Abstract
Introduction
Studies have been reported that frequent use of methamphetamine (MA) is associated with brain function impairment, mood disorders and excessive free radical production accompanied by the decreased level of the antioxidant response elements, but no study investigated their correlations simultaneously. In the current study, the correlation of brain function, depression and anxiety levels, and the serum levels of PON1 (an antioxidant) in MA-dependent patients were investigated.
Methods
Nineteen active MA abusers and 18 control subjects performed color-word Stroop task during fMRI and the state of their depression, anxiety, and stress were measured by the Depression, Anxiety and Stress Scale-21 Items (DASS-21) questionnaire. Their blood samples were collected to measure the level of PON1 by the human enzyme-linked immunosorbent assay (ELISA) kit and its correlation with the measured variables was studied.
Results
Analysis of fMRI findings showed frontocingulate dysfunction in Stroop effect condition, including left anterior cingulate cortex, paracingulate gyrus, superior frontal gyrus, and frontal pole in MA-dependent patients, which was associated with a higher level of depression and decreased level of serum PON1 in these patients.
Discussion
The results of the current study showed that MA-dependency is associated with frontocingulate dysfunction, decreased serum PON1 concentration, and increased depression/anxiety, which is worth to be more studied to elucidate their roles in the pathophysiology of MA addiction.
Keywords: anxiety, depression, fMRI, PON1, Stroop task
Introduction
Methamphetamine (MA) releases dopamine (DA), serotonin (5HT) and norepinephrine (NE) from the storage vesicles into the cytoplasm by reversing the vesicular monoamine transporter 2 (VMAT2).1 Dopamine quickly autoxidizes and produces DA quinones and several reactive oxygen species such as hydroxyl radicals (OH−), hydrogen peroxide (H2O2), and the superoxide anion (O2−) resulting in oxidative stress and dopaminergic terminals damage.2,3 Chronic exposure to MA can result in neurotoxicity and long-lasting damage to DAergic and 5HTergic terminals by a reduction in DA transporters (DAT), serotonin transporters (SERTs), VMAT2, tyrosine hydroxylase (TH), and tryptophan hydroxylase.2
Paraoxonase1 (PON1) is a Ca2+-dependent antioxidative enzyme which is a member of a family of glycoproteins capable of hydrolyzing several lactones, cyclic carbonates, thiolactones, aryl esters, and organophosphate pesticides. PON1 is synthesized in the liver and is secreted into the circulating system, where it is accompanied by high-density lipoproteins (HDLs).4–6 PON1 and PON3 are mostly located in the plasma and protect the circulating lipids from oxidation, probably due to their ability to hydrolyze oxidized lipids.7 Serum PON1 levels are primarily determined by the liver production and release. However, enzymatic turnover, protein stability, and the level of PON1 gene expression is a major determinant of PON1 status.8 A variety of genetic and nongenetic factors have been shown to influence PON1 gene expression, and its serum levels and activity.8 Moderate daily consumption of vitamin C and E,9 wine,10 or pomegranate juice,11 increase PON1 serum levels in animals and in humans but the involved molecular mechanisms regulating PON1 gene expression are less explored.
Human serum PON1 plays a role in the pathogenesis of several human diseases, including atherosclerosis, diabetes mellitus, kidney disease, inflammatory bowel disease, obesity, neurodegenerative disorders, in particular, Alzheimer’s disease (AD) and mood disorders.12–16 Decreased PON1 level in individuals with tobacco use disorder and nicotine dependence have been reported,17,18 but no study has been performed to investigate serum PON1 content in MA and other drug users.
Brain is very sensitive to oxidative stress due to its high oxygen use, lipid-rich content and poor antioxidant systems.19–21 Neuroimaging data, including positron emission tomography (PET)22,23 and single-photon emission computed tomography (SPECT)24 have reported MA neurotoxicity. Functional magnetic resonance imaging (fMRI) data have also provided support for prolonged neurotoxicity following repeated MA use. These studies have shown that MA-dependents have impairments in prefrontal and striatal activation during tests of executive function.25–28
MA use usually is associated with mental illness, particularly depression, anxiety and psychotic symptoms. Cognitive impairments, including learning, memory, and executive functioning have also been reported in chronic MA users.29–31
As it was mentioned, functional impairment in different brain areas in MA-dependents and depressed patients were reported separately in previous papers. Besides, the increased level of oxidative stress and decreased level of anti-oxidants in major depression were explained before,32,33 but no study has been conducted to study all the correlations in MA-dependent patients. The aim of this study was to investigate the relationship between brain function, depression/anxiety/stress, and serum antioxidant PON1 in MA-dependent patients.
Methods and Materials
Subjects
A total of 19 MA-dependent patients from substance abuse treatment programs and 18 control subjects were studied. There were no significant differences in the age, gender and education levels of these two groups. The MA abusers met DSM-5 criteria for MA dependency (according to DSM-5, most of them were moderate to severe users), their ages were between 20 and 50 years, and had the ability of reading and writing. Addiction Severity Index (ASI) questionnaire was applied to drug use history. Urine test was done for checking the drugs used by the subjects and if they were positive for MA and negative for other drugs, they were included in the study. All of MA subjects were active users and did not have withdrawal time. Subjects were asked about the history of psychiatric drug use. None of the subjects had a history of psychiatric illness or psychiatric drug use. Exclusion criteria were: use of addictive drugs except MA, cigarette and methadone during the past four months, contraindication for MRI, history of brain damage, brain surgery, neurological disease, serious psychiatric illness, and chronic diseases.
