Abstract
On 13 June 2018, Genentech, Inc. issued a press release announcing that the US FDA had approved the antiangiogenesis drug, bevacizumab, in combination with chemotherapy for frontline and maintenance therapy for women with newly diagnosed ovarian cancer. Regulatory approval was based on the National Cancer Institute-sponsored Gynecologic Oncology Group (GOG) protocol 0218, the Phase III, randomized, placebo-controlled, double-blind, multi-center and multi-national clinical trial that met its primary end point, progression-free survival. Bevacizumab is now approved in the frontline, platinum-sensitive recurrent and platinum-resistant recurrent settings for epithelial ovarian cancer. This review will address the broad range of clinical trials addressing the efficacy of bevacizumab use in ovarian cancer.
Keywords: : bevacizumab, biological therapy, clinical trials, gynecologic/ovarian
The formation of new blood vessels, known as angiogenesis, is essential for solid tumor growth and metastasis. VEGF is a protein-signaling molecule that acts on endothelial cells to promote angiogenesis. VEGF is overexpressed in epithelial ovarian cancers making targeting of angiogenesis through the VEGF-signaling pathway an attractive therapeutic option [1]. Bevacizumab is a recombinant humanized monoclonal antibody against VEGF-A that directly binds and neutralizes VEGF-A preventing activation of the VEGF receptor. In 2004, bevacizumab was the first antiangiogenesis agent approved by the US FDA for metastatic colon cancer and since then it has been approved for multiple indications, including ovarian cancer. This review aims to provide a comprehensive overview of the use bevacizumab in ovarian, fallopian tube and primary peritoneal cancers, hereafter referred to collectively as ovarian cancer.
The role of bevacizumab in primary treatment of epithelial ovarian cancer
For the past two decades, platinum- and taxane-based doublet therapy has been the preferred adjuvant chemotherapy regimen [2]. Bevacizumab is theorized to works synergistically with standard chemotherapy in targeting angiogenesis [3,4]. Carboplatin induces VEGF expression in the tumor microenvironment potentiating the effect of bevacizumab and paclitaxel is antiangiogenic inhibiting endothelial cell proliferation and migration. Bevacizumab also normalizes tumor vasculature to facilitate delivery of chemotherapeutic agents. Early Phase II studies of single-agent bevacizumab as salvage therapy in heavily pretreated ovarian cancer patients demonstrated the safety and efficacy of bevacizumab and provided the preliminary data to launch a Phase III trial [5–7]. In 2011, the results of two randomized, Phase III clinical trials, GOG-0218 and ICON-7, were published simultaneously and showed a significant increase in progression-free survival (PFS) when bevacizumab was added to standard adjuvant chemotherapy with carboplatin and paclitaxel (Table 1).
Table 1. . The five principle Phase III randomized trials of bevacizumab in advanced ovarian carcinoma.
| Study (year) | Trial design | Eligibility | Treatment arms | Bevacizumab dose | n | Median PFS, months (HR, 95% CI) | Median OS, months (HR, 95% CI) | Comments |
|---|---|---|---|---|---|---|---|---|
| Bevacizumab for primary EOC treatment | ||||||||
| GOG-0218 (2011) | Double-blind, Placebo-controlled | Newly diagnosed stage III or IV EOC† with gross residual disease after maximal debulking effort | Arm (1) C AUC 6, T 175 mg/m2 q3 weeks for six cycles + placebo q3 weeks cycles 2–22 Arm (2) C AUC 6, T 175 mg/m2 q3 weeks for six cycles + B 15 mg/kg q3 weeks cycles 2–6 AND placebo q3 weeks cycles 7–22 Arm (3) C AUC 6, T 175 mg/m2 q3 weeks for six cycles + B 15 mg/kg q3 weeks cycles 2–22 |
15 mg/kg | 1873 | Arm (1) 10.3 (ref.) Arm (2) 11.2 (0.91, 0.80–1.04) Arm (3) 14.1 (0.72, 0.63–0.82)¶ |
Arm (1) 40.6 (ref.) Arm (2) 38.7 (1.07, 0.91–1.26) Arm (3) 43.8 (0.88, 0.75– 1.04) |
Overall poor prognosis; 40% were suboptimally debulked and another 25% had stage IV cancer |
| ICON7 (2011) | Open-label | Newly diagnosed stage I–IIA grade 3 EOC, any stage with clear cell histology and stage III or IV EOC all after maximal debulking effort | Arm (1) C AUC 5 or 6, T 175 mg/m2 q3 weeks for six cycles + placebo q3 weeks cycles 1 or 2 through cycle 18 Arm (2) C AUC 5 or 6, T 175 mg/m2 q3 weeks for six cycles + B 7.5 mg/kg q3 weeks cycles 1 or 2 through cycle 18 |
7.5 mg/kg | 1528 | Total cohort Arm (1) 17.5 (ref.) Arm (2) 19.9 (0.93, 0.83–1.05) High-risk progression‡ Arm (1) 10.5 (ref.) Arm (2) 16.0 (0.73, 0.61–0.88)¶ |
Total cohort Arm (1) 58.6 (ref.) Arm (2) 58.0 (0.99, 0.85–1.14) High-risk progression‡ Arm (1) 30.2 (ref.) Arm (2) 39.7 (0.78, 0.63–0.97)¶ |
High-risk early-stage limited to 9% of study population. Only 26% had suboptimal debulking and 12% had stage IV cancer |
| Bevacizumab for recurrent EOC treatment | ||||||||
| OCEANS (2012) | Double-blind, Placebo-controlled | Platinum-sensitive (>6 month PFI), one prior anticancer regimen | Arm (1) C AUC 4 day 1, G 1000 mg/m2 on day 1, day 8 q3 weeks for six to ten cycles + placebo day 1 q3 weeks until progression or toxicity Arm (2) C AUC 4 day 1, G 1000 mg/m2 on day 1, day 8 q3 weeks for six to ten cycles + B 15 mg/kg day 1 q3 weeks until progression or toxicity |
15 mg/kg | 484 | Arm (1) 8.4 (ref.) Arm (2) 12.4 (0.48, 0.39–0.61)¶ |
Arm (1) 32.9 Arm (2) 33.6 (0.95, 0.77–1.18) |
PFI >12 months in 58% of patients |
| GOG-0213 (2017) | Open-label | Platinum-sensitive (>6 month PFI), one prior anticancer regimen. Patients enrolled after 08/2011 needed to also meet surgical eligibility criteria | Arm (1) C AUC 5, T 175 mg/m2 q3 weeks for six to eight cycles Arm (2) C AUC 5, T 175 mg/m2 q3 weeks for six to eight cycles + B 15 mg/kg q3 weeks until progression or toxicity (prior to chemorandomization, patient may have been randomized to secondary cytoreductive surgery) |
15 mg/kg | 674 | Pre-treatment stratification Arm (1) 10.4 (ref.) Arm (2) 13.8 (0.63, 0.53–0.74) |
Pre-treatment stratification Arm (1) 37.3 (ref.) Arm (2) 42.2 (0.83, 0.68–1.01) Sensitivity analysis for TFI§ HR: 0.823 (95% CI: 0.680–0.996)¶ |
PFI >12 months in 75% of patients. 10% of patients had prior bevacizumab treatment. 16% of patients were eligible for secondary cytoreduction |
| AURELIA (2014) | Open-label | Platinum-resistant (<6 month PFI), up to two prior anticancer regimens. Platinum refractory patients excluded | Arm (1) Chemotherapy alone, either T 80 mg/m2 weekly q4 weeks or PLD 40 mg/m2 IV q4 weeks or TPT 4 mg/m2 IV on days 1, 8, and 15 q4 weeks or TPT 1.25 mg/m2 on days 1–5 q3 weeks until progression or toxicity Arm (2) Chemotherapy (as in Arm 1) plus B 10 mg/kg q2 weeks until progression or B 15 mg/kg q3 weeks if on TPT q3 weeks until progression or toxicity |
10 mg/kg or 15 mg/kg | 361 | Arm (1) 3.4 (ref.) Arm (2) 6.7 (0.48, 0.38–0.60)¶ |
Arm (1) 13.3 (ref.) Arm (2) 16.6 (0.85, 0.66–1.08) |
25% of patients had PFI <3 months. 7% had prior antiangiogenic therapy, 40% had two prior chemotherapy lines. By study design, 40% of patients in Arm (1) crossed over to single-agent B 15 mg/kg q3 weeks after progression on chemotherapy alone |
EOC includes all epithelial ovarian, fallopian tube and primary peritoneal carcinoma.
High risk of progression was defined as stage IV disease, inoperable stage III disease or suboptimally debulked (>1 cm residual) stage III disease.
Patients were stratified based on platinum TFI, however, in 7% of patients the TFI was calculated from the last cycle of any chemotherapy instead of from the last cycle of platinum-based primary chemotherapy. The sensitivity analysis corrected for this error.
Significant statistical findings (p-value <0.05).
Most recent survival analysis as of June 2018 included. Final OS data may not yet be reported.
AUC: Area under the curve; B: Bevacizumab; C: Carboplatin; EOC: Epithelial ovarian cancer; G: Gemcitabine; HR: Hazard ratio; OS: Overall survival; PFI: Platinum-free interval; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin; ref: Reference group; T: Paclitaxel; TFI: Treatment-free interval; TPT: Topotecan.
Reproduced with permission from [8–12].
GOG-0218
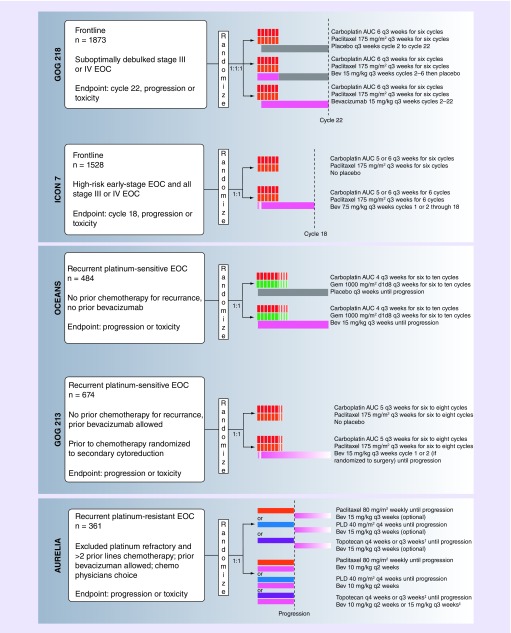
In GOG-0218, a multicenter, Phase III randomized, placebo-controlled study, patients with stage III or IV epithelial ovarian cancer (EOC) and measurable disease after surgical debulking were randomized to three treatment arms: six cycles of carboplatin/paclitaxel, plus placebo through 22 cycles; six cycles of carboplatin/paclitaxel with bevacizumab on cycles 2–6, plus placebo through 22 cycles (‘Bev-initiation’); or six cycles of carboplatin/paclitaxel with bevacizumab on cycles 2–6, plus bevacizumab maintenance through 22 cycles (‘Bev-throughout’; Figure 1) [8]. Initially, patients who were optimally debulked (<1 cm residual disease) were excluded, resulting in a cohort with an overall poor prognosis. After a protocol amendment, patients with any gross residual disease were included and approximately a third of all study patients were optimally debulked.
