Although many chronic inflammatory diseases share the feature of elevated IL-17 production, therapeutic targeting of IL-17 has vastly different clinical outcomes. Here the authors summarize the recent progress in understanding the protective and pathogenic role of the IL-23/IL-17 axis in preclinical models and human inflammatory diseases.
Abstract
Chronic inflammatory diseases like psoriasis, Crohn’s disease (CD), multiple sclerosis (MS), rheumatoid arthritis (RA), and others are increasingly recognized as disease entities, where dysregulated cytokines contribute substantially to tissue-specific inflammation. A dysregulation in the IL-23/IL-17 axis can lead to inflammation of barrier tissues, whereas its role in internal organ inflammation remains less clear. Here we discuss the most recent developments in targeting IL-17 for the treatment of chronic inflammation in preclinical models and in patients afflicted with chronic inflammatory diseases.
The IL-17 family
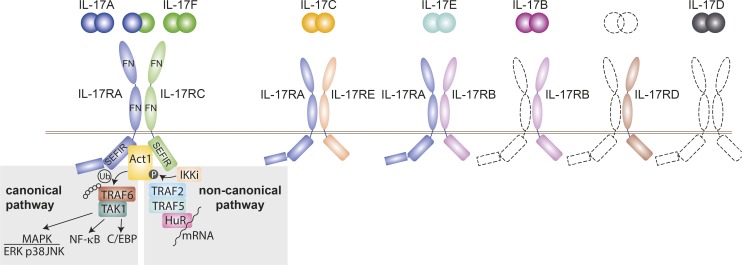
IL-17A, the founding member of the IL-17 family, was first discovered in mice in 1993 (Rouvier et al., 1993) and 2 yr later in humans (Yao et al., 1995b). The IL-17 family is evolutionary conserved within vertebrates and composes six structurally related members in mammals, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F (Malagoli, 2016). All cytokines bind to their receptors as disulfide-linked homodimers, with the exception of IL-17A and IL-17F, which can also form heterodimers (Wright et al., 2007; Fig. 1).
Figure 1.
IL-17 cytokine and receptor family. Schematic overview of the known heterodimeric IL-17 receptor complexes with their respective IL-17 cytokines. Unknown coreceptors and ligands are displayed with dashed lines. IL-17 receptors share a common structure with two extracellular fibronectin II–like (FN) domains and an intracellular SEFIR domain. Downstream signaling events depend on the adaptor Act1. In the canonical pathway Act1 ubiquitinates TRAF6, leading to recruitment of TAK1 and triggering of NF-κB, MAPK pathways, and the CCAAT-enhancer-binding proteins (C/EBP) pathway. The noncanonical pathway is initiated by phosphorylation of Act1 by inducible IκB kinase (IKKi) and the subsequent formation of a complex with TRAF2, TRAF5, and mRNA stabilizing factor human antigen R (HuR), which increases the half-life of several mRNAs. Ub, ubiquitin; P, phosphoryl group.
Five IL-17 receptor subunits have been identified (IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE). All are single-pass transmembrane receptors with two extracellular fibronectin II–like domains and a cytoplasmatic “SEFIR” motif for activation of downstream signaling pathways (McGeachy et al., 2019). The receptor for IL-17A and IL-I7F homodimers and IL-17A/F heterodimers consists of a heterodimeric receptor complex composed of IL-17RA and IL-17RC subunits. IL-17RA can also pair with IL-17RE or IL-17RB, which then bind IL-17C and IL-17E, respectively. IL-17B has been shown to engage with IL-17RB, but a coreceptor is still missing, as well as the cognate receptors for IL-17D and a cytokine that binds to IL-17RD.
IL-17R signal transduction has been best studied for IL-17RA/IL-17RC complexes after IL-17A ligation (Gu et al., 2013). Receptor engagement leads to a conformational change, which allows the adaptor protein Act1 to form a homotypic interaction between the receptor’s “SEFIR” domain and its own. The E3 ligase activity of Act1 initiates the canonical pathway via ubiquitylation of TNF receptor-associated factor 6 (TRAF6), recruitment of TAK1 and subsequent activation of the canonical NF-κB and MAPK pathways including ERK, p38, and JNK, as well as the CCAAT-enhancer-binding proteins pathway (Monin and Gaffen, 2018). An alternative pathway depends on the phosphorylation of Act1 by inducible IκB kinase, which leads to the assembly of TRAF2 and TRAF5 with mRNA stabilizing factor human antigen R, thereby increasing stability of multiple mRNAs (Amatya et al., 2017). IL-17A and F lie in close proximity in the genome on chromosome 1 in mice and chromosome 6 in humans, share the highest structural identity among all IL-17 family members, and have been shown to have largely overlapping biological activities (Akimzhanov et al., 2007; Monin and Gaffen, 2018). Hereafter, we focus on IL-17A/F and their role in chronic inflammatory disorders and use IL-17 as shorthand.
T helper (Th) 17 cell polarization and other sources of IL-17
Mosmann et al. (1986) were the first to propose a bifurcation in CD4+ Th cells. Th1 cells were defined as mediators of “cellular immunity” directed against intracellular pathogens and were characterized mainly by their production of IL-2 and IFN-γ, but also other cytokines such as GM-CSF and TNF. Th2 cells were described as IL-4, IL-5, IL-13, and IL-10–secreting cells involved in “humoral immunity” directed against extracellular pathogens (reviewed in Raphael et al., 2015). The discovery of IL-17 initially did not influence the Th1/Th2 paradigm prevailing in the field at this time even though numerous reports indicated that it could be involved in several inflammatory disorders (Kotake et al., 1999; Kostulas et al., 1999; Antonysamy et al., 1999; Chabaud et al., 1998, 1999). Langrish et al. (2005) found that among many other mediators, IL-17A is produced by Th cells upon engagement of their IL-23R. In vitro, IL-17 secretion could be elicited from naive CD4 T cells upon polarization with TGF-β and IL-6 (Veldhoen et al., 2006). In humans, IL-6 and IL-1β and IL-21 were later also identified as promoters of the IL-17 expression in Th cells (reviewed in Sallusto et al., 2012). IL-17 expression is driven by the transcription factor RORγt (via a STAT3-dependent mechanism; Ivanov et al., 2006). The term Th17 cell was coined, and for some time Th17 polarization and their role in immunity became the core interest of the immunology community. The discovery and characterization of Th17 cells are reviewed in more detail elsewhere (Korn et al., 2009; McGeachy and Cua, 2008). Over the years, it crystallized that the commonly called “IL-23/IL-17 axis” is critically involved in the regulation of tissue inflammation, in the context of both host defense and chronic inflammatory disorders.
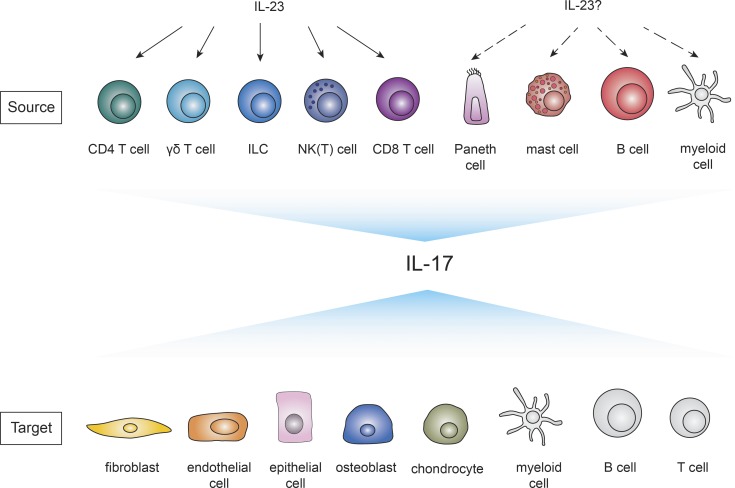
In addition to Th17 cells, innate cells located at epithelial and mucosal barriers such as CD27− γδ T cells, NK(T) cells, and innate lymphoid cells (ILCs) emerged as potent IL-17 producers (Happel et al., 2003; Ferretti et al., 2003; Zheng et al., 2008; Fig. 2). Their constitutive IL-23R and RORγt expression allows them to respond quickly (within hours) to injury or pathogenic triggers (reviewed in Cua and Tato, 2010). Also, other leukocytes such as CD8 T cells and B cells and even Paneth cells have been reported as cellular sources of IL-17 (He et al., 2006; Schlegel et al., 2013; Vazquez-Tello et al., 2012; Takahashi et al., 2008; Fig. 2). Whether myeloid cells such as neutrophils or macrophages produce IL-17 is still controversially discussed (see Fig. 2).
Figure 2.
Cellular sources and targets of IL-17A/F. Overview of described cellular sources (top row) and targets (bottom row) of IL-17A/F. IL-23 induces IL-17 production in CD4 T cells (Aggarwal et al., 2003), γδ T cells (Sutton et al., 2009), NK(T) cells (Michel et al., 2007; Passos et al., 2010), ILCs (Buonocore et al., 2010), and CD8 T cells (Happel et al., 2003). The dependence of IL-23 on other IL-17–producing cells as Paneth cells (Takahashi et al., 2008), mast cells (Lin et al., 2011; Mashiko et al., 2015), and B cells (Schlegel et al., 2013) has to be determined. Whether myeloid cells (depicted in gray) produce IL-17 is still controversially discussed (Ferretti et al., 2003; Song et al., 2008; Li et al., 2010; Tamassia et al., 2018). IL-17RA is ubiquitously expressed, but the main targets of IL-17 include endothelial cells, epithelial cells, fibroblasts (Fossiez et al., 1996), osteoblasts (Kotake et al., 1999), and chondrocytes (Shalom-Barak et al., 1998). Less-studied potential cellular targets of IL-17 (depicted in gray) are myeloid cells and B and T cells.
IL-17 is a pleiotropic cytokine that acts mainly on cells of mesenchymal origin such as endothelial cells, epithelial cells, and fibroblasts but has also effects on immune cells including dendritic cells and macrophages (reviewed in Xu and Cao, 2010; Fig. 2). IL-17 executes key functions to prevent pathogen invasion by the secretion of proinflammatory cytokines, chemokines, matrix metalloproteinases, growth factors, and antimicrobial peptides (reviewed in Onishi and Gaffen, 2010). Hereby IL-17 often acts in synergy with other mediators such as TNF and IL-22 (Shen et al., 2005; Albanesi et al., 1999). Conversely, excessive IL-17 production contributes via similar mechanisms to pathogenic inflammation.
IL-17 in chronic inflammation
Most of our understanding of IL-17 and its role in inflammatory disorders was gained through various preclinical models of disease and has prompted the development of new drugs targeting IL-17. Several drugs were designed to block the IL-17 pathway either selectively or in combination with other inflammatory cytokines, or cytokines involved in the maintenance of IL-17 producing cells such as IL-23. Table 1 summarizes the therapeutic agents that are already approved or in clinical development. In the following section, we will discuss preclinical experimental findings about the role of IL-17 in prototypic chronic inflammatory disorders and compare the results to data from clinical trials.
