Abstract
Background
Selenium (Se) is an indispensable trace element required for animals and human beings, whereas Se-deficiency can accelerate the development of acute gastric injury induced by over-consumption of alcohol. Selenium nanoparticles (SeNPs), as a special Se-supplement with favorable properties and unique bioactivities, are expected to play a passive role in gastroprotection. To the best of our knowledge, the gastroprotective potential of SeNPs is unknown and also, a rapid preparation of orally stable SeNPs available for prospective commercial application in the clinic is needed. Thus, SeNPs-embedded chitosan microspheres (SeNPs-CM) were developed to deliver SeNPs, and their gastroprotective potential was evaluated.
Results
Herein, a rapid, eco-friendly and economic preparation process, composed of synthesis of SeNPs decorated by chitosan (CS), purification of CS-SeNPs by ultra-filtration (UF) and spray-drying of the purified CS-SeNPs, was introduced to prepare SeNPs-CM. The uniformly distributed SeNPs with a nanosize range of 60 nm were loaded into CS-microspheres, and they could be released from the microspheres in gastric conditions. In addition, SeNPs-CM were safer than selenite in terms of Se dose, with a LD50 of around 8-fold of that of selenite, and it could efficiently enhance the Se retention in Se-deficient Wistar rats. Furthermore, SeNPs-CM pre-treatment might significantly attenuate the ethanol-induced gastric mucosal damage, based on histological evaluation. It might be partly attributed to the systematic antioxidant activities of SeNPs-CM, reflected by the reduction in lipid peroxidation, the augmentation in antioxidant enzymatic activity as well as decreasing aggressive nitric oxides (NO).
Conclusion
SeNPs-CM could be taken into consideration as a prospective Se-supplement for the oral delivery of SeNPs, with prominent gastroprotective effect against ethanol-induced mucosal injury.
Keywords: selenium nanoparticles, gastric mucosal injury, gastroprotection, antioxidant
Introduction
Gastric mucosal ulcer is a common gastrointestinal disorder all over the world. Chronic gastric ulcer is closely associated with smoking, bacteria infection, irregular eating habits, chronic bile reflux, autoimmune disorders and stress, whereas acute gastric ulcer is usually caused by excessive consumption of alcohol or high dose of non-steroidal anti-inflammatory drugs (NSAIDs).1–6 High concentration of alcohol (>40%, v/v) and overconsumption of alcoholic beverages can lead to acute gastric ulcers characterized by powerful oxidative stress.1–4,6–8
Selenium (Se) is an indispensable trace element required for most of the living organisms including animals and human beings.9 Se-deficiency might condone the development of acute gastric mucosal injury induced by alcohol, especially for those living in Se-deficient area.7,10–12 Low-Se diet affects both inflammatory cytokine production and histological characteristics, especially in the digestive system.11 As reported, selenium inhibits the formation of ethanol-induced gastric mucosal lesions in rats through prevention of lipid peroxidation and activation of enzymatic radical scavenging.7 As well, selenium either alone or in combination with N-acetylcysteine and vitamin E has a protective effect against ethanol-caused gastric mucosal injury in rats, by increasing gastric glutathione (GSH) and lowering gastric lipid peroxide (LPO) levels.12 Besides, Se-supplement can be gastroprotective against the gastric mucosal damage induced by water-immersion restraint stress in rats.13 Apart from its gastroprotective effects, Se can accelerate the healing of gastric mucosal injury caused by chemicals. For instance, this element has a curative effect on gastric ulcer induced by indomethacin.14 As well, it can accelerate the healing of acetic acid-induced gastric ulcer by facilitating mucosal regeneration, reducing LPO, increasing antioxidant activity and altering mucus secretion response.15 Collectively, the aforementioned studies indicate that Se-supplement has protective and curative effects upon acute gastric injury, involving the antioxidant activity of this element.
Selenium nanoparticles (SeNPs), a kind of elemental Se particles at a nano-size scale with bright red color, have attracted increasing attention in recent years.16,17 The nanoparticles, famous of their low toxicity,16,17 have been considered as a prospective Se formulation for nutritional supplement use, chemoprevention and chemical therapy against cancer, due to their unique properties and excellent biological activities such as free radicals scavenging, immunomodulation, growth promotion, anti-tumor, antimicrobial and anti-inflammatory effects.16–21 Thus, the passive role of SeNPs-supplement in dealing with the gastric injury caused by oxidative stress is anticipated. However, the potential of SeNPs as a gastroprotective agent has not been reported yet. Also, and bare SeNPs are unavailable for commercial applications in the clinic due to their awful stability.19–23 From this perspective, the development of feasible oral SeNPs-supplement with gastroprotective potential is needed.
In some previous reports,19–24 SeNPs were synthesized in protein or polysaccharides, and then, dialysis was applied to purify these nanoparticles, followed by a lyophilization to obtain the final SeNPs product. However, these methods are impracticable in industrial production due to their small production scale, low efficiency and high cost. In this study, a simple way comprising of fast synthesis of SeNPs in the presence of chitosan (CS) and rapid purification of CS-SeNPs by ultra-filtration (UF) was introduced. Thereafter, the purified CS-SeNPs was spray-dried to obtain selenium nanoparticles-embedded chitosan microspheres (SeNPs-CM) designed for the oral delivery of SeNPs. Furthermore, the biosafety, Se retention capacity and gastroprotective effects of SeNPs-CM against ethanol-induced gastric mucosal injury were investigated.
Materials and Methods
Reagents and Animals
CS (>90% deacetylated, average molecular weight of 37 kDa) of food grade was purchased from Aoxin Pharmaceutical Co. Ltd (Taizhou, People’s Republic of China). The assay kits for measuring glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), nitric oxide (NO), prostaglandin E2 (PGE2), thiobarbituric acid-reactive substances (TBARS), lipid peroxide (LPO), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT) and protein content were provided by Jiancheng Bio-engineering Institute (Nanjing, People’s Republic of China). Sucralfate suspensoid was obtained from Kunming Jida Pharmaceutical Co. Ltd (Kunming, People’s Republic of China). Ethanol, chloral hydrate, 40% Formaldehyde, hematoxylin, eosin and other agents of AR grade, were supplied by commercial suppliers.
Male Wistar rats of specific-pathogen-free (SPF) grade, 5–6 weeks old, 180–220 g body weight (bw), were supplied by Laboratory Animal Center, Shenyang Pharmaceutical University (Shenyang, People’s Republic of China) with the license No. SCXK (Liaoning) 2015–0001. Kunming (KM) mice of SPF grade, half male and half female, 6–8 weeks old, weighing 18–22 g, were provided by the same supplier with the license No. SCXK (Liaoning) 2014–0004. Animals were housed in a standardized sterile animal room with controlled temperature (25 ± 2°C) and humidity (50 ± 10%) and a 12 hr light/dark cycle.
Synthesis of SeNPs and Preparation of SeNPs-CM
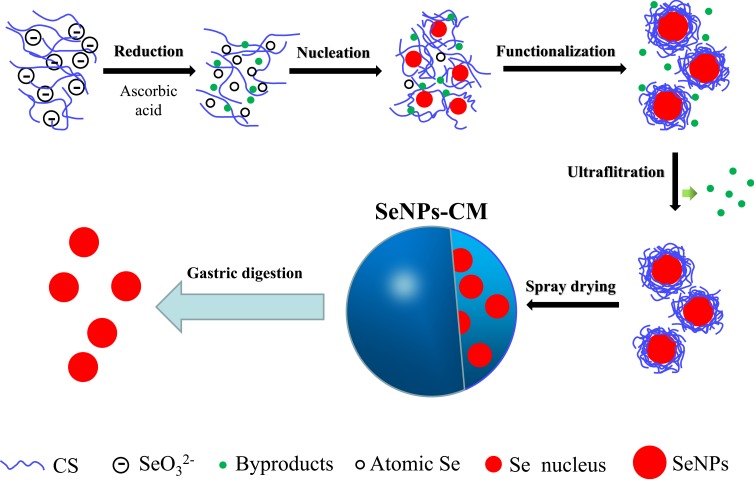
The synthesis of SeNPs and preparation of SeNPs-CM were carried out as shown in Scheme 1. SeNPs were prepared by reducing selenite as described in our previous studies19,20 with some modification. Briefly, CS (200 g), Vc (0.8 molar), acetic acid (300 mL) and deionized water (5700 mL) were mixed well to attain CS/Vc solution. After that, selenium dioxide or sodium selenite (0.2 molar) was dissolved in 500 mL of deionized water, and the resultant mixture was dropwise added to the CS/Vc solution and stirred (150 rpm).
Scheme 1.
The preparation of SeNPs-CM and the oral delivery of SeNPs in the stomach. In brief, aqueous selenite used as the Se precursor was reduced by ascorbic acid (Vc) in the presence of chitosan (CS), generating atomic selenium (Se). Atomic Se nucleated to form Se nucleus, and these Se nuclei assembled into SeNPs. SeNPs was surface-decorated by CS, benefiting to its purification performed by using ultra-filtration (UF). The purified CS-SeNPs colloid was spay-dried to obtain spherical SeNPs-CM with acceptable storage stability, also allowing the expected release of SeNPs in the stomach.
