IL-15 supports NK, NK-T, γδ, ILC1, and memory CD8 T cell function, and dysregulated IL-15 is associated with many autoimmune diseases. Striking IL-15–driven increases in NK and CD8 T cells in patients highlight the potential for combination therapy of cancers.
Abstract
IL-15, a pleiotropic cytokine, stimulates generation of NK, NK-T, γδ, ILC1, and memory CD8 T cells. IL-15 disorders play pathogenetic roles in organ-specific autoimmune diseases including celiac disease. Diverse approaches are developed to block IL-15 action. IL-15 administered to patients with malignancy yielded dramatic increases in NK numbers and modest increases in CD8 T cells. Due to immunological checkpoints, to achieve major cancer therapeutic efficacy, IL-15 will be used in combination therapy, and combination trials with checkpoint inhibitors, with anti-CD40 to yield tumor-specific CD8 T cells, and with anticancer monoclonal antibodies to increase ADCC and antitumor efficacy, have been initiated.
Introduction
IL-15 is a 14–15-kD 4-α helix bundle family cytokine member (Waldmann and Tagaya, 2000; Fehniger and Caligiuri, 2001). 25 yr ago, IL-15 was identified by our group and that of Grabstein in culture supernatants from two cell lines (Cv-1/EBNA and HuT-102) that stimulated proliferation of the cytokine-dependent T cell line CTLL-2 in the presence of anti–IL-2 antibodies (Bamford et al., 1994; Burton et al., 1994; Grabstein et al., 1994). Since that discovery, there have been >6,000 papers and >170 clinical trials involving IL-15, which are beyond the scope of this review and extensively covered in numerous reviews (Tagaya et al., 1996; Waldmann 2003, 2006, 2014, 2015, 2018; Waldmann et al., 1998, 2001; Fehniger and Caligiuri, 2001; Fehniger et al., 2002; Lodolce et al., 2002; Becknell and Caligiuri, 2005; Ma et al., 2006; Overwijk and Schluns, 2009; Rochman et al., 2009; Jakobisiak et al., 2011; Steel et al., 2012; Mishra et al., 2014; Anthony and Schluns, 2015; Pilipow et al., 2015; Patidar et al., 2016; Chłopek et al., 2017; Robinson and Schluns, 2017; Lin and Leonard, 2018). Rather, we present a discussion of compelling topics that focuses on IL-15 in the pathogenesis of autoimmune disorders and select malignancies and analyzes approaches to block disordered IL-15 actions. The second theme presented focuses on immunostimulatory aspects and translation of the dramatic effects of IL-15 on natural killer (NK) and CD8 T cells generation and function in development of rational combination therapies for cancer.
IL-15 mRNA is expressed by many tissues; however, IL-15 protein is largely limited to monocytes, macrophages, and dendritic cells (Bamford et al., 1996a). Although some regulation of IL-15 protein production occurs with transcription, most control of expression is at translation (Bamford et al., 1996a, 1996b). Transcription of IL-15 is stimulated by type I and II interferons, CD40 ligation, and TLR stimuli. IL-15 translation is impeded by multiple 5′-untranslated region AUG sequences, a long signal peptide, and a negative regulatory element in the coding sequence C-terminus (Bamford et al., 1996b). Tight regulation of IL-15 expression is required because of its potency as an inflammatory cytokine. The heterotrimeric IL-15 receptor is composed of common gamma chain (γc) subunit (CD132) shared with IL-2, IL-4, IL-7, IL-9, and IL-21; β chain (βc) subunit (IL-2R/IL-15R, CD122) shared with the IL-2 receptor; and a private IL-15–specific α subunit IL-15Rα (CD215; Fehniger and Caligiuri, 2001; Waldmann, 2006). IL-15 binding to IL-2/IL-15Rβ/γc heterodimer induces JAK1 activation that phosphorylates STAT3 via the β chain and JAK3 that phosphorylates STAT5 (STAT5A, STAT5B) via the γ chain (Fig. 1; Mishra et al., 2014).
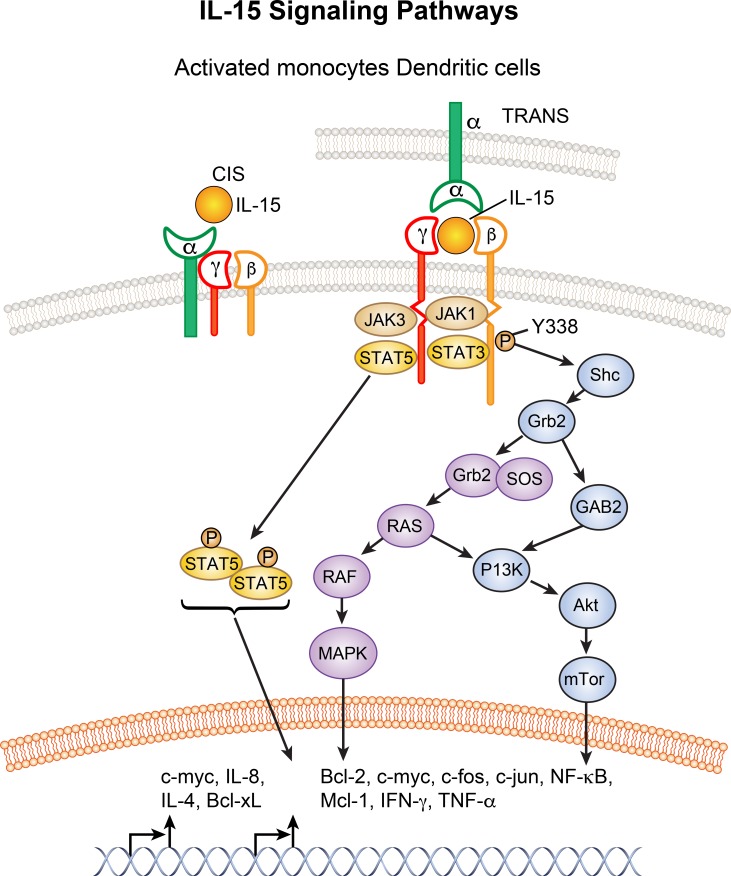
Figure 1.
IL-15 signaling pathways. IL-15, in one scenario at left, binds to the heterotrimeric receptor in cis (Zanoni et al., 2013; Mishra et al., 2014). At right, IL-15 binds to the high-affinity IL-15Rα subunit expressed on antigen-presenting cells and is presented in trans to IL-2/IL-15Rβγ heterodimers on NK or CD8 T cells (Dubois et al., 2002). Activation proceeds via three pathways. The first involves JAK1/3/STAT3/5 activation, with phosphorylated STAT proteins forming dimers trafficking to the nucleus for transcriptional activation. In the second IL-15 pathway, adaptor protein Shc binds to a phosphotyrosine residue on IL-2/IL-15Rβ, resulting in activation of the Shc, Grb2, GAB2, P13K, and AkT signaling pathway (Mishra et al., 2014). In the third pathway, IL-15 signaling is associated with activation of Grb2 and SOS to form a Grb2/SOS complex that activates the RAS-RAF MAPK pathway involved in cellular proliferation. Collectively, these signaling pathways induce expression and activation of c-Myc, c-Fos, c-Jun, Bcl-2, Bcl-xL, Mcl-1, NF-κB, and TNFα (modified from Mishra et al., 2014).
IL-15 and IL-2 have several common functions including facilitating development of NK cells that reflect their sharing of receptor components IL-2/IL-15Rβ and γc and their use of common JAK1/3 and STAT3/5 signaling (Carson et al., 1997; Geginat et al., 2003; Farag and Caligiuri, 2006; Waldmann, 2006; Huntington, 2014). However, NK cell development is fairly normal in the absence of IL-2, whereas IL-15 is required for normal NK development. There are also distinctions between IL-2 and IL-15 in adaptive immune responses. IL-2 acts as a growth factor during initiation of immune responses but also has crucial roles in preventing immunity to self by termination of T cell immune responses by activation-induced cell death (AICD) and by action of regulatory T cells (T reg cells; Lenardo et al., 1999; Snow et al., 2010; Sakaguchi, 2011).
In contrast, IL-15 has no major net effect on maintenance of T reg cell fitness. IL-15 provides a sustained immune response to invading pathogens by being an antiapoptotic factor; in particular, in IL-15 transgenic mice, IL-2–induced AICD is inhibited (Marks-Konczalik et al., 2000). In addition, IL-15 promotes maintenance of CD44hi CD8 T cell memory phenotype cells and renewal of viral specific memory CD8 T cells (Becker et al., 2002; Burkett et al., 2003; Schluns and Lefrançois, 2003; Munks et al., 2006; Purton et al., 2007; Boyman et al., 2009; Burrack et al., 2018). Furthermore, IL-15 is critical in development of tissue memory phenotype CD103+CD28−CD8+ T cells (Mackay et al., 2013; Mayassi and Jabri, 2018) and for the maintenance of CD4+ T cells, CD8αα (+) intraepithelial lymphocytes (Kwong and Lazarevic, 2014; Klose et al., 2014), and ILC1, 2, and 3 innate lymphoid cells (Robinette et al., 2017; Fuchs et al., 2013), and innate cells that express CD103+, CD56+, and CD44+ (Sciumè et al., 2017).
IL-2 is a predominantly secreted molecule with wider effects, whereas IL-15 is secreted only in small quantities; membrane-bound IL-15 induces signaling in cell–cell contact at an immunological synapse. IL-15 and IL-15Rα coexpressed by monocytes and dendritic cells become associated on cell surfaces where IL-15 is presented in trans to NK and CD8 memory T cells (Dubois et al., 2002; Kobayashi et al., 2005; Lucas et al., 2007; Huntington et al., 2009; Castillo and Schluns, 2012). In addition, Zanoni et al. (2013) demonstrated that IL-15 cis presentation is required for optimal NK cell activation in LPS-mediated inflammatory conditions.