This research was performed in accordance with the latest version of the Declaration of Helsinki and with the approval of the research ethics committee of Neuroscience Research Center, Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.139). All subjects who were recruited for the study participated voluntarily and signed the written informed consent forms.
Clinical Assessments
Socio-demographic, Addiction Severity Index (ASI) and the Persian version of Depression, Anxiety and Stress Scale-21 Items (DASS-21)34,35 questionnaires were completed through the interview subjects. ASI was used to determine medical, drug use, family/social, employment and psychiatric status of MA-dependent subjects. These variables were asked from controls using the socio-demographic questionnaire. DASS questionnaire also measured the state of the depression, anxiety, and stress of all the subjects.
Stroop Color-Word Interference Task
Stroop task was combined with neuroimaging fMRI to study the function of brain areas. The Stroop task stimuli consisted of a random presentation of three color names (RED, BLUE and GREEN) which were presented in congruent (C) (eg, the word RED displayed in red ink) and incongruent (IC) (eg, the word “RED” displayed in green ink) conditions. Congruent means non-conflict and incongruent means conflict trails. Stimuli were presented via magnet-compatible goggles. Subjects who were checked for color-blindness should respond to the ink-color of the Stroop stimuli by pressing a button on a response device which was handled by both hands (left thumb finger for red color, left index finger for blue color and right thumb finger for green). Subjects were trained on how to work with this device before performing the task.
This study used one run which consisted of 16 task blocks (eight congruent and eight incongruent trials) and 16 rest blocks which were white circles in the center of the screen and with a duration of 15 seconds. There were 12 words per task block. Presentation and interval times of stimuli were, respectively, 2000 and 500 milliseconds. The total task time was 12 mins. Reaction time and error rates of responses were measured.
Imaging
Functional images were acquired on a 3 T Siemens (Prisma, Germany) head-only MRI scanner. The first, for anatomical localization T1 MPRAGE acquisition, was collected. Then, T2* weighted, gradient-recalled echo-echo planar imaging sequence was used for functional images with 3000 ms repetition time, 30 ms echo time, 46 slices, 3 mm slice thickness with a 3.0-mm slice spacing, 90-degree flip angle, 658×658 image columns, and rows, and 2.5×2.5×2.5 mm voxel size. During each run of the task, 240 brain volumes were collected.
For acquiring the magnetic stability in the preprocessing fMRI analysis, we removed the 4 initial volumes (12 seconds), afterward, data were preprocessed and analyzed using FSL toolbox. Image pre-processing steps were: high pass filtering slice timing correction, motion correction, normalization to MNI space and spatial smoothing.
Measurement of Circulating PON1
To measure the PON1 level, three mL of blood samples was collected from each subject; samples were allowed to clot for two hours at room temperature then they were centrifuged for 15 min at 1500×g at 4°C. The supernatant of each tube was collected, and was frozen at −80°C until analysis.
Human enzyme-linked immunosorbent assay (ELISA) kit was applied for the measurement of serum PON1and was done duplicate (Catalog No: MBS177320).
Statistical Analysis
Statistical Analysis of fMRI imaging data: The analyses were performed using a General Linear Model (GLM). First of all, individual subjects’ GLMs were built and fitted to each subject's functional data based on congruent (C) and incongruent trials (IC) as well as the Stroop effect (IC-C). Then, two samples t-test using a mixed effect was conducted for group analysis. Age and the right/left-handedness were eliminated as a covariate; the contrast images were thresholded. A mask was built using the activated cluster in the anterior cingulate cortex (ACC) and prefrontal cortex. Finally, the average activation level of each subject was calculated in the defined mask.
Comparison of socio-demographic data, depression and anxiety scores, fMRI/Stroop task factors and serum PON1 levels between MA-dependent patients and controls were conducted by t-test or Mann Whitney test. To investigate data correlation, Pearson and Spearman’s tests were performed. Multiple linear regression analysis was also used to evaluate the association of depression, anxiety, PON1 with fMRI. A value of less than 0.05 was considered statistically significant.
Results
Socio-Demographic Information of Included Subjects
After removing two MA abusers and one control subject due to the head motion/rotation and inappropriate MRI signals, data of 19 MA-dependents and 18 controls were finally used for the functional MRI and serum PON1 evaluations. Socio-demographic data and drug use information have been shown in Table 1. Both groups were smokers, but the amount of cigarette use differed significantly between the two groups (p-value=0.01).
Table 1.