Figure 1. . Schema for GOG-0218, ICON 7, OCEANS, GOG-0213, and AURELIA.
†Topotecan 4 mg/m2 IV on days 1, 8, and 15 q4 week or 1.25 mg/m2 on days 1–5 q3 week
‡Bev 15mg/kg q3 weeks only for q3 weeks topotecan regimen
AUC: Area under the curve; Bev: Bevacizumab; EOC: Epithelial ovarian cancer; Gem: Gemcitabine; PLD: Pegylated liposomal doxorubicin.
A total of 1873 patients were enrolled and the median follow-up time for PFS was 17.4 months. When compared with the control group, patients who received bevacizumab as upfront and maintenance therapy (Bev-throughout) had a significant 3.8-month improvement in PFS (14.1 vs 10.3 months; hazard ratio [HR]: 0.72; 95% CI: 0.63–0.82). Using bevacizumab only concurrently with carboplatin/paclitaxel (Bev-initiation) resulted in a 1-month improvement in PFS, which was not statistically significant (11.2 vs 10.3 months; HR: 0.91; 95% CI: 0.80–1.04). When calculating PFS with radiologic progression only, the median PFS was 18.2 months, compared with 12.0 months for the control group (HR: 0.62; 95% CI: 0.52–0.75), doubling the survival benefit to 6.2 months [13]. The secondary end point for overall survival (OS) was reached after a median follow-up of 103 months after which 80% of patients had died. In final analysis, there was no difference in OS between the three arms, with all arms having an approximately 41–43-month OS in the intent-to-treat group (HR: 0.96; 95% CI: 0.85–1.09 for Bev-initiation vs control; HR: 1.06; 95% CI: 0.94–1.20 for Bev-throughout vs control) [14]. However, patients with stage IV disease receiving Bev-throughout had an OS of 42.8 months, compared with stage IV control (32.6 months) and stage IV Bev-initiation (34.5 months), indicating a larger treatment effect with more advanced disease.
ICON-7
ICON-7 was an international, open-label, randomized Phase III study of 1528 women with EOC. In contrast to GOG-0218, ICON-7 was not blinded, included patients with high-risk early-stage disease and optimally debulked stage III–IV EOC, had only two arms and defined disease progression using RECIST criteria alone. Participants were randomized to either: six cycles of carboplatin/paclitaxel or six cycles of carboplatin/paclitaxel with bevacizumab on cycles 2–6 (option to include in cycle 1 if chemotherapy starting >1 month after surgery), plus bevacizumab maintenance through 18 total cycles [9]. At a median follow-up of 19.4 months, the median PFS was significantly improved by 2 months in the bevacizumab group compared with the control group (19.0 vs 17.3 months; HR: 0.81; 95% CI: 0.70–0.94). In an updated analysis after a median follow-up of 48.9 months, there was no statistically significant difference in PFS or OS in the overall patient cohort. Patients in ICON-7 had an overall more favorable prognosis compared with the population studied in GOG-0218, which may explain a difference in the findings. In subgroup analysis of 502 patients with a high-risk of disease progression, which mirrored the population in GOG-0218, the median PFS was 10.5 months for the control group and 16 months for the bevacizumab group (HR: 0.73; 95% CI: 0.61–0.88; p < 0.001). OS was also significantly improved in the high-risk group for patients who received bevacizumab compared with the control (39.7 vs 30.2 months; HR: 0.78; 95% CI: 0.63–0.97) [15].
The improvement in PFS seen in these two clinical trials has been affirmed in meta-analyses [16]. There are several interesting observations to come from these trials. First, there seems to be a relationship with bevacizumab where patients with a poorer prognosis have a more sizeable improvement in PFS. In GOG-0218 and the ICON-7 high-risk subgroup, the difference in median PFS is approximately 4 months, but in the ICON-7 lower-risk subgroup there was no difference in PFS. That the size of effect is larger with patients with suboptimally debulked disease makes sense intuitively in that microscopic tumor cells (those <1–2 mm) would be less active in angiogenesis and produce less VEGF-A. Second, the maximum separation of the PFS curves in GOG-0218 and ICON-7 occurred at 15 and 12 months, respectively, when bevacizumab maintenance therapy was stopped. Since bevacizumab does not change the tumor potential to generate VEGF-A and is cleared after administration, discontinuing bevacizumab is expected to result in resumption of angiogenesis and tumor regrowth. A discussion on the duration of bevacizumab maintenance will be addressed later in this review.
Safety & quality of life in GOG-0218 & ICON-7
GOG-0218 and ICON-7 demonstrated a safety profile of bevacizumab similar to other cancers. The most common side effects associated with bevacizumab treatment are hypertension, proteinuria and nasal mucosal disorder (epistaxis). Additional side effects of bevacizumab include impaired wound healing, gastrointestinal (GI) perforation or fistula, arterial or venous thrombosis and rarer serious outcomes (Table 2) [17]. Other side effects during chemotherapy such as neutropenia, thrombocytopenia, neuropathy and hypersensitivity reactions occurred commonly in both studies but were not thought to be due to bevacizumab. Bevacizumab package labeling has a black box warning for the risk of GI perforation, wound-healing complications and hemorrhage. In general, most adverse events occur during the combination chemotherapy phase of bevacizumab administration. Bevacizumab in well tolerated and in trials of prolonged maintenance exposure to bevacizumab the median time to discontinuation from unacceptable toxicity in 9.9 months [18]. In patients with more serious toxicities, bevacizumab administration can be delayed or discontinued altogether; there are no recommended dose reductions.
Table 2. . Adverse events of interest reported in GOG-0218, ICON 7, OCEANS, GOG-0213, and AURELIA.
| Adverse event† | GOG-0218‡ | ICON7 | OCEANS | GOG-0213 | AURELIA | Relative risk (95% CI)§ |
|---|---|---|---|---|---|---|
| Hypertension, (grade ≥2) | 22.9% | 18% | 18.2% (grade ≥3) | 32% | 20% | 4.09 (3.84–6.25) |
| Proteinuria, (grade ≥3) | 1.6% | 1% | 10.9% | 8% | 10.6% | 6.63 (3.17–13.88) |
| GI events, (grade ≥2, perforation, fistula, necrosis, anastamotic leak) | 2.6% | 1% | 2.4% | 2% | 4.4% | Perforation 2.90 (1.45–5.82) Fistula or abscess 1.70 (0.7–3.97) |
| Thromboembolic events, (all grades) | 7.4% (6.7% VTE) | 11% | 6.9% (4.5% VTE) | 7% (all ATE) | 5% | ATE 2.29 (1.33–3.75) VTE 1.32 (0.995–1.82) |
| Non-CNS bleeding, (grade ≥3) | 2.1% | 1% | 6.5% | 1.8% | 1.1% | 3.63 (1.81–7.29) |
| CNS bleeding, (all grades) | 0.3% | <1% | 0.8% | 0.3% | Not reported | 3.42 (0.72–16.35) |
| PRES | 0.2% | 0 | 0.8% | 0 | 0.6% | 4.23 (0.72–25.03) |
| Impaired wound healing, (all grades) | 3.6% | 5% | 0.8% | 3% | 0 | 1.48 (0.85–2.57) |
Patients randomized to bevacizumab arm.
Bev-throughout group (Arm 3).
Bevacizumab containing arm compared with chemotherapy alone. Excludes GOG-0213 as results not available at time of analysis.
ATE: Arterial thromboembolism; CNS: Central nervous system; GI: Gastrointestinal; PRES: Posterior reversible encephalopathy syndrome; VTE: Venous thromboembolism.
Ovarian cancer patients treated with bevacizumab have the highest bowel perforation rate of all solid cancers studied [20]. The cause is multifactorial but bevacizumab is thought to impair the ability of intestinal mucosa to heal after an insult. In an early clinical trial of recurrent EOC (ROC) patients treated with single-agent bevacizumab, five patients (11%) developed an event attributed to a GI perforation, one which resulted in a patient death. Each of these patients had evidence of bowel involvement prior to the study and had received three or more prior chemotherapy regimens. After the results of this study were published, clinical trials of bevacizumab use in EOC incorporated strict criteria excluding patients with a current or history of bowel obstruction, bowel involvement seen on imaging, as well as history of GI fistula, GI perforation or intra-abdominal abscess [8,10]. The mortality of bowel perforation is approximately 25–50% and prevention is paramount. In GOG-0218, there were 18 GI perforations in the two bevacizumab arms and in ICON-7 there were 10 GI perforations in the bevacizumab arm. Multiple studies, including GOG-0218, have looked a risk factors and predictors of GI adverse events [21,22]. The risk of a GI adverse event is increased with evidence of ileus/bowel obstruction, prior bowel resection with anastomosis, bowel resection at primary cytoreductive surgery with upfront therapy, history of inflammatory bowel disease and rectovaginal nodularity [20]. The risk of anastomotic leak after bowel resection is increased by two- to threefold in EOC treated with bevacizumab. Neoadjuvant chemotherapy (NACT) can decrease the risk of bowel resection by 50%, which ultimately might lead to fewer GI complications [23], and bevacizumab incorporated into NACT regimens (although held for 28 days prior to interval debulking surgery [IDS]) does not appear to result in an increased risk of adverse GI events [24]. Interestingly, prior bowel surgery is not always the direct cause of bowel perforation, as the anastomosis is only involved in 30% of cases, but rather may be a surrogate for bowel obstruction and bowel disease [25]. Most GI perforations occur within 50 days of initiation of bevacizumab. There is evidence that bevacizumab is tolerable in patients with three or more prior cytotoxic regimens but it is not approved by the FDA for this indication given the early safety signals related to GI perforation [26].
Despite the additional side effects, maintenance bevacizumab does not affect quality of life (QOL). In GOG-0218, QOL survey scores were initially lower for the bevacizumab arm but there was no difference in QOL during the maintenance phase [27]. In ICON-7, there was no overall difference in QOL during the study period.