IL-15 in autoimmune diseases
IL-15 is a proinflammatory cytokine that McInnes et al. (1996) suggested is at the apex of the proinflammatory cytokine cascade preceding expression of TNFα and inflammatory cytokines. In an elegant opinion article, Jabri and Abadie (2015) proposed that IL-15 functions as a danger signal to regulate tissue-resident T cells and limit tissue destruction. IL-15 contributes to tissue protection by promoting elimination of infected cells, but chronically dysregulated IL-15 promotes organ-specific autoimmune diseases. IL-15 is constitutively up-regulated in a wide variety of autoimmune diseases: rheumatoid arthritis (Harada et al., 1999; Benito-Miguel et al., 2009), multiple sclerosis (Vaknin-Dembinsky et al., 2008; Rentzos and Rombos, 2012), systemic lupus erythematosus (Aringer et al., 2001; Robak et al., 2002), alopecia areata (Xing et al., 2014), vitiligo (Richmond et al., 2018), psoriasis (Villadsen et al., 2003; Bouchaud et al., 2013), type 1 diabetes (Chen et al., 2013), and celiac disease (Abadie and Jabri, 2014; Korneychuk et al., 2014; Meresse et al., 2015). Disordered expression of IL-15 by resident cells is often associated with up-regulated expression of ligands for activating NK receptors including NKG2D. The severity of rheumatoid arthritis was related to IL-15–induced expansion of CD4+ CD28− T cells expressing NKG2D and stress-induced MHC class I–related chain ligands on rheumatoid synoviocytes (Baslund et al., 2005). An antibody to IL-2Rβ that targets IL-15 and IL-2 was effective in a preclinical model of vitiligo (Richmond, et al., 2018). Celiac disease is characterized by induction of NKG2D ligands and HLA-E (Yokoyama et al., 2009; Abadie and Jabri, 2014; Ettersperger et al., 2016). IL-15 promotes expansion of intestinal intraepithelial lymphocytes, which have cytotoxic activity and kill epithelial cells. Alopecia areata was shown to be driven by cytotoxic CD8 T cells that express NKG2D responding to IL-15 (Xing et al., 2014). Administration of ruxolitinib, a JAK1 and JAK2 inhibitor, to patients with alopecia areata restored near complete hair growth, supporting IL-15’s pathogenic role (Mackay-Wiggan et al., 2016). To investigate the role of IL-15/IL-15Rα in the pathogenesis of type 1 diabetes, we generated double transgenic mice with pancreatic β cells expressing IL-15 and IL-15Rα (Chen et al., 2013). The mice developed hyperglycemia, mononuclear infiltration, β cell destruction, and anti-insulin autoantibodies mimicking early human type 1 diabetes, and hyperglycemia was reversed by inhibiting IL-15 signaling with anti-IL-2/IL-15Rβ (anti-CD122) or pan JAK inhibitor tofacitinib. We demonstrated data supporting IL-15’s role in human type 1 diabetes showing pancreatic β cell expression of IL-15 and IL-15Rα in diabetic individuals but not in control subjects (Chen et al., 2013). Abadie and Jabri (Abadie and Jabri 2014; Jabri and Abadie, 2015) propose that two signals are required for tissue destruction in type 1 diabetes and celiac disease. The latent potential for autoimmune diseases like celiac disease and diabetes in adults are characterized by dysregulated immune responses to gluten and β islet cell antigens, respectively, with preservation of functional tissue. IL-15 up-regulation is absent in intestinal epithelium and β islet cells of these patients, which supports the hypothesis that tissue disruption by cytotoxic T cells requires IL-15 signals to license them to kill target cells (Jabri and Abadie, 2015).
IL-15 plays a role in diverse T cell malignancies
IL-15 transgenic mice develop fatal lymphocytic leukemias with CD8 phenotype and with NK surface markers (Fehniger et al., 2001; Sato et al., 2011). IL-15 treatment up-regulates NK markers in both mice and humans.
Retrovirus HTLV-1 infection results in adult T cell leukemia (ATL), a leukemia of T reg cells in 2–5% of infected individuals (Cook et al., 2019). HTLV-1 associated protein Tax transactivates two autocrine (IL-2/IL-2R and IL-15/IL-15R) and one paracrine (IL-9) pathway, yielding activating JAK1/3 and STAT3/5, resulting in spontaneous leukemic cell proliferation that is inhibitable ex vivo by anti-cytokine antibodies or JAK inhibitors (Chen et al., 2008; Ju et al., 2011), which is being evaluated in a clinical trial by our group (NCT01712659) with the JAK inhibitor ruxolitinib. Döbbeling et al. (1998) demonstrated that IL-15 acts as a growth and viability factor in cutaneous T cell lymphoma (CTCL). Mishra and coworkers (Mishra et al., 2012; Mishra et al., 2016) showed that ZEB1 is a transcriptional repressor of IL-15 in T cells and that in CTCL, hypermethylation of the ZEB1 binding region within the IL-15 promoter prevented ZEB1 binding and caused increased IL-15 transcription.
IL-15Rα/IL-15 levels were elevated in T cell large granular lymphocytic (LGL) leukemia (Chen et al., 2012), and a model of survival signaling in T cell LGL leukemia suggested that IL-15 and platelet-derived growth factor are sufficient to reproduce deregulations in T cell LGL leukemia (Zhang et al., 2008).
Therapeutic targeting of IL-15, its receptor, or signaling pathway
IL-15–inhibiting agents developed include soluble IL-15Rα, mutant IL-15 molecules (Kim et al., 1998; Ferrari-Lacraz et al., 2004; Zhao et al., 2016), anti-IL-15 or IL-2/IL-15Rβ antibodies (Morris et al., 2006; Waldmann et al., 2013; Sestak et al., 2018), and modifications of IL-2 that block IL-15 interaction with its receptor (Mitra et al., 2015; Nata et al., 2015), as well as JAK inhibitors (Zhang et al., 2015; Fig. 2).
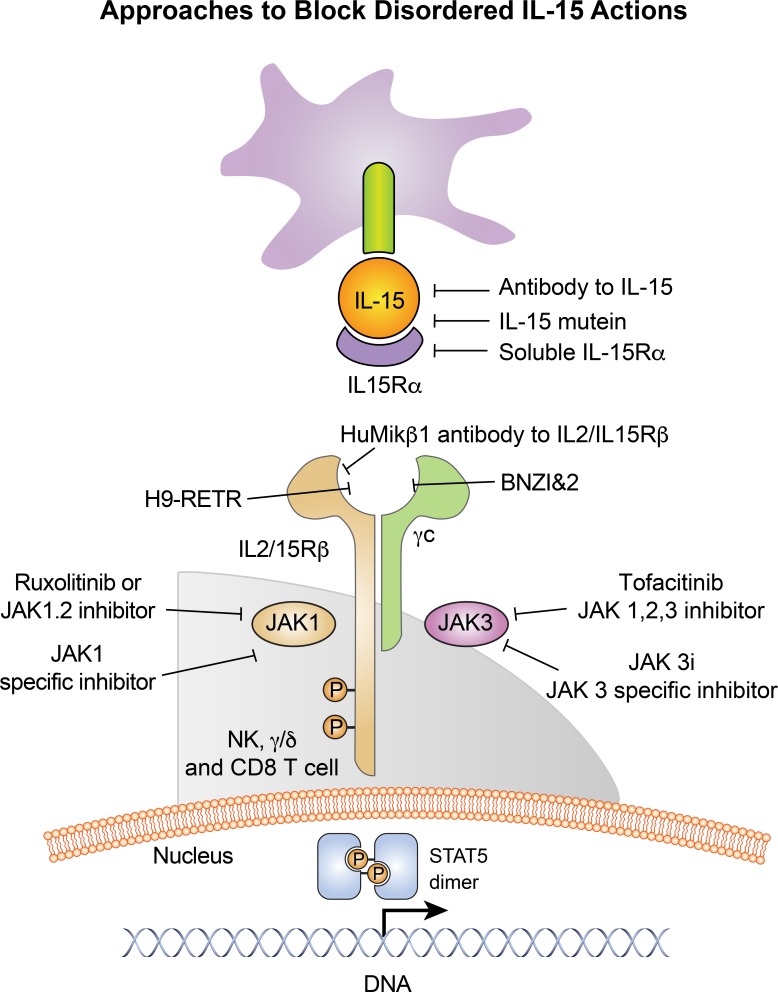
Figure 2.
Approaches to block disordered IL-15 actions. Several agents that inhibit IL-15 activity were developed including soluble IL-15Rα, IL-15 mutein (Kim et al., 1998), antibodies specific for IL-15 or IL-2/IL-15Rβ, mutant modifications of IL-2 (BNZ-1 and 2, H9-RETR), to block IL-15 interaction with its receptors (Mitra et al., 2015; Nata et al., 2015), and JAK inhibitors. IL-15 antagonists produced by mutating a glutamine residue to aspartic acid residue at the C-terminus of IL-15 increased survival of pancreatic islet cell allografts. Soluble high-affinity IL-15Rα prevented development of collagen-induced arthritis in mice. An antibody specific for IL-15 was effective in mouse models of autoimmune diseases. We developed humanized antibody (Hu-Mik-Beta-1) specific for IL-2/IL-15Rβ. This antibody blocked trans presentation of IL-15 by antigen-presenting cells to target NK cells and CD8 T cells and prolonged cardiac allograft survival in cynomolgus monkeys (Morris et al., 2006). Clinical studies with Hu-Mik-Beta-1 are underway in patients with refractory celiac disease (NCT01893775) and HTLV-1–associated tropical spastic paraparesis (NCT00076180).
Engineered modifications of IL-2 that block binding of IL-2 and IL-15 to IL-2/IL-15Rβ and γ chains, simultaneously inhibiting actions of both IL-2 and IL-15 (Mitra et al., 2015; Nata et al., 2015; Wang et al., 2019), were generated. The RETR mutant of H9 super IL-2 (denoted H9-RETR) binds to IL-2/IL-15Rβ but not γ chain. BNZ-1, a 24-aa agent analogous to shared elements of the D helix of IL-2 and IL-15, binds to γc and not to IL-2/IL-15Rβ. Both agents prevent IL-2- and IL-15–mediated heterodimerization of IL-2/15R β with γc, which is required for signaling.
IL-15 in the immunotherapy of cancer
Recombinant IL-2 was approved in 1992 by the US Food and Drug Administration (Rosenberg, 2014). However, although IL-2 is involved in cancer cell death by immune activation, it also suppresses immune responses by maintenance of CD25+Foxp3+ T reg cells and participates in AICD (Snow et al., 2010; Sakaguchi, 2011). Furthermore, IL-2 is associated with multiple serious side effects such as capillary leak syndrome, hypotension, hypoxia, and oliguric renal failure. These issues prompted the search for immunotherapeutics with benefits of IL-2 but fewer negative effects. Whereas IL-15 has immune enhancing properties like IL-2, it does not have major effects on T reg cells but suppresses AICD and has less capillary leak syndrome and vascular complications in mice, nonhuman primates, and patients. Multiple immunotherapy studies in murine models indicated that IL-15 may be valuable in therapy of neoplasia (Brentjens et al., 2003; Munger et al., 1995; Klebanoff et al., 2004; Villinger et al., 2004; Teague et al., 2006). 10-d 20 µg/kg/d administration of IL-15 to rhesus macaques by continuous i.v. infusion (CIV) was associated with 10-fold increases in the number of circulating NK cells and 80–100-fold increases in the number of circulating effector memory CD8 T cells (Sneller et al., 2011; Waldmann et al., 2011).