Socio-Demographic Data and Drug Use Measures of 19 MA Abusers and 18 Control Subjects
| Variables | Control Subjects Mean (±SEM) |
MA Abusers Mean (±SEM) |
P-value |
|---|---|---|---|
| Age, Year | 36.9 (±1.9) | 35.5 (±1.8) | 0.58 |
| Educational level, Year | 8.5 (±1.1) | 7.0 (±0.7) | 0.24 |
| Cigarette use, PackYear | 7.1 (±2.4) | 13.1 (±1.9) | 0.01* |
| Mean daily MA use, Grams | – | 0.8 (±0.14) | |
| MA use Duration, Year | – | 7.8 (±0.9) | |
| Age of first MA use, Year | – | 27.5 (±1.9) |
Note: *Significantly different from the control group. P<0.05.
DASS Questionnaire Data of Included Subjects
Results of DASS questionnaire data analysis are presented in Table 2. As it has been shown depression and anxiety levels were significantly higher in MA-dependents (p-value=0.002 and p-value=0.0003, respectively) but the mean of stress level in MA abusers was not significantly different compared to the control subjects (Table 2).
Table 2.
DASS Questionnaire Results from MA-Dependents and Control Subjects
| DASS Questionnaire Mean (±SEM) |
Control Subjects (n=18) | MA Abusers (n=19) | P-value |
|---|---|---|---|
| Depression | 8.5 (±0.6) | 11.7 (±0.8) | 0.005* |
| Anxiety | 7.7 (±0.2) | 9.7 (±0.5) | 0.0003* |
| Stress | 10.5 (±0.7) | 12.3 (±1.0) | 0.15 |
Note: *Significantly different from the control group, P <0.01.
Abbreviation: DASS, depression anxiety stress scales.
Functional MRI Results
In the Stroop task, mean (±SEM) of incongruent errors in MA-dependents and controls were 21.7 (±5.9) and 5.1 (±1.1), respectively (p-value=0.03). Stroop effects in MA-dependents and controls were 9.8 (±2.7) and 3.2 (±1.0), respectively (p-value=0.04). Congruent errors were not significantly different between two groups (p-value=0.14).
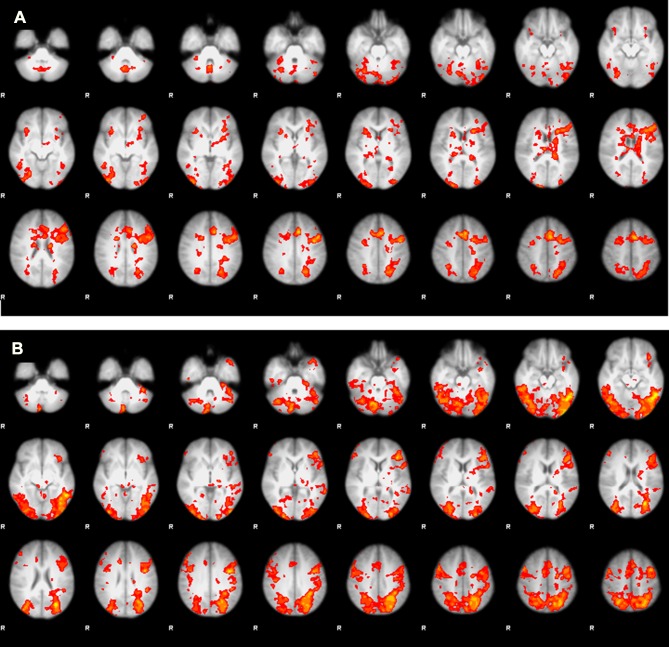
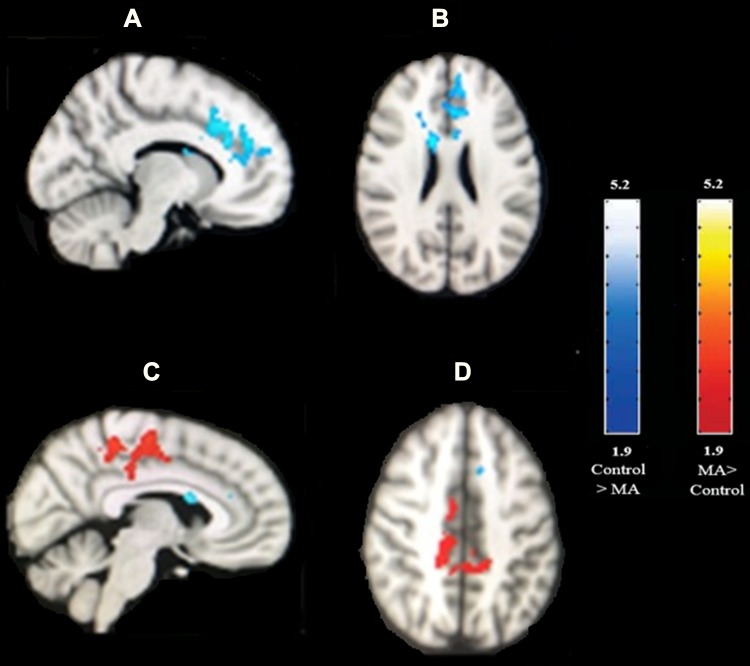
We performed t-test analyses between two groups to investigate the group differences in the blood-oxygen-level-dependent (BOLD) activation for three conditions of interest (congruent, incongruent, and Stroop effect). There were significant differences in the Stroop effect, but not in the congruent or incongruent conditions. Average BOLD activation for Stroop effect condition (IC-C) in the control subjects and MA-dependents following the performance of Stroop color-word task has shown in Figure 1. Comparing the BOLD activation in MA-dependent patients and controls after unpaired t-test analysis revealed significant differences in the Stroop effect condition of fMRI so that control subjects significantly showed higher activation in the left anterior cingulate cortex, paracingulate gyrus, superior frontal gyrus and frontal pole (p-value<0.05) compared to the MA-dependent group. In addition, MA abusers had significantly higher activation in the right posterior cingulate cortex, inferior and middle temporal gyrus, right precentral gyrus, supplementary motor cortex, cuneal cortex, precuneus cortex, and superior parietal lobule (p value<0.05) (threshold = 1.9) (Figure 2).