Bevacizumab in conjunction with NACT
NACT is a treatment option for women with advanced ovarian cancer, with the intent to reduce the morbidity of IDS and increase the likelihood of obtaining complete cytoreduction [23]. There is enthusiasm that neoadjuvant bevacizumab may further improve cytoreduction at IDS given the enhanced efficacy seen with bulky disease but this must be weighed against concerns for major GI complications and impaired wound healing, which are known side effects of bevacizumab. Neither GOG-0218 or ICON-7 protocols allowed for NACT and to date studies of neoadjuvant bevacizumab have been limited. In a case–control study of 75 patients with advanced ovarian cancer treated with a median of four cycles of carboplatin/paclitaxel/bevacizumab compared with carboplatin/paclitaxel alone, the ORR was 88% for patients receiving bevacizumab compared with 72.3 % in the control group (p = 0.054), there was no significant difference in complications and the PFS was 18 months for cases compared with 10 months for controls (p = 0.001) [28]. In a subsequent multicenter open-label, noncomparative Phase II study of NACT, 95 patients with stage IIIC or IV EOC with unresectable disease were randomized to receive four cycles of neoadjuvant carboplatin/paclitaxel with or without bevacizumab on cycles 1 through 3 (Table 3) [24]. More patients in the bevacizumab arm were candidates for IDS after NACT (69 vs 60%). Neoadjuvant bevacizumab was tolerable with no significant difference in completion rates of NACT between the two groups. In the bevacizumab group, the complete resection rate (CRR) at time of IDS was 58.6% (with a lower CI of 47.0%) compared with 46.3% in the Phase III EORTC study group [23], with no significant difference in adverse events. However, the 51.4% CRR in the control arm was also higher than the EORTC population suggesting the surgeons in this study were overall more aggressive [23]. The CRR for patients who received bevacizumab and were candidates for IDS was 85%, with a lower CI of 72.5%. In the MITO16A-MaNGO OV2A on prognostic biomarkers for upfront bevacizumab, an unplanned analysis was performed on 74 patients who received NACT with bevacizumab. The median cycles received were three and 86.5% of cases were optimally debulked and 63.5% of cases had no residual disease after IDS. Wound-healing complications occurred in 3% of patients and GI fistula in 1% [29]. There is currently an ongoing clinical trial (NCT01847677) of bevacizumab in the neoadjuvant setting that is nearing completion.
Table 3. . Additional trials of interest using bevacizumab in ovarian carcinoma.
| Frontline regimen strategy | Study name | Trial design | Eligibility | Treatment arms | n | Outcome | Results | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | ANTHALYA (NCT01739218) | Phase II, multicenter, randomized, open label | Stage IIIC or IV EOC considered non-optimally resectable | Arm 1) C AUC 6, T 175 mg/m2 q3 weeks for four cycles, followed by IDS, then C AUC 6, T 175 mg/m2 q3 weeks for four additional cycles Arm 2) C AUC 6, T 175 mg/m2 q3 weeks for four cycles with B 15mg/kg q3 weeks cycles 1–3, followed by IDS, then C AUC 6, T 175 mg/m2 q3 weeks for cycles 5–8 with B 15mg/kg q3 weeks cycles 6–26 |
95 | CRR | Intention to treat: Arm 1) CRR: 51.8% Arm 2) CRR: 58.6% IDS only patients: Arm 1) CRR: 86.4% Arm 2) CRR: 85.5% |
IDS performed in 65% of patients CRR >45% considered statistically significant |
[24] |
| MITO16A-MaNGO OV2A (NCT01706120) | Phase IV, multicenter, non-randomized | Overall Cohort: stage IIIB, IIIC or IV EOC Subgroup limited to patients who underwent IDS |
C AUC 5, T 175 mg/m2 + B 15mg/kg q3 weeks followed by IDS, then C AUC 5, T 175 mg/m2 q3 weeks + B 15mg/kg q3 weeks adjuvant | 79 | Descriptive subgroup analysis | CRR: 63.5% CR <1cm: 86.5% |
79 patients (19.8%) of overall study population underwent IDS | [29] | |
| Dose dense paclitaxel | OCTAVIA (NCT00937560) | Phase II, single arm, open label | High-risk early-stage disease, and stage III or IV any grade EOC | C AUC 6 day 1, T 80mg/m2 days 1, 8, 15 and B 7.5mg/kg day 1 q3 weeks for 6–8 total cycles with B maintenance q3 weeks through 17 cycles | 189 | PFS | Median PFS: 23.7 months (95% CI, 19.8–26.4 months) | High risk early stage: stage I–IIA with grade 3 histology PFS >18 months prespecified as clinically meaningful |
[33] |
| GOG-0262 (NCT01167712) | Phase III, multicenter, randomized, open label | Stage II to IV EOC | Arm 1) C AUC 6, T 175 mg/m2 q3 weeks for six cycles Arm 2) C AUC 6 day 1, T 80mg/m2 days 1, 8, 15 q3 weeks for six cycles Both arms stratified by optional B 15 mg/kg q3 week |
580 | PFS | Arm 1) PFS w/ B: 14.7 months PFS w/o B: 10.3 months Arm 2) PFS w/ B: 14.9 months PFS w/o B: 14.2 months |
580 patients (84%) of overall cohort received bevacizumab Median PFS in patients who opted for bevacizumab was not different in patients who received paclitaxel weekly versus every 3 weeks (14.9 vs 14.7 months, HR: 0.99; 95% CI: 0.83 to 1.20; p = 0.60) |
[4] | |
| Intraperitoneal chemotherapy | GOG-0252 (NCT00951496) | Phase III, multicenter, randomized, open label | Stage II to IV EOC | Arm 1) IV C AUC 6 day 1, IV T 80mg/m2 days 1, 8, 15 q3 weeks for cycles 1–6 and B 15 mg/kg q3 weeks cycle 2–22 Arm 2) IP C AUC 6 day 1, IV T 80mg/m2 days 1, 8, 15 q3 weeks for cycles 1–6 and B 15 mg/kg q3 weeks cycles 2–22 Arm 3) IV T 135 mg/m2 day 1, IP cisplatin 75 mg/m2 day 2, IP T 60 mg/m2 day 8 q3 weeks for cycles 1–6 and B 15 mg/kg q3 weeks cycles 2–22 |
1560 | PFS | Arm 1) 24.9 months (ref.) Arm 2) 27.4 months (HR: 0.925; 95% CI: 0.802 to 1.07) Arm 3) 26.2 months (HR: 0.977; 95% CI: 0.847 to 1.13) |
There was no benefit of IP therapy in this trial IP cisplatin with bevacizumab increased hypertension and led to bevacizumab discontinuation and overall fewer cycles of chemotherapy |
[37] |
| Alternative platinum and taxane based therapy | TEACO (NCT00296816) | Phase II, single arm, open label | Stage IB to IV EOC | Oxaliplatin 85 mg/m2, docetaxel 75 mg/m2, and B 15 mg/kg q3 week, followed by maintenance B for up to 1 year | 132 | PFS rate | PFS rate, 12 months: 65.7% (95% CI: 53.4% to 76.7%) Median PFS: 16.3 months |
PFS rate at 12 months considered statistically significant | [40] |
EOC: Epithelial ovarian cancer; C: Carboplatin; T: Paclitaxel; B: Bevacizumab; IDS: Interval debulking surgery; CRR: Complete resection rate; CR: Complete resection; PFS: Progression-free survival; IP: Intraperitoneal; IV: Intravenous.
Bevacizumab with dose-dense paclitaxel
The results of Phase III clinical trials on the efficacy of dose-dense paclitaxel have been mixed. In JGOG-3016 (NOVEL), the median PFS was 28.0 in patients receiving dose-dense paclitaxel, compared with 17.2 months for patients using conventional q3 weeks dosing [30] but subsequent trials such as MITO-7 [31], GOG-0262 [4] and ICON-8 [32] have shown no improvement in PFS. There have been two large studies of carboplatin/dose-dense paclitaxel in combination with bevacizumab but neither protocol involved randomization limiting the strength of the findings.
In the single-arm OCTAVIA study, 189 patients with high-risk early-stage disease, or stage III or IV EOC (similar to the ICON-7 cohort) were treated with carboplatin/dose-dense paclitaxel plus bevacizumab, followed by bevacizumab maintenance. The median PFS was 23.7 months (95% CI: 19.8–26.4 months) for the overall cohort and 18.1 months (16.0–19.6 months) for the high-risk progression group. The 1-year progression-free rate was 86%. Median OS was unable to be calculated due to low number of events after 2 years; 1- and 2-year OS rates were 97.8% (95% CI: 95.7–99.9%) and 92.3% (95% CI: 88.4–96.2%). The rate of adverse events was similar to ICON-7 [33,34].
In GOG-0262, a study of carboplatin/dose-dense paclitaxel, 84% of participants elected to have upfront bevacizumab. When the results were stratified based on bevacizumab treatment, patients who did not receive bevacizumab had a statistically significant improvement in PFS of 3.9 months in the weekly paclitaxel group (14.2 vs 10.3 months; HR: 0.62). Interestingly, in the bevacizumab treatment group, there was no difference in PFS between the dose-dense and conventional chemotherapy regimens (14.9 vs 14.7 months). Thus, the PFS benefit of dose-dense paclitaxel and bevacizumab is 4 months each when used independently, but the effect is not additive. Results should be interpreted with caution as patients were not randomized to bevacizumab and the nonbevacizumab group was only 16% of the study population. These finding may be further validated in the ICON8B clinical trial, which is currently enrolling patients. In ICON8B, 660 patients will be enrolled and randomized to either carboplatin/dose dense paclitaxel plus bevacizumab or conventional q3 weeks carboplatin/paclitaxel plus bevacizumab.
Bevacizumab with intraperitoneal chemotherapy
In optimally debulked patients with stage III ovarian cancer randomized to intraperitoneal (IP) cisplatin/IV paclitaxel or IV cisplatin/IV paclitaxel, there is an improvement in PFS (23.8 vs 18.3 months; HR: 0.80; 95% CI: 0.64–1.0) and OS (65.6 vs 49.7 months; HR: 0.75; 95% CI: 0.58–0.97) in patients who receive IP chemotherapy [35]. This survival advantage was seen despite only 42% of patients completing all six cycles of IP chemotherapy due to significant toxicity. The first study to include bevacizumab in an IP chemotherapy regimen was a Phase II trial of 41 patients treated with IP cisplatin, IV paclitaxel and IV bevacizumab, notably the dose of cisplatin in this trial was reduced compared with GOG-172 and IV paclitaxel was given over 3 h instead of 24 h [36]. The median PFS was estimated to be 28.6 months (95% CI: 19.1–38.9 months). More importantly, the addition of bevacizumab to IP chemotherapy was shown to be feasible, with 73% of patients were able to receive all six cycles, although safety concerns were raised over an increased number of bowel obstructions and hypertension. GOG-0252 is a randomized Phase III trial of 1560 patients with stage II–IV optimally debulked ovarian cancer with three arms: IP carboplatin/IV paclitaxel/IV bevacizumab; IP cisplatin/paclitaxel and IV paclitaxel/bevacizumab; or the control group IV carboplatin/paclitaxel/bevacizumab. There was no statistically significant difference in median PFS (27.3 vs 26.0 vs 24.9 months) or OS (74.2 vs 67.6 vs 75.4 months) among the three arms [37]. The study protocol used a reduced dose of IP cisplatin in an attempt to limit toxicity, however, this dose was still less well tolerated than IP or IV carboplatin with lower patient reported outcome scores and an increase in bevacizumab-induced hypertension. GOG-0252 is noteworthy as it is the first of the four IV/IP Phase III clinical trials to isolate the effect of IP chemotherapy. While OS data are not mature, it is unlikely that IP intervention in GOG-0252 will result in a survival advantage. Stated differently, with bevacizumab in each of the arms of GOG-0252, IP therapy is no longer a clinically meaningful contribution in the population studied.
