IL-17 plays versatile roles during tumorigenesis. Here, Vitiello and Miller summarize current knowledge in harnessing IL-17–producing γδ and Th17 cells for successful cancer immunotherapy.
Abstract
The role of IL-17 in cancer remains controversial. Emerging evidence suggests that during early oncogenesis IL-17 supports tumor growth, whereas in established tumors IL-17 production by γδ and Th17 cells potentiates antitumor immunity. Consequently, γδ and Th17 cells are attractive targets for immunotherapy in the IL-17 immune axis. To optimize IL-17–based immunotherapy, a deeper understanding of the cytokines dictating IL-17 production and the polarity of γδ and Th17 cells is critical. Here, we delve into the dichotomous roles of IL-17 in cancer and provide insight into the tumor microenvironment conducive for successful IL-17–based γδ and Th17 cell immunotherapy.
Introduction
Immunotherapy has changed the landscape of cancer treatment (Couzin-Frankel, 2013). Remarkable response rates to immune checkpoint blockade were observed in patients whose malignancies previously carried a dismal prognosis, providing hope for those with advanced and metastatic melanoma, renal, and lung cancers (Borghaei et al., 2015; Brahmer et al., 2015; Eggermont et al., 2016; Hodi et al., 2010; Robert et al., 2015; Brahmer, J., et al. 2017. American Association for Cancer Research Annual Meeting. Abstr. CT077). The lack of response to immunotherapy, however, has been disappointing in a variety of other malignancies, including pancreatic cancer, in which an unfavorable, immunosuppressive tumor microenvironment precludes immunotherapy success (Brahmer et al., 2012; Royal et al., 2010). While tumor mutational burden, heterozygosity in patient HLA genotypes, and “immunogenic” gene expression–based tumor sequencing profiles have predicted immunotherapy response in select cancers (Chowell et al., 2018; Prat et al., 2017; Samstein et al., 2019), a deeper understanding of the biochemical microenvironment contributing to resistance is necessary.
IL-17 has been described as a prevalent cytokine in the tumor microenvironment, where it can play dichotomous roles in both cancer growth and tumor elimination (Murugaiyan and Saha, 2009). IL-17 regulates the immune response to microbes, balancing both cytotoxic and tolerant immune profiles that foster symbiosis, but also results in chronic inflammation (Gaffen et al., 2014; Ivanov and Manel, 2010). Subversion of the IL-17 immune axis may be one mechanism by which cancer utilizes the immunosuppressive environment associated with a chronic inflammatory response to undermine the efficacy of immune checkpoint blockade. In this review, we first highlight both the tumor-promoting and tumor-protective properties of IL-17 in the tumor microenvironment. Next, we focus on the generation, function, and polarity of the major inflammatory cells shaping the IL-17 immune axis, namely γδ T cells and T helper 17 (Th17) cells. Finally, we discuss γδ and Th17 immune cell receptor-based approaches and adoptive cell transfer (ACT) strategies that may be used to augment the IL-17 immune axis for cancer immunotherapy.
The roles of IL-17 in the tumor microenvironment
The IL-17 family comprises six cytokines (IL-17A through IL-17F) that ligate five receptors (IL-17RA through IL-17RE; Kolls and Lindén, 2004). For simplification, we refer to IL-17 as the entire group of cytokines and do not differentiate among the six subtypes, which have been shown to have divergent cells of origin and tissue specificity (Iwakura et al., 2011). Furthermore, there are functional discrepancies among IL-17 cytokine subtypes that add complexity to the relationship between IL-17 and the host immune response. IL-17A signaling strength, for example, is nearly 10–30 times as potent as IL-17F signaling, which may explain why IL-17A has been implicated in global immune function, while IL-17F participates more peripherally in mucosal immunity (Gaffen, 2009; Zhou et al., 2007). Regardless, the overarching function of IL-17 is to mediate the response to pathogenic and commensal organisms through varying effects and targets, all of which balance the inflammatory response of the immune system (Iwakura et al., 2011; Kolls and Lindén, 2004). In the “Tumor-promoting functions of IL-17” and “Tumor-protective functions of IL-17” sections below, we highlight the most pertinent roles of IL-17 with regard to cancer initiation, progression, and immunotherapy (Fig. 1).