Clinical trials using IL-15 in the treatment of cancer
Over 170 clinical trials have been initiated in treatment of cancer using different IL-15 preparations. The results of six of these trials have been published in referenced journals (Table 1). We initiated a first-in-human phase I trial of recombinant IL-15 administered by i.v. bolus daily for 12 d to adults with metastatic malignancy (Conlon et al., 2015). This study started with an initial dose of 3 µg/kg/d. However, after initial patients developed grade 3 hypotension and thrombocytopenia, doses of 1.0 and 0.3 µg/kg/d were added. All nine patients at the 0.3 µg/kg/d dose level received 12 doses without dose-limiting toxicity.
Table 1. IL-15 clinical trials in patients with metastatic malignancy.
| IL-15 agent | MTD or expansion dose/dosing schedule | Study population | Serious and notable adverse events | Maximum fold increase in total NK cells at MTD | Maximum fold increase in CD56bright NK cells | Maximum fold increase in CD8 T cells | Best clinical response | References |
|---|---|---|---|---|---|---|---|---|
| E. coli rhIL-15 | 0.3 µg/kg/d bolus i.v. for 12 d consecutively | 18 patients with malignant melanoma or renal cell cancer | Grade 3 hypotension; grade 3 thrombocytopenia; grade 3 ALT and AST elevations | 2–3 | 3–4 | 3 | Stable disease (five patients had 10–30% decrease in marker lesions and two had disappearance of lung lesions) | Conlon et al., 2015 |
| E. coli rhIL-15 | 2 µg/kg/d CIV for 10 d | 27 patients with metastatic solid tumors | Two deaths (one due to gastrointestinal ischemia and one due to disease progression); grade 3 bleeding; grade 3 papilledema; grade 3 uveitis; grade 3 hepatic encephalopathy | 38 | 358 | 5.8 | Stable disease | Conlon et al., 2019 |
| E. coli rhIL-15 | 2 µg/kg/d s.c. on days 1–5 and 8–12 | 19 patients with advanced solid tumors | Grade 2 pancreatitis; grade 3 cardiac/chest pain | 10.8 | 39.7 | 3.3 | Stable disease | Miller et al., 2018 |
| ALT-803 | 10 µg/kg i.v. or s.c. weekly for 4 wk | 33 patients with hematological malignancies | Two deaths (one due to sepsis, one due to intracranial hemorrhage); grade 4 sepsis; grade 2 pemphigus | 8 | 8 | 2 | 1 CR, 1 PR, 3 SD | Romee et al., 2018 |
| ALT-803 | 20 µg/kg s.c. for 4 wk consecutively every 6 wk | 24 patients (11 i.v., 13 s.c.) with solid tumors | Grade 4 congestive heart failure; grade 4 neutropenia; injection site reaction | 3.3 | 6.3 | 1.3 | No PR or CR | Margolin et al., 2018 |
| ALT-803 + nivolumab | 20 µg/kg ALT-803 s.c. combination with i.v. nivolumab every 2 wk | 21 patients with metastatic non-small cell lung cancer | Grade 3 myocardial infarction; injection site reaction | 3 | NA | Minor response | 6 PR, 10 SD | Wrangle et al., 2018 |
CR, complete response; NA, not available; PR, partial response; SD, stable disease.
Flow cytometry revealed a 10-fold increase in absolute NK cell numbers with the 3 µg/kg/d dose level, as well as significant increases in circulating CD8 T and γδ T cell numbers. The best response was stable disease. Inflammatory cytokines IL-6 and IFNγ were markedly elevated, which coincided with acute clinical toxicities of fever, chills, and blood pressure changes. To reduce toxicity with the goal of reducing maximum serum concentration, excess cytokine release, and macrophage activation syndrome, two additional clinical trials were initiated. One involved subcutaneous recombinant human IL-15 (rhIL-15) given daily five times a week for 2 wk consecutively (Miller et al., 2018). There were two serious adverse events among 19 patients treated: grade 2 pancreatitis, and grade 3 cardiac chest pain, hypotension, and elevated troponin (a dose-limiting toxicity). No objective responses were observed; however, several patients had disease stabilization. The treatment induced expansion of circulating NK cells, especially CD56bright cells (Fig. 3). There was a proportional but less dramatic increase of CD8+ T cells.
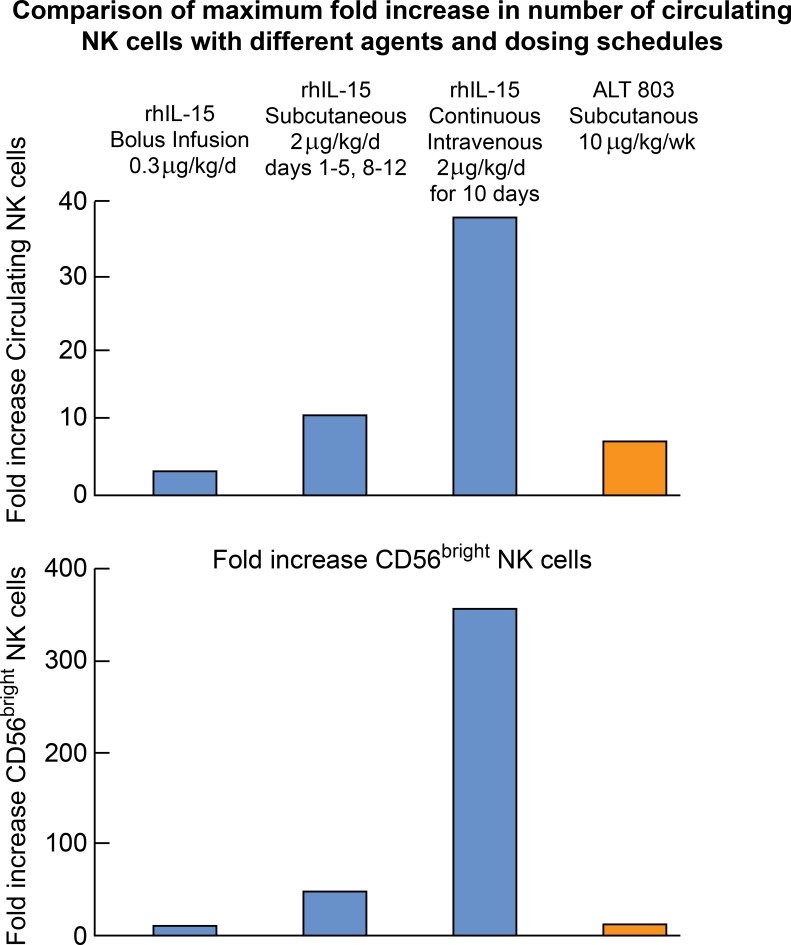
Figure 3.
Comparison of maximum fold increase in number of circulating NK cells with different agents and dosing schedules. rhIL-15 administered by bolus infusions at the MTD (0.3 μg/kg/d) yielded only a two to threefold increase in NK cells (Conlon et al., 2015). rhIL-15 administered s.c. at the expansion dose of 2 μg/kg/d on days 1–5 and 8–12 produced a 10.8-fold increase in total NK cells and a 39.7-fold increase in CD56bright NK cells (Miller et al., 2018). ALT-803 mutant (IL-15/IL-15Rα/IgFc) at 10 μg/kg/wk elicited an eightfold increase in NK cells (Romee et al., 2018). rhIL-15 by CIV at 2 μg/kg/d for 10 d resulted in the greatest increase, with a 38-fold increase in circulating total NK cells and a 358-fold increase in CD56bright NK cells (Conlon et al., 2019).
In a parallel trial, we administered rhIL-15 to patients with metastatic malignancy by CIV for 10 d or in a subsequent trial for 5 d (Conlon et al., 2019). We observed two deaths at 4.0 µg/kg/d, one disease progression, and a grade 5 visceral arterial ischemia. An expansion cohort of nine patients was subsequently treated at maximum tolerated dose of 2.0 µg/kg/d. In the CIV trial following maximum serum concentration at 48 h, there was a gradual decline of serum IL-15 concentration to 8% of the maximum level by days 8–10 of the IL-15 infusions. This decline may reflect induction of IL-15 receptor–bearing cells and an increase in the number of IL-2/IL-15Rβ (CD122) receptors per cell that acted as a sink binding rhIL-15. Within 1–3 d of CIV infusion initiation, there was a profound decline in the number of circulating NK and CD8 memory phenotype T cells, followed by a gradual increase until termination of infusions. During 1–3 d following termination of infusions, there was a 38-fold increase in the number of circulating NK cells and a 358-fold increase in the number of circulating CD56bright NK cells (Fig. 3). Studying purified NK cells in vitro, Felices et al. (2018) suggested that continuous treatment with IL-15 exhausts purified NK cells, resulting in decreased viability and a cell cycle arrest gene expression pattern. Furthermore, they proposed that their findings should inform IL-15 dosing strategies. Our studies with IL-15 in vivo by CIV to humans do not support these conclusions (Dubois et al., 2017; Conlon et al., 2019). The proliferation rate of different subsets of NK cells at termination of 10-d IL-15 CIV assayed by Ki67 was >90%. The cytolytic capacity of the CD56bright NK subset was very effective. In particular, lytic activity was markedly increased by IL-15 CIV, including antibody-dependent cellular cytotoxicity (ADCC) assessed with CD20 antibody-coated Raji cells, natural cytotoxicity to K562 cells mediated by NKp30 and NKp46, and MICA/NKG2D-mediated cytotoxicity (Dubois et al., 2017). These observations on the effects of IL-15 on NK subsets support the view that after 5- or 10-d rhIL-15 CIV, NK cells remain effective and do not support the hypothesis that such strategies would be associated with NK cell exhaustion.
Although rhIL-15 may show efficacy in metastatic malignancy, a particular challenge is that it has a short in vivo survival. Indeed, true IL-15 may not be an IL-15 monomer but may rather be an IL-15Rα/IL-15 heterodimer (Dubois et al., 2008; Bergamaschi et al., 2012). Therefore, an array of IL-15 agents with IL-15Rα were introduced clinically (Fig. 4).
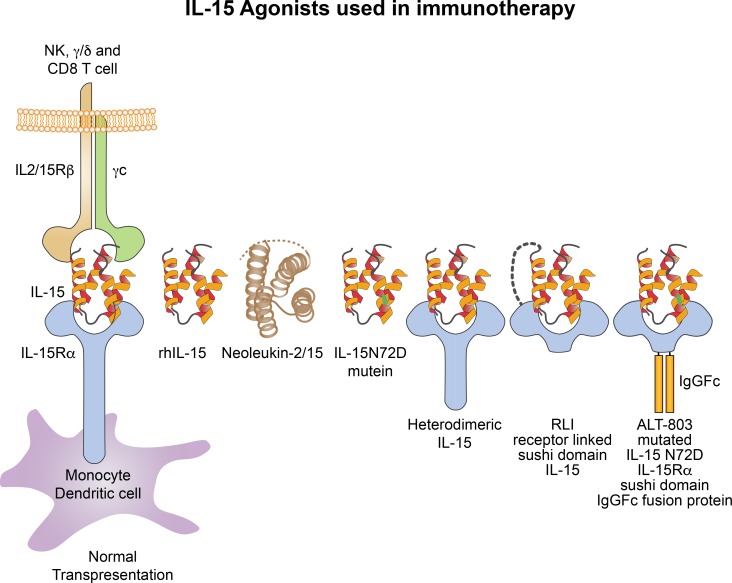
Figure 4.