Figure 1.
Average BOLD activation for Stroop effect condition (IC-C) following the performance of Stroop color-word task. (A) In the control subjects and (B) in the MA-dependents.
Figure 2.
Higher BOLD activation for Stroop effect condition (IC-C) after unpaired t-test analysis in MA abusers (red color) and control subjects (blue color). (A) Sagittal and (B) axial view of the brain: regions (blue color) show increased BOLD activity in controls relative to the MA abusers including the left anterior cingulate cortex, paracingulate gyrus, superior frontal gyrus, and frontal pole. (C) Sagittal and (D) axial view of the brain: regions (red color) show increased BOLD activity in MA abusers relative to the controls by using unpaired t-test analysis for the Stroop effect, including right posterior cingulate cortex, right precentral gyrus, supplementary motor cortex, precuneus cortex, and superior parietal lobule. Image threshold using clusters determined by Z>1.9 and a corrected cluster significance level of p<0.05. The scale represents the color (from dark to light) of the cluster corresponding to the increasing Z-statistic.
Serum PON1 Levels in the Studied Subjects
Comparing the level of serum PON1in MA-dependent patients and controls showed significantly lower levels of serum PON1 in MA abusers compared to the controls. The mean (±SEM) of serum PON1 in MA-dependents and controls were 50.64 (±4.5) and 122.83 (±14.3) pg/mL, respectively (p-value<0.0001, df=34).
Correlation of Imaging Data, Stroop Task Results, DASS Data, and PON1 Level
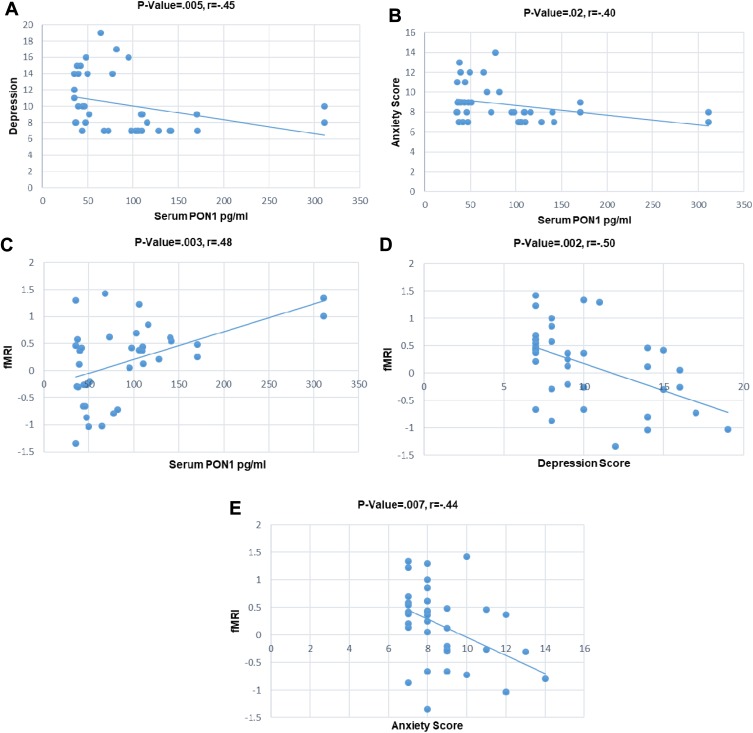
A mask was built using the activated fMRI cluster in the control group, including the anterior cingulate cortex, paracingulate gyrus, superior frontal gyrus, and frontal pole. Then, the average level of activation of each subject was calculated in the defined mask and was considered as fMRI result. Correlations of fMRI results, depression, anxiety, and serum PON1 concentration were studied using Pearson and Spearman correlation analyses for all the studied subjects (both MA abusers and controls). Results showed a negative correlation between PON1 level and depression (Figure 3A), as well as PON1 level and anxiety (Figure 3B). A positive correlation between fMRI results and PON1 (Figure 3C) has been revealed after data analysis. Results of fMRI and depression (Figure 3D) as well as fMRI results and anxiety (Figure 3E) showed negative correlation. There was no significant correlation between the pattern of MA use parameters (years and amount of MA use) and depression and anxiety levels as well as serum PON1concentration and fMRI activation.
Figure 3.
