Abstract
Radiation therapy to treat cancer has evolved significantly since the discovery of x-rays. Yet, radiation therapy still has room for improvement in reducing side effects and improving control of cancer. Safer and more effective delivery of radiation has led us to novel techniques and use of biomaterials. Biomaterials in combination with radiation and chemotherapy have started to appear in pre-clinical explorations and clinical applications, with many more on the horizon. Biomaterials have revolutionized the field of diagnostic imaging, and now are being cultivated into the field of theranostics, combination therapy, and tissue protection. This review summarizes recent development of biomaterials in radiation therapy in several application areas.
Keywords: Biomaterials, Theranostics, Nanoparticles, Radiation therapy, Self-assembly
Graphical abstract
1. Introduction
Radiation therapy (RT), one of the main cancer treatment modalities, has evolved significantly since it was initially discovered through refinement in radiation localization and image-guided technologies [1]. Despite modern improvements in RT, there are still significant limitations to its use. Radiation utilizes ionizing energy delivered through external machines or internal radioisotopes to disrupt double-stranded DNA, inducing apoptosis [2]. This is not without complications as the damage to DNA is non-discriminatory to healthy and malignant tissue alike. Acute and chronic toxicities have been well documented in many systems (head and neck, anal, base of skull tumors, etc.) [3], which has led to limitation in radiation dosage and thus its therapeutic effects. Radiation resistance can also develop in some tumors, aiding to incomplete response or recurrence [4]. Thus, new breakthroughs are critical to improve efficacy and safety of radiation therapy. One potential area of development is using biomaterials in RT to combine the effects of RT with other treatment modalities and to improve efficacy of RT itself.
Biomaterials include natural or synthetic materials traditionally used in medical devices, but use of biomaterials has recently expanded to more diverse medical applications [5,6]. Biomaterials have been widely used for targeted delivery of therapeutics and local sustained delivery in preclinical and clinical research due to their unique chemical, physical and biological properties [7]. Biomaterials have been a focus of contemporary research and clinical applications for various medical purposes, such as angiogenesis [8], tissue regeneration [9] and drug delivery [10,11]. Cancer diagnosis and therapy have also benefitted immensely from the use of biomaterials leading to a better understanding of cancer biology [6], enhanced imaging techniques [12], and catapulted delivery of therapeutics such as chemotherapy [13] and immunotherapy [6]. As drug delivery vehicles, nano-scale biomaterials have the advantage of prolonged blood circulation time, enabling them to target tumor vasculature more effectively due to the enhanced permeability and retention (EPR) effect in solid tumors where vasculature exhibits different flow dyanmics and architecture compared to normal vasculature [14].
As such, nanomaterials can be utilized in RT for the delivery of radioisotope, radiosensitizer, chemoradiotherapy, and imaging agents. In addition to systemic delivery with nanomaterials, polymer hydrogels and other in situ scaffolds can support sustained local delivery of therapeutics, increasing local drug concentration while reducing systemic side effects [15,16].
Currently in clinical radiation oncology, biomaterials are mostly limited in use as removable radioactive implants for image-guided radiation therapy, markers and spacers in brachytherapy. The purpose of this review is to examine various biomaterials that have been developed and tested in clinical and preclinical stages and discuss future directions currently being investigated. We focus on several main applications of biomaterials in 1) aiding delivery of radioisotopes, 2) enhancing imaging techniques for accurate tumor localization, 3) used in combination chemo-radiotherapeutics, and 4) potential use as protective and healing agents.
2. Targeted radiation therapy through systemic delivery
2.1. Alpha particles
Instead of traditional radio waves used for RT, recent development of alpha particles in targeted delivery have resulted in several clinical studies in leukemia, prostate cancer [17], and more recently advanced solid tumors [18]. Alpha emitter elements such as 223radium offer the potential of targeted therapy when conjugated with suitable antibodies or small molecules that target cancer tissues/cells. Broadly speaking, ionizing radiation can be classified as alpha, beta, or gamma rays. Gamma rays are massless, made entirely of photons, and can travel through most materials. Beta rays are made mostly of electrons, but affect tissue in a similar manner, albeit a little less penetrative. Alpha particles comparatively, are made of neutrons and protons, which make it difficult for them to penetrate tissue. Alpha particles are the least ionizing in terms of radiation in an environmental sense. However, they can have higher potency and specificity locally, which is ideal for targeted therapy. Their poor penetration limits their range of treatment, approximately 50–90 μM, reducing the unwanted detrimental effects to healthy tissue. In addition, their linear transfer of energy is much higher (>20 times) than beta or gamma rays [19]. These combined properties preclude their use in EBRT (external beam radiation therapy), but make it an ideal choice for local radiotherapy. The two main disadvantages of alpha particles are their delivery and limited availability [20].
Even with the limited range of alpha particles, excessive radiation exposure has the potential for severe unwanted effects. As such, the International Commission on Radiological Protection (ICRP) has developed guidelines for the protection from radiation exposure which focus mainly on developing radiation monitoring, health surveillance and managing contaminated commodities [21]. One way to circumvent this delivery limitation is through use of antibodies that are specific to a cancer. Antibodies, that have been approved for use in immunotherapy, can be tagged with alpha particles in what is sometimes referred to as immunoradiotherapy [22]. This in particular has applications in hematological malignancies such as Non-Hodgkin's Lymphoma, which has a number of known monoclonal antibodies that can be used to target it [[23], [24], [25], [26], [27], [28]]. However, solid organ tumors are more difficult to target, as they do not have as well-developed target receptors and related antibodies. Future research is still needed in order to overcome this particular hurdle.
2.2. Peptide receptor therapy
Peptide receptor radionuclide therapy, such as somatostatin, is another emerging field with potential utility in imaging and therapeutics [29]. This peptide hormone has been used for the treatment of neuroendocrine tumors [30]. Specifically, peptide receptor radionuclide therapy is comprised of a peptide that targets the tumor, a pharmacokinetic modifier, bifunctional chelating agent that binds both moieties, and a radionuclide. Some of the main limitations are related to the short half-life of the peptides and sequestration in organs rich in macrophages, such as the liver, spleen, and kidneys [29]. There are some potential solutions, which will be expanded below. Additionally, other studies have looked into treatment with radioisotopes tagged to ibritumomab tiuxetan, a monoclonal antibody, in refractory follicular lymphoma to standard of care [[31], [32], [33], [34], [35]]. Wiseman et al. showed in a phase I/II trial safe and potential use for such therapies in patients with lymphomas unresponsive [36]. These promising results warrant future studies in the field.
2.3. Nanoparticles
Attempts have been made in using biomaterials with tumor-seeking properties to deliver radioisotopes to tumor cells. Due to their unique pharmacological properties, liposomes and polymers have been investigated for potential use in targeted delivery of radioisotopes and radiosensitizers in radioisotope therapy. Nanoparticles have also been studied in that regard [37]. In vitro testing has shown some promise with liposomal 124-Iodine radiotherapy injected intravenously. In these studies, liposomal preparations not only had longer blood circulation time, but also had less sequestration in the spleen, kidneys and liver [38]. Other nanoparticles have been implemented in specific protein deliveries tagged with specific tumor inhibitors, such as raltitrexed, to attack specific targeted tumor cells. They demonstrated increased uptake using molecules such as hyaluronic acid on the peptide. This type of specific drug delivery has been shown to stunt tumor growth [39]. Nanoparticles can also be combined with lipid molecules to increase the penetration into the blood-brain barrier. This might prove beneficial in targeting cells in difficult drug delivery locations, such as brain metastasis [40].