Table 1. Clinical development of IL-17/IL-23 inhibitors in chronic inflammatory diseases.
| Barrier tissues | Nonbarrier tissues | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Drug and trade names | Plaque psoriasis | IBD | Other diseases | PsA | Ankylosing spondylitis | RA | MS | Other diseases |
| IL-17A | Secukinumab (AIN457; Cosentyx) | Approved | Phase II, terminated (ineffective); NCT01009281 (Hueber et al., 2012) | Asthma: Phase II, terminated (ineffective); NCT01478360 | Approved | Approved | Phase III, terminated; NCT01377012 | Phase II, terminated; NCT01051817 (Havrdová et al., 2016) | Diabetes mellitus type 1: Phase II, terminated (business reasons); NCT02044848 |
| Atopic dermatitis: Phase II; NCT02594098 | |||||||||
| Hidradenitis suppurativa: Phase II; NCT03713632 | Giant cell arteritis: Phase II; NCT03765788 | ||||||||
| Pityriasis rubra pilaris: Phase I; NCT03342573 | |||||||||
| IL-17A | Ixekizumab (Taltz) | Approved | Approved | Phase III; NCT02696785 (van der Heijde et al., 2018) | Phase II; NCT00966875 (Genovese et al., 2014) | ||||
| IL-17A | Netakimab (BCD-085) | Phase III; NCT03390101 | Phase III; NCT03598751 | Phase III; NCT03447704 | Primary biliary cirrhosis: Phase II; NCT03476993 | ||||
| IL-17A | CNTO 6785 | Phase II (ineffective); NCT01909427 (Mease et al., 2018b) | |||||||
| IL-17A | CJM112 | Phase I, terminated; NCT01828086 | Asthma: Phase II; NCT03299686 | ||||||
| Hidradenitis suppurativa: Phase II; NCT02421172 | |||||||||
| IL-17A /IL-17F | Bimekizumab | Phase III; NCT03598790 | Hidradenitis suppurativa: Phase II; NCT03248531 | Phase III; NCT03896581; NCT03895203 | Phase III; NCT03928743 | ||||
| IL-17A/IL-17F | M1095 (ALX-0761) | Phase II NCT03384745 | |||||||
| IL-17RA | Brodalumab (Siliq; Kyntheum) | Approved | Phase II, terminated (disease worsening); NCT00966875 (Targan et al., 2016) | Asthma: Phase II, terminated (ineffective); NCT01902290 | Phase III, terminated (sponsor decision); NCT02029495 (Mease et al., 2014) | Phase II, withdrawn (sponsor decision); NCT02429882 | Phase II, terminated (ineffective); NCT00950989 (Pavelka et al., 2015) | ||
| Phase II (ineffective); NCT01199289 (Busse et al., 2013) | |||||||||
| IL-17A/TNF | ABT-122 | Phase II, (ineffective); NCT02349451 (Mease et al., 2018a) | Phase II, (ineffective); NCT02433340, NCT02349451 (Khatri et al., 2019) | ||||||
| IL-17A/TNF | COVA 322 | Phase II, terminated (safety profile); NCT02243787 | |||||||
| IL-17/IL-6 | MT-6194 | Preclinical (Lyman et al., 2018) | |||||||
| IL-17/BAFF | LY3090106 | Phase I; NCT01925157 | Sjögren’s syndrome: Phase I; NCT02614716 | ||||||
| IL-23p19 | Risankizumab (Skyrizi) | Approved | Crohn’s disease: Phase III; NCT03104413, NCT03105102, NCT03105128 | Asthma: Phase II; NCT02443298 | Phase III; NCT03675308, NCT03671148 | Phase II (ineffective); NCT02047110 (Baeten et al., 2018) | |||
| Ulcerative colitis: Phase III; NCT03398148, NCT03398135 | Atopic dermatitis: Phase II; NCT03706040 | ||||||||
| Hidradenitis suppurativa: Phase II; NCT03926169 | |||||||||
| IL-23p19 | Tildrakizumab (Ilumetri; Ilumya) | Approved | Phase II; NCT03552276, NCT02980692 | Phase III; NCT03552276 | |||||
| IL-23p19 | Guselkumab (Tremfya) | Approved | Crohn’s disease: Phase III; NCT03466411 | Phase III; NCT03796858, NCT03158285, NCT03162796 | Phase II (ineffective); NCT01645280 (Smolen et al., 2017) | ||||
| IL-23p19 | Brazikumab (AMG 139) | Phase I; NCT01094093 | Crohn’s disease: Phase III; NCT03961815, NCT03759288 | ||||||
| Ulcerative colitis: Phase II; NCT03616821 | |||||||||
| IL-23p19 | Mirikizumab (LY 3074828) | Phase III; NCT03535194 | Crohn’s disease: Phase III; NCT03926130 | ||||||
| Ulcerative colitis: Phase II; NCT03524092 | |||||||||
Source: https://clinicaltrials.gov if not otherwise specified. NCT, Clinicaltrials.gov identifier.
Targeting IL-17 in inflammatory disorders affecting skin and mucosal barriers
Psoriasis
Psoriasis is a chronic immune-mediated skin disease, characterized by the proliferation of keratinocytes and infiltration of T cells (Nikaein et al., 1991). In xenograft models of psoriasis, nonlesional skin from psoriasis patients is transplanted onto immunocompromised mice (Boehncke et al., 1994; Boyman et al., 2004). The model uncovered a pathogenic role of IL-23 (Tonel et al., 2010), IFN-α (Nestle et al., 2005), and TNF (Boyman et al., 2004) in psoriasis and paved the path for the successful introduction of TNF inhibitors as therapeutic options for psoriasis vulgaris (Leonardi et al., 2003). In 2009, van der Fits et al. (2009) introduced the Aldara (5% Imiquimod)-model of psoriasis, which very closely mimics human psoriasiform lesions. We and others then found that psoriasiform inflammation greatly depends on the IL-23/IL-17 axis (Pantelyushin et al., 2012; Cai et al., 2011). Skin of Aldara-treated mice expressed high levels of IL-17A/F (Pantelyushin et al., 2012; Cai et al., 2011) and IL-22 (Pantelyushin et al., 2012). In line with this, it was reported that IL-17A, IL-22, and IL-23 are up-regulated in the serum of patients with active psoriasis, and increased levels of IL-17A, F, and C were found in psoriatic skin lesions (Johansen et al., 2009; Fotiadou et al., 2015). Surprisingly, γδ T cells and not Th17 cells were found to be the primary source of IL-17A/F in the Aldara-mouse model, and their ablation led to disease protection (Cai et al., 2011; Pantelyushin et al., 2012). Similarly, increased numbers of IL-17 producing γδ T cells were also found in the skin of psoriasis patients (Laggner et al., 2011). There is some debate as to which cells in developing lesions initially deliver IL-17 as CD8+ IL-17 producing cells were also identified in psoriatic lesions (Ortega et al., 2009). The disease-promoting role of IL-17 in psoriasiform inflammation was shown to be mainly mediated via the activation of epithelial cells and keratinocytes and their increased secretion of antimicrobial peptides, cytokines, and chemokines mobilizing the recruitment of neutrophils (reviewed in Perera et al., 2012).
After the identification of the IL-23/IL-17 axis as a key factor in the pathogenesis of psoriasis, Ustekinumab, the first p40 inhibitor targeting both IL-12 and IL-23, was introduced to the clinic with success (Papp et al., 2008). However, we found that only IL-23 is disease-promoting in psoriasiform inflammation, whereas IL-12 has a protective role (Kulig et al., 2016), suggesting that collateral neutralization of IL-12 instead of IL-23 alone limited the effectiveness of these drugs.
Recently, the first three selective IL-23 inhibitors targeting the IL-23p19 subunit were licensed for treatment of plaque psoriasis after successful completion of several phase III clinical trials: Tildrakizumab, Risankizumab, and Guselkumab (Reich et al., 2017; Gordon et al., 2018; Blauvelt et al., 2017b). IL-23p19 inhibitors were more efficient in improving skin lesions than TNF inhibitors (Reich et al., 2017) and in line with findings from the Aldara-model of psoriasiform inflammation, superior compared with IL-12/IL-23 inhibitor Ustekinumab (Gordon et al., 2018). The selective IL-23 inhibitor Guselkumab was also effective in psoriasis patients where combined IL-12/IL-23 inhibition had failed (Langley et al., 2018), emphasizing that sparing IL-12 is necessary for resolution of skin inflammation in some psoriasis patients.
Between 2015 and 2017, three IL-17 pathway inhibitors reached the market. Secukinumab and Ixekizumab are monoclonal antibodies specific for IL-17A and thus inhibit both IL-17A homodimers as well as IL-17A/F heterodimers (Liu et al., 2016), while IL-17F homodimers remain unaffected. In contrast, the monoclonal antibody Brodalumab targets IL-17RA, and thus blocks the activity of IL-17A, IL-17F, IL-17C, and IL-17E. All three biologicals are approved by the Food and Drug Administration and European Medicine Agency for the treatment of moderate to severe plaque psoriasis. Several pivotal phase III studies demonstrated rapid and sustained skin improvement in the treated patients compared with placebo (Langley et al., 2014; Gordon et al., 2016; Lebwohl et al., 2015). Depending on the design of the studies, up to 42% of patients who received one of the IL-17 pathway inhibitors achieved disease-free skin (Papp et al., 2016). Clinical responses are paralleled by decreased IL-17A, IL-17F, and IL-22 expression and downmodulation of leukocyte infiltration-promoting chemoattractants in the skin (Kolbinger et al., 2017).
IL-17 inhibitors showed superior efficacy in skin clearance than previously approved psoriasis therapy with IL-12/IL-23 inhibitor Ustekinumab (Blauvelt et al., 2017c; Paul et al., 2019; Lebwohl et al., 2015) or TNF inhibitor Etanercept (Griffiths et al., 2015; Langley et al., 2014). In addition, Ixekizumab demonstrated efficacy in Etanercept nonresponders (Blauvelt et al., 2017a) and Brodalumab in Ustekinumab nonresponders (Langley et al., 2014). Head-to-head trials comparing the efficacy between the available IL-17 and IL-23p19 inhibitors have not yet been performed, but a recent meta-analysis of 77 randomized controlled trials failed to detect a difference between the drug classes, as Brodalumab, Ixekizumab, Guselkumab, and Risankizumab were equally efficient to achieve skin improvement (Sawyer et al., 2019).
Besides its presence in diseased skin, IL-17 is also observed in healthy skin and has a nonredundant role in tissue protection and immunity against fungi (Naik et al., 2015, 2017; Linehan et al., 2018; Okada et al., 2016; Sparber et al., 2019). In spite of this, the safety profile of IL-17 pathway inhibitors is favorable over long-term treatment with a consistent spectrum of adverse events across all three therapies (Deodhar et al., 2019; Langley et al., 2019; Lebwohl et al., 2015). Upper respiratory tract infections occur more frequently, followed by a higher susceptibility to mucocutaneous Candida spp. infections (Saunte et al., 2017).