IL-15 agonists used in immunotherapy. IL-15 preparations in clinical use include rhIL-15 produced in Escherichia coli (Conlon et al., 2015, 2019), an IL-15N72D mutein with a four to fivefold increase in biological activity (Zhu et al., 2009), and heterodimeric mammalian IL-15 (Chertova et al., 2013; Bergamaschi et al., 2008, 2012, 2018). Anti-CD20-RLI (Cytune Pharma) is a fusion protein consisting of IL-15 linked to the cytokine-binding (sushi) domain of IL-15Rα (Vincent et al., 2014). ALT-803 (Altor Pharmaceutical) represents a mutated (N72D) IL-15 (asparagine replacing aspartic residue) linked to the sushi domain of IL-15Rα that is fused to an IgG-Fc fragment to increase in vivo survival (Liu et al., 2016; Furuya et al., 2019; Chen, X., et al. 2015. 30th Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer. Abstr. P347). Not shown, ALT-803 scaffold has been fused to four single chains of the tumor-targeting monoclonal antibody rituximab (Schmohl et al., 2016). This ALT-803 monoclonal fusion protein prolonged survival in murine models of cancer.
ALT-803 (IL-15 mutant/IL-15Rα/Ig1fusion protein) was administered i.v. or s.c. to 33 patients with hematological malignancies once weekly for four doses (Romee et al., 2018), and pharmacokinetic analysis revealed prolonged serum concentrations following s.c. compared with i.v. infusion. There were two deaths, one due to sepsis and one due to intracranial hemorrhage deemed to be unrelated to ALT-803. Administrations of ALT-803 or hetIL-15 (mammalian IL-15/IL-15Rα) by s.c. injection were associated with large (30 cm) erythematous plaques, mimicking cellulitis (Romee et al., 2018). The rash was associated with an infiltrate predominantly of CD56+ NKp46− γδ T cells. Development of this complication with both ALT-803 and hetIL-15 precluded further increases in IL-15 doses. As noted in Fig. 3, rhIL-15 by CIV yielded by far the greatest increases in total NK and CD56bright NK cells.
IL-15 in combination therapy
Although diverse forms of IL-15 monotherapy augment the number of NK and CD8 T cells, due to immunological checkpoints, IL-15 will have to be used in combination therapy if it is to become a major factor in the cancer therapy armamentarium (Evans et al., 1997; Romee et al., 2012; Ochoa et al., 2017a, 2017b; Johnson and Miller, 2018).
Agents to relieve checkpoints on the immune system to optimize IL-15 action
IL-15 is associated with expression of immune checkpoints, including the inhibitory cytokine IL-10, TIM3, and TIGIT, and expression of PD-1 on CD8 T cells (Yu et al., 2010, 2012). Furthermore, IL-15 is critical in maintenance of CD122+CD8+ T reg cells (Rifa’i et al., 2008). The combination of IL-15 superagonist with anti–PD-L1 therapy was more effective than either agent alone in murine tumor models (Kowalsky et al., 2018; Knudson et al., 2019; Zhao et al., 2019). Furthermore, Desbois et al. (2016) demonstrated that IL-15 trans-signaling with the receptor-linker-IL-15 (RLI) that consists of human IL-15 covalently linked to the human IL-15Rα sushi domain promotes effector/memory CD8+ T cell responses and enhances the antitumor activity of PD-1 antagonists. With ALT-803 in combination with nivolumab in patients with metastatic nonsmall cell lung cancer, 6 of 21 patients manifested an objective response (Wrangle et al., 2018).
To address checkpoints, we administered IL-15 in combination with antibodies to cytotoxic T lymphocyte antigen-4 (CTLA-4) and PD-L1 (Yu et al., 2012). In the CT26 or MC38 colon carcinoma or TRAMP-C2 prostatic cancer syngeneic tumor models, IL-15 alone provided modest antitumor activity. Addition of either anti-CTLA-4 or anti–PD-L1 alone in association with IL-15 did not increase efficacy. However, tumor-bearing mice receiving IL-15 in combination with both anti-checkpoint antibodies manifested a marked prolongation of survival. In translation, a phase I trial is initiated that involves IL-15 (rhIL-15) in combination with nivolumab and ipilimumab in refractory cancers (NCT 03388632).
Combination of IL-15 plus optimized agonistic anti-CD40
As noted above, in rhesus macaques, CIV of IL-15 at 20 µg/kg/d for 10 d led to an 80–100-fold increase in circulating effector memory CD8 T cells. rhIL-15 by CIV to humans led to a lower CD8 T cell response with an up to eightfold increase in the number of circulating activated CD38+, MHC Class II+ CD8+ T cells. However, there was little evidence that the CD8 T cells generated had antitumor activity. IL-15 induces a complex of intracellular regulatory suppressor of cytokine signaling (SOCS) agents (Alexander and Hilton 2004). High concentrations of IL-15 increase expression of CIS encoded by cytokine-inducible SH2-containing protein, a potent checkpoint of NK-mediated tumor immunity (Bottino et al., 2016; Delconte et al., 2016). Furthermore, SOCS1 attenuates IL-15 receptor signaling of CD8 thymocytes and CD8+ CD44high memory T lymphocytes (Ilangumaran et al., 2003a, 2003b; Calabrese et al., 2009). In addition, SOCS2 up-regulation following IL-15 stimulation enhances IL-15–primed human NK cell function via control of phosphorylated Pyk2 (Lee et al., 2010). Sckisel et al. (2015) demonstrated that administration of γc cytokines was often ineffective in cancer immunotherapy because it led to paralysis/depression of CD4 but not CD8 T cells that was mediated through transient expression of SOCS3, which inhibited the STAT5B signaling pathway (Alexander and Hilton, 2004; Sckisel et al., 2015). This paralysis of primary CD4 T cell helper activity prevented generation of tumor-specific CD8 T cells. The role of CD4 helper cell interaction with dendritic cells and CD8 T cells was shown to be alternatively mediated by CD40 agonists (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998). In our studies in the murine syngeneic tumor model, TRAMP-C2 treatment with either IL-15 or agonistic anti-CD40 antibody alone prolonged survival (Zhang et al., 2009, 2012). However, combination of IL-15 with agonistic anti-CD40 produced markedly additive effects when compared with either agent alone. IL-15 or anti-CD40 alone did not augment the number of tumor-specific CD8 T cells, whereas administration of a combination of IL-15 with anti-CD40 antibody yielded a 10-fold increase in the number of TRAMP-C2 tumor-specific Spas-/SCNC 9H tetramer-positive CD8 T cells. A clinical trial is being initiated using an optimized intralesional anti-CD40 FcγRII-binding antibody in combination with CIV rhIL-15 (Li and Ravetch, 2011; Dahan et al., 2016).
IL-15 in combination with anticancer monoclonal antibodies
rhIL-15 administration leads to increases in the number of activated NK cells. However, such increases alone were not sufficient to produce antitumor efficacy, likely because most tumors express self-MHC class I molecules that inhibit NK effector functions. Nevertheless, ex vivo cytokine (IL-12, -15, and -18)-induced memory-like natural killer cells exhibited clinical responses in five of nine evaluable patients with acute myeloid leukemia including four complete remissions (Robak et al., 2002; Berrien-Elliott et al., 2015; Romee et al., 2016). Furthermore, Cursons et al. (2019) demonstrated that patients with metastatic cutaneous melanoma have improved survival rates if their tumor shows evidence of NK cell infiltration. Furthermore, these survival effects were enhanced in tumors that show higher expression of genes that encode NK stimuli such as the cytokine IL-15. Their results provide evidence that NK cells play a role in the regulation of human tumors and highlight potential survival effects associated with increased NK cell activity.
IL-15 preparations have been reported to be of value in combination with in vivo administered monoclonal antibodies (Moga et al., 2011; Wrangle et al., 2018; Zhang et al., 2018; Chen, X., et al. 2015. 30th Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer. Abstr. P347). In particular, an engineered fusion protein linking a soluble form of human IL-15Rα sushi with an antibody demonstrated antitumor responses (Liu et al., 2016). Furthermore, there was enhancement of ADCC and anti-breast cancer efficacy of cetuximab by a chimeric protein encompassing human interleukin-15 (Roberti et al., 2012; Ochoa et al., 2017a, 2017b). In addition, IL-15 enhanced rituximab ADCC against chronic lymphocytic leukemia cells (Moga et al., 2011).
We investigated combination therapy of IL-15 with rituximab in a syngeneic mouse model of EL-4 lymphoma transfected with human CD20 and with alemtuzumab (CAMPATH-1H) in a xenograft model of human ATL (Zhang et al., 2018). IL-15 enhanced therapeutic efficacy of both antibodies. Both NK cells and macrophages were critical elements in the chain of interacting effectors involved in augmented ADCC and optimal therapeutic responses. These results provided the scientific basis for a phase I trial of IL-15 combined with alemtuzumab (anti-CD52) for patients with ATL (NCT02689453). Additional trials were initiated with chronic lymphocytic leukemia with rhIL-15 in combination with obinutuzumab (anti-CD20; NCT03759184) and avelumab (anti-PD-L1; NCT03905135).
Ethics
All studies were performed under the conditions of the University of Helsinki. All patients signed a written informed consent for participation in clinical studies. Clinical studies were approved by the Intramural Review Board of the National Cancer Institute. Animal studies were approved by the National Cancer Institute Animal Care and Use Committee.
Conclusions and future perspectives
Specific disorders of IL-15 play pathogenic roles in diverse organ-specific autoimmune disorders. With the alternative goal, IL-15 administration to patients with malignancy dramatically increased circulating NK cells and increased CD8 T cell numbers. However, with the exception of JAK inhibitors, none of the IL-15–related study agents directed toward autoimmunity or cancer has received US Food and Drug Administration approval. Therefore, it will be critical to translate outstanding opportunities suggested in animal models into clinical trials. In particular, many strategies to block IL-15 action for autoimmunity that were effective in models or with ex vivo spontaneously proliferating cytokine autocrine cells gave disappointing results in clinical trials. When evaluating efficacy in inhibiting IL-15 action in in vivo approaches, a valuable biomarker of sustained inhibition of IL-15 signaling is a dramatic reduction in the number of circulating NK cells. It is critical for an effective antibody to IL-15 that it inhibit IL-15 administered in vivo as well as in vitro. Two of three antibodies to IL-2 studied by Boyman et al. (2006) that functioned as inhibitors in vitro were superagonists in vivo. We propose that lack of a marked reduction of NK cells and relative failure of an antibody to IL-15 in treatment of celiac disease may reflect that this antibody was not effective at inhibiting IL-15 in vivo.