Recently, the results of a randomized, open-label, Phase III study of the use of hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with stage III EOC were published and showed a 3.5-month improvement in recurrence-free survival (10.7 vs 14.2 months; HR: 0.66; 95% CI: 0.50–0.87) and a 12-month OS advantage (45.7 vs 33.9 months; HR: 0.67; 95% CI: 0.48–0.94) in patients receiving one dose of HIPEC with cisplatin in addition to NACT [38]. There are very limited data on the use of bevacizumab with upfront HIPEC. In one Phase I trial where bevacizumab therapy was planned to start 10–14 weeks after cytoreductive surgery and HIPEC treatment, only two-thirds of patients could be safely started on bevacizumab maintenance and only seven patients could complete all 22 cycles. There were two (10%) GI adverse events [39]. There are no current ongoing clinical trials involving bevacizumab and HIPEC for first-line treatment, however, HIPEC with secondary cytoreductive surgery is being investigated in the recurrent setting (NCT01376752) with the addition of bevacizumab (NCT03220932).
Bevacizumab maintenance therapy duration
In both GOG-0218 and ICON-7, the maximum separation of the survival curves occurred at the point when bevacizumab maintenance therapy was stopped per protocol suggesting that angiogenesis resumes unaltered after bevacizumab is discontinued. This raises questions about the optimal duration of bevacizumab therapy. The ROSiA study evaluated the safety and efficacy of extended maintenance bevacizumab. In this study, patients with newly diagnosed stage II–IV EOC were treated with carboplatin/paclitaxel/bevacizumab for four to eight cycles and then bevacizumab was continued until disease progression [18]. The patient population was similar to ICON-7, with 24% of patients with stage IV disease and 34% of patients suboptimally debulked. The median time of bevacizumab exposure was 15.5 months and 29% percent of patients were on maintenance bevacizumab for more than 2 years. Seventeen percent of patients discontinued bevacizumab due to toxicity and the median time to toxicity-related discontinuation was 9.9 months. The overall median PFS was 25.5 months (95% CI: 23.7–27.6 months) and in high-risk patients was 18.3 months. The median OS was unable to be determined due to the low rate of events (2-year OS was 85%). Although comparisons between studies are problematic, the data suggest that there is a PFS and OS survival benefit with extended bevacizumab maintenance. The ENGOT Ov-15 Trial AGO-OVAR 17 (BOOST) trial is a prospective randomized Phase III trial to evaluate optimal maintenance bevacizumab treatment duration in newly diagnosed ovarian cancer (NCT01462890). Patients are randomized to receive either 22 or 44 cycles of bevacizumab. The primary outcome measure is PFS and estimated study completion is in 2021.
Bevacizumab with alternate platinum & taxane-based regimens
An alternative adjuvant chemotherapy regimen of oxaliplatin, docetaxel and bevacizumab was investigated in a Phase II clinical trial of 132 patients with EOC [40]. Oxaliplatin and docetaxel is thought to be less neurotoxic than carboplatin/paclitaxel. The antiangiogenic activity of docetaxel might also be greater than paclitaxel. 90% of patients were advanced stage and 37% were suboptimally debulked. The ORR was 73%, median PFS was 16.3 months and median OS was 47.2 months, demonstrating similar efficacy to carboplatin/paclitaxel.
Bevacizumab for ROC
Despite a favorable response to initial treatment, over 80% of EOC will recur and after recurrence the most important prognostic factor for the success of future therapies is the platinum-free interval (PFI). Improved survival has been noted in patient with longer PFI [41]. In patients who do recur, the probability of responding to a second platinum-based regimen is 0% for refractory, less than 10% for resistant, 30% for partially sensitive and more than 50% for sensitive. In general, platinum-containing chemotherapy doublets are used to treat platinum-sensitive recurrence and single-agent chemotherapy is used to treat platinum-resistant ovarian cancer recurrence. The use of bevacizumab for recurrent platinum-sensitive and platinum-resistant ROC has been explored in three Phase III clinical trials (Table 1) and numerous Phase II trials.
Bevacizumab for platinum-sensitive EOC
For platinum-sensitive ovarian cancer, three carboplatin-based chemotherapy regimens have been shown to be equally efficacious: carboplatin/paclitaxel [42], carboplatin/gemcitabine [43] and carboplatin/pegylated liposomal doxorubicin (PLD) [44], although treatment regimens should be suited to the individual. Additionally, maintenance therapy with poly(ADP-ribose) polymerase (PARP) inhibitors are the FDA approved for the treatment of recurrent platinum-sensitive chemotherapy.
OCEANS was a randomized, double-blind Phase III trial of bevacizumab in addition to chemotherapy for patients with platinum-sensitive ROC at first recurrence [11]. In this study, 484 patients were randomized to receive six to ten cycles of either gemcitabine/carboplatin (GC) or gemcitabine/carboplatin/bevacizumab followed by bevacizumab maintenance until disease progression. 42% of the cohort had platinum partially sensitive disease and 11% had secondary cytoreductive surgery. Patients received a median number of 12 (range: 1–43) cycles of bevacizumab and at time of final analysis, 12% of patients were not known to have discontinued bevacizumab. After 24 months of follow-up, the addition of bevacizumab to carboplatin/gemcitabine improved PFS by 4 months (12.4 vs 8.4 months; HR: 0.48; 95% CI: 0.39–0.61) and improved the ORR by 21% (78.5 vs 57.4%; p < 0.001). In platinum partially sensitive patients, the median PFS was 12.5 for the bevacizumab arm compared with 7.4 months for the control arm (HR: 0.36; 95% CI: 0.25–0.53). The results were confirmed in an independent radiologic review of the data [45]. After 58 months of follow-up, the median OS was similar between the two arms (GC plus bevacizumab: 33.6 months; GC only: 32.9 months; HR: 0.95; 95% CI: 0.77–1.18). In subgroup analysis (stratified by age, PFI and tumor size), there was also no OS benefit [19]. Of note, after progression approximately 90% of patients received an alternative chemotherapy agent, and the median number of subsequent therapies was five, which confounds the interpretation of OS. There was a noted increase in adverse events in the bevacizumab containing group including hypertension, proteinuria and serious adverse events, which resulted in drug discontinuation in 20% of patients compared with 5% in the placebo arm.
In GOG-0213, an open-label randomized Phase III trial of EOC patient with first recurrence of platinum-sensitive cancer, the objective was to evaluate the efficacy of both bevacizumab and secondary cytoreduction on OS [12]. For the bevacizumab objective, 674 patients were randomized to receive either carboplatin/paclitaxel or carboplatin/paclitaxel/bevacizumab for six to eight cycles followed by bevacizumab maintenance until disease progression or toxicity. A separate cohort of patients eligible for secondary cytoreduction could be randomized to this objective, and 16% of patients had secondary cytoreductive surgery. Prior treatment regimens for the cohort included prior IP chemotherapy (18%), maintenance chemotherapy (13%) and prior bevacizumab treatment (10%). Although there was initial misclassification of disease-free interval leading to an overestimation of platinum partially sensitive patients, 25% of patients were ultimately determined to be platinum partially sensitive. The regimen was well tolerated and maintenance bevacizumab continued for a median of 16 cycles. The median OS in the bevacizumab group was 49.6 months, compared with 37.3 months in the chemotherapy only group (HR: 0.823; 95% CI: 0.680–0.996). After controlling for patients who were additionally randomized to secondary cytoreduction, were platinum partially sensitive and who had received prior bevacizumab treatment, median PFS was significantly increased by 3 months in the chemotherapy plus bevacizumab group (13.8 vs 10.4 months; HR: 0.63; 95% CI: 0.53–0.74). There was a 14% increase in complete response rate (32 vs 18%) in patients in the bevacizumab arm. As expected, there was overall greater toxicity with incorporation of bevacizumab, however, there were no differences in QOL scores among the two arms at any time in the study. After disease progression, over 80% of patients received additional treatment, with almost half of patients receiving three or more lines of treatment. Secondary cytoreduction was ultimately found to have no improvement in PFS or OS, though there was improved PFS seen in patients with complete surgical debulking compared with patients with suboptimal debulking (HR: 0.51; 95% CI: 0.36–0.72) or no debulking surgery (HR: 0.68; 95% CI: 0.51–0.90).
Both treatment regimens used in OCEANS and GOG-0213 have been approved by the US FDA for this use. More recently studies have looked into the combination of carboplatin, PLD and bevacizumab. The Phase III AGO OVAR 2.21 that was presented in abstract form at ESMO in November 2018 demonstrated significantly superior PFS with carboplatin/PLD/bevacizumab (CG-BEV) versus carboplatin/gemcitabine/bevacizumab (CG-BEV; HR: 0.81; 95% CI: 0.68–0.96). Median PFS was 11.7 months in the control arm versus 13.3 months in the CG-BEV experimental arm [46]. The median OS was 28.2 months with CG-BEV vs 33.5 months with CD-BEV (HR: 0.83; p = 0.079). Toxicities and QOL scores were additionally similar between the two arms.
Bevacizumab for platinum-resistant EOC
Patients with platinum-resistant disease have a poorer response rate to chemotherapy (typically 10–20%) compared with platinum-sensitive disease (ORR typically 50–60%) and thus a poorer prognosis. With enough recurrences, all patients are thought to develop platinum-resistant disease [47]. Standard treatment for recurrent platinum-resistant EOC is single-agent cytotoxic therapy, commonly with paclitaxel, PLD, gemcitabine, topotecan or etoposide, but other agents are active as well. With the results of a Phase III study of bevacizumab in addition to single-agent therapy in platinum-resistant EOC, bevacizumab is the FDA approved as a combination therapy for this population.
The AURELIA trial is a Phase III, open-label randomized trial of three different single-agent chemotherapies (PLD, paclitaxel, topotecan) alone or in addition to bevacizumab in patients with recurrent platinum-resistant ovarian cancer who had received no more than two prior lines of chemotherapy [10]. Single-agent chemotherapy was investigators choice and patients were randomized to bevacizumab. The PLD cohort was the first to be fully recruited as this was the preferred regimen by participating physicians. A total of 361 patients were enrolled, and 25% had a PFI of less than 3 months, 40% had two prior chemotherapy lines and 7% had prior antiangiogenic therapy. A median of six cycles of chemotherapy was received by the bevacizumab arm prior to progression or toxicity, compared with a median of three cycles in the chemotherapy alone arm. The median PFS in patient receiving chemotherapy plus bevacizumab compared with chemotherapy alone was 6.7 versus 3.4 months (HR: 0.42; 95% CI: 0.32–0.53). These results were confirmed on independent radiologic review [48]. This effect remained regardless of PFI or size of recurrent lesion. The ORR was 30.9% in the chemotherapy plus bevacizumab group compared with 12.6% for chemotherapy alone. There was no statistically significant difference in OS between the regimens. In the chemotherapy alone arm, 40% of participants were switched to single-agent bevacizumab after progression. This crossover likely confounded the OS results. In an exploratory analysis, patients who received bevacizumab with chemotherapy had a 32% reduction in risk of death (HR: 0.68; 95% CI: 0.52–0.90) when compared with patients who never crossed over to bevacizumab. Similarly, patients who crossed over to bevacizumab after progression on chemotherapy alone had a 40% decrease in risk of death (HR: 0.60; 95% CI: 0.43–0.86) compared with patients with no crossover [49]. There was greater improvement in PFS and OS in patients who were secondarily platinum-resistant, compared with those who were primarily resistant [50]. Bevacizumab was well tolerated, safe and the addition of bevacizumab to the treatment regimen resulted in a greater than 15% improvement in GI symptoms on QOL questionnaires in 22% of patients [51].
