Abstract
Distinct neuronal and glial tau pathologies define corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). Additional Alzheimer disease, TDP-43, and Lewy body copathologies are also common. The interplay of these pathologies with clinical symptoms remains unclear as individuals can present with corticobasal syndrome, frontotemporal dementia, PSP, or atypical Parkinsonism and may have additional secondary impairments. We report clinical, pathological, and genetic interactions in a cohort of CBD and PSP cases. Neurofibrillary tangles and plaques were common. Apolipoprotein E (APOE)ε4 carriers had more plaques while PSP APOEε2 carriers had fewer plaques. TDP-43 copathology was present and age-associated in 14% of PSP, and age-independent in 33% of CBD. Lewy body copathology varied from 9% to 15% and was not age-associated. The primary FTD-Tau burden—a sum of the neuronal, astrocytic and oligodendrocytic tau—was not age-, APOE-, or MAPT-related. In PSP, FTD-Tau, independent of copathology, associated with executive, language, motor, and visuospatial impairments, while PSP with Parkinsonism had a lower FTD-Tau burden, but this was not the case in CBD. Taken together, our results indicate that the primary tauopathy burden is the strongest correlate of clinical PSP, while copathologies are principally determined by age and genetic risk factors.
Keywords: Corticobasal degeneration, FTLD-Tau, Progressive supranuclear palsy
INTRODUCTION
Clinicobasal syndrome (CBS) and Richardson syndrome (RS) are the classic clinical phenotypes of neuropathologically defined corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), respectively. CBS is the clinical phenotype in less than 50% of autopsy-confirmed CBD cases (1). The most recent CBD consensus criteria describe the major CBD clinical phenotypes as CBS, PSP syndrome, frontal behavioral-spatial syndrome, and nonfluent/agrammatic variant of primary progressive aphasia. Similarly, while the RS phenotype is observed in more than half of PSP cases, other clinical phenotypes of PSP include parkinsonism subtype, gait freezing subtypes, corticobasal syndrome, primary nonfluent aphasia or speech and/or language disorders, and behavioral variant frontotemporal dementia (FTD) or predominant frontal presentations (2).
Tau proteins are encoded by the MAPT gene and alternative splicing of MAPT exons gives rise to 6 tau isoforms with either 3R or 4R repeats (3). The tau isoforms in CBD and PSP are primarily comprised tau with 4 microtubule binding repeats (4R) and both diseases are described as 4R tauopathies. Hence, the diagnosis of CBD or PSP depends on the demonstration of histologically distinct 4R-positive neuronal and glial tau pathologies (4, 5). Astrocytic plaques are characteristic for CBD which has also 4R tau-positive tangles (NFTs), oligodendroglial coiled bodies and abundant white matter threads (6). PSP has tufted astrocytes, NFTs and globose tangles, and oligodendroglial coiled bodies (7). In addition to the abundant distribution of diverse tau pathologies, CBD frequently has TDP-43 copathology (8), PSP frequently has Alzheimer disease (AD) and Lewy body (LB) copathologies (9), and both groups have age-related NFTs (10).
The overall diagnostic accuracy for PSP is ∼70% while CBD correlates with CBS less than 40% of the time (11, 12). These discordances in clinicopathological correlation may be due to the clinical heterogeneity, the pathological heterogeneity, or both. In this retrospective analysis, patients with autopsy-confirmed CBD or PSP were re-examined according to the latest consensus criteria. Three CBD clinical subtypes were assigned: CBD-CBS for CBS, CBD-RS for PSP syndrome, and CBD-FTD for frontal behavioral-spatial syndrome, primary aphasia, or dementia cases. Similarly, 4 PSP clinical subtypes were assigned: PSP-RS for the RS phenotype, PSP-CBS for corticobasal syndrome, PSP-FTD for primary nonfluent aphasia, speech and/or other language disorders, frontal behavioral-spatial syndromes or dementia cases, and PSP-P for parkinsonism and gait freezing subtypes. Using histopathology techniques and markers, we assessed the burden of each tau pathology, AD Aβ plaques and NFTs, Lewy bodies and TDP-43 inclusions, and asked which pathologies best associate with the clinical syndromes of each disease. In addition, since multiple clinical phenotypes may exist in 1 individual, we also asked if individual deficits in behavioral, language, memory, motor, executive, and visuospatial domains associate with the underlying pathologies.
MATERIALS AND METHODS
Participants
Demographic, clinical, and neuropathological data were obtained for all autopsy cases with a primary neuropathologic diagnosis of CBD (n = 45) or PSP (n = 108) (Table 1) with available slides for microscope analysis at the Center for Neurodegenerative Disease Research (CNDR) for the period from January 1986 to December 2018 (13). Most patients were clinically evaluated longitudinally and invited to participate in the brain donation program by clinicians at the University of Pennsylvania, although 29 cases were evaluated clinically at the University of California San Francisco. Informed consent for autopsy was obtained in accordance with state laws and protocols approved by the University of Pennsylvania and University of California San Francisco. Using the final clinical diagnosis as the main criteria, CBD-CBS and CBD-RS were assigned for CBS and RS, respectively. CBD manifesting as FTD (CBS-FTD) included all forms of FTD (n = 15) including behavioral variant FTD, primary progressive aphasia, primary nonfluent aphasia, FTD not otherwise specified, AD (n = 3), and dementia with Lewy bodies (DLBs) (n = 1). PSP-RS and PSP-CBS were similarly assigned. PSP-FTD included FTD (n = 21), AD (n = 2), and DLB (n = 1). The PSP-P group included Parkinson disease (PD), PD with dementia, multiple system atrophy, Parkinsonism not otherwise specified, motor neuron disease, and clinically suspected amyotrophic lateral sclerosis.