In addition, the use of ruxolitinib to interrupt IL-15 signaling through inhibition of JAK1 and JAK2 in patients with cytokine-dependent ATL was not optimal. Due to the action of HTLV-1–associated Tax transactivation of autocrine and paracrine cytokine pathways in leukemic ATL cells, such cells proliferate ex vivo in 6-d cultures. This proliferation was inhibited when ruxolitinib with JAK1 and off-target JAK2 inhibition was added to these cultures. However, ruxolitinib gave disappointing results both in maintaining inhibition of STAT5 phosphorylation and in clinical response when administered to ATL patients. Pharmacokinetic and pharmacodynamic factors contributed to this result in that when administered orally, ruxolitinib inhibited phosphorylation of STAT5 of peripheral blood mononuclear cells only at the 1-h time point and modestly at 3 h but were relatively ineffective in the remaining hours of the day. To address this issue and the off-target JAK2 inhibition of ruxolitinib, present studies are focusing on specific JAK3 and JAK1 inhibitors.
Alternative inhibitors of IL-15 receptor binding include PEGylated BNZ-1, which blocks IL-2 and IL-15 binding when administered to controls, had a survival t1/2 of ∼5 d, and led to a 70–80% decline in the number of circulating NK cells and an 80–93% decline in T reg cells. This agent is under clinical trial in patients with T cell LGL and with CTCL (Nata et al., 2015; Wang et al., 2019). A second trial of BNZ-2 that blocks IL-15 and IL-21 is being initiated in treatment of patients with refractory celiac disease. Nevertheless, even in this case, it is likely that inhibition of IL-15 receptor binding or signaling pathway alone will not be optimal and that combinations will be of value. To identify combinations, we employed a high-throughput assay to define agent combinations that are additive, including the Bcl-2/Bcl-xL inhibitor navitoclax (Zhang et al., 2015). The combination of ruxolitinib with navitoclax demonstrated synergy in murine models of ATL. In addition, the combination manifested activation of caspase 3 and 7 and conversion of Mcl-1 from its 40-kD anti-apoptotic form to a 24-kD pro-apoptotic form. In the future, such combinations of effective IL-15 action inhibitors may be of value in treatment of autoimmune diseases and select T cell malignancies.
With the alternative goal of treating patients with cancer and developing more effective vaccines, novel approaches with IL-15 are being developed to yield desired pharmacokinetics with one dosing per week provided by IL-15/IL-15Rα combinations along with maximal increases in NK and CD8 T cells provided by CIV rhIL-15. ARMO BioSciences is developing a PEGylated IL-15 to prolong survival. Furthermore, Tan and Waldmann are exploring a long-acting rhIL-15 depot for enhanced cancer immunotherapy in which IL-15 is mixed with an aqueous solution of polylactic-co-glycolic acid–PEG, a copolymer that is in solution at room temperature but transitions into a hydrogel at body temperature (Kim et al., 2001). In murine models, rhIL-15 hydrogel yielded immunotherapeutic IL-15 concentrations that persisted for days.
Despite their augmentation of NK cells and CD8 T cells, all IL-15 preparations administered as monotherapy of solid tumors were ineffective due to action of immunological checkpoints that prevented immune responses to self. In particular, there was inhibition of NK cell action by interactions of KIRS and NKG2A with self-class I MHC. There was parallel inhibition of CD8 T cells stimulated by IL-15 due to induction of SOCS3 in CD4 helper T cells, thereby yielding “helpless” CD8 T cells. To circumvent those latter checkpoints, combination trials of IL-15 and multiple other agents are being initiated. These combinations include IL-15 with checkpoint inhibitors, with anti-CD40 to yield tumor-specific CD8 T cells, and with cancer-directed monoclonal antibodies to increase their ADCC and anticancer efficacy.
In summary, our expanding understanding of the role of IL-15 in the life and death of lymphoid cells in health and disease is providing new perspectives for treatment of autoimmune disorders and malignancies.
Acknowledgments
This study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The authors declare no competing financial interests.
Author contributions: T.A. Waldmann designed, wrote, and edited the manuscript. M.D. Miljkovic and K.C. Conlon provided critical concepts and insights. All authors read and approved the manuscript prior to submission.
References
- Abadie V., and Jabri B.. 2014. IL-15: a central regulator of celiac disease immunopathology. Immunol. Rev. 260:221–234. 10.1111/imr.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander W.S., and Hilton D.J.. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22:503–529. 10.1146/annurev.immunol.22.091003.090312 [DOI] [PubMed] [Google Scholar]
- Anthony S.M., and Schluns K.S.. 2015. Emerging roles for IL-15 in the activation and function of T-cells during immune stimulation. Res. Rep. Biol. 6:25–37. [Google Scholar]
- Aringer M., Stummvoll G.H., Steiner G., Köller M., Steiner C.W., Höfler E., Hiesberger H., Smolen J.S., and Graninger W.B.. 2001. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford). 40:876–881. 10.1093/rheumatology/40.8.876 [DOI] [PubMed] [Google Scholar]
- Bamford R.N., Grant A.J., Burton J.D., Peters C., Kurys G., Goldman C.K., Brennan J., Roessler E., and Waldmann T.A.. 1994. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 91:4940–4944. 10.1073/pnas.91.11.4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford R.N., Battiata A.P., and Waldmann T.A.. 1996a IL-15: the role of translational regulation in their expression. J. Leukoc. Biol. 59:476–480. 10.1002/jlb.59.4.476 [DOI] [PubMed] [Google Scholar]
- Bamford R.N., Battiata A.P., Burton J.D., Sharma H., and Waldmann T.A.. 1996b Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA. 93:2897–2902. 10.1073/pnas.93.7.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslund B., Tvede N., Danneskiold-Samsoe B., Larsson P., Panayi G., Petersen J., Petersen L.J., Beurskens F.J., Schuurman J., van de Winkel J.G., et al. 2005. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 52:2686–2692. 10.1002/art.21249 [DOI] [PubMed] [Google Scholar]
- Becker T.C., Wherry E.J., Boone D., Murali-Krishna K., Antia R., Ma A., and Ahmed R.. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becknell B., and Caligiuri M.A.. 2005. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 86:209–239. 10.1016/S0065-2776(04)86006-1 [DOI] [PubMed] [Google Scholar]
- Benito-Miguel M., García-Carmona Y., Balsa A., Pérez de Ayala C., Cobo-Ibáñez T., Martín-Mola E., and Miranda-Carús M.E.. 2009. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25- responder T cells. J. Immunol. 183:8268–8279. 10.4049/jimmunol.0900007 [DOI] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F., and Heath W.R.. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 393:478–480. 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- Bergamaschi C., Rosati M., Jalah R., Valentin A., Kulkarni V., Alicea C., Zhang G.M., Patel V., Felber B.K., and Pavlakis G.N.. 2008. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 283:4189–4199. 10.1074/jbc.M705725200 [DOI] [PubMed] [Google Scholar]
- Bergamaschi C., Bear J., Rosati M., Beach R.K., Alicea C., Sowder R., Chertova E., Rosenberg S.A., Felber B.K., and Pavlakis G.N.. 2012. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood. 120:e1–e8. 10.1182/blood-2011-10-384362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi C., Watson D.C., Valentin A., Bear J., Peer C.J., Figg W.D. Sr., Felber B.K., and Pavlakis G.N.. 2018. Optimized administration of hetIL-15 expands lymphocytes and minimizes toxicity in rhesus macaques. Cytokine. 108:213–224. 10.1016/j.cyto.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrien-Elliott M.M., Wagner J.A., and Fehniger T.A.. 2015. Human cytokine-induced memory-like natural killer cells. J. Innate Immun. 7:563–571. 10.1159/000382019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C., Dondero A., Moretta A., and Castriconi R.. 2016. CIS is a negative regulator of IL-15-mediated signals in NK cells. Transl. Cancer Res. 5(S4):S875–S877. 10.21037/tcr.2016.10.79 [DOI] [Google Scholar]
- Bouchaud G., Gehrke S., Krieg C., Kolios A., Hafner J., Navarini A.A., French L.E., and Boyman O.. 2013. Epidermal IL-15Rα acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J. Exp. Med. 210:2105–2117. 10.1084/jem.20130291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Kovar M., Rubinstein M.P., Surh C.D., and Sprent J.. 2006. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 311:1924–1927. 10.1126/science.1122927 [DOI] [PubMed] [Google Scholar]
- Boyman O., Létourneau S., Krieg C., and Sprent J.. 2009. Homeostatic proliferation and survival of naïve and memory T cells. Eur. J. Immunol. 39:2088–2094. 10.1002/eji.200939444 [DOI] [PubMed] [Google Scholar]
- Brentjens R.J., Latouche J.B., Santos E., Marti F., Gong M.C., Lyddane C., King P.D., Larson S., Weiss M., Rivière I., and Sadelain M.. 2003. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9:279–286. 10.1038/nm827 [DOI] [PubMed] [Google Scholar]
- Burkett P.R., Koka R., Chien M., Chai S., Chan F., Ma A., and Boone D.L.. 2003. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 100:4724–4729. 10.1073/pnas.0737048100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack K.S., Huggins M.A., Taras E., Dougherty P., Henzler C.M., Yang R., Alter S., Jeng E.K., Wong H.C., Felices M., et al. 2018. Interleukin-15 complex treatment protects mice from cerebral malaria by inducing interleukin-10-producing natural killer cells. Immunity. 48:760–772.e4. 10.1016/j.immuni.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.D., Bamford R.N., Peters C., Grant A.J., Kurys G., Goldman C.K., Brennan J., Roessler E., and Waldmann T.A.. 1994. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 91:4935–4939. 10.1073/pnas.91.11.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V., Mallette F.A., Deschênes-Simard X., Ramanathan S., Gagnon J., Moores A., Ilangumaran S., and Ferbeyre G.. 2009. SOCS1 links cytokine signaling to p53 and senescence. Mol. Cell. 36:754–767. 10.1016/j.molcel.2009.09.044 [DOI] [PubMed] [Google Scholar]
