This review discusses the growing literature on the pathogenic role of IL-17 in cancer, focusing on recent studies that place IL-17 as a nexus linking inflammation, tissue repair, and cancer.
Abstract
IL-17, a potent proinflammatory cytokine, has been shown to intimately contribute to the formation, growth, and metastasis of a wide range of malignancies. Recent studies implicate IL-17 as a link among inflammation, wound healing, and cancer. While IL-17–mediated production of inflammatory mediators mobilizes immune-suppressive and angiogenic myeloid cells, emerging studies reveal that IL-17 can directly act on tissue stem cells to promote tissue repair and tumorigenesis. Here, we review the pleotropic impacts of IL-17 on cancer biology, focusing how IL-17–mediated inflammatory response and mitogenic signaling are exploited to equip its cancer-promoting function and discussing the implications in therapies.
Introduction
IL-17A and IL-17F, hereafter refer to as IL-17, are signature proinflammatory cytokines of the CD4+ T helper 17 (Th17) cells. They function either as a homodimer or heterodimer and signal through a heterodimeric receptor complex that consists of IL-17 receptor A (IL-17RA) and IL-17RC (Chang and Dong, 2007; Chen and Kolls, 2017; Ely et al., 2009; Fossiez et al., 1996; Gaffen, 2009; Gu et al., 2013; Hymowitz et al., 2001; Kuestner et al., 2007; Toy et al., 2006; Wright et al., 2008; Wright et al., 2007; Yao et al., 1995).
While IL-17 is essential for the protection against extracellular bacterial infection and fungal infection, dysregulation of IL-17 production and/or signaling often results in unresolved inflammation, leading to the autoimmune response and tissue destruction. Attesting to its role in autoimmunity, the anti–IL-17A neutralizing antibody secukinumab showed >80% response rate in patients with moderate-to-severe psoriasis (Baeten et al., 2015; Langley et al., 2014; Mease et al., 2015), which led to its approval by the US Food and Drug Administration. Since then, secukinumab has also been approved for the treatment of psoriatic arthritis and ankylosing spondylitis, with additional autoimmune conditions under active clinical investigation as potential indications (McGeachy et al., 2019).
In addition to autoimmunity, dysregulated IL-17 is emerging as a major pathogenic factor involved in both the early and late stages of cancer development. Ablation of IL-17 blunts tumorigenesis in a wide range of organs in mouse models, including colon (Chung et al., 2018; Grivennikov et al., 2012; Wang et al., 2014; Zepp et al., 2017), liver (Ma et al., 2014; Sun et al., 2016), pancreas (McAllister et al., 2014; Zhang et al., 2018), lung (Chang et al., 2014; Jin et al., 2019), and skin (Chen et al., 2019; Wu et al., 2015). Inhibition of IL-17 has also been shown to suppress metastasis and improve the sensitivity to both chemotherapy and radiation therapy in preclinical cancer models (Coffelt et al., 2015; Lee et al., 2014; Lotti et al., 2013; Wang et al., 2014). In support of these preclinical findings, higher levels of serum IL-17 are associated with poor prognosis for a variety of solid tumors in cancer patients (Punt et al., 2015); several IL-17A polymorphisms have been associated with cancer susceptibility (Al Obeed et al., 2018; Bedoui et al., 2018; Elshazli et al., 2018; Samiei et al., 2018), which would benefit from validation in independent cohorts.
Despite the growing evidence on the pathogenic role of IL-17 in cancer, the underlying molecular and cellular mechanisms are still not completely understood. One emerging concept is that chronic tissue damage and the associated tissue repair process may lead to cancer (Karin and Clevers, 2016; Shalapour and Karin, 2015). Early studies indeed suggested that “tumor production is a possible overhealing” or, alternatively, that “tumors are wounds that do not heal” (Dvorak, 1986; Shalapour and Karin, 2015). IL-17 has been shown to play a critical role in tissue repair in the mucosal surfaces, implicating this cytokine as a link between wound healing and cancer development (Chen et al., 2019). While the relationship between chronic inflammation and cancer is well recognized, knowledge regarding the mechanisms in these processes continues to evolve. In this review, we examine the increasing body of literature supporting the multifaceted role of IL-17 in promoting tumor development, progression, and therapy resistance.
Acute and chronic IL-17 production
A number immune cell types, including Th17 cells (Harrington et al., 2005; Langrish et al., 2005), γδ T cells (Papotto et al., 2017), cytotoxic T cells (CD8+ αβ T cells; Ciric et al., 2009; Hamada et al., 2009), natural killer cells (Cupedo et al., 2009; Michel et al., 2007), and innate lymphoid cells (Buonocore et al., 2010) are capable of producing IL-17. These populations are collectively termed type 17 cells to indicate this unique function (Gaffen et al., 2014). The majority of type 17 cells are subjected to the similar regulatory axis: IL-17 production responds to IL-23 and IL-1 stimulation (Chung et al., 2009; Ivanov et al., 2006; Langrish et al., 2005; Revu et al., 2018; Sutton et al., 2009). At the transcriptional level, while the expression of IL-17 is controlled by the transcription factor STAT3 (Zhou et al., 2007) and RORγt (Ivanov et al., 2006) in all type 17 cells, c-Maf is emerging as another transcriptional factor required specifically for IL-17 production from γδ T cells (Zuberbuehler et al., 2019). While different type 17 cells play spatially and temporally distinct roles in physiological responses, they have all been implicated as sources of IL-17 in chronic inflammatory conditions, including cancer (Cua and Tato, 2010; McGeachy et al., 2019).
Despite the focus of this review, it is important to recognize the vital functions of IL-17 in physiological responses, especially in host defense and barrier protection (Table 1). For example, homeostatic IL-17 activity in the gut critically controls the composition and colonization of gut-resident microbiota by mediating the release of antimicrobial peptides (e.g., defensins; Kumar et al., 2016). Similarly, local and transient IL-17 activity defends the host against opportunistic pathogen, such as Candida albicans in the skin (Naik et al., 2015). Additionally, IL-17–induced expansion and recruitment of neutrophils are central for the control and early clearance of invasive extracellular bacteria and fungi (Cho et al., 2010; Conti et al., 2009; DeLyria et al., 2009; Khader et al., 2007; Sparber et al., 2018). Furthermore, IL-17 helps to maintain the tight junctions between intestinal epithelial cells and promote their proliferation in response to wounding (Lee et al., 2015; Zepp et al., 2017). These activities collectively ensure and enhance the barrier function of mucosal surfaces that are in direct contact with trillions of commensals. In human, blockade of IL-17 activity has been shown to exacerbate disease activity in Crohn’s disease patients (Hueber et al., 2012; Targan et al., 2016). Rare genetic defects in IL-17 signaling components are associated with increased susceptibility to opportunistic infections by extracellular bacteria and fungi (Li et al., 2018).
Table 1. Beneficial and pathogenic activities of IL-17.
| Cause | IL-17–induced response | Outcome | References |
|---|---|---|---|
| Beneficial functions | |||
| C. albicans infection | Neutrophilia (via the induction of G-CSF, CXCL1, etc.) and production of antimicrobial peptides | Clearance of invading fungi | Bär et al., 2014; Conti et al., 2009; Huang et al., 2004; Kagami et al., 2010; Sparber et al., 2018; |
| Staphylococcus aureus, Helicobacter pylori, Mycobacterium tuberculosis, Pseudomonas aeruginosa infections | Neutrophilia (via the induction of G-CSF, CXCL1, etc.) and production of antimicrobial peptides | Clearance of invading extracellular bacteria | Cho et al., 2010; DeLyria et al., 2009; Ferreira et al., 2009; Khader et al., 2007; Priebe et al., 2008 |
| SFB colonization | Production of α-defensin and induction of Pigir, which increased IgA trancytosis | Limiting the SFB expansion | Kumar et al., 2016 |
| Staphylococcus epidermidis colonization | Upregulation of S100A8 and S100A9, and recruitment of neutrophils | Preventing fungal infection | Naik et al., 2015 |
| Colonization by mucosal-resident commensals | Production of RegIIIγ and induction of Pigir, increasing IgA transcytosis | Reinforced intestinal immune barrier | Martínez-López et al., 2019 |
| Acute ETBF colonization | Mucosal proliferation and recruitment of leukocyte | Fight infection and restore the barrier integrity | Geis et al., 2015 |
| Mechanical injury to the skin | Expression of antimicrobial molecules, including RegIIIγ; activation of Lrig1+ skin stem cells and induction of progenies from Lrig1+ cells for tissue repair | Wound closure | Chen et al., 2019; MacLeod et al., 2013 |
| Damage to intestinal epithelium | Enhanced tight junctions among epithelial cells; induction of Plet1+ progenitor cells for tissue repair | Reinforced intestinal physical barrier, restoration of intestinal epithelium | Lee et al., 2015; Song et al., 2015; Zepp et al., 2017 |
| CDE-induced liver inflammation | Liver progenitor cell expansion and differentiation | Liver regeneration | Guillot et al., 2018 |
| Bone injury | Activation of osteoblast | Bone regeneration | Ono et al., 2016 |
| Pathogenic functions | |||
| Chronic ETBF colonization in mice with oncogenic mutation | Recruitment of polymorphonuclear myeloid cells | Colon tumorigenesis | Chung et al., 2018; Wu et al., 2009; Housseau and Sears, 2010; Geis et al., 2015 |
| Oncogenic mutation (Kras, loss of p53)–induced dysbiosis in the lung | Recruitment of neutrophils | Formation of lung adenocarcinoma | Jin et al., 2019 |
| Chemical-induced liver damage | Recruitment of MDSCs | Liver tumorigenesis | Sun et al., 2016 |
| Chemical/wounding-induced skin inflammation and injury | Proliferation of Lrig1+ skin stem, expansion and migration of progenies of Lrig1+ stem cells for tissue repair | Skin tumorigenesis | Chen et al., 2019; Wang et al., 2009; Wu et al., 2015 |
| Damage to intestinal epithelium | Induction of Plet1+ progenitor cells for tissue repair | Colon tumorigenesis | Zepp et al., 2017 |
| Compromised intestinal barrier integrity from loss of tumor suppressor gene Apc | Proliferation of transformed enterocytes, induction of IL-6 | Colon tumorigenesis | Wang et al., 2014; Grivennikov et al., 2012 |
| Oncogenic mutation (Kras) | Induction of stem cell phenotype in transformed pancreatic cells | Pancreatic tumorigenesis | McAllister et al., 2014; Zhang et al., 2018 |
Gray shading indicates induction of inflammatory mediators; yellow shading indicates activation of cell proliferation. CDE, ethionine-supplemented; SFB, segmented filamentous bacteria.