TABLE 1.
Clinical and Genetic Characteristics
| Group | CBD | PSP |
|---|---|---|
| Demographics | ||
| n | 45 | 108 |
| Male:female ratio | 17:18 | 67:41 |
| Age of onset (year)* | 62 ± 9.4 | 68 ± 7.3 |
| Disease duration (year) | 6 ± 2.4 | 7 ± 3.9 |
| Age at death (year) | 67 ± 9.7 | 76 ± 7.4 |
| Clinical syndrome | ||
| CBS | 35% (16) | 10% (11) |
| FTD† | 42% (19) | 22% (23) |
| Parkinsonism‡ | — | 10% (11) |
| RS | 22% (10) | 58% (63) |
| Genetics | ||
| APOEε2§ | 18% (8) | 19% (21) |
| APOEε4§ | 13% (6) | 19% (21) |
| MAPT h1h1 | 80% (36) | 92% (99) |
| MAPT h1c | ||
| 0 alleles | 29% (13) | 27% (29) |
| 1 alleles | 53% (24) | 42% (45) |
| 2 alleles | 18% (8) | 31% (34) |
Mean ± SD.
FTD includes bvFTD, FTLD-PPA (PNFA), FTLD-PPA (Logopenic) or FTLD-NOS, AD, Dementia with Lewy bodies, or dementia NOS.
Parkinsonism includes PD, PD with dementia, MSA, Parkinsonism NOS, Motor neuron disease, and clinically suspected ALS.
One or more copies.
Copathology
Eighteen regions are routinely examined in a CNDR neuropathology evaluation with each region assigned a semiquantitative score (i.e. 0–3 for none, rare, mild, moderate, or severe) for β-amyloid (Nab228 antibody, CNDR), hyperphosphorylated tau (PHF1 antibody, a gift from Dr Peter Davies), α-synuclein (Syn303 antibody, CNDR), and phosphorylated TDP-43 (1D3 antibody, a gift from Dr Manuela Neumann)-positive pathologies (13). These scores determined the extent of each pathology and were used to categorize cases based on published criteria: Thal phases 0–5 for Aβ plaques; CERAD score (C 0–3) for neuritic plaques; and LB pathology distribution in amygdala only [1], brainstem [1], limbic [2], or neocortical [3] distributions (LB 0–3) (14, 15). Braak stage (0–6) was assessed using GT-38 antibody against AD-specific NFTs by examining the locus coeruleus, entorhinal cortex, hippocampus, and occipital cortex as described (10). TDP-43 pathology was staged for amygdala [1], hippocampal [2], or neocortical [3] involvement (16) as described (17) or for subcortical or brainstem [4] involvement in CBD (8) (TDP 0–4). All regions were stained with all antibodies except for pre-2012 cases where only 4 neocortical regions were screened for Aβ amyloid and, if positive, the remaining 14 regions were stained and assessed, but if the cortical regions were negative, the remaining regions were assigned a score of presumed 0.
Primary FTD-Tau Pathology
Tau scores were assigned for each region on a semiquantitative scale for each type: neuronal, astrocytic, oligodendrocytic, and white matter (Table 2) by examination of PHF1-immunohistochemically stained regions. For each case, 62 individual tau scores were summed to yield a FTD-Tau score.
TABLE 2.
FTD-Tau Pathology Regional Means
| CBD |
PSP |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Astro | Neuron | Oligo | WM | Astro | Neuron | Oligo | WM | ||
| Cortex | Mid fro | 2.6 | 2.0 | 1.0 | 1.8 | 1.3 | 0.8 | 0.6 | 0.7 |
| Motor | 2.6 | 1.8 | 1.6 | 2.1 | 2.0 | 1.1 | 1.1 | 1.1 | |
| Sup temp | 2.1 | 2.0 | 0.8 | 1.5 | 1.0 | 0.8 | 0.5 | 0.5 | |
| Angular | 2.4 | 2.0 | 1.2 | 1.7 | 1.3 | 0.7 | 0.7 | 0.6 | |
| Visual | 1.0 | 0.6 | 0.4 | 0.7 | 0.5 | 0.2 | 0.4 | 0.2 | |
| Ant cing | 2.2 | 2.1 | 1.1 | 1.8 | 1.5 | 1.3 | 0.8 | 0.9 | |
| MTL | Amygdala | 2.5 | 2.5 | 1.3 | — | 1.6 | 1.7 | 0.9 | — |
| Hipp DG | — | 2.5 | — | — | — | 1.0 | — | — | |
| Hipp CA | 2.0 | 2.1 | 0.7 | — | 0.9 | 1.7 | 0.5 | — | |
| EC | 1.8 | 2.0 | 0.9 | — | 0.9 | 1.7 | 0.7 | — | |
| Sub-cortical | Putamen | 2.0 | 1.1 | 1.4 | — | 2.1 | 0.8 | 1.0 | — |
| G pallidus | 1.6 | 1.2 | 1.4 | — | 1.8 | 1.1 | 1.7 | — | |
| Thalamus | 2.1 | 2.0 | 0.9 | — | 1.8 | 1.9 | 2.0 | — | |
| Brainstem | S. Nigra | 1.2 | 2.6 | 1.1 | — | 1.4 | 2.1 | 1.1 | — |
| Midbrain | 1.5 | 1.5 | 1.3 | — | 1.7 | 1.8 | 1.6 | — | |
| LC | 0.5 | 2.3 | 0.9 | — | 0.7 | 2.3 | 0.9 | — | |
| Pons basis | 1.4 | 1.3 | 0.9 | — | 0.6 | 1.9 | 0.9 | — | |
| Medulla | 0.9 | 1.3 | 0.8 | — | 0.9 | 2.0 | 1.1 | — | |
| Cereb | D. nucleus | 1.1 | 1.4 | 0.6 | 0.7 | 1.0 | 1.3 | 1.3 | 1.0 |
Mid fro, mid frontal cortex; Motor, motor cortex; Sup temp, superior temporal cortex; Angular, angular gyrus; Visual, visual sulcus; Ant cing, anterior cingulated gyrus; Hipp DG, hippocampal dentate gyrus; Hipp CA, hippocampal CA1-3 regions; EC, entorhinal cortex; G pallidus, globus pallidus; S. Nigra, substantia nigra; LC, locus coeruleus; D. nucleus, dentate nucleus of the cerebellum. Semi-quantative scale: 0–1.0 (light), 1.1–2.0 (medium), 2.1–3.0 (dark).