We speculate that IL-17 production in cells associated with homeostasis in the skin is largely independent of IL-23R signaling, suggesting that the blockade of IL-23 would trigger fewer adverse effects compared with the general inhibition of IL-17, which may serve a function in homeostatic barrier maintenance and the control of the commensal flora. Indeed, the risk of Candida infections was lower when targeting IL-23p19 alone compared with studies with IL-17 inhibitors (Blauvelt et al., 2018). Whether IL-23 may be required for homeostatic IL-22 remains unclear.
Inflammatory bowel disease (IBD)
IBDs, which comprise of Crohn’s disease and ulcerative colitis, are inflammatory conditions of the colon and small intestine (Abraham and Cho, 2009). Single nucleotide polymorphisms in IL23R are associated with the susceptibility to IBD, and both increased IL-23 and IL-17 levels were found in colon biopsies of IBD patients, especially during active disease (Fujino et al., 2003; Duerr et al., 2006; Hölttä et al., 2008).
Also in mouse models of IBD, IL-23 was clearly associated with colitis, and as for psoriasis and experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS), IL-23 and not IL-12 is critically driving the disease (Buonocore et al., 2010; Ahern et al., 2010; Yen et al., 2006; Hue et al., 2006; Elson et al., 2007). In contrast, targeting IL-17 or its receptors led to controversial results in different animal models of colitis. Already in 2004, Ogawa et al. (2004) showed that neutralization of IL-17 leads to increased disease severity in the dextrane sulfate sodium (DSS) model. Several studies confirmed this result in the DSS as well as in other models of IBD (O’Connor et al., 2009; Song et al., 2015; Lee et al., 2015; Maxwell et al., 2015) and are in conflict with the few reports showing a disease promoting role of IL-17 (Feng et al., 2011; Leppkes et al., 2009; Yen et al., 2006). Despite the description of a protective role of IL-17 in different murine models of colitis, IL-17A (Hueber et al., 2012) and IL-17RA inhibitors (Targan et al., 2016) were tested for therapeutic efficacy in patients with Crohn’s disease. The treatment failed and the studies had to be terminated due to exacerbation of colitis. In fact, it was already proposed in the early 2000s that IL-17 has an important role in the maintenance of barrier properties of epithelial tissues in vitro and later also in vivo (Kinugasa et al., 2000; Lee et al., 2015). Two independent reports using different colitis models demonstrated that IL-17A increases the barrier integrity in epithelial cells and demonstrated that tissue-protective IL-17 is produced by γδ T cells in the absence of IL-23 (even though they do express IL-23R; Lee et al., 2015; Maxwell et al., 2015).
Interestingly, new-onset IBD is a rare adverse event in psoriasis patients treated with IL-17 inhibitors (Schreiber et al., 2019), and mucosal inflammation is also not part of the disease spectrum in patients with inborn errors of the IL-17 pathway (Okada et al., 2016). However, during an established mucosal inflammation like in Crohn’s disease, the beneficial function of IL-17 on the integrity of the epithelial barrier might prevail (Maxwell et al., 2015).
While IL-17 inhibition resulted in alarming disease exacerbation, IL-23 inhibition has revealed promising results in clinical trials for Crohn’s disease (Feagan et al., 2017, 2018; Sands et al., 2017). These clinical data suggest that selective targeting of IL-23 allows the IL-23–independent IL-17 production in the gut needed to keep the epithelial barrier intact, while IL-23–driven disease-promoting IL-17–producing cells decrease, mirroring results from previous animal studies (Lee et al., 2015).
Given the critical role of IL-23 in the maintenance of disease-promoting IL-17–producing cells, selective IL-23 class inhibitors hold the promise to interfere especially with the development of these pathogenic IL-17–producing cells while sparing the IL-23–independent IL-17–producing cells involved in host defense against Candida and maintenance of the gut barrier. Several reports indicated that the protective effect of IL-17 could be mediated via the control of the commensal microflora (Tang et al., 2018; Song et al., 2015; Kumar et al., 2016), which were already earlier shown to induce IL-17 in the colon (Ivanov et al., 2009). Interestingly, disrupted IL-17R signaling in gut epithelial cells and the following dysbiosis was also connected to a higher predisposition to neuroinflammation, indicating that homeostatic IL-17R signaling in the gut is vital for a normal gut microflora, which in turn can control the autoimmune potential of lymphocytes (Kumar et al., 2016). Furthermore, it was shown that only deletion of IL-17F but not IL-17A prevents disease in both DSS and T cell–induced colitis models and that IL-17F promotes colitis by inhibiting the colonization of the gut by regulatory T cell–promoting commensals (Tang et al., 2018). These findings indicate that specific IL-17F inhibitors also could be a potential option for the treatment of Crohn’s disease.
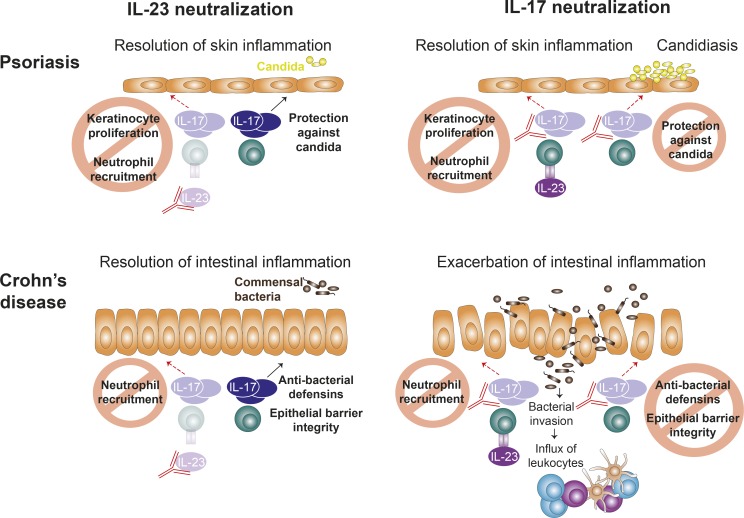
Fig. 3 summarizes the divergent roles of IL-23 and IL-17 inhibition in Crohn’s disease and psoriasis.
Figure 3.
Effects of targeting IL-17 and IL-23 in psoriasis and Crohn’s disease. The neutralization of IL-23 cures skin inflammation in psoriasis by interfering with the IL-23/IL-17 axis. Abrogation of pathogenic IL-23–dependent IL-17 production prevents IL-17–driven keratinocyte proliferation and neutrophil recruitment. IL-23–independent IL-17 production is preserved, which is needed for protection from Candida infections. Direct inhibition of IL-17 also reduces skin inflammation, but compromises host defense against Candida, leading to increased rates of candidiasis. Intestinal inflammation characteristic of Crohn’s disease is improved upon IL-23 neutralization, which inhibits mainly IL-23–dependent IL-17 production and thus interferes with disease-driving mechanisms like neutrophil attraction. IL-23 neutralization spares IL-23–independent IL-17 production important for maintenance of epithelial barrier function and control of the commensal bacteria in the gut. In contrast, direct IL-17 inhibition exacerbates intestinal skin inflammation due to abrogation of the protective functions of IL-17 on epithelial integrity and microbiota control. This leads to invasion of luminal bacteria with a subsequent influx of immune cells and secretion of other pro-inflammatory cytokines to mount an immune response, which perpetuates intestinal inflammation.
Other chronic inflammatory disorders afflicting barrier tissues
The success of IL-17 inhibition in psoriasis encouraged the initiation of clinical trials in other chronic inflammatory diseases with elevated IL-17 expression. Serum IL-17 and IL-23 levels are increased in children with atopic dermatitis and correlated with disease severity (Leonardi et al., 2015). IL-17 and IL-23 inhibitors are currently in phase II trials; however, two trails with the combined IL-12/IL-23 inhibitor Ustekinumab failed to show superiority compared with placebo (Khattri et al., 2017; Saeki et al., 2017), suggesting redundancy of the IL-23/IL-17 axis in atopic dermatitis. Several case reports reported successful outcomes of patients suffering from Hidradenitis suppurativa (Jørgensen et al., 2018) and Pityriasis rubra pilaris (Wain et al., 2018) with IL-17 inhibitors, indicating shared pathomechanisms of these inflammatory skin diseases with psoriasis despite different clinical presentation. IL-17 pathway inhibition did not show a benefit over placebo for the treatment of asthma in multiple trials (Busse et al., 2013; see Table 1), suggesting that the IL-23/IL-17 axis is not a major pathological driver in asthmatic airway inflammation.
Targeting IL-17 in inflammatory disorders affecting nonbarrier tissues
Overexpression of IL-17 in mice leads to inflammation of epithelial barriers as well as granulocytosis (Croxford et al., 2014; Haak et al., 2009). However, no inflammation-related symptoms of other, nonbarrier tissues are induced (Haak et al., 2009). This argues against the notion that the dysregulation of IL-17 would elicit immunopathology in nonbarrier tissues. The exception seems to be psoriasis arthritis (PsA), where inflammation of the skin usually precedes joint pathology and has a very different disease etiology than rheumatoid arthritis (RA). Nevertheless, the role of IL-17 in inflammatory disorders of nonbarrier tissues was extensively studied using preclinical models, and IL-17 inhibitors were tested in the clinics.
PsA
PsA is the most common psoriasis-associated disorder, found in up to 30% of psoriasis patients (Ritchlin et al., 2017; Villani et al., 2015) and is among others associated to polymorphisms in IL23R (Bowes et al., 2015). Increased numbers of IL-17 producing ILCs as well as CD4 and CD8 T cells are found in the synovial fluid of PsA patients (Benham et al., 2013; Leijten et al., 2015; Menon et al., 2014). In the joints, IL-17 seems to act on osteoblasts inducing osteoclast differentiation and thereby increases bone resorption (Sato et al., 2006). Until now, no specific murine model for has PsA existed. However, in some models for psoriasis, the development of arthritis was observed and demonstrated that changes in the skin can be sufficient to induce pathological changes in the joints (Zenz et al., 2005; Yamamoto et al., 2015; Croxford et al., 2014). In line with this, most drugs that are effective to treat psoriatic skin lesions also inhibit arthritis in PsA patients, indicating a shared pathophysiology (McInnes et al., 2013, 2015; Mease et al., 2015). However, it is still unclear how inflammatory processes in the skin, which often precede PsA, lead to arthritis (Eder et al., 2016).
Soon after the success in treating plaque psoriasis, Secukinumab and Ixekizumab received the Food and Drug Administration/European Medicine Agency approval for treatment of PsA (McInnes et al., 2015; Mease et al., 2017); Secukinumab has extended the approved indication spectrum even for ankylosing spondylitis (Baeten et al., 2015). Both IL-17A inhibitors have shown clinical efficacy and long-term improvements, even in patients unresponsive to first-line therapy with TNF inhibitors (Coates et al., 2018; Nash et al., 2017). However, compared with the high rates of complete skin clearance in plaque psoriasis, effects on PsA are relatively modest, possibly due to a less pronounced IL-17 signature in affected joints compared with skin (Belasco et al., 2015). The safety profile in the setting of both diseases is comparable to IL-17 pathway inhibition in plaque psoriasis (Mease et al., 2019; Deodhar et al., 2019).