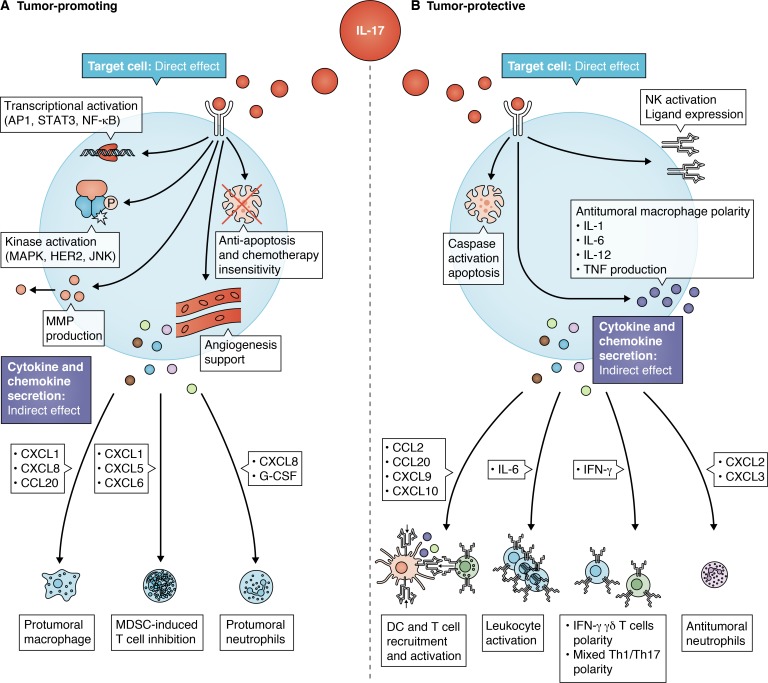
Figure 1.
Functions of IL-17 in cancer. (A) Tumor-promoting effects of IL-17 are directly attributable to increased molecular signaling, tissue remodeling, and angiogenesis while indirectly related to the recruitment of immunosuppressive immune cells. (B) Conversely, the tumor-protective effects of IL-17 are directly related to cancer cell apoptosis, antitumoral immune cell activation, and the induction of IFN-γ+γδ T cells and mixed Th1/Th17 cells. MDSC, myeloid-derived suppressor cell.
Tumor-promoting functions of IL-17
IL-17 has tumor-promoting effects by directly stimulating cancer cells as well as by indirectly inducing an immunosuppressive tumor environment. IL-17 binds IL-17R on tumor cells, signaling the downstream activation of transcription factors (NF-κB, STAT, and AP-1), kinases (MAPK and HER1), tissue remodeling matrix metalloproteinases (MMPs), and anti-apoptotic proteins (Akt, Erk, mTOR, Bcl-2, and Bax) in a myriad of cancers. For example, IL-17 ligation stimulates the proliferation and self-renewal of ovarian cancer stem cells in a dose-dependent fashion via the NF-κB and MAPK pathways (Xiang et al., 2015). Similarly, IL-17 ligation up-regulates NF-κB signaling in a dose-dependent fashion in glioblastoma cell lines (Kehlen et al., 1999); mediates intracellular NF-κB, MAPK, and AP-1 activity in gastric cancer (Zhou et al., 2007); and promotes hepatocellular carcinoma invasion and prostate cancer epithelial to mesenchymal transition in vivo via MMP-2, MMP-7, MMP-9, and NF-κB signal transduction (Li et al., 2011a; Liu et al., 2016). Finally, IL-17 directly contributes to the proliferation of keratinocytes via the IL-17R-Act1-TRAF4-MEKK3-ERK5 circuit in skin cancer, and promotes MMP-dependent cell invasion, supports angiogenesis, inhibits TGF-β–dependent cellular apoptosis, and enhances MEK-, ERK-, JNK-, and STAT3-mediated cell proliferation in breast cancer (Fabre et al., 2018; Wu et al., 2015b). Thus, IL-17 has been implicated in the oncogenesis of many tumor types.