Genetics
Genomic DNA was extracted from brain tissue using QIAamp DNA mini kit (Qiagen, Germantown, MD) and genotyped for APOE rs429358 and rs7412, which define the ε2, ε3, and ε4 alleles and MAPT rs1052553 and rs242557 which determine H1/H2 and H1c haplotypes, respectively, using TaqMan allelic discrimination assays (Thermo Fisher, Waltham, MA).
Statistics
All statistical analyses were performed in R version 3.5.0. Demographic characteristics were compared across groups using Pearson χ2 or post hoc t-tests. All statistical tests were 2-sided, with significance levels of ≤0.01 to reduce false-positive findings in lieu of formal multiple testing adjustment due to the exploratory nature of the study (18). Ward’s method was used to cluster cases into high-tau and low-tau groups and comparisons between regional tau scores were performed. Linear regressions with a single predictor were used to assess associations among copathology scores and demographic variables. Scores with fewer than 7 categories (Thal 0–5, LB 0–3, TDP 0–4 for all groups) were treated as ordinal categorical variables in the regression outcome, and proportional odds regression was performed in lieu of linear regression for these variables. To explore effects of genetics on copathology scores, we performed either linear regression (for continuous scores) or proportional odds regression (for ordinal scores), using a separate model for each genetic predictor (APOEε4 carrier, APOEε2carrier, MAPT H1H1 haplotype, MAPT H1c haplotype), and controlling for age at death and sex. Clinicopathological correlations (see Results) were assessed using multinomial regressions with a single pathological or demographic predictor in each model, and outcome variable of CBD categories (with CBD-CBS as reference group) or PSP categories (with PSP-RS as reference group). Finally, clinical deficits were measured as binary outcomes, and associations with copathology scores were analyzed using logistic regression.
RESULTS
Demographics
FTLD-Tau affected individuals are often younger than 65 years old and this is true in our cohort (Table 1). The average age of onset was 62 (44–86 range) in the CBD group and higher at 68 (48–96 range) in the PSP group. More than half the CBD patients (n = 24, 53%) had an onset before 65, but this was not true of the PSP group (n = 30, 28%). Men and women were equally affected consistent with most population-based studies (19). The clinical presentation in each group varied. CBD-CBS was observed in 35% (n = 16), CBD-FTD in 42% (n = 19), and CBD-RS in 22% (n = 10). In the PSP group, PSP-RS was diagnosed in the majority of cases (58%, n = 63), but PSP-FTD was not uncommon (21%, n = 23), while PSP-CBS (10%, n = 11), and PSP-P (10%, n = 11) affected a smaller number of individuals.
The MAPT genomic region produces 2 extended MAPT haplotypes H1 and H2 that influence the risk for disease. The MAPT H1H1 haplotype occurs at a frequency of 60%–70% and genetic studies reveal that a further MAPT regulatory region—H1c—may increase 4R tau containing transcripts in the human brain (20). Genetically, the MAPT H1H1 haplotype associates with the 4R tauopathies and in our cohort 80% of CBD cases and 92% of PSP cases had the MAPT H1H1 haplotype. The MAPT H1c allele was also prevalent in CBD and PSP. Seventy-one percent of CBD and 73% of PSP cases had 1 or more MAPT H1c alleles.
Primary Tau Pathology
Tau pathology was observed in almost all regions of the CBD and PSP groups (Fig. 1; Table 2). We describe here the most frequent distribution of neuronal, astrocytic, and oligodendrocytic tau pathologies for CBD and PSP.
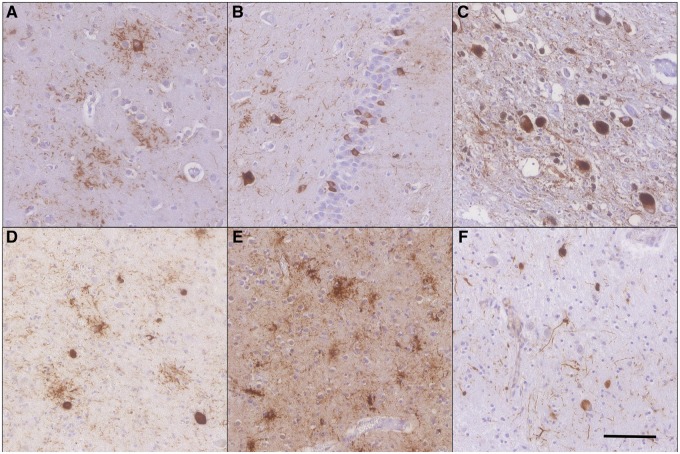
FIGURE 1.