Consequently, the CS-SeNPs colloid was rapidly purified through ultrafiltration (UF) by using a membrane separation device (FlowMem-0015, Xiamen Starmem Scitechnology Co. Ltd, People’s Republic of China) equipped with a piece of polyethersulfone (PES) UF membrane (UE008, GE, USA) with its molecular weight cut-off (MWCO) of 8 kDa. In each round of UF, the component intercepted by the previous round of UF was mixed with aqueous acetic acid (0.5%, v/v) at the ratio of 1:3 (v/v), and the mixture was ultra-filtrated till the volume of the intercepted section decreased to 1/4 of that of the mixture. After few rounds of UF, the by-products with low molecular weight, such as selenite, Vc and its oxidation products, were almost removed, which was evidenced by the fact the final permeatee did not fade 1 μmol/L of potassium permanganate (KMnO4) solution (containing 18.4 mmol L−1 of sulphuric acid) within 20 min.
Finally, this red colloid was spay-dried by using a centrifugal spray dryer (LPG-5; Shiyuan Biological Equipment Engineering Co. Ltd., Shanghai, People’s Republic of China) with a typical working condition: inlet temperature 155°C, inlet temperature 105°C, rotation rate of centrifugal atomization device 20,000 rpm, pumping flow rate 4–5 L−1, and drying air flow discharge 860–1160 m3.h−1 The ratio of SeNPs/CS could be adjusted before spay-drying. The blank chitosan microspheres (BCM) without any SeNP were prepared by replacing Se (IV) with deionized water.
Characterization of SeNPs and SeNPs-CM
The morphology of nanoparticles or microspheres was observed by means of transmission electron microscopy (TEM) and scanning electron microscopy (SEM). A TEM device (JEM-2100; JEOL, Tokyo, Japan) and a SEM machine (S-4800; Hitachi, Tokyo, Japan) both equipping an energy-dispersive X-ray spectroscopy (EDS) apparatus were used.19,20 The size distribution of SeNPs was measured basing on their TEM image, and the zeta-potential of the nanoparticles was determined by using a Zetasizer Nano ZS particle analyzer (VEM3600; Malvern Instruments, Malvern, UK) with a 173° scattering angle.20 The size of microspheres was also investigated by a particulate size analyzer (LS-POP(6); Zhuhai OMIC Instruments Co. Ltd., Zhuhai, P.R. China).19,20 The Se content of SeNPs-CM was determined by utilizing inductively coupled plasma mass spectrometry (ICP-MS).25 A Nicolet Nexus 470 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to record the infrared spectra acquired at 400–4000 cm−1 wavenumbers with a 4 cm−1 resolution.19,20 XPS measurement was conducted by a photoelectron spectrograph (Escalab 250Xi; Thermo Fisher Scientific) equipped with a monochromatic Al Kα X-ray source with an energy resolution of 0.05 eV.19,20 A dual-beam charge neutralization system composed of a low-energy electron flood gun (~1 eV) and an argon ion gun (≤10 eV) was used.19,20
Release Study
The release of SeNPs from their vehicles was studied in a HCl solution (pH 1.2) stimulating the pH environment of the stomach. Briefly, SeNPs-CM was mixed with the HCl solution (pH 1.2) and stirred at 37°C for 1–2 hrs. The mixture was filtrated with a filter membrane (pore size of 0.22 μm), and the filtrate was dropped onto copper grids and dried in clean air, followed by the TEM observation and the EDS determination as previously described.19,20
Animal Experiment
Acute Lethal Test in vivo
The acute lethal properties of SeNPs-CM were determined as previously described19,20 with little change. Briefly, 60 KM mice were randomly divided into 6 groups with 10 mice per group after adaption for 3 days. Each group was given saline or SeNPs-CM (5.00, 3.75, 2.81, 2.10, 1.58 mg kg−1 bw) by single intragastric administration, and cumulative mortality within 14 days was recorded to calculate median lethal dose (or LD50) by Bliss method.26
Ulcer Experiment
Seventy male Wistar rats, as presented in Table 1, were randomly divided into 7 groups (each containing 10 animals): Control (saline), Model (ethanol), Sucralfate (ethanol + sucralfate), BCM (ethanol + BCM) and three Se groups (ethanol + SeNPs-CM) comprised of L-Se, M-Se and H-Se. Control and Model groups were given normal saline by gavage (10 mL kg−1 bw), while Sucralfate group acting as a positive group was intragastrically administered sucralfate suspensoid (200 mg kg−1 bw). L-Se, M-Se and H-Se groups were orally administrated with 0.6, 1.2 and 2.4 mg kg−1 bw of SeNPs-CM, respectively. Meanwhile, BCM at a comparable dose (1.2 mg kg−1 bw) acting as a vehicle control was utilized to evaluate the contribution of chitosan vehicle to the bioactivity of SeNPs-CM. The daily administration and body weighting were performed for 30 days. All rats were allowed free access to a low-Se diet (<0.1 mg Se kg−1 diet) and water during the experiments.
Table 1.
The Administration During the Gastroprotective Experiment (Wistar Rats, n=10)
| Group | Pre-Treatment (Once Daily, Day 1–30) | Treatment (Once, Day 31) |
|---|---|---|
| Control | Normal saline | Normal saline |
| Model | Normal saline | Ethanol (1mL each) |
| Sucralfate | Sucralfate Suspensoid (200 mg kg−1 bw) | Ethanol (1mL each) |
| BCM | BCM (1.2 mg kg−1 bw) | Ethanol (1mL each) |
| L-Se | SeNPs-CM (0.6 mg kg−1 bw) | Ethanol (1mL each) |
| M-Se | SeNPs-CM (1.2 mg kg−1 bw) | Ethanol (1mL each) |
| H-Se | SeNPs-CM (2.4 mg kg−1 bw) | Ethanol (1mL each) |
Abbreviation: bw, body weight.
After the last administration, all the rats were deprived of food. Twenty-four hours later, the fasting animals were administrated with absolute ethanol (1 ml per rat) by gavage except for the Control group. At 60 min after the ethanol treatment, the anaesthesia of rats was carried out through intraperitoneal injection of aqueous chloral hydrate (0.5 mg kg−1 bw). The blood in aorta abdominalis was collected into a heparin-free tube, and the animals were euthanized by cervical dislocation. Quickly, serum was obtained after immediate centrifugation (1000×g, 4°C, 10 min) and stored at −80°C until analysis, while some important organs such as stomach and liver were collected for further investigation. The viscera index, defined as the ration of organ weight to body weight,27 was also measured.
Macroscopic Evaluation
The freshly excised stomach was opened along the greater curvature and rinsed in ice-cold normal saline to remove the gastric contents and blood clots. The samples were photographed to explore the gross gastric mucosal lesions. The injury incidence was calculated following Equation (1). Gross mucosal lesions were recognized as hemorrhage spot or damage strip (erosions) on the mucosal surface.6 The areas of stomach tissue and gross lesions were approximately measured by using Image J (v1.48) software (developed by National Institutes of Health, USA).28 The results were expressed as the gross ulcer index-1 (GUI-1) calculated by using Equation (2). Macroscopic scoring of gastric mucosal lesions was also performed by an independent viewer blinded to the treatment as described by Kan et al6 with limited modification. In brief, scoring gastric injury was carried out basing on the severity of hyperemia and hemorrhagic erosions, and the gross ulcer index-2 (GUI-2) was defined as the weighted sum of individual scores according to Kan.6,30
 |
Equation\(1) |
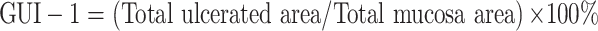 |
Equation\ (2) |
Histological Examination
Samples from the comparable region of the stomach or liver were excised and fixed in a 10% of buffered formalin solution, routinely followed by paraffin embedding. Sections (5 μm thick) were stained with hematoxylin & eosin (HE) dual-dye and examined under a light microscope.1,12,19 The sections were observed and photos were captured to explore the histological change.
Based on the severity of histological disorder, the histological damage in gastric mucosa was graded by using two methods. In the first method, each field was scored histologically on a scale from 0 to 4 in accordance with previously described criteria29 with limited modification: 0 – normal; 1 – epithelial cell damage; 2 – glandular disruption, vasocongestion or edema in the upper mucosa; 3 – mucosal disruption, vasocongestion or edema in the mild lower mucosa; 4 – extensive mucosal disruption throughout the mucosa. The overall mean value of the scores for each of the fields was taken as the histological ulcer index-1 (HUI-1) for the section. In the second method, grading the gastric injury was achieved on a 1–5 scale according to Kan et al6 based on the percentage of the damage area. The damage score called the histological ulcer index-2 (HUI-2), was defined as the weighted sum of individual scores.6 All determinations were performed in a randomized manner and histological sections were coded to eliminate an observer bias.
Se Retention Determination
Se concentration in blood or organ was determined by ICP-MS assay as described in previous studies.19,20,25 Briefly, serum or organ sample was mixed with hydrogen nitrate/hydrogen peroxide (HNO3/H2O2) solution. The mixture was then digested by means of microwave heating. The Se concentration of each digested sample was determined by using an ICP-MS device (7700X, Agilent, USA).
Biochemical Analysis
The fresh samples of the liver or stomach were rinsed with cold normal saline, and the homogenization in ice-cold saline was conducted. After immediate centrifugation (10,000×g, 4°C, 10 min), the supernatant was collected. After that, the serum and the supernatant were used to measure the levels of GOT, GPT, GSH, TBARS (malondialdehyde equivalent), GSH-Px, CAT, SOD, PGE2, NO and LPO, following the instructions of commercial kits listed previously.
Statistical Analysis
In all the experiments, data were presented as mean ± SD. The difference between the two groups was analyzed by Student’s t-test, while the difference between three or more groups was analyzed by one-way analysis of variance (one-way ANOVA) test followed by multiple comparisons. SPSS software program (version 17.0 for Windows) was utilized, and a P value of <0.05 was considered statistically significant.