Correlation scatter plots of some covariates in the all subjects (MA abusers and control subjects); (A) Inverse relation between serum PON1 concentration and depression, p-value= 0.005, r = –0.45; (B) inverse relation between serum PON1 concentration and anxiety, p-value=0.02, r= –0.40; (C) positive relation between fMRI and serum PON1 concentration, p-value=0.003, r=0.48; (D) inverse relation between fMRI and depression, p-value=0.002, r= –0.50; (E) inverse relation between fMRI and anxiety, p-value=0.007, r= –0.44.
The association between depression, PON1 and anxiety with fMRI was also investigated by linear regression analysis (Table 3). Variables were fMRI as the dependent variable; depression, PON1 and anxiety as the predictor variables; age and smoking as covariates. After multiple regression analysis (adjusted for age and smoking), significant correlations between depression and PON1 concentration with fMRI were observed, while no significant correlations were found between fMRI and anxiety.
Table 3.
Linear Regression Analysis Results Showing the Association Between Depression, PON1 and Anxiety with fMRI
| Univariable Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| B (SE) | β | P | B (SE) | β | P | |
| Depression | −0.099 (0.030) | −0.49 | 0.002* | −0.075 (0.033) | −0.373 | 0.030* |
| PON1 | 0.005 (0.002) | 0.48 | 0.003* | 0.004 (0.002) | 0.329 | 0.030* |
| Anxiety | −0.165 (0.057) | −0.44 | 0.007* | −0.066 (0.064) | −0.176 | 0.305 |
| Smoking | −0.015 (0.012) | −0.20 | 0.234 | −0.009 (0.012) | −0.13 | 0.45 |
| Age | −0.005 (0.015) | −0.05 | 0.75 | −0.019 (0.016) | −0.20 | 0.25 |
Notes: Dependent Variable: fMRI; B: unstandardized coefficients; β: standardized coefficients; *p < 0.05 indicates statistically significant difference.
Discussion
The results of the current study indicated the higher levels of depression and anxiety and lower level of serum PON1 in MA abusers compared to the control subjects. Moreover, fMRI findings showed less activation of the left anterior cingulate cortex, paracingulate gyrus, superior frontal gyrus and frontal pole in MA-dependents as well as significantly higher activation of right posterior cingulate cortex, right precentral gyrus, supplementary motor cortex, precuneus cortex, and superior parietal lobule in these subjects. Brain function (frontocingulate cluster) was inversely correlated with depression and anxiety, but showed a direct correlation with the serum PON1 concentration. There was also a significant negative correlation between depression and anxiety levels and serum PON1 concentration in all subjects. Results showed no significant correlation between the pattern of MA use parameters and depression and anxiety levels as well as serum PON1concentration. After multiple regression analysis, significant correlations between depression and PON1 concentration with fMRI were also observed, while no significant correlation was found between fMRI and anxiety.
Frontocingulate Dysfunction in MA-Dependents
Frontocingulate region is critical for controlling the impulses of drug seeking.36 ACC region is a part of the executive network,37 which has been linked to the functions such as attention,38,39 inhibition control,40 error detection, conflict resolution, and selection of an appropriate response.41–43 Imaging studies, including a positron-emission tomography (PET) study, also showed the negative correlation of the anterior cingulate cortex activation and recent MA use.44 In another study by Nordahl et al, magnetic resonance spectroscopy imaging revealed the abnormal low function of ACC in MA users.45 Cognitive control impairment accompanied by frontal and cingulate regions dysfunction during the fMRI and Stroop task performance by abstinent MA-dependent subjects have also been reported by Nestor et al.28 However, Salo et al did not find any difference between abstinent MA-dependents and controls in ACC function related to the Stroop effect.46 Consistent with the previous studies, in our study, the posterior cingulate cortex (PCC) and superior parietal lobule are areas that have higher activation in the Stroop effect condition in MA abusers compared to the controls.44,47 MA abuse seems to alter anterior callosal white matter (WM) microstructure with less evidence of change within the posterior callosal WM microstructure.48 Axons within the genu are usually thinner in diameter and less myelinated than those in the splenium and thus might be more susceptible to damage after long-term drug abuse.49,50
Higher Depression and Anxiety in MA-Dependents
Observed higher depression and anxiety in MA abusers in this study is also consistent with other studies,29,30,44,51 reflecting neurochemical abnormalities following MA use. It has been reported that MA reduces the density of dopamine and serotonin transporters in the adult brain22,52 leading to dysfunction of the serotonin and dopamine neurotransmitter systems and depression.53,54 It is reported that MA exposure increases depression-like behaviors in mice, and it may be related to MA-induced alterations in the hypothalamic-pituitary-adrenal axis.55 We have not found any association between anxiety and depression symptoms and the pattern of MA use (MA use level and duration) which is consistent with the study of Shetty et al.56 Moreover, statistical analyses detected a negative correlation of frontocingulate function in fMRI with depression, confirming the Pizzagalli hypothesis proposing dysfunction of the frontocingulate area in depressed patients during a variety of executive tasks.57 After multiple regression analysis (adjusted for age and smoking), no significant correlation was found between fMRI and anxiety. This indicates that anxiety does not affect frontocingulate function.