Representative tau pathology in corticobasal degeneration (CBD) (A–C) and progressive supranuclear palsy (PSP) (D–F). Tauopathy in CBD includes neuronal tau and astrocytic plaques in the frontal cortex (A), neuronal inclusions in the granular cells of the hippocampal dentate gyrus (B), and globose tangles affecting the substantia nigra (C). PSP pathology was frequently neuronal inclusions and tufted astrocytes in the thalamus (D); both tufted astrocytes and oligodendrocytic coiled bodies are present in the frontal cortex (E); the astrocytic and neuronal tau pathology affect the dentate nucleus of the cerebellum with oligodendrocytic coiled bodies common in the surrounding white matter (F). Scale bar in (F) is used in all 160× images. All images are PHF1 immunohistochemistry.
In CBD, the characteristic astrocytic plaque was the most prominent pathology in the gray matter while thread-like tau-positive processes and oligodendroglial coiled bodies affected the cortical white matter (Table 2). The moderate to severe astrocytic burden was usually accompanied by a moderate burden of neuronal inclusions resembling small NFTs and a mild burden of oligodendroglial coiled bodies. Cortical regions were consistently affected included the mid-frontal cortex (Fig. 1A), motor cortex, superior temporal cortex, angular gyrus, and anterior cingulate cortex, while the visual cortex was only infrequently affected.
In the medial temporal lobe (MTL), abundant tau deposition occurred in the neurons of the hippocampal granule cells (Fig. 1B), hippocampal CA fields, entorhinal cortex, and the amygdala. Astrocytic plaques and thorn-shaped astrocytes also severely affected the amygdala and nearby MTL regions. Subcortically, the thalamus was affected by a moderate astrocytic and neuronal burden. The tau burden in the basal ganglia was primarily a moderate level of astrocytic plaques while the neuronal tau inclusions were more limited. In the brainstem, the substantia nigra neurons frequently contained large globose tangles accompanied by a milder astrocytic burden of tau pathology (Fig. 1C). Neuronal pathology also accumulated in other brainstem nuclei in the surrounding midbrain and the locus coeruleus of the pons. The cerebellar dentate nucleus and white matter were consistently affected.
PSP is primarily characterized by distinct neuronal, astrocytic, and oligodendrocytic tau pathology including globose and smaller flame-shaped NFTs, tufted astrocytes with ramified processes and oligodendroglia coiled bodies. In the majority of PSP cases, cortical pathology was infrequent and mild although the cases with a higher tau burden frequently had a more moderate burden of tufted astrocytes (Fig. 1E). In cortical regions, only the motor cortex and anterior cingulate consistently displayed moderate tufted astrocytes and mild neuronal tau. In the MTL, neuronal tau inclusions were common although frequently mild, except the amygdala, which harbored a moderate burden of NFTs, tufted astrocytes and thorn-shaped astrocytes. Subcortical white matter involvement was mild. In the cerebellum, tau pathology affected all 3 cell types and the white matter (Fig. 1F).
A FTD-Tau measure was generated by summing all tau scores across all regions. In CBD, FTD-Tau ranged from 55.5 to 117.5 with an average burden of 91.8 (Table 3). In PSP, FTD-Tau ranged from 26.0 to 117.0 with an average burden of 68.1. In CBD, the FTD-Tau burden did not differ with age at death (p > 0.40), but, in PSP, FTD-Tau trended toward being higher at younger ages (Beta −0.56, CI −1.16 to 0.04, p = 0.018). Clustering of the groups by FTD-Tau (see Materials and Methods) revealed that the majority of CBD cases had a similar tau burden throughout the brain. After clustering into high and low FTD-Tau subgroups, only 7 of the 62 individual tau scores were significantly different (p < 0.01). In PSP however, 52 of the regional tau scores differed (p < 0.01).
TABLE 3.
Copathology in CBD and PSP is Primarily Age-Related
| Group | Age | n | Onset | Duration | Age | FTD-Tau* | Braak Stage† (%) | CERAD Plaques (%) | Thal Phase (%) | LB (%) | TDP-43 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CBD | All | 45 | 62.0 | 5.6 | 67.3 | 91.8 | 51 | 33 | 42 | 9 | 33 |
| 70− | 27 | 55.4 | 5.3 | 61.0 | 89.7 | 33 | 19 | 30 | 4 | 30 | |
| 70+ | 18 | 70.7 | 6.1 | 76.8 | 94.9 | 78 | 56 | 61 | 17 | 39 | |
| PSP | All | 108 | 68.3 | 7.1 | 75.5 | 68.1 | 74 | 41 | 63 | 15 | 14 |
| 70− | 21 | 60.2 | 5.2 | 65.4 | 70.2 | 35 | 24 | 43 | 24 | 0 | |
| 70+ | 56 | 67.2 | 7.5 | 74.6 | 70.2 | 77 | 38 | 68 | 11 | 9 | |
| 80+ | 31 | 76.0 | 7.7 | 84.0 | 62.7 | 94 | 58 | 68 | 16 | 32 |
By PHF1 immunohistochemistry.
By GT-38 immunohistochemistry.
Copathology
We staged the AD and LB copathologies for CBD and PSP according to consensus criteria (14, 15) and scored the amount of TDP-43 pathology in each region (Table 3; Supplementary Data Table S1). Logistic regression or proportional odds regression was performed to determine the relationships between each pathology and age (see Materials and Methods).