Results and Discussion
Design of SeNPs-CM
CS is the only positively charged natural polysaccharide with excellent biodegradable and biocompatible characteristics, having been extensively examined in the pharmaceutical fields for its potential in developing medicine delivery systems.30 SeNPs (Se(0)) can be synthesized in the presence of CS.19–22 However, it was a process of laboratory level, with its small scale far from practical production. Even worse, the purification of SeNPs by means of dialysis19,22 was impracticable in industrial production because of the low efficiency and high cost. Besides, bare SeNPs and CS-SeNPs were unavailable for commercial applications in oral administration systems due to their awful stability.19,20 Therefore, SeNPs-CM aiming at both the stability and oral delivery of SeNPs was developed, and a rapid preparation process designed for the industrial production of SeNPs-CM was also introduced (Scheme 1). The physicochemical characters and bioactivities of SeNPs might be retained if the design worked.
Physicochemical Properties of SeNPs and SeNPs-CM
Morphology and Formation of SeNPs
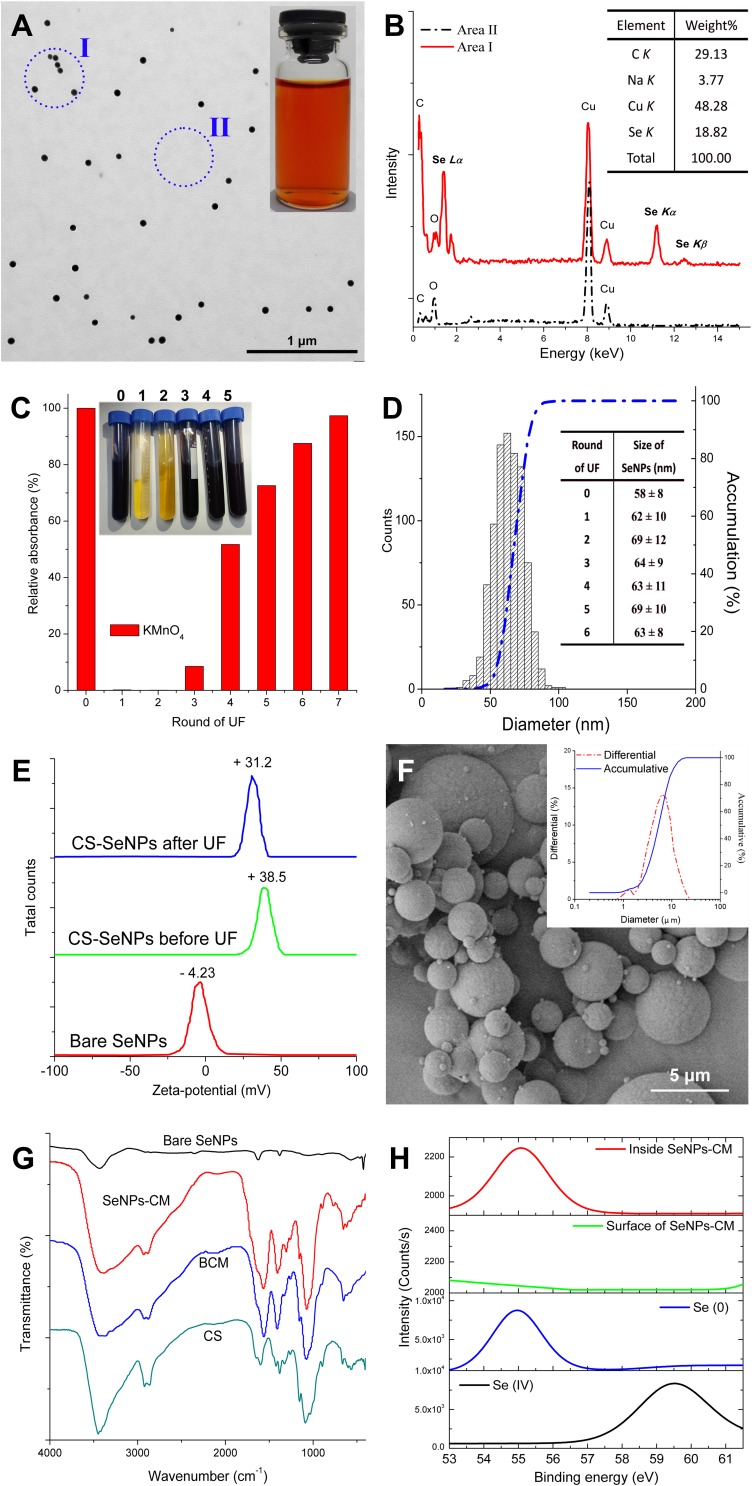
The synthesis of SeNPs was performed on a much bigger scale (>1000-fold) and at a significantly lower stirring speed (150 rpm) as compared with previous studies.19–22 As a result, monodisperse spherical CS-SeNPs in high uniform was obtained, with red appearance and the mean size of around 60 nm (Figure 1A). The typical Se peaks (1.37, 11.22 and 12.49 keV), identified as Se Lα, Se Kα and Se Kβ, respectively, were found in the EDS spectra of CS-SeNPs, strongly confirming the elemental nature of these nanoparticles (Figure 1B). It indicated small CS-SeNPs can be fabricated even in such a condition stimulating industrial production.
Figure 1.
The physicochemical properties of CS-SeNPs. (A) Transmission electron microscopy (TEM) image of CS-SeNPs and their appearance (inset). (B) Typical energy-dispersive X-ray spectroscopy (EDS) spectra of CS-SeNPs (found at Area I, Panel (A) and their elemental composition (inset). (C) The absorbance (525 nm) of potassium permanganate (KMnO4) solution when coming across with UF filtrate and their appearance (inset). (D) The size distribution of CS-SeNPs measured basing on TEM results, and the influence of UF on the size of these nanoparticles (inset). (E) Zeta-potentials of bare SeNPs and CS-SeNPs (before or after 6 rounds of UF). (F) SEM image of SeNPs-CM and their size distribution (inset). (G) FTIR spectra of bare SeNPs, BCM and SeNPs-M. (H) Se 3d XPS patterns of Se (IV) (selenite or selenium dioxide), Se (0) (bare SeNPs or crystal Se) and SeNPs-CM, obtained with or without argon etching.
Influence of UF Upon CS-SeNPs
Ultrafiltration (UF) was used instead of dialysis to purify CS-SeNPs. The PES membrane (MWCO of 8 kDa) used could retain CS and SeNPs, but it allowed free access of soluble compounds with low molecular-weight (such as Vc and its oxidates), of which might fade aqueous acidic KMnO4 solution.31 The content of the byproduct in the retentate, in theory, could be reduced by geometric progression in accordance with the frequency of the UF process. After six or seven round of UF treatment, aqueous KMnO4 (1 μmol L−1) was unable to fade within 20 min when mixed with the corresponding filtration, indicating little byproduct could be transported to the filtrate (Figure 1C). It implied UF can remove the unwanted inclusion efficiently. More importantly, limited modification of the shape or size of SeNPs was observed during rounds of the UF process (shown in Figure 1D), also suggesting the feasibility of UF used in the purification of SeNPs. Significantly, CS might guarantee the stability of SeNPs during the UF process. It could be partly explained by the viscosity of CS.30 The surface decoration by CS on SeNPs, resulting in the elevation of zeta-potential of these nanoparticles,19,22 also contributed to the stability (Figure 1E). The limited change of zeta-potential during UF processing made UF suitable for the rapid purification of CS-SeNPs.
Characterization of SeNPs-M
The purified CS-SeNPs colloid was finally mixed with another CS solution, and the mixture was spray-dried to remove moisture and acetic acid, generating solid SeNPs-CM with their average size of around 6 μm (Figure 1F). The SeNPs-CM seemed to be a simple collection of SeNPs and CS, without any new functional group found in the FTIR spectra (Figure 1G). Furthermore, XPS patterns of SeNPs-CM were recorded to explore the status of Se. As presented in Figure 1H, the peaks at 55.3 eV and 59.5 eV were identified as the typical Se 3d signals of Se (0) and Se (IV), respectively, confirming the Se within SeNPs-CM was in elementary status.19,20 However, the Se 3d signals on the surface of SeNPs-CM were significantly weaker than that inside SeNPs-CM, which was found by using argon ion etching to expose the elements inside. It implied most of the SeNPs are enclosed inside SeNPs-CM. Apparently, the physical stabilization of SeNPs was carried out, and the content of SeNPs within SeNPs-CM could be easily adjusted by modifying material ratio.