IL-17–mediated ERK activation and HER1 phosphorylation also promote resistance to docetaxel-based chemotherapy and tyrosine kinase inhibition, highlighting the role of IL-17 not only in cancer cell growth but also as a mechanism of treatment resistance (Cochaud et al., 2013; Merrouche et al., 2016). IL-17 ligation on pancreatic cancer cells directly up-regulates ERK signaling, which increases cancer cell invasion and endothelial cell migration and supports the survival of cancer cells at distant organs (Wu et al., 2015a). Notably, treatment with an antagonistic IL-17 antibody blocks the development of pancreatic cancer metastasis in a murine xenograft model. In colorectal cancer, both secretory and membrane-bound forms of IL-17 can contribute to cell cycle progression and oncogenesis (Al-Samadi et al., 2016; Cui et al., 2012; Do Thi et al., 2016). The amount of IL-17 also correlates directly with the severity of dysplasia in the colonic adenoma-to-carcinoma sequence, making IL-17 an attractive cytokine for colon cancer diagnosis and severity (Wu et al., 2013). Together, these results suggest that IL-17 supports tumor growth, tumor progression, treatment resistance, and metastasis.
IL-17 also indirectly shapes the immune cell microenvironment through chemokines and cytokines to support cancer cell proliferation. Specifically, IL-17 induces the expression of CXCL1, CXCL5, CXCL6, and CXCL8, which enhances the immune suppressive function of myeloid-derived suppressor cells in breast cancer, inhibits T cell infiltration in lymphoma models, recruits macrophages in pancreatic cancer, and supports angiogenesis and in vivo tumor growth in lung cancer (He et al., 2010; Laan et al., 1999; Novitskiy et al., 2011; Numasaki et al., 2005; Wu et al., 2015a). IL-17 potentiates systemic G-CSF expression in breast cancer in vivo, which attracts immunosuppressive neutrophils and maximizes the potential for breast cancer metastasis (Coffelt et al., 2015). Similarly, cervical carcinoma cells respond to IL-17 by increasing IL-6 and CXCL8 expression, which recruit tumor-promoting macrophages and neutrophils in vivo and result in increased tumor size (Tartour et al., 1999). IL-17–induced CXCL8 expression from colonic epithelial cells in colon cancer not only attracts immunosuppressive neutrophils, but also synergizes with TNF to activate oncogenic epidermal growth factor receptor and ERK signaling pathways (Lee et al., 2008). Finally, IL-17 represses the expression of the Th1-activating chemokines CXCL10, CXCL11, and CCR5, limiting overall cytotoxicity within the tumor environment. In summary, the direct and indirect oncogenic properties of IL-17 complement each other to stimulate early tumor growth and suppress the immune system.
Regardless of the mechanism by which IL-17 promotes tumor growth, compiled data support our hypothesis that the protumoral properties of IL-17 impact the early stages of carcinogenesis and oncogenesis rather than the later stages in an established tumor. IL-17 signaling has been shown to drive the early stages of pancreatic and colorectal cancer formation (Chae et al., 2010; McAllister et al., 2014), and inhibition of IL-17 signaling prevented neoplastic initiation. Yet, the role of IL-17 blockade in established cancer models has been consistently less clear (Martinez et al., 2008). This may be because the established tumor is a complex microenvironment, rich in immune cells, stromal components, and redundant signaling pathways that make the effect of any individual cytokine less predictable (Balachandran et al., 2019; Egeblad et al., 2010). This is an important distinction that requires further investigation, suggesting that cytokine-based IL-17 inhibition may be more useful during early tumor growth.
Tumor-protective functions of IL-17
IL-17 expression correlates with a better prognosis and improvement in patient survival in a variety of cancers, supporting a role for IL-17 in antitumor immunity. In chronic lymphocytic leukemia, for example, an increased number of peripherally circulating IL-17+ Th17 cells is associated with better prognostic markers and improved patient survival (Jain et al., 2012). Analysis of IL-17 in gastric cancer surgical specimens reveals that greater immunohistochemical IL-17 expression independently reduces the likelihood of death by 48% at 5 yr (Chen et al., 2011). IL-17 expression also correlates with a greater number of cytotoxic IFN-γ+CD4+ and IFN-γ+CD8+ T cells in ovarian cancer (Kryczek et al., 2009a). Conversely, a reduction in the number of tumor-infiltrating Th17 cells or the amount of IL-17 in ascitic fluid predicts a worse patient outcome (Kryczek et al., 2009a). In cervical cancer, an increased number of IL-17+ cells correlates significantly with smaller tumors, reduced depth of tumor infiltration, and less frequent vascular invasion (Punt et al., 2015). Finally, in colorectal and lung cancer, IL-17 recruits antitumoral neutrophils to the tumor environment, which stimulates a T cell response and correlates with better overall survival (Eruslanov et al., 2014; Lin et al., 2015).