Lower Serum PON1 Concentration in MA-Dependents
Free oxidant radicals and antioxidant system imbalance can induce brain damage since the brain is very sensitive to oxidative stress.19 Studies have shown lower serum concentrations of PON1 protein in the coronary heart disease (CHD), mixed connective tissue disease and hemodialyzed patients,58–60 Alzheimer disease,61,62 autism,63 type II diabetes mellitus64 and adverse spectrum of disease including tumors, immune disease and obesity,65 while no study has been conducted on serum PON1 level in MA-dependents and its correlation with frontocingulate function. Our findings showed low level of serum PON1 which correlated directly to their frontocingulate function. The only related report was an animal PET study showed that pre-treatment with N-acetyl cysteine as an antioxidant in MA-dependent animals significantly which improved the density DAT in the monkey striatum.66 Besides, we showed that the depression and anxiety status of subjects have a significant negative correlation with the serum PON1 concentration. Consistent with our findings, a meta-analysis reported that in depressed patients the antioxidant and serum PON1 levels decreased while the serum-free radical levels increased.32 Other studies have shown that comorbidity of tobacco use disorder or smoking with mood disorders are associated with the lower anti-oxidants and PON1 activity as well as higher levels of oxidative stress markers such as nitric oxide and lipid hydroperoxides.16,67
This study had some limitations which the first one was the small sample size, so replication of this study with a larger sample is required. Second, MA-dependent patients usually use other drugs with MA, so we have considered methadone use as an inclusion criteria. However, only three included subjects were used methadone during the past four months. Third, we chose controls from smoker subjects to remove the effects of smoking on the results. However, comparison of cigarette use showed a significant difference between two groups which needs further Investigations to study its impact on the results.
Conclusions
Altogether, our results showed frontocingulate dysfunction, higher levels of depression/anxiety and lower levels of serum PON1 in MA-dependent patients. Frontocingulate dysfunction was associated with depression and decreased serum PON1. Further studies with larger sample size need to elucidate the causality of the studied variables and the role of serum PON1 concentration, depression and frontocingulate dysfunction in MA addiction.
Acknowledgments
This article has been extracted from the thesis written by Nooshin Ghavidel in School of Medicine, Shahid Beheshti University of Medical Sciences (Registration No: 79). This study was supported by Neuroscience Research Center, SBMU and Iran National Science Foundation (Grant no. 96015957). We greatly appreciate the collaboration of National Brain Mapping Lab, Tehran, Iran for providing data recording services.
Abbreviations
MA, methamphetamine; fMRI, functional magnetic resonance imaging; DASS-21, depression, anxiety and stress scale-21 items; ELISA, human enzyme-linked immunosorbent assay; DA, dopamine; PON1, paraoxonase1; HDLs, high-density lipoproteins; AD, Alzheimer’s disease; ASI, addiction severity index; C, congruent; IC, incongruent; GLM, general linear model; ACC, anterior cingulate cortex; PET, positron-emission tomography; PCC, posterior cingulate cortex; WM, white matter; CHD, coronary heart disease; DAT, DA transporter.
Author Contributions
NG was responsible for the acquisition of clinical data including imaging data, DASS questionnaire and serum PON1 experiment as well as statistical analysis. All authors contributed to study design, data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest in this work.
References
- 1.Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose‐binge regimen followed by single‐day multiple‐dose methamphetamine challenges. J Neurochem. 2008;105(5):1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x [DOI] [PubMed] [Google Scholar]
- 2.Moszczynska A, Callan SP. Molecular, behavioral, and physiological consequences of methamphetamine neurotoxicity: implications for treatment. J Pharmacol Exp Ther. 2017;362(3):474–488. doi: 10.1124/jpet.116.238501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Wang Y, Li Q, et al. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front Mol Neurosci. 2018;11:186. doi: 10.3389/fnmol.2018.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deakin S, Leviev I, Gomaraschi M, Calabresi L, Franceschini G, James RW. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem. 2002;277(6):4301–4308. doi: 10.1074/jbc.M107440200 [DOI] [PubMed] [Google Scholar]
- 5.Sorenson RC, Bisgaier CL, Aviram M, Hsu C, Billecke S, La Du BN. Human serum paraoxonase/arylesterase’s retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: apolipoprotein AI stabilizes activity. Arterioscler Thromb Vasc Biol. 1999;19(9):2214–2225. doi: 10.1161/01.ATV.19.9.2214 [DOI] [PubMed] [Google Scholar]
- 6.Grdic Rajkovic M, Rumora L, Barisic K. The paraoxonase 1, 2 and 3 in humans. Biochem Med. 2011;21(2):122–130. doi: 10.11613/issn.1846-7482 [DOI] [PubMed] [Google Scholar]