Release of SeNPs from SeNPs-CM
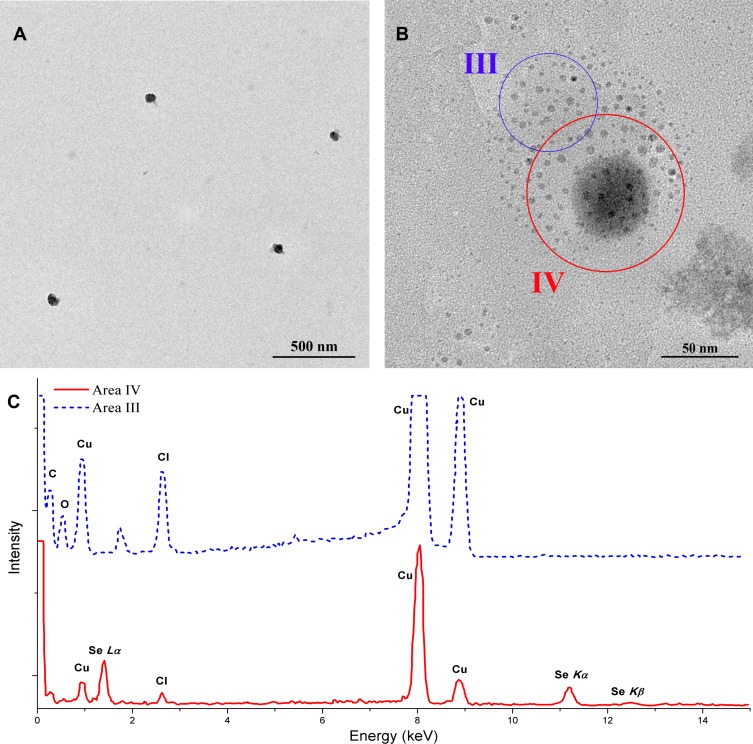
The release of nanoparticles from their vehicle was very important to their absorption and their bioactivities.17,20 In the study, SeNPs-CM was mixed with HCl solution (pH 1.2) and stirred at 37°C, simulating the gastric digestion of this Se-supplement. Intact SeNPs could be observed in the mixture after the incubation (shown in Figure 2A and B), suggesting SeNPs can be released from SeNPs-CM in the mammalian stomach. Interestingly, many small beads of few nanometres were found around much bigger single ones in the TEM image (Figure 2B). EDS performed on the area containing the small or big beads (shown in Figure 2C) indicated the bigger ones were SeNPs while the smaller ones might be CS. Perhaps CS in SeNPs-CM might collapse into small colloid drop when mixing with HCl solution. It was also possible the aqueous CS might turn to solid CS beads when sampling for TEM investigation. Although how the SeNPs escaped from SeNPs-CM was unknown, it was certain SeNPs can be released from their vehicles in the digestive tract. In other words, the embedding by CS did not change the size and morphology of SeNPs, two important properties of which could profoundly affect the bio-activities of the nanoparticles.32,33 It also proved SeNPs were stable in the vehicle during storage.
Figure 2.
Release of SeNPs from SeNPs-CM in HCl solution. SeNPs-CM was mixed with HCl solution (pH 1.2). After 1–2 hrs, the mixture was filtrated by using a filter membrane (with the aperture size of 0.45 μm), and the filtrate was sampled for TEM observation and EDS investigation. (A) TEM image of SeNPs and (B) the details. (C) Typical EDS spectra obtained in Figure 2B (Area III versus Area IV).
Safety of SeNPs-CM
Acute Toxicity of SeNPs-CM in KM Mice
The safety of SeNPs-CM was considered before its potential in the clinic. As reported in some previous work,19,20,34 SeNPs were safer than selenite or selenomethionine, mainly responsible for the whole toxicity of SeNPs/vehicle complexes. In this study, SeNPs-CM was much safer than selenite19 in turns of Se dose, with a LD50 of around 8-fold of that of selenite in KM mice (Table 2). The finding was in accordance with some reports,19,20,34 though the model animals (KM mice or ICR mice) or the vehicle (chitosan, or chitosan/citrate, or BSA) might be different. Perhaps, SeNPs loaded by protein or polysaccharide might present comparable toxicity, when (1) they shared similar basic physicochemical properties35,36 such as shape, size, chemical composition and surface properties and (2) they could escape from their vehicles in the stomach.19
Table 2.
Acute Lethal Results by Single Oral Administration in KM Mice (n=10)
| Sample | Se Content (mg g−1) |
LD50 # (mg kg−1 bw) |
LD50 (Se) * (mg Se kg−1 bw) |
|---|---|---|---|
| Sodium selenitea | 456.7 | 19.2 (16.2–22.7)$ |
8.8 (7.4–10.4)^ |
| BCMa | – | > 15 × 103 | – |
| SeNPs-CM | 30 | 2.44 × 103 (1.81×103 - 3.03×103)$ |
73.2 (54.3–90.9)^ |
Notes: #LD50 = median lethal dose; *LD50 (Se) = LD50 × Se content. $The LD50 of 95% confidence interval. ^The LD50(Se) of 95% confidence interval. aThe results of sodium selenite and BCM were reported in previous work Reference.19
Abbreviation: bw, body weight.
Influence of SeNPs-CM on the Growth, Viscera and Aminotransferases of Wistar Rats
In the ulcer experiment, another SeNPs-CM sample containing 20 mg g−1 of Se (measured by ICP-MS25) was administrated at the doses of 0.6, 1.2, 2.4 mg/kg bw (shown in Table 1) based on the daily recommendation dose of Se.37,38 During the experiment, BCM (1.2 mg kg−1 bw), SeNPs-CM and sucralfate (200 mg kg−1 bw) did not exhibit any visible negative effects on rats’ behavior and body weight (See Table S1, Supplementary materials). Besides, the viscera indexes of heart, liver, spleen and kidney were normal in each group (See Table S2, Supplementary materials). Moreover, little visible modification could be found in the structure of the liver (See Figure S1, Supplementary materials).
The levels of GOT and GPT in serum can help people diagnose body tissues are injured or not, especially the heart and the liver.39 Serum GPT is sensitive to acute hepatic injury since it can be released from hepatic cells when acute destruction of cytomembrane happens.40 The jump of serum GOT is closely associated with the damage to endochylema and the dysfunction of the mitochondrial membrane, implying severe injury of an organelle.40 In this study, GOT in each group was normal (Table 3), indicating 30-day administrations of SeNPs, BCM and sucralfate at their setting doses were safe. Besides, the ethanol-caused elevation of the GPT level might be retarded by all the pretreatments, suggesting the liver protection conducted by the three compounds.
Table 3.
The Serum Aminotransferases in Wistar Rats at the End of Ulcer Experiment (n=10)
| Group | GOT (U L−1) | GPT (U L−1) | Ratio of GOT/GPT |
|---|---|---|---|
| Control | 127.07 ± 55.76 | 24.27 ± 17.78a,b | 5.24 |
| Model | 119.27 ± 47.42 | 59.77 ± 16.43c | 2.00 |
| Sucralfate | 137.86 ± 69.10 | 25.17 ± 16.23a,b | 5.48 |
| BCM | 103.49 ± 44.37 | 29.15 ± 11.31a,b | 3.55 |
| L-Se | 141.31 ± 52.21 | 17.37 ± 13.92a | 8.14 |
| M-Se | 151.37 ± 37.11 | 41.70 ± 11.03b | 3.63 |
| H-Se | 157.85 ± 131.21 | 37.48 ± 18.60a,b | 4.21 |
Notes: a–cMeans within a column with different letters differ significantly (P < 0.05).
Selenium Retention in vivo
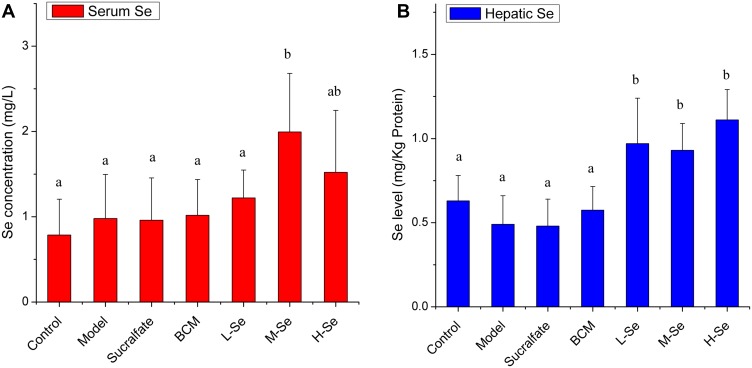
The Se level in the body was monitored to study the Se-supplying ability of SeNPs-CM in rats. As presented in Figure 3, serum Se concentrations in M-Se group and hepatic Se levels in the Se-supplement groups were significantly higher than that of no Se-supplement groups (P < 0.05), indicating SeNPs-CM at the doses could benefit to the Se stock in animals. Markedly, SeNPs-CM was actually the main Se supplement, in consideration of the extremely low Se content of the feed (<0.1 μg Se g−1 diet) and the estimable daily intake of adult Wistar rats (about 20–30 g diet day−1 each).41–43 Additionally, visible difference in Se levels was found between M-Se and BCM (P < 0.05). It was the SeNPs within SeNPs-CM contributed to the improvement of Se retention.
Figure 3.
The serum Se concentration (A) and hepatic Se level (B) in Wistar rats. The daily administration of each group was performed as shown in Table 1. After the last administration, rats were deprived of food, allowed free access to water. Twenty-four hours later, rats were given ethanol or saline, and they were sacrificed in 1 hr to obtain serum, liver and other organs. The samples were digested in hydrogen nitrate/hydrogen peroxide (HNO3/H2O2) solution, and then, the Se content in the digestion was determined by using ICP-MS assay. a–bMeans within a panel with different letters differ significantly (P < 0.05).
The deposition preference of SeNPs was studied. Interestingly, hepatic Se seemed to be more sensitive to the supplement of SeNPs-CM than serum Se (Figure 3). The Se-boost in serum was latter than that in the liver, in line with a previous study20 reporting a faster increase of hepatic Se than that of plasma Se in mice. It suggested the Se from SeNPs-CM is apt to be stored in the liver. Additionally, Se accumulation in the liver was found in mice or fish when BSA-SeNPs34,44,45 or selenite34 or selenate46 were given. Possibly, inorganic Se was inclined to be collected in hepatic tissue.