AD NFTs and Aβ plaques represent common age-related pathologies whose distribution and severity are captured by Braak stage and Thal phase, respectively (9, 21). In the cohorts studied here, 51% of the CBD cases and 74% of the PSP cases had NFTs as determined by GT-38 immunohistochemistry. While prevalent, NFTs were generally limited to the entorhinal cortex and hippocampal CA regions. Braak stages I and II represented 42% of the CBD cases and 49% of the PSP cases. Braak stage significantly associated with age of onset (p = 0.003) but not age at death in CBD (p = 0.011). PSP NFTs significantly associated with both age of onset (p < 0.0001) and age at death (p < 0.0001). Aβ plaques were present in 42% of the CBD cases and 63% of the PSP cases. In most cases, the distribution of plaques was limited to the neocortical and allocortical areas. Thal phases 1–2 represented 26% of the CBD cases and 31% of the PSP cases. Thal phase significantly associated with both age of onset (p = 0.005) and age in CBD (p = 0.004). In PSP, Aβ plaques were common at all ages and did not associate with age of onset (p = 0.081) or age at death (p = 0.018). The prevalence of LB pathology varied from 9% to 15% of the groups, a prevalence consistent with age-related incidental LB disease and not associated in these cohorts (p > 0.01) (22).
TDP-43 pathology was a relatively common copathology in CBD and was not infrequent in PSP (Table 3; Supplementary Data Table S1). In both diseases, TDP-43 lesions included neuronal cytoplasmic inclusions, dystrophic neurites and glial cytoplasmic inclusions, but TDP-43 morphology and distribution were distinct in CBD (8, 23). Overall, TDP-43 pathology was observed in 33% of CBD cases, an amount that did not vary with age at death (p = 0.99). The MTL, primarily the amygdala, was affected in 20% of the cases; 22% had cortical pathology, including astrocytic plaque-like inclusions in the frontal and temporal lobes; the subcortical regions including the basal ganglia and thalamus were consistently affected in 24%; the brainstem had TDP-43 inclusions in 24%, primarily the substantia nigra and midbrain. In PSP, TDP-43 pathology was observed in 14% of cases and significantly associated with age of onset (p = 0.001) and age at death (p < 0.0001). The distribution of TDP-43 pathology in PSP was primarily observed in a limbic predominant age-related TDP-43 encephalopathy (LATE) pattern (16).
Genetic Interactions
APOEε4 allele carriers are at greater risk of developing AD and the ε2 allele has been shown to have a protective effect (24). Recently, APOE has been directly implicated in the spread and presence of both AD NFTs and FTLD-Tau primary tau pathology (25–27). Therefore, we hypothesized that APOE ε4 carriers would be more at risk for AD and an increased FTD-Tau burden, while APOE ε2 carriers would be less likely to have AD pathology and have a lower FTD-Tau burden (Table 4). APOEε4 carriers were more likely to have a higher plaque burden in CBD (p = 0.007) and PSP (p = 0.004), but not a higher NFT burden (CBD p = 0.320; PSP p = 0.165). APOEε2 carriers were not associated with a lower plaque burden in CBD (p = 0.121), but they were in PSP (p = 0.009). FTD-Tau in both CBD and PSP did not differ by APOE allele (all p > 0.12).
TABLE 4.
Pathological Interactions With APOE Alleles
| APOE | Interaction | Group | Beta | 99% CI | SE | p Value |
|---|---|---|---|---|---|---|
| ε2 | Braak stage | CBD | −0.61 | −1.57 - 0.35 | 0.37 | 0.111 |
| PSP | 0.19 | −0.59 - 0.97 | 0.30 | 0.535 | ||
| Thal phase | CBD | −0.79 | −2.08 - 0.50 | 0.50 | 0.121 | |
| PSP | −0.94 | −1.85 - −0.04 | 0.35 | 0.009 | ||
| FTD-Tau | CBD | 8.73 | −5.60 - 23.06 | 5.56 | 0.124 | |
| PSP | −4.19 | −15.46 - 7.08 | 4.38 | 0.341 | ||
| ε4 | Braak stage | CBD | 0.44 | −0.68 - 1.55 | 0.43 | 0.320 |
| PSP | 0.42 | −0.35 - 1.19 | 0.30 | 0.165 | ||
| Thal phase | CBD | 1.54 | 0.15 - 2.92 | 0.54 | 0.007 | |
| PSP | 1.05 | 0.14 - 1.96 | 0.35 | 0.004 | ||
| FTD-Tau | CBD | −6.32 | −22.97 - 10.34 | 6.47 | 0.335 | |
| PSP | 4.81 | −6.58 - 16.20 | 4.42 | 0.279 |
The presence of the MAPT H1 haplotype is associated with CBD and PSP (28) and the MAPT H1c risk haplotype has been implicated in tau levels in primary tauopathies (29). We asked whether either MAPT H1 or H1c associated with an increase in AD NFTs, Aβ plaques, or FTD-Tau (Supplementary Data Table S2). In CBD and PSP, neither MAPT haplotype was related to an increase in AD or FTD-Tau pathology (all p > 0.18).
Pathological Correlations
The pathological correlation between NFTs and Aβ plaques is well documented (30). While NFTs frequently accumulate by age 50, the latter accumulation of Aβ plaques is associated with the deposition of an additional tau burden in the form of neuritic plaques (21, 31). Since NFTs and Aβ plaques were common copathologies in both cohorts (Table 3), we asked if NFTs, plaques and FTD-Tau correlate in CBD and PSP (Table 5). Unsurprisingly, increases in Thal phase correlated with increases in the CERAD neuritic plaque score (p < 0.001) while an increase in Braak stage also related to increases in Thal phase and CERAD neuritic plaque scores (p < 0.013). Surprisingly, increases in Braak stage, Thal phase, or even the CERAD neuritic plaque score were not associated with an increase in FTD-Tau (p > 0.09).
TABLE 5.
