DG structural abnormalities (e.g. focal granule cell bilamination) could alter limbic forebrain excitability and cause lethal seizures. Alternatively, hippocampal abnormalities could result from febrile or afebrile seizures, some or all of which may have been unrecognized since ictal features may be subtle in infants and young children, and can cause apnea or life-threatening events without motor phenomena (21, 22). Further, DG granule cell migration occurs in animal models as a consequence of experimentally induced seizures in vivo or in hippocampal slices exposed to chemoconvulsants (i.e. from epileptiform activity, not seizures) (23–25). The inconsistent identification and classification of hippocampal lesions between neuropathologists undermines the association, and even more so the pathogenic relevance, of these findings. Our failure to identify a significant relationship between the frequency or type of hippocampal lesions among patients with and without a history of seizure, or with lifetime seizure frequency, suggests that pathophysiological conclusions should remain tentative (Fig. 2D–G). Although limited by low numbers, similar hippocampal microscopic findings in most of our possibly explained deaths, similar to the San Diego cohort (8), also cast doubt on the significance of these findings. The prevalence of hippocampal microscopic findings in the general pediatric population is not known, although imaging studies suggest minor developmental lesions such as malrotation are common, identified in 23% of MRI scans (26). Discordance between reviewers for some hippocampal microscopic findings in our cohort may reflect a lack of specificity of these findings for distinguishing normal variants from pathological features. Thus, whether these hippocampal microscopic findings are pathologic or form part of a normal variant spectrum remains unknown.
The prevalence of 1 or more febrile seizures in our cohort was 60%, compared with a population prevalence of 2%–5% in the United States up to age 6 years and nearly double that of other SUDC cohorts (5, 7). The high febrile seizure prevalence in our cohort may reflect referral bias as forensic pathologists likely referred SUDC associated with a febrile seizure history to our study since we performed ex vivo MRI and a board-certified neuropathologist was available to examine and report on brain findings. However, we did not find any specific hippocampal pathology associated with a history of seizure.
Molecular analyses identified pathogenic genetic cardiac variants that may have either caused or contributed to death for 3 (15%) of our cases. Two of these cases had single gene variants in cardiac associated genes identified by whole exome sequencing without a febrile seizure history, one of whom had cardiomegaly at autopsy. One patient (No. 37) was heterozygous for a sequence variant in TNNI3 (c.574C>T), a variant reported as likely pathogenic in ClinVar and associated with a protein change (p.Arg192Cys) predicted to be probably deleterious or disease causing based on PolyPhen-2, SIFT, and MutationTaster amino acid substitution prediction analysis (27–30). Mutations in the TNNI3 gene are associated with hypertrophic and restrictive cardiomyopathy (31). The other single gene variant case (No. 100) was heterozygous for a variant (c.6737C>T) in the RYR2 gene, a change predicted to result in an amino acid substitution (p.Ser2246Leu), which is known to be causative of catecholaminergic polymorphic ventricular tachycardia (32, 33). The third case (No. 74) had acute viral pneumonitis positive for adenovirus and rhinovirus/enterovirus, in addition to a complex chromosomal aberrancy. This patient had a history of febrile seizures with a recent viral prodrome. Of the 4 copy number variants detected in this patient, 1 deletion (1q43) incorporated intron 1 of the RYR2 gene. The resolution of the microarray meant it was not possible to exclude a further extension of the deletion into exon 2 of this gene, mutations in which are associated with arrhythmogenic right ventricular dysplasia type 2 and catecholaminergic polymorphic ventricular tachycardia (32, 34). Large sudden cardiac death cohorts have yielded positive molecular diagnostic findings in up to 25% of negative autopsy cases (35, 36). These observations underscore the need for complete and thorough ancillary testing including toxicologic, microbiologic, and molecular studies amongst others to accurately identify a cause and manner of death in those unexpected pediatric deaths where a cause can be ascertained.