When exploring the mechanism by which IL-17 expression is tumor protective, there appears to be evidence for both direct and indirect antitumoral function. Nonmalignant mammary epithelial cells secrete IL-17, which ligates IL-17R on breast cancer cells and signals apoptosis via receptor-mediated caspase activation (Furuta et al., 2011). IL-17 can also activate individual immune cells, as evidenced by enhanced CD107a, TNF, IFN-γ, perforin, NKp46, NKG2D, and NKp44 expression in natural killer (NK) cells, or antitumoral macrophage polarization via expression of IL-1, IL-6, IL-12, and TNF (Al Omar et al., 2013; Jovanovic et al., 1998; Lu et al., 2013). The most potent tumor-protective property of IL-17, however, results from indirect, immune-mediated phenomena where IL-17 instructs the innate and adaptive immune system to become cytotoxic (Benchetrit et al., 2002). Endogenous IL-17 production suppresses tumor progression via increased IFN-γ+ T cell activity in an immunocompetent mouse model of colon cancer (Kryczek et al., 2009b). IL-17 stimulates CXCL2 and CXCL3 production from squamous esophageal cancer cells, which attracts myeloperoxidase+IFN-γ+ antitumoral neutrophils in vivo and inhibits tumor growth (Chen et al., 2017a). Similarly, IL-17 induces CCL2, CCL20, CXCL9, and CXCL10 production from esophageal squamous cancer cells that recruits and activates T cells, dendritic cells (DCs), and NK cells, correlating with an improvement in overall survival in 181 patients with esophageal squamous cell carcinoma (Lu et al., 2013). IL-17 also coaxes IL-6 production from a variety of cells in the tumor microenvironment, including macrophages and tumor cells. Ultimately, the role of IL-17–induced IL-6 expression in antitumor immunity is rooted in the survival, proliferation, recruitment, and cytotoxicity of leukocytes (Fisher et al., 2014a). Thus, in established tumors, IL-17 both directly activates immune cells and indirectly nurtures a cytotoxic cytokine environment.
Consequently, there are a few important observations to recognize when characterizing the role of IL-17 in antitumor immunity. First, the antitumor effect associated with exogenous or genetic overexpression of IL-17 is dependent on CD4+ and CD8+ T cells (Benchetrit et al., 2002). Similarly, endogenous IL-17 production supports tumor growth in an immune-deficient mouse model of colorectal cancer but potentiates an antitumor response in an immunocompetent mouse model, strongly suggesting that immune cells play an essential role in IL-17–mediated cytotoxicity (Kryczek et al., 2009b). Next, the antitumoral effects of IL-17 appear to be related to cytokine shifts and the expression of IFN-γ in the tumor microenvironment (Muranski et al., 2008), with Th1-like IFN-γ–producing Th17 cells being the major mediators of tumor destruction (Kryczek et al., 2009a). Finally, IL-17–producing immune cells are not constitutive IFN-γ producers, but are instead activated by the tumor environment to produce IFN-γ in the appropriate setting (Bailey et al., 2014; Chien et al., 2013). These observations not only highlight the significance of the tumor microenvironment in dictating IL-17 function, but also suggest the potential for IL-17–producing immune cell modulation in cancer immunotherapy. Therefore, a detailed understanding of the cytokine environment that heralds IFN-γ production from IL-17–producing immune cells is paramount.
Cytokines and immune cell polarity in the IL-17 axis
Immune cells are functionally complicated and express different effector molecules, including IL-17. Many animal-based IL-17 models only explore the function of exogenous IL-17 in the tumor environment, and extrapolating these functions to include IL-17–producing immune cells needs further validation. However, some IL-17–producing immune cells, namely γδ and Th17 T cells, have been directly implicated in antitumor immunity. Since γδ and Th17 T cells are the two inflammatory cell types primarily responsible for the production of IL-17 in the tumor microenvironment, exploration into how the surrounding cytokines shape their antitumoral phenotype is important for ultimately predicting immunotherapy response (Fig. 2).