Pathological Interactions
| Pathology | Interaction | Group | Beta | 99% CI | SE | p Value |
|---|---|---|---|---|---|---|
| Braak stage | Thal phase | CBD | 0.29 | 0.01–0.57 | 0.11 | 0.010 |
| PSP | 0.28 | 0.07–0.50 | 0.08 | <0.001 | ||
| CERAD plaques | CBD | 0.50 | 0.01–0.98 | 0.19 | 0.011 | |
| PSP | 0.43 | 0.14–0.72 | 0.11 | <0.001 | ||
| Thal phase | CERAD plaques | CBD | 1.45 | 1.09–1.82 | 0.14 | <0.001 |
| PSP | 1.05 | 0.83–1.27 | 0.09 | <0.001 | ||
| FTD-Tau | Braak stage | CBD | −3.31 | −8.33 - 1.71 | 1.95 | 0.097 |
| PSP | 1.47 | −1.92 - 4.86 | 1.32 | 0.267 | ||
| Thal phase | CBD | 0.47 | −3.51 - 4.46 | 1.55 | 0.761 | |
| PSP | 1.71 | −1.29 - 4.71 | 1.17 | 0.145 | ||
| CERAD plaques | CBD | 0.73 | −6.13 - 7.60 | 2.67 | 0.785 | |
| PSP | 1.00 | −3.15 - 5.16 | 1.61 | 0.535 |
Clinicopathological Correlations
Recent studies have highlighted a correlation between the primary clinical diagnosis and the underlying tau pathology or copathology. Specifically, CBD-RS may have more TDP-43 pathology than other clinical subgroups of CBD (23) and PSP-P may have less tau pathology than PSP-RS (32, 33). In our CBD cohort, we asked if the CBD-FTD and CBD-RS groups differed in comparison to the CBD-CBS group in any of the pathological measures. Each group’s FTD-Tau score was similar and no significant difference was observed in any copathology (data not shown; all p values >0.17). In the PSP cohort, we asked if the PSP-CBD, PSP-FTD, and PSP-P groups differed in comparison to the PSP-RS group in any of the pathological measures. Only PSP-P was different from FTD-Tau with a score significantly lower than the typical PSP-RS case (OR 0.90, p = 0.0006). FTD-Tau in PSP-RS was 68.9 ± 16.7, while it was lower in PSP-P (46.4 ± 18.6), but not significantly different in PSP-FTD (71.9 ± 14.8, p = 0.46) or PSP-CBS (76.8 ± 19.7, p = 0.16). Copathologies did not differ between groups (data not shown; all p values >0.08).
Clinical Deficits
While only PSP-P was substantially lower than PSP-RS by FTD-Tau burden and other clinical subgroups were not distinguished pathologically, we hypothesized that there may still be a correlation between individual clinical symptoms and the underlying pathologies (Table 6). Clinical records were available for the majority of patients and reviewed by a neurologist. Symptoms were categorized as behavioral, executive, language, memory, motor, or visuospatial deficits. We assessed if there was a correlation between each deficit and the major pathologies observed in each disease: FTD-Tau and NFTs (Table 6), and the Aβ plaque and TDP-43 copathologies (Supplementary Data Table S3).
TABLE 6.
Clinical Deficit Correlation With Primary and Secondary Tau Burden
| Group | Deficit | % Affected | FTD-Tau |
Braak Stage |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 99% CI | p Value | OR | 99% CI | p Value | |||
| CBD | Behavioral | 46 | 0.98 | 0.92–1.05 | 0.437 | 1.07 | 0.48–2.40 | 0.824 |
| Executive | 83 | 0.95 | 0.86–1.04 | 0.144 | 2.03 | 0.52–7.96 | 0.184 | |
| Language | 77 | 1.02 | 0.95–1.10 | 0.456 | 0.55 | 0.21–1.49 | 0.123 | |
| Memory | 49 | 1.02 | 0.96–1.09 | 0.421 | 1.06 | 0.47–2.36 | 0.859 | |
| Motor | 69 | 1.03 | 0.96–1.11 | 0.284 | 1.07 | 0.45–2.55 | 0.848 | |
| Visuospatial | 63 | 1.00 | 0.93–1.07 | 0.912 | 1.29 | 0.54–3.05 | 0.451 | |
| PSP | Behavioral | 44 | 1.02 | 0.99–1.05 | 0.043 | 0.95 | 0.65–1.39 | 0.724 |
| Executive | 45 | 1.04 | 1.01–1.07 | 0.002 | 0.74 | 0.49–1.12 | 0.060 | |
| Language | 50 | 1.04 | 1.01–1.08 | <0.001 | 0.93 | 0.64–1.36 | 0.638 | |
| Memory | 32 | 1.03 | 1.00–1.06 | 0.020 | 1.03 | 0.69–1.53 | 0.854 | |
| Motor | 66 | 1.05 | 1.01–1.09 | <0.001 | 1.12 | 0.75–1.68 | 0.475 | |
| Visuospatial | 26 | 1.04 | 1.00–1.08 | 0.007 | 0.88 | 0.56–1.38 | 0.458 | |
The majority of CBD patients were affected by executive, language, motor, and visuospatial deficits and many had behavioral and memory impairments as well. In CBD, these deficits did not associate with an increase in either FTD-Tau or Braak stage (all p values >0.12). The significant TDP-43 burden in CBD also did not associate with any deficit (p > 0.22), nor did the Thal phase (p > 0.34) except the Thal phase trended toward significance with visuospatial deficits (p = 0.04).
The majority of PSP patients had motor and language impairments, while behavioral, executive, memorial, and visuospatial deficits were less common. In PSP, FTD-Tau associated with executive (p = 0.002), language (p < 0.001), motor (p < 0.001), and visuospatial (p = 0.007) impairments. Behavior (p = 0.04) and memory (p = 0.02) impairments also trended toward being significantly associated with the primary tau burden in PSP. None of these impairments associated with Braak stage (p > 0.06) or Thal phase (p > 0.20). Similarly, TDP-43 copathology did not associate with any deficit (all p values >0.11) except motor impairments trended toward significance (p = 0.05).