- Carson W.E., Fehniger T.A., Haldar S., Eckhert K., Lindemann M.J., Lai C.F., Croce C.M., Baumann H., and Caligiuri M.A.. 1997. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99:937–943. 10.1172/JCI119258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo E.F., and Schluns K.S.. 2012. Regulating the immune system via IL-15 transpresentation. Cytokine. 59:479–490. 10.1016/j.cyto.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Petrus M., Bryant B.R., Phuc Nguyen V., Stamer M., Goldman C.K., Bamford R., Morris J.C., Janik J.E., and Waldmann T.A.. 2008. Induction of the IL-9 gene by HTLV-I Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood. 111:5163–5172. 10.1182/blood-2007-09-113654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Petrus M., Bamford R., Shih J.H., Morris J.C., Janik J.E., and Waldmann T.A.. 2012. Increased serum soluble IL-15Rα levels in T-cell large granular lymphocyte leukemia. Blood. 119:137–143. 10.1182/blood-2011-04-346759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Feigenbaum L., Awasthi P., Butcher D.O., Anver M.R., Golubeva Y.G., Bamford R., Zhang X., St Claire M.B., Thomas C.J., et al. 2013. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc. Natl. Acad. Sci. USA. 110:13534–13539. 10.1073/pnas.1312911110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E., Bergamaschi C., Chertov O., Sowder R., Bear J., Roser J.D., Beach R.K., Lifson J.D., Felber B.K., and Pavlakis G.N.. 2013. Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer. J. Biol. Chem. 288:18093–18103. 10.1074/jbc.M113.461756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chłopek M., Kowalik A., Góźdź S., and Koziak K.. 2017. The role of interleukin 15 in neoplasia. Postepy Hig. Med. Dosw. 71:5–19. 10.5604/17322693.1228266 [DOI] [PubMed] [Google Scholar]
- Conlon K.C., Lugli E., Welles H.C., Rosenberg S.A., Fojo A.T., Morris J.C., Fleisher T.A., Dubois S.P., Perera L.P., Stewart D.M., et al. 2015. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33:74–82. 10.1200/JCO.2014.57.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon K.C., Potter E.L., Pittaluga S., Lee C.R., Miljkovic M.D., Fleisher T.A., Dubois S., Bryant B.R., Petrus M., Perera L.P., et al. 2019. IL-15 by continuous i.v. infusion to adult patients with solid tumors in a Phase I trial induced dramatic NK cell subset expansion. Clin. Cancer Res. 25:4945–4954. 10.1158/1078-0432.CCR-18-3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L.B., Fuji S., Hermine O., Bazarbachi A., Ramos J.C., Ratner L., Horwitz S., Fields P., Tanase A., Bumbea H., et al. 2019. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 37:677–687. 10.1200/JCO.18.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursons J., Souza-Fonseca-Guimaraes F., Foroutan M., Anderson A., Hollande F., Hediyeh-Zadeh S., Behren A., Huntington N.D., and Davis M.J.. 2019. A gene signature predicting natural killer cell infiltration and improved survival in melanoma patients. Cancer Immunol. Res. 7:1162–1174. 10.1158/2326-6066.CIR-18-0500 [DOI] [PubMed] [Google Scholar]
- Dahan R., Barnhart B.C., Li F., Yamniuk A.P., Korman A.J., and Ravetch J.V.. 2016. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell. 29:820–831. 10.1016/j.ccell.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delconte R.B., Kolesnik T.B., Dagley L.F., Rautela J., Shi W., Putz E.M., Stannard K., Zhang J.G., Teh C., Firth M., et al. 2016. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 17:816–824. 10.1038/ni.3470 [DOI] [PubMed] [Google Scholar]
- Desbois M., Le Vu P., Coutzac C., Marcheteau E., Béal C., Terme M., Gey A., Morisseau S., Teppaz G., Boselli L., et al. 2016. IL-15 trans-signaling with the superagonist RL1 promotes effector/memory CD8+ T cell responses and enhances antitumor activity of PD-1 antagonists. J. Immunol. 197:168–178. 10.4049/jimmunol.1600019 [DOI] [PubMed] [Google Scholar]
- Döbbeling U., Dummer R., Laine E., Potoczna N., Qin J.Z., and Burg G.. 1998. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 92:252–258. 10.1182/blood.V92.1.252.413k08_252_258 [DOI] [PubMed] [Google Scholar]
- Dubois S., Mariner J., Waldmann T.A., and Tagaya Y.. 2002. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 17:537–547. 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- Dubois S., Patel H.J., Zhang M., Waldmann T.A., and Müller J.R.. 2008. Preassociation of IL-15 with IL-15R α-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 180:2099–2106. 10.4049/jimmunol.180.4.2099 [DOI] [PubMed] [Google Scholar]
- Dubois S., Conlon K.C., Müller J.R., Hsu-Albert J., Beltran N., Bryant B.R., and Waldmann T.A.. 2017. IL-15 infusion of cancer patients expands the subpopulation of cytotoxic CD56bright NK cells and increases NK-cell cytokine release capabilities. Cancer Immunol. Res. 5:929–938. 10.1158/2326-6066.CIR-17-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettersperger J., Montcuquet N., Malamut G., Guegan N., Lopez-Lastra S., Gayraud S., Reimann C., Vidal E., Cagnard N., Villarese P., et al. 2016. Interleukin-15-dependent T-cell-like innate intraepithelial lymphocytes develop in the intestine and transform into lymphomas in celiac disease. Immunity. 45:610–625. 10.1016/j.immuni.2016.07.018 [DOI] [PubMed] [Google Scholar]
- Evans R., Fuller J.A., Christianson G., Krupke D.M., and Troutt A.B.. 1997. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell. Immunol. 179:66–73. 10.1006/cimm.1997.1132 [DOI] [PubMed] [Google Scholar]
- Farag S.S., and Caligiuri M.A.. 2006. Human natural killer cell development and biology. Blood Rev. 20:123–137. 10.1016/j.blre.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Fehniger T.A., and Caligiuri M.A.. 2001. Interleukin 15: biology and relevance to human disease. Blood. 97:14–32. 10.1182/blood.V97.1.14 [DOI] [PubMed] [Google Scholar]
- Fehniger T.A., Suzuki K., VanDeusen J.B., Cooper M.A., Freud A.G., and Caligiuri M.A.. 2001. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol. Dis. 27:223–230. 10.1006/bcmd.2001.0379 [DOI] [PubMed] [Google Scholar]
- Fehniger T.A., Cooper M.A., and Caligiuri M.A.. 2002. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13:169–183. 10.1016/S1359-6101(01)00021-1 [DOI] [PubMed] [Google Scholar]
- Felices M., Lenvik A.J., McElmurry R., Chu S., Hinderlie P., Bendzick L., Geller M.A., Tolar J., Blazar B.R., and Miller J.S.. 2018. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 3:96219 10.1172/jci.insight.96219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari-Lacraz S., Zanelli E., Neuberg M., Donskoy E., Kim Y.S., Zheng X.X., Hancock W.W., Maslinski W., Li X.C., Strom T.B., and Moll T.. 2004. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J. Immunol. 173:5818–5826. 10.4049/jimmunol.173.9.5818 [DOI] [PubMed] [Google Scholar]
- Fuchs A., Vermi W., Lee J.S., Lonardi S., Gilfillan S., Newberry R.D., Cella M., and Colonna M.. 2013. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 38:769–781. 10.1016/j.immuni.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya H., Chan O.T.M., Pagano I., Zhu C., Kim N., Peres R., Hokutan K., Alter S., Rhode P., and Rosser C.J.. 2019. Effectiveness of two different dose administration regimens of an IL-15 superagonist complex (ALT-803) in an orthotopic bladder cancer mouse model. J. Transl. Med. 17:29 10.1186/s12967-019-1778-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J., Sallusto F., and Lanzavecchia A.. 2003. Cytokine-driven proliferation and differentiation of human naïve, central memory and effector memory CD4+ T cells. Pathol. Biol. (Paris). 51:64–66. 10.1016/S0369-8114(03)00098-1 [DOI] [PubMed] [Google Scholar]
- Grabstein K.H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M.A., Ahdieh M., et al. 1994. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 264:965–968. 10.1126/science.8178155 [DOI] [PubMed] [Google Scholar]
- Harada S., Yamamura M., Okamoto H., Morita Y., Kawashima M., Aita T., and Makino H.. 1999. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 42:1508–1516. [DOI] [PubMed] [Google Scholar]
- Huntington N.D. 2014. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol. Cell Biol. 92:210–213. 10.1038/icb.2014.1 [DOI] [PubMed] [Google Scholar]
- Huntington N.D., Legrand N., Alves N.L., Jaron B., Weijer K., Plet A., Corcuff E., Mortier E., Jacques Y., Spits H., and Di Santo J.P.. 2009. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 206:25–34. 10.1084/jem.20082013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangumaran S., Ramanathan S., La Rose J., Poussier P., and Rottapel R.. 2003a Suppressor of cytokine signaling 1 regulates IL-15 receptor signaling in CD8+CD44high memory T lymphocytes. J. Immunol. 171:2435–2445. 10.4049/jimmunol.171.5.2435 [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., Ramanathan S., Ning T., La Rose J., Reinhart B., Poussier P., and Rottapel R.. 2003b Suppressor of cytokine signaling 1 attenuates IL-15 receptor signaling in CD8+ thymocytes. Blood. 102:4115–4122. 10.1182/blood-2003-01-0175 [DOI] [PubMed] [Google Scholar]
- Jabri B., and Abadie V.. 2015. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 15:771–783. 10.1038/nri3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobisiak M., Golab J., and Lasek W.. 2011. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 22:99–108. 10.1016/j.cytogfr.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Johnson J.K., and Miller J.S.. 2018. Current strategies exploiting NK-cell therapy to treat haematologic malignancies. Int. J. Immunogenet. 45:237–246. 10.1111/iji.12387 [DOI] [PubMed] [Google Scholar]
- Ju W., Zhang M., Jiang J.K., Thomas C.J., Oh U., Bryant B.R., Chen J., Sato N., Tagaya Y., Morris J.C., et al. 2011. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood. 117:1938–1946. 10.1182/blood-2010-09-305425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Maslinski W., Zheng X.X., Stevens A.C., Li X.C., Tesch G.H., Kelley V.R., and Strom T.B.. 1998. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fc γ2a protein blocks delayed-type hypersensitivity. J. Immunol. 160:5742–5748. [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Choi S., Koh J.J., Lee M., Ko K.S., and Kim S.W.. 2001. Controlled release of insulin from injectable biodegradable triblock copolymer. Pharm. Res. 18:548–550. 10.1023/A:1011074915438 [DOI] [PubMed] [Google Scholar]
- Klebanoff C.A., Finkelstein S.E., Surman D.R., Lichtman M.K., Gattinoni L., Theoret M.R., Grewal N., Spiess P.J., Antony P.A., Palmer D.C., et al. 2004. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA. 101:1969–1974. 10.1073/pnas.0307298101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C.S., Blatz K., d’Hargues Y., Hernandez P.P., Kofoed-Nielsen M., Ripka J.F., Ebert K., Arnold S.J., Diefenbach A., Palmer E., and Tanriver Y.. 2014. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8αα(+) intraepithelial lymphocyte development. Immunity. 41:230–243. 10.1016/j.immuni.2014.06.018 [DOI] [PubMed] [Google Scholar]
- Knudson K.M., Hicks K.C., Alter S., Schlom J., and Gameiro S.R.. 2019. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer. 7:82 10.1186/s40425-019-0551-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Dubois S., Sato N., Sabzevari H., Sakai Y., Waldmann T.A., and Tagaya Y.. 2005. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 105:721–727. 10.1182/blood-2003-12-4187 [DOI] [PubMed] [Google Scholar]