The dose of SeNPs needed more attention. Comparable hepatic Se levels were achieved in all the Se groups, suggesting a possible “plateau” (Figure 3B). A similar plateau was reported by Barnes47 and Raines,48 who pointed out haptic Se level was very sensitive to Se content in food. Se-supplementation resulted in a sigmoidal response in the liver Se concentration of rats: hepatic Se quickly increased to a plateau at 0.08 μg Se g−1 diet, remained at this level until 0.24 μg Se g−1 diet, and then increased gradually to 1.80-fold of Se adequate levels (0.08–0.24 μg Se g−1 diet) in rats fed 0.8 μg Se g−1 diet;47 hepatic Se increased fast if the Se content in diet was too high (>0.8 μg Se g−1 diet).48 Herein, the daily Se intakes in the Se groups reached the plateau, indicating the daily doses of SeNPs were able to meet the requirement of adequate or super-nutritional Se. That was, the doses of SeNPs-CM were acceptable, far from the toxic status according to Barnes47 and Raines.48
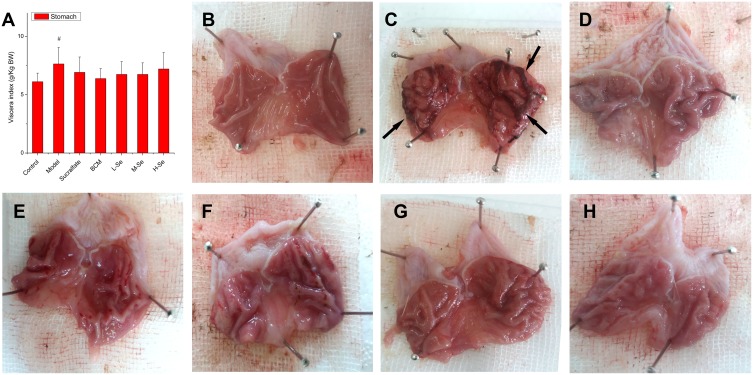
Macroscopic Gastric Damage
Ethanol resulted in gastric edema, which was evidenced by the significantly higher stomach index (P < 0.05, Model versus Control) as shown in Figure 4A and B. Apart from the edema, high injury incidence (≥90%) was observed in the groups given ethanol (Table 4), suggesting the ethanol-induced lesions are very common in animals. The damage was highlighted in the congestion and hemorrhagic lesions in the gastric mucosa (Figure 4C). Sucralfate, an efficient anti-ulcer medicine widely used to treat gastric ulcer,49 strongly resisted the gastric disorder induced by ethanol (Figure 4D). BCM also retarded the formation of lesions (Figure 4E), while strong gastroprotection was found in all the groups pretreated by SeNPs-CM (Figure 4F–H).
Figure 4.
The viscera index of the stomach and the gross appearance of gastric mucosa in Wistar rats. Wistar rats were pre-treated as described in Table 1. After the pre-treatment, gastric mucosal injury was induced by the oral administration of ethanol. One hour after the ethanol challenge, the rats were sacrificed. The stomach was opened along the greater curvature and washed in ice-cold normal saline, followed by macroscopic observation. The stomach was weighted to measure (A) viscera index, while typical appearances of gastric mucosa in different groups: (B) Control, (C) Model, (D) Sucralfate, (E) BCM, (F) L-Se, (G) M-Se and (H) H-Se, were recorded. Severe hemorrhages (arrow) were found in Model group. # P < 0.05, versus Control.
Table 4.
The Gross Ulcer Indexes (GUIs) of Gastric Mucosa in Wistar Rats (n=10)
| Group | Injury Incidence (%) | GUI-1 * (% of Area) |
Inhibition of GUI-1^ (%) | GUI-2$ (Point) |
Inhibition of GUI-2^ (%) |
|---|---|---|---|---|---|
| Control | 0 | 0 ± 0a | - | 1.5 ± 0.8a | - |
| Model | 100% | 14.36 ± 8.92d | 0 | 67.4 ± 12.7d | 0 |
| Sucralfate | 90% | 1.54 ± 1.46a,b | 89.3 | 14.7 ± 11.2a,b | 78.3 |
| BCM | 90% | 7.73 ± 4.10c | 46.2 | 37.7 ± 25.7c | 44.2 |
| L-Se | 100% | 5.72 ± 2.04b,c | 60.2 | 34.3 ± 18.9b,c | 49.2 |
| M-Se | 100% | 2.50 ± 1.75a,b | 82.6 | 14.1 ± 8.4a,b | 79.1 |
| H-Se | 100% | 2.03 ± 1.72a,b | 85.8 | 20.4 ± 14.0a,b,c | 69.8 |
Notes: a–dMeans within a column with different letters differ significantly (P < 0.05). *The score was grading in accordance with Reference.28 $The score was grading based on the criteria listed in Reference.6 ^The percentage of ulcer inhibition was calculated as the formula: (GUIModel - GUIexperimental group)/GUIModel × 100%.
The methods of grading gastric injury were various.6,28 Herein, grading was performed basing on scar area28 or scar severity,6 and the results were expressed as GUI-1 or GUI-2 (Table 4). As expected, SeNPs-CM was able to inhibit the forming of gastric mucosal injury in a dose-dependent manner. Amazingly, the inhibition of both GUIs (about 70% - 85%) by SeNPs-CM (1.2 or 2.4 mg kg−1 bw) was comparable with that by sucralfate (200 mg kg−1 bw), suggesting the potent gastric protection by the Se-supplement. Furthermore, more inhibition of GUIs was found in M-Se group as compared with BCM group (P < 0.05), though BCM itself also had a passive influence on gastric protection.50 It implied SeNPs contribute greatly to the gastroprotection of SeNPs-CM against ethanol.
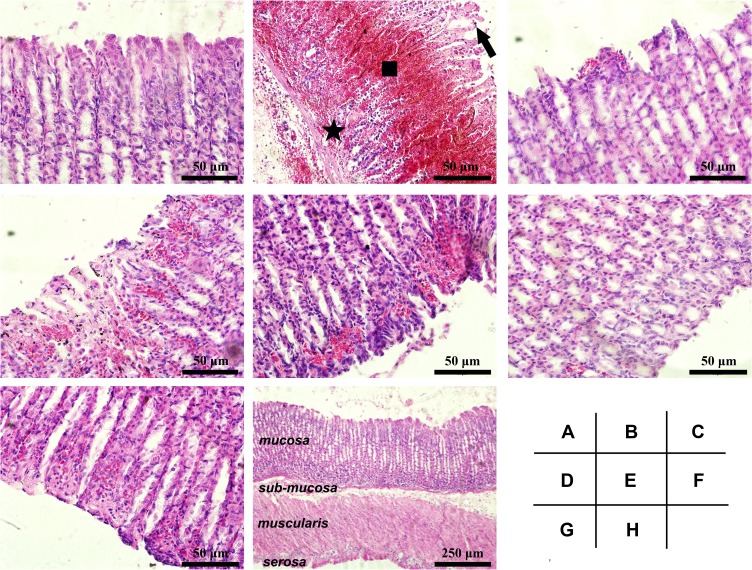
Histological Findings
The histological property of gastric mucosa was investigated for more details. Normal structures were found in Control (Figure 5A). However, extensive damage in the gastric mucosa was found in Model group, characterized by epithelial degeneration, cell desquamation, hemorrhage, and inflammatory cell infiltration (shown in Figure 5B), in line with previous reports.6,29 Pretreatment with sucralfate or SeNPs-CM revealed the protective effects highlighted in the reduction in hyperaemia, haemorrhage, disrupted surface epithelium and leucocytes infiltration (Figure 5C,5E–5G), while BCM also showed some weak protection (Figure 5D). Other typical responses to ethanol, such as submucosal edema, deeply penetrated necrotic lesions into the mucosa and weak mucous secretion, were also weakened in all the pretreatment groups. Particularly, nearly normal structure was found throughout the full thickness of gastric section in M-Se group (presented in Figure 5H), implying SeNPs-CM can protect the whole gastric tissue from ethanol-caused damage.
Figure 5.
Histological details of the gastric mucosa in Wistar rats. The microscopic examination was performed on the gastric mucosa obtained from different groups: (A) Control, (B) Model, (C) Sucralfate, (D) BCM, (E) L-Se, (F, H) M-Se and (G) H-Se. Briefly, Wistar rats were treated as presented in Table 1, and they were sacrificed after the ethanol challenge. The stomach was immediately obtained, part of which was quickly fixed in formaldehyde solution, routinely embedded by paraffin, sectioned (5 μm thick) and finally stained by hematoxylin and eosin (H&E) duel-dye. The Sections were examined under a light microscope. Typical gastric mucosal damage including epithelial exfoliation (arrow), mucosal hemorrhage (square) and inflammatory cell infiltration (star) was found in Model group. (A–G) magnification, × 200; (H) magnification, × 40.
HUI-1 and HUI-2 were measured to grade the microscopic lesions in gastric mucosa, depending on scar area and scar severity.6,29 As shown in Table 5, Model ranked the highest HUI-1 and HUI-2. However, the pretreatment groups exhibited lower HUIs when comparing with Model (P < 0.05). Among the pretreatments, SeNPs-CM at the dose of 1.2 mg kg−1 bw presented potent inhibition against the ethanol-caused lesions, while the inhibition by BCM was weaker (P < 0.05, M-Se versus BCM). This was consistent with the results shown in Table 4. It also confirmed the contribution of SeNPs to the gastroprotection of SeNPs-CM.