- 7.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37(9):1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman B. Regulation of hepatic paraoxonase-1 expression. J Lipids. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvik GP, Tsai NT, McKinstry LA, et al. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler Thromb Vasc Biol. 2002;22(8):1329–1333. doi: 10.1161/01.ATV.0000027101.40323.3A [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman B, Aviram M. Preservation of paraoxonase activity by wine flavonoids: possible role in protection of LDL from lipid peroxidation. Ann N Y Acad Sci. 2002;957(1):321–324. doi: 10.1111/nyas.2002.957.issue-1 [DOI] [PubMed] [Google Scholar]
- 11.Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am J Clin Nutr. 2000;71(5):1062–1076. doi: 10.1093/ajcn/71.5.1062 [DOI] [PubMed] [Google Scholar]
- 12.Moya C, Máñez S. Paraoxonases: metabolic role and pharmacological projection. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(4):349–359. doi: 10.1007/s00210-018-1473-9 [DOI] [PubMed] [Google Scholar]
- 13.Shunmoogam N, Naidoo P, Chilton R. Paraoxonase (PON)-1: a brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc Health Risk Manag. 2018;14:137. doi: 10.2147/VHRM.S165173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackness M, Mackness B. Current aspects of paraoxonase-1 research In: The HDL Handbook—Biological Functions and Clinical Implications, 2nd ed Academic Press: London, UK: Elsevier; 2014:273–291. [Google Scholar]
- 15.Cervellati C, Valacchi G, Tisato V, Zuliani G, Marsillach J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2018;110(3):238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunes SOV, de Castro MRP, Moreira EG, et al. Association of paraoxonase (PON) 1 activity, glutathione S-transferase GST T1/M1 and STin. 2 polymorphisms with comorbidity of tobacco use disorder and mood disorders. Neurosci Lett. 2015;585:132–137. doi: 10.1016/j.neulet.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Bortolasci CC, Vargas HO, Souza-Nogueira A, et al. Lowered plasma paraoxonase (PON) 1 activity is a trait marker of major depression and PON1 Q192R gene polymorphism – smoking interactions differentially predict the odds of major depression and bipolar disorder. J Affect Disord. 2014;159:23–30. doi: 10.1016/j.jad.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 18.Nunes SOV, Vargas HO, Prado E, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev. 2013;37(8):1336–1345. doi: 10.1016/j.neubiorev.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 19.Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butterfield DA, Lange MLB, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine Y, Minabe Y, Ouchi Y, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160(9):1699–1701. doi: 10.1176/appi.ajp.160.9.1699 [DOI] [PubMed] [Google Scholar]
- 23.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Liu XD, Han M, et al. Comparison of striatal dopamine transporter levels in chronic heroin‐dependent and methamphetamine‐dependent subjects. Addict Biol. 2017;22(1):229–234. doi: 10.1111/adb.2017.22.issue-1 [DOI] [PubMed] [Google Scholar]
- 25.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015;1628:174–185. doi: 10.1016/j.brainres.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res Neuroimaging. 2013;211(3):234–238. doi: 10.1016/j.pscychresns.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53. doi: 10.1016/S0893-133X(01)00334-7 [DOI] [PubMed] [Google Scholar]
- 28.Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res Neuroimaging. 2011;194(3):287–295. doi: 10.1016/j.pscychresns.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13(2):181–190. doi: 10.1080/10550490490436055 [DOI] [PubMed] [Google Scholar]
- 30.Hermens DF, Lubman DI, Ward PB, Naismith SL, Hickie IB. Amphetamine psychosis: a model for studying the onset and course of psychosis. Med J Aust. 2009;190(4):S22. doi: 10.5694/j.1326-5377.2009.tb02370.x [DOI] [PubMed] [Google Scholar]
- 31.Potvin S, Pelletier J, Grot S, Hebert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav. 2018;80:154–160. doi: 10.1016/j.addbeh.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10(10):e0138904. doi: 10.1371/journal.pone.0138904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreira EG, Correia DG, Bonifácio KL, et al. Lowered PON1 activities are strongly associated with depression and bipolar disorder, recurrence of (hypo) mania and depression, increased disability and lowered quality of life. World J Biol Psychiatry. 2019;20(5):368–380. doi: 10.1080/15622975.2017.1322219 [DOI] [PubMed] [Google Scholar]
- 34.Lovibond S, Lovibond P. Manual for the Depression Anxiety Stress Scale. Sydney: The Psychological Foundation of Australia. Inc; 1995. [Google Scholar]
- 35.Asghari A, Saed F, Dibajnia P. Psychometric properties of the depression anxiety stress scales-21 (DASS-21) in a non-clinical Iranian sample. Int J Psychol. 2008;2(2):82–102. [Google Scholar]
- 36.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/PL00005483 [DOI] [PubMed] [Google Scholar]
- 37.Heinonen J, Numminen J, Hlushchuk Y, Antell H, Taatila V, Suomala J. Default mode and executive networks areas: association with the serial order in divergent thinking. PLoS One. 2016;11(9):e0162234. doi: 10.1371/journal.pone.0162234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benedict RH, Shucard DW, Maria MPS, et al. Covert auditory attention generates activation in the rostral/dorsal anterior cingulate cortex. J Cogn Neurosci. 2002;14(4):637–645. doi: 10.1162/08989290260045765 [DOI] [PubMed] [Google Scholar]