The effect of each individual chemotherapy regimen was explored in a subsequent analysis [52]. Since patients were not randomized to chemotherapy arm, results should be interpreted with caution, although analysis of baseline characteristics showed that the arms only differed in the proportion of patients who had received two prior lines of chemotherapy. The PFS was longer with the addition of bevacizumab to each chemotherapy cohort with median PFS of 10.4 versus 3.9 months in the paclitaxel cohort (HR: 0.46; 95% CI: 0.30–0.71), 5.4 versus 3.5 months in the PLD cohort (HR: 0.57; 95% CI: 0.39–0.83) and 5.8 versus 2.1 months in the topotecan cohort (HR: 0.32; 95% CI: 0.21–0.49). While no chemotherapy arm reached significance with respect to OS, paclitaxel plus bevacizumab had a noticeable effect, with a median OS of 22.4 versus 13.2 months (HR: 0.65; 95% CI: 0.42–1.02). Likewise, the ORR was increased in the chemotherapy plus bevacizumab arms with paclitaxel having a 23.1% increase in ORR (53.3 vs 30.2%), topotecan having a 17.0% improvement in ORR (17.0 vs 0%) and PLD having a 5.9% difference in ORR (13.7 vs 7.8%). Thus, in this study paclitaxel was most active regimen.
Numerous Phase II trials have been conducted on alternative single-agent chemotherapy plus bevacizumab regimens. Chemotherapy agents that are shown to have more than 20% ORR with addition of bevacizumab include oral metronomic cyclophosphamide (24%) [7], nabpaclitaxel (50%) [53], PLD with q3 weeks bevacizumab dosing (30%) [54], weekly PLD with weekly bevacizumab dosing (33%) [55], pemetrexed (41%, only 33% of patients platinum-resistant) [56] and irinotecan (27.9%, two-thirds of patient platinum-resistant) [57]. While these studies have shown treatment with a single-agent chemotherapy agent plus bevacizumab appear to be safe and effective, randomized trials will need to be conducted before any of these findings can be validated.
The initial studies of bevacizumab in ovarian cancer were with bevacizumab as a single agent in pretreated patients. In GOG-0170D, 62 patients with recurrent ovarian cancer and up to two prior lines of chemotherapy were treated with single-agent bevacizumab until disease progression or toxicity [5]. Fifty-eight percent of patients were platinum resistant. Single-agent bevacizumab resulted in an ORR of 21%, the median PFS was 4.7 months and the OS was 17 months. There were no major adverse events and patients received a median of seven cycles. The results of an industry-sponsored study with a similar design were published simultaneously [6]. In this study, patients were allowed to have received three prior chemotherapy regimens and 84% were platinum resistant. After enrolling 44 patients, the study was stopped prematurely due to safety concerns as there was an 11.4% rate of GI perforation. The ORR was 15.9% (all in platinum-resistant patients) and 61.4% had stable disease. The median PFS was 4.4 months (95% CI: 3.1–5.5 months) and the median OS was 10.7 months. In another study of 32 patients with heavily pretreated ROC and a median of five prior lines of chemotherapy, the addition of single-agent bevacizumab resulted in an ORR of 16% with 62.5% of patients with stable disease, a median PFS of 5.5 months and a median OS of 6.9 months [58]. These studies show that single-agent bevacizumab has an activity similar to other single-agent chemotherapies in recurrent platinum-resistant EOC, but less than the benefit of combination therapy [59].
There have been few studies in patients who are heavily pretreated and most are retrospective in nature. Tillmanns et al. reported a substantial improvement in ORR (50%; 95% CI: 34.8–65.1%) and PFS (8.8 months; 95% CI: 5.8–10.2 months) in patients with up to six prior lines of chemotherapy (median two lines chemotherapy) using albumin-bound paclitaxel and bevacizumab [53]. In a review of 15 patients with recurrent platinum-resistant ovarian cancer and a median of five prior lines of chemotherapy, single-agent bevacizumab had an ORR of 13% with 40% stable disease, the median PFS was 6.6 months and the median OS was 15.0 months [60]. There were no GI perforations. In another retrospective study, the addition of bevacizumab to weekly paclitaxel in patients with a median of four prior chemotherapy regimens, resulted in a 15% increase in ORR (63 vs 48%) and a 7-month increase in median PFS (13.2 vs 6.2 months; p < 0.01) [61]. After one retrospective study of combination low-dose metronomic oral cyclophosphamide and bevacizumab in ROC patients with a median of four prior lines showed an ORR of 40%, the same investigators then prospectively studied combination low-dose metronomic oral cyclophosphamide and bevacizumab in 15 patients with a median number of eight prior chemotherapy regimens, and reported an ORR of 53% without noting any major GI complications [62].
Timing & readministration of bevacizumab
The benefit of bevacizumab therapy in the upfront maintenance setting and also in the recurrent ovarian cancer setting raises questions about the timing of bevacizumab therapy and the benefit of retreatment with bevacizumab after an initial complete response to a bevacizumab-containing regimen. Bevacizumab in the upfront setting has shown an improvement in PFS, but the HRs for PFS are smaller for recurrent ovarian cancer indicating a potentially larger treatment effect for ROC [63]. There is limited evidence that bevacizumab given upfront may also alter recurrence patterns such that there is a higher rate of lung and pleural recurrence and lower rate of liver recurrence, possibly due to a longer PFI for peritoneal cavity disease with bevacizumab [64].
The readministration of bevacizumab in patient previously treated with bevacizumab has shown efficacy in breast cancer [65] and metastatic colon cancer [66]. The biologic rationale behind retreating is that in addition to the antiangiogenic effects of bevacizumab, there might also be a normalization of tumor vasculature that improves chemotherapeutic drug delivery to the tumor cells. Recent clinical trials of recurrent ovarian cancer include subsets of patient who have had prior treatment with bevacizumab. However, in these studies, patients were stratified by prior bevacizumab therapy to avoid confounding and this limits analysis of the effect of prior bevacizumab treatment on PFS. In one study of 29 patients with heavily pretreated ovarian cancer (median five prior regimens), nearly half of patients had previously received bevacizumab [57]. The patients with prior bevacizumab who were treated with combination irinotecan and bevacizumab demonstrated similar ORR to patients who were bevacizumab naïve, suggesting that prior bevacizumab treatment may not alter the efficacy of subsequent treatment. The ENGOT Ov-17; MITO 16b; MANGO-OV2b trial was a multicenter, Phase III randomized study of second-line chemotherapy with bevacizumab in 405 patients with platinum-sensitive ROC treated with prior bevacizumab in first-line chemotherapy. 36% of patients were platinum partially sensitive. Patients rechallenged with platinum-based doublet therapy and bevacizumab demonstrated a significant improvement in PFS (11.8 vs 8.8 months; HR: 0.51; 95% CI: 0.41–0.64), but there was no difference in OS [67]. Retreatment with bevacizumab was additionally explored in AGO OVAR 2.21 in which 50% of patients enrolled had prior treatment with bevacizumab. Based on available data, retreatment with bevacizumab was both safe and efficacious with a significant PFS improvement seen in the subgroup of patients with previous antiangiogenic treatment [46].
Response to bevacizumab as first-line therapy is not predictive of response to retreatment [68]. One retrospective study has studied the role of readministering bevacizumab therapy after cancer progression and found that there was a 25% response rate in patients who had responded to the prior bevacizumab therapy and also an 18% response rate in patients who did not respond to their first-line chemotherapy with bevacizumab [69]. Similar to PFI, a retrospective study found that the bevacizumab-free interval may be prognostic, raising the question if prolonging the bevacizumab-free interval with a non-antiangiogenic agent may improve response to treatment. Patients with an interval greater than 9 months had a median OS of 24.3 months compared with 6.8 months in patient with an interval less than 9 months (p = 0.003) [68].
Palliative use of bevacizumab for malignant ascites
Bevacizumab helps to decrease ascites by normalizing the vasculature in otherwise leaky tumors. In AURELIA, treatment with bevacizumab reduced the proportion of patients requiring a paracentesis compared with single-agent chemotherapy (2 vs 17%). Patients reported improved QOL with less symptomatic ascites. Treatment of ascites with IP bevacizumab (5 mg/kg monthly) has been reported with resolution of ascites for at least 2 months and minimal side effects [70]. Another study of combination cisplatin and bevacizumab 300 mg q2 weeks for 6 weeks, patients randomized to IP cisplatin/bevacizumab compared with cisplatin only reported a very high ORR (90.3 vs 59.3 %; p < 0.05) with improved QOL scores (93.55 vs 48.15 %; p < 0.05) [71]. Similarly, bevacizumab has been reported to resolve pericardial and pleural effusions in ovarian cancer patients.
Bevacizumab in combination with other targeted therapies
Recently, there has been significant advancement in the use of targeted therapies for ovarian cancer including PARP inhibitors, immune checkpoint inhibitors and antiangiogenic agents. There are several clinical trials investigating the interaction between bevacizumab and other targeted therapies (Table 4).