Table 5.
The Histological Ulcer Indexes (HUIs) of Gastric Mucosa in Wistar Rats (n=10)
| Group | HUI-1* (Point) |
Inhibition of HUI-1^ (%) |
HUI-2$ (Point) |
Inhibition of HUI-2^ (%) |
|---|---|---|---|---|
| Control | 0.00 ± 0.00a | - | 0.00 ± 0.00a | - |
| Model | 3.40 ± 0.70d | 0 | 14.30 ± 3.47d | 0 |
| Sucralfate | 1.70 ± 1.06b,c | 50.0 | 8.50 ± 3.41b,c | 40.6 |
| BCM | 1.80 ± 1.11c | 47.1 | 10.30 ± 4.62c,d | 28.0 |
| L-Se | 2.00 ± 0.82c | 41.2 | 9.80 ± 2.44b,c,d | 31.5 |
| M-Se | 0.65 ± 0.63a,b | 80.9 | 4.80 ± 2.97a,b | 66.4 |
| H-Se | 2.10 ± 1.52c | 38.2 | 9.40 ± 6.15b,c,d | 34.3 |
Notes: *The score was grading in accordance with Reference.32 $The score was grading based on the criteria reported in Reference.6 ^The percentage of ulcer inhibition was calculated as the formula: (HUIModel - HUIexperimental group)/HUIModel × 100%. a–d Means within a column with different letters differ significantly (P < 0.05).
Relieving Effect of SeNPs-CM Against Ethanol-Induced Biochemical in Stomach
Antioxidant Ability of SeNPs-CM in Stomach
Exogenous ethanol acting as a potent aggressive agent not only causes serious lesions in gastric mucosal by its direct toxic effect,51 but also lead to excessive lipid peroxidation in gastric mucosa by intriguing radical oxygen species (ROS) reaction.52 As shown in Table 6, both LPO (typical intermediate product in lipid peroxidation)53,54 and TBARS (typical end-product in lipid peroxidation)53,54 were increased by ethanol, indicating oxidative damage in cytomembrane. However, the lipid peroxidation could be retarded by the pretreatment with BCM, SeNPs-CM and Sucralfate, as evidenced by the decrease of LPO or TBARS (Table 6). Interestingly, SeNPs-CM might be superior to sucralfate (200 mg kg−1 bw) in attenuating lipid peroxidation, since the former could decrease both LPO and TBARS, whereas the latter might not reduce LPO. Probably, SeNPs-CM inhibited some oxidation steps throughout the lipid peroxidation.
Table 6.
The Levels of LPO, TBARS, GSH-Px, PGE2 and NO in Stomach (Wistar Rats, n=10)
| Group | LPO (μmol g−1 Prot) |
TBARS (μmol g−1 Prot) |
GSH-Px (U mg−1 Prot) |
PGE2 (ng mg−1 Prot) |
NO (μmol g−1 Prot) |
|---|---|---|---|---|---|
| Control | 2.32 ± 1.43a,b | 0.94 ± 0.52a | 836 ± 206c | 1.67 ± 0.84a | 0.45 ± 0.26a |
| Model | 4.31 ± 1.88b,c | 1.93 ± 0.81b | 427 ± 45a,b | 1.59 ± 0.51a | 1.32 ± 0.43b |
| Sucralfate | 5.16 ± 1.52c | 0.91 ± 0.37a | 385 ± 120a,b | 4.81 ± 0.62b | 1.07 ± 0.35a,b |
| BCM | 2.03 ± 1.39a,b | 1.35 ± 0.81a,b | 181 ± 86a | 1.04 ± 0.54a | 1.05 ± 0.54a,b |
| L-Se | 3.52 ± 2.25a,b,c | 1.08 ± 0.67a,b | 616 ± 203b,c | 1.26 ± 0.48a | 0.76 ± 0.38a,b |
| M-Se | 2.29 ± 1.71a,b | 1.19 ± 0.60a,b | 731 ± 302c | 1.03 ± 0.49a | 0.67 ± 0.48a |
| H-Se | 1.11 ± 0.79a | 1.17 ± 0.39a,b | 444 ±182b | 1.06 ± 0.54a | 0.50 ± 0.16a |
Notes: a–cMeans within a column with different letters differ significantly (P < 0.05).
Abbreviation: prot, protein.
GSH-Px is a family of enzymes reducing lipid hydroperoxides to their corresponding alcohols and also reducing free hydrogen peroxide to water, whose catalytic site contains Selenocysteine (Se-Cys).16–20,34 Dramatic decrease of gastric GSH-Px was induced by ethanol, suggesting the dysfunction of the antioxidant enzyme system (Table 6). Pretreatment with sucralfate or BCM could not stop the loss of GSH-Px. In contrast, oral administration of SeNPs-CM (1.2 mg kg−1 bw) was able to suppress the reduction of GSH-Px caused by ethanol (P < 0.05, versus Model). The boost of GSH-Px might benefit to the stability of some bioactive gastric mucosal defensive factors such as endogenous nitric oxides (NO) by lowering ROS (i.e., hydrogen peroxide),55 and it could also retard the oxidative damage to cells,16–20,34 resulting in the reduction of TBARS and LPO. Furthermore, SeNPs might contribute mainly to the retention of GSH-Px by SeNPs-CM (P < 0.05, M-Se versus BCM), in consideration of the negative influence of BCM on GSH-Px level. Perhaps SeNPs-CM could offer Se to the synthesis of GSH-Px, consistent with its contribution to Se retention in vivo (Figure 3). Undoubtedly, the improvement of GSH-Px by SeNPs took an important part in the gastroprotection mechanism of SeNPs-CM.
Influence of SeNPs-CM on Inflammatory Mediators
PGE2 and NO are proved to be important mediators of the inflammatory process in gastric ulcer or acute gastric injury.56 As an important member of prostaglandins (PGs), PGE2 can protect the gastric mucosa by activating its different EP receptors, which can enhance the secretion of mucus and bicarbonate, increase blood flow, and decrease acid secretion.56,57 Moreover, PGE2 in ulcer tissue specimens has been reported to play a passive role in ulcer repairing.56,57 However, little change of PGE2 could be found among the groups except Sucralfate group in the present study (shown in Table 6), implying the gastroprotection of SeNPs-CM might be independent of endogenous PGE2. It was quite different from that of sucralfate.
The physiological role of NO in gastric tissue is very complicated. Not only is NO a free radical acting as a precursor of different reactive nitrogen species (RNS),58 but it is a critical signaling molecule in the inflammatory process.56,58 In mammals, synthesis of NO is catalyzed by a set of enzymes called nitric oxide synthases (NOS), and three distinct isoforms of NOS have been identified – neuronal, macrophage and endothelial types.56,58 A high level of NO is released by the inducible isoform (iNOS, macrophage type) as compared to the amount generated by the constitutive isoforms (neuronal or endothelial types), when wound healing, burn injury, endotoxin exposure, arthritis and inflammatory bowel diseases happen.56,58 In acute gastric injury caused by ethanol, great up-regulation of iNOS takes place at both gene and protein levels,56,59,60 whereas the levels of other NOS are strongly down-regulated.1,59 As a result, a dramatic jump of NO can be found hand in hand with the up-regulation of iNOS in the gastric tissue damaged by ethanol, resulting in inflammation56,58,61 and immunostimulation.58,61,62 Herein, nitrate and nitrite (NO3/NO2), a marker of NO production, was determined to evaluate the perturbation in NO level of the stomach. When an excessive amount of absolute ethanol was given, an acute rise of NO generation was observed in the gastric tissue (P < 0.05, Model versus Control, shown in Table 6), accompanied by the inflammatory cell infiltration (Figure 5B). Sucralfate and BCM were unable to control the increase of NO concentration, but SeNPs-CM, as presented in Table 6, might retard the generation of NO (P < 0.05, versus Model). It suggested the reduction of NO is probably involved in the gastroprotective action of SeNPs-CM.
Systematic Antioxidant Activity of SeNPs-CM
Excessive consumption of high concentration of ethanol might lead to oxidative damage in other tissues63 when inducing gastric mucosal lesions. Ethanol triggered marked oxidative stress in the serum and liver of rats, as characterized by the increase of TBARS and the decrease of GSH (Tables 7 and 8), resulting in the release of GPT (Table 3). It meant the ethanol-caused oxidative stress extend quickly throughout the body of rats. However, the oxidative effects were significantly attenuated by SeNPs-CM, which might be attributed to the capacity of SeNPs-CM in up-regulating the levels of SOD, GSH-Px and CAT (Tables 7 and 8). More precisely, SeNPs was the main reason for the improvement of these antioxidant enzymatic activities, which might be deduced from the significant difference between M-Se and BCM (Tables 7 and 8). Apparently, SeNPs-pretreatment established a defense system against ethanol in other tissues in addition to the stomach.
Table 7.
The Levels of TBARS, GSH and GSH-Px in the Serum of Wistar Rats (n=10)
| Group | TBARS (μmol L−1) |
GSH (μmol L−1) |
GSH-Px (U mL−1) |
|---|---|---|---|
| Control | 3.95 ± 0.53a | 31.6 ± 18.1a,b | 39.2 ± 4.4a |
| Model | 5.06 ± 0.71b | 11.2 ± 9.4a | 38.4 ± 6.0a |
| Sucralfate | 3.80 ± 0.44a | 25.6 ± 12.4a,b | 41.8 ± 5.3a |
| BCM | 4.44 ± 0.74a,b | 18.5 ± 10.7a,b | 36.3 ± 5.0a |
| L-Se | 3.92 ± 0.54a | 20.8 ± 9.7a,b | - |
| M-Se | 4.32 ± 0.39a,b | 26.7 ± 15.1a,b | 81.1 ± 8.4b |
| H-Se | 4.23 ± 0.56a | 34.3 ± 17.4b | 76.4 ± 6.9b |
Notes: a–bMeans within a column with different letters differ significantly (P < 0.05).