IL-23 inhibition is effective in phase II clinical studies in PsA (Deodhar et al., 2018), and phase III studies are ongoing (NCT03552276), while there was no evidence of clinically meaningful improvements in ankylosing spondylitis (Baeten et al., 2018), although both diseases respond well to IL-17 blockade. A hypothesis for the dichotomy of clinical efficacy is that in contrast to PsA, IL-17–producing cells become IL-23–independent in the chronic phase of joint inflammation in ankylosing spondylitis as shown in an animal model (van Tok et al., 2018). Counterintuitive to the proposed synergistic action of TNF and IL-17A in PsA, a recent study failed to show superiority of dual neutralization of TNF and IL-17A with a bi-specific antibody over TNF inhibition alone (Mease et al., 2018a).
RA
Early after the discovery of IL-17, several reports showed an association between IL-17 and RA, which is a systemic autoimmune disorder characterized by the chronic inflammation of several organs including the synovia of joints (Scott et al., 2010). Those reports demonstrated the presence of IL-17 and IL-17R in synovial fluids and tissue from RA patients, and synovial IL-17 expression was identified as being predictive for joint damage progression (Ziolkowska et al., 2000; Honorati et al., 2001; Kirkham et al., 2006; Chabaud et al., 1999, 1998). The main producers of IL-17 in synovial tissues of RA patients were identified as CD4+ memory T cells (Kotake et al., 1999). IL-17 was shown to potently induce cytokines such as TNF, IL-1β, and GM-CSF from different joint cells as synovial fibroblasts, chondrocytes, and macrophages (Yao et al., 1995a, 1995b; Fossiez et al., 1996; Chabaud et al., 1998; Jovanovic et al., 1998) and was associated to bone and cartilage degradation (Chabaud et al., 2001; Cai et al., 2001; Chabaud and Miossec, 2001; Kim et al., 2015; Osta et al., 2015).
The disease-promoting role of IL-17 in RA was also described in mice. In wild-type mice, inflammatory arthritis can be induced by injecting recombinant IL-17 into the intra-articular space of knee joints (Chabaud et al., 2001; Cai et al., 2001). With rare exceptions (Doodes et al., 2008), the large majority of reports on IL-17 using different mouse models of RA showed that ablation of IL-17 reduced disease severity (Lubberts et al., 2001; Nakae et al., 2003; Katayama et al., 2013; Wu et al., 2010; Hirota et al., 2007; Duarte et al., 2010; Chabaud et al., 2001). The ablation of IL-17 was associated with reduced systemic levels of IL-6 as well as IL-1β and RANKL (receptor activator of NF-κB ligand)–producing cells in the synovium (Lubberts et al., 2004). Also, the transdifferentiation of Foxp3 regulatory T cells into IL-17–producing cells was associated to arthritis (Komatsu et al., 2014). Furthermore, it was proposed that changes in the microbiome and IL-17 in the gut are early events in RA (Wu et al., 2010), supporting the mucosal origins hypothesis suggesting that the disease-promoting immunity in RA is initiated at mucosal sites (leading to the circulation of auto-antibodies before clinical symptoms are observed) and only later spreads to the synovial joints generating symptomatic inflammation (reviewed in Holers et al., 2018).
Despite the extensive rationale from preclinical studies in mice and human to target IL-17 in RA, results from clinical studies fell far short of expectations. Although IL-17A inhibition was superior to placebo and improved symptoms in TNF nonresponders (Blanco et al., 2017; Genovese et al., 2014), response rates were still lower compared with an established second-line biological CTLA4-Ig, Abatacept (Blanco et al., 2017). As these studies provided evidence that IL-17A is a central pathogenic driver only in a small subgroup of RA patients and other available substance classes are more effective, clinical development was terminated after phase III (NCT01377012).
Many of the preclinical findings on IL-17 in RA were made using the collagen-induced arthritis (CIA) model where DBA-1 mice are immunized against type 2 collagen and immune response is boosted with CFA (Courtenay et al., 1980). Despite its valuable contributions to a better understanding of RA, in the CIA model, γδ T cells are the main producers of IL-17, while these cells were hardly detected in joints of RA patients (Ito et al., 2009). We speculate that the CFA used in the CIA model induces the excessive IL-17 expression, thus inflating the role of IL-17 in the model compared with human RA.
Furthermore, it was recently shown that the transition of disease initiation to symptomatic arthritis could be mediated by IL-23 rather than IL-17. Pfeifle et al. (2017) found that IL-23–activated Th17 cells promoted the production of pro-inflammatory antibodies by B cells via IL-22 and IL-21, but not IL-17. However, at least in established RA, a recent study with a selective IL-23 inhibitor showed no improvement in disease symptoms (Smolen et al., 2017).
MS
As for other inflammatory disorders, dysregulated cytokines play an important role in MS. In 2002, it was shown that, as for psoriasis, IL-23 and not IL-12 is disease-promoting in the EAE model of MS (Cua et al., 2003; Becher et al., 2002). IL-23–deficient mice were shown to be resistant to EAE and had reduced levels of IL-17, while the transfer of IL-17–producing T cells led to disease exacerbation (Langrish et al., 2005). The presence of IL-17–producing cells in active brain lesions of MS patients (Tzartos et al., 2008) and increased IL-17 levels in cerebrospinal fluid especially during clinical exacerbation (Matusevicius et al., 1999), supported the idea that IL-17 might be implicated in MS pathogenesis.
Surprisingly, blockade of IL-17A or F in EAE only led to a disease reduction in SJL/J mice, but had only a modest effect in C57/BL6 mice (Park et al., 2005; Hofstetter et al., 2005; Komiyama et al., 2006; Langrish et al., 2005; Haak et al., 2009). McGeachy et al. (2007) reported that IL-17–producing Th cells are only pathogenic when they are driven by IL-23, but not by IL-6 and TGFβ. In addition, IL-23 decreased the IL-10 expression by IL-17–producing cells, promoting their encephalitogenic capacity. In support of this, we found that IL-23 authorizes T cells to invade the central nervous system (CNS) and to polarize to Th17 cells (Gyülvészi et al., 2009). Furthermore, several reports suggested the existence of nonpathogenic Th17 cells (Yang et al., 2009; Esplugues et al., 2011). All these findings demonstrate that while IL-23 is essential for the development of EAE, IL-17 is largely redundant for disease development, and IL-23 seems to induce other pathogenic mediators. Indeed, we and others reported that IL-23 converts nonpathogenic into pathogenic T cells by the induction of GM-CSF (Codarri et al., 2011; El-Behi et al., 2011). Within the CNS of EAE mice, GM-CSF is produced almost exclusively by neuro-antigen–specific Th cells (Komuczki et al., 2019), and systemic dysregulation of GM-CSF leads to severe and fatal CNS inflammation dominated by the invasion into the CNS of monocytes (Spath et al., 2017). Lee et al. (2012) found that T-bet, which was already associated to the expression of IL-23R in Th17 cells (Gocke et al., 2007) and protection from EAE (Panitch et al., 1987), is driving the pathogenic functions of Th17 cells independently of their IL-17 production (Lee et al., 2012). In line with this, clinically stable MS patients had higher expression of IL-10 in Th17 cells, and single-cell clones from patients with MS showed enhanced production of IL-17, GM-CSF, or IFN-γ, while in healthy controls, IL-10 dominated (Cao et al., 2015; Hu et al., 2017).
In summary, targeting of IL-17 does not efficiently inhibit disease formation in mice, whereas targeting IL-23 selectively blocks disease-promoting Th cells, independently of IL-17. The role of IL-17 in MS remains controversial. Although IL-17 pathway inhibition slowed down the formation of new Gd-enhancing T1 brain lesions in a phase II study, the trial was terminated because the cumulative number of lesions was not significantly different compared with placebo at the primary endpoint (Havrdová et al., 2016). Although according to preclinical data the new selective IL-23 inhibitors offer a promising strategy for MS, clinical trials have not been initiated yet. Combined IL-12/IL-23 inhibition failed to show clinical efficacy in patients (Segal et al., 2008; Vollmer et al., 2011), possibly by a negative effect of the concomitant IL-12 pathway inhibition.
Other nonbarrier–associated chronic inflammatory diseases
There are ongoing trials exploring the possibility of IL-17 inhibitors in primary biliary cirrhosis, giant cell arteritis, and Sjögren’s syndrome (Table 1). The rational for such trials came from observations that IL-17–producing cells were present in the inflammatory infiltrates of the respective affected organs of patients (Katt et al., 2013; Deng et al., 2010; Zhang et al., 2018). A clinical trial investigating IL-17 inhibition in diabetes mellitus type 1 patients was terminated during phase II (Table 1).
Concluding remarks
It is becoming increasingly clear that dysregulated cytokine networks are fundamentally involved in chronic inflammatory diseases and that targeting cytokines therapeutically has great value in treating patients suffering from these disorders. Of note, excessive production of single cytokines in transgenic mice actually led to tissue-specific inflammation (Keffer et al., 1991; Haak et al., 2009; Spath et al., 2017). IL-17 in particular is connected with barrier function, and dysregulation of IL-17 is closely linked to psoriasiform skin inflammation. The most likely explanation for the skin tropism of IL-17–mediated inflammation is the fact that the receptor complex for IL-17 is highly abundant in the skin compared with other tissues. The normal steady-state function is likely the control of the commensal microflora, and as a quasi-innate cytokine, IL-17 can rapidly recruit other leukocytes in response to dysbiosis or pathogen invasion through barriers. So, neutralization of the IL-17/IL-23 axis has been shown to be beneficial in treating psoriasis, while in Crohn's disease only the targeting of IL-23 shows clinical benefit, possible because the steady-state function of IL-17 in gut barrier function is too fragile for external intervention. Nevertheless, the impact of the IL-23/IL-17 axis on the microbiome is abundantly clear. The nature of the commensal flora in the steady-state has in turn a dramatic effect on immunity in general. We thus propose that the conflicting data regarding the role of IL-17 in preclinical models of inflammation (outside of barrier tissues) is likely the result of the altered gut immune axis observed in IL-17–deficient mice. The clinical success of targeting the IL-17/IL-23 axis in chronic inflammation of the body lining but not of diseases of internal organs (e.g., RA, diabetes mellitus type 1, MS, etc.), was largely predicted in the preclinical studies, which provides further support for integrative and comprehensive bench to bedside research.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (310030_146130, 316030_150768, and 310030_170320 to B. Becher), the European Union FP7 Project ATECT (Advanced T-cell Engineered for Cancer Therapy; to B. Becher), the European Union H2020 Project iPC (Individualized Paediatric Cure), and the University Research Priority Project Translational Cancer Research (to B. Becher).
The authors declare no competing financial interests.
Author contributions: S. Unger, P. Zwicky, and B. Becher wrote the manuscript.