DISCUSSION
Both CBD and PSP are rare disorders with complicated clinical symptoms, pathology, and genetics. The primary FTD-Tau pathology we observed in PSP did not distinguish the clinicopathological groups of PSP-RS, PSP-CBS, and PSP-FTD, but was lower in PSP-P and PSP patients with high FTD-Tau were more likely to have multiple clinical deficits. In CBD, on the other hand, FTD-Tau pathology was more abundant and less likely to vary between clinical groups.
In general, the abundant and variable copathologies we report also did not associate with clinical symptoms. Instead, the copathologies are primarily age-associated or linked to APOE allele status. AD neuropathologic change is the most prevalent copathology in CBD and is especially common in PSP. Importantly, the plaque burden in FTLD-Tau did not associate with an increase in primary FTD-Tau pathology. Instead, the AD-related NFTs and plaque measures correlated with each other implying that the AD pathology—both NFTs and plaques—are independent but co-occurring events in CBD and PSP. TDP-43 pathology is also a common copathology and, in PSP, is age-related and may represent LATE. In CBD, the TDP-43 inclusions and neurites are not age-related and are distinct from the TDP-43 pathology that occurs in FTLD-TDP or LATE (5, 8, 16). Other copathologies did not associate with any clinical diagnosis or specific deficit.
The primary tau burden in CBD and PSP is composed of multiple tau pathologies, but it remains a challenge to describe the progression of tau inclusions in each of these diseases. While there is still no consensus for the staging of FTD-Tau in CBD, our data is consistent with astrocytic plaques developing first in cortical areas with neuronal tau pathology developing later (Supplementary DataFig. S1) consistent with previous work (34). The earliest PSP pathology is speculated to be NFTs in the substantia nigra before tufted astrocytes develop (35). In our PSP cohort, subcortical and brainstem tau may deposit earlier before cortical tau NFTs and tufted astrocytes accumulate. In neurodegenerative diseases in general, clinical deficits are thought to be associated with neuronal dysfunction with the contribution of the glial tau burden unclear (36). Nonetheless, in CBD and PSP, the progressive clinical deficits may be the consequence of a tight interplay among neuronal, astrocytic, oligodendroglial, and axonal pathologies that differentially propagate through the diseased human brain. Clearly, further staging efforts are needed.
If FTD-Tau is suspected as the underlying pathology, the heterogeneous mix of clinical symptoms, pathologies and copathologies, age and genetics may nonetheless be of some value in identifying the primary tau pathology associated with each disease (Table 7). In our cohort, several features may help distinguish the CBD-CBS from PSP-CBS. Compared to CBD-CBS, PSP-CBS cases are more likely to have an intermediate or high level of AD neuropathological change (69% vs 31%). Genetically, the presence of 2 MAPT H1c alleles associated with PSP-CBS than CBD-CBS (64% vs 19%). Intriguingly, several features were common in the CBS groups. CBS affected women more than men regardless of the underlying pathology with similar sex ratios reported in other CBS cohorts including AD-CBS (37). CBS patients were more likely to have an APOEε2 haplotype (13% and 23%, for CBD-CBS and PSP-CBS, respectively, compared to a population average of 6%) (38).
TABLE 7.
Clinicopathological Groups
| Clinical |
CBS |
FTD |
RS |
P |
|||
|---|---|---|---|---|---|---|---|
| FTLD-Tau | CBD | PSP | CBD | PSP | CBD | PSP | PSP |
| Demographics | |||||||
| n | 16 | 11 | 19 | 23 | 10 | 63 | 11 |
| % Male | 31 | 38 | 37 | 91 | 50 | 52 | 82 |
| Age of onset | 67 | 71 | 61 | 69 | 58 | 68 | 68 |
| Duration | 5.6 | 6.1 | 6.4 | 6.0 | 4.4 | 7.0 | 10.7 |
| Age at death | 71 | 77 | 67 | 75 | 62 | 75 | 80 |
| Pathology | |||||||
| FTD-Tau | 86.9 | 74.0 | 95.5 | 71.9 | 92.5 | 68.9 | 46.4 |
| NFT (%) | 63 | 85 | 53 | 74 | 30 | 73 | 64 |
| AB (%) | 44 | 77 | 47 | 65 | 30 | 59 | 73 |
| LB (%) | 6 | 15 | 11 | 22 | 10 | 11 | 18 |
| TDP-43 (%) | 31 | 8 | 26 | 17 | 50 | 10 | 36 |
| Deficits (%) | |||||||
| Behavioral | 18 | 36 | 53 | 59 | 67 | 48 | 9 |
| Executive | 91 | 64 | 73 | 68 | 89 | 41 | 9 |
| Language | 64 | 64 | 100 | 68 | 56 | 52 | 0 |
| Memory | 45 | 27 | 47 | 50 | 56 | 28 | 18 |
| Motor | 91 | 91 | 33 | 59 | 100 | 72 | 27 |
| Visuospatial | 64 | 36 | 60 | 27 | 67 | 30 | 0 |
| Genetics (%) | |||||||
| ApoE e2 | 13 | 23 | 26 | 9 | 10 | 21 | 27 |
| ApoE e4 | 19 | 15 | 16 | 22 | 0 | 19 | 18 |
| MAPT H1H1 | 81 | 77 | 84 | 91 | 70 | 95 | 82 |
| MAPT H1c 2 alleles | 19 | 64 | 16 | 35 | 20 | 29 | 18 |
Clinical features and demographics more than pathology may best distinguish CBD-FTD from PSP-FTD. CBD-FTD was likely in men (37%) but was strongly associated with men (91%) in PSP-FTD. A later age of onset was also more likely in the PSP-FTD group. Two variants of FTD are recognized in CBD: frontal behavioral-spatial syndrome or nonfluent/agrammatic variant of primary progressive aphasia (1). Language deficits affect all CBD-FTD cases, but not necessarily all PSP-FTD (68%) cases. Similarly, visuospatial deficits may also be more common in CBD-FTD (60%) compared to PSP-FTD (27%).