Table 4. . Clinical trials of bevacizumab with other novel targeted agents.
| Therapy | Setting | Study name | Clinical trials ID | Phase | Design | Arms | Primary outcome | Anticipated accrual | Anticipated primary completion date |
|---|---|---|---|---|---|---|---|---|---|
| Bev and PARPi | Frontline maintenance | OVARIO | NCT03326193 | II | Single-arm, open-label | Bev q21 days and niraparib daily | PFS at 18 months | 90 | Dec 2020 |
| Frontline maintenance | PAOLA-1 (ENGOT-ov25) | NCT02477644 | III | Randomized, triple-blinded | (1) CT and Bev, and Bev maintenance (2) CT and Bev, and Bev and olaparib maintenance |
PFS | 612 | Jun 2022 | |
| Frontline maintenance | MITO25 | NCT03462212 | II | Randomized, open-label | (1) CT and Bev, and Bev maintenance (2) CT and Bev, and Bev and rucaparib maintenance (3) CT and rucaparib maintenance |
MTD and PFS | 234 | Nov 2018 | |
| Frontline maintenance | GOG-9923 | NCT00989651 | I | Nonrandomized, open-label | (1) CT, and Bev and veliparib × six cycles, followed by Bev maintenance (2) C, dose dense T, Bev and veliparib × six cycles, followed by Bev maintenance (3) IP cisplatin, T, and Bev and veliparib × six cycles, followed by Bev maintenance |
DLT | 474 | Sept 2020 | |
| Bev and immunotherapy | Frontline maintenance | IMagyn050/GOG 3015/ENGOT-OV39 | NCT03038100 | III | Randomized, double-blinded | (1) CT, and Bev and atezolizumab maintenance (2) CT and Bev maintenance and placebo |
PFS | 1300 | Apr 2020 |
| Platinum sensitive | GINECO-OV236b (ATALANTE) | NCT02891824 | III | Randomized, triple-blinded | (1) Platinum doublet and Bev and placebo maintenance (2) Platinum doublet and Bev and atezolizumab maintenance |
PFS | 405 | Sept 2020 | |
| Platinum resistant | EORTC-1508 | NCT02659384 | II | Randomized, triple-blinded | (1) Bev monotherapy (2) Atezolizumab and placebo (3) Atezolizumab and acetylsalicylic acid (4) Atezolizumab and bevacizumab and placebo (5) Atezolizumab and bevacizumab and acetylsalicylic acid |
PFS at 6 months | 160 | Jan 2021 | |
| Platinum resistant | NRG-GY009 | NCT02839707 | II/III | Randomized, open-label | (1) PLD, atezolizumab (2) PLD, bevacizumab, atezolizumab (3) PLD, bevacizumab |
DLT and PFS | 488 | Jun 2023 | |
| Platinum resistant | AGO-OVAR 2.29 | NCT03353831 | III | Randomized, triple-blinded | (1) Weekly T or PLD and Bev and placebo (2) Weekly T or PLD and Bev and atezolizumab |
PFS | 664 | Jul 2021 | |
| Triplet therapy | Frontline maintenance | DUO-O | NCT03737643 | III | Randomized, quadruple-blinded | (1) CT and Bev, and Bev maintenance (2) CT and Bev, and Bev and durvalumab maintenance (3) CT and Bev, and Bev, durvalumab and olaparib maintenance |
PFS | 1056 | May 2022 |
| Frontline maintenance | ENGOT-0V44 (FIRST) | NCT03602859 | III | Randomized, triple-blinded | (1) CT and/or Bev maintenance (2) CT and/or Bev maintenance and niraparib maintenance (3) CT and/or Bev maintenance and niraparib and TSR042 (anti-PD-1) maintenance |
PFS | 912 | Nov 2021 | |
| Platinum sensitive | ENGOT-OV42-NSGO/AVANOVA-Triplet | NCT03806049 | III | Randomized, open-label | (1) Niraparib and Bev and TSR042 (anti-PD-1) (2) Niraparib and Bev (3) CT |
PFS | 337 | Jun 2022 |
Bev: Bevacizumab; C: Carboplatin; DLT: Dose-limiting toxicity; MTD: Maximum tolerated dose; PARPi: Poly-ADP ribose polymerase inhibitor; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin; T: Paclitaxel.
PARP inhibitors & bevacizumab
PARP inhibition has been shown to reduce angiogenesis in vivo [72]. Likewise, antiangiogenic agents are thought to increase the efficacy of PARP inhibitors by decreasing the expression of genes and proteins that are involved in homologous recombination repair (HRR). The efficacy of olaparib and a different antiangiogenic agent, cediranib, have shown to improve PFS [73], and there is believed to be a synergistic effect of antiangiogenesis and PARP inhibition. The PARP inhibitors niraparib, olaparib and rucaparib have all shown efficacy as maintenance therapy in recurrent platinum-sensitive ovarian cancer, regardless of BRCA status. Olaparib and rucaparib are additionally the FDA approved for recurrent platinum-resistant EOC in patients with a known germline BRCA mutation. Most recently, the publication of the Phase III randomized, blinded study of maintenance olaparib in germline BRCA-mutated patients with newly diagnosed advanced ovarian cancer (SOLO-1) showed the risk of disease progression or death was 70% lower with maintenance olaparib than with placebo and the median PFS was not reached after 41 months [74]. With the results of SOLO-1, maintenance olaparib in patients with germline BRCA mutations is a new standard of care, however, patients who received bevacizumab were excluded from SOLO-1. There are several ongoing clinical trials specifically addressing the efficacy of combination PARP inhibitors and bevacizumab. PAOLA-1 (ENGOT-ov25) is a Phase III randomized, controlled trial of olaparib in patients with newly diagnosed EOC on bevacizumab maintenance therapy (NCT02477644). The results of PAOLA-1 after a first prespecified data cutoff were presented in September 2019. The addition of olaparib to bevacizumab maintenance in patients with newly diagnosed, advanced stage EOC showed an increase in median PFS compared to bevacizumab maintenance alone (22.1 months vs 16.6 months, HR 0.59 (95% CI 0.49–0.72; p < 0.0001). The combined maintenance regimen was well tolerated. Subgroup analysis showed patients with a BRCA mutation or homologous recombination deficiency had the greatest benefit in PFS [75]. ENGOT-OV24/AVANOVA (NCT02354131) is a Phase II trial comparing both bevacizumab and niraparib to niraparib alone in platinum-sensitive recurrent EOC. The combination of both bevacizumab and niraparib improved progression-free survival compared to niraparib alone (11.9 months vs 5.5 months, HR 0.35; p < 0.0001) and may offer an option for combined molecular therapy in patients who cannot receive platinum-based chemotherapy [76,77]. Similar trials of niraparib (NCT03326193) and rucaparib (NCT03462212) in combination with bevacizumab in newly diagnosed EOC are recently underway. The PARP inhibitor veliparib has been shown to sensitize alkylating agents and is generally well tolerated. The use of veliparib with carboplatin/PLD/bevacizumab was investigated in a Phase I trial, however, the combination bevacizumab and veliparib led to a dose limiting toxicity in 75% of patients.
Immune checkpoint inhibition & bevacizumab
The PD-1 pathway prevents cell death from the immune system through a negative feedback loop and is overexpressed in many cancers. Inactivation of the PD-1 receptor or binding of the PD-L1 ligand allows T cells to target the tumor cells. There is thought to be a synergistic effect between antiangiogenic agents and immune checkpoint inhibitors as abnormal tumor vasculature may prevent immune cell trafficking. There are many upcoming studies with immune checkpoint inhibitors and bevacizumab including the PD-L1 inhibitors atezolizumab (NCT03038100, NCT03353831, NCT02659384, NCT02891824, NCT03363867) and avelumab (NCT03197584) and the PD-1 inhibitors pembrolizumab (NCT02853318, NCT03275506) and nivolumab (NCT02873962).
Dosing
The dosing regimen chosen in GOG-0218 is 15 mg/kg q3 weeks based on a half-life of 20 days when given intravenously, and is the dosing approved for non-small-cell lung cancer [8]. ICON7 used the 7.5 mg/kg q3 weeks dosing that is approved for metastatic colorectal cancer in Europe. For recurrent ovarian cancer, the FDA has approved dosing based on the study protocols for OCEANS, GOG-0213 and AURELIA; for platinum-sensitive cancer, bevacizumab is given 15 mg/kg q3 weeks. In platinum-resistant cancer, bevacizumab 15 mg/kg is given every 3 weeks with topotecan and bevacizumab is given 10 mg/kg q2 weeks with weekly paclitaxel, PLD and weekly topotecan. The decision to give bevacizumab either every 3 weeks or 2 weeks takes into consideration the dosing schedule of the concurrent chemotherapy for ease of administration, as both regimens are 5 mg/kg per week and achieve a similar steady-state exposure [76]. While high-quality studies are limited, there appears to be no difference in efficacy between the high-dose (5 mg/kg per week) and low-dose regimens (2.5 mg/kg per week) and the low-dose regimen may have fewer dose-related side effects.
Predictors of response to bevacizumab
Studies of biomarkers and genetic polymorphisms associated with survival outcomes with bevacizumab have been explored in many small studies, as well as subset analysis in many randomized, controlled trials. Although these studies need further validation, they nonetheless provide candidate markers for individualized care.
Predictors of response
Despite early promising results, serum biomarkers of angiogenesis, including expression of VEGF-A and VEGFR, have not been shown to predict survival [77–81]. Both GOG-0218 and ICON7 investigated candidate biomarkers. In GOG-0218, patients with the highest quartile of tumor VEGF-A levels demonstrated an OS advantage, but no difference in PFS, and plasma VEGF-A was not correlated with response [82]. Higher microvessel density of tumor samples in GOG-0218 was associated with greater efficacy of bevacizumab [82]. In ICON-7, a combination of high ANG-1 and low TIE-2 were predictive of improved PFS, but 13 other angiogenesis-related factors including various isoforms of VEGF and VEGFR had no predictive value [83]. Another ICON-7 study sought to identify candidate biomarkers by comparing protein expression between responders and nonresponders; a combination of CA-125, FLT4 (VEGFR3), AGP and mesothelin levels was predictive of OS [84]. Several other predictive biomarkers have been explored in small studies with positive results but no biomarker has been validated in large prospective studies [85–88] HRR gene mutations, including BRCA1 and BRCA2, were shown to be predictive of survival in the GOG-0218 cohort, but there was not shown to be an interaction between HRR gene mutations and response to bevacizumab [89].
Imaging biomarkers of bevacizumab response have been explored as proof of concept with promising results. Contrast-enhanced computed tomography (CT) can be used to measure tumor blood flow, blood volume and vessel permeability surface product and can be incorporated into standard CT-based treatment monitoring protocols. ACRIN 6695 was an exploratory study of the GOG-0262 cohort using CT perfusion-based biomarkers to predict survival outcomes [90]. In this study, increased tumor blood flow and blood volume were associated with shorter PFS. One disadvantage of radiographic biomarkers is that they require a targetable lesion, however, this is less of a concern for bevacizumab front-line therapy where suboptimally debulked patients derive the greatest treatment benefit. CT perfusion is currently being prospectively studied in a Phase II open-label study by the ECOG-ACRIN Cancer Research Group (NCT03412630).
Prior number of chemotherapy regimens, treatment-free interval, platinum sensitivity and ascites are all well-studied prognostic factors for PFS in EOC [91]. Increased adiposity is associated with poor prognosis as adipose cells are known to produce VEGF. In patients dichotomized to BMI above and below the median BMI of 28.4, median PFS was shorter for patients with high BMI (9.8 vs. 24.7 months; p = 0.03) in the group receiving first-line chemotherapy with bevacizumab [92]. The MITO-16 - MANGO-OV2 is a Phase IV exploratory study prognostic clinical factors and biomarkers with patients receiving upfront chemotherapy with bevacizumab (NCT01706120).
Monitoring response to bevacizumab
Measurement of disease with CT imaging is the most reliable measurement of recurrence. Data on the correlation of CA-125 and radiologic response in patients treated with bevacizumab are limited but the two are generally shown to correlate [93,94]. CA-125 may become a less reliable marker with extended bevacizumab treatment [95]. Early-onset HTN [96] and possibly resolution of ascites are clinical indicators of tumor response.