In contrast to the self-limiting and acute IL-17 activity during a physiological response, chronic IL-17 production has been shown to drive tumor formation and progression (Table 1). Dysregulated IL-17 production can be triggered by an intractable pathogenic microbiota, which prompts a continued attempt by the immune system to reign in the uncontrolled and invasive colonization. For instance, persistent dysbiosis promotes the formation of lung adenocarcinoma by triggering sustained IL-17 production from γδ T cells. Another example is the intestinal colonization by the enterotoxigenic Bacteroides fragilis (ETBF), a carcinogenic bacteria that enhances IL-17–dependent colon tumorigenesis in genetically susceptible mice bearing defective tumor-suppressor gene Apc (Chung et al., 2018). Interestingly, mucosa tissue from familial adenomatous polyposis patients was associated with the presence of ETBF and a subtype of Escherichia coli that was also implicated in colon carcinogenesis (Dejea et al., 2018). Furthermore, combined colonization by these two bacterial strains induced robust colon tumorigenesis in an IL-17–dependent manner in recipient mice, pointing to a role of IL-17 in early tumorigenesis in human. In addition to aberrant commensals, repeated tissue injury is also known to enhance IL-17–dependent tumorigenesis in mouse models of skin and colon cancer. In this case, sustained tissue repair instigates the proliferation of premalignant cells, leading to tumor formation. These studies suggest the very same IL-17–orchestrated responses that mediate host defense and barrier protection can turn into pathogenic driving tumor formation when the reaction becomes chronic (Table 1). In the following sections, we discuss how two major IL-17–induced cellular responses, the production of inflammatory mediators and activation of cell proliferation, contribute to tumor formation and progression.
IL-17–induced inflammatory response and cancer
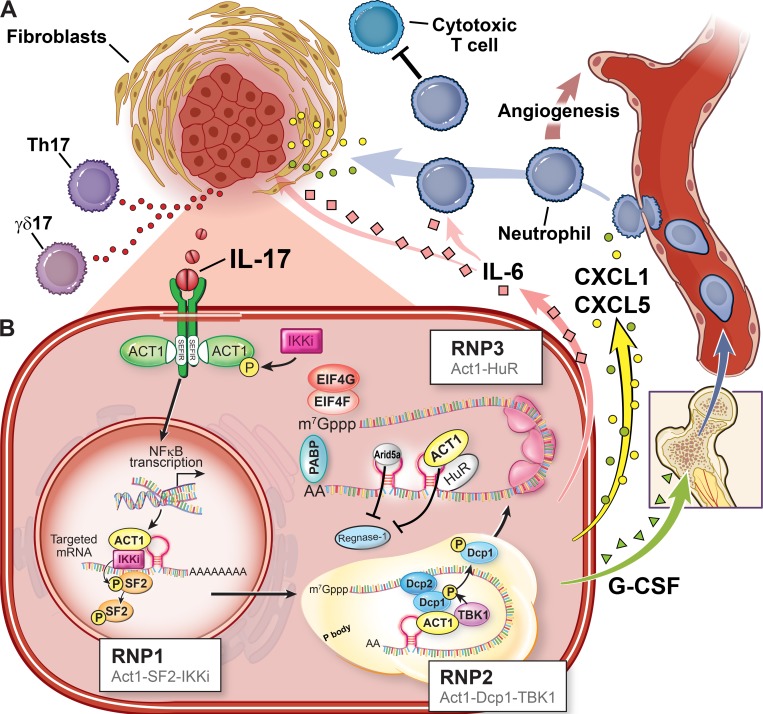
Chronic IL-17 activity leads to a protumor microenvironment (Fig. 1 A). This effect is dependent on its ability to induce the production of inflammatory mediators, mobilizing myeloid cells and reshaping the phenotype of stromal cells.
Figure 1.
IL-17 induces inflammatory mediators to promote tumor progression. (A) IL-17 stimulates the production of myeloid-mobilizing cytokines (e.g., G-CSF) to expand myeloid cells, predominantly neutrophils or granulocytic MDSCs. These expanded myeloid cells are subsequently recruited to the tumor tissue by IL-17–induced chemokines (e.g., CXCL1 and CXCL5). The recruited myeloid cells can promote tumor progression by augmenting angiogenesis and suppressing antitumor immunity. In addition, IL-17–induced protumoral cytokines such as IL-6 function in a paracrine manner to enhance tumor growth and survival. (B) IL-17 induces the production of inflammatory mediators by activating transcription (e.g., NF-κB) and posttranscriptional regulation of gene expression. While Act1 is the adaptor protein for IL-17R, it also functions as a crucial RNA-binding protein that directs the formation of compartmentally distinct RNA–protein complexes to regulate the fate of otherwise unstable mRNAs. As part of the feedforward, self-reinforcing mechanism, Arid5a is induced by IL-17 to suppress the nuclease Regnase-1. Additionally, Regnase-1 is phosphorylated by TBK1/IKKi and thereby removed from the polysomes in an Act1-dependent manner. AA represents the poly A tail; P indicates a phosphorylation event. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2019. All rights reserved.
IL-17 induces inflammatory mediators at both transcriptional and posttranscriptional levels
IL-17 binds the IL-17R to trigger inflammatory response by inducing proinflammatory cytokines and chemokines from epithelial and stromal cells. This is achieved through a combination of weak transcriptional changes (activation of NF-κB and C/EBP) and less well-defined but more robust posttranscriptional changes that include stabilization of specific mRNAs and protein translation. Cytokine and chemokine mRNAs have short half-lives because of conserved cis elements within the 3′ untranslated regions that can be recognized by RNA-binding proteins (e.g., SF2) and mediate the sequential deadenylation, decapping, and ultimately exonucleolytic degradation of the RNA (Amatya et al., 2018; Bulek et al., 2011; Garg et al., 2015; Herjan et al., 2013, 2018; Somma et al., 2015; Sun et al., 2011). While multiple mRNA destabilizing mechanisms have been discovered, little is understood about the stabilization of mRNAs encoding inflammatory factors. Act1 is the key adaptor molecule directly recruited to IL-17R and is required for both the transcriptional and posttranscriptional changes induced by IL-17 (Chang et al., 2006; Herjan et al., 2018; Qian et al., 2007).
The mechanisms by which extrinsic signals are relayed to specific RNA-binding proteins to regulate select cohorts of mRNAs remain poorly understood. A recent progress was the unanticipated discovery that Act1 directly binds RNA (Herjan et al., 2018). This finding provides an example of a receptor-interacting adaptor molecule, Act1, playing a direct role in mRNA metabolism, orchestrating receptor-mediated selectivity of mRNA stabilization and translation (Fig. 1 B). The SEFIR (similar expression to fibroblast growth factor genes and IL-17R) domain of Act1 recognizes and binds to unique stem-loop structures (termed SEFIR-binding elements) in IL-17 target transcripts, enabling Act1 to direct the formation of three compartmentally distinct protein–RNA complexes that prevent mRNA decay in the nucleus, inhibit mRNA decapping in P-bodies, and promote client mRNA translation in the polyribosomes. The posttranscriptional regulation of mRNA is part of a self-reinforcing and feedforward mechanism that potentiates IL-17 activity. This is illustrated by IL-17–induced expression of Arid5a, which in turn counteracts ribonuclease-mediated degradation of labile transcripts and promotes mRNA translation (Amatya et al., 2018). A set of RNA destabilizers, including SF2, Dcp1/2, Regnase-1, and Roquins, has been shown to balance IL-17–mediated mRNA stabilization. IL-17 was shown to induce the interactions of Act1 with the kinases IKKi and TBK1, and Act1 binding to 3′ untranslated regions stem loops delivers these kinases to specific mRNAs, where they phosphorylate the RNA destabilizers that control mRNA fate (Bulek et al., 2011; Herjan et al., 2018; Tanaka et al., 2019). In the nucleus, the binding of Act1 competes off SF2 from the mRNAs by bringing IKKi to phosphorylate SF2, preventing SF2-mediated mRNA decay. In the cytoplasm, RNA binding of the Act1–TBK1 complex results in phosphorylation of the Dcp1 subunit of the mRNA 5′ decapping complex, resulting in loss of decapping activity and mRNA stabilization. Moreover, TBK1 and IKKi were also able to induce phosphorylation of Regnase-1 in an Act1–dependent manner, followed by the release of Regnase-1 from the ER into the cytosol, thereby losing its mRNA degradation function and promoting expression of IL-17 target genes (Tanaka et al., 2019). The mechanisms for the interplay between the stabilizers and destabilizers under IL-17 will continue to evolve, especially regarding the feedforward mode of action governed by Arid5a.