Additionally, the biodistribution of nanoparticles and its subsequent biodegradability are important parameters to evaluate practicality of nanoparticle delivery. For high Z-number metal and chelate nanoparticles, the percentage of possible sequestration in the liver and kidneys (which can be irradiated with up to 30–35 Gy) and kidneys (15–17 Gy) must be taken into consideration. Heavy metal related acute side effects are partly due to mimicking of other metal ions that are essential for homeostasis [41]. And as they are elements, they cannot be broken down and excreted through physiological means. So, they are frequently engulfed by macrophages and sequestered in various organs such as the liver, causing progressive accumulation and organ failure [42].
2.4. Reactive oxygen species
Reactive oxygen species (ROS) are a potent cytotoxic component for eliminating tumor cells, but hypoxic tumor conditions sometimes pose difficulties to generating ROS. Prasad et al., however, were able to replenish oxygen into the tumor environment by using MnO2-bound albumin nanoparticles (A–MnO2) [43]. When the MnO2 reacts with ROS such as H2O2, it produces O2 and can increase vascular saturated O2 levels by 45%. The difficulty here is that ultraviolet or visible light-based photosensitizers, that are needed to activate ROS, may not penetrate deeply enough into the desired tissues. Photodynamic therapy has been used for specific conditions that can be exposed to light waves, for e.g. Barrett's Esophagus [44] or skin cancer [45]. These agents stay attached to cancer cells longer than normal cells [[46], [47], [48], [49], [50], [51]]. Each family of light-based photosensitizers (porphyrins, chlorophylls, and dyes) requires a specific wavelength to be activated [52]. First, the patient is injected with a photosensitizing agent. Then, the required wavelength light is then exposed to these cells, for e.g. through an endoscope or through laser therapy on the skin. However, exposure to the light is location dependent and is not always possible for all types of malignancies. There have been some advances in semiconductor particles that can convert X-ray energy into ultraviolet or visible light energy, which would be sufficient to activate the photosensitizers [43]. In time, these advances may provide another way to attack difficult to treat malignancies such as cholangiocarcinoma or advanced pancreatic cancer, but that still remains to be seen.
2.5. Metal-organic frameworks
Metal-organic frameworks (MOFs) are formed from metal ions and organic linkers. Such particles might have potential beneficial uses in gas storage, separations, catalysis and chemical sensing [53]. MOFs might also have a potential to improve radiation therapy effect by enabling radiodynamic therapy [54], as they forego the need of visible light reaching a tumor [55]. Alternatively, photodynamic therapy can be used in conjunction with radiodynamic therapy, reducing the exposure to radiation and its dose related side effects, as shown by several groups [56,57]. Similar models have been tested in animals and showed promising results. A 2019 study by Ma et al. used a Quercetin and Zirconium based MOF with the hope of enhancing radiation effects in mice models. The Quercetin was used as a radiosensitizer, and the Zirconium was used as a carbonic anhydrase inhibitor to reduce hypoxic induced resistance. The following combination showed an enhanced effect of radiation in animal models, while maintaining minimal toxicities [58]. Furthermore, combining low dose radiation therapy with MOFs might also help potentiate the effects of immunotherapy. Specifically, a Hafnium (Hf) based MOF was developed as a radiosensitizer in mice [54]. The combination of Hf-MOF with programmed cell death inhibitors – a class of immunotherapy drugs with extensive promising clinical results – has shown extension of the local effects of radiation therapy to distant tumors, without increasing any radiation toxicity.
2.6. Liposomes
Another therapy with much anticipated optimism is liposomal delivery. Liposomes are a phospholipid bilayer around an aqueous fluid (Fig. 1) that can be designed to transport a variety of drugs, molecules, and/or peptides. Their versatile use in nearly every type of drug delivery has been well investigated [59]. This will be discussed further in the section “Combined therapies.” Liposomes have been extensively utilized for radiosensitizer deliveries (Fig. 4). Targeted delivery of radioisotopes can potentially increase radiation efficacy at the tumor site and reduce toxicity to normal tissue. Delivery of radiosensitizers can help overcome radiation resistance, which is a common cause of radiation therapy failure [60]. The main challenges to overcome with liposomal delivery are related to achieving maximum drug release at the site of the tumor and minimizing systemic injury. One way to counter this is using the EPR effect, as mentioned previously. The EPR effect is characterized by larger intercellular openings between endothelial cells in tumor vessel wall. This could be hundreds of nanometers in diameter in tumor endothelial cells, compared to normal endothelial gap junctions which are usually less than 10 nm [61]. Thus, theoretically, nanoparticles or liposomal could be designed to be larger than the intercellular cleft, which would let them migrate specifically into tumor vasculature with ease while being denied entry through normal endothelial tight junctions.
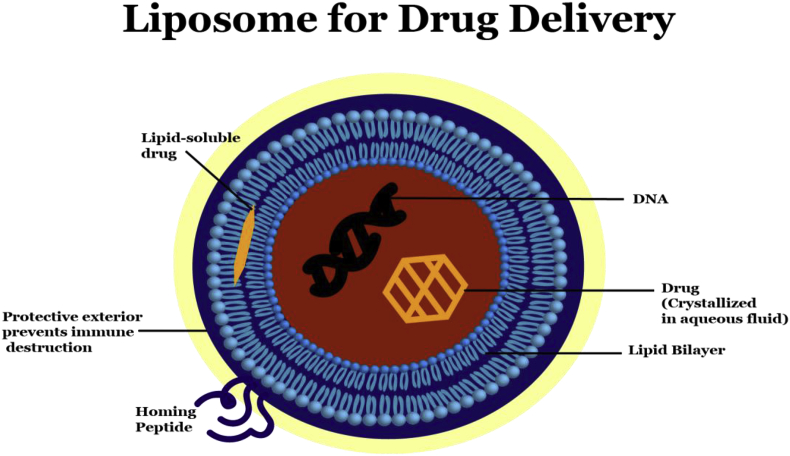
Fig. 1.
A Liposome structure is demonstrated below. A homing peptide acts like a chemoattractant for the liposome moiety. The liposome can interact with this homing peptide and enter the cell cytoplasm, where the intended carload is released/activated. Liposome can be used for delivery of drug or other therapeutics, including DNA, mRNA, peptides etc.
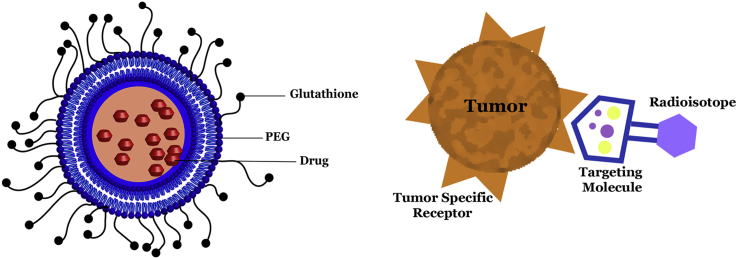
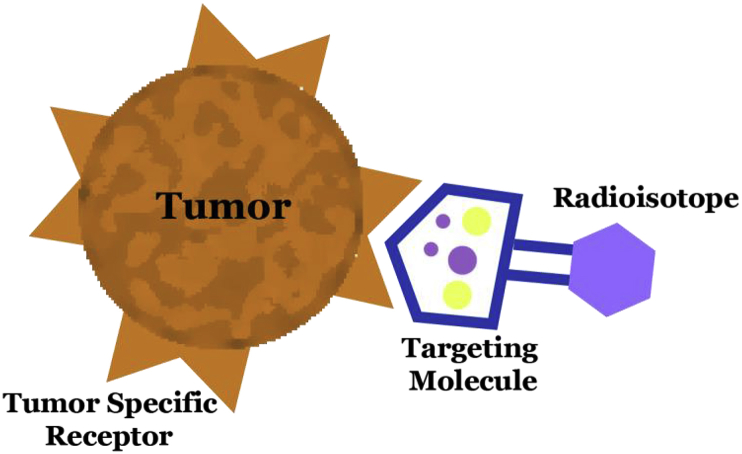
Fig. 4.