Limitations of our study include the small number of cases and very low number of controls. This reflects that in the United States, forensic pathologists often cannot actively engage in referring cases for prospective enrollment for academic research due to legal and other constraints. SUDC is a diagnosis of exclusion and referral of potential cases for prospective analysis may be limited when forensic pathologists need to first exclude other competing forensic diagnoses. Although cases with a definitively explained cause of death would represent optimal age-matched controls, the manner of death in those cases is often unnatural, resulting in limited availability of whole brains as controls for prospective blinded analysis. Assessment of suspected SUDC is complex and challenging and may require a team of multidisciplinary experts to exclude rarer causes, which could also foster better understanding of pathomechanisms and standardized evaluation of these cases. Despite these limitations, this study confirmed the high prevalence of hippocampal findings following standardized and uniform neuropathologic analysis, and the crucial role of ancillary testing to identify a cause of death in some cases.
Conclusion
Although uncommon, SUDC is an important public health condition and a major cause of unexpected death in toddlers that claims 28 000 potential life-years in the United States each year (23). A careful and standardized approach to autopsy, including detailed ancillary studies with multi-disciplinary input, is often required to identify natural deaths that have a potential underlying explanation. Hippocampal findings including alterations of the DG are common in unexplained pediatric deaths, as well as in cases that have a mechanistic explanation. Therefore, the significance of hippocampal findings in SUDC is unclear. However, hippocampal findings may be a result of seizures, a contributor to seizures, an unrelated phenomenon, or a normal anatomic variant in children. Further research is required to understand the relevance of these hippocampal findings in SUDC.
ACKNOWLEDGMENTS
We wish to thank the families, clinicians, and forensic pathologists for their participation.
This work was supported by the SUDC Foundation, Finding A Cure for Epilepsy and Seizures (FACES), The Shaw Family Foundation, and NIH grant P30AG008051.
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Krous HF, Chadwick AE, Crandall L, et al. Sudden unexpected death in childhood: A report of 50 cases. Pediatr Dev Pathol 2005;8:307–19 [DOI] [PubMed] [Google Scholar]
- 2. Haas E. Sudden Unexplained Death in Childhood: An Overview. Adelaide: University of Adelaide Press; 2018 [PubMed] [Google Scholar]
- 3. Kinney HC, Chadwick AE, Crandall LA, et al. Sudden death, febrile seizures, and hippocampal and temporal lobe maldevelopment in toddlers: A new entity. Pediatr Dev Pathol 2009;12:455–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGarvey CM, O'Regan M, Cryan J, et al. Sudden unexplained death in childhood (1-4 years) in Ireland: An epidemiological profile and comparison with SIDS. Arch Dis Child 2012;97:692–7 [DOI] [PubMed] [Google Scholar]
- 5. Hesdorffer DC, Crandall LA, Friedman D, et al. Sudden unexplained death in childhood: A comparison of cases with and without a febrile seizure history. Epilepsia 2015;56:1294–300 [DOI] [PubMed] [Google Scholar]
- 6. Kinney HC, Armstrong DL, Chadwick AE, et al. Sudden death in toddlers associated with developmental abnormalities of the hippocampus: A report of five cases. Pediatr Dev Pathol 2007;10:208–23 [DOI] [PubMed] [Google Scholar]
- 7. Hefti MM, Kinney HC, Cryan JB, et al. Sudden unexpected death in early childhood: General observations in a series of 151 cases: Part 1 of the investigations of the San Diego SUDC Research Project. Forensic Sci Med Pathol 2016;12:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hefti MM, Cryan JB, Haas EA, et al. Hippocampal malformation associated with sudden death in early childhood: A neuropathologic study: Part 2 of the investigations of The San Diego SUDC Research Project. Forensic Sci Med Pathol 2016;12:14–25 [DOI] [PubMed] [Google Scholar]
- 9. Narula N, Tester DJ, Paulmichl A, et al. Post-mortem whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: A case series. Pediatr Cardiol 2015;36:768–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holm IA, Poduri A, Crandall L, et al. Inheritance of febrile seizures in sudden unexplained death in toddlers. Pediatr Neurol 2012;46:235–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halvorsen M, Petrovski S, Shellhaas R, et al. Mosaic mutations in early-onset genetic diseases. Genet Med 2016;18:746–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Gama AM, Walsh CA.. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci 2018;21:1504–14 [DOI] [PubMed] [Google Scholar]