Because IL-17 is consistently found to be a modest transcriptional activator in vitro, the ability to control the post-transcriptional mRNA metabolism is believed to underlie its potent pro-inflammatory activity in vivo (McGeachy et al., 2019). The impact on mRNA metabolism also enables IL-17 to synergize with other cytokines such as TNF to amplify the inflammatory response (Chiricozzi et al., 2011). Interestingly, IL-17 has been shown to cooperate with a wide range of signaling activators, including IFN-γ, IL-13, TGF-β, and even microbial products (Fabre et al., 2014a; Hall et al., 2017; Kaiko et al., 2019; Teunissen et al., 1998; Verma et al., 2017). Such promiscuity is highly relevant but poorly understood in the context of intratumoral inflammation, which is usually driven by a myriad of factors and exhibits considerable heterogeneity, even among tumors of the same tissue origin. With accumulating evidence demonstrating a fundamental role of intratumoral inflammation in cancer progression and response to therapies, extensive investigations are warranted to determine whether different synergizing partners of IL-17 drive divergent inflammatory outcomes in tumors.
IL-17–induced inflammatory mediators engage myeloid cells to promote cancer progression
IL-17 is exalted as the orchestrator of immunity partly because it promotes the production of inflammatory mediators, predominantly neutrophils, that stimulate the expansion and tissue infiltration of myeloid cells (Veldhoen, 2017). While the recruitment of neutrophils critically contributes to IL-17–mediated host defense (Ye et al., 2001), a population of pathogenic myeloid cells are generated from sustained IL-17 activity as a result of nonresolving inflammation-associated chronic wounding, persistent infection, autoimmune response, or carcinogenesis (Veglia et al., 2018). These IL-17–dependent tumor-promoting myeloid populations are a hallmark in IL-17–sculpted tumor microenvironment (Fig. 1 A). Available evidence indicates that IL-17 mobilizes the myeloid cells via two steps. IL-17 was shown to induce G-CSF expression to promote the expansion of granulocytes in several cancer models (Chung et al., 2013; Coffelt et al., 2015). Additionally, IL-17–mediated production of proinflammatory cytokines such as IL-6 and TNF may play important roles in conferring a suppressive phenotype in the recruited myeloid cells (Veglia et al., 2018).
Referred to as myeloid-derived suppressor cells (MDSCs) or tumor-induced neutrophils, IL-17–dependent myeloid cells are mostly granulocytes that share similar phenotypical markers with neutrophils (Coffelt et al., 2015; Jin et al., 2019; Ma et al., 2014; Wu et al., 2014; Zhuang et al., 2012). In human, the frequencies of intratumoral granulocytic polymorphonuclear MDSCs were found to correlate with IL-17–producing cells in both gastric and colorectal cancers (Wu et al., 2014; Zhuang et al., 2012). The induction of pathogenic myeloid cells is associated with tumor progression in a broad range of IL-17–dependent murine cancer models, including colon cancer (Chung et al., 2018; Thiele Orberg et al., 2017), lung cancer (Chang et al., 2014; Jin et al., 2019), liver cancer (Ma et al., 2014), and breast cancer (Coffelt et al., 2015). In a mouse model of lung adenocarcinoma driven by oncogenic KRAS and IL-17–dependent airway inflammation, depletion of Gr1+CD11b+ cells suppressed tumor growth in the lungs (Chang et al., 2014). Likewise, anti-Gr1 antibody–mediated depletion of granulocytic MDSCs attenuated IL-17–induced tumor growth in a subcutaneous model of hepatocellular carcinoma (Ma et al., 2014). In addition, in a mouse model of spontaneous breast cancer, abrogation of tumor-induced, Ly6G+ neutrophils resulted in significant reductions of pulmonary and lymph node metastases (Coffelt et al., 2015). Collectively, these studies demonstrate that myeloid cells accumulated in IL-17–dependent cancer models contribute to IL-17–mediated tumor progression.
Two main mechanisms have been proposed to underlie the tumor progression mediated by IL-17–mobilized myeloid cells. First, several in vivo studies support the idea that the IL-17–dependent myeloid cells promote tumor progression via the inhibition of antitumor immunity (He et al., 2010; Hayata et al., 2013; Coffelt et al., 2015). For instance, while depletion of tumor-induced neutrophils in the IL-17–dependent model of spontaneous breast cancer metastases improved cytotoxic CD8 T cell function with limited metastases, simultaneous abrogation of cytotoxic T cells in neutrophil-depleted mice restored cancer dissemination (Coffelt et al., 2015). Second, IL-17–mobilized myeloid cells have been shown to express angiogenic factors including Bv8 and MMP9 and promote angiogenesis in several tumor types in mouse (Chang et al., 2014; Chung et al., 2013), fueling tumor progression by enhancing tumor vascularization.
A possible role for IL-17 in remodeling the stromal architecture of tumor microenvironment
An underexplored aspect of the IL-17 activity in tumor microenvironment is its impact on the cancer-associated fibroblasts (CAFs). Accumulating data suggest that CAFs can be a driving force behind cancer progression (Su et al., 2018; Yamauchi et al., 2018). IL-17 has been shown to promote pathological fibrosis in the lung (Park et al., 2018), intestine (Honzawa et al., 2014), and the liver (Meng et al., 2012; Tan et al., 2013). At the cellular level, IL-17 can activate many primary and immortalized fibroblasts (Hata et al., 2002; Qian et al., 2007) and promote their proliferation, providing a potential mechanism for IL-17 to mediate fibrosis (Majumder et al., 2019). Moreover, IL-17 can synergize with suboptimal doses of TGF-β in mediating the expression of profibrotic genes (Fabre et al., 2014b). Therefore, it is possible that IL-17 might remodel stromal architecture in the tumor to promote tumor growth as well as resistance to therapy. Future studies are required to elucidate the cell type–specific role of IL-17 signaling in CAFs during tumorigenesis and tumor progression.
In summary, via the transcriptional activation and receptor-mediated stabilization of select mRNAs, IL-17 critically fosters a favorable microenvironment for tumor progression by inducing the production of inflammatory mediators in cooperation with a wide range of ligands abundant in the tumor microenvironment.
IL-17–induced mitogenic signaling and cancer
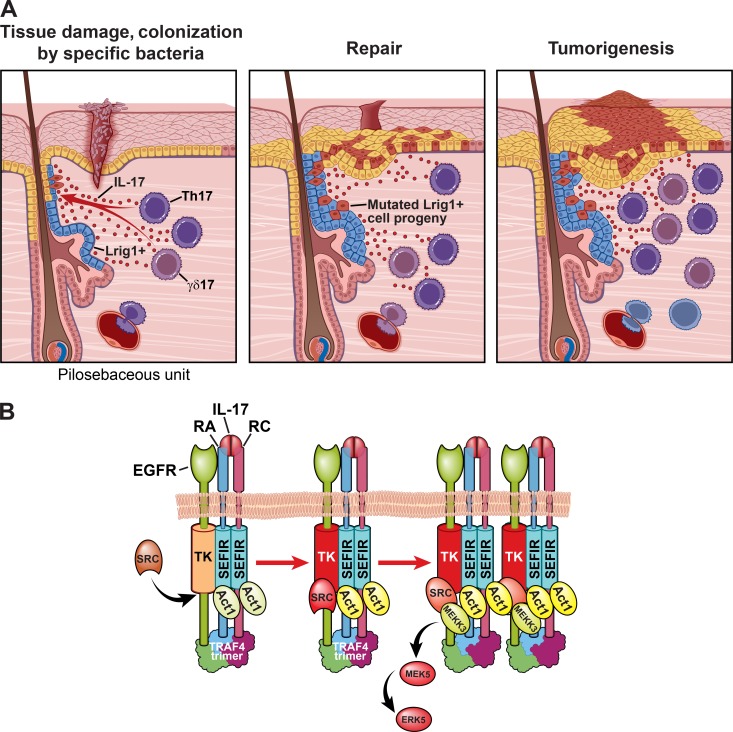
Besides the impact on tumor microenvironment, recent studies have discovered new dimensions of IL-17 activity that directly promotes the proliferation of premalignant cells, which plays a crucial role in the early stage of tumorigenesis (Fig. 2 A).
Figure 2.
IL-17 signaling links wound healing to tumor growth. (A) IL-17 stimulates the proliferation of Lrig1+ cells and promotes the expansion and migration of their progeny. Expanded Lrig1+ progeny migrate out of the hair follicle and participate in reepithelialization. In the presence of oncogenic mutations such as KrasG12D, the IL-17–expanded progeny of Lrig1+ cells contribute majorly to wound- or inflammation-induced tumor tissue. (B) IL-17 stimulation in Lrig1+ cells leads to the recruitment of EGFR by TRAF4 to the IL-17R complex. The IL-17R adaptor Act1 then recruits Src to the receptor complex, resulting in the transactivation of EGFR. EGFR subsequently phosphorylates MEKK3, initiating the MEKK3–MEK5–ERK5 cascade. RA, IL-17 receptor A; RC, IL-17 receptor C; SRC, proto-oncogene tyrosine-protein kinase Src; TK, tyrosine kinase domain. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2019. All rights reserved.
Mitogenic IL-17 signaling choreographs stem cell activity
Normal tissue homeostasis at mucosal surfaces such as the skin and intestine is maintained by a steady turnover of epithelial cells generated by adult tissue stem cells (Clevers, 2013; Ge and Fuchs, 2018). The dynamic turnover is tightly controlled to prevent abnormal tissue growth. The action of mitogenic factors (growth factors and morphogens) required for tissue stem cell self-renewal is often anatomically restricted in a niche, such as the crypt in the intestine and hair follicle in the skin, to limit the proliferative activity to a confined compartment (Farin et al., 2016; Yang et al., 2017). Moreover, adult tissue stem cells also express negative regulators that restrict the activity of potent mitogenic stimuli (Page et al., 2013; Powell et al., 2012; Wong et al., 2012). For example, intradermal injection of epidermal growth factor (EGF) or transgenic overexpression of epidermal growth factor receptor (EGFR) ligands does not induce epidermal growth in adult mice (Cohen, 1962; Cohen and Elliott, 1963; Vassar and Fuchs, 1991). In contrast, infection and tissue injury can readily accelerate the proliferation of tissue stem cells in order to rapidly supply new epithelial cells that migrate to and repair a breached barrier. While inflammatory response has been proposed to be contribute to tissue repair (Karin and Clevers, 2016), it remains unclear whether and how inflammatory cytokines influence stem cells during tissue regeneration.