References
- Abraham C., and Cho J.H.. 2009. Inflammatory bowel disease. N. Engl. J. Med. 361:2066–2078. 10.1056/NEJMra0804647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., and Gurney A.L.. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. 10.1074/jbc.M207577200 [DOI] [PubMed] [Google Scholar]
- Ahern P.P., Schiering C., Buonocore S., McGeachy M.J., Cua D.J., Maloy K.J., and Powrie F.. 2010. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 33:279–288. 10.1016/j.immuni.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimzhanov A.M., Yang X.O., and Dong C.. 2007. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 282:5969–5972. 10.1074/jbc.C600322200 [DOI] [PubMed] [Google Scholar]
- Albanesi C., Cavani A., and Girolomoni G.. 1999. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 162:494–502. [PubMed] [Google Scholar]
- Amatya N., Garg A.V., and Gaffen S.L.. 2017. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 38:310–322. 10.1016/j.it.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy M.A., Fanslow W.C., Fu F., Li W., Qian S., Troutt A.B., and Thomson A.W.. 1999. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol. 162:577–584. [PubMed] [Google Scholar]
- Baeten D., Sieper J., Braun J., Baraliakos X., Dougados M., Emery P., Deodhar A., Porter B., Martin R., Andersson M., et al. MEASURE 2 Study Group . 2015. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 373:2534–2548. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- Baeten D., Østergaard M., Wei J.C.-C., Sieper J., Järvinen P., Tam L.-S., Salvarani C., Kim T.-H., Solinger A., Datsenko Y., et al. . 2018. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 77:1295–1302. 10.1136/annrheumdis-2018-213328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Durell B.G., and Noelle R.J.. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497. 10.1172/JCI0215751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J., Louie J.S., Gulati N., Wei N., Nograles K., Fuentes-Duculan J., Mitsui H., Suárez-Fariñas M., and Krueger J.G.. 2015. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 67:934–944. 10.1002/art.38995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham H., Norris P., Goodall J., Wechalekar M.D., FitzGerald O., Szentpetery A., Smith M., Thomas R., and Gaston H.. 2013. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 15:R136 10.1186/ar4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F.J., Möricke R., Dokoupilova E., Codding C., Neal J., Andersson M., Rohrer S., and Richards H.. 2017. Secukinumab in Active Rheumatoid Arthritis: A Phase III Randomized, Double-Blind, Active Comparator- and Placebo-Controlled Study. Arthritis Rheumatol. 69:1144–1153. 10.1002/art.40070 [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Papp K.A., Griffiths C.E.M., Puig L., Weisman J., Dutronc Y., Kerr L.F., Ilo D., Mallbris L., and Augustin M.. 2017a Efficacy and Safety of Switching to Ixekizumab in Etanercept Non-Responders: A Subanalysis from Two Phase III Randomized Clinical Trials in Moderate-to-Severe Plaque Psoriasis (UNCOVER-2 and -3). Am. J. Clin. Dermatol. 18:273–280. 10.1007/s40257-016-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A., Papp K.A., Griffiths C.E.M., Randazzo B., Wasfi Y., Shen Y.-K., Li S., and Kimball A.B.. 2017b Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 76:405–417. 10.1016/j.jaad.2016.11.041 [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Reich K., Tsai T.-F., Tyring S., Vanaclocha F., Kingo K., Ziv M., Pinter A., Vender R., Hugot S., et al. . 2017c Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J. Am. Acad. Dermatol. 76:60–69.e9. 10.1016/j.jaad.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Blauvelt A., Reich K., Papp K.A., Kimball A.B., Gooderham M., Tyring S.K., Sinclair R., Thaçi D., Li Q., Cichanowitz N., et al. . 2018. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br. J. Dermatol. 179:615–622. 10.1111/bjd.16724 [DOI] [PubMed] [Google Scholar]
- Boehncke W.H., Sterry W., Hainzl A., Scheffold W., and Kaufmann R.. 1994. Psoriasiform architecture of murine epidermis overlying human psoriatic dermis transplanted onto SCID mice. Arch. Dermatol. Res. 286:325–330. 10.1007/BF00402223 [DOI] [PubMed] [Google Scholar]
- Bowes J., Budu-Aggrey A., Huffmeier U., Uebe S., Steel K., Hebert H.L., Wallace C., Massey J., Bruce I.N., Bluett J., et al. . 2015. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat. Commun. 6:6046 10.1038/ncomms7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Hefti H.P., Conrad C., Nickoloff B.J., Suter M., and Nestle F.O.. 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199:731–736. 10.1084/jem.20031482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., and Powrie F.. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 464:1371–1375. 10.1038/nature08949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse W.W., Holgate S., Kerwin E., Chon Y., Feng J., Lin J., and Lin S.-L.. 2013. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 188:1294–1302. 10.1164/rccm.201212-2318OC [DOI] [PubMed] [Google Scholar]
- Cai L., Yin J.P., Starovasnik M.A., Hogue D.A., Hillan K.J., Mort J.S., and Filvaroff E.H.. 2001. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 16:10–21. 10.1006/cyto.2001.0939 [DOI] [PubMed] [Google Scholar]
- Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V.R., Zhang H.G., Wang T., Zheng J., and Yan J.. 2011. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 35:596–610. 10.1016/j.immuni.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Goods B.A., Raddassi K., Nepom G.T., Kwok W.W., Love J.C., and Hafler D.A.. 2015. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 7:287ra74 10.1126/scitranslmed.aaa8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M., and Miossec P.. 2001. The combination of tumor necrosis factor α blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 44:1293–1303. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Fossiez F., Taupin J.L., and Miossec P.. 1998. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 161:409–414. 10.4049/jimmunol.165.9.5332 [DOI] [PubMed] [Google Scholar]
- Chabaud M., Durand J.M., Buchs N., Fossiez F., Page G., Frappart L., and Miossec P.. 1999. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42:963–970. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Lubberts E., Joosten L., van Den Berg W., and Miossec P.. 2001. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 3:168–177. 10.1186/ar294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates L.C., Gladman D.D., Nash P., FitzGerald O., Kavanaugh A., Kvien T.K., Gossec L., Strand V., Rasouliyan L., Pricop L., et al. FUTURE 2 study group . 2018. Secukinumab provides sustained PASDAS-defined remission in psoriatic arthritis and improves health-related quality of life in patients achieving remission: 2-year results from the phase III FUTURE 2 study. Arthritis Res. Ther. 20:272 10.1186/s13075-018-1773-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., and Becher B.. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12:560–567. 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- Courtenay J.S., Dallman M.J., Dayan A.D., Martin A., and Mosedale B.. 1980. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 283:666–668. 10.1038/283666a0 [DOI] [PubMed] [Google Scholar]
- Croxford A.L., Karbach S., Kurschus F.C., Wörtge S., Nikolaev A., Yogev N., Klebow S., Schüler R., Reissig S., Piotrowski C., et al. . 2014. IL-6 regulates neutrophil microabscess formation in IL-17A-driven psoriasiform lesions. J. Invest. Dermatol. 134:728–735. 10.1038/jid.2013.404 [DOI] [PubMed] [Google Scholar]
- Cua D.J., and Tato C.M.. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489. 10.1038/nri2800 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. . 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Deng J., Younge B.R., Olshen R.A., Goronzy J.J., and Weyand C.M.. 2010. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 121:906–915. 10.1161/CIRCULATIONAHA.109.872903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodhar A., Gottlieb A.B., Boehncke W.-H., Dong B., Wang Y., Zhuang Y., Barchuk W., Xu X.L., and Hsia E.C.. CNTO1959PSA2001 Study Group . 2018. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 391:2213–2224. 10.1016/S0140-6736(18)30952-8 [DOI] [PubMed] [Google Scholar]
- Deodhar A., Mease P.J., McInnes I.B., Baraliakos X., Reich K., Blauvelt A., Leonardi C., Porter B., Das Gupta A., Widmer A., et al. . 2019. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res. Ther. 21:111 10.1186/s13075-019-1882-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doodes P.D., Cao Y., Hamel K.M., Wang Y., Farkas B., Iwakura Y., and Finnegan A.. 2008. Development of Proteoglycan-Induced Arthritis Is Independent of IL-17. J. Immunol. 10.4049/jimmunol.181.1.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J., Agua-Doce A., Oliveira V.G., Fonseca J.E., and Graca L.. 2010. Modulation of IL-17 and Foxp3 expression in the prevention of autoimmune arthritis in mice. PLoS One. 5:e10558 10.1371/journal.pone.0010558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J., Steinhart A.H., Abraham C., Regueiro M., Griffiths A., et al. . 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 314:1461–1463. 10.1126/science.1135245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder L., Haddad A., Rosen C.F., Lee K.A., Chandran V., Cook R., and Gladman D.D.. 2016. The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis Rheumatol. 68:915–923. 10.1002/art.39494 [DOI] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., and Rostami A.. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575. 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C.O., Cong Y., Weaver C.T., Schoeb T.R., McClanahan T.K., Fick R.B., and Kastelein R.A.. 2007. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 132:2359–2370. 10.1053/j.gastro.2007.03.104 [DOI] [PubMed] [Google Scholar]
- Esplugues E., Huber S., Gagliani N., Hauser A.E., Town T., Wan Y.Y., O’Connor W. Jr., Rongvaux A., Van Rooijen N., Haberman A.M., et al. . 2011. Control of TH17 cells occurs in the small intestine. Nature. 475:514–518. 10.1038/nature10228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan B.G., Sandborn W.J., D’Haens G., Panés J., Kaser A., Ferrante M., Louis E., Franchimont D., Dewit O., Seidler U., et al. . 2017. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 389:1699–1709. 10.1016/S0140-6736(17)30570-6 [DOI] [PubMed] [Google Scholar]
- Feagan B.G., Panés J., Ferrante M., Kaser A., D’Haens G.R., Sandborn W.J., Louis E., Neurath M.F., Franchimont D., Dewit O., et al. . 2018. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol. Hepatol. 3:671–680. 10.1016/S2468-1253(18)30233-4 [DOI] [PubMed] [Google Scholar]
- Feng T., Qin H., Wang L., Benveniste E.N., Elson C.O., and Cong Y.. 2011. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 186:6313–6318. 10.4049/jimmunol.1001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti S., Bonneau O., Dubois G.R., Jones C.E., and Trifilieff A.. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170:2106–2112. 10.4049/jimmunol.170.4.2106 [DOI] [PubMed] [Google Scholar]
- Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J.J., Garrone P., Garcia E., Saeland S., et al. . 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593–2603. 10.1084/jem.183.6.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadou C., Lazaridou E., Sotiriou E., Gerou S., Kyrgidis A., Vakirlis E., and Ioannides D.. 2015. IL-17A, IL-22, and IL-23 as markers of psoriasis activity: A cross-sectional, hospital-based study. J. Cutan. Med. Surg. 19:555–560. 10.1177/1203475415584503 [DOI] [PubMed] [Google Scholar]
- Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., and Fujiyama Y.. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. 10.1136/gut.52.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M.C., Greenwald M., Cho C.S., Berman A., Jin L., Cameron G.S., Benichou O., Xie L., Braun D., Berclaz P.Y., and Banerjee S.. 2014. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 66:1693–1704. 10.1002/art.38617 [DOI] [PubMed] [Google Scholar]
- Gocke A.R., Cravens P.D., Ben L.-H., Hussain R.Z., Northrop S.C., Racke M.K., and Lovett-Racke A.E.. 2007. T-bet Regulates the Fate of Th1 and Th17 Lymphocytes in Autoimmunity. J. Immunol. 10.4049/jimmunol.178.3.1341 [DOI] [PubMed] [Google Scholar]
- Gordon K.B., Blauvelt A., Papp K.A., Langley R.G., Luger T., Ohtsuki M., Reich K., Amato D., Ball S.G., Braun D.K., et al. UNCOVER-3 Study Group . 2016. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 375:345–356. 10.1056/NEJMoa1512711 [DOI] [PubMed] [Google Scholar]
- Gordon K.B., Strober B., Lebwohl M., Augustin M., Blauvelt A., Poulin Y., Papp K.A., Sofen H., Puig L., Foley P., et al. . 2018. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 392:650–661. 10.1016/S0140-6736(18)31713-6 [DOI] [PubMed] [Google Scholar]
- Griffiths C.E.M., Reich K., Lebwohl M., van de Kerkhof P., Paul C., Menter A., Cameron G.S., Erickson J., Zhang L., Secrest R.J., et al. UNCOVER-2 and UNCOVER-3 investigators . 2015. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 386:541–551. 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- Gu C., Wu L., and Li X.. 2013. IL-17 family: cytokines, receptors and signaling. Cytokine. 64:477–485. 10.1016/j.cyto.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyülvészi G., Haak S., and Becher B.. 2009. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur. J. Immunol. 39:1864–1869. 10.1002/eji.200939305 [DOI] [PubMed] [Google Scholar]
- Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., and Waisman A.. 2009. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119:61–69. 10.1172/JCI35997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel K.I., Zheng M., Young E., Quinton L.J., Lockhart E., Ramsay A.J., Shellito J.E., Schurr J.R., Bagby G.J., Nelson S., and Kolls J.K.. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436. 10.4049/jimmunol.170.9.4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrdová E., Belova A., Goloborodko A., Tisserant A., Wright A., Wallstroem E., Garren H., Maguire R.P., and Johns D.R.. 2016. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J. Neurol. 263:1287–1295. 10.1007/s00415-016-8128-x [DOI] [PubMed] [Google Scholar]
- He D., Wu L., Kim H.K., Li H., Elmets C.A., and Xu H.. 2006. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 177:6852–6858. 10.4049/jimmunol.177.10.6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., and Sakaguchi S.. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204:41–47. 10.1084/jem.20062259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H.H., Ibrahim S.M., Koczan D., Kruse N., Weishaupt A., Toyka K.V., and Gold R.. 2005. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237:123–130. 10.1016/j.cellimm.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Holers V.M., Demoruelle M.K., Kuhn K.A., Buckner J.H., Robinson W.H., Okamoto Y., Norris J.M., and Deane K.D.. 2018. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheumatol. 14:542–557. 10.1038/s41584-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä V., Klemetti P., Sipponen T., Westerholm-Ormio M., Kociubinski G., Salo H., Räsänen L., Kolho K.L., Färkkilä M., Savilahti E., and Vaarala O.. 2008. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm. Bowel Dis. 14:1175–1184. 10.1002/ibd.20475 [DOI] [PubMed] [Google Scholar]
- Honorati M.C., Meliconi R., Pulsatelli L., Canè S., Frizziero L., and Facchini A.. 2001. High in vivo expression of interleukin-17 receptor in synovial endothelial cells and chondrocytes from arthritis patients. Rheumatology (Oxford). 40:522–527. 10.1093/rheumatology/40.5.522 [DOI] [PubMed] [Google Scholar]
- Hu D., Notarbartolo S., Croonenborghs T., Patel B., Cialic R., Yang T.H., Aschenbrenner D., Andersson K.M., Gattorno M., Pham M., et al. . 2017. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 8:1600 10.1038/s41467-017-01571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S., Ahern P., Buonocore S., Kullberg M.C., Cua D.J., McKenzie B.S., Powrie F., and Maloy K.J.. 2006. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203:2473–2483. 10.1084/jem.20061099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D.R., Wehkamp J., Feagan B.G., Yao M.D., Karczewski M., et al. Secukinumab in Crohn’s Disease Study Group . 2012. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 61:1693–1700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Usui T., Kobayashi S., Iguchi-Hashimoto M., Ito H., Yoshitomi H., Nakamura T., Shimizu M., Kawabata D., Yukawa N., et al. . 2009. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 60:2294–2303. 10.1002/art.24687 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., and Littman D.R.. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. . 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139:485–498. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen C., Usher P.A., Kjellerup R.B., Lundsgaard D., Iversen L., and Kragballe K.. 2009. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 160:319–324. 10.1111/j.1365-2133.2008.08902.x [DOI] [PubMed] [Google Scholar]
- Jørgensen A.R., Yao Y., and Thomsen S.F.. 2018. Therapeutic Response to Secukinumab in a 36-Year-Old Woman with Hidradenitis Suppurativa. Case Rep. Dermatol. Med. 2018:8685136 10.1155/2018/8685136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., and Pelletier J.P.. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160:3513–3521. [PubMed] [Google Scholar]
- Katayama M., Ohmura K., Yukawa N., Terao C., Hashimoto M., Yoshifuji H., Kawabata D., Fujii T., Iwakura Y., and Mimori T.. 2013. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 8:e62231 10.1371/journal.pone.0062231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katt J., Schwinge D., Schoknecht T., Quaas A., Sobottka I., Burandt E., Becker C., Neurath M.F., Lohse A.W., Herkel J., and Schramm C.. 2013. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 58:1084–1093. 10.1002/hep.26447 [DOI] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., and Kollias G.. 1991. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 10:4025–4031. 10.1002/j.1460-2075.1991.tb04978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri A., Klünder B., Peloso P.M., and Othman A.A.. 2019. Exposure-response analyses demonstrate no evidence of interleukin 17A contribution to efficacy of ABT-122 in rheumatoid or psoriatic arthritis. Rheumatology (Oxford). 58:352–360. 10.1093/rheumatology/key312 [DOI] [PubMed] [Google Scholar]
- Khattri S., Brunner P.M., Garcet S., Finney R., Cohen S.R., Oliva M., Dutt R., Fuentes-Duculan J., Zheng X., Li X., et al. . 2017. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp. Dermatol. 26:28–35. 10.1111/exd.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.W., Kim H.R., Kim B.M., Cho M.L., and Lee S.H.. 2015. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 185:3011–3024. 10.1016/j.ajpath.2015.07.017 [DOI] [PubMed] [Google Scholar]
- Kinugasa T., Sakaguchi T., Gu X., and Reinecker H.C.. 2000. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 118:1001–1011. 10.1016/S0016-5085(00)70351-9 [DOI] [PubMed] [Google Scholar]
- Kirkham B.W., Lassere M.N., Edmonds J.P., Juhasz K.M., Bird P.A., Lee C.S., Shnier R., and Portek I.J.. 2006. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 54:1122–1131. 10.1002/art.21749 [DOI] [PubMed] [Google Scholar]
- Kolbinger F., Loesche C., Valentin M.A., Jiang X., Cheng Y., Jarvis P., Peters T., Calonder C., Bruin G., Polus F., et al. . 2017. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 139:923–932.e8. 10.1016/j.jaci.2016.06.038 [DOI] [PubMed] [Google Scholar]
- Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-hora M., Kodama T., Tanaka S., Bluestone J.A., and Takayanagi H.. 2014. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20:62–68. 10.1038/nm.3432 [DOI] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., and Iwakura Y.. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573. 10.4049/jimmunol.177.1.566 [DOI] [PubMed] [Google Scholar]
- Komuczki J., Tuzlak S., Friebel E., Hartwig T., Spath S., Rosenstiel P., Waisman A., Opitz L., Oukka M., Schreiner B., et al. . 2019. Fate-Mapping of GM-CSF Expression Identifies a Discrete Subset of Inflammation-Driving T Helper Cells Regulated by Cytokines IL-23 and IL-1β. Immunity. 50:1289–1304.e6. 10.1016/j.immuni.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., and Kuchroo V.K.. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485–517. 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- Kostulas N., Pelidou S.H., Kivisäkk P., Kostulas V., and Link H.. 1999. Increased IL-1β, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 30:2174–2179. 10.1161/01.STR.30.10.2174 [DOI] [PubMed] [Google Scholar]
- Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., Saito S., Inoue K., Kamatani N., Gillespie M.T., et al. . 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103:1345–1352. 10.1172/JCI5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulig P., Musiol S., Freiberger S.N., Schreiner B., Gyülveszi G., Russo G., Pantelyushin S., Kishihara K., Alessandrini F., Kündig T., et al. . 2016. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 7:13466 10.1038/ncomms13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Monin L., Castillo P., Elsegeiny W., Horne W., Eddens T., Vikram A., Good M., Schoenborn A.A., Bibby K., et al. . 2016. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 44:659–671. 10.1016/j.immuni.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggner U., Di Meglio P., Perera G.K., Hundhausen C., Lacy K.E., Ali N., Smith C.H., Hayday A.C., Nickoloff B.J., and Nestle F.O.. 2011. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J. Immunol. 187:2783–2793. 10.4049/jimmunol.1100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E.M., Papp K., Puig L., Nakagawa H., Spelman L., Sigurgeirsson B., et al. FIXTURE Study Group . 2014. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371:326–338. 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- Langley R.G., Tsai T.-F., Flavin S., Song M., Randazzo B., Wasfi Y., Jiang J., Li S., and Puig L.. 2018. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 178:114–123. 10.1111/bjd.15750 [DOI] [PubMed] [Google Scholar]
- Langley R.G., Kimball A.B., Nak H., Xu W., Pangallo B., Osuntokun O.O., Agada N., and Reich K.. 2019. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J. Eur. Acad. Dermatol. Venereol. 33:333–339. 10.1111/jdv.15242 [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., and Cua D.J.. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M., Strober B., Menter A., Gordon K., Weglowska J., Puig L., Papp K., Spelman L., Toth D., Kerdel F., et al. . 2015. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N. Engl. J. Med. 373:1318–1328. 10.1056/NEJMoa1503824 [DOI] [PubMed] [Google Scholar]
- Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A., et al. . 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13:991–999. 10.1038/ni.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Tato C.M., Joyce-Shaikh B., Gulen M.F., Cayatte C., Chen Y., Blumenschein W.M., Judo M., Ayanoglu G., McClanahan T.K., et al. . 2015. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 43:727–738. 10.1016/j.immuni.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijten E.F.A., van Kempen T.S., Boes M., Michels-van Amelsfort J.M.R., Hijnen D., Hartgring S.A.Y., van Roon J.A.G., Wenink M.H., and Radstake T.R.D.J.. 2015. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol. 67:2673–2678. 10.1002/art.39261 [DOI] [PubMed] [Google Scholar]