- Korneychuk N., Ramiro-Puig E., Ettersperger J., Schulthess J., Montcuquet N., Kiyono H., Meresse B., and Cerf-Bensussan N.. 2014. Interleukin 15 and CD4+ T cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology. 146:1017–1027. 10.1053/j.gastro.2013.12.023 [DOI] [PubMed] [Google Scholar]
- Kowalsky S.J., Liu Z., Feist M., Berkey S.E., Ma C., Ravindranathan R., Dai E., Roy E.J., Guo Z.S., and Bartlett D.L.. 2018. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 26:2476–2486. 10.1016/j.ymthe.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong B., and Lazarevic V.. 2014. T-bet orchestrates CD8αα IEL differentiation. Immunity. 41:169–171. 10.1016/j.immuni.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Yun S., Piao Z.H., Jeong M., Kim D.O., Jung H., Lee J., Kim M.J., Kim M.S., Chung J.W., et al. 2010. Suppressor of cytokine signaling 2 regulates IL-15-primed human NK cell function via control of phosphorylated Pyk2. J. Immunol. 185:917–928. 10.4049/jimmunol.1000784 [DOI] [PubMed] [Google Scholar]
- Lenardo M., Chan K.M., Hornung F., McFarland H., Siegel R., Wang J., and Zheng L.. 1999. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221–253. 10.1146/annurev.immunol.17.1.221 [DOI] [PubMed] [Google Scholar]
- Li F., and Ravetch J.V.. 2011. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 333:1030–1034. 10.1126/science.1206954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.X., and Leonard W.J.. 2018. The common cytokine receptor γ chain family of cytokines. Cold Spring Harb. Perspect. Biol. 10:a028449 10.1101/cshperspect.a028449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Kong L., Han K., Hong H., Marcus W.D., Chen X., Jeng E.K., Alter S., Zhu X., Rubinstein M.P., et al. 2016. A novel fusion of ALT-803 (interleukin (IL)-15 superagonist) with an antibody demonstrates antigen-specific antitumor responses. J. Biol. Chem. 291:23869–23881. 10.1074/jbc.M116.733600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce J., Burkett P., Koka R., Boone D., Chien M., Chan F., Madonia M., Chai S., and Ma A.. 2002. Interleukin-15 and the regulation of lymphoid homeostasis. Mol. Immunol. 39:537–544. 10.1016/S0161-5890(02)00211-0 [DOI] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., and Diefenbach A.. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26:503–517. 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A., Koka R., and Burkett P.. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657–679. 10.1146/annurev.immunol.24.021605.090727 [DOI] [PubMed] [Google Scholar]
- Mackay L.K., Rahimpour A., Ma J.Z., Collins N., Stock A.T., Hafon M.L., Vega-Ramos J., Lauzurica P., Mueller S.N., Stefanovic T., et al. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14:1294–1301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Mackay-Wiggan J., Jabbari A., Nguyen N., Cerise J.E., Clark C., Ulerio G., Furniss M., Vaughan R., Christiano A.M., and Clynes R.. 2016. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 1:e89790 10.1172/jci.insight.89790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K., Morishima C., Velcheti V., Miller J.S., Lee S.M., Silk A.W., Holtan S.G., Lacroix A.M., Fling S.P., Kaiser J.C., et al. 2018. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin. Cancer Res. 24:5552–5561. 10.1158/1078-0432.CCR-18-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Konczalik J., Dubois S., Losi J.M., Sabzevari H., Yamada N., Feigenbaum L., Waldmann T.A., and Tagaya Y.. 2000. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA. 97:11445–11450. 10.1073/pnas.200363097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayassi T., and Jabri B.. 2018. Human intraepithelial lymphocytes. Mucosal Immunol. 11:1281–1289. 10.1038/s41385-018-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I.B., al-Mughales J., Field M., Leung B.P., Huang F.P., Dixon R., Sturrock R.D., Wilkinson P.C., and Liew F.Y.. 1996. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 2:175–182. 10.1038/nm0296-175 [DOI] [PubMed] [Google Scholar]
- Meresse B., Korneychuk N., Malamut G., and Cerf-Bensussan N.. 2015. Interleukin-15, a master piece in the immunological jigsaw of celiac disease. Dig. Dis. 33:122–130. 10.1159/000369521 [DOI] [PubMed] [Google Scholar]
- Miller J.S., Morishima C., McNeel D.G., Patel M.R., Kohrt H.E.K., Thompson J.A., Sondel P.M., Wakelee H.A., Disis M.L., Kaiser J.C., et al. 2018. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res. 24:1525–1535. 10.1158/1078-0432.CCR-17-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Liu S., Sams G.H., Curphey D.P., Santhanam R., Rush L.J., Schaefer D., Falkenberg L.G., Sullivan L., Jaroncyk L., et al. 2012. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 22:645–655. 10.1016/j.ccr.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Sullivan L., and Caligiuri M.A.. 2014. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 20:2044–2050. 10.1158/1078-0432.CCR-12-3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., La Perle K., Kwiatkowski S., Sullivan L.A., Sams G.H., Johns J., Curphey D.P., Wen J., McConnell K., Qi J., et al. 2016. Mechanism, consequences and therapeutic targeting of abnormal IL15 signaling in cutaneous T-cell lymphoma. Cancer Discov. 6:986–1005. 10.1158/2159-8290.CD-15-1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Ring A.M., Amarnath S., Spangler J.B., Li P., Ju W., Fischer S., Oh J., Spolski R., Weiskopf K., et al. 2015. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity. 42:826–838. 10.1016/j.immuni.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga E., Cantó E., Vidal S., Juarez C., Sierra J., and Briones J.. 2011. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp. Hematol. 39:1064–1071. 10.1016/j.exphem.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Morris J.C., Janik J.E., White J.D., Fleisher T.A., Brown M., Tsudo M., Goldman C.K., Bryant B., Petrus M., Top L., et al. 2006. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc. Natl. Acad. Sci. USA. 103:401–406. 10.1073/pnas.0509575103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger W., DeJoy S.Q., Jeyaseelan R. Sr., Torley L.W., Grabstein K.H., Eisenmann J., Paxton R., Cox T., Wick M.M., and Kerwar S.S.. 1995. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell. Immunol. 165:289–293. 10.1006/cimm.1995.1216 [DOI] [PubMed] [Google Scholar]
- Munks M.W., Cho K.S., Pinto A.K., Sierro S., Klenerman P., and Hill A.B.. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450–458. 10.4049/jimmunol.177.1.450 [DOI] [PubMed] [Google Scholar]
- Nata T., Basheer A., Cocchi F., van Besien R., Massoud R., Jacobson S., Azimi N., and Tagaya Y.. 2015. Targeting the binding interface on a shared receptor subunit of a cytokine family enables the inhibition of multiple member cytokines with selectable target spectrum. J. Biol. Chem. 290:22338–22351. 10.1074/jbc.M115.661074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa M.C., Minute L., López A., Pérez-Ruiz E., Gomar C., Vasquez M., Inoges S., Etxeberria I., Rodriguez I., Garasa S., et al. 2017a Enhancement of antibody-dependent cellular cytotoxicity of cetuximab by a chimeric protein encompassing interleukin-15. OncoImmunology. 7:e1393597 10.1080/2162402X.2017.1393597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa M.C., Minute L., Rodriguez I., Garasa S., Perez-Ruiz E., Inogés S., Melero I., and Berraondo P.. 2017b Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 95:347–355. 10.1038/icb.2017.6 [DOI] [PubMed] [Google Scholar]
- Overwijk W.W., and Schluns K.S.. 2009. Functions of γC cytokines in immune homeostasis: current and potential clinical applications. Clin. Immunol. 132:153–165. 10.1016/j.clim.2009.03.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patidar M., Yadav N., and Dalai S.K.. 2016. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 31:49–59. 10.1016/j.cytogfr.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Pilipow K., Roberto A., Roederer M., Waldmann T.A., Mavilio D., and Lugli E.. 2015. IL15 and T-cell stemness in T-cell-based cancer immunotherapy. Cancer Res. 75:5187–5193. 10.1158/0008-5472.CAN-15-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton J.F., Tan J.T., Rubinstein M.P., Kim D.M., Sprent J., and Surh C.D.. 2007. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 204:951–961. 10.1084/jem.20061805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M., and Rombos A.. 2012. The role of IL-15 in central nervous system disorders. Acta Neurol. Scand. 125:77–82. 10.1111/j.1600-0404.2011.01524.x [DOI] [PubMed] [Google Scholar]
- Richmond J.M., Strassner J.P., Zapata L. Jr., Garg M., Riding R.L., Refat M.A., Fan X., Azzolino V., Tovar-Garza A., Tsurushita N., et al. 2018. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 10:eaam7710 10.1126/scitranslmed.aam7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa F., and Matzinger P.. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–478. 10.1038/30989 [DOI] [PubMed] [Google Scholar]
- Rifa’i M., Shi Z., Zhang S.Y., Lee Y.H., Shiku H., Isobe K., and Suzuki H.. 2008. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int. Immunol. 20:937–947. 10.1093/intimm/dxn052 [DOI] [PubMed] [Google Scholar]
- Robak E., Robak T., Wozniacka A., Zak-Prelich M., Sysa-Jedrzejowska A., and Stepien H.. 2002. Proinflammatory interferon-gamma--inducing monokines (interleukin-12, interleukin-18, interleukin-15)--serum profile in patients with systemic lupus erythematosus. Eur. Cytokine Netw. 13:364–368. [PubMed] [Google Scholar]
- Roberti M.P., Rocca Y.S., Amat M., Pampena M.B., Loza J., Coló F., Fabiano V., Loza C.M., Arriaga J.M., Bianchini M., et al. 2012. IL-2- or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res. Treat. 136:659–671. 10.1007/s10549-012-2287-y [DOI] [PubMed] [Google Scholar]