Radioisotope delivery to tumor cells using a targeting molecule. Targeting molecule preferentially binds to tumor specific receptors on tumor cells, thereby delivering radioisotope with high specificity and lower toxicity to normal tissue.
Adding to this intrigue, Besse et al. investigated another interesting utility of liposomes with thermosensitivity, which are triggered by a set temperature to have a fast release and lead to a stronger radiosensitizer effect [62]. Their results showed that tumor response was greater when treated with radiation and heat after injection of thermosensitive liposomes. Thermodox® is a liposomal formulation that is currently in Phase 3 trials with FDA approval under the OPTIMA trial. Previously, Thermodox® had been investigated in the HEAT trial (NCT00617981) with mixed results. The drug failed to reach its primary outcome of progression free survival of 33% in inoperable hepatocellular carcinoma (HCC), but a subgroup of patients who underwent at least 45 min of radiofrequency ablation had a marked improvement in progression free survival [63]. The OPTIMA trial is now looking to investigate this subgroup of patients in a randomized fashion to delineate the role of thermosensitive radiotherapy.
3. Tumor imaging
Within the area of tumor imaging, radionucleotide scans are a mainstay in detection and surveillance of malignancies. Positron emission tomography (PET) uses radionuclide 18Fluorine in fluorodeoxyglucose (FDG), which is a glucose analogue. Its uptake has been benchmarked in normal healthy tissue, and thus an abnormal uptake is related to pathophysiological states. 68Gallium dotatate has also been similarly used for detection of neuroendocrine tumors. Other forms of tumor imaging and advances in imaging technology have greatly improved the clinical use of radiotherapy. This is demonstrated by technologies such as stereotactic radiosurgery that deliver localized radiation with extremely high precision, especially to tumors that are difficult to surgically resect [64]. To further improve accuracy of tumor imaging, researchers have investigated biomaterials such as liposomes, metal/polymeric nanoparticles, and hydrogel fiducial markers [65]. A fiducial is a marker placed at the area of measurement or treatment to provide a point of reference. Liposomes (Fig. 1) and nanoparticles have been implicated in imaging advances for this reason. Liposomes, as discussed above, have been used extensively in delivering active molecules to their site of action [66]. Additionally, metal nanoparticles are solid colloidal particles with varying sizes, which can be utilized to improve drug delivery efficiency and specificity, and thereby to reduce drug resistance [67]. Hydrogel fiducial markers are a novel FDA approved radio-opaque absorbable particles, with a high water and iodine content. They are used to better visualize tumors or normal tissues on radiological imaging (whether on Computed Tomography, Magnetic Resonance Imaging or Ultrasound) [68]. While traditional fiducial markers were made of inert metals, more novel fiducials offer advantages like decreased image artifacts, decreased migration and the ability to create 3D structures with varying sizes [69]. Advances in tumor imaging are perhaps the best example of the evolving role of biomaterials, and we expect this will only continue to improve with time.
3.1. Liposomes
Many new applications in clinical testing utilize liposomes for topical and intravenous delivery of pharmaceutics in various conditions, such as cancer therapy, viral vaccines, photodynamic therapy, analgesics and anti-fungals [65,70]. Thus, intravenous injections of liposomal-based therapies have been used in the management of ovarian cancer, multiple myeloma and Kaposi sarcoma [[71], [72], [73]]. Versatility in chemical functionalization of liposomes offers the advantage of engineering liposomes to specifically target cancer cells and increase visibility of the malignant lesion in radionuclide imaging. Liposome-mediated tumor imaging was first tested in patients thirty years ago, but high uptake of liposomes in the liver and spleen created concerns for toxicity and halted further clinical testing. Yet, liposomal technologies have been used for tumor imaging in patients suspected to have carcinoma, melanoma, sarcoma or lymphoma. Currently, a new generation of liposomes as targeted delivery systems for imaging agents is still under pre-clinical research [74].
3.2. Composite nanoparticles
Composite nanoparticles are nanoparticles made with multiple materials rather than a single one [75]. As examples, a combination of silica/inorganic, silica/polymer or polymer/inorganic can be used [76]. There are several advantages to this technique such as but not limited to improved solubility, decreased toxicity with lower loading dose requirements, enhanced optical clarity, and overall increased functionalization [[75], [76], [77]]. The use of contrast agents, such as paramagnetic complexes (Ga3+ or Mn2+ based chelates), paramagnetic ion nanoparticles (Gd2O3 and MnO), and superparamagnetic iron oxide nanoparticles (Fe3O4, FeCO, and MnFe2O4) has greatly improved the diagnostic abilities of MRI and CT [78]. Copper sulfide (CuS) nanoparticles have also contributed greatly to photoacoustic imaging, MRI and other combinatorial therapy due to its low toxicity and cost [79]. They can participate in a wide variety of procedures, including chemophototherapy, immunotherapy, and photo-radiotherapy.
To take this further, incorporating nanoparticles in imaging has been shown to improve radiation localization and tumor-free survival rates in preclinical animal models [80,81]. Jolck et al. experimented with gold-nanoparticles in a carbohydrate-based gelation matrix as an intravenous liquid fiducial to radiologically locate a mast cell tumor in a canine model [80]. Hainfeld et al. injected similar gold-nanoparticles for localization of malignant gliomas in mice, which showed good uptake in the tumor, but did not cross the physiologic blood-brain barrier [81]. This is likely due to the altered permeability near the tumor, which allows its unimpeded growth. The downside to the use of nanoparticles is not well delineated, but one potential concern is the over-accumulation of toxic foreign metal nanoparticles that can potentially cause damage to human organs. Historically, similar studies in rats have shown pulmonary emphysema, interstitial hyperemia and lung inflammation with abnormally high levels of localized neutrophils, lymphocytes and eosinophils [82]. However, no studies have been performed comparing the in vivo macrophage processing and clearance between liposomal formulations and metallic nanoparticles or other nanoparticle formulations. This is an area that will require further investigation as biomaterials in radiation continue to develop.
Metallic nanoparticles containing high-Z elements (e.g. gadolinium, bismuth, and gold) also have been proven to augment the efficacy of radiotherapy due to their interactions with low-energy photons, and are used as positive contrast agents in MRI and radiosensitizers as mentioned earlier [83]. Duk et al. have shown that 2 Gy radiation treatment with Gadolinium-based nanoparticles, AGuIX® (Activation and Guidance of Irradiation by X-ray), increased the period of uptake and therefore treatment timeframe, allowing higher treatment efficacy [84]. In addition, gadolinium nanoparticles produced superior results compared to their conventionally used molecular chelate form due to their higher longitudinal relaxivity and longer lifespan in blood circulation [84,85]. Au nanoparticles have also supported multiple imaging modalities including MRI, Raman spectroscopy, photoacoustic imaging, and CT scans. They have been extensively used in photothermal therapy, in which they are structured into nanocages that transport therapeutic agents in response to exogenously applied laser light [86].
Finally, nanoparticles are also used as radiotracers. The following allows nanoparticles to have the potential to be used in conjunction with liposomal radiation therapy to enhance imaging accuracy. In particular, 64Cu nanoparticles can conjugate to liposomes as narrow as 100-nm and accumulate in malignant tumor cells that have enhanced retention and vascular permeability [87]. Compared to the current gold standard 18F-FDG, which can only be used to detect tumors of 1 cm, these nanoparticles can amass in much smaller volumes. While further research and examination is warranted, the reduced lower-limit of tumor sizes that the nanoparticles can detect makes them an attractive alternate to standard radiotracers for PET scans [87]. Nanoparticles as radiotracers also have the potential to be used in liposomal radiation therapy. Cu nanoparticles can conjugate to liposomes as narrow as 100-nm and accumulate in malignant tumor cells that have enhanced retention and vascular permeability [87]. Previously described liposomal 124-Iodine conjugated complex is also another example of theranostics [38]. Pending further research and examination, it could be a potential replacement for PET scans as the current gold standard, which (18F-FDG) can only be used to detect tumors of 1 cm3 or more [87].