Accumulating evidence indicates that cytokines can directly regulate of stem cell activity (Biton et al., 2018; Gronke et al., 2019; Hanash et al., 2012). In particular, IL-17 was recently found to be a critical inflammatory signal that activates a group of Lrig1+ stem cells that normally residing in the hair follicle to participate in wound healing in the skin (Chen et al., 2019). A novel IL-17–induced EGFR-mediated Act1–TRAF4–ERK5 axis was discovered in the Lrig1+ stem cells (Chen et al., 2019; Wu et al., 2015). Upon IL-17 stimulation, IL-17R recruits EGFR to the receptor complex. IL-17R hijacks the tyrosine kinase activity of EGFR to activate a MEK3–MEK5 complex that, in turn, phosphorylates the effector kinase ERK5 to promote the Lrig1+ stem cell proliferation and migration (Fig. 2 B). Notably, the recruitment of EGFR to the IL-17R complex is driven by TRAF4, which binds to motifs in IL-17Rs and EGFRs and brings the receptors in close proximity. The close proximity of IL-17R and EGFR allows the adaptor protein Act1 to recruit c-Src for IL-17A–induced EGFR transactivation, enabling the activation of the MEK3–MEK5–ERK5 axis. Additionally, a substantial body of literature indicates that IL-17 stimulation is able to directly promote cell proliferation (e.g., keratinocytes and intestinal epithelial cells) by activating mitogenic signaling pathways such as ERK1/2 (Göktuna et al., 2016; Ha et al., 2014; Qian et al., 2007; Shen et al., 2009; Song et al., 2015; Wang et al., 2014; Zepp et al., 2017). Notably, ERK1/2 and ERK5 were shown to regulate distinct sets of genes (Schweppe et al., 2006). The in vivo distinct functions of ERK1/2 versus ERK5 have been demonstrated by the obligatory role of IL-17A–induced ERK5 activation in the KRAS G12D-driven, wound-induced tumorigenesis model (Chen et al., 2019). Future studies are required to delineate the possible cooperativity between IL-17A–induced ERK1/2 and ERK5 activation in orchestrating stem cell activity during tissue repair and regeneration.
Interestingly, Lrig1 is a negative regulator of EGF-induced EGFR activation (Gur et al., 2004), and Lrig1+ cells do not contribute to the homeostasis of the epidermis outside of the hair follicle in the steady state (Jensen et al., 2009; Page et al., 2013; Schepeler et al., 2014). However, in response to wounding or inflammation, these cells are enlisted to generate progenies that proliferate and migrate out of the hair follicle to contribute to reepithelialization (Page et al., 2013). Since Lrig1 suppresses EGFR signaling in these cells, the ability of IL-17 to transactivate EGFR is crucial in calling this population of cells into action, as inflammation is usually caused by environmental insults that represent a state of emergency. The impact of IL-17 on Lrig1+ cells is an example of how a proinflammatory cytokine coordinates the activity of stem cells in response to wounding for tissue repair and tumorigenesis. An array of different stem cells marked by distinct receptors (e.g., Lgr5 and Lgr6) can be found in the skin (Kretzschmar et al., 2016; Yang et al., 2017). It is possible that the other stem cells may be enlisted by additional inflammatory cytokines to contribute to repairing the wounded skin.
In addition to the skin, the intestinal crypts have Lrig1+ cells (Powell et al., 2012; Wong et al., 2012) overlapping with a subgroup of Lgr5+ cells (Poulin et al., 2014), which is thought to be a reserved stem cell population that can be engaged for intestinal regeneration (Bankaitis et al., 2018). In the stomach, Lrig1 also marks a group of progenitor cells that contribute to damage recovery (Choi et al., 2018). Although further studies are required to determine whether IL-17–induced EGFR-mediated ERK5 activity also operates in these Lrig1+ cells, available evidence shows that IL-17 activity induces the emergence of a highly proliferative progenitor cell population marked by the protein Plet1 from Lgr5+ cells during intestinal inflammation (Zepp et al., 2017). Hence, choreographing stem cell activity is emerging as a new paradigm of IL-17 function in mucosal surfaces during inflammation and tissue repair.
IL-17 signaling links wound healing to tumor growth
The mitogenic signal of IL-17 is crucial for the maintenance and repair of tissue barrier function. This protective role of IL-17 was first appreciated in patients with inflammatory bowel disease, whose symptoms were paradoxically aggravated by blockade of IL-17 activity that was intended to quench the chronic intestinal inflammation (Hueber et al., 2012; Targan et al., 2016). It is now understood that IL-17 is indispensable for tissue regeneration in response to injury in the gut (Lee et al., 2015; Song et al., 2015; Zepp et al., 2017). In addition, impaired IL-17 response has been shown to inhibit liver regeneration after hepatectomy (Furuya et al., 2013; Rao et al., 2014) and delay the reepithelialization of incisional wounds in mouse models (Chen et al., 2019; MacLeod et al., 2013).
Tumors have been proposed to be the wounds that do not heal (Dvorak, 1986). Accumulating evidence suggests that IL-17 links inflammation, wound healing, and tumorigenesis. Incisional wounds in mice that carry the oncogenic Kras in Lrig1+ cells drive skin tumorigenesis (Page et al., 2013). While IL-17–induced progenies of Lrig1+ cells critically contributes to wound healing in the presence of the oncogene, the IL-17–induced expansion and migration of Lrig1+ progeny are required for wounding-induced skin tumorigenesis (Chen et al., 2019; Fig. 2 B). In a chemical-induced skin cancer model driven by IL-17 signaling, lineage tracing has shown that the Lrig1+ progeny comprise the majority of tumor mass, indicating that the same cellular process mediates inflammation-associated tumorigenesis (Chen et al., 2019). In addition, IL-17A regulates the development of stem cell features in both mouse and human pancreatic cancer models and critically contributes to tumor growth and progression (McAllister et al., 2014; Zhang et al., 2018). Intriguingly, IL-17 can also promote the expansion of liver progenitor cells to promote liver regeneration (Guillot et al., 2018); the absence of IL-17 signaling ablates tumorigenesis in a chemical-induced hepatocellular carcinoma (Sun et al., 2016), an inflammation-driven liver cancer model. This IL-17–driven cellular mechanism linking tissue repair to tumorigenesis probably also applies to colon cancer. While IL-17 signaling in transformed enterocytes promotes adenoma formation (Wang et al., 2014), IL-17 signaling induced the expansion of intestinal stem cells contributing the repair of intestinal epithelium (Song et al., 2015; Zepp et al., 2017). Therefore, IL-17–mediated choreographing of stem cell activity appears to be a recurring paradigm underlying both IL-17–mediated tissue repair and tumorigenesis.
In addition to directly engaging mitogenic kinases such as ERK5, IL-17 has also been shown to promote tumor cell proliferation via its target genes. IL-17–induced IL-6 production enhanced the growth of implanted syngeneic tumors (Wang et al., 2009) and was shown to partially contribute to tumorigenesis in the colon (Wang et al., 2014). Notably, IL-17–induced IL-6 production from the tumor microenvironment activates tumor-intrinsic STAT3 to promote its growth (Wang et al., 2009). Of interest, IL-6 it also engages the Src–YAP module to promote epithelial regeneration (Taniguchi et al., 2015) and colonic tumorigenesis (Gregorieff et al., 2015; Taniguchi et al., 2017), implicating the multiple downstream effector functions of the IL-17–IL-6 axis. Additionally, in a mouse model of prostate cancer, IL-17 drives growth and progression of prostate adenocarcinoma by instigating MMP7 production (Zhang et al., 2012, 2017). Taken together, these studies indicate that IL-17 can employ a multitude of mechanisms to support early stages of tumor formation as well as tumor progression.
IL-17 in anticancer therapies
Resistance to chemotherapy and radiation therapy
Despite the growing number of new therapeutic modalities, chemotherapy and radiotherapy remain the mainstays in the standard of care for many advanced-stage malignancies (André et al., 2015; Berry et al., 2005; Karagkounis et al., 2018; Pignon et al., 2009). These conventional treatments are more than palliative, as they prevent disease recurrence and provide survival benefit when the disease is responsive (Karagkounis et al., 2018). Unfortunately, only a small percentage of patients are complete responders. The resistant residual viable tumor cells can be a source of recurrence and metastasis. Hence, there is strong clinical interest in identifying biological factors that enhance or hinder tumor response. Because intratumoral inflammation is implicated in driving therapy failure (Ritter and Greten, 2019), IL-17 is now being examined as a cause of chemoresistance. Supporting evidence includes the presence of IL-17–activated prosurvival and mitogenic signaling and conferred resistance to clinically used cytotoxic agents in a variety of cancer cell lines (Bi et al., 2016; Cochaud et al., 2013; Lotti et al., 2013; Sui et al., 2019). In addition, low-dose radiation induced IL-17 in the tumor beds and enhanced the growth of subsequently implanted tumor in an IL-17–dependent manner in a mouse model (Lee et al., 2014). These results suggest that IL-17 may indeed contribute to therapy resistance.