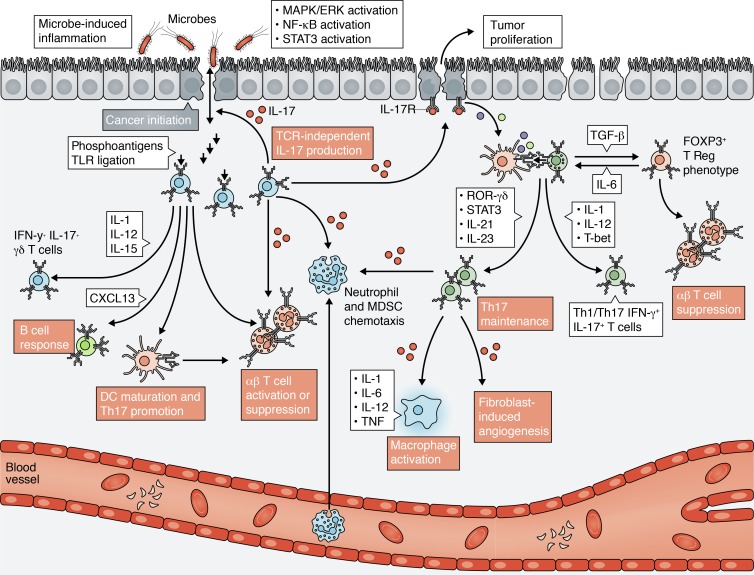
Figure 2.
Th17 and γδ T cell function in the IL-17 immune axis. The roles of IL-17, Th17, and γδ T cells in the tumor microenvironment. Th17 and γδ T cell polarity is dictated by the presence or absence of specific cytokines. IL-1, IL-12, IL-15, TLR ligation, and microbial antigens potentiate an antitumoral γδ T phenotype. IL-1, IL-6, IL-12, and T-bet expression promotes a cytotoxic and mixed Th1/Th17 cell phenotype. MDSC, myeloid-derived suppressor cell.
γδ T cells
γδ T cells are non-MHC restricted T cells functioning at the interface of innate and adaptive immunity. After being generated in the thymus and developing an antigen-specific, monoclonal TCR, γδ T cells migrate to well-defined and anatomically distinct immunological tissues (e.g., intestinal mucosa and dermis) in preparation for rapid education of an immune response to commensal pathogens or inflammation (Vantourout and Hayday, 2013). Activation of γδ T cells leads to a coordinated antigen- and cytokine-mediated immune response that dictates αβ CD8 T cell, CD4 T cell, B cell, NK cell, and DC activity (Fig. 2). B cell germination, recruitment, maturation, and antibody production ensue following γδ T cell activation and subsequent CXCL13 production (Vermijlen et al., 2007). Depending on their polarity, γδ T cells also help refine DC antigen presentation, directly induce NK cell activity, and activate or inhibit αβ CD8 T cell activity.
The cytokines and receptor ligands in the tumor environment dictate γδ T cell polarity. IL-17–producing γδ T cells rely on IL-7 and IL-4 to engender a tumor-promoting phenotype (Corpuz et al., 2016; Skeen and Ziegler, 1995; Wesch et al., 2001). Similarly, exposure to TGF-β and IL-15 can stimulate γδ T cells to become more like FOXP3+ immunosuppressive T regulatory cells (Casetti et al., 2009). On the other hand, antitumoral IFN-γ–producing γδ T cells rely heavily on IL-1, IL-12, and IL-15 (Corpuz et al., 2016; Skeen and Ziegler, 1995; Wesch et al., 2001). Microbial antigens, glycolipids, phosphoantigens, and tumor cell ligation of NKG2B receptors expressed on γδ T cells can also polarize and potentiate an antitumoral immune response orchestrated by the early activation of IFN-γ+ γδ T cells (Lafont et al., 2014; Lo Presti et al., 2018). Ligation of TLR8 on γδ T cells can unleash an antitumoral phenotype via DC maturation and αβ T cell activation (Peng et al., 2007). Finally, TCR-specific ligation on γδ T cells can lead to direct tumor cell lysis via perforin-granzyme secretion (Alexander et al., 2008; Viey et al., 2005). Additional mechanisms of γδ T cell–mediated cytotoxicity rely on the expression and ligation of CD16, FasL, and TRAIL (Dieli et al., 2007; Fisher et al., 2014c; Todaro et al., 2009).