- Leonardi C.L., Powers J.L., Matheson R.T., Goffe B.S., Zitnik R., Wang A., and Gottlieb A.B.. Etanercept Psoriasis Study Group . 2003. Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 349:2014–2022. 10.1056/NEJMoa030409 [DOI] [PubMed] [Google Scholar]
- Leonardi S., Cuppari C., Manti S., Filippelli M., Parisi G.F., Borgia F., Briuglia S., Cannavò P., Salpietro A., Arrigo T., and Salpietro C.. 2015. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): association with clinical severity and phenotype. Allergy Asthma Proc. 36:74–81. 10.2500/aap.2015.36.3808 [DOI] [PubMed] [Google Scholar]
- Leppkes M., Becker C., Ivanov I.I., Hirth S., Wirtz S., Neufert C., Pouly S., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., et al. . 2009. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 136:257–267. 10.1053/j.gastro.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Li L., Huang L., Vergis A.L., Ye H., Bajwa A., Narayan V., Strieter R.M., Rosin D.L., and Okusa M.D.. 2010. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120:331–342. 10.1172/JCI38702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.M., Rubin C.J., Khandpur R., Wang J.Y., Riblett M., Yalavarthi S., Villanueva E.C., Shah P., Kaplan M.J., and Bruce A.T.. 2011. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187:490–500. 10.4049/jimmunol.1100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan J.L., Harrison O.J., Han S.-J., Byrd A.L., Vujkovic-Cvijin I., Villarino A.V., Sen S.K., Shaik J., Smelkinson M., Tamoutounour S., et al. . 2018. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell. 172:784–796.e18. 10.1016/j.cell.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Lu J., Allan B.W., Tang Y., Tetreault J., Chow C.K., Barmettler B., Nelson J., Bina H., Huang L., et al. . 2016. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J. Inflamm. Res. 9:39–50. 10.2147/JIR.S100940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E., Joosten L.A., Oppers B., van den Bersselaar L., Coenen-de Roo C.J., Kolls J.K., Schwarzenberger P., van de Loo F.A., and van den Berg W.B.. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167:1004–1013. 10.4049/jimmunol.167.2.1004 [DOI] [PubMed] [Google Scholar]
- Lubberts E., Koenders M.I., Oppers-Walgreen B., van den Bersselaar L., Coenen-de Roo C.J.J., Joosten L.A.B., and van den Berg W.B.. 2004. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50:650–659. 10.1002/art.20001 [DOI] [PubMed] [Google Scholar]
- Lyman M., Lieuw V., Richardson R., Timmer A., Stewart C., Granger S., Woods R., Silacci M., Grabulovski D., and Newman R.. 2018. A bispecific antibody that targets IL-6 receptor and IL-17A for the potential therapy of patients with autoimmune and inflammatory diseases. J. Biol. Chem. 293:9326–9334. 10.1074/jbc.M117.818559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagoli D. 2016. The evolution of the immune system : conservation and diversification. Elsevier, London. 384 pp. [Google Scholar]
- Mashiko S., Bouguermouh S., Rubio M., Baba N., Bissonnette R., and Sarfati M.. 2015. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 136:351–9.e1. 10.1016/j.jaci.2015.01.033 [DOI] [PubMed] [Google Scholar]
- Matusevicius D., Kivisäkk P., He B., Kostulas N., Özenci V., Fredrikson S., and Link H.. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5:101–104. 10.1177/135245859900500206 [DOI] [PubMed] [Google Scholar]
- Maxwell J.R., Zhang Y., Brown W.A., Smith C.L., Byrne F.R., Fiorino M., Stevens E., Bigler J., Davis J.A., Rottman J.B., et al. . 2015. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 43:739–750. 10.1016/j.immuni.2015.08.019 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., and Cua D.J.. 2008. Th17 cell differentiation: the long and winding road. Immunity. 28:445–453. 10.1016/j.immuni.2008.03.001 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., and Cua D.J.. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397. 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J., and Gaffen S.L.. 2019. The IL-17 Family of Cytokines in Health and Disease. Immunity. 50:892–906. 10.1016/j.immuni.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I.B., Kavanaugh A., Gottlieb A.B., Puig L., Rahman P., Ritchlin C., Brodmerkel C., Li S., Wang Y., Mendelsohn A.M., and Doyle M.K.. PSUMMIT 1 Study Group . 2013. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 382:780–789. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- McInnes I.B., Mease P.J., Kirkham B., Kavanaugh A., Ritchlin C.T., Rahman P., van der Heijde D., Landewé R., Conaghan P.G., Gottlieb A.B., et al. FUTURE 2 Study Group . 2015. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 386:1137–1146. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- Mease P.J., Genovese M.C., Greenwald M.W., Ritchlin C.T., Beaulieu A.D., Deodhar A., Newmark R., Feng J., Erondu N., and Nirula A.. 2014. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 370:2295–2306. 10.1056/NEJMoa1315231 [DOI] [PubMed] [Google Scholar]
- Mease P.J., McInnes I.B., Kirkham B., Kavanaugh A., Rahman P., van der Heijde D., Landewé R., Nash P., Pricop L., Yuan J., et al. FUTURE 1 Study Group . 2015. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 373:1329–1339. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- Mease P.J., van der Heijde D., Ritchlin C.T., Okada M., Cuchacovich R.S., Shuler C.L., Lin C.-Y., Braun D.K., Lee C.H., and Gladman D.D.. SPIRIT-P1 Study Group . 2017. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J., Genovese M.C., Weinblatt M.E., Peloso P.M., Chen K., Othman A.A., Li Y., Mansikka H.T., Khatri A., Wishart N., and Liu J.. 2018a Phase II Study of ABT-122, a Tumor Necrosis Factor- and Interleukin-17A-Targeted Dual Variable Domain Immunoglobulin, in Patients With Psoriatic Arthritis With an Inadequate Response to Methotrexate. Arthritis Rheumatol. 70:1778–1789. 10.1002/art.40579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J., Jeka S., Jaller J.J., Kitumnuaypong T., Louthrenoo W., Mann H., Matsievskaia G., Soriano E.R., Jia B., Wang C., et al. . 2018b CNTO6785, a fully human antIInterleukin 17 monoclonal antibody, in patients with rheumatoid arthritis with inadequate response to methotrexate: A randomized, placebo-controlled, phase II, dose-ranging study. J. Rheumatol. 45:22–31. 10.3899/jrheum.161238 [DOI] [PubMed] [Google Scholar]
- Mease P., Roussou E., Burmester G.-R., Goupille P., Gottlieb A., Moriarty S.R., Benichou O., Adams D.H., Xu W., and Nash P.. 2019. Safety of Ixekizumab in Patients With Psoriatic Arthritis: Results From a Pooled Analysis of Three Clinical Trials. Arthritis Care Res. (Hoboken). 71:367–378. 10.1002/acr.23738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon B., Gullick N.J., Walter G.J., Rajasekhar M., Garrood T., Evans H.G., Taams L.S., and Kirkham B.W.. 2014. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 66:1272–1281. 10.1002/art.38376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M.-L., Keller A.C., Paget C., Fujio M., Trottein F., Savage P.B., Wong C.-H., Schneider E., Dy M., and Leite-de-Moraes M.C.. 2007. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995–1001. 10.1084/jem.20061551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L., and Gaffen S.L.. 2018. Interleukin 17 Family Cytokines : Signaling and Therapeutic Implications. Cold Spring Harb. Perspect. Biol. 10 10.1101/cshperspect.a028522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., and Coffman R.L.. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357. [PubMed] [Google Scholar]
- Naik S., Bouladoux N., Linehan J.L., Han S.J., Harrison O.J., Wilhelm C., Conlan S., Himmelfarb S., Byrd A.L., Deming C., et al. . 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 520:104–108. 10.1038/nature14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Larsen S.B., Gomez N.C., Alaverdyan K., Sendoel A., Yuan S., Polak L., Kulukian A., Chai S., and Fuchs E.. 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 550:475–480. 10.1038/nature24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S., Nambu A., Sudo K., and Iwakura Y.. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177. 10.4049/jimmunol.171.11.6173 [DOI] [PubMed] [Google Scholar]
- Nash P., Kirkham B., Okada M., Rahman P., Combe B., Burmester G.R., Adams D.H., Kerr L., Lee C., Shuler C.L., and Genovese M.. SPIRIT-P2 Study Group . 2017. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 389:2317–2327. 10.1016/S0140-6736(17)31429-0 [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y.-J., and Gilliet M.. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143. 10.1084/jem.20050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaein A., Phillips C., Gilbert S.C., Savino D., Silverman A., Stone M.J., and Menter A.. 1991. Characterization of skin-infiltrating lymphocytes in patients with psoriasis. J. Invest. Dermatol. 96:3–9. 10.1111/1523-1747.ep12514646 [DOI] [PubMed] [Google Scholar]
- O’Connor W. Jr., Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y., Kolls J.K., and Flavell R.A.. 2009. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10:603–609. 10.1038/ni.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A., Andoh A., Araki Y., Bamba T., and Fujiyama Y.. 2004. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110:55–62. 10.1016/j.clim.2003.09.013 [DOI] [PubMed] [Google Scholar]
- Okada S., Puel A., Casanova J.-L., and Kobayashi M.. 2016. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunology. 5:e114 10.1038/cti.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi R.M., and Gaffen S.L.. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129:311–321. 10.1111/j.1365-2567.2009.03240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega C., Fernández-A S., Carrillo J.M., Romero P., Molina I.J., Moreno J.C., and Santamaría M.. 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86:435–443. 10.1189/JLB.0109046 [DOI] [PubMed] [Google Scholar]
- Osta B., Roux J.P., Lavocat F., Pierre M., Ndongo-Thiam N., Boivin G., and Miossec P.. 2015. Differential effects of IL-17A and TNF-α on osteoblastic differentiation of isolated synoviocytes and on bone explants from arthritis patients. Front. Immunol. 6:151 10.3389/fimmu.2015.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch H.S., Hirsch R.L., Schindler J., and Johnson K.P.. 1987. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 37:1097–1102. 10.1212/WNL.37.7.1097 [DOI] [PubMed] [Google Scholar]
- Pantelyushin S., Haak S., Ingold B., Kulig P., Heppner F.L., Navarini A.A., and Becher B.. 2012. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122:2252–2256. 10.1172/JCI61862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N., Guzzo C., Hsu M.C., Wang Y., Li S., et al. PHOENIX 2 study investigators . 2008. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 371:1675–1684. 10.1016/S0140-6736(08)60726-6 [DOI] [PubMed] [Google Scholar]
- Papp K.A., Reich K., Paul C., Blauvelt A., Baran W., Bolduc C., Toth D., Langley R.G., Cather J., Gottlieb A.B., et al. . 2016. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 175:273–286. 10.1111/bjd.14493 [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., and Dong C.. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos S.T., Silver J.S., O’Hara A.C., Sehy D., Stumhofer J.S., and Hunter C.A.. 2010. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J. Immunol. 184:1776–1783. 10.4049/jimmunol.0901843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Griffiths C.E.M., van de Kerkhof P.C.M., Puig L., Dutronc Y., Henneges C., Dossenbach M., Hollister K., and Reich K.. 2019. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J. Am. Acad. Dermatol. 80:70–79.e3. 10.1016/j.jaad.2018.06.039 [DOI] [PubMed] [Google Scholar]