- 39.London ED, Berman SM, Voytek B, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58(10):770–778. doi: 10.1016/j.biopsych.2005.04.039 [DOI] [PubMed] [Google Scholar]
- 40.Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35(2):248–275. doi: 10.1016/j.neubiorev.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 41.Dreher J-C, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13(4):329–339. doi: 10.1093/cercor/13.4.329 [DOI] [PubMed] [Google Scholar]
- 42.Carter CS, Macdonald AM, Botvinick M, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang G-J, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 2013;23(4):639–648. doi: 10.1016/j.conb.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73 [DOI] [PubMed] [Google Scholar]
- 45.Nordahl TE, Salo R, Natsuaki Y, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62(4):444–452. doi: 10.1001/archpsyc.62.4.444 [DOI] [PubMed] [Google Scholar]
- 46.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(8):706–709. doi: 10.1016/j.biopsych.2008.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jan RK, Lin JC, McLaren DG, Kirk IJ, Kydd RR, Russell BR. The effects of methylphenidate on cognitive control in active methamphetamine dependence using functional magnetic resonance imaging. Front Psychiatry. 2014;5:20. doi: 10.3389/fpsyt.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salo R, Nordahl TE, Buonocore MH, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry. 2009;65(2):122–128. doi: 10.1016/j.biopsych.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7(11):1761–1764. doi: 10.1097/00001756-199607290-00013 [DOI] [PubMed] [Google Scholar]
- 50.LaMantia A, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10(7):2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma inflammatory factors are associated with anxiety, depression, and cognitive problems in adults with and without methamphetamine dependence: an exploratory protein array study. Front Psychiatry. 2015;6:178. doi: 10.3389/fpsyt.2015.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699. doi: 10.1038/nm0696-699 [DOI] [PubMed] [Google Scholar]
- 53.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- 54.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40(2):288–295. doi: 10.1093/clinchem/40.2.288 [DOI] [PubMed] [Google Scholar]
- 55.Joca L, Zuloaga DG, Raber J, Siegel JA. Long-term effects of early adolescent methamphetamine exposure on depression-like behavior and the hypothalamic vasopressin system in mice. Dev Neurosci. 2014;36(2):108–118. doi: 10.1159/000360001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shetty V, Mooney LJ, Zigler CM, Belin TR, Murphy D, Rawson R. The relationship between methamphetamine use and increased dental disease. J Am Dent Assoc. 2010;141(3):307–318. doi: 10.14219/jada.archive.2010.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183. doi: 10.1038/npp.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domagała T, Łacinski M, Trzeciak W, Mackness B, Mackness M, Jakubowski H. The correlation of homocysteine-thiolactonase activity of the paraoxonase (PON1) protein with coronary heart disease status. Cell Mol Biol (Noisy-Le-Grand). 2006;52(5):4–10. [PubMed] [Google Scholar]
- 59.Suehiro T, Ikeda Y, Shiinoki T, et al. Serum paraoxonase (PON1) concentration in patients undergoing hemodialysis. J Atheroscler Thromb. 2002;9(3):133–138. doi: 10.5551/jat.9.133 [DOI] [PubMed] [Google Scholar]
- 60.Bodolay E, Seres I, Szodoray P, et al. Evaluation of paraoxonase activity in patients with mixed connective tissue disease. J Rheumatol. 2008;35(2):237–243. [PubMed] [Google Scholar]
- 61.Paragh G, Balla P, Katona E, Seres I, Égerházi A, Degrell I. Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2002;252(2):63–67. doi: 10.1007/s004060200013 [DOI] [PubMed] [Google Scholar]
- 62.Zengi O, Karakas A, Ergun U, Senes M, Inan L, Yucel D. Urinary 8-hydroxy-2′-deoxyguanosine level and plasma paraoxonase 1 activity with Alzheimer’s disease. Clin Chem Lab Med. 2012;50(3):529–534. doi: 10.1515/cclm.2011.792 [DOI] [PubMed] [Google Scholar]
- 63.Paşca SP, Nemeş B, Vlase L, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78(19):2244–2248. doi: 10.1016/j.lfs.2005.09.040 [DOI] [PubMed] [Google Scholar]
- 64.Juretić D, Motejlkova A, Kunovic B, et al. Paraoxonase/arylesterase in serum of patients with type II diabetes mellitus. Acta Pharm. 2006;56(1):59–68. [PubMed] [Google Scholar]
- 65.Goswami B, Tayal D, Gupta N, Mallika V. Paraoxonase: a multifaceted biomolecule. Clin Chim Acta. 2009;410(1–2):1–12. doi: 10.1016/j.cca.2009.09.025 [DOI] [PubMed] [Google Scholar]
- 66.Hashimoto K, Tsukada H, Nishiyama S, et al. Effects of N‐Acetyl‐l‐Cysteine on the reduction of brain dopamine transporters in monkey treated with methamphetamine. Ann N Y Acad Sci. 2004;1025(1):231–235. doi: 10.1196/annals.1316.028 [DOI] [PubMed] [Google Scholar]
- 67.Vargas HO, Nunes SOV, de Castro MRP, et al. Oxidative stress and inflammatory markers are associated with depression and nicotine dependence. Neurosci Lett. 2013;544:136–140. doi: 10.1016/j.neulet.2013.03.059 [DOI] [PubMed] [Google Scholar]