As a result, γδ T cells can have both protumoral and antitumoral roles within the tumor environment (Zhao et al., 2018). γδ T cells promote tumor growth via angiogenesis in gallbladder cancer, pancreatic adenocarcinoma, and fibrosarcoma (McAllister et al., 2014; Patil et al., 2016; Wakita et al., 2010). γδ T cells also elicit antitumoral properties by directly lysing breast, renal, squamous, and osteosarcoma cells (Alexander et al., 2008; Dhar and Chiplunkar, 2010; Li et al., 2011b; Viey et al., 2005). Some evidence suggests that γδ T cells produce either IL-17 or IFN-γ, which is predictive of their protumoral or antitumoral function, respectively (Rei et al., 2015). However, there is a distinct phenotypic difference between naive IL-17–producing γδ T cells and activated IFN-γ–producing γδ T cells, the latter of which are the earliest producers of IFN-γ in the tumor environment and “set the stage” for a cytotoxic immune response (Gao et al., 2003). Moreover, IL-17 production by γδ T cells appears to be independent of TCR ligation, and immunotherapeutic augmentation of γδ T cells can lead to tumor destruction despite an unchanged production in IL-17 (Daley et al., 2016; Vantourout and Hayday, 2013). These data support an “IL-17 default position” within the origin of γδ T cells (Vantourout and Hayday, 2013), where TCR-independent IL-17–producing γδ T cells are more innate-like while adaptive IFN-γ+ γδ T cells are experienced in TCR ligation. Ultimately, this suggests that when licensing γδ T cells for cancer immunotherapy, reliance on functional studies rather than IL-17 expression alone should be considered to characterize γδ T cell polarity.
Th17 cells
Th17 cells are a subset of CD4 helper T cells that play an essential role in immunological development and response to pathogens (Asadzadeh et al., 2017; Zou and Restifo, 2010). Distinct from the cytotoxic Th1 and immunosuppressive Th2 CD4 helper cell phenotypes, Th17 cells are characterized by the lineage transcription factor RORγT, which with epithelial or tumor cell production of TGF-β or IL-6 promotes Th17 cell maintenance (Fig. 2). RORγT drives a transcriptional program that leads to the downstream expression of STAT3 and IL-17, which together coordinate IL-17 production and immune function.
Similar to γδ T cells, Th17 cells exhibit “plasticity,” where the presence or absence of specific cytokines directs differentiation and function (Guéry and Hugues, 2015; Majchrzak et al., 2016). The presence of TGF-β, IL-23, and IL-6 maintains IL-17 production and Th17 differentiation, while TGF-β alone may shift differentiation toward an immunosuppressive T regulatory phenotype (Fig. 2). TGF-β and IL-6 co-culture also induces the expression of the immunosuppressive ectonucleotidases CD39 and CD73 on Th17 cells, perpetuating a protumoral phenotype (Chalmin et al., 2012). Contrastingly, exposure to IL-12, IL-23, and IL-1 in the tumor environment enables IL-17–producing Th17 cells to shift toward a Th1-type phenotype, where IFN-γ alone or IFN-γ and IL-17 are coproduced (Lee et al., 2009; Revu et al., 2018). IL-12 also potentiates a transcriptional profile controlled by T-bet, enabling Th17 cells to mimic the high IFN-γ production of Th1 cells. Notably, Th1-like Th17 cells remain distinguishable from Th1 cells by a unique receptor profile characterized by the expression of CD161, ICOS, and IL23R (Bailey et al., 2014). As a result of their functional plasticity, Th17 cells exhibit a stem cell–like transcriptional profile in which a Th1-like phenotype is acquired over time and persists in vivo (Muranski et al., 2011). Furthermore, Th17 cells display an increased proliferative and self-renewal capacity upon antigen restimulation when compared with Th1 cells, which makes them attractive candidates for ACT and