Both chemotherapy and radiotherapy are external insults designed to cause injury, albeit intended malignant tissues. Accordingly, the very same signaling and cellular mechanisms by which IL-17 drives tissue repair and tumorigenesis may also contribute to the “healing” of tumor in response to chemotherapy and radiotherapy. Interestingly, the choreographing of stem cell activity by IL-17 signaling in tissue repair and tumorigenesis appears to hold true in the highly tumorigenic, stem-like cancer-initiating cells, which have been shown in certain cancers to be the culprit of metastasis and disease recurrence (Prager et al., 2019). IL-17 promotes the self-renewal and thereby the maintenance of cancer-initiating cells in colorectal and ovarian cancer (Lotti et al., 2013; Xiang et al., 2015). In addition, IL-17 induced quiescent gastric cancer stem cells to acquire features of epithelial-to-mesenchymal transition (Xiang et al., 2015), a cellular process associated with cancer metastasis as well as therapy resistance (Aiello and Kang, 2019). Moreover, since Lrig1 can be induced in cancer cells undergoing epithelial-to-mesenchymal transition (Voon et al., 2013; Wong et al., 2013), expression of Lrig1 would set stage for the IL-17–EGFR–ERK5 axis and bestow a survival advantage in response to the chemotherapy and radiotherapy.
IL-17 in immunotherapy
Cancer treatment is now in the era of immunotherapy. While the indications continue to expand, only a small subset of patients may benefit from checkpoint inhibitors such as anti-PD1. Although there is limited information as to the role of IL-17 in modifying the response to checkpoint inhibitors, correlative evidence suggests that IL-17 activity may drive resistance to antitumor immunity and contribute to the therapeutic failure. Th17 signature was found to associate with poor prognosis in colorectal cancer patients (Tosolini et al., 2011). Intriguingly, Th17 cells exist in much higher frequency in microsatellite stable tumors than in tumors with microsatellite instability in colorectal cancer patients (Pushalkar et al., 2018). Incidentally, recent studies have identified microsatellite instability as a predicative marker for response to checkpoint inhibitors (Le et al., 2015, 2017; Vilar and Gruber, 2010). Thus, there is possibly an unexplored negative association between Th17 cells and response to checkpoint inhibitors. In support of this idea, data from recent clinical analysis implicated IL-17 signature in the resistance to anti-PD1 therapies in colorectal cancer (Llosa et al., 2019) and melanoma patients (Gopalakrishnan et al., 2018). The evidence raises the possibility that anti–IL-17 may help to improve the response to checkpoint inhibitors.
Conclusions and perspectives
This review summarized the literature regarding how IL-17–mediated inflammatory response and mitogenic signaling exert the various impacts on tumor development, progression, and resistance to therapies. IL-17–induced inflammatory mediators such as G-CSF, IL-6, and CXCL1 stimulate the expansion and recruitment of dysfunctional myeloid cells to establish a proangiogenic and immune suppressive tumor environment that enhances tumor growth and metastasis. Discoveries of receptor-directed mRNA metabolism via mRNA stabilizers (e.g., Act1 and Aria5) of inflammatory mRNAs provide possible new therapeutic approaches to intervene IL-17–dependent inflammatory response in cancer. Another major recent development in the mechanism by which IL-17 contributes to tumor formation and progression is the discovery of IL-17–mediated direct mitogenic signaling in tissue stem cells such as Lrig1+ cells in the skin and colon. The activation of a unique IL-17–mediated EGFR–ERK5 axis in the tissue stem cells via the integration of IL-17 with EGFR signaling helps to explain the critical link of IL-17 in tissue repair and cancer. While the tissue repair response is called into action when conventional cytotoxic therapies are applied to the tumor tissue, future studies are required to elucidate the potential roles of IL-17–mediated inflammatory response and mitogenic signaling in therapy resistance.
Several biologics that effectively block IL-17 activity in humans have been approved by the US Food and Drug Administration for treating autoinflammatory diseases. These therapeutic agents may potentially be employed as adjuvant treatment to overcome resistance to chemotherapies and radiotherapies. To this end, preclinical studies are still needed to warrant clinical trials, and biomarkers for intratumor IL-17 activity may be required to identify responsive patients. Cancer treatment is now in the era of immunotherapy. Although there is limited information as to the role of IL-17 in regulating the response to checkpoint inhibitors, based on the studies discussed in this review, it is important to examine whether neutralizing IL-17 sensitizes resistant tumors to cancer immunotherapy. Since IL-17 is emerging as a driver of immune-related adverse events in checkpoint inhibitor–treated patients, adding anti–IL-17 to standard checkpoint inhibitor regimen offers the enticing potential of killing both the tumor and autoimmune side effects with “one” shot.
Acknowledgments
X. Li was supported by National Institutes of Health grants P01CA062220 and P01HL103453 and National Multiple Sclerosis Society grant RG5130A2/1.
The authors declare no competing financial interests.
Author contributions: J. Zhao, X. Chen, T. Herjan, and X. Li wrote the manuscript.
References
- Aiello N.M., and Kang Y.. 2019. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 216:1016–1026. 10.1084/jem.20181827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Obeed O.A., Vaali-Mohamed M.A., Alkhayal K.A., Bin Traiki T.A., Zubaidi A.M., Arafah M., Harris R.A., Khan Z., and Abdulla M.H.. 2018. IL-17 and colorectal cancer risk in the Middle East: gene polymorphisms and expression. Cancer Manag. Res. 10:2653–2661. 10.2147/CMAR.S161248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya N., Childs E.E., Cruz J.A., Aggor F.E.Y., Garg A.V., Berman A.J., Gudjonsson J.E., Atasoy U., and Gaffen S.L.. 2018. IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a. Sci. Signal. 11:eaat4617 10.1126/scisignal.aat4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André T., de Gramont A., Vernerey D., Chibaudel B., Bonnetain F., Tijeras-Raballand A., Scriva A., Hickish T., Tabernero J., Van Laethem J.L., et al. . 2015. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 33:4176–4187. 10.1200/JCO.2015.63.4238 [DOI] [PubMed] [Google Scholar]
- Baeten D., Sieper J., Braun J., Baraliakos X., Dougados M., Emery P., Deodhar A., Porter B., Martin R., Andersson M., et al. MEASURE 2 Study Group . 2015. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 373:2534–2548. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- Bankaitis E.D., Ha A., Kuo C.J., and Magness S.T.. 2018. Reserve Stem Cells in Intestinal Homeostasis and Injury. Gastroenterology. 155:1348–1361. 10.1053/j.gastro.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär E., Whitney P.G., Moor K., Reis e Sousa C., and LeibundGut-Landmann S.. 2014. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 40:117–127. 10.1016/j.immuni.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Bedoui S.A., Barbirou M., Stayoussef M., Dallel M., Mokrani A., Makni L., Mezlini A., Bouhaouala B., Yacoubi-Loueslati B., and Almawi W.Y.. 2018. Association of interleukin-17A polymorphisms with the risk of colorectal cancer: A case-control study. Cytokine. 110:18–23. 10.1016/j.cyto.2018.04.017 [DOI] [PubMed] [Google Scholar]
- Berry D.A., Cronin K.A., Plevritis S.K., Fryback D.G., Clarke L., Zelen M., Mandelblatt J.S., Yakovlev A.Y., Habbema J.D., and Feuer E.J.. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators . 2005. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 353:1784–1792. 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- Bi L., Wu J., Ye A., Wu J., Yu K., Zhang S., and Han Y.. 2016. Increased Th17 cells and IL-17A exist in patients with B cell acute lymphoblastic leukemia and promote proliferation and resistance to daunorubicin through activation of Akt signaling. J. Transl. Med. 14:132 10.1186/s12967-016-0894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton M., Haber A.L., Rogel N., Burgin G., Beyaz S., Schnell A., Ashenberg O., Su C.W., Smillie C., Shekhar K., et al. . 2018. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell. 175:1307–1320.e22. 10.1016/j.cell.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulek K., Liu C., Swaidani S., Wang L., Page R.C., Gulen M.F., Herjan T., Abbadi A., Qian W., Sun D., et al. . 2011. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat. Immunol. 12:844–852. 10.1038/ni.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., and Powrie F.. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 464:1371–1375. 10.1038/nature08949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.H., and Dong C.. 2007. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17:435–440. 10.1038/cr.2007.35 [DOI] [PubMed] [Google Scholar]
- Chang S.H., Park H., and Dong C.. 2006. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 281:35603–35607. 10.1074/jbc.C600256200 [DOI] [PubMed] [Google Scholar]
- Chang S.H., Mirabolfathinejad S.G., Katta H., Cumpian A.M., Gong L., Caetano M.S., Moghaddam S.J., and Dong C.. 2014. T helper 17 cells play a critical pathogenic role in lung cancer. Proc. Natl. Acad. Sci. USA. 111:5664–5669. 10.1073/pnas.1319051111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., and Kolls J.K.. 2017. Interluekin-17A (IL17A). Gene. 614:8–14. 10.1016/j.gene.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Cai G., Liu C., Zhao J., Gu C., Wu L., Hamilton T.A., Zhang C.J., Ko J., Zhu L., et al. . 2019. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells. J. Exp. Med. 216:195–214. 10.1084/jem.20171849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A., Guttman-Yassky E., Suárez-Fariñas M., Nograles K.E., Tian S., Cardinale I., Chimenti S., and Krueger J.G.. 2011. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131:677–687. 10.1038/jid.2010.340 [DOI] [PubMed] [Google Scholar]
- Cho J.S., Pietras E.M., Garcia N.C., Ramos R.I., Farzam D.M., Monroe H.R., Magorien J.E., Blauvelt A., Kolls J.K., Cheung A.L., et al. . 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120:1762–1773. 10.1172/JCI40891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E., Lantz T.L., Vlacich G., Keeley T.M., Samuelson L.C., Coffey R.J., Goldenring J.R., and Powell A.E.. 2018. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut. 67:1595–1605. 10.1136/gutjnl-2017-313874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., and Dong C.. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A.S., Wu X., Zhuang G., Ngu H., Kasman I., Zhang J., Vernes J.M., Jiang Z., Meng Y.G., Peale F.V., et al. . 2013. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 19:1114–1123. 10.1038/nm.3291 [DOI] [PubMed] [Google Scholar]
