Abstract
Current interventions to reduce skeletal fragility are insufficient at enhancing both the quantity and quality of bone when attempting to improve overall mechanical integrity. Bisphosphonates, such as Zoledronate (ZOL), are used to treat a variety of bone disorders by increasing bone mass to decrease fracture risk, but long-term use has been shown in some settings to compromise bone quality. Alternatively, Raloxifene (RAL) has recently been demonstrated to improve tissue quality and overall mechanical properties in a cell-independent manner by binding to collagen and increasing tissue hydration. We hypothesized that a combination of RAL and ZOL would improve mechanical and material properties of bone more than either monotherapy alone by enhancing both quantity and quality. In this study, wildtype (WT) and heterozygous (OIM+/−) male mice from the Osteogenesis Imperfecta (OI) murine model were treated with either RAL, ZOL, or both from 8 weeks to 16 weeks of age. Using the OIM model allows for investigation of therapeutic effects on a quality-based bone disease. Combination treatment resulted in higher trabecular architecture, cortical mechanical properties, and cortical fracture toughness in diseased mouse bone. Two fracture toughness properties, which are direct measures of the tissue's ability to resist the initiation and propagation of a crack, were significantly improved with combination treatment in OIM+/− compared to control. There was no significant effect on fracture toughness with either monotherapy alone in either genotype. Following the mass-based effects of ZOL, trabecular bone volume fraction was significantly higher with combination treatment in both genotypes. Combination treatment resulted in higher ultimate stress in both genotypes. RAL and combination treatment in OIM+/− also increased resilience compared to the control. In conclusion, this study demonstrates the beneficial effects of using combination drug treatments to increase bone mass while simultaneously improving tissue quality, especially to enhance the mechanical integrity of diseased bone. Combination therapies could be a potential method to improve bone health and combat skeletal fragility on both the microscopic and macroscopic levels.
Keywords: Osteogenesis Imperfecta, SERM, Bisphosphonate, Bone quality, Fracture toughness
1. Introduction
Bisphosphonates (BPs) have been the gold standard to treat skeletal fragility and numerous bone disorders for the past 30 years. BPs increase bone mineral density which leads to decreased fracture risk in diseases such as postmenopausal osteoporosis, Paget's disease, metastatic osteolytic lesions, and more recently, Osteogenesis Imperfecta (OI) [1-3]. BPs target osteoclasts and decrease their activity, which ultimately leads to the disruption of the bone remodeling process [4]. However, long term BP use may have unintended consequences. The disruption of bone remodeling reduces the bone repair mechanism which can lead to the accrual of microdamage in the tissue, making the tissue more susceptible to failure [5]. BP use has also demonstrated an increase of non-enzymatic cross linking in the collagen matrix, which has been correlated with reduced post-yield mechanical properties [6-8]. Despite the positive mass-based effects BPs have in bone, tissue quality may not be optimal, making these treatments insufficient to overcome mechanical deficits that commonly manifest with disease. There is a need to simultaneously improve tissue quality while increasing mass.
Raloxifene (RAL) is in a different class of FDA-approved drugs used to treat osteoporosis in post-menopausal women. The drug primarily acts in a cell-dependent manner as a Selective Estrogen Receptor Modulator (SERM) and combats bone loss by binding and signaling through estrogen receptors on osteoblasts [9]. Clinically, RAL reduces fractures by ~50% but with modest changes in remodeling and bone mineral density (BMD) [10-12]. This observation suggests that the changes in mechanical integrity are driven by tissue quality changes versus altered mass or architecture. Recent work has demonstrated that RAL also exhibits cell-independent behavior by binding to collagen and increasing tissue hydration, leading to enhanced mechanical properties and fracture resistance [13,14]. These cell-independent, material-based changes provide a unique opportunity to beneficially alter bone fracture resistance through changes in tissue quality, especially in disease states that are driven by inferior tissue properties.
OI is a genetic disease in bone caused by a mutation in Type I collagen or related proteins. The mutated collagen leads to poor formation of the triple helical structure, driving quality-based deficiencies in the collagen-mineral composite [15-17]. These microscopic changes induce macroscopic effects and cause brittle bones and frequent fractures in patients suffering from the disease. The majority of fractures in OI patients occur at cortical regions of their long bones, and the femoral mid-diaphysis is particularly at risk due to load bearing during ambulation [18,19]. Targeting quality at these regions is crucial to clinically reduce fractures in patients affected by OI.
Previously, clinical research has shown potential benefits of using the combination of RAL and ZOL in osteoporotic women [20]. However, combination treatment has not been assessed in a disease where the mechanical deficits root from microscopic-level deficiencies in quality. In this study, the osteogenesis imperfecta murine (oim) model of OI allowed for investigation of how combination treatments impact the phenotype of a quality-based disease state [21], where adding more, poor quality, tissue with BPs might not be enough to overcome mechanical deficiencies. It was hypothesized that using the mass-based effects of BPs, in conjunction with the tissue level improvements noted with RAL, would improve bone mechanical properties and fracture resistance more than either monotherapy alone.
2. Materials and methods
2.1. Animals and treatment
All protocols and procedures were performed with prior approval from the IU School of Medicine IACUC. Male wild-type (WT) and heterozygous (OIM +/−) mice were bred from heterozygous parental strains on a C57BL/6 background [22]. Beginning at 8 weeks of age, mice (n = 13–15 per group) were injected subcutaneously with either RAL (0.5 mg/kg; 5×/week), zoledronate (ZOL; 80 μg/kg; at 8 weeks and 12 weeks of age), or the combination. Untreated controls were also included. These dosages were chosen based on previous research showing efficacy in vivo [23-26]. At 16 weeks of age, the mice were euthanized by CO2 asphyxiation, and the right femora and tibiae were harvested, stripped of soft tissue, and frozen wrapped in phosphate buffered saline (PBS)-soaked gauze at −20 °C.