Resistance to bevacizumab
Studies on resistance to bevacizumab have been limited to date. Resistance to bevacizumab therapy may be mediated by alternative angiogenesis pathways [97]. In in vitro models, it is hypothesized that tumors may bypass the VEGF pathway and instead promote angiogenesis through PI3K/Akt activation of the proangiogenic factor FGF2 [98]. Another study showed that tumor cells secrete hypoxia-induced microseminoprotein that triggers MAPK signaling of angiogenesis in endothelial cells [99]. Capecitabine, a prodrug of the chemotherapeutic agent 5-FU (5-fluorouracil) that is thought to be antiangiogenic through alternative pathways, has been shown to overcome bevacizumab resistance [100].
Conclusion
Bevacizumab has demonstrated a modest survival benefit in the frontline, platinum-sensitive and platinum-recurrent settings but questions remain as to when to administer bevacizumab. Until ongoing clinical trials of combination-targeted maintenance therapies are completed, the decision on which maintenance strategy to use will depend on multiple patient-related factors such as platinum-sensitivity, BRCA status, presence of homologous recombination deficiency, disease burden, associated symptoms and anticipated toxicities. In some cases, such as the BRCA-mutated population, PARP inhibitor therapy is the clear choice for maintenance therapy. In the frontline setting, bevacizumab seems best suited for BRCA-negative patients with high-risk disease. For platinum-sensitive disease, adding bevacizumab to carboplatin/paclitaxel has shown an OS benefit. For platinum-resistant recurrent disease, the largest survival benefit was seen with the weekly paclitaxel/bevacizumab group. Taken together, there may be a synergistic effect in administering paclitaxel and bevacizumab. Previously, consideration was given on whether to ‘save’ bevacizumab for recurrent disease, although more recent data support that readministration of bevacizumab is safe and efficacious. Ultimately, there is a strong need for biomarkers to individualize maintenance therapy and until such biomarkers are discovered, the decision on when to administer bevacizumab will remain at the discretion of the provider based on a multitude of factors.
Executive summary.
The addition of bevacizumab maintenance to standard frontline cytotoxic chemotherapy results in a 3–4-month increase in progression-free survival. The survival advantage is most pronounced in high-risk subgroups.
GOG-0213 was the only study to show an overall survival advantage of incorporating bevacizumab to standard treatment regimens.
In AURELIA, the largest survival benefit was seen with the weekly paclitaxel plus bevacizumab group, suggesting a synergistic response between the two agents.
There may be a survival advantage to readministering bevacizumab treatment and prior bevacizumab in the upfront setting should not preclude bevacizumab in the recurrent setting.
There are multiple studies currently underway on the combination of bevacizumab plus poly(ADP-ribose) polymerase inhibitors and immune checkpoint inhibitors. The therapeutic effect may be synergistic.
The most common toxicities are hypertension and proteinuria. There are no dose adjustments for bevacizumab.
Currently, there are no prospectively validated biomarkers for response to bevacizumab.
Footnotes
Financial & competing interests disclosure
This paper is funded in or in part, from direct NIH funding, grant number: 2T32CA060396. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Company review disclosure
In addition to the peer-review process, with the authors’ consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the authors at their discretion and based on scientific or editorial merit only. The authors maintained full control over the manuscript, including content, wording and conclusions.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol. Oncol. 94(3), 630–635 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bundy BN, Greer BE. et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 21(17), 3194–3200 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Wild R, Dings RP, Subramanian I, Ramakrishnan S. Carboplatin selectively induces the VEGF stress response in endothelial cells: potentiation of antitumor activity by combination treatment with antibody to VEGF. Int. J. Cancer 110(3), 343–351 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Chan JK, Brady MF, Penson RT. et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 374(8), 738–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a gynecologic oncology group study. J. Clin. Oncol. 25(33), 5165–5171 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Cannistra SA, Matulonis UA, Penson RT. et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 25(33), 5180–5186 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Garcia AA, Hirte H, Fleming G. et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital Phase II Consortia. J. Clin. Oncol. 26(1), 76–82 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Burger RA, Brady MF, Bookman MA. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365(26), 2473–2483 (2011). [DOI] [PubMed] [Google Scholar]; •• Published Phase III clinical trials reviewed in this paper.
- 9.Perren TJ, Swart AM, Pfisterer J. et al. A Phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365(26), 2484–2496 (2011). [DOI] [PubMed] [Google Scholar]; •• Published Phase III clinical trials reviewed in this paper.
- 10.Pujade-Lauraine E, Hilpert F, Weber B. et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized Phase III trial. J. Clin. Oncol. 32(13), 1302–1308 (2014). [DOI] [PubMed] [Google Scholar]; •• Published Phase III clinical trials reviewed in this paper.
- 11.Aghajanian C, Blank SV, Goff BA. et al. OCEANS: a randomized, double-blind, placebo-controlled Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30(17), 2039–2045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Published Phase III clinical trials reviewed in this paper.
- 12.Coleman RL, Brady MF, Herzog TJ. et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, Phase 3 trial. Lancet Oncol. 18(6), 779–791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Published Phase III clinical trials reviewed in this paper.
- 13.Burger RA, Brady MF, Rhee J. et al. Independent radiologic review of the Gynecologic Oncology Group Study 0218, a Phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer. Gynecol. Oncol. 131(1), 21–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger RA, Enserro D, Tewari KS. et al. Final overall survival (OS) analysis of an international randomized trial evaluating bevacizumab (BEV) in the primary treatment of advanced ovarian cancer: a NRG oncology/Gynecologic Oncology Group (GOG) study. J. Clin. Oncol. 36(15 Suppl.), 5517–5517 (2018). [Google Scholar]
- 15.Oza AM, Cook AD, Pfisterer J. et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a Phase 3 randomised trial. Lancet Oncol. 16(8), 928–936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan G, Ye L, Liu G, An J, Sehouli J, Sun P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: an updated systematic review and meta-analysis. Onco Targets Ther. 11, 521–528 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Yu P, Qu X, Liu Y, Zhang J. Phase III trials of standard chemotherapy with or without bevacizumab for ovarian cancer: a meta-analysis. PLoS ONE 8(12), e81858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This meta-analysis forms the basis of Table 2.
- 18.Oza AM, Selle F, Davidenko I. et al. Efficacy and safety of bevacizumab-containing therapy in newly diagnosed ovarian cancer: ROSiA single-arm Phase 3B study. Int. J. Gynecol. Cancer 27(1), 50–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a Phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 139(1), 10–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson DL, Backes FJ, Hurt JD. et al. Which factors predict bowel complications in patients with recurrent epithelial ovarian cancer being treated with bevacizumab? Gynecol. Oncol. 118(1), 47–51 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Burger RA, Brady MF, Bookman MA. et al. Risk factors for GI adverse events in a Phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: a gynecologic oncology group study. J. Clin. Oncol. 32(12), 1210–1217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz JP, Tew WP, Zivanovic O. et al. Incidence and management of bevacizumab-associated gastrointestinal perforations in patients with recurrent ovarian carcinoma. Gynecol. Oncol. 116(3), 335–339 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Vergote I, Tropé CG, Amant F. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363(10), 943–953 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Rouzier R, Gouy S, Selle F. et al. Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: results from the ANTHALYA trial. Eur. J. Cancer 70, 133–142 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Tanyi JL, McCann G, Hagemann AR. et al. Clinical predictors of bevacizumab-associated gastrointestinal perforation. Gynecol. Oncol. 120(3), 464–469 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Martin JY, Urban RR, Liao JB, Goff BA. Bevacizumab toxicity in heavily pretreated recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers. J. Gynecol. Oncol. 27(5), e47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monk BJ, Huang HQ, Burger RA. et al. Patient reported outcomes of a randomized, placebo-controlled trial of bevacizumab in the front-line treatment of ovarian cancer: a Gynecologic Oncology Group Study. Gynecol. Oncol. 128(3), 573–578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrillo M, Paris I, Vizzielli G. et al. Neoadjuvant chemotherapy followed by maintenance therapy with or without bevacizumab in unresectable high-grade serous ovarian cancer: a case-control study. Ann. Surg. Oncol. 22(3), 952–958 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Daniele G, Lorusso D, Scambia G. et al. Feasibility and outcome of interval debulking surgery (IDS) after carboplatin-paclitaxel-bevacizumab (CPB): a subgroup analysis of the MITO-16A-MaNGO OV2A Phase 4 trial. Gynecol. Oncol. 144(2), 256–259 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Katsumata N, Yasuda M, Takahashi F. et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a Phase 3, open-label, randomised controlled trial. Lancet 374(9698), 1331–1338 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Pignata S, Scambia G, Katsaros D. et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 15(4), 396–405 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Clamp AR, McNeish I, Dean A. et al. 929O_PRICON8: a GCIG Phase III randomised trial evaluating weekly dose- dense chemotherapy integration in first-line epithelial ovarian/fallopian tube/primary peritoneal carcinoma (EOC) treatment: results of primary progression- free survival (PFS) analysis. Ann. Oncol. 28(Suppl. 5), mdx440.039–mdx440.039 (2017). [Google Scholar]
- 33.Gonzalez-Martin A, Gladieff L, Tholander B. et al. Efficacy and safety results from OCTAVIA, a single-arm Phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur. J. Cancer 49(18), 3831–3838 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Martin A, Gladieff L, Tholander B. et al. Updated results from OCTAVIA (front-line bevacizumab, carboplatin and weekly paclitaxel therapy for ovarian cancer). Eur. J. Cancer 50(4), 862–863 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Armstrong DK, Bundy B, Wenzel L. et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354(1), 34–43 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Konner JA, Grabon DM, Gerst SR. et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J. Clin. Oncol. 29(35), 4662–4668 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker JL, Wenzel L, Huang H, Brady MF. Patient-reported outcomes in GOG 252: NRG oncology study of IV vs IP chemotherapy for ovarian, fallopian, or peritoneal carcinoma. Gynecol. Oncol. 141, 208 (2016). [Google Scholar]
- 38.van Driel WJ, Koole SN, Sikorska K. et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 378(3), 230–240 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Gouy S, Ferron G, Glehen O. et al. Results of a multicenter Phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer. Gynecol. Oncol. 142(2), 237–242 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Herzog TJ, Monk BJ, Rose PG. et al. A Phase II trial of oxaliplatin, docetaxel, and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol. Oncol. 132(3), 517–525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreas DB, Alexander R, Eric PL, Philipp H, Isabelle RC, Jacobus P. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized Phase 3 multicenter trials. Cancer 115(6), 1234–1244 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Parmar MK, Ledermann JA, Colombo N. et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361(9375), 2099–2106 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Pfisterer J, Plante M, Vergote I. et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J. Clin. Oncol. 24(29), 4699–4707 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E. et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 28(20), 3323–3329 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Aghajanian C, Goff B, Nycum LR, Wang Y, Husain A, Blank S. Independent radiologic review: bevacizumab in combination with gemcitabine and carboplatin in recurrent ovarian cancer. Gynecol. Oncol. 133(1), 105–110 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Pfisterer J, Shannon C, Hein A. et al. 933OCarboplatin/pegylated liposomal doxorubicin/bevacizumab (CD-BEV) vs. carboplatin/gemcitabine/bevacizumab (CG-BEV) in patients with recurrent ovarian cancer: a prospective randomized Phase III ENGOT/GCIG-Intergroup study (AGO study group, AGO-Austria, ANZGOG, GINECO, ANZGOG, GINECO, GINECO, SGCTG). Ann. Oncol. 29(Suppl. 8), viii332–viii358 (2018). [Google Scholar]