Table 8.
The Levels of TBARS, SOD, CAT and GSH-Px in the Liver of Wistar Rats (n=10)
| Group | TBARS (μmol g−1 Prot) |
SOD (U mg−1 Prot) |
CAT (U mg−1 Prot) |
GSH-Px (U mg−1 Prot) |
|---|---|---|---|---|
| Control | 1.28 ± 0.31a | 141 ± 51a | 74 ± 39a | 180 ± 42a,b |
| Model | 1.78 ± 0.46a | 154 ± 31a | 79 ± 29a | 150 ± 29a |
| Sucralfate | 1.18 ± 0.40a | 202 ± 30a | 63 ± 25a | 232 ± 60a,b,c |
| BCM | 1.72 ± 0.46a | 211 ± 21a | 78 ± 37a | 150 ± 42a |
| L-Se | 1.77 ± 0.91a | 414 ± 138b | 76 ± 45a | 340 ± 145c,d |
| M-Se | 1.58 ± 0.84a | 410 ± 75b | 123 ± 64a | 289 ± 38b,c,d |
| H-Se | 1.79 ± 0.48a | 370 ± 70b | 88 ± 27a | 361 ± 60d |
Notes: a–dMeans within a column with different letters differ significantly (P < 0.05).
Abbreviation: prot, protein.
The antioxidant ability of SeNPs, highlighted in the improvement of both Se retention and antioxidant enzymatic activities, had been found in many organs of animals.16,19,20,33,34,44 The results shown in Tables 6–8 implied the antioxidant activity of SeNPs is systematic but not local, superior to that of Sucralfate. The antioxidant effect in the stomach, contributing to gastric protection, was merely a part of the total antioxidant activity of SeNPs. The gastroprotective function of SeNPs-CM might be supported by its systematic antioxidant activities. Besides, oxidative stress is one of the major contributors to the development of stomach diseases,52 and it is very common in acute gastric mucosal injury caused by ethanol,28,59 emotional stress,13,28 bacteria infection64 or NSAIDs.14,28 Possibly, the antioxidant activities of SeNPs might be used to treat or prevent other gastric diseases or damages. It deserved more investigation.
Conclusion
In this study, a simple preparation process composed of synthesis of CS-SeNPs, ultra-filtration of CS-SeNPs and spray-drying of purified CS-SeNPs was introduced to prepare SeNPs-CM. The SeNPs of around 60 nm were embedded in SeNPs-CM, allowing their stabilization in storage and their release in the gastric environment. SeNPs-CM at their recommended daily intake doses (0.6–2.4 mg kg−1 bw) were safe to Wistar rats, and they might benefit the Se retention in rats. SeNPs-CM were able to protect rats from the ethanol-induced gastric mucosal injury, due to their potent capacities of improving antioxidant enzymatic activity, inhibiting lipid peroxidation and attenuating inflammatory NO generation. SeNPs made the main contribution to the gastric protection of SeNPs-CM. SeNPs-CM possessing the potential in nutrient supplement and gastroprotection, deserved further development.
Acknowledgments
We thank Yanhua Mu from Shenyang Pharmaceutical University offered great help in the animal experiments. We also gratefully acknowledge assistance by Li Gu (Third Institute of Oceanography, MNR), Binbin Xu and Yuanfei Wu (both Xiamen University) when measuring TEM, SEM and EDS.
Funding Statement
The project was sponsored by the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources, People’s Republic of China (No. 2019014), the Scientific and Technological Projects of Fujian Province, People’s Republic of China (No. 2016N0018), and the Science &Technology Major Projects of Fujian Province, People’s Republic of China (No. 2014NZ0001).
Abbreviations
BCM, blank chitosan microspheres; Blank-C/C, blank chitosan/citrate complex; BSA, bovine serum albumin; bw, body weight; CAT, catalase; CS, chitosan; EDS, energy-dispersive X-ray spectroscopy; FTIR, Fourier transform infrared spectroscopy; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase; GSH, glutathione; GSH-Px, glutathione peroxidase; GUI, gross ulcer index; HE, hematoxylin and eosin; ICR, Institute of Cancer Research; HUI, histological ulcer index; ICP-MS, inductively coupled plasma mass spectrometry; KM, Kunming; KMnO4, potassium permanganate; LD50, median lethal dose; LPO, lipid peroxide; MWCO, molecular weight cut-off; NO, nitric oxides; PGE2, prostaglandin E2; RH, relative humidity; ROS, radical oxygen species; Se, selenium; SEM, scanning electron microscopy; SeNPs, selenium nanoparticles; SeNPs-CM, selenium nanoparticles-embedded chitosan microspheres; SOD, superoxide dismutase; SPF, specific-pathogen-free; TBARS, thiobarbituric acid-reactive substances; TEM, transmission electron microscopy; UF, ultra-filtration; Vc, ascorbic acid; XPS, X-ray photoelectron spectroscopy.
Ethics Approval and Consent to Participate
The procedures used in animal experiments were approved by the Animal Ethics Committee of Shenyang Pharmaceutical University, and they were also compliant with the Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article and its supplementary files.
Consent for Publication
All authors agreed to submit this manuscript.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Zhao X, Sun P, Li G, Yi R, Qian Y, Park K. Polyphenols in Kuding tea help prevent HCl/ethanol-induced gastric injury in mice. Food Funct. 2018;9:1713–1725. doi: 10.1039/C7FO01754E [DOI] [PubMed] [Google Scholar]
- 2.Li W, Wang X, Zhang H, et al. Anti-ulcerogenic effect of cavidine against ethanol-induced acute gastric ulcer in mice and possible underlying mechanism. Int Immunopharmacol. 2016;38:450–459. doi: 10.1016/j.intimp.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Mendoza ME, López-Lorenzo Y, Cruz-Antonio L, Matus-Meza A, Sánchez-Mendoza Y, Arrieta J. Gastroprotection of Calein D against ethanol-induced gastric lesions in mice: role of prostaglandins. Nitric oxide and sulfhydryls. Molecules. 2019;24:622–629. doi: 10.3390/molecules24030622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Liao H, Liu Y, et al. Protective effects of pogostone from Pogostemonis Herba against ethanol-induced gastric ulcer in rats. Fitoterapia. 2015;100:110–117. doi: 10.1016/j.fitote.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Sabatino A, Lenti MV, Giuffrida P, Vanoli A, Corazza GR. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun Rev. 2015;14(12):1161–1169. doi: 10.1016/j.autrev.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Kan J, Hood M, Burns C, et al. A novel combination of wheat peptides and fucoidan attenuates ethanol-induced gastric mucosal damage through anti-oxidant, anti-inflammatory, and pro-survival mechanisms. Nutrients. 2017;9:978–989. doi: 10.3390/9090978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Park SH, Nam SW, Choi YH. Gastroprotective Effect of Selenium on Ethanol-Induced Gastric Damage in Rats. Int J Mol Sci. 2012;13:5740–5750. doi: 10.3390/ijms13055740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loguercio C, Tuccillo C, Federico A, Fogliano V, Del Vecchio Blanco C, Romano M. Alcoholic beverages and gastric epithelial cell viability: effect on oxidative stress-induced damage. J Physiol Pharmacol. 2009;60(Suppl 7):87–92. doi: 10.1007/BF03185938 [DOI] [PubMed] [Google Scholar]
- 9.Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. 2008;400:115–141. doi: 10.1016/j.scitotenv.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Yao H, Gao X, Zhang Z, Wang J-F, Xu S-W. The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res. 2015;163:144–153. doi: 10.1007/s12011-014-0164-8 [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Xu Z, Huan K. Effects of dietary selenium on inflammation and hydrogen sulfide in the gastrointestinal tract in chickens. Biol Trace Elem Res. 2016;174:428–435. doi: 10.1007/s12011-016-0735-y [DOI] [PubMed] [Google Scholar]
- 12.Ozkol H, Bulut G, Balahoroglu R, Tuluce Y, Ozkol HU. Protective effects of selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175:177–185. doi: 10.1007/s12011-016-0762-8 [DOI] [PubMed] [Google Scholar]
- 13.Sadau Y, Adelaiye AB, Magaji RA, Ayo JO, Mabrouk MA, Isa AI. Role of selenium and vitamin E on gastric mucosal damage induced by water-immersion restraint stress in wistar rats. IOSR J Pharm Biol Sci. 2015;10(1):34–39. doi: 10.9790/3008-10133439 [DOI] [Google Scholar]
- 14.Kim JH, Kim BW, Kwon HJ, Nam SW. Curative effect of selenium against indomethacin-induced gastric ulcers in rats. J Microbiol Biotechnol. 2011;21(4):400–404. doi: 10.4014/jmb.1012.12019 [DOI] [PubMed] [Google Scholar]
- 15.Stephen AO, James O, Ikoojo ER, Sunday AO. Effects of selenium treatment on healing of acetic acid induced gastric ulcer in albino wistar rats. Am J Biomed Res. 2016;4(1):18–22. doi: 10.12691/ajbr-4-1-4 [DOI] [Google Scholar]