4. Combination therapies
Synchronous chemoradiotherapy started gaining traction more than 20 years ago, especially in squamous cell cancers [88]. Similar tumors were of particular interest as they were surgically unresectable, which made chemotherapy and radiation therapy the only options. Since then, there have been multiple studies indicating the benefit in providing combination treatments [89]. This synergistic effect is hypothesized to be due to the chemotherapeutic radiosensitization of the tumor [90]. However, multiple studies also showed increased toxicity from the combination of both treatment modalities [91]. For these reasons, novel techniques are necessary to maximize the available benefits of synchronous chemoradiotherapy, while minimizing the complications. This is where biomaterials may present yet another utility.
4.1. Liposomes
As it is apparent, liposomes have broad utility as delivery vehicles. Besides tumor imaging, liposomal materials have been used to deliver chemotherapy in combined treatments to various oncological targets. Their use in multiple fields of pharmacotherapeutics is due to their potential in lowering toxicity [63]. The earliest liposomal chemotherapy, liposomal doxorubicin, has been approved to treat cancer of the ovary or breast, myeloma and Human Immunodeficiency Virus/Acquired Immune deficiency Syndrome-related Kaposi's sarcoma (Fig. 2) [92]. Currently a number of liposomal chemotherapies have FDA approval and under study including Doxil® (liposomal doxorubicin), DaunoXome® (daunorubicin), DepoCyt® (cytarabine), Myocet® (doxorubicin citrate), Lipo-dox® (generic doxorubicin hydrochloride), Marqibo® (vincristine) etc, [63]. Chemoradiotherapy combinations with liposomal doxorubicin and RT were tested, in the hope of improving response of high-risk tumors after traditional chemoradiotherapy [93]. However, past attempts in preclinical and clinical studies have been complicated by a combined toxicity of co-delivery of liposomal chemotherapeutics and radiation. Currently, liposomal biomaterials are being studied in combination therapies specifically targeting radiation-resistant tumors, such as hypoxic prostate tumors studied in xenograft animal models [94]. Liposomal biomaterials are increasingly used in the clinical setting, with an expected growing market, as more generic versions may be approved soon.
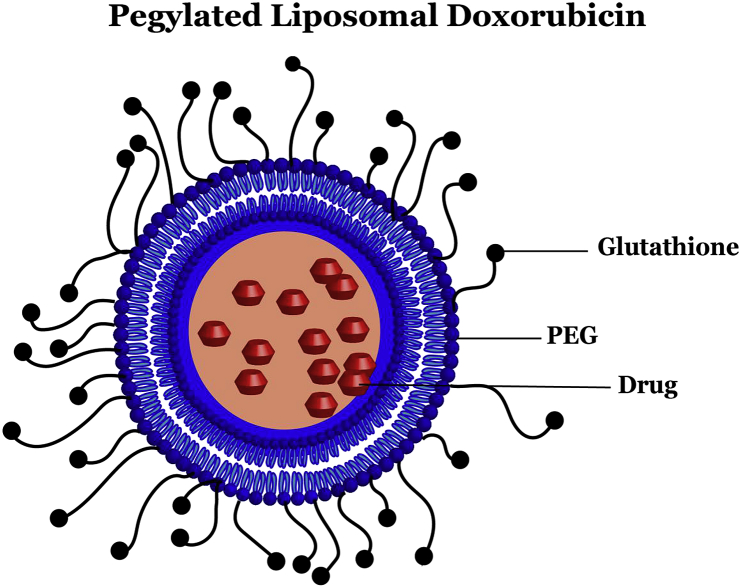
Fig. 2.
Schematic outlining Doxorubicin within a Pegylated Liposome. Glutathione facilitates delivery of the liposome complex through various cell junctions, including blood-brain barrier. Pegylated liposomes stably encapsulate doxorubicin, enabling extended release, passive accumulation in tumors, and decreased delivery to cardiac tissue due to size.
4.2. Polymeric nanoparticles
Other biomaterials, such as polymer-based nanoparticles, allow more complicated architecture of biomaterial design and represent a new class of biomaterials entering clinical trials [95]. Furthermore, polymeric nanoparticles are similar to liposomes, but differ in key aspects. They are also lipid-based, but they vary according to the function they are designed for. Structurally, they are prepared as a solid lipid nanomaterial or with a liquid core. The necessary drug can be attached on the outside or can be housed inside the nanoparticle. In contrast, in liposomes (as seen in Fig. 2), the aqueous core houses the drug surrounded by a phospholipid bilayer. Additionally, polymeric nanoparticles have theoretical advantages in clinical medicine when compared to liposomes, but have yet to be proven. Their greatest advantage lies in our ability to alter their design, allowing researchers to customize specific materials for the required task. This ability helps tailor the drug's pharmacodynamic and pharmacokinetic profile by changing the polymeric nanoparticle profile, resulting in more effective targeting of organ or tumor [96]. They also have a reliable profile in terms of degradation or metabolization, many of which can bypass hepatic metabolization and even the reticuloendothelial systems. However, liposomes have been in use much longer and are approved by the FDA for multiple drug formulations, currently conferring them an advantage for clinical use. Poly(l-glutamic acid)-paclitaxel, which can be functionalized to prolong circulation time, showed promising results in phase I/II trials but was halted at phase III due to lack of efficacy [97].
Other systems of nanoparticle delivery of other chemotherapeutics with adjuvant radiotherapy are currently being tested under clinical trials. Sanoff et al. provided their phase 1b and 2 results identifying a nanoparticle cyclodextrin-based polymer conjugated to campothecin that demonstrated initial success in safe dosing, limiting side effect profile [98]. Results from such clinical trials may provide us with answers regarding optimizing dosing, minimizing side effects, and lastly prove their efficacy against the current standard of treatment. While the toxicities associated with the use of combined biomaterials-mediated chemoradiation can significantly limit the use of biomaterials in drug delivery, recent studies have suggested a synergistic effect between biomaterial-delivered chemotherapeutics and radiation, which can be leveraged to reduce toxicity. Drugs such as cisplatin, doxorubicin, and paclitaxel have been shown to have radio-sensitizing effects [37]. Therefore, delivery of chemotherapeutics can be combined with delivery of radiosensitizers and/or radioisotopes through co-conjugation with the same nanoparticles, increasing the efficacy of the radioisotopes, reducing dosage, and thus decreasing the potential toxicities of chemotherapy and radiation agents.
In addition to systemic delivery, polymers can be used as a local co-delivery platform for sustained release of a multitude of therapeutic agents. For example, polymers have been used to release gold nanoparticles in preclinical studies for CT-guided RT, photothermal therapy, and theranostics. Mukherjee et al. describe yet another polymer based material that combines the best of liposomes and nanoparticles [63]. Lipid-polymer hybrid nanoparticles (LPHNP) consist of a polymer core around the active agent, contained in a liquid core, with an outer lipid layer. This allows for multiple potential uses such as combination chemoradiotherapy, siRNA vector, Au-conjugated MRI imaging, folate, etc. The advantage lies in their ability to deliver multiple agents at once, and can even be used for targeted therapy if conjugated with folate or monoclonal antibodies. Simple organic carbon-based compounds may also be used in conjunction with near infrared radiation to cause photothermal damage as the molecules absorb the light [99]. The ability to cause photothermal damage in a short duration with high localization capability of the carbon based drug carriers makes this combination therapy of radiation and biomaterials potentially superior to standard practices [100].
Polymers such as polysaccharide hydrogels can be used to deliver immunostimulatory radioisotopes, combining immunotherapy and radiation therapy [101]. Polymers can be functionalized with cytotoxic and radiosensitizer moieties [102]. Biomaterials have been used to deliver therapeutics that may have limited bioavailability and distribution in the body alone. One application of this technology is the use of biomaterials to deliver siRNA to a defined tissue area to overcome radiation resistance [103]. Other methods are emerging, such as ultrasound [104], thermal ablation trigger [105], X-ray trigger, and near infrared laser control drug delivery, but yet to transition into their incipient clinical phases. Mechanistically, through thermal and mechanical effects after ultrasound administration, it would allow targeted drug release from thermosensitive liposomes [106]. Additionally, radiation from light, neutrons, ultrasound and X-rays can be used to control drug delivery through biomaterials including liposomes and other nanoparticles [107]. Radiation-guided drug delivery could play an active role in nanoparticle drug delivery [108], inducing receptor expression via radiation in tumor blood vessels that are then recognized by peptides conjugated on nanoparticles [109].
5. Tissue healing and tissue protection
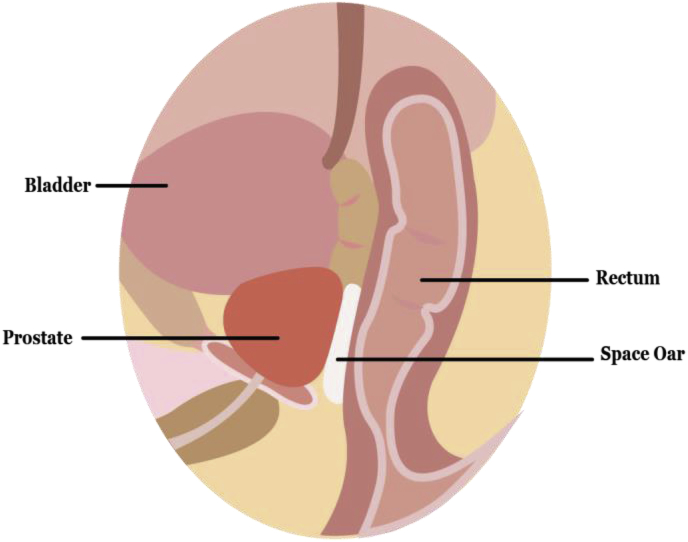
RT can produce serious side effects on normal tissue due to the high cytotoxicity of radiation. Skin reaction is a common side-effect caused by RT to various cancers [110,111]. Biomaterials in general have been studied for tissue healing after RT [112]. Specifically, polymers can be used to protect normal tissue [113] and promote skin healing [114]. For example, RT-induced oral mucositis is a side effect especially common in patients treated for head-and-neck cancer and can be treated with polymer oral rinse, which accelerates healing by forming a protective layer over the wounds [115]. Such applications in tissue healing and protection can improve quality of life among cancer patients and expand use of radiation in frail patients susceptible to side effects. RT can produce serious side effects on normal tissue due to its high cytotoxicity. In addition to tissue healing, polymers have also been used for tissue protection during RT (Fig. 3). For example, SpaceOAR®, a hydrogel prostate-rectum spacer, has been tested clinically and approved by FDA for rectal radioprotection during radiotherapy targeting prostate [116]. Polymer hydrogels are injectable through minimally invasive procedures and can be integrated into clinical care during radiation treatment, a promising area for further development.
Fig. 3.
Biomaterials such as a hydrogel spacer is shown placed between the prostate and rectum to reduce brachytherapy or external beam radiation therapy related radiation proctitis. Spacer is injected in situ with formation of hydrogel upon delivery, forming a temporary protection to rectum during radiation therapy.
6. Challenges in translation of biomaterials into RT
There are still many hurdles to cross for biomaterials to be integrated into effective therapies. As with most therapies, it takes years, if not decades, for proper evaluation and application. As we stand, there are many theoretical benefits supported by clinical data presented above. The toxicity of the biomaterials alone is minimal, but may be altered when combined with a drug. Addition of yet another drug or radioisotope can further change the pharmacokinetic profile. This adds a layer of complexity that has perhaps not yet been encountered in modern medicine. With this level of complexity, in vivo interactions may not be easily comprehended. It is obviously extremely important to maintain a level of caution, but also to remain optimistic as we uncover the potential of novel biomaterials.
Acknowledgements
This work was supported by NIH R15 EY029504 to VAK.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Bernier J., Hall E.J., Giaccia A. Radiation oncology: a century of achievements. Nat. Rev. Canc. 2004;4:737. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 2.Hill R., Healy B., Holloway L., Kuncic Z., Thwaites D., Baldock C. Advances in kilovoltage x-ray beam dosimetry. Phys. Med. Biol. 2014;59:R183. doi: 10.1088/0031-9155/59/6/R183. [DOI] [PubMed] [Google Scholar]
- 3.Harrington C.B., Hansen J.A., Moskowitz M., Todd B.L., Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors—a systematic review. Int. J. Psychiatr. Med. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 4.Pajonk F., Vlashi E., McBride W.H. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cell. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Health Science education - biomaterials. Natl. Inst. Biomed. Imag. Bioeng. September 2017 https://www.nibib.nih.gov/science-education/science-topics/biomaterials [Google Scholar]
- 6.Gu L., Mooney D.J. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat. Rev. Canc. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langer R., Peppas N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. [Google Scholar]
- 8.Petrak K., Vissapragada R., Shi S., Siddiqui Z., Kim K.K., Sarkar B., Kumar V.A. Challenges in translating from bench to bed-side: pro-angiogenic peptides for ischemia treatment. Mol. Basel Switz. 2019;24 doi: 10.3390/molecules24071219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V.A., Taylor N.L., Shi S., Wang B.K., Jalan A.A., Kang M.K., Wickremasinghe N.C., Hartgerink J.D. Highly angiogenic peptide nanofibers. ACS Nano. 2015;9:860–868. doi: 10.1021/nn506544b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Nguyen P.K., Cabral H.J., Diez-Barroso R., Derry P.J., Kanahara S.M., Kumar V.A. Development of peptide inhibitors of HIV transmission. Bioact. Mater. 2016;1:109–121. doi: 10.1016/j.bioactmat.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V.A., Shi S., Wang B.K., Li I.-C., Jalan A.A., Sarkar B., Wickremasinghe N.C., Hartgerink J.D. Drug-triggered and cross-linked self-assembling nanofibrous hydrogels. J. Am. Chem. Soc. 2015;137:4823–4830. doi: 10.1021/jacs.5b01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo S., Zhang E., Su Y., Cheng T., Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C. A targeted approach to cancer imaging and therapy. Nat. Mater. 2014;13:110. doi: 10.1038/nmat3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maya S., Sarmento B., Nair A., Rejinold N.S., Nair S.V., Jayakumar R. Smart stimuli sensitive nanogels in cancer drug delivery and imaging: a review. Curr. Pharmaceut. Des. 2013;19:7203–7218. doi: 10.2174/138161281941131219124142. [DOI] [PubMed] [Google Scholar]
- 16.Methachan B., Thanapprapasr K. Polymer-based materials in cancer treatment: from therapeutic carrier and ultrasound contrast agent to theranostic applications. Ultrasound Med. Biol. 2017;43:69–82. doi: 10.1016/j.ultrasmedbio.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Sartor O.A., Maalouf B.N., Hauck C.R., Macklis R.M. Targeted use of alpha particles: current status in cancer therapeutics. 2012. [DOI]
- 18.Fusion pharma announces first patient dosing in a phase 1 clinical trial of [225Ac]-FPI-1434 injection in patients with advanced solid tumors. https://www.biospace.com/article/fusion-pharma-announces-first-patient-dosing-in-a-phase-1-clinical-trial-of-225ac-fpi-1434-injection-in-patients-with-advanced-solid-tumors/ BioSpace. (n.d.) (accessed April 9, 2019)
- 19.Miller R.C., Marino S.A., Brenner D.J., Martin S.G., Richards M., Randers-Pehrson G., Hall E.J. The biological effectiveness of radon-progeny alpha particles. II. Oncogenic transformation as a function of linear energy transfer. Radiat. Res. 1995;142:54–60. [PubMed] [Google Scholar]
- 20.Elgqvist J., Frost S., Pouget J.-P., Albertsson P. The potential and hurdles of targeted alpha therapy – clinical trials and beyond. Front. Oncol. 2014;3 doi: 10.3389/fonc.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentin J. Elsevier International Commission on Radiological Protection; 2008. The 2007 Recommendations of the International Commission on Radiological Protection. [Google Scholar]
- 22.Sharkey R.M., Goldenberg D.M. Cancer radioimmunotherapy. Immunotherapy. 2011;3:349–370. doi: 10.2217/imt.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller R.A., Maloney D.G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N. Engl. J. Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 24.Davis T.A., White C.A., Grillo-López A.J., Velásquez W.S., Link B., Maloney D.G., Dillman R.O., Williams M.E., Mohrbacher A., Weaver R., Dowden S., Levy R. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: results of a phase II trial of rituximab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999;17:1851–1857. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 25.DeNardo G.L., DeNardo S.J., Goldstein D.S., Kroger L.A., Lamborn K.R., Levy N.B., McGahan J.P., Salako Q., Shen S., Lewis J.P. Maximum-tolerated dose, toxicity, and efficacy of (131)I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998;16:3246–3256. doi: 10.1200/JCO.1998.16.10.3246. [DOI] [PubMed] [Google Scholar]
- 26.Witzig T.E., Gordon L.I., Cabanillas F., Czuczman M.S., Emmanouilides C., Joyce R., Pohlman B.L., Bartlett N.L., Wiseman G.A., Padre N., Grillo-López A.J., Multani P., White C.A. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 27.Horning S.J., Younes A., Jain V., Kroll S., Lucas J., Podoloff D., Goris M. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:712–719. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 28.Hagenbeek A., Gadeberg O., Johnson P., Pedersen L.M., Walewski J., Hellmann A., Link B.K., Robak T., Wojtukiewicz M., Pfreundschuh M., Kneba M., Engert A., Sonneveld P., Flensburg M., Petersen J., Losic N., Radford J. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111:5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 29.Dash A., Chakraborty S., Pillai M.R.A., Knapp F.F.R. Peptide receptor radionuclide therapy: an overview. Canc. Biother. Radiopharm. 2015;30:47–71. doi: 10.1089/cbr.2014.1741. [DOI] [PubMed] [Google Scholar]
- 30.Bodei L., Ferone D., Grana C.M., Cremonesi M., Signore A., Dierckx R.A., Paganelli G. Peptide receptor therapies in neuroendocrine tumors. J. Endocrinol. Invest. 2009;32:360–369. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 31.Gordon L.I., Molina A., Witzig T., Emmanouilides C., Raubtischek A., Darif M., Schilder R.J., Wiseman G., White C.A. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 32.Witzig T.E., Molina A., Gordon L.I., Emmanouilides C., Schilder R.J., Flinn I.W., Darif M., Macklis R., Vo K., Wiseman G.A. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 33.Morschhauser F., Radford J., Van Hoof A., Botto B., Rohatiner A.Z.S., Salles G., Soubeyran P., Tilly H., Bischof-Delaloye A., van Putten W.L.J., Kylstra J.W., Hagenbeek A. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:1977–1983. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 34.Berinstein N.L., Pennell N.M., Weerasinghe R., Buckstein R., Piliotis E., Imrie K.R., Chodirker L., Cussen M.-A., Miles E., Reis M.D., Ghorab Z., Cheung M.C. Management of newly diagnosed high-risk and intermediate-risk follicular lymphoma with 90 Y ibritumomab tiuxetan in a phase II study. Hematol. Oncol. 2018 doi: 10.1002/hon.2513. [DOI] [PubMed] [Google Scholar]
- 35.Provencio M., Franco F., Gómez-Codina J., Quero Blanco C., Llanos M., Garcia-Arroyo F., de la Cruz L., Gumá J., Delgado J.R., Álvarez R., Chacón J.I., Royuela A., Rueda A. Consolidation treatment with yttrium-90 ibritumomab tiuxetan after new induction regimen in advanced stage follicular lymphoma: update results from the Spanish Lymphoma Oncology Group trial after a median follow-up of 8.5-years. Leuk. Lymphoma. 2019;60:856–859. doi: 10.1080/10428194.2018.1509322. [DOI] [PubMed] [Google Scholar]
- 36.Wiseman G.A., White C.A., Stabin M., Dunn W.L., Erwin W., Dahlbom M., Raubitschek A., Karvelis K., Schultheiss T., Witzig T.E. Phase I/II 90 Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin’s lymphoma. Eur. J. Nucl. Med. 2000;27:766–777. doi: 10.1007/s002590000276. [DOI] [PubMed] [Google Scholar]
- 37.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Engudar G., Schaarup-Jensen H., Fliedner F.P., Hansen A.E., Kempen P., Jølck R.I., Kjæer A., Andresen T.L., Clausen M.H., Jensen A.I., Henriksen J.R. Remote loading of liposomes with a 124I-radioiodinated compound and their in vivo evaluation by PET/CT in a murine tumor model. Theranostics. 2018;8:5828–5841. doi: 10.7150/thno.26706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosch J.G., Landry M.R., Thomas C.R., Sun C. Enhancing chemoradiation of colorectal cancer through targeted delivery of raltitrexed by hyaluronic acid coated nanoparticles. Nanoscale. 2019;11:13947–13960. doi: 10.1039/c9nr04320a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T., Lip H., He C., Cai P., Wang Z., Henderson J.T., Rauth A.M., Wu X.Y. Multitargeted nanoparticles deliver synergistic drugs across the blood-brain barrier to brain metastases of triple negative breast cancer cells and tumor-associated macrophages. Adv. Healthc. Mater. 2019 doi: 10.1002/adhm.201900543. e1900543. [DOI] [PubMed] [Google Scholar]
- 41.Jan A.T., Azam M., Siddiqui K., Ali A., Choi I., Mohd Q., Haq R. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015;16:29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad P., Gordijo C.R., Abbasi A.Z., Maeda A., Ip A., Rauth A.M., DaCosta R.S., Wu X.Y. Multifunctional albumin-MnO₂ nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano. 2014;8:3202–3212. doi: 10.1021/nn405773r. [DOI] [PubMed] [Google Scholar]
- 44.Qumseya B.J., David W., Wolfsen H.C. Photodynamic therapy for Barrett's esophagus and esophageal carcinoma. Clin. Endosc. 2013;46:30. doi: 10.5946/ce.2013.46.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton C., Szeimies R., Basset‐Seguin N., Calzavara‐Pinton P., Gilaberte Y., Hædersdal M., Hofbauer G., Hunger R., Karrer S., Piaserico S. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications–actinic keratoses, Bowen's disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019;33:2225–2238. doi: 10.1111/jdv.16017. [DOI] [PubMed] [Google Scholar]
- 46.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Canc. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 47.Wilson B.C. Photodynamic therapy for cancer: principles. Chin. J. Gastroenterol. Hepatol. 2002;16:393–396. doi: 10.1155/2002/743109. [DOI] [PubMed] [Google Scholar]
- 48.Vrouenraets M.B., Visser G., Snow G.B. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23:505–522. [PubMed] [Google Scholar]
- 49.Gudgin E.D., Goyan R., Pottier R. New directions in photodynamic therapy. Cell. Mol. Biol. Noisy--Gd. Fr. 2002;48:939–954. [PubMed] [Google Scholar]
- 50.Capella M.A.M., Capella L.S. A light in multidrug resistance: photodynamic treatment of multidrug-resistant tumors. J. Biomed. Sci. 2003;10:361–366. doi: 10.1007/BF02256427. [DOI] [PubMed] [Google Scholar]
- 51.Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J., Peng Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allison R.R., Downie G.H., Cuenca R., Hu X.-H., Childs C.J., Sibata C.H. Photosensitizers in clinical PDT, photodiagnosis photodyn. Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 53.Yuan S., Feng L., Wang K., Pang J., Bosch M., Lollar C., Sun Y., Qin J., Yang X., Zhang P. Stable metal–organic frameworks: design, synthesis, and applications. Adv. Mater. 2018;30:1704303. doi: 10.1002/adma.201704303. [DOI] [PubMed] [Google Scholar]
- 54.Ni K., Lan G., Veroneau S.S., Duan X., Song Y., Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat. Commun. 2018;9:4321. doi: 10.1038/s41467-018-06655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Zhao Y., Chen X. Bioengineering of metal-organic frameworks for nanomedicine. Theranostics. 2019;9:3122. doi: 10.7150/thno.31918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J., Gao J., Wei Q. Combination of photodynamic therapy with radiotherapy for cancer treatment. J. Nanomater. 2016 2016. [Google Scholar]
- 57.Mayahi S., Neshasteh-Riz A., Pornour M., Eynali S., Montazerabadi A. Investigation of combined photodynamic and radiotherapy effects of gallium phthalocyanine chloride on MCF-7 breast cancer cells. JBIC J. Biol. Inorg. Chem. 2019:1–10. doi: 10.1007/s00775-019-01730-w. [DOI] [PubMed] [Google Scholar]
- 58.Ma T., Liu Y., Wu Q., Luo L., Cui Y., Wang X., Chen X., Tan L., Meng X. Quercetin-modified metal–organic frameworks for dual sensitization of radiotherapy in tumor tissues by inhibiting the carbonic anhydrase IX. ACS Nano. 2019;13:4209–4219. doi: 10.1021/acsnano.8b09221. [DOI] [PubMed] [Google Scholar]
- 59.Olusanya T.O., Ahmad H., Rushdi R., Ibegbu D.M., Smith J.R., Elkordy A.A. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23:907. doi: 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwatra D., Venugopal A., Anant S. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013;2:330–342. [Google Scholar]
- 61.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Contr. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 62.Besse H.C., Bos C., Zandvliet M.M., van der Wurff-Jacobs K., Moonen C.T., Deckers R. Triggered radiosensitizer delivery using thermosensitive liposomes and hyperthermia improves efficacy of radiotherapy: an in vitro proof of concept study. PloS One. 2018;13 doi: 10.1371/journal.pone.0204063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dou Y., Hynynen K., Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J. Contr. Release. 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 64.Driver D., Dobbs H.J. Improvements in radiotherapy practice: the impact of new imaging technologies. Canc. Imag. 2004;4:142. doi: 10.1102/1470-7330.2004.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamichhane N., Udayakumar T.S., D'Souza W.D., Simone C.B., II, Raghavan S.R., Polf J., Mahmood J. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:288. doi: 10.3390/molecules23020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad M.Z., Akhter S., Jain G.K., Rahman M., Pathan S.A., Ahmad F.J., Khar R.K. Metallic nanoparticles: technology overview & drug delivery applications in oncology, Expert Opin. Drug Deliv. 2010;7:927–942. doi: 10.1517/17425247.2010.498473. [DOI] [PubMed] [Google Scholar]
- 68.Bair R., Cimbak N., Wakefield C., Bair E., Viswanathan A.N. Radiopaque polymer hydrogel used as a fiducial marker in gynecologic brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2014;90:S505. [Google Scholar]
- 69.de Souza Lawrence L., Ford E., Gilbert C., Yarmus L., Meneshian A., Feller-Kopman D., Hales R. Novel applications of an injectable radiopaque hydrogel tissue marker for management of thoracic malignancies. Chest. 2013;143:1635–1641. doi: 10.1378/chest.12-1691. [DOI] [PubMed] [Google Scholar]
- 70.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muggia F.M., Hainsworth J.D., Jeffers S., Miller P., Groshen S., Tan M., Roman L., Uziely B., Muderspach L., Garcia A. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol. 1997;15:987–993. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 72.Bogner J.R., Kronawitter U., Rolinski B., Truebenbach K., Goebel F.-D. Liposomal doxorubicin in the treatment of advanced AIDS-related Kaposi-sarcoma. J. Acquir. Immune Defic. Syndr. 1994:463–468. [PubMed] [Google Scholar]
- 73.Orlowski R.Z., Nagler A., Sonneveld P., Bladé J., Hajek R., Spencer A., San Miguel J., Robak T., Dmoszynska A., Horvath N. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J. Clin. Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 74.Erdogan S. Liposomal nanocarriers for tumor imaging. J. Biomed. Nanotechnol. 2009;5:141–150. doi: 10.1166/jbn.2009.1016. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y., Huang L. In: Composite Nanoparticles for Gene Delivery. Genet Adv., editor. Elsevier; 2014. pp. 111–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janczak C.M., Aspinwall C.A. Composite nanoparticles: the best of two worlds. Anal. Bioanal. Chem. 2012;402:83–89. doi: 10.1007/s00216-011-5482-5. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt G., Malwitz M.M. Properties of polymer–nanoparticle composites. Curr. Opin. Colloid Interface Sci. 2003;8:103–108. [Google Scholar]
- 78.Blasiak B., van Veggel F.C., Tomanek B. Applications of nanoparticles for MRI cancer diagnosis and therapy. J. Nanomater. 2013;2013:12. [Google Scholar]
- 79.Poudel K., Gautam M., Jin S.G., Choi H.-G., Yong C.S., Kim J.O. Copper sulfide: an emerging adaptable nanoplatform in cancer theranostics. Int. J. Pharm. 1 May 2019;562:135–150. doi: 10.1016/j.ijpharm.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 80.Jølck R.I., Rydhög J.S., Christensen A.N., Hansen A.E., Bruun L.M., Schaarup‐Jensen H., von Wenck A.S., Børresen B., Kristensen A.T., Clausen M.H. Injectable colloidal gold for use in intrafractional 2D image‐guided radiation therapy. Adv. Healthc. Mater. 2015;4:856–863. doi: 10.1002/adhm.201400651. [DOI] [PubMed] [Google Scholar]
- 81.Hainfeld J.F., Smilowitz H.M., O’connor M.J., Dilmanian F.A., Slatkin D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomed. 2013;8:1601–1609. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadeghi L., Yousefi V.B., Espanani H. Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl. Lek. Listy. 2015;116:373–378. doi: 10.4149/bll_2015_071. [DOI] [PubMed] [Google Scholar]
- 83.Kotb S., Detappe A., Lux F., Appaix F., Barbier E.L., Tran V.-L., Plissonneau M., Gehan H., Lefranc F., Rodriguez-Lafrasse C. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: proof of concept before phase I trial. Theranostics. 2016;6:418. doi: 10.7150/thno.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Duc G., Roux S., Paruta-Tuarez A., Dufort S., Brauer E., Marais A., Truillet C., Sancey L., Perriat P., Lux F. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Canc. Nanotechnol. 2014;5:4. doi: 10.1186/s12645-014-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia J., Liu S.Z., Louie A.Y. Biological effects of MRI contrast agents: gadolinium retention, potential mechanisms and a role for phosphorus. Philos. Transact. A Math. Phys. Eng. Sci. 2017;375 doi: 10.1098/rsta.2017.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riley R.S., Day E.S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1449. doi: 10.1002/wnan.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahakian L.M., Farwell D.G., Zhang H., Seo J.W., Poirier B., Tinling S.P., Afify A.M., Haynam E.M., Shaye D., Ferrara K.W. Comparison of PET imaging with 64 Cu-liposomes and 18 F-FDG in the 7, 12-dimethylbenz [a] anthracene (DMBA)-Induced hamster buccal pouch model of oral dysplasia and squamous cell carcinoma. Mol. Imag. Biol. 2014;16:284–292. doi: 10.1007/s11307-013-0676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tobias J.S., Ball D. Synchronous chemoradiation for squamous carcinomas. BMJ. 2001;322:876–878. doi: 10.1136/bmj.322.7291.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernando I., Bowden S., Brookes C., Grieve R., Spooner D., Agrawal R., Brunt A., Churn M., Rea D., Canney P. Synchronous chemo-radiation can reduce local recurrence in early stage breast cancer: results of the SECRAB Trial (ISRCTN: 84214355) presented on behalf of the SECRAB Steering Committee. Eur. J. Canc. 2011;47:2. [Google Scholar]
- 90.Maier P., Hartmann L., Wenz F., Herskind C. Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int. J. Mol. Sci. 2016;17:102. doi: 10.3390/ijms17010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu Y., Huang H., Yang H., Chen D. Randomized controlled trial of late-course concurrent versus sequential chemoradiotherapy after mastectomy and axillary surgery in locally advanced breast cancer. Medicine (Baltim.) 2017;96 doi: 10.1097/MD.0000000000008252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treat J., Damjanov N., Huang C., Zrada S., Rahman A. Liposomal-encapsulated chemotherapy: preliminary results of a phase I study of a novel liposomal paclitaxel. Oncol. Williston Park NY. 2001;15:44–48. [PubMed] [Google Scholar]
- 93.Eblan M.J., Wang A.Z. Improving chemoradiotherapy with nanoparticle therapeutics. Transl. Cancer Res. 2013;2:320. doi: 10.3978/j.issn.2218-676X.2013.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagtvet E., Røe K., Olsen D.R. Liposomal doxorubicin improves radiotherapy response in hypoxic prostate cancer xenografts. Radiat. Oncol. 2011;6:135. doi: 10.1186/1748-717X-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeFrates K., Markiewicz T., Gallo P., Rack A., Weyhmiller A., Jarmusik B., Hu X. Protein polymer-based nanoparticles: fabrication and medical applications. Int. J. Mol. Sci. 2018;19:1717. doi: 10.3390/ijms19061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mukherjee A., Waters A.K., Kalyan P., Achrol A.S., Kesari S., Yenugonda V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019;14:1937–1952. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wire. Authors . Adv. Sci. News.; 2017. Chemotherapy or Not? Clinical Studies of Poly(L-Glutamic Acid)-Paclitaxel.https://www.advancedsciencenews.com/chemotherapy-not-clinical-studies-polyl-glutamic-acid-paclitaxel/ [Google Scholar]
- 98.Wang A., McRee A.J., Blackstock A.W., O'Neil B.H., Moore D.T., Calvo B.F., Lee M.S., Murphy C., Caliri K., Tynan M.T. 2017. Phase Ib/II Study of Neoadjuvant Chemoradiotherapy with CRLX101 and Capecitabine for Locally Advanced Rectal Cancer. [Google Scholar]
- 99.Estelrich J., Busquets M.A. Iron oxide nanoparticles in photothermal therapy. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018;23 doi: 10.3390/molecules23071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y., Shan Y., Cong H., Shen Y., Yu B. Advanced carbon-based nanoplatforms combining drug delivery and thermal therapy for cancer treatment. Curr. Pharmaceut. Des. 2018;24:4060–4076. doi: 10.2174/1381612825666181120160959. [DOI] [PubMed] [Google Scholar]
- 101.Chao Y., Xu L., Liang C., Feng L., Xu J., Dong Z., Tian L., Yi X., Yang K., Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018:1. doi: 10.1038/s41551-018-0262-6. [DOI] [PubMed] [Google Scholar]
- 102.Yilmaz G., Guler E., Barlas F.B., Timur S., Yagci Y. Polymeric thioxanthones as potential anticancer and radiotherapy agents. Macromol. Rapid Commun. 2016;37:1046–1051. doi: 10.1002/marc.201600189. [DOI] [PubMed] [Google Scholar]
- 103.Kievit F.M., Stephen Z.R., Wang K., Dayringer C.J., Sham J.G., Ellenbogen R.G., Silber J.R., Zhang M. Nanoparticle mediated silencing of DNA repair sensitizes pediatric brain tumor cells to γ‐irradiation. Mol. Oncol. 2015;9:1071–1080. doi: 10.1016/j.molonc.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boissenot T., Bordat A., Fattal E., Tsapis N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: from theoretical considerations to practical applications. J. Control. Release Off. J. Control. Release Soc. 2016;241:144–163. doi: 10.1016/j.jconrel.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 105.Rossmann C., McCrackin M.A., Armeson K.E., Haemmerich D. Temperature sensitive liposomes combined with thermal ablation: effects of duration and timing of heating in mathematical models and in vivo. PloS One. 2017;12 doi: 10.1371/journal.pone.0179131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qi X., Xiong L., Peng J., Tang D. Near infrared laser-controlled drug release of thermoresponsive microgel encapsulated with Fe3O4 nanoparticles. RSC Adv. 2017;7:19604–19610. [Google Scholar]
- 107.Stacy D.R., Lu B., Hallahan D.E. Radiation-guided drug delivery systems. Expert Rev. Anticancer Ther. 2004;4:283–288. doi: 10.1586/14737140.4.2.283. [DOI] [PubMed] [Google Scholar]
- 108.Wang A.Z., Tepper J.E. Nanotechnology in radiation oncology. J. Clin. Oncol. 2014;32:2879. doi: 10.1200/JCO.2014.55.0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hariri G., Han Z., Hallahan D. Radiation-guided drug delivery of nanoparticle albumin-bound paclitaxel to lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:S705–S706. [Google Scholar]
- 110.Porock D., Kristjanson L. Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. Eur. J. Canc. Care. 1999;8:143–153. doi: 10.1046/j.1365-2354.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 111.Lee N., Chuang C., Quivey J.M., Phillips T.L., Akazawa P., Verhey L.J., Xia P. Skin toxicity due to intensity-modulated radiotherapy for head-and-neck carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:630–637. doi: 10.1016/s0360-3016(02)02756-6. [DOI] [PubMed] [Google Scholar]
- 112.Leu A., Stieger S.M., Dayton P., Ferrara K.W., Leach J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng. 2008;15:877–885. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nambiar S., Yeow J.T. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces. 2012;4:5717–5726. doi: 10.1021/am300783d. [DOI] [PubMed] [Google Scholar]
- 114.Zhong S., Zhang Y., Lim C. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2:510–525. doi: 10.1002/wnan.100. [DOI] [PubMed] [Google Scholar]
- 115.Nakayama M., Fujiwara M., Nakamura T., Azuma T., Matzno S., Kamikonya N., Kimura T., Matsuyama K., Kawabata A. Stability of polaprezinc-containing oral rinse and its clinical effectiveness against radiotherapy-induced oral mucositis. Iyakuhin Johogaku. 2013;15:133–138. [Google Scholar]
- 116.Fischer-Valuck B.W., Chundury A., Gay H., Bosch W., Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract. Radiat. Oncol. 2017;7:195–202. doi: 10.1016/j.prro.2016.10.004. [DOI] [PubMed] [Google Scholar]