- 47.Hanker LC, on behalf of the AGOaGsg, Loibl S. et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 23(10), 2605–2612 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Husain A, Wang Y, Hanker LC. et al. Independent radiologic review of AURELIA, a Phase 3 trial of bevacizumab plus chemotherapy for platinum-resistant recurrent ovarian cancer. Gynecol. Oncol. 142(3), 465–470 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Bamias A, Gibbs E, Khoon Lee C. et al. Bevacizumab with or after chemotherapy for platinum-resistant recurrent ovarian cancer: exploratory analyses of the AURELIA trial. Ann. Oncol. 28(8), 1842–1848 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Trillsch F, Mahner S, Hilpert F. et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Ann. Oncol. 27(9), 1733–1739 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Stockler MR, Hilpert F, Friedlander M. et al. Patient-reported outcome results from the open-label Phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J. Clin. Oncol. 32(13), 1309–1316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poveda AM, Selle F, Hilpert F. et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized Phase III AURELIA Trial. J. Clin. Oncol. 33(32), 3836–3838 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Tillmanns TD, Lowe MP, Walker MS, Stepanski EJ, Schwartzberg LS. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecol. Oncol. 128(2), 221–228 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Verschraegen CF, Czok S, Muller CY. et al. Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann. Oncol. 23(12), 3104–3110 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Kudoh K, Takano M, Kouta H. et al. Effects of bevacizumab and pegylated liposomal doxorubicin for the patients with recurrent or refractory ovarian cancers. Gynecol. Oncol. 122(2), 233–237 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Hagemann AR, Novetsky AP, Zighelboim I. et al. Phase II study of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol. Oncol. 131(3), 535–540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musa F, Pothuri B, Blank SV. et al. Phase II study of irinotecan in combination with bevacizumab in recurrent ovarian cancer. Gynecol. Oncol. 144(2), 279–284 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Monk BJ, Han E, Josephs-Cowan CA, Pugmire G, Burger RA. Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol. Oncol. 102(2), 140–144 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Fuh KC, Secord AA, Bevis KS. et al. Comparison of bevacizumab alone or with chemotherapy in recurrent ovarian cancer patients. Gynecol. Oncol. 139(3), 413–418 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Pietzner K, Richter R, Chekerov R. et al. Bevacizumab in heavily pre-treated and platinum resistant ovarian cancer: a retrospective study of the North-Eastern German Society of Gynaecologic Oncology (NOGGO) Ovarian Cancer Study Group. Anticancer Res. 31(8), 2679–2682 (2011). [PubMed] [Google Scholar]
- 61.O’Malley DM, Richardson DL, Rheaume PS. et al. Addition of bevacizumab to weekly paclitaxel significantly improves progression-free survival in heavily pretreated recurrent epithelial ovarian cancer. Gynecol. Oncol. 121(2), 269–272 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Chura JC, Van Iseghem K, Downs LS, Carson LF, Judson PL. Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol. Oncol. 107(2), 326–330 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Monk BJ, Pujade-Lauraine E, Burger RA. Integrating bevacizumab into the management of epithelial ovarian cancer: the controversy of front-line versus recurrent disease. Ann. Oncol. 24(Suppl. 10), x53–x58 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Rauh-Hain JA, Guseh SH, Esselen KM. et al. Patterns of recurrence in patients treated with bevacizumab in the primary treatment of advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 23(7), 1219–1225 (2013). [DOI] [PubMed] [Google Scholar]
- 65.von Minckwitz G, Puglisi F, Cortes J. et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised Phase 3 trial. Lancet Oncol. 15(11), 1269–1278 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Bennouna J, Sastre J, Arnold D. et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised Phase 3 trial. Lancet Oncol. 14(1), 29–37 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Pignata S, Lorusso D, Joly F. et al. Chemotherapy plus or minus bevacizumab for platinum-sensitive ovarian cancer patients recurring after a bevacizumab containing first line treatment: the randomized Phase 3 trial MITO16B-MaNGO OV2B-ENGOT OV17. J. Clin. Oncol. 36(15 Suppl.), 5506–5506 (2018). [Google Scholar]
- 68.Laskey RA, Richard SD, Smith AL. et al. Retreatment with bevacizumab in patients with gynecologic malignancy is associated with clinical response and does not increase morbidity. Onco Targets Ther. 7, 469–476 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Backes FJ, Richardson DL, McCann GA. et al. Should bevacizumab be continued after progression on bevacizumab in recurrent ovarian cancer? Int. J. Gynecol. Cancer 23(5), 833–838 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Eskander RN, Tewari KS. Emerging treatment options for management of malignant ascites in patients with ovarian cancer. Int. J. Womens Health 4, 395–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao H, Li X, Chen D. et al. Intraperitoneal administration of cisplatin plus bevacizumab for the management of malignant ascites in ovarian epithelial cancer: results of a Phase III clinical trial. Med. Oncol. 32(2), 292 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Tentori L, Lacal PM, Muzi A. et al. Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur. J. Cancer 43(14), 2124–2133 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Liu JF, Barry WT, Birrer M. et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised Phase 2 study. Lancet Oncol. 15(11), 1207–1214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore K, Colombo N, Scambia G. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379(26), 2495–2505 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Mirza MR, Wang J, Mau-Sørensen M. et al. 953PA Phase 1 study to evaluate the safety and tolerability of bevacizumab-niraparib combination therapy and determine the recommended Phase 2 dose (RP2D) in women with platinum-sensitive epithelial ovarian cancer (ENGOT-OV24/AVANOVA1). Ann. Oncol. 28(Suppl. 5), mdx372.024–mdx372.024 (2017). [Google Scholar]
- 76.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 62(5), 779–786 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Smerdel MP, Steffensen KD, Waldstrøm M, Brandslund I, Jakobsen A. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol. Oncol. 118(2), 167–171 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Wimberger P, Chebouti I, Kasimir-Bauer S. et al. Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol. Oncol. 133(3), 467–472 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Raja FA, Hook JM, Ledermann JA. Biomarkers in the development of anti-angiogenic therapies for ovarian cancer. Cancer Treat. Rev. 38(6), 662–672 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Schultheis AM, Lurje G, Rhodes KE. et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin. Cancer Res. 14(22), 7554–7563 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han ES, Burger RA, Darcy KM. et al. Predictive and prognostic angiogenic markers in a gynecologic oncology group Phase II trial of bevacizumab in recurrent and persistent ovarian or peritoneal cancer. Gynecol. Oncol. 119(3), 484–490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bais C, Mueller B, Brady MF. et al. Tumor microvessel density as a potential predictive marker for bevacizumab benefit: GOG-0218 biomarker analyses. J. Natl Cancer Inst. 109(11), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backen A, Renehan AG, Clamp AR. et al. The combination of circulating Ang1 and Tie2 levels predicts progression-free survival advantage in bevacizumab-treated patients with ovarian cancer. Clin. Cancer Res. 20(17), 4549–4558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collinson F, Hutchinson M, Craven RA. et al. Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection. Clin. Cancer Res. 19(18), 5227–5239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karihtala P, Mäenpää J, Turpeenniemi-Hujanen T, Puistola U. Front-line bevacizumab in serous epithelial ovarian cancer: biomarker analysis of the FINAVAST trial. Anticancer Res. 30(3), 1001–1006 (2010). [PubMed] [Google Scholar]
- 86.Boisen MK, Madsen CV, Dehlendorff C, Jakobsen A, Johansen JS, Steffensen KD. The prognostic value of plasma YKL-40 in patients with chemotherapy-resistant ovarian cancer treated with bevacizumab. Int. J. Gynecol. Cancer 26(8), 1390–1398 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Steffensen KD, Madsen CV, Andersen RF, Waldstrøm M, Adimi P, Jakobsen A. Prognostic importance of cell-free DNA in chemotherapy resistant ovarian cancer treated with bevacizumab. Eur. J. Cancer 50(15), 2611–2618 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Chan JK, Kiet TK, Blansit K. et al. MiR-378 as a biomarker for response to anti-angiogenic treatment in ovarian cancer. Gynecol. Oncol. 133(3), 568–574 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Norquist BM, Brady MF, Harrell MI. et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: an NRG Oncology/Gynecologic Oncology Group Study. Clin. Cancer Res. 24(4), 777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng CS, Zhang Z, Lee SI. et al. CT perfusion as an early biomarker of treatment efficacy in advanced ovarian cancer: an ACRIN and GOG study. Clin. Cancer Res. 23(14), 3684–3691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Previs RA, Bevis KS, Huh W. et al. A prognostic nomogram to predict overall survival in women with recurrent ovarian cancer treated with bevacizumab and chemotherapy. Gynecol. Oncol. 132(3), 531–536 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Slaughter KN, Thai T, Penaroza S. et al. Measurements of adiposity as clinical biomarkers for first-line bevacizumab-based chemotherapy in epithelial ovarian cancer. Gynecol. Oncol. 133(1), 11–15 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Azad NS, Annunziata CM, Steinberg SM. et al. Lack of reliability of CA125 response criteria with anti‐VEGF molecularly targeted therapy. Cancer 112(8), 1726–1732 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Randall LM, Sill MW, Burger RA, Monk BJ, Buening B, Sorosky JI. Predictive value of serum CA-125 levels in patients with persistent or recurrent epithelial ovarian cancer or peritoneal cancer treated with bevacizumab on a Gynecologic Oncology Group Phase II trial. Gynecol. Oncol. 124(3), 563–568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dao MD, Alwan LM, Gray HJ, Tamimi HK, Goff BA, Liao JB. Recurrence patterns after extended treatment with bevacizumab for ovarian, fallopian tube, and primary peritoneal cancers. Gynecol. Oncol. 130(2), 295–299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mir O, Coriat R, Cabanes L. et al. An observational study of bevacizumab-induced hypertension as a clinical biomarker of antitumor activity. Oncologist 16(9), 1325–1332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int. J. Mol. Sci. 19(4), 1232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guerrouahen BS, Pasquier J, Kaoud NA. et al. Akt-activated endothelium constitutes the niche for residual disease and resistance to bevacizumab in ovarian cancer. Mol. Cancer Ther. 13(12), 3123–3136 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Mitamura T, Pradeep S, McGuire M. et al. Induction of anti-VEGF therapy resistance by upregulated expression of microseminoprotein (MSMP). Oncogene 37, 722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwai T, Sugimoto M, Harada S, Yorozu K, Kurasawa M, Yamamoto K. Continuous administration of bevacizumab plus capecitabine, even after acquired resistance to bevacizumab, restored anti-angiogenic and antitumor effect in a human colorectal cancer xenograft model. Oncol. Rep. 36(2), 626–632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]