- Chung L., Thiele Orberg E., Geis A.L., Chan J.L., Fu K., DeStefano Shields C.E., Dejea C.M., Fathi P., Chen J., Finard B.B., et al. . 2018. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe. 23:203–214.e5. 10.1016/j.chom.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric B., El-behi M., Cabrera R., Zhang G.X., and Rostami A.. 2009. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J. Immunol. 182:5296–5305. 10.4049/jimmunol.0900036 [DOI] [PubMed] [Google Scholar]
- Clevers H. 2013. The intestinal crypt, a prototype stem cell compartment. Cell. 154:274–284. 10.1016/j.cell.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Cochaud S., Giustiniani J., Thomas C., Laprevotte E., Garbar C., Savoye A.M., Curé H., Mascaux C., Alberici G., Bonnefoy N., et al. . 2013. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 3:3456 10.1038/srep03456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., Verstegen N.J.M., Ciampricotti M., Hawinkels L.J.A.C., Jonkers J., and de Visser K.E.. 2015. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 522:345–348. 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. 1962. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 237:1555–1562. [PubMed] [Google Scholar]
- Cohen S., and Elliott G.A.. 1963. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J. Invest. Dermatol. 40:1–5. 10.1038/jid.1963.1 [DOI] [PubMed] [Google Scholar]
- Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W., et al. . 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206:299–311. 10.1084/jem.20081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua D.J., and Tato C.M.. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489. 10.1038/nri2800 [DOI] [PubMed] [Google Scholar]
- Cupedo T., Crellin N.K., Papazian N., Rombouts E.J., Weijer K., Grogan J.L., Fibbe W.E., Cornelissen J.J., and Spits H.. 2009. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10:66–74. 10.1038/ni.1668 [DOI] [PubMed] [Google Scholar]
- Dejea C.M., Fathi P., Craig J.M., Boleij A., Taddese R., Geis A.L., Wu X., DeStefano Shields C.E., Hechenbleikner E.M., Huso D.L., et al. . 2018. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 359:592–597. 10.1126/science.aah3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLyria E.S., Redline R.W., and Blanchard T.G.. 2009. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 136:247–256. 10.1053/j.gastro.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H.F. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315:1650–1659. 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- Elshazli R.M., Salman D.O., Kamel M.M., Toraih E.A., and Fawzy M.S.. 2018. Genetic polymorphisms of IL-17A rs2275913, rs3748067 and IL-17F rs763780 in gastric cancer risk: evidence from 8124 cases and 9873 controls. Mol. Biol. Rep. 45:1421–1444. 10.1007/s11033-018-4202-z [DOI] [PubMed] [Google Scholar]
- Ely L.K., Fischer S., and Garcia K.C.. 2009. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 10:1245–1251. 10.1038/ni.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre T., Kared H., Friedman S.L., and Shoukry N.H.. 2014a IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 193:3925–3933. 10.4049/jimmunol.1400861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre T., Kared H., Friedman S.L., and Shoukry N.H.. 2014b IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 193:3925–3933. 10.4049/jimmunol.1400861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V., de Punder K., Angers S., Peters P.J., Maurice M.M., and Clevers H.. 2016. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 530:340–343. 10.1038/nature16937 [DOI] [PubMed] [Google Scholar]
- Ferreira D.M., Darrieux M., Silva D.A., Leite L.C.C., Ferreira J.M.C. Jr., Ho P.L., Miyaji E.N., and Oliveira M.L.S.. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16:636–645. 10.1128/CVI.00395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J.J., Garrone P., Garcia E., Saeland S., et al. . 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593–2603. 10.1084/jem.183.6.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S., Kono H., Hara M., Hirayama K., Tsuchiya M., and Fujii H.. 2013. Interleukin-17A plays a pivotal role after partial hepatectomy in mice. J. Surg. Res. 184:838–846. 10.1016/j.jss.2013.03.033 [DOI] [PubMed] [Google Scholar]
- Gaffen S.L. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556–567. 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S.L., Jain R., Garg A.V., and Cua D.J.. 2014. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14:585–600. 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A.V., Amatya N., Chen K., Cruz J.A., Grover P., Whibley N., Conti H.R., Hernandez Mir G., Sirakova T., Childs E.C., et al. . 2015. MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity. 43:475–487. 10.1016/j.immuni.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y., and Fuchs E.. 2018. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat. Rev. Genet. 19:311–325. 10.1038/nrg.2018.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis A.L., Fan H., Wu X., Wu S., Huso D.L., Wolfe J.L., Sears C.L., Pardoll D.M., and Housseau F.. 2015. Regulatory T-cell Response to Enterotoxigenic Bacteroides fragilis Colonization Triggers IL17-Dependent Colon Carcinogenesis. Cancer Discov. 5:1098–1109. 10.1158/2159-8290.CD-15-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göktuna S.I., Shostak K., Chau T.L., Heukamp L.C., Hennuy B., Duong H.Q., Ladang A., Close P., Klevernic I., Olivier F., et al. . 2016. The Prosurvival IKK-Related Kinase IKKε Integrates LPS and IL17A Signaling Cascades to Promote Wnt-Dependent Tumor Development in the Intestine. Cancer Res. 76:2587–2599. 10.1158/0008-5472.CAN-15-1473 [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. . 2018. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M.R., Khomchuk Y., and Wrana J.L.. 2015. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 526:715–718. 10.1038/nature15382 [DOI] [PubMed] [Google Scholar]
- Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., Taniguchi K., Yu G.Y., Osterreicher C.H., Hung K.E., et al. . 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258. 10.1038/nature11465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke K., Hernández P.P., Zimmermann J., Klose C.S.N., Kofoed-Branzk M., Guendel F., Witkowski M., Tizian C., Amann L., Schumacher F., et al. . 2019. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 566:249–253. 10.1038/s41586-019-0899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Wu L., and Li X.. 2013. IL-17 family: cytokines, receptors and signaling. Cytokine. 64:477–485. 10.1016/j.cyto.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A., Gasmi I., Brouillet A., Ait-Ahmed Y., Calderaro J., Ruiz I., Gao B., Lotersztajn S., Pawlotsky J.M., and Lafdil F.. 2018. Interleukins-17 and 27 promote liver regeneration by sequentially inducing progenitor cell expansion and differentiation. Hepatol Commun. 2:329–343. 10.1002/hep4.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur G., Rubin C., Katz M., Amit I., Citri A., Nilsson J., Amariglio N., Henriksson R., Rechavi G., Hedman H., et al. . 2004. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 23:3270–3281. 10.1038/sj.emboj.7600342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H.L., Wang H., Pisitkun P., Kim J.C., Tassi I., Tang W., Morasso M.I., Udey M.C., and Siebenlist U.. 2014. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl. Acad. Sci. USA. 111:E3422–E3431. 10.1073/pnas.1400513111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.L., Baker T., Lajoie S., Richgels P.K., Yang Y., McAlees J.W., van Lier A., Wills-Karp M., Sivaprasad U., Acciani T.H., et al. . 2017. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J. Allergy Clin. Immunol. 139:462–471.e14. 10.1016/j.jaci.2016.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Garcia-Hernandez M.L., Reome J.B., Misra S.K., Strutt T.M., McKinstry K.K., Cooper A.M., Swain S.L., and Dutton R.W.. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182:3469–3481. 10.4049/jimmunol.0801814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash A.M., Dudakov J.A., Hua G., O’Connor M.H., Young L.F., Singer N.V., West M.L., Jenq R.R., Holland A.M., Kappel L.W., et al. . 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 37:339–350. 10.1016/j.immuni.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., and Weaver C.T.. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- Hata K., Andoh A., Shimada M., Fujino S., Bamba S., Araki Y., Okuno T., Fujiyama Y., and Bamba T.. 2002. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G1035–G1044. 10.1152/ajpgi.00494.2001 [DOI] [PubMed] [Google Scholar]
- Hayata K., Iwahashi M., Ojima T., Katsuda M., Iida T., Nakamori M., Ueda K., Nakamura M., Miyazawa M., Tsuji T., et al. . 2013. Inhibition of IL-17A in tumor microenvironment augments cytotoxicity of tumor-infiltrating lymphocytes in tumor-bearing mice. PLoS ONE. 8:e53131 10.1371/journal.pone.0053131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Li H., Yusuf N., Elmets C.A., Li J., Mountz J.D., and Xu H.. 2010. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 184:2281–2288. 10.4049/jimmunol.0902574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herjan T., Yao P., Qian W., Li X., Liu C., Bulek K., Sun D., Yang W.P., Zhu J., He A., et al. . 2013. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. 191:640–649. 10.4049/jimmunol.1203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herjan T., Hong L., Bubenik J., Bulek K., Qian W., Liu C., Li X., Chen X., Yang H., Ouyang S., et al. . 2018. IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nat. Immunol. 19:354–365. 10.1038/s41590-018-0071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honzawa Y., Nakase H., Shiokawa M., Yoshino T., Imaeda H., Matsuura M., Kodama Y., Ikeuchi H., Andoh A., Sakai Y., et al. . 2014. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn’s disease. Gut. 63:1902–1912. 10.1136/gutjnl-2013-305632 [DOI] [PubMed] [Google Scholar]
- Housseau F., and Sears C.L.. 2010. Enterotoxigenic Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/-) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle. 9:3–5. 10.4161/cc.9.1.10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Na L., Fidel P.L., and Schwarzenberger P.. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190:624–631. 10.1086/422329 [DOI] [PubMed] [Google Scholar]
- Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D., Wehkamp J., Feagan B.G., Yao M.D., Karczewski M., et al. Secukinumab in Crohn’s Disease Study Group . 2012. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 61:1693–1700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz S.G., Filvaroff E.H., Yin J.P., Lee J., Cai L., Risser P., Maruoka M., Mao W., Foster J., Kelley R.F., et al. . 2001. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20:5332–5341. 10.1093/emboj/20.19.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., and Littman D.R.. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Jensen K.B., Collins C.A., Nascimento E., Tan D.W., Frye M., Itami S., and Watt F.M.. 2009. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 4:427–439. 10.1016/j.stem.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Lagoudas G.K., Zhao C., Bullman S., Bhutkar A., Hu B., Ameh S., Sandel D., Liang X.S., Mazzilli S., et al. . 2019. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 176:998–1013.e16. 10.1016/j.cell.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S., Rizzo H.L., Kurtz S.E., Miller L.S., and Blauvelt A.. 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J. Immunol. 185:5453–5462. 10.4049/jimmunol.1001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko G.E., Chen F., Lai C.W., Chiang I.L., Perrigoue J., Stojmirović A., Li K., Muegge B.D., Jain U., VanDussen K.L., et al. . 2019. PAI-1 augments mucosal damage in colitis. Sci. Transl. Med. 11:eaat0852 10.1126/scitranslmed.aat0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagkounis G., Thai L., Mace A.G., Wiland H., Pai R.K., Steele S.R., Church J.M., and Kalady M.F.. 2018. Prognostic Implications of Pathological Response to Neoadjuvant Chemoradiation in Pathologic Stage III Rectal Cancer. Ann. Surg. 10.1097/SLA.0000000000002719 [DOI] [PubMed] [Google Scholar]
- Karin M., and Clevers H.. 2016. Reparative inflammation takes charge of tissue regeneration. Nature. 529:307–315. 10.1038/nature17039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., et al. . 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377. 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K., Weber C., Driskell R.R., Calonje E., and Watt F.M.. 2016. Compartmentalized Epidermal Activation of β-Catenin Differentially Affects Lineage Reprogramming and Underlies Tumor Heterogeneity. Cell Reports. 14:269–281. 10.1016/j.celrep.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner R.E., Taft D.W., Haran A., Brandt C.S., Brender T., Lum K., Harder B., Okada S., Ostrander C.D., Kreindler J.L., et al. . 2007. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179:5462–5473. 10.4049/jimmunol.179.8.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Monin L., Castillo P., Elsegeiny W., Horne W., Eddens T., Vikram A., Good M., Schoenborn A.A., Bibby K., et al. . 2016. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 44:659–671. 10.1016/j.immuni.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K., Puig L., Nakagawa H., Spelman L., Sigurgeirsson B., et al. FIXTURE Study Group . 2014. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371:326–338. 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., and Cua D.J.. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. . 2015. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372:2509–2520. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. . 2017. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 357:409–413. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.J., Park H.J., Lee I.J., Kim W.W., Ha S.J., Suh Y.G., and Seong J.. 2014. Inhibition of IL-17A suppresses enhanced-tumor growth in low dose pre-irradiated tumor beds. PLoS One. 9:e106423 10.1371/journal.pone.0106423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Tato C.M., Joyce-Shaikh B., Gulen M.F., Cayatte C., Chen Y., Blumenschein W.M., Judo M., Ayanoglu G., McClanahan T.K., et al. . 2015. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 43:727–738. 10.1016/j.immuni.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Casanova J.L., and Puel A.. 2018. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol. 11:581–589. 10.1038/mi.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa N.J., Luber B., Tam A.J., Smith K.N., Siegel N., Awan A.H., Fan H., Oke T., Zhang J., Domingue J., et al. . 2019. Intratumoral Adaptive Immunosuppression and Type 17 Immunity in Mismatch Repair Proficient Colorectal Tumors. Clin. Cancer Res. 25:5250–5259. 10.1158/1078-0432.CCR-19-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti F., Jarrar A.M., Pai R.K., Hitomi M., Lathia J., Mace A., Gantt G.A. Jr., Sukhdeo K., DeVecchio J., Vasanji A., et al. . 2013. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 210:2851–2872. 10.1084/jem.20131195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Cheng Q., Cai Y., Gong H., Wu Y., Yu X., Shi L., Wu D., Dong C., and Liu H.. 2014. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 74:1969–1982. 10.1158/0008-5472.CAN-13-2534 [DOI] [PubMed] [Google Scholar]
- MacLeod A.S., Hemmers S., Garijo O., Chabod M., Mowen K., Witherden D.A., and Havran W.L.. 2013. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Invest. 123:4364–4374. 10.1172/JCI70064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S., Amatya N., Revu S., Jawale C.V., Wu D., Rittenhouse N., Menk A., Kupul S., Du F., Raphael I., et al. . 2019. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat. Immunol. 20:534–545. 10.1038/s41590-019-0367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-López M., Iborra S., Conde-Garrosa R., Mastrangelo A., Danne C., Mann E.R., Reid D.M., Gaboriau-Routhiau V., Chaparro M., Lorenzo M.P., et al. . 2019. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and -22 Production and Promotes Intestinal Barrier Integrity. Immunity. 50:446–461.e9. 10.1016/j.immuni.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F., Bailey J.M., Alsina J., Nirschl C.J., Sharma R., Fan H., Rattigan Y., Roeser J.C., Lankapalli R.H., Zhang H., et al. . 2014. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 25:621–637. 10.1016/j.ccr.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J., and Gaffen S.L.. 2019. The IL-17 Family of Cytokines in Health and Disease. Immunity. 50:892–906. 10.1016/j.immuni.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J., McInnes I.B., Kirkham B., Kavanaugh A., Rahman P., van der Heijde D., Landewé R., Nash P., Pricop L., Yuan J., et al. FUTURE 1 Study Group . 2015. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 373:1329–1339. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- Meng F., Wang K., Aoyama T., Grivennikov S.I., Paik Y., Scholten D., Cong M., Iwaisako K., Liu X., Zhang M., et al. . 2012. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 143:765–776.e3. 10.1053/j.gastro.2012.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M.L., Keller A.C., Paget C., Fujio M., Trottein F., Savage P.B., Wong C.H., Schneider E., Dy M., and Leite-de-Moraes M.C.. 2007. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995–1001. 10.1084/jem.20061551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Bouladoux N., Linehan J.L., Han S.J., Harrison O.J., Wilhelm C., Conlan S., Himmelfarb S., Byrd A.L., Deming C., et al. . 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 520:104–108. 10.1038/nature14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T., Okamoto K., Nakashima T., Nitta T., Hori S., Iwakura Y., and Takayanagi H.. 2016. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 7:10928 10.1038/ncomms10928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.E., Lombard P., Ng F., Göttgens B., and Jensen K.B.. 2013. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 13:471–482. 10.1016/j.stem.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papotto P.H., Ribot J.C., and Silva-Santos B.. 2017. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18:604–611. 10.1038/ni.3726 [DOI] [PubMed] [Google Scholar]
- Park M.-J., Moon S.-J., Lee E.-J., Jung K.-A., Kim E.-K., Kim D.-S., Lee J.-H., Kwok S.-K., Min J.-K., Park S.-H., and Cho M.L.. 2018. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front. Immunol. 9:1611 10.3389/fimmu.2018.01611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon J.P., le Maître A., Maillard E., and Bourhis J.. MACH-NC Collaborative Group . 2009. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92:4–14. 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Poulin E.J., Powell A.E., Wang Y., Li Y., Franklin J.L., and Coffey R.J.. 2014. Using a new Lrig1 reporter mouse to assess differences between two Lrig1 antibodies in the intestine. Stem Cell Res. (Amst.). 13(3 Pt A, 3 pt A):422–430. 10.1016/j.scr.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A.E., Wang Y., Li Y., Poulin E.J., Means A.L., Washington M.K., Higginbotham J.N., Juchheim A., Prasad N., Levy S.E., et al. . 2012. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 149:146–158. 10.1016/j.cell.2012.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager B.C., Xie Q., Bao S., and Rich J.N.. 2019. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell. 24:41–53. 10.1016/j.stem.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe G.P., Walsh R.L., Cederroth T.A., Kamei A., Coutinho-Sledge Y.S., Goldberg J.B., and Pier G.B.. 2008. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 181:4965–4975. 10.4049/jimmunol.181.7.4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt S., Langenhoff J.M., Putter H., Fleuren G.J., Gorter A., and Jordanova E.S.. 2015. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. OncoImmunology. 4:e984547 10.4161/2162402X.2014.984547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S., Hundeyin M., Daley D., Zambirinis C.P., Kurz E., Mishra A., Mohan N., Aykut B., Usyk M., Torres L.E., et al. . 2018. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 8:403–416. 10.1158/2159-8290.CD-17-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Liu C., Hartupee J., Altuntas C.Z., Gulen M.F., Jane-Wit D., Xiao J., Lu Y., Giltiay N., Liu J., et al. . 2007. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8:247–256. 10.1038/ni1439 [DOI] [PubMed] [Google Scholar]
- Rao R., Graffeo C.S., Gulati R., Jamal M., Narayan S., Zambirinis C.P., Barilla R., Deutsch M., Greco S.H., Ochi A., et al. . 2014. Interleukin 17-producing γδT cells promote hepatic regeneration in mice. Gastroenterology. 147:473–84.e2. 10.1053/j.gastro.2014.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revu S., Wu J., Henkel M., Rittenhouse N., Menk A., Delgoffe G.M., Poholek A.C., and McGeachy M.J.. 2018. IL-23 and IL-1β Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Reports. 22:2642–2653. 10.1016/j.celrep.2018.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B., and Greten F.R.. 2019. Modulating inflammation for cancer therapy. J. Exp. Med. 216:1234–1243. 10.1084/jem.20181739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiei G., Yip W.K., Leong P.P., Jabar M.F., Dusa N.M., Mohtarrudin N., and Seow H.F.. 2018. Association between polymorphisms of interleukin-17A G197A and interleukin-17F A7488G and risk of colorectal cancer. J. Cancer Res. Ther. 14(9, Supplement):S299–S305. 10.4103/0973-1482.235345 [DOI] [PubMed] [Google Scholar]