- 13. Kinney HC, Cryan JB, Haynes RL, et al. Dentate gyrus abnormalities in sudden unexplained death in infants: Morphological marker of underlying brain vulnerability. Acta Neuropathol 2015;129:65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinney HC, Poduri AH, Cryan JB, et al. Hippocampal formation maldevelopment and sudden unexpected death across the pediatric age spectrum. J Neuropathol Exp Neurol 2016;75:981–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thorn M. Neuropathologic findings in postmortem studies of sudden death in epilepsy. Epilepsia 1997;38:S32–4 [DOI] [PubMed] [Google Scholar]
- 16. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol 2013;12:966–77 [DOI] [PubMed] [Google Scholar]
- 17. Ackerman MJ, Andrew TA, Baker AM, et al. An Association of hippocampal malformations and sudden death? We need more data. Forensic Sci Med Pathol 2016;12:229–31 [DOI] [PubMed] [Google Scholar]
- 18. Hoch MJ, Bruno MT, Faustin A, et al. 3T MRI whole-brain microscopy discrimination of subcortical anatomy, part 1: Brain stem. AJNR Am J Neuroradiol 2019;40:401–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoch MJ, Bruno MT, Faustin A, et al. 3T MRI whole-brain microscopy discrimination of subcortical anatomy, part 2: Basal forebrain. AJNR Am J Neuroradiol 2019;40:1095–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folkerth RD, Nunez J, Georgievskaya Z, McGuone D.. Neuropathologic examination in sudden unexpected deaths in infancy and childhood: Recommendations for highest diagnostic yield and cost-effectiveness in forensic settings. Acad Forensic Pathol 2017;7:182–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crandall LG, Lee JH, Stainman R, et al. Potential role of febrile seizures and other risk factors associated with sudden deaths in children. JAMA Netw Open 2019;2:e192739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewertson J, Poets CF, Samuels MP, et al. Epileptic seizure-induced hypoxemia in infants with apparent life-threatening events. Pediatrics 1994;94:148–56 [PubMed] [Google Scholar]
- 23. Crandall L, Devinsky O.. Sudden unexplained death in children. Lancet Child Adolesc Health 2017;1:8–9 [DOI] [PubMed] [Google Scholar]
- 24. Heinrich C, Nitta N, Flubacher A, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci 2006;26:4701–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chai X, Munzner G, Zhao S, et al. Epilepsy-induced motility of differentiated neurons. Cereb Cortex 2014;24:2130–40 [DOI] [PubMed] [Google Scholar]
- 26. Cury C, Toro R, Cohen F, et al. Incomplete hippocampal inversion: A comprehensive MRI study of over 2000 subjects. Front Neuroanat 2015;9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng PC, Henikoff S.. Predicting deleterious amino acid substitutions. Genome Res 2001;11:863–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwarz JM, Rodelsperger C, Schuelke M, et al. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010;7:575–6 [DOI] [PubMed] [Google Scholar]
- 29. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landrum MJ, Lee JM, Benson M, et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res 2018;46:D1062–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Wijngaard A, Volders P, Van Tintelen JP, et al. Recurrent and founder mutations in the Netherlands: Cardiac Troponin I (TNNI3) gene mutations as a cause of severe forms of hypertrophic and restrictive cardiomyopathy. Neth Heart J 2011;19:344–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001;103:196–200 [DOI] [PubMed] [Google Scholar]
- 33. Kawamura M, Ohno S, Naiki N, et al. Genetic background of catecholaminergic polymorphic ventricular tachycardia in Japan. Circ J 2013;77:1705–13 [DOI] [PubMed] [Google Scholar]
- 34. Tiso N, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet 2001;10:189–94 [DOI] [PubMed] [Google Scholar]
- 35. Tester DJ, Medeiros-Domingo A, Will ML, et al. Cardiac channel molecular autopsy: Insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 2012;87:524–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bagnall RD, Weintraub RG, Ingles J, et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med 2016;374:2441–52 [DOI] [PubMed] [Google Scholar]