A 73-year-old woman presented with generalized fatigue, back pain and gait disturbance 4 weeks after pembrolizumab (200 mg) administration for lung adenocarcinoma. A neurological examination revealed right ptosis, proximal dominant limb and truncal weakness and diplopia that worsened with rightward gaze. Spinal magnetic resonance images (MRIs) showed neither metastases nor spinal canal stenosis. However, paraspinal muscle hyperintensities appeared on short-tau inversion recovery (Fig. 1a) and gadolinium-enhanced (Fig. 1b) images. These findings suggested myositis and we performed a detailed examination.
Figure 1.
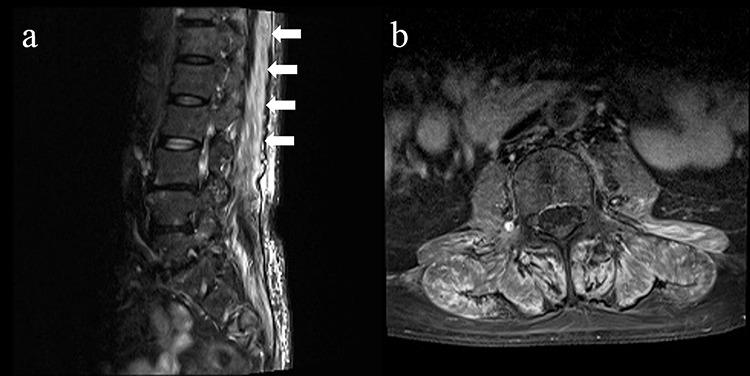
Spinal MRIs show erector spinae muscle, multifidus muscle and quadratus lumborum muscle hyperintensities on short-tau inversion recovery (a) and gadolinium-enhanced (b) images.
The patient was negative for myositis-specific autoantibodies, but her blood tests showed an elevated creatine kinase level (2427 U/L; reference, 41–153 U/L); her upper and lower limbs showed hyperintense muscle signals in fat-suppressed T2-weighted MRIs. Diplopia and right ptosis are associated with myasthenia gravis (MG). However, she tested negative for anti-acetylcholine receptor antibodies and muscle specific kinase, and a repeated nerve stimulation test detected no muscle abnormalities. Further, normal electrocardiogram and echocardiography excluded myocarditis.
The patient was diagnosed with pembrolizumab-induced myositis with myasthenic symptoms. Based on our findings, we determined that it was an immune-related adverse event. Her symptoms gradually improved after oral prednisolone therapy (15 mg/day), although lower than the recommended dose [1].
Pembrolizumab, an immune checkpoint inhibitor, has been increasingly reported to cause myositis and/or MG for which immunosuppressive therapy is effective [1–3]. Involvement of the paraspinal muscles is occasionally observed with all forms of systemic myositis, so our findings were not specific to pembrolizumab-related myositis. However, spinal MRIs are usually performed on cancer patients with back pain or gait disturbances to rule out bone metastases or narrow canals, so the paraspinal areas are easily overlooked. Awareness of muscle signal changes is key to correct diagnosis. Thus, evaluation of spinal MRI should include paraspinal areas. Myositis should be considered if abnormal findings similar to this case are found.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ETHICAL APPROVAL
No approval was required.
CONSENT
The patient provided written informed consent.
GUARANTOR
Yasunobu Nosaki.
REFERENCES
- 1. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, et al. . Neurological complications associated with anti-programmed death 1 (Pd-1) antibodies. JAMA Neurol 2017;74:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takamatsu K, Nakane S, Suzuki S, Kosaka T, Fukushima S, Kimura T, et al. . Immune checkpoint inhibitors in the onset of myasthenia gravis with hyperCKemia. Ann Clin Transl Neurol 2018;5:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