- Schepeler T., Page M.E., and Jensen K.B.. 2014. Heterogeneity and plasticity of epidermal stem cells. Development. 141:2559–2567. 10.1242/dev.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe R.E., Cheung T.H., and Ahn N.G.. 2006. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J. Biol. Chem. 281:20993–21003. 10.1074/jbc.M604208200 [DOI] [PubMed] [Google Scholar]
- Shalapour S., and Karin M.. 2015. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 125:3347–3355. 10.1172/JCI80007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Li N., Gade P., Kalvakolanu D.V., Weibley T., Doble B., Woodgett J.R., Wood T.D., and Gaffen S.L.. 2009. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2:ra8 10.1126/scisignal.2000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma D., Mastrovito P., Grieco M., Lavorgna A., Pignalosa A., Formisano L., Salzano A.M., Scaloni A., Pacifico F., Siebenlist U., and Leonardi A.. 2015. CIKS/DDX3X interaction controls the stability of the Zc3h12a mRNA induced by IL-17. J. Immunol. 194:3286–3294. 10.4049/jimmunol.1401589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Dai D., He X., Zhu S., Yao Y., Gao H., Wang J., Qu F., Qiu J., Wang H., et al. . 2015. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 43:488–501. 10.1016/j.immuni.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Sparber F., Dolowschiak T., Mertens S., Lauener L., Clausen B.E., Joller N., Stoitzner P., Tussiwand R., and LeibundGut-Landmann S.. 2018. Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog. 14:e1007069 10.1371/journal.ppat.1007069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Chen J., Yao H., Liu J., Yu S., Lao L., Wang M., Luo M., Xing Y., Chen F., et al. . 2018. CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 172:841–856.e16. 10.1016/j.cell.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Sui G., Qiu Y., Yu H., Kong Q., and Zhen B.. 2019. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol. Lett. 17:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Novotny M., Bulek K., Liu C., Li X., and Hamilton T.. 2011. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat. Immunol. 12:853–860. 10.1038/ni.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Kono H., Furuya S., Hara M., Hirayama K., Akazawa Y., Nakata Y., and Fujii H.. 2016. Interleukin-17A Plays a Pivotal Role in Chemically Induced Hepatocellular Carcinoma in Mice. Dig. Dis. Sci. 61:474–488. 10.1007/s10620-015-3888-1 [DOI] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., and Mills K.H.. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341. 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Tan Z., Qian X., Jiang R., Liu Q., Wang Y., Chen C., Wang X., Ryffel B., and Sun B.. 2013. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J. Immunol. 191:1835–1844. 10.4049/jimmunol.1203013 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Arima Y., Kamimura D., Tanaka Y., Takahashi N., Uehata T., Maeda K., Satoh T., Murakami M., and Akira S.. 2019. Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. J. Exp. Med. 216:1431–1449. 10.1084/jem.20181078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Wu L.W., Grivennikov S.I., de Jong P.R., Lian I., Yu F.X., Wang K., Ho S.B., Boland B.S., Chang J.T., et al. . 2015. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 519:57–62. 10.1038/nature14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Moroishi T., de Jong P.R., Krawczyk M., Grebbin B.M., Luo H., Xu R.H., Golob-Schwarzl N., Schweiger C., Wang K., et al. . 2017. YAP-IL-6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc. Natl. Acad. Sci. USA. 114:1643–1648. 10.1073/pnas.1620290114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan S.R., Feagan B., Vermeire S., Panaccione R., Melmed G.Y., Landers C., Li D., Russell C., Newmark R., Zhang N., et al. . 2016. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn’s Disease. Am. J. Gastroenterol. 111:1599–1607. 10.1038/ajg.2016.298 [DOI] [PubMed] [Google Scholar]
- Teunissen M.B., Koomen C.W., de Waal Malefyt R., Wierenga E.A., and Bos J.D.. 1998. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111:645–649. 10.1046/j.1523-1747.1998.00347.x [DOI] [PubMed] [Google Scholar]
- Thiele Orberg E., Fan H., Tam A.J., Dejea C.M., Destefano Shields C.E., Wu S., Chung L., Finard B.B., Wu X., Fathi P., et al. . 2017. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 10:421–433. 10.1038/mi.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini M., Kirilovsky A., Mlecnik B., Fredriksen T., Mauger S., Bindea G., Berger A., Bruneval P., Fridman W.-H., Pagès F., et al. . 2011. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 71:1263–1271. 10.1158/0008-5472.CAN-10-2907 [DOI] [PubMed] [Google Scholar]
- Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., and Peschon J.. 2006. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177:36–39. 10.4049/jimmunol.177.1.36 [DOI] [PubMed] [Google Scholar]
- Vassar R., and Fuchs E.. 1991. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 5:714–727. 10.1101/gad.5.5.714 [DOI] [PubMed] [Google Scholar]
- Veglia F., Perego M., and Gabrilovich D.. 2018. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19:108–119. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M. 2017. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18:612–621. 10.1038/ni.3742 [DOI] [PubMed] [Google Scholar]
- Verma A.H., Richardson J.P., Zhou C., Coleman B.M., Moyes D.L., Ho J., Huppler A.R., Ramani K., McGeachy M.J., Mufazalov I.A., et al. . 2017. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2:eaam8834 10.1126/sciimmunol.aam8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar E., and Gruber S.B.. 2010. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 7:153–162. 10.1038/nrclinonc.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon D.C., Wang H., Koo J.K., Chai J.H., Hor Y.T., Tan T.Z., Chu Y.S., Mori S., and Ito Y.. 2013. EMT-induced stemness and tumorigenicity are fueled by the EGFR/Ras pathway. PLoS One. 8:e70427 10.1371/journal.pone.0070427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yi T., Kortylewski M., Pardoll D.M., Zeng D., and Yu H.. 2009. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 206:1457–1464. 10.1084/jem.20090207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Kim M.K., Di Caro G., Wong J., Shalapour S., Wan J., Zhang W., Zhong Z., Sanchez-Lopez E., Wu L.W., et al. . 2014. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 41:1052–1063. 10.1016/j.immuni.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V.W., Stange D.E., Page M.E., Buczacki S., Wabik A., Itami S., van de Wetering M., Poulsom R., Wright N.A., Trotter M.W., et al. . 2012. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat. Cell Biol. 14:401–408. 10.1038/ncb2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.E., Yu J.S., Quigley D.A., To M.D., Jen K.Y., Huang P.Y., Del Rosario R., and Balmain A.. 2013. Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression. Genes Dev. 27:670–682. 10.1101/gad.210427.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.F., Guo Y., Quazi A., Luxenberg D.P., Bennett F., Ross J.F., Qiu Y., Whitters M.J., Tomkinson K.N., Dunussi-Joannopoulos K., et al. . 2007. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282:13447–13455. 10.1074/jbc.M700499200 [DOI] [PubMed] [Google Scholar]
- Wright J.F., Bennett F., Li B., Brooks J., Luxenberg D.P., Whitters M.J., Tomkinson K.N., Fitz L.J., Wolfman N.M., Collins M., et al. . 2008. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181:2799–2805. 10.4049/jimmunol.181.4.2799 [DOI] [PubMed] [Google Scholar]
- Wu P., Wu D., Ni C., Ye J., Chen W., Hu G., Wang Z., Wang C., Zhang Z., Xia W., et al. . 2014. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 40:785–800. 10.1016/j.immuni.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Chen X., Zhao J., Martin B., Zepp J.A., Ko J.S., Gu C., Cai G., Ouyang W., Sen G., et al. . 2015. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212:1571–1587. 10.1084/jem.20150204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Rhee K.-J., Albesiano E., Rabizadeh S., Wu X., Yen H.-R., Huso D.L., Brancati F.L., Wick E., McAllister F., et al. . 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15:1016–1022. 10.1038/nm.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Long H., He L., Han X., Lin K., Liang Z., Zhuo W., Xie R., and Zhu B.. 2015. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 34:165–176. 10.1038/onc.2013.537 [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Barker T.H., Gibbons D.L., and Kurie J.M.. 2018. The fibrotic tumor stroma. J. Clin. Invest. 128:16–25. 10.1172/JCI93554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Adam R.C., Ge Y., Hua Z.L., and Fuchs E.. 2017. Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell. 169:483–496.e13. 10.1016/j.cell.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., and Spriggs M.K.. 1995. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 3:811–821. 10.1016/1074-7613(95)90070-5 [DOI] [PubMed] [Google Scholar]
- Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., et al. . 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527. 10.1084/jem.194.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J.A., Zhao J., Liu C., Bulek K., Wu L., Chen X., Hao Y., Wang Z., Wang X., Ouyang W., et al. . 2017. IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J. Immunol. 199:3849–3857. 10.4049/jimmunol.1601540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu S., Ge D., Zhang Q., Xue Y., Xiong Z., Abdel-Mageed A.B., Myers L., Hill S.M., Rowan B.G., et al. . 2012. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 72:2589–2599. 10.1158/0008-5472.CAN-11-3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu S., Parajuli K.R., Zhang W., Zhang K., Mo Z., Liu J., Chen Z., Yang S., Wang A.R., et al. . 2017. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 36:687–699. 10.1038/onc.2016.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zoltan M., Riquelme E., Xu H., Sahin I., Castro-Pando S., Montiel M.F., Chang K., Jiang Z., Ling J., et al. . 2018. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology. 155:210–223.e3. 10.1053/j.gastro.2018.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ivanov I.I., Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., and Littman D.R.. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Peng L.S., Zhao Y.L., Shi Y., Mao X.H., Chen W., Pang K.C., Liu X.F., Liu T., Zhang J.Y., et al. . 2012. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 143:951–62.e8. 10.1053/j.gastro.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Zuberbuehler M.K., Parker M.E., Wheaton J.D., Espinosa J.R., Salzler H.R., Park E., and Ciofani M.. 2019. The transcription factor c-Maf is essential for the commitment of IL-17-producing γδ T cells. Nat. Immunol. 20:73–85. 10.1038/s41590-018-0274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]