- Robinette M.L., Bando J.K., Song W., Ulland T.K., Gilfillan S., and Colonna M.. 2017. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 8:14601 10.1038/ncomms14601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.O., and Schluns K.S.. 2017. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 190:159–168. 10.1016/j.imlet.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y., Spolski R., and Leonard W.J.. 2009. New insights into the regulation of T cells by γ(c) family cytokines. Nat. Rev. Immunol. 9:480–490. 10.1038/nri2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Schneider S.E., Leong J.W., Chase J.M., Keppel C.R., Sullivan R.P., Cooper M.A., and Fehniger T.A.. 2012. Cytokine activation induces human memory-like NK cells. Blood. 120:4751–4760. 10.1182/blood-2012-04-419283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Rosario M., Berrien-Elliott M.M., Wagner J.A., Jewell B.A., Schappe T., Leong J.W., Abdel-Latif S., Schneider S.E., Willey S., et al. 2016. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8:357ra123 10.1126/scitranslmed.aaf2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E., Weisdorf D.J., Blazar B.R., Ustun C., DeFor T.E., et al. 2018. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 131:2515–2527. 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. 2014. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192:5451–5458. 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. 2011. Regulatory T cells: history and perspective. Methods Mol. Biol. 707:3–17. 10.1007/978-1-61737-979-6_1 [DOI] [PubMed] [Google Scholar]
- Sato N., Sabzevari H., Fu S., Ju W., Petrus M.N., Bamford R.N., Waldmann T.A., and Tagaya Y.. 2011. Development of an IL-15-autocrine CD8 T-cell leukemia in IL-15-transgenic mice requires the cis expression of IL-15Rα. Blood. 117:4032–4040. 10.1182/blood-2010-09-307504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S., and Lefrançois L.. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269–279. 10.1038/nri1052 [DOI] [PubMed] [Google Scholar]
- Schmohl J.U., Felices M., Taras E., Miller J.S., and Vallera D.A.. 2016. Enhanced ADCC and NK cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol. Ther. 24:1312–1322. 10.1038/mt.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E., van der Voort E.I., Offringa R., and Melief C.J.. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- Sciumè G., Le M.T., and Gadina M.. 2017. HiJAKing innate lymphoid cells? Front. Immunol. 8:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sckisel G.D., Bouchlaka M.N., Monjazeb A.M., Crittenden M., Curti B.D., Wilkins D.E., Alderson K.A., Sungur C.M., Ames E., Mirsoian A., et al. 2015. Out-of-sequence signal 3 paralyzes CD4+ T-cell-dependent immunity. Immunity. 43:240–250. 10.1016/j.immuni.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K., Dufour J.P., Liu D.X., Rout N., Alvarez X., Blanchard J., Faldas A., Laine D.J., Clarke A.W., and Doyle A.G.. 2018. Beneficial effects of human anti-interleukin-15 antibody in gluten-sensitive rhesus macaques with celiac disease. Front. Immunol. 9:1603 10.3389/fimmu.2018.01603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M.C., Kopp W.C., Engelke K.J., Yovandich J.L., Creekmore S.P., Waldmann T.A., and Lane H.C.. 2011. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 118:6845–6848. 10.1182/blood-2011-09-377804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A.L., Pandiyan P., Zheng L., Krummey S.M., and Lenardo M.J.. 2010. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 236:68–82. 10.1111/j.1600-065X.2010.00917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J.C., Waldmann T.A., and Morris J.C.. 2012. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 33:35–41. 10.1016/j.tips.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y., Bamford R.N., DeFilippis A.P., and Waldmann T.A.. 1996. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 4:329–336. 10.1016/S1074-7613(00)80246-0 [DOI] [PubMed] [Google Scholar]
- Teague R.M., Sather B.D., Sacks J.A., Huang M.Z., Dossett M.L., Morimoto J., Tan X., Sutton S.E., Cooke M.P., Ohlén C., and Greenberg P.D.. 2006. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat. Med. 12:335–341. 10.1038/nm1359 [DOI] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A., Brass S.D., Gandhi R., and Weiner H.L.. 2008. Membrane bound IL-15 is increased on CD14 monocytes in early stages of MS. J. Neuroimmunol. 195:135–139. 10.1016/j.jneuroim.2008.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen L.S., Schuurman J., Beurskens F., Dam T.N., Dagnaes-Hansen F., Skov L., Rygaard J., Voorhorst-Ogink M.M., Gerritsen A.F., van Dijk M.A., et al. 2003. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 112:1571–1580. 10.1172/JCI200318986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger F., Miller R., Mori K., Mayne A.E., Bostik P., Sundstrom J.B., Sugimoto C., and Ansari A.A.. 2004. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 22:3510–3521. 10.1016/j.vaccine.2003.07.022 [DOI] [PubMed] [Google Scholar]
- Vincent M., Teppaz G., Lajoie L., Solé V., Bessard A., Maillasson M., Loisel S., Béchard D., Clémenceau B., Thibault G., et al. 2014. Highly potent anti-CD20-RLI immunocytokine targeting established human B lymphoma in SCID mouse. MAbs. 6:1026–1037. 10.4161/mabs.28699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A. 2003. IL-15 in the life and death of lymphocytes: immunotherapeutic implications. Trends Mol. Med. 9:517–521. 10.1016/j.molmed.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Waldmann T.A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6:595–601. 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- Waldmann T.A. 2014. Interleukin-15 in the treatment of cancer. Expert Rev. Clin. Immunol. 10:1689–1701. 10.1586/1744666X.2014.973856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A. 2015. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol. Res. 3:219–227. 10.1158/2326-6066.CIR-15-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A. 2018. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 10:a028472 10.1101/cshperspect.a028472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A., and Tagaya Y.. 2000. IL-15 cytokine. In The Cytokine Reference. A Compendium of Cytokines and Other Mediators of Host Defense. Oppenheim J.J. and Feldmann M., editors. Academic Press, London. 213–224 pp. [Google Scholar]
- Waldmann T., Tagaya Y., and Bamford R.. 1998. Interleukin-2, interleukin-15, and their receptors. Int. Rev. Immunol. 16:205–226. 10.3109/08830189809042995 [DOI] [PubMed] [Google Scholar]
- Waldmann T.A., Dubois S., and Tagaya Y.. 2001. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 14:105–110. [PubMed] [Google Scholar]
- Waldmann T.A., Lugli E., Roederer M., Perera L.P., Smedley J.V., Macallister R.P., Goldman C.K., Bryant B.R., Decker J.M., Fleisher T.A., et al. 2011. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 117:4787–4795. 10.1182/blood-2010-10-311456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T.A., Conlon K.C., Stewart D.M., Worthy T.A., Janik J.E., Fleisher T.A., Albert P.S., Figg W.D., Spencer S.D., Raffeld M., et al. 2013. Phase 1 trial of IL-15 trans presentation blockade using humanized Mikβ1 mAb in patients with T-cell large granular lymphocytic leukemia. Blood. 121:476–484. 10.1182/blood-2012-08-450585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Yang J., Zhang Y., Zhang M., Dubois S., Conlon K.C., Tagaya Y., Hamele C.E., Dighe S., Olson T.L., et al. 2019. IL-2 and IL-15 blockade by BNZ-1, an inhibitor of selective γ-chain cytokines, decreases leukemic T-cell viability. Leukemia. 33:1243–1255. 10.1038/s41375-018-0290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangle J.M., Velcheti V., Patel M.R., Garrett-Mayer E., Hill E.G., Ravenel J.G., Miller J.S., Farhad M., Anderton K., Lindsey K., et al. 2018. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19:694–704. 10.1016/S1470-2045(18)30148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Dai Z., Jabbari A., Cerise J.E., Higgins C.A., Gong W., de Jong A., Harel S., DeStefano G.M., Rothman L., et al. 2014. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 20:1043–1049. 10.1038/nm.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe N., Sato N., Perera P.Y., Filkoski L., Tanaka T., Miyasaka M., Waldmann T.A., Hiroi T., and Perera L.P.. 2009. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc. Natl. Acad. Sci. USA. 106:15849–15854. 10.1073/pnas.0908834106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Steel J.C., Zhang M., Morris J.C., and Waldmann T.A.. 2010. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin. Cancer Res. 16:6019–6028. 10.1158/1078-0432.CCR-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Steel J.C., Zhang M., Morris J.C., Waitz R., Fasso M., Allison J.P., and Waldmann T.A.. 2012. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc. Natl. Acad. Sci. USA. 109:6187–6192. 10.1073/pnas.1203479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I., Spreafico R., Bodio C., Di Gioia M., Cigni C., Broggi A., Gorletta T., Caccia M., Chirico G., Sironi L., et al. 2013. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Reports. 4:1235–1249. 10.1016/j.celrep.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Zhang R., Shah M.V., Yang J., Nyland S.B., Liu X., Yun J.K., Albert R., and Loughran T.P. Jr. 2008. Network model of survival signaling in large granular lymphocyte leukemia. Proc. Natl. Acad. Sci. USA. 105:16308–16313. 10.1073/pnas.0806447105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Yao Z., Dubois S., Ju W., Müller J.R., and Waldmann T.A.. 2009. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc. Natl. Acad. Sci. USA. 106:7513–7518. 10.1073/pnas.0902637106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Ju W., Yao Z., Yu P., Wei B.R., Simpson R.M., Waitz R., Fassò M., Allison J.P., and Waldmann T.A.. 2012. Augmented IL-15Rα expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J. Immunol. 188:6156–6164. 10.4049/jimmunol.1102604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Mathews Griner L.A., Ju W., Duveau D.Y., Guha R., Petrus M.N., Wen B., Maeda M., Shinn P., Ferrer M., et al. 2015. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl. Acad. Sci. USA. 112:12480–12485. 10.1073/pnas.1516208112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wen B., Anton O.M., Yao Z., Dubois S., Ju W., Sato N., DiLillo D.J., Bamford R.N., Ravetch J.V., and Waldmann T.A.. 2018. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl. Acad. Sci. USA. 115:E10915–E10924. 10.1073/pnas.1811615115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Hu B., Zhang Y., Song Y., Lin D., Liu Y., Mei Y., Sandikin D., Sun W., Zhuang M., and Liu H.. 2016. An activation-induced IL-15 isoform is a natural antagonist for IL-15 function. Sci. Rep. 6:25822 10.1038/srep25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Luo M., Xie Y., Jiang H., Cagliero C., Li N., Ye H., Wu M., Hao S., Sun T., et al. 2019. Development of a recombinant human IL-15·sIL-15Rα/Fc superagonist with improved half-life and its antitumor activity alone or in combination with PD-1 blockade in mouse model. Biomed. Pharmacother. 112:108677 10.1016/j.biopha.2019.108677 [DOI] [PubMed] [Google Scholar]
- Zhu X., Marcus W.D., Xu W., Lee H.I., Han K., Egan J.O., Yovandich J.L., Rhode P.R., and Wong H.C.. 2009. Novel human interleukin-15 agonists. J. Immunol. 183:3598–3607. 10.4049/jimmunol.0901244 [DOI] [PMC free article] [PubMed] [Google Scholar]