- 16.Hosnedlova B, Kepinska M, Skalickova S, et al. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed. 2018;13:2107–2128. doi: 10.2147/IJN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi: 10.1016/j.nut.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zhang Y, Yuan Y, Yue T. Immunomodulatory of selenium nano-particles decorated by sulfated Ganoderma lucidum polysaccharides. Food Chem Toxicol. 2014;68:183–189. doi: 10.1016/j.fct.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Bai K, Hong B, Hong Z, Sun J, Wang C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in d-galactose-induced aging mice. J Nanobiotechnol. 2017;15:92–105. doi: 10.1186/s12951-017-0324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai K, Hong B, He J, Hong Z, Tan R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int J Nanomed. 2017;12:4527–4539. doi: 10.2147/IJN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhai X, Zhang C, Zhao G, Stoll S, Ren F, Leng X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J Nanobiotechnol. 2017;15:4–15. doi: 10.1186/s12951-016-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Zhai X, Zhao G, Ren F, Leng X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr Polym. 2015;134:158–166. doi: 10.1016/j.carbpol.2015.07.065 [DOI] [PubMed] [Google Scholar]
- 23.Kong H, Yang J, Zhang Y, Fang Y, Nishinari K, Phillips GO. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int J Biol Macromol. 2014;65:155–162. doi: 10.1016/j.ijbiomac.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 24.Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. Biofactors. 2001;15:27–38. doi: 10.1002/biof.5520150103 [DOI] [PubMed] [Google Scholar]
- 25.Dufailly V, Noël L, Guérin T. Determination of chromium, iron and selenium in foodstuffs of animal origin by collision cell technology, inductively coupled plasma mass spectrometry (ICP-MS), after closed vessel microwave digestion. Anal Chim Acta. 2006;565(2):214–221. doi: 10.1016/j.aca.2006.02.046 [DOI] [Google Scholar]
- 26.Bliss CI. The calculation of the dosage-mortality curve. Ann Appl Biol. 1935;22(1):134–167. doi: 10.1111/aab.1935.22.issue-1 [DOI] [Google Scholar]
- 27.Zheng G, Xu X, Zheng J, Liu A. Protective effect of seleno-β-lactoglobulin (Se-β-lg) against oxidative stress in d-galactose-induced aging mice. J Funct Foods. 2016;27:310–318. doi: 10.1016/j.jff.2016.09.015 [DOI] [Google Scholar]
- 28.Zheng Y-F, Xie J-H, Xu Y-F, et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem-Biol Interact. 2014;222:27–36. doi: 10.1016/j.cbi.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Dokmeci D, Akpolat M, Aydogdu N, Doganay L, Turan FN. L-carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacol Rep. 2005;57:481–488. doi: 10.1016/j.ejca.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 30.Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–632. doi: 10.1016/j.progpolymsci.2006.06.001 [DOI] [Google Scholar]
- 31.Wang L, Jiang J, Pang SY, et al. Further insights into the combination of permanganate and peroxymonosulfate as an advanced oxidation process for destruction of aqueous organic contaminants. Chemosphere. 2019;228(33):602–610. doi: 10.1016/j.chemosphere.2019.04.149 [DOI] [PubMed] [Google Scholar]
- 32.Huang B, Zhang J, Hou J, Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic Biol Med. 2003;35(7):805–813. doi: 10.1016/S0891-5849(03)00428-3 [DOI] [PubMed] [Google Scholar]
- 33.Peng D, Zhang J, Liu Q, Taylor EW. Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem. 2007;101(10):1457–1463. doi: 10.1016/j.jinorgbio.2007.06.021 [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radical Bio Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Gil P, Jimenez de Aberasturi D, Wulf V, et al. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc Chem Res. 2013;46(3):743–749. doi: 10.1021/ar300039j [DOI] [PubMed] [Google Scholar]
- 36.Gil PR, Oberdörster G, Elder A, Puntes V, Parak WJ. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano. 2010;4(10):5527–5531. doi: 10.1021/nn1025687 [DOI] [PubMed] [Google Scholar]
- 37.Xia Y, Hill HE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81(4):829–834. doi: 10.1093/ajcn/81.4.829 [DOI] [PubMed] [Google Scholar]
- 38.Levander OA, Burk RF. Update of human dietary standards for selenium In: Hatfield DL, Berry MJ, Gladyshev VN, editors. Selenium. Boston: Springer; 2006:399–400. [Google Scholar]
- 39.Huang XJ, Choi YK, Im HS, Yarimaga O, Yoon E, Kim HS. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) detection techniques. Sensors. 2006;6:756–782. doi: 10.3390/s6070756 [DOI] [Google Scholar]
- 40.Vroon DH, Israili Z. Aminotransferases In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990:492–493. [PubMed] [Google Scholar]
- 41.Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obesity. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079 [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/S0006-8993(99)01905-8 [DOI] [PubMed] [Google Scholar]
- 43.Owu DU, Osim EE, Ebong PE. Serum liver enzymes profile of Wistar rats following chronic consumption of fresh or oxidized palm oil diets. Acta Trop. 1998;69:65–73. doi: 10.1016/S0001-706X(97)00115-0 [DOI] [PubMed] [Google Scholar]
- 44.Li H, Zhang J, Wang T, Luo W, Zhou Q, Jiang G. Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquatic Toxicol. 2008;89:251–256. doi: 10.1016/j.aquatox.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Wang H, Bao Y, Zhang L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004;75:237–244. doi: 10.1016/j.lfs.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 46.Lennicke C, Rahn J, Kipp AP, et al. Individual effects of different selenocompounds on the hepatic proteome and energy metabolism of mice. BBA-Gen Subjects. 2017;861:3323–3334. doi: 10.1016/j.bbagen.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 47.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139:199–206. doi: 10.3945/jn.108.098624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raines AM, Sunde RA. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics. 2011;12:26–40. doi: 10.1186/1471-2164-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollander D, Tarnawski A, Krause WJ, Gergely H. Protective effect of sucralfate against alcohol-induced gastric-mucosal injury in the rat - macroscopic, histologic, ultrastructural, and functional time sequence-analysis. Gastroenterology. 1985;88(1):366–374. doi: 10.1016/S0016-5085(85)80191-8 [DOI] [PubMed] [Google Scholar]
- 50.Carvalho JE, Pintado ME, Ana Lúcia TG, Ruiz JMP, Tavaria FK. Anti-proliferative, anti-inflammatory, anti-ulcerogenic and wound healing properties of chitosan. Curr Bioact Comp. 2016;12(2):114–122. doi: 10.2174/1573407212666160330204522 [DOI] [Google Scholar]
- 51.Jafri MA, Farah JK, Singh S. Evaluation of the gastric antiulcerogenic effect of large cardamom (fruits of Amomum subulatum Roxb). J Ethnopharmacol. 2001;75(2–3):89–94. doi: 10.1016/S0378-8741(00)00398-6 [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2011;50(1):35–39. doi: 10.3164/jcbn.11-115SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolesnikova LI, Darenskaya MA, Grebenkina LA, et al. Activity of lipid peroxidation in infertile women from different populations. B Exp Biol Med. 2012;154(2):203–205. doi: 10.1007/s10517-012-1912-4 [DOI] [PubMed] [Google Scholar]
- 54.Baltacıoğlu E, Yuva P, Aydın G, et al. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: a new biomarker for periodontal disease? J Periodontol. 2014;85(10):1432–1441. doi: 10.1902/jop.2014.130654 [DOI] [PubMed] [Google Scholar]
- 55.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch Eur J Phy. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2 [DOI] [PubMed] [Google Scholar]
- 56.Wang QS, Zhu XN, Jiang HL, Wang GF, Cui YL. Protective effects of alginate-chitosan microspheres loaded with alkaloids from Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth. (Zuojin Pill) against ethanol-induced acute gastric mucosal injury in rats. Drug Des Dev Ther. 2015;9:6151–6165. doi: 10.2147/DDDT.S96056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konturek SJ, Konturek PC, Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol. 2005;56(Suppl 5):5–31. [PubMed] [Google Scholar]
- 58.Kumar S, Singhc RK, Bhardwaj TR. Therapeutic role of nitric oxide as emerging molecule. Biomed Pharmacother. 2017;85:182–201. doi: 10.1016/j.biopha.2016.11.125 [DOI] [PubMed] [Google Scholar]
- 59.Long X, Zhao X, Wang W, et al. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J Sci Food Agr. 2019;99:2974–2986. doi: 10.1002/jsfa.2019.99.issue-6 [DOI] [PubMed] [Google Scholar]
- 60.El-Naga RN. Apocynin protects against ethanol-induced gastric ulcer in rats by attenuating the upregulation of NADPH oxidases 1 and 4. Chem Biol Interact. 2015;242:317–326. doi: 10.1016/j.cbi.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 61.Zheng H, Chen Y, Zhang J, et al. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem Biol Interact. 2016;250:68–77. doi: 10.1016/j.cbi.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 62.Li W, Huang H, Niu X, Fan T, Mu Q, Li H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol App Pharm. 2013;272:21–29. doi: 10.1016/j.taap.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 63.Molina PE, Hoek JB, Nelson S, et al. Mechanisms of alcohol-induced tissue injury. Alcohol Clin Exp Res. 2003;27(3):563–575. doi: 10.1097/01.ALC.0000057946.57330.F7 [DOI] [PubMed] [Google Scholar]
- 64.Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997–1003. doi: 10.1007/s00011-010-0245-x [DOI] [PubMed] [Google Scholar]