The classic clinical features of PSP are described as RS and PSP-RS was common in our cohort, but RS also associated with CBD. PSP-RS generally occurs a decade later in life than CBD-RS (68 vs 58) and may have a longer disease duration (7.0 vs 4.4 years). In terms of copathology, PSP-RS is more likely to have an intermediate or high level of AD neuropathologic change compared to CBD-RS (48%–10%) while CBD-RS has a higher incidence of TDP-43 pathology (50% vs 10%). While not statistically significant in our cohort, this connection between CBD-RS and TDP-43 pathology is in agreement with larger studies (23). Executive impairments may also be more common in CBD-RS, than in PSP-RS (89% vs 41%).
PSP cases also presented with atypical Parkinsonism. Overall, the burden of FTD-Tau was lower in PSP-P than in PSP-RS, consistent with previous studies (32, 33). PSP cases with a higher FTD-Tau score were more likely to have additional executive, language, motor, and visuospatial deficits. Similarly, PSP-P cases had fewer additional clinical deficits than the PSP-CBD, PSP-FTD, and PSP-RS cases.
Understanding the interplay between the progression of each disease’s morphologically distinct tau pathologies, variable copathologies, genetic, and age-related risk factors and the progression of clinical symptoms remains a long-term goal. The analysis presented here represents a small step along the way to a better treatment for all patients affected by these diseases.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank the additional contributions of Theresa Schuck and Catherine Herrman.
This work was supported by NIH grants AG053036, AG10124, AG17586, AG000255 and the Wyncote Foundation.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 2017;32:853–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee VM, Goedert M, Trojanowski JQ.. Neurodegenerative tauopathies. Annu Rev Neurosci 2001;24:1121–59 [DOI] [PubMed] [Google Scholar]
- 4. Dickson DW, Kouri N, Murray ME, et al. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-Tau). J Mol Neurosci 2011;45:384–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol 2015;129:469–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickson DW, Bergeron C, Chin SS, et al. Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002;61:935–46 [DOI] [PubMed] [Google Scholar]
- 7. Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–9 [DOI] [PubMed] [Google Scholar]
- 8. Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008;67:555–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain J Neurol 2018;141:2181–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibbons GS, Kim S-J, Robinson JL, et al. Detection of Alzheimer’s disease (AD) specific tau pathology with conformation-selective anti-tau monoclonal antibody in co-morbid frontotemporal lobar degeneration-tau (FTLD-Tau). Acta Neuropathol Commun 2019;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: Clinicopathological correlations. Ann Neurol 2006;59:952–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain J Neurol 2017;140:3329–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement 2014;10:477–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain J Neurol 2019;142:1503–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 2014;127:441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bender R, Lange S.. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001;54:343–9 [DOI] [PubMed] [Google Scholar]
- 19. Onyike CU, Diehl-Schmid J.. The epidemiology of frontotemporal dementia. Int Rev Psychiatry Abingdon Engl 2013;25:130–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kouri N, Ross OA, Dombroski B, et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 2015;6:7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braak H, Thal DR, Ghebremedhin E, et al. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70:960–9 [DOI] [PubMed] [Google Scholar]
- 22. Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson’s disease. Ann Neurol 2012;71:258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koga S, Kouri N, Walton RL, et al. Corticobasal degeneration with TDP-43 pathology presenting with progressive supranuclear palsy syndrome: A distinct clinicopathologic subtype. Acta Neuropathol 2018;136:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol 2017;74:1178–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toledo JB, Da X, Weiner MW, et al. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol 2014;127:621–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017;549:523–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao N, Liu C-C, Van Ingelgom AJ, et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat Commun 2018;9:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokoyama JS, Karch CM, Fan CC, et al. Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol 2017;133:825–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myers AJ, Pittman AM, Zhao AS, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis 2007;25:561–70 [DOI] [PubMed] [Google Scholar]
- 30. Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011;121:571–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He Z, Guo JL, McBride JD, et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med 2018;24:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams DR, Holton JL, Strand C, et al. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain J Neurol 2007;130:1566–76 [DOI] [PubMed] [Google Scholar]
- 33. Jellinger KA. Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis 2008;5:339–46 [DOI] [PubMed] [Google Scholar]
- 34. Ling H, Kovacs GG, Vonsattel JPG, et al. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain J Neurol 2016;139:3237–52 [DOI] [PubMed] [Google Scholar]
- 35. Nogami A, Yamazaki M, Saito Y, et al. Early stage of progressive supranuclear palsy: A neuropathological study of 324 consecutive autopsy cases. J Nippon Med Sch 2015;82:266–73 [DOI] [PubMed] [Google Scholar]
- 36. Kovacs GG, Xie SX, Robinson JL, et al. Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun 2018;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hassan A, Whitwell JL, Josephs KA.. The corticobasal syndrome-Alzheimer’s disease conundrum. Expert Rev Neurother 2011;11:1569–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eisenberg DTA, Kuzawa CW, Hayes MG.. Worldwide allele frequencies of the human apolipoprotein E gene: Climate, local adaptations, and evolutionary history. Am J Phys Anthropol 2010;143:100–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.