- Pavelka K., Chon Y., Newmark R., Lin S.-L., Baumgartner S., and Erondu N.. 2015. A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J. Rheumatol. 42:912–919. 10.3899/jrheum.141271 [DOI] [PubMed] [Google Scholar]
- Perera G.K., Di Meglio P., and Nestle F.O.. 2012. Psoriasis. Annu. Rev. Pathol. 7:385–422. 10.1146/annurev-pathol-011811-132448 [DOI] [PubMed] [Google Scholar]
- Pfeifle R., Rothe T., Ipseiz N., Scherer H.U., Culemann S., Harre U., Ackermann J.A., Seefried M., Kleyer A., Uderhardt S., et al. . 2017. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18:104–113. 10.1038/ni.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael I., Nalawade S., Eagar T.N., and Forsthuber T.G.. 2015. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 74:5–17. 10.1016/j.cyto.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K., Papp K.A., Blauvelt A., Tyring S.K., Sinclair R., Thaçi D., Nograles K., Mehta A., Cichanowitz N., Li Q., et al. . 2017. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 390:276–288. 10.1016/S0140-6736(17)31279-5 [DOI] [PubMed] [Google Scholar]
- Ritchlin C.T., Colbert R.A., and Gladman D.D.. 2017. Psoriatic Arthritis. N. Engl. J. Med. 376:957–970. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M.F., Mattéi M.G., Denizot F., and Golstein P.. 1993. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 150:5445–5456. [PubMed] [Google Scholar]
- Saeki H., Kabashima K., Tokura Y., Murata Y., Shiraishi A., Tamamura R., Randazzo B., and Imanaka K.. 2017. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br. J. Dermatol. 177:419–427. 10.1111/bjd.15493 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Zielinski C.E., and Lanzavecchia A.. 2012. Human Th17 subsets. Eur. J. Immunol. 42:2215–2220. 10.1002/eji.201242741 [DOI] [PubMed] [Google Scholar]
- Sands B.E., Chen J., Feagan B.G., Penney M., Rees W.A., Danese S., Higgins P.D.R., Newbold P., Faggioni R., Patra K., et al. . 2017. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology. 153:77–86.e6. 10.1053/j.gastro.2017.03.049 [DOI] [PubMed] [Google Scholar]
- Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., Tanaka S., Kodama T., Akira S., Iwakura Y., et al. . 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203:2673–2682. 10.1084/jem.20061775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunte D.M., Mrowietz U., Puig L., and Zachariae C.. 2017. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 177:47–62. 10.1111/bjd.15015 [DOI] [PubMed] [Google Scholar]
- Sawyer L.M., Cornic L., Levin L.Å., Gibbons C., Møller A.H., and Jemec G.B.. 2019. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J. Eur. Acad. Dermatol. Venereol. 33:355–366. 10.1111/jdv.15277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel P.M., Steiert I., Kötter I., and Müller C.A.. 2013. B cells contribute to heterogeneity of IL-17 producing cells in rheumatoid arthritis and healthy controls. PLoS One. 8:e82580 10.1371/journal.pone.0082580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Colombel J.-F., Feagan B.G., Reich K., Deodhar A.A., McInnes I.B., Porter B., Das Gupta A., Pricop L., and Fox T.. 2019. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 78:473–479. 10.1136/annrheumdis-2018-214273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.L., Wolfe F., and Huizinga T.W.J.. 2010. Rheumatoid arthritis. Lancet. 376:1094–1108. 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- Segal B.M., Constantinescu C.S., Raychaudhuri A., Kim L., Fidelus-Gort R., and Kasper L.H.. Ustekinumab MS Investigators . 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7:796–804. 10.1016/S1474-4422(08)70173-X [DOI] [PubMed] [Google Scholar]
- Shalom-Barak T., Quach J., and Lotz M.. 1998. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J. Biol. Chem. 273:27467–27473. 10.1074/jbc.273.42.27467 [DOI] [PubMed] [Google Scholar]
- Shen F., Ruddy M.J., Plamondon P., and Gaffen S.L.. 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-α-induced genes in bone cells. J. Leukoc. Biol. 77:388–399. 10.1189/jlb.0904490 [DOI] [PubMed] [Google Scholar]
- Smolen J.S., Agarwal S.K., Ilivanova E., Xu X.L., Miao Y., Zhuang Y., Nnane I., Radziszewski W., Greenspan A., Beutler A., and Baker D.. 2017. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 76:831–839. 10.1136/annrheumdis-2016-209831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Luo L., Lei Z., Li B., Liang Z., Liu G., Li D., Zhang G., Huang B., and Feng Z.-H.. 2008. IL-17-Producing Alveolar Macrophages Mediate Allergic Lung Inflammation Related to Asthma. J. Immunol. 181:6117–6124. 10.4049/jimmunol.181.9.6117 [DOI] [PubMed] [Google Scholar]
- Song X., Dai D., He X., Zhu S., Yao Y., Gao H., Wang J., Qu F., Qiu J., Wang H., et al. . 2015. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 43:488–501. 10.1016/j.immuni.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Sparber F., De Gregorio C., Steckholzer S., Ferreira F.M., Dolowschiak T., Ruchti F., Kirchner F.R., Mertens S., Prinz I., Joller N., et al. . 2019. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe. 25:389–403.e6. 10.1016/j.chom.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Spath S., Komuczki J., Hermann M., Pelczar P., Mair F., Schreiner B., and Becher B.. 2017. Dysregulation of the Cytokine GM-CSF Induces Spontaneous Phagocyte Invasion and Immunopathology in the Central Nervous System. Immunity. 46:245–260. 10.1016/j.immuni.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., and Mills K.H.G.. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341. 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L.A., Lubberts E., van den Berg W.B., and Libert C.. 2008. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205:1755–1761. 10.1084/jem.20080588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamassia N., Arruda-Silva F., Calzetti F., Lonardi S., Gasperini S., Gardiman E., Bianchetto-Aguilera F., Gatta L.B., Girolomoni G., Mantovani A., et al. . 2018. A reappraisal on the potential ability of human neutrophils to express and produce IL-17 family members in vitro: Failure to reproducibly detect it. Front. Immunol. 9:795 10.3389/fimmu.2018.00795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Kakuta S., Shimizu K., Kadoki M., Kamiya T., Shimazu T., Kubo S., Saijo S., Ishigame H., Nakae S., and Iwakura Y.. 2018. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat. Immunol. 19:755–765. 10.1038/s41590-018-0134-y [DOI] [PubMed] [Google Scholar]
- Targan S.R., Feagan B., Vermeire S., Panaccione R., Melmed G.Y., Landers C., Li D., Russell C., Newmark R., Zhang N., et al. . 2016. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am. J. Gastroenterol. 111:1599–1607. 10.1038/ajg.2016.298 [DOI] [PubMed] [Google Scholar]
- Tonel G., Conrad C., Laggner U., Di Meglio P., Grys K., McClanahan T.K., Blumenschein W.M., Qin J.-Z., Xin H., Oldham E., et al. . 2010. Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 185:5688–5691. 10.4049/jimmunol.1001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., and Fugger L.. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172:146–155. 10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J.S.A., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.-M., Florencia E., Prens E.P., and Lubberts E.. 2009. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182:5836–5845. 10.4049/jimmunol.0802999 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Cheng-Chung Wei J., Dougados M., Mease P., Deodhar A., Maksymowych W.P., Van den Bosch F., Sieper J., Tomita T., Landewé R., et al. COAST-V study group . 2018. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 392:2441–2451. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- van Tok M.N., Na S., Lao C.R., Alvi M., Pots D., van de Sande M.G.H., Taurog J.D., Sedgwick J.D., Baeten D.L., and van Duivenvoorde L.M.. 2018. The Initiation, but Not the Persistence, of Experimental Spondyloarthritis Is Dependent on Interleukin-23 Signaling. Front. Immunol. 9:1550 10.3389/fimmu.2018.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Tello A., Halwani R., Li R., Nadigel J., Bar-Or A., Mazer B.D., Eidelman D.H., Al-Muhsen S., and Hamid Q.. 2012. IL-17A and IL-17F expression in B lymphocytes. Int. Arch. Allergy Immunol. 157:406–416. 10.1159/000329527 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., and Stockinger B.. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Villani A.P., Rouzaud M., Sevrain M., Barnetche T., Paul C., Richard M.A., Beylot-Barry M., Misery L., Joly P., Le Maitre M., et al. . 2015. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 73:242–248. 10.1016/j.jaad.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Vollmer T.L., Wynn D.R., Alam M.S., and Valdes J.. 2011. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Mult. Scler. 17:181–191. 10.1177/1352458510384496 [DOI] [PubMed] [Google Scholar]
- Wain T., Choy B., Satchell A.C., Woods J.A., and Frew J.W.. 2018. Secukinumab in pityriasis rubra pilaris: A case series demonstrating variable response and the need for minimal clinical datasets. JAAD Case Rep. 4:500–505. 10.1016/j.jdcr.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.F., Guo Y., Quazi A., Luxenberg D.P., Bennett F., Ross J.F., Qiu Y., Whitters M.J., Tomkinson K.N., Dunussi-Joannopoulos K., et al. . 2007. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282:13447–13455. 10.1074/jbc.M700499200 [DOI] [PubMed] [Google Scholar]
- Wu H.-J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., and Mathis D.. 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 32:815–827. 10.1016/j.immuni.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., and Cao X.. 2010. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 7:164–174. 10.1038/cmi.2010.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Nakajima K., Takaishi M., Kitaba S., Magata Y., Kataoka S., and Sano S.. 2015. Psoriatic inflammation facilitates the onset of arthritis in a mouse model. J. Invest. Dermatol. 135:445–453. 10.1038/jid.2014.426 [DOI] [PubMed] [Google Scholar]
- Yang Y., Weiner J., Liu Y., Smith A.J., Huss D.J., Winger R., Peng H., Cravens P.D., Racke M.K., and Lovett-Racke A.E.. 2009. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206:1549–1564. 10.1084/jem.20082584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., and Spriggs M.K.. 1995a Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 3:811–821. 10.1016/1074-7613(95)90070-5 [DOI] [PubMed] [Google Scholar]
- Yao Z., Painter S.L., Fanslow W.C., Ulrich D., Macduff B.M., Spriggs M.K., and Armitage R.J.. 1995b Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155:5483–5486. [PubMed] [Google Scholar]
- Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., et al. . 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316. 10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenz R., Eferl R., Kenner L., Florin L., Hummerich L., Mehic D., Scheuch H., Angel P., Tschachler E., and Wagner E.F.. 2005. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 437:369–375. 10.1038/nature03963 [DOI] [PubMed] [Google Scholar]
- Zhang L.-W., Zhou P.-R., Wei P., Cong X., Wu L.-L., and Hua H.. 2018. Expression of interleukin-17 in primary Sjögren’s syndrome and the correlation with disease severity: A systematic review and meta-analysis. Scand. J. Immunol. 87:e12649 10.1111/sji.12649 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., and Ouyang W.. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- Ziolkowska M., Koc A., Luszczykiewicz G., Ksiezopolska-Pietrzak K., Klimczak E., Chwalinska-Sadowska H., and Maslinski W.. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832–2838. 10.4049/jimmunol.164.5.2832 [DOI] [PubMed] [Google Scholar]