2.2. Microcomputed tomography (μCT) and architectural analysis
To determine the effects of treatment on bone architecture, right femora were scanned using a nominal voxel size of 10 μm (Skyscan 1172, Bruker). Scans were performed using a 0.7-degree angle increment, two frames averaged, through a 0.5 mm Al filter (V = 60 kV, I = 167 μA). Images were reconstructed (nRecon) and calibrated to hydroxyapatite-mimicking phantoms (0.25 and 0.75 g/cm3 Ca-HA). For each femur, a cancellous region was selected at the distal metaphysis, extending 1-mm proximally from the most proximal portion of the growth plate, and then quantified using CT Analyzer (CTAn). To obtain cortical architectural properties, a 1-mm cortical region was selected at approximately 50% length of the femur, then analyzed with a custom MATLAB script [27]. Additionally, right tibiae were scanned at the mid-diaphysis to obtain cortical geometry used for fracture toughness testing as indicated below.
2.3. Three-point bending mechanical testing to failure
Right femora were tested to failure in three-point bending (support span at 8 mm) with the anterior surface in tension. The bones were loaded at a displacement control rate of 0.25 mm/s while the sample remained hydrated with PBS. Cross-sectional cortical properties at the femoral mid-diaphysis were obtained from μCT images as described above. These properties were used to map load-displacement data into stress-strain data using standard engineering equations as previously reported [28].
2.4. Fracture toughness testing
Fracture toughness of the right tibiae was measured using a linear elastic fracture mechanics approach, as described previously [29,30]. Briefly, a notch was made on the anteromedial aspect of the tibia, at approximately 50% of the length, using a scalpel blade lubricated with a 1 μm diamond suspension. The tibiae were notched into the medullary cavity to a depth not exceeding the bone's midpoint. They were then tested to failure in 3-point bending at a displacement control rate of 0.001 mm/s with the notched surface in tension. The load point was positioned directly above the notch site.
After mechanical testing, the bones were cleansed of marrow and dehydrated using an ethanol gradient (70–100%) and a vacuum desiccator. Following sputter-coating with gold, the cross-sectional fracture surface was imaged with scanning electron microscopy (SEM). The angles of stable and unstable crack growth were obtained from the images and, along with geometric properties from μCT data, a custom MATLAB script calculated stress intensity factors for crack initiation, maximum load, and fracture instability.
2.5. Statistical analysis
All data were checked for assumptions of normality and homogeneity of variance, and violations were corrected using transformations. For each genotype independently, a One-Way ANOVA with post-hoc Dunnett's test was used to statistically analyze the effect of each treatment versus control. Analysis was performed using GraphPad Prism (v.8) with a significance level at α = 0.05.
3. Results
3.1. Treatment contributed to gains in trabecular architecture and mineralization
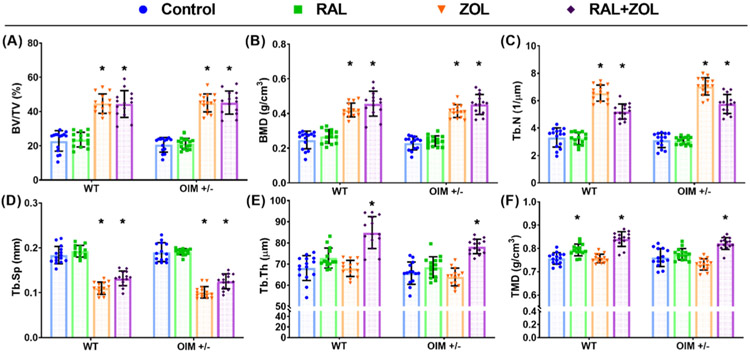
Bone volume fraction and bone mineral density in the femoral distal metaphysis were significantly greater compared to control in both genotypes with ZOL monotherapy and combination treatment, but not with RAL monotherapy (Fig. 1, Supplemental Tables 1 and 3). Similarly, in both genotypes, trabecular number was significantly greater and trabecular spacing significantly lower with ZOL alone and with combinatorial therapy but not with RAL alone. Although trabecular thickness trended upward with RAL in both WT (+7.1%) and OIM+/− (+5.8%), it only reached significance with combination treatment in both genotypes (WT: +24.7%; OIM+/−: +20.9%). Tissue mineral density (TMD) was significantly elevated with the combination treatment in both genotypes. Additionally, TMD significantly increased in WT mice with RAL monotherapy. Although the property trended upward in OIM+/− with RAL alone, it did not reach significance. ZOL had no effect on TMD in either genotype.
Fig. 1.
Treatment effects on (A) bone volume fraction (BV/TV), (B) bone mineral density (BMD), (C) trabecular number (Tb.N), (D) trabecular separation (Tb.Sp), (E) trabecular thickness (Tb.Th), and (F) tissue mineral density (TMD) in the distal femoral metaphysis. Significant change from control indicated by ‘*’ at p < 0.05.
3.2. Treatment had no effect on cortical geometry at the femoral mid-diaphysis
Despite the large changes in cancellous bone architecture, cortical geometric properties showed no significant changes with either monotherapy or combinatorial treatment at the femoral mid-diaphysis (Table 1).
Table 1.
Cortical geometry at the femoral mid-diaphysis.
| WT |
OIM+/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | RAL | ZOL | RAL + ZOL | p-Value | Control | RAL | ZOL | RAL + ZOL | p-Value | |
| Total CSA (mm2) | 2.031 ± 0.174 | 2.068 ± 0.193 | 2.011 ± 0.216 | 2.053 ± 0.19 | 0.877 | 1.897 ± 0.267 | 1.837 ± 0.167 | 1.965 ± 0.31 | 1.834 ± 0.194 | 0.712 |
| Marrow Area (mm2) | 1.051 ± 0.1 | 1.047 ± 0.116 | 1.027 ± 0.104 | 1.028 ± 0.102 | 0.891 | 0.974 ± 0.128 | 0.902 ± 0.096 | 0.979 ± 0.157 | 0.906 ± 0.116 | 0.273 |
| Cortical Area (mm2) | 0.98 ± 0.113 | 1.02 ± 0.084 | 0.984 ± 0.129 | 1.024 ± 0.107 | 0.583 | 0.923 ± 0.179 | 0.935 ± 0.092 | 0.987 ± 0.176 | 0.929 ± 0.091 | 0.951 |
| Cortical Thickness (mm) | 0.227 ± 0.021 | 0.235 ± 0.01 | 0.23 ± 0.021 | 0.238 ± 0.017 | 0.371 | 0.225 ± 0.04 | 0.231 ± 0.017 | 0.238 ± 0.036 | 0.229 ± 0.013 | 0.939 |
| Periosteal perimeter (mm) | 5.868 ± 0.231 | 5.921 ± 0.239 | 5.852 ± 0.271 | 5.898 ± 0.24 | 0.881 | 5.684 ± 0.357 | 5.63 ± 0.25 | 5.789 ± 0.395 | 5.611 ± 0.254 | 0.758 |
| Endocortical perimeter (mm) | 4.581 ± 0.224 | 4.533 ± 0.242 | 4.53 ± 0.282 | 4.5 ± 0.218 | 0.839 | 4.414 ± 0.301 | 4.286 ± 0.265 | 4.513 ± 0.543 | 4.247 ± 0.256 | 0.327 |
| Imax (mm4) | 0.346 ± 0.064 | 0.366 ± 0.061 | 0.347 ± 0.074 | 0.366 ± 0.067 | 0.739 | 0.3 ± 0.084 | 0.301 ± 0.058 | 0.329 ± 0.081 | 0.292 ± 0.058 | 0.839 |
| Imin (mm4) | 0.168 ± 0.031 | 0.177 ± 0.035 | 0.165 ± 0.041 | 0.174 ± 0.035 | 0.826 | 0.151 ± 0.045 | 0.139 ± 0.025 | 0.166 ± 0.073 | 0.142 ± 0.032 | 0.738 |
| TMD (g/cm3) | 1.272 ± 0.021 | 1.267 ± 0.048 | 1.277 ± 0.024 | 1.28 ± 0.021 | 0.698 | 1.309 ± 0.032 | 1.314 ± 0.031 | 1.307 ± 0.035 | 1.314 ± 0.037 | 0.954 |
Values are presented as mean ± standard deviation. CSA - cross sectional area; Imax - maximum moment of inertia; Imin - minimum moment of inertia; TMD - tissue mineral density. There was not a main ANOVA effect in any of the properties.
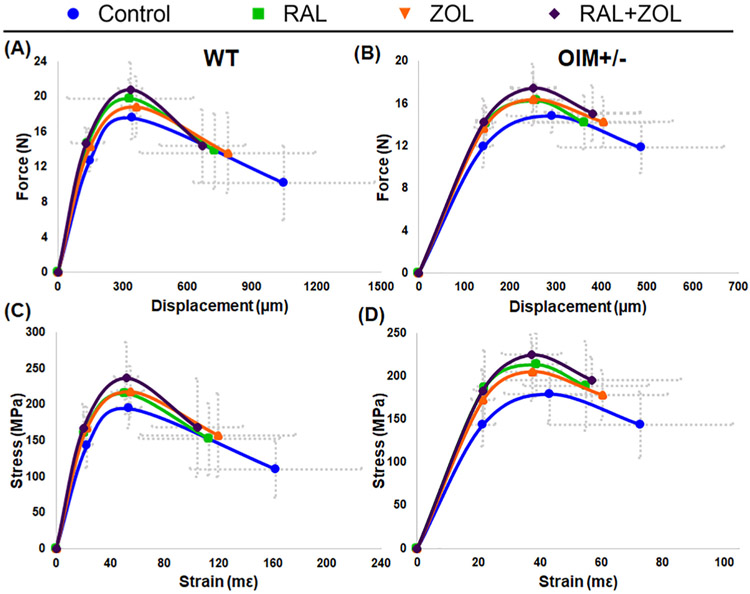
3.3. Treatments increased femoral mechanical strength and stiffness
The majority of significant effects noted in femoral mechanical properties occurred in strength parameters (Fig. 2, Tables 2 and 3, and Supplemental Table 3). At the structural level, RAL monotherapy and combination treatment resulted in higher yield force in both genotypes. ZOL monotherapy also significantly increased the property but only in WT. Ultimate force was significantly elevated compared to control with combination treatment in both genotypes. In WT, total displacement was significantly lower with RAL and combination treatment compared to control.
Fig. 2.
Schematic force-displacement curves for WT (A) and OIM +/− (B). Schematic stress-strain curves for WT (C) and OIM +/− (D).
Table 2.
Structural mechanical properties from 3-point bending of the femoral mid-diaphysis.
| WT |
OIM+/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | RAL | ZOL | RAL + ZOL | p-Value | Control | RAL | ZOL | RAL + ZOL | p-Value | |
| Yield force (N)*# | 12.7 ± 1.3 | 14.7 ± 1.6** | 14.3 ± 2* | 14.7 ± 1.8** | 0.007 | 11.9 ± 2 | 14.1 ± 1.7** | 13.7 ± 1.8 | 14.3 ± 2.2** | 0.008 |
| Ultimate force (N)*# | 17.6 ± 2.4 | 19.8 ± 2.1 | 18.8 ± 3.4 | 20.8 ± 3.1** | 0.023 | 14.8 ± 1.6 | 16.2 ± 1.6 | 16.4 ± 2.5 | 17.4 ± 2.2** | 0.011 |
| Displ. to yield (μm) | 148.4 ± 30.6 | 138.5 ± 21.9 | 148.8 ± 17.3 | 130.6 ± 12.8 | 0.092 | 142.9 ± 18.9 | 147 ± 12.8 | 142.1 ± 20.4 | 142.8 ± 16.1 | 0.862 |
| Post yield Displ. (μm)* | 899.9 ± 417 | 590.8 ± 291.5 | 638.9 ± 395.6 | 539.6 ± 196* | 0.029 | 343.8 ± 177.3 | 215.7 ± 120.3 | 261.7 ± 156.5 | 238.2 ± 100.6 | 0.096 |
| Total displ. (μm)* | 1048.3 ± 421.5 | 729.3 ± 292.5* | 787.8 ± 407.1 | 670.2 ± 197.6* | 0.024 | 486.6 ± 181.4 | 362.7 ± 121.6 | 403.9 ± 152 | 381 ± 104.3 | 0.111 |
| Stiffness (N/mm)*# | 97.7 ± 18.9 | 120.1 ± 19.7** | 107.9 ± 21.7 | 125.8 ± 14.5*** | <0.001 | 92.4 ± 9.9 | 107 ± 14.5* | 107.9 ± 17* | 111.5 ± 16** | 0.006 |
| Work to yield (mJ) | 1.02 ± 0.23 | 1.11 ± 0.23 | 1.15 ± 0.17 | 1.05 ± 0.2 | 0.386 | 0.93 ± 0.25 | 1.13 ± 0.19 | 1.06 ± 0.25 | 1.12 ± 0.24 | 0.103 |
| Post yield work (mJ) | 11.2 ± 4.29 | 9.46 ± 4.33 | 9.36 ± 4.27 | 9.62 ± 3.52 | 0.587 | 4.61 ± 2.49 | 3.1 ± 1.51 | 3.97 ± 2.48 | 3.79 ± 1.69 | 0.289 |
| Total work (mJ) | 12.22 ± 4.22 | 10.57 ± 4.37 | 10.51 ± 4.37 | 10.67 ± 3.6 | 0.633 | 5.54 ± 2.55 | 4.23 ± 1.47 | 5.03 ± 2.5 | 4.9 ± 1.82 | 0.431 |
Values are presented as mean ± standard deviation. In the property column, a significant main effect of treatment is indicated by ‘*’ in WT and ‘#’ in OIM+/−. A significant difference from control within each genotype is indicated by ‘*’ for p < 0.05, ‘**’ for p < 0.01, and ‘***’ for p < 0.001. Displ. – displacement.
Table 3.
Estimated tissue-level mechanical properties from 3-point bending of the femoral mid-diaphysis.
| WT |
OIM+/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | RAL | ZOL | RAL + ZOL | p-Value | Control | RAL | ZOL | RAL + ZOL | p-Value | |
| Yield stress (MPa)# | 143 ± 30.8 | 161 ± 24.3 | 167.2 ± 29.5 | 167.3 ± 33.7 | 0.104 | 143.3 ± 24.4 | 187 ± 42.5** | 172.5 ± 35.6* | 183.2 ± 17.4** | 0.002 |
| Ultimate stress (MPa)*# | 194.3 ± 26.7 | 215.5 ± 20.3 | 217.7 ± 35.5 | 236.6 ± 49.3** | 0.020 | 179.3 ± 30.5 | 213.3 ± 37.1* | 205.4 ± 34 | 224.7 ± 23.3** | 0.003 |
| Strain to yield (mε) | 22.9 ± 3.9 | 21.0 ± 3.5 | 22.7 ± 2.8 | 20.1 ± 1.9 | 0.060 | 21.3 ± 4.1 | 21.9 ± 2.3 | 21.4 ± 3.8 | 21.3 ± 3.3 | 0.949 |
| Total strain (mε) * | 162.3 ± 63.5 | 113.2 ± 52 | 119.6 ± 57.2 | 104.3 ± 33.4* | 0.023 | 72.9 ± 30.1 | 55 ± 20.5 | 60.2 ± 21.4 | 57 ± 16.8 | 0.162 |
| Modulus (GPa)* | 6.92 ± 1.19 | 8.65 ± 1.6 | 8.33 ± 2.54 | 9.39 ± 2.34** | 0.013 | 7.74 ± 2.19 | 9.69 ± 3.2 | 9.09 ± 2.11 | 9.76 ± 1.82 | 0.106 |
| Resilience (MPa)# | 1.8 ± 0.62 | 1.85 ± 0.49 | 2.03 ± 0.35 | 1.83 ± 0.39 | 0.567 | 1.64 ± 0.38 | 2.2 ± 0.43** | 2.03 ± 0.69 | 2.13 ± 0.4* | 0.017 |
| Toughness (MPa) | 21.3 ± 7.6 | 17.34 ± 6.27 | 18.53 ± 7.63 | 18.28 ± 5.71 | 0.456 | 9.66 ± 4.03 | 8.12 ± 2.68 | 9.15 ± 3.84 | 9.23 ± 2.78 | 0.650 |
Values are presented as mean ± standard deviation. In the property column, a significant main One-Way ANOVA effect of treatment is indicated by ‘*’ in WT and ‘#’ in OIM+/−. A significant difference from control within each genotype is indicated by ‘*’ for p < 0.05, ‘**’ for p < 0.01, and ‘***’ for p < 0.001.
At the tissue level, ultimate stress was significantly higher with combination treatment compared to control in both genotypes. RAL alone increased ultimate stress in OIM+/− but not in WT. Yield stress significantly increased with all treatments in OIM+/−. Although the property trended up in WT, it did not reach significance (p = 0.10). Higher yield stress led to greater resilience in OIM+/− with RAL and combination treatment versus control, but not with ZOL alone. Following the displacement trend, total strain in WT was significantly lower with combination treatment.
A few non-strength parameters were also impacted by treatment. Stiffness was significantly higher with all treatments in OIM +/−, but in WT, only increased with RAL monotherapy and combination therapy. The combination treatment in WT also resulted in a greater modulus and decreased total strain compared to control.
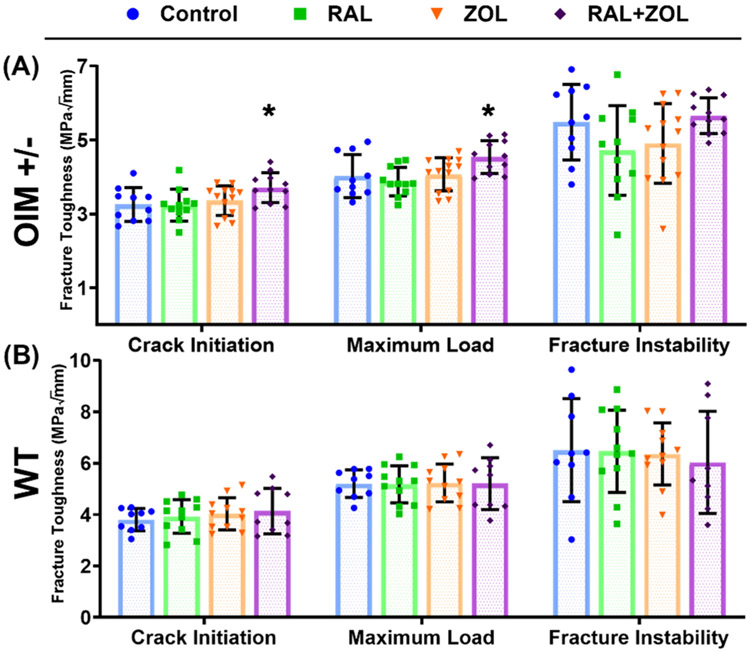
3.4. Fracture toughness was elevated in OIM+/− with combination treatment
Combination treatment in OIM+/− led to significantly higher stress intensity factors at crack initiation and maximum load at the tibial mid-diaphysis (Fig. 3, Supplemental Tables 2 and 3). Neither monotherapy significantly changed fracture toughness in OIM+/−. Treatment in WT produced no significant effects in the stress intensity factors.
Fig. 3.
Treatment effects on the stress intensity factor for crack initiation, maximum load, and fracture instability for OIM+/− (A) and WT (B). Significant change from control indicated by ‘*’ at p < 0.05.
4. Discussion
Current therapeutics used to combat bone fragility are able to increase bone mass but are insufficient on their own to enhance bone quality and improve fracture resistance in some diseased states. Targeting different mechanisms with a combination treatment could be a powerful technique to enhance bone on both a macroscopic (mass) and microscopic tissue (quality) level. The goal of this study was to investigate how the mass-based effects of bisphosphonates, combined with the tissue-level improvements seen with Raloxifene, would improve bone quantity, quality, and fracture resistance. The results demonstrate that ZOL and RAL could be used together to enhance trabecular architecture, cortical mechanical properties, and tissue fracture toughness more than either treatment alone, a finding that was more pronounced in diseased bone.
For trabecular architecture, the combination treatment appeared to be driven by the dominant effects of ZOL, with greater bone volume fraction, greater trabecular number, and lower trabecular spacing. ZOL alone did not impact trabecular thickness or TMD. These trabecular findings are consistent with previous research investigating BP use in adolescent OI mice [31-34]. The results are unsurprising as at this relatively young age, trabecular bone is often lost with longitudinal growth coupled with resorptive modeling in mice. BP use effectively inhibited this modeling and bone volume was maintained.
Although RAL exhibited an overall minimal effect on trabecular structure, there was a trend toward increased trabecular thickness and TMD. Similar changes to thickness and TMD have previously been noted with RAL use [26,32]. The properties did not change with ZOL alone, but with combination therapy, the improvements were synergistic in both genotypes, producing greater than three times the effect of either monotherapy in most cases. Increased thickness with RAL was likely initiated by the cellular anabolic effects of RAL as a SERM drug. When adding ZOL, the resorptive modeling that would have otherwise occurred at the struts was inhibited, and thickness was maintained and elevated compared to control. It is unclear why TMD was amplified with combination treatment, but this positive effect deserves additional investigation.
Although trabecular architecture was enhanced and this positive effect cannot be overlooked, clinical fractures typically occur at cortical locations in the diaphysis rather than the metaphyses, and improved trabecular features does not necessarily address the problem of fracture resistance. In this current study, there were no significant effects on cortical geometry with any treatment in either genotype. Previous work has shown that, in cortical bone, metabolic activity is lower, and it was expected that ZOL would have little effect on mass [31-34]. Likewise, RAL is expected to have limited geometric effect in cortical locations. However, previous work has demonstrated significant increases in cortical thickness that were not reproduced in this study [26,32]. This discrepancy could be due to differences in animal age, as one of the studies investigated mice at from 16 weeks to 24 weeks, or phenotype severity, as the other study used homozygous OI mice bred on a mixed B6/C3H background.
Regardless, the lack of geometric changes suggests that any differences in mechanical properties are most likely driven by changes at the intrinsic tissue level. Greater intracortical porosity has been demonstrated in pediatric OI bone, which negatively impacts cortical mechanical integrity [35,36], but intracortical porosity was not observed here. Given that the majority of clinical fractures occur at cortical regions in OI pediatric patients, further insight on pharmacological improvements of cortical bone porosity and quality should be investigated.
The significant effects of treatment versus control on mechanical behavior were mostly related to strength parameters. The tissues had increased stiffness with all the treatments. ZOL had a modest impact on its own, significantly increasing yield force in WT and yield stress in OIM+/−. The effects of RAL alone were more compelling and drove the changes seen with combination treatment. Yield stress increased in OIM+/− with RAL and combination treatment which led to a significant increase in resilience, indicating the bones were able to absorb more energy before yielding.
Previous work with RAL has demonstrated enhanced post-yield mechanical behavior with bone soaked ex-vivo, one year of treatment in female beagles, and with RAL administration after first treating with ZOL in mice [13,23,32]. In this study, treatment groups did not show increased post-yield properties and, in some cases, surprisingly decreased. This is common with BP use, but a significant decrease in post-yield behavior with RAL was unexpected. Previous work with OI mice at this age range demonstrated no significant effect of in-vivo RAL treatment on any whole bone mechanical properties [26]. In the current study, control groups from both genotypes showed far more total deformation than expected, causing treatment effects to appear significantly lower compared to control. The reason behind these effects is not clear, but it removed the possibility of detecting any potential post-yield effects of treatment.
Perhaps the most important result here is that the combination treatment improved stress intensity factors at crack initiation and maximum load in the diseased mice. This type of test reflects true material-level properties in bone and indicates improved fracture resistance in diseased bone with this combined treatment. It was hypothesized that increasing mass at the same time as improving quality would benefit fracture resistance more than either monotherapy alone. The argument for this is that when more bone is formed in the presence of a compound that enhances quality, the combined tissue-level impact should be large. Because ZOL did not improve cortical mass as expected, and RAL alone did not improve fracture toughness, the improvement with combined treatment is curious. The mechanism behind this change is not known, but future investigation of tissue quality changes should help to clarify. Treatment did not impact WT mice, likely because it is difficult to improve bone that is already of good quality. Similar to the results of this study, fracture toughness has not been shown to improve with BP treatment. In skeletally mature rabbits, ZOL injections did not improve fracture toughness in ulnar sections [37]. In humans, long term BP use has additionally been shown to compromise fracture toughness on femoral corticocancellous biopsies [38].
BPs are the only FDA approved drug for pharmaceutical treatment of Osteogenesis Imperfecta. A recent review paper compiled over 15 years of clinical trials investigating BPs use in patients with OI. It was determined that while both oral and IV BPs substantially improved BMD, it was unclear whether BPs consistently improve fracture resistance and decrease fracture risk [39]. Continuous use of BPs in children and teenagers with OI has revealed some negative impacts on bone health, including microdamage accumulation and delayed healing [40-42]. These findings confirm the need for treatments that focus on improving bone quality, in addition to quantity, to improve skeletal fragility and prevent fracture. The fact that we have shown a positive combined treatment effect on measures of fracture resistance in diseased bone, effectively returning these properties to near WT control levels, is promising and future work will focus on treatment strategies to optimize this effect.
There are some limitations to our study. Male mice were chosen in attempt to reduce the metabolic effects of RAL and utilize its cell-independent mechanism. This was intentional but could limit the findings. The study also did not directly compare the effects of treatment on bone cell activity or on extracellular matrix quality. Future work should be conducted to assess treatment effects on both sexes along with further investigation of cellular and matrix activity in the diseased condition. With fracture toughness testing, sample sizes were not consistent across groups. Numerous bones were accidentally broken during the notching process. It was also difficult to determine the transition lines of crack propagation for several samples. Homozygous (OIM−/−) mice were originally included in this study. However, the severity of the phenotype caused numerous spontaneous fractures in the untreated mice, and only 2 control samples were usable for analysis. Lastly, the age of the mice used here could be controversial. The mice received treatment from 8 weeks to 16 weeks. The age group was chosen to mirror the rapid growth during human adolescence, and potentially when treatment might be started in humans with OI. This age could have led to the high deformation in the control groups due to rapid modeling and slow mineralization. It is also problematic because it does not coincide with an age that humans receive RAL, as RAL is typically only administered in older adults. Future studies will also investigate the effects of combined treatment in older animals, following skeletal maturity.
The use of RAL has some limitations in itself. Although RAL possesses beneficial intrinsic effects, it may not necessarily be the most ideal drug to pursue for combinatorial therapy. RAL suppresses bone loss as a selective estrogen receptor modulator (SERM), but this estrogen therapy produces adverse effects such as hot flashes and increased thrombosis risk, making it a problematic treatment. The estrogen receptor binding also prevents usage in some at-risk patient populations including children, specifically those with OI [43,44]. Other drugs could be considered for a combination treatment. Recent work has shown positive outcomes in OI patients and OI mice treated with sclerostin antibody and denosumab [34,45,46]. A more appropriate therapeutic for combination treatment might be parathyroid hormone (PTH) as its anabolic mechanism has been shown to create better quality new bone [47]. Combination of PTH and alendronate has previously been studied clinically for osteoporosis treatment, but results did not indicate any remarkable benefit compared to either monotherapy [48-50]. In OI patients, PTH has been shown to positively impact BMD, but comparison to BP use or a combination treatment was not studied [51]. These compounds, amongst others, should be investigated deeper to determine an ideal combination, concentration, and dosing schedule. Ideally, there is a need to develop pharmaceuticals that, like RAL, directly target collagen and the extracellular matrix to improve quality at the microscopic level, without acting as a SERM [14].
In conclusion, the current study shows beneficial effects of combination therapy, enhancing quality of diseased bone neither treatment could accomplish alone. Utilizing the mass-based effects of ZOL with the tissue material changes of RAL, combined therapy improved fracture toughness and led to increases in trabecular architecture and cortical mechanics in diseased animals. Combinatorial treatments should be considered for future therapies to optimize patient care and bone health.
Supplementary Material
Acknowledgements
We gratefully acknowledge Charlotte Phillips for the OI breeding colony. This work was supported, in part, by the NSF (AGB: DGE1333468) and NIH (JMW: AR067221, AR072609). We would like to acknowledge the Integrated Nanosystems Development Institute (INDI) for use of their JEOL7800F Field Emission Scanning Electron Microscope, which was awarded through NSF grant MRI-1229514.
Footnotes
Declaration of Competing Interest
There are no known conflicts of interest associated with this publication, and there has been no financial support for this work that could have influenced its outcome.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bone.2019.06.018.
References
- [1].Russell RG, Bisphosphonates: the first 40 years, Bone 49 (2011) 2–19 July. [DOI] [PubMed] [Google Scholar]
- [2].NIH, Osteoporosis prevention, diagnosis, and therapy, JAMA 285 (2001) 785–795 February 14. [DOI] [PubMed] [Google Scholar]
- [3].Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R, Cyclic administration of pamidronate in children with severe osteogenesis imperfecta, N. Engl. J. Med 339 (October 1 1998) 947–952. [DOI] [PubMed] [Google Scholar]
- [4].Russell RG, Watts NB, Ebetino FH, Rogers MJ, Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy, Osteoporos. Int 19 (2008) 733–759 June. [DOI] [PubMed] [Google Scholar]
- [5].Allen MR, Burr DB, Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know, Bone 49 (2011) 56–65 July. [DOI] [PubMed] [Google Scholar]
- [6].Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D, Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate, Osteoporos. Int 20 (2009) 887–894 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD, Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra, Osteoporos. Int 19 (2008) 329–337 March. [DOI] [PubMed] [Google Scholar]
- [8].Acevedo C, Bale H, Gludovatz B, Wat A, Tang SY, Wang M, et al. , Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone, Bone 81 (2015) 352–363 December. [DOI] [PubMed] [Google Scholar]
- [9].Bryant HU, Mechanism of action and preclinical profile of raloxifene, a selective estrogen receptor modulation, Rev. Endocr. Metab. Disord 2 (2001) 129–138 January. [DOI] [PubMed] [Google Scholar]
- [10].Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD, Anti-vertebral fracture efficacy of raloxifene: a meta-analysis, Osteoporos. Int 17 (2006) 313–316 February. [DOI] [PubMed] [Google Scholar]
- [11].Recker RR, Mitlak BH, Ni X, Krege JH, Long-term raloxifene for postmenopausal osteoporosis, Curr. Med. Res. Opin 27 (2011) 1755–1761 September. [DOI] [PubMed] [Google Scholar]
- [12].Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. , Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) investigators, JAMA 282 (1999) 637–645. [DOI] [PubMed] [Google Scholar]
- [13].Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, et al. , Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties, Bone 61 (2014) 191–200. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bivi N, Hu H, Chavali B, Chalmers MJ, Reutter CT, Durst GL, et al. , Structural features underlying raloxifene's biophysical interaction with bone matrix, Bioorg. Med. Chem. 24 (2016) 759–767 February 15. [DOI] [PubMed] [Google Scholar]
- [15].Kuivaniemi H, Tromp G, Prockop DJ, Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels, Hum. Mutat 9 (1997) 300–315. [DOI] [PubMed] [Google Scholar]
- [16].Pihlajaniemi T, Dickson LA, Pope FM, Korhonen VR, Nicholls A, Prockop DJ, et al. , Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation, J. Biol. Chem 259 (1984) 12941–12944. [PubMed] [Google Scholar]
- [17].Rowe DW and Shapiro JR, "Osteogenesis Imperfecta," in Metabolic Bone Disease and Clinically Related Disorders, 3 ed: Academic Press, 1998, pp. 651–683. [Google Scholar]
- [18].Nicolaou N, Agrawal Y, Padman M, Fernandes JA, Bell MJ, Changing pattern of femoral fractures in osteogenesis imperfecta with prolonged use of bisphosphonates, J. Child. Orthop 6 (2012) 21–27 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fritz JM, Guan Y, Wang M, Smith PA, Harris GF, A fracture risk assessment model of the femur in children with osteogenesis imperfecta (OI) during gait, Med. Eng. Phys 31 (2009) 1043–1048 2009/November/01/. [DOI] [PubMed] [Google Scholar]
- [20].Johnell O, Scheele WH, Lu Y, Reginster JY, Need AG, Seeman E, Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis, J. Clin. Endocrinol. Metab 87 (2002) 985–992 March. [DOI] [PubMed] [Google Scholar]
- [21].Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, et al. , How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone, J. Bone Miner. Res 29 (2014) 1392–1401 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, et al. , Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim), Bone 42 (2008) 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Allen MR, Hogan HA, Hobbs WA, Koivuniemi AS, Koivuniemi MC, Burr DB, Raloxifene enhances material-level mechanical properties of femoral cortical and trabecular bone, Endocrinology 148 (2007) 3908–3913. August. [DOI] [PubMed] [Google Scholar]
- [24].Allen MR, Territo PR, Lin C, Persohn S, Jiang L, Riley AA, et al. , In vivo UTE-MRI reveals positive effects of raloxifene on skeletal-bound water in skeletally mature beagle dogs, J. Bone Miner. Res 30 (2015) 1441–1444. August. [DOI] [PubMed] [Google Scholar]
- [25].Aref MW, McNerny EM, Brown D, Jepsen KJ, Allen MR, Zoledronate treatment has different effects in mouse strains with contrasting baseline bone mechanical phenotypes, Osteoporos. Int 27 (2016) 3637–3643 December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berman AG, Wallace JM, Bart ZR, Allen MR, Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta, Matrix Biol. 52-54 (2016) 19–28 May-Jul. [DOI] [PubMed] [Google Scholar]
- [27].Berman AG, Clauser CA, Wunderlin C, Hammond MA, Wallace JM, Structural and mechanical improvements to bone are strain dependent with axial compression of the tibia in female C57BL/6 mice, PLoS One 10 (2015) e0130504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wallace JM, Golcuk K, Morris MD, Kohn DH, Inbred strain-specific response to biglycan deficiency in the cortical bone of C57BL6/129 and C3H/He mice, J. Bone Miner. Res 24 (2009) 1002–1012 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hammond MA, Berman AG, Pacheco-Costa R, Davis HM, Plotkin LI, Wallace JM, Removing or truncating connexin 43 in murine osteocytes alters cortical geometry, nanoscale morphology, and tissue mechanics in the tibia, Bone 88 (2016) 85–91 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ritchie RO, Koester KJ, Ionova S, Yao W, Lane NE, Ager JW 3rd, Measurement of the toughness of bone: a tutorial with special reference to small animal studies, Bone 43 (2008) 798–812 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bargman R, Posham R, Boskey AL, DiCarlo E, Raggio C, Pleshko N, Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta, Osteoporos. Int 23 (2012) 1141–1150 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meixner CN, Aref MW, Gupta A, McNerny EMB, Brown D, Wallace JM, et al. , Raloxifene improves bone mechanical properties in mice previously treated with zoledronate, Calcif. Tissue Int 101 (2017) 75–81 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Misof BM, Roschger P, Baldini T, Raggio CL, Zraick V, Root L, et al. , Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse, Bone 36 (2005) 150–158 January. [DOI] [PubMed] [Google Scholar]
- [34].Olvera D, Stolzenfeld R, Marini JC, Caird MS, Kozloff KM, Low dose of bisphosphonate enhances sclerostin antibody-induced trabecular bone mass gains in Brtl/+ osteogenesis imperfecta mouse model, J. Bone Miner. Res. 33 (2018) 1272–1282 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Imbert L, Aurégan J-C, Pernelle K, Hoc T, Microstructure and compressive mechanical properties of cortical bone in children with osteogenesis imperfecta treated with bisphosphonates compared with healthy children, J. Mech. Behav. Biomed. Mater 46 (2015) 261–270 (2015/June/01/). [DOI] [PubMed] [Google Scholar]
- [36].Vardakastani V, Saletti D, Skalli W, Marry P, Allain JM, Adam C, Increased intra-cortical porosity reduces bone stiffness and strength in pediatric patients with osteogenesis imperfecta, Bone 69 (2014) 61–67 December. [DOI] [PubMed] [Google Scholar]
- [37].Hunckler MD, Chu ED, Baumann AP, Curtis TE, Ravosa MJ, Allen MR, et al. , The fracture toughness of small animal cortical bone measured using arc-shaped tension specimens: effects of bisphosphonate and deproteinization treatments, Bone 105 (2017) 67–74 December. [DOI] [PubMed] [Google Scholar]
- [38].Lloyd AA, Gludovatz B, Riedel C, Luengo EA, Saiyed R, Marty E, et al. , Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 8722–8727. August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dwan K, Phillipi CA, Steiner RD, Basel D, Bisphosphonate therapy for osteogenesis imperfecta (in eng), Cochrane Database Syst. Rev 7 (2014) p. Cd005088. [DOI] [PubMed] [Google Scholar]
- [40].Anam EA, Rauch F, Glorieux FH, Fassier F, Hamdy R, Osteotomy healing in children with osteogenesis imperfecta receiving bisphosphonate treatment, J. Bone Miner. Res 30 (2015) 1362–1368. August. [DOI] [PubMed] [Google Scholar]
- [41].Papapoulos SE, Cremers SC, Prolonged bisphosphonate release after treatment in children, N. Engl. J. Med 356 (2007) 1075–1076 March 8. [DOI] [PubMed] [Google Scholar]
- [42].Vasanwala RF, Sanghrajka A, Bishop NJ, Hogler W, Recurrent proximal femur fractures in a teenager with osteogenesis imperfecta on continuous bisphosphonate therapy: are we overtreating? J. Bone Miner. Res 31 (2016) 1449–1454 July. [DOI] [PubMed] [Google Scholar]
- [43].Qaseem A, Forciea MA, McLean RM, Denberg TD, Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians, Ann. Intern. Med 166 (2017) 818–839 June 6. [DOI] [PubMed] [Google Scholar]
- [44].Reid IR, Efficacy, effectiveness and side effects of medications used to prevent fractures, J. Intern. Med 277 (2015) 690–706 June. [DOI] [PubMed] [Google Scholar]
- [45].Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, et al. , BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial, J. Bone Miner. Res 32 (2017) 1496–1504 July. [DOI] [PubMed] [Google Scholar]
- [46].Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. , Safety and efficacy of denosumab in children with osteogenesis imperfect–a first prospective trial, J. Musculoskelet. Neuronal Interact 16 (2016) 24–32 March. [PMC free article] [PubMed] [Google Scholar]
- [47].Sato M, Zeng GQ, Turner CH, Biosynthetic human parathyroid hormone (1–34) effects on bone quality in aged ovariectomized rats, Endocrinology 138 (1997) 4330–4337. [DOI] [PubMed] [Google Scholar]
- [48].Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. , The Effects of Parathyroid Hormone and Alendronate Alone or in Combination in Postmenopausal Osteoporosis, vol. 349, (2003), pp. 1207–1215. [DOI] [PubMed] [Google Scholar]
- [49].Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM, The Effects of Parathyroid Hormone, Alendronate, or Both in Men with Osteoporosis, vol. 349, (2003), pp. 1216–1226. [DOI] [PubMed] [Google Scholar]
- [50].Khosla S, Parathyroid Hormone plus Alendronate — A Combination That Does Not Add up, vol. 349, (2003), pp. 1277–1279. [DOI] [PubMed] [Google Scholar]
- [51].Orwoll ES, Shapiro J, Veith S, Wang Y, Lapidus J, Vanek C, et al. , Evaluation of teriparatide treatment in adults with osteogenesis imperfecta, J. Clin. Invest 124 (2014) 491–498 February/03/. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.