Abstract
Extra-articular synovial chondromatosis is a rare entity in the foot and ankle. We present a case of a 49-year-old female who presented for evaluation of a palpable concern following trauma; which was found to represent synovial chondromatosis. This case demonstrates the multimodality imaging findings, including ultrasound and MRI, with histopathologic correlation.
Keywords: Synovial chondromatosis, Extra-articular, Ankle, MRI, Ultrasound, Pathology
Introduction
Extra-articular synovial chondromatosis (SC) is a rare, benign mass that arises from synovium found in joints and tendon sheaths. This condition is most commonly observed in the knee joint (50%), but any synovium containing site can be affected. Although benign, the abnormal synovium in this condition can calcify, and generate loose bodies within the joint. Both the gritty calcified synovial surface and the loose bodies can damage cartilage, causing a secondary osteoarthritis. In severe instances, the loose bodies can occupy the entire joint and penetrate into adjacent tissues. It is not clear if SC is a neoplastic or metaplastic condition, since a specific genetic abnormality has not yet been identified. SC occurs in men twice as often as women, and most commonly in patients 30-50 years of age. Primary SC is known as Reichel syndrome, whereas secondary SC is the result of degenerative changes from other intra-articular conditions. Malignant degeneration to chondrosarcoma has been reported, however is rare [1], [2], [3].
Case report
A 49-year-old female sustained a traumatic ankle injury as the result of a twist and fall. She was seen at an urgent care immediately following the injury where initial x-rays were taken, and negative for fracture. A noncontrast MRI was ordered.
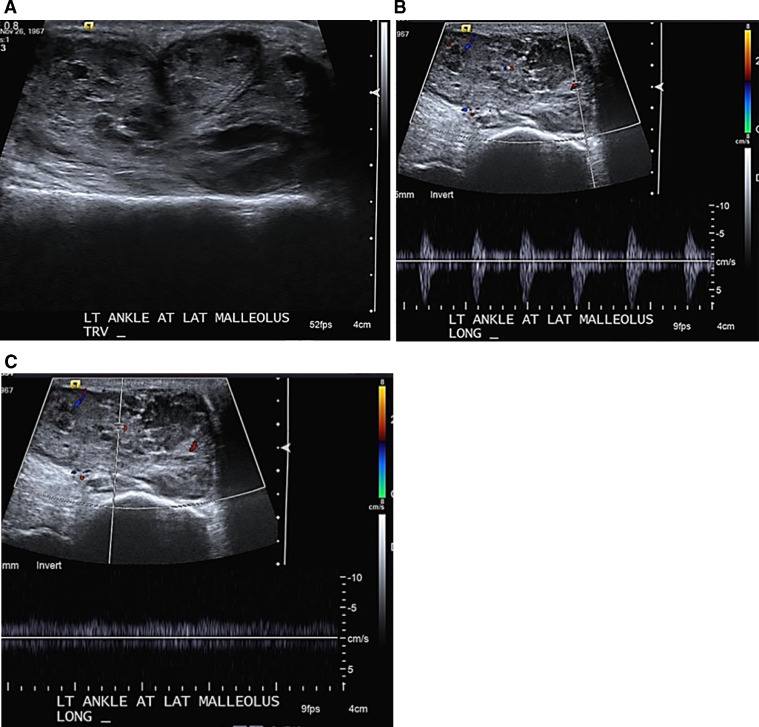
The patient then presented to our office for further care. She had no history of prior ankle abnormality. A palpable mass was noted at the lateral ankle on clinical exam which patient stated appeared following the injury. The MRI findings showed a large multinodular, presumably cystic, lesion measuring approximately 5.0 × 2.2 × 3.8 cm in the inferolateral ankle and was reported as probable large ganglion (Fig. 1A, B, C). The patient was referred for an ultrasound-guided aspiration which revealed a 5.0 × 5.0 × 5.0 × 3.0 cm lobulated highly heterogeneous and vascular soft tissue mass with both arterial and venous waveforms; which was reported as suggestive of a neurogenic tumor or sarcoma (Fig. 2A, B, C). A follow-up MRI with intravenous contrast (Fig. 1D, E) revealed a lobulated mass located in close proximity to the tendon sheath of the peroneal tendons, with a peripheral and mildly nodular and lace-like internal enhancement pattern. Proposed diagnoses included a myxoid neoplasm or a synovial sarcoma based on location in the foot. The mass was measured at 6.0 × 4.0 × 3.0 cm (Fig. 2). The decision was made to perform an intraoperative ultrasound guided biopsy of the lesion. Surgical pathology suggested a benign cartilaginous lesion, consistent with SC and complete surgical excision of the mass was advised and subsequently performed, in the same setting. During surgery, the mass was noted superior to and arising from the tendon sheath.
Fig. 1.
MRI of the ankle. (A) Axial (long-axis) T1-weighted image demonstrates a large, lobulated soft tissue lesion at the lateral aspect of the ankle, of intermediate signal intensity, deforming the skin surface. The externally placed vitamin marker (arrow) demarcates the area of palpable concern. (B) Coronal (short-axis) proton density, fat saturated image further demonstrates the lobulated, septated nature of the lesion, and confirms the intimate relationship with the underlying peroneal tendons. (C) Sagittal, T2-weighted, fat saturated image, demonstrating the increased, fluid-like signal within the lesion, consistent with a high fluid content, and also demonstrates the normal, adjacent peroneal tendons. Following the intravenous administration of gadolinium-based contrast, (D) axial (long-axis) and (E) sagittal T1-weighted, fat saturated images demonstrate a peripheral and internal, “lace-like” pattern of enhancement, suggestive of a chronic, inflammatory process as opposed to neoplasm.
Fig. 2.
Ultrasound of the lateral ankle. (A) Grey-scale image demonstrates the lobulated contour of the lesion, with heterogeneous, internal echogenicity, not consistent with a simple ganglion or tenosynovitis. Doppler interrogation of the mass demonstrates (B) arterial and (C) venous blood flow; together indicating neovascularity, due to an inflammatory and/or neoplastic process.
Histologic evaluation noted well-circumscribed lobular fragments of cartilaginous proliferation with low cellularity and low nuclear-to-cytoplasmic ratios. There was no nuclear atypia, mitoses, or necrosis. The findings were compatible with a benign cartilaginous proliferation, most consistent with SC (Fig. 3).
Fig. 3.
Histologic findings of the ankle lesion (A and B). Well circumscribed lobular fragments of cartilaginous proliferation (C and D). The cellularity is low, with no nuclear atypia, mitosis, or necrosis.(A. at 10×; B. at 20×; C. at 100×; D. at 200×.)
At her initial 2-week postoperative visit, the patient had full range of motion, normal strength, and normal neurovascular status. At 4 and 10-month follow ups, the patient had no complaints or limitations.
Discussion
Extra-articular SC is rare in the foot and ankle and pathogenesis remains unclear. It is usually monoarticular and the occurrence rate is approximately 1 per 100,000 [3]. Literature review reveals 7 reported cases in the foot and ankle, with locations including the tibiotalar, calcaneocuboid, naviculocuneiform, tarsometatarsal, metatarsophalangeal, and interphalangeal joints [4]. Most cases of extra-articular SC indicate patients have symptoms for several years before diagnosis and surgical intervention. Symptoms usually include pain, locking, instability, and palpable mass [1]. This patient was not symptomatic: however, the findings were discovered incidentally as a result of a traumatic injury which led to early diagnosis and treatment.
The disease can be classified into 3 phases based on maturation. Milgram described this classification system in 1977: early, transitional, and late. Early phase is described as active synovial disease without the presence of loose bodies. Transitional phase is described as the presence of synovial disease and loose bodies. Late phases is described as loose bodies without synovial involvement [5].
The MRI appearance of SC has been reported as variable, including loose bodies, which were absent in our case, proliferative synovium and pressure erosions. The use of intravenous contrast is helpful in distinguishing synovial fluid from highly vascular synovial tissue. The enhancement pattern in our case primarly suggested synovial fluid with a lining of synvovial tissue. MRI often also shows areas of signal void on all pulse sequences that correspond to calcification [6], [7], [8], which was notably absent in our case. SC on MRI can be mistaken for pigmented villonodular synovitis since both appear with signal voids, however, the synovial lining in pigmented villonodular synovitis will typically show low MRI signal because of hemosiderin deposits [6,9]. CT imaging can be useful in detecting calcified loose bodies that are a result of primary SC and distinguish from more localized loose bodies that are secondary to degenerative osteoarthritis (OA).
Histologic analysis via biopsy and/or surgical frozen section is helpful in determining the surgical procedure before total excision. SC has some histologic features that are also found in low-grade chondrosarcoma including binucleated chondrocytes, although this does not imply malignancy. Five features have been described in successfully differentiating SC from chondrosarcoma. These include (1) chondrocytes arranged in sheets in SC (rather than clusters in cases of chondrosarcoma), (2) myxoid change in SC (vs enhancing, solid matrix in chondrosarcoma), (3) the absence of hypercellularity and crowding and/or spindling of nuclei at the periphery in SC, but present in chondrosarcoma, (4) the absence of necrosis in SC, which is seen in chondrosarcoma, and finally (5) SC involvement of adjacent bone margins is described as “pushing” (aka—without infiltrative features), whereas chondrosarcoma demonstrates permeation and/or infiltration of trabecular bone [4,10,11].
It has been reported that the relative risk of malignant transformation in SC can be as high as 5%. It is not known whether intra-articular and extra-articular variants carry the same risk of malignant degeneration. Recurrence has been reported to range from 37.5% to over 50%; although these reported rates can be biased due to incomplete resection or misdiagnosis [2,12]. This patient's early diagnosis and treatment has allowed for a quick return to normal active lifestyle without evidence of malignancy or recurrence.
Curative treatment of extra-articular SC is typically performed with en bloc, complete surgical excision. Recurrence, which is attributed to incomplete excision, is extremely low. In keeping with the benign histology, there is currently no role for either preoperative or postoperative, adjuvant radiation or chemotherapy. If, following surgical pathologic evaluation, malignant features are found, treatment includes wider surgical resection (if needed) and adjuvant chemotherapy and/or radiation, depending on institutional protocols [2], [3], [4],10].
Footnotes
Competing Interests: None.
REFERENCES
- 1.Dorfman HD, Czerniak B. Bone tumors. Mosby; St Louis, Mo: 1998. Synovial lesions; pp. 1041–1086. [Google Scholar]
- 2.Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67:155–162. doi: 10.1002/1097-0142(19910101)67:1<155::aid-cncr2820670127>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Villacin AB, Brigham LN, Bullough PG. Primary and secondary synovial chondrometaplasia: histopathologic and clinicoradiologic differences. Hum Pathol. 1979;10:439–451. doi: 10.1016/s0046-8177(79)80050-7. [DOI] [PubMed] [Google Scholar]
- 4.Galat D, Ackerman D, Spoon D, Turner N, Shives T. Synovial chondromatosis of the Foot and Ankle. Foot Ankle Int. 2008;29(3):312–317. doi: 10.3113/FAI.2008.0312. [DOI] [PubMed] [Google Scholar]
- 5.Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59:792–801. [PubMed] [Google Scholar]
- 6.Harish S, Saifuddin A, Cannon S, Flanagan A. Synovial chondromatosis of the foot presenting with Lisfranc dislocation. Skeletal Radiol. 2005;34:736–739. doi: 10.1007/s00256-005-0923-x. [DOI] [PubMed] [Google Scholar]
- 7.Kramer J., Recht M., Deely D.M., Schweitzer M., Pathria M.N., Gentili A. MR appearance of idiopathic synovial osteochondromatosis. J Comput Assist Tomogr. 1993;17:772–776. doi: 10.1097/00004728-199309000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Narvaes JA, Narvaez J, Aguilera C, DeLama E, Portabella F. MR imaging of synovial tumors and tumor-like lesions. Eur Radiol. 2001:2549–2560. doi: 10.1007/s003300000759. [DOI] [PubMed] [Google Scholar]
- 9.Hughes TH, Sartoris DJ, Schweitzer ME, Resnick DL. Pigmented villonodular synovitis: MRI characteristics. Skeletal Radiol. 1995;24:7–12. doi: 10.1007/BF02425937. [DOI] [PubMed] [Google Scholar]
- 10.Davis RJ, Hamilton A, Biggart JD. Primary synovial chrondromatosis: a clinicopathological review and assessment of malignant potential. Hum Pathol. 1998;29:683–688. doi: 10.1016/s0046-8177(98)90276-3. [DOI] [PubMed] [Google Scholar]
- 11.Hermann G, Klein M, Abdelwahab I. Synovial Chondrosarcoma arising in synovial chondromatosis of the right hip. Skeletal Radiol. 1997;26:366–369. doi: 10.1007/s002560050249. [DOI] [PubMed] [Google Scholar]
- 12.Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29:683–688. doi: 10.1016/s0046-8177(98)90276-3. [DOI] [PubMed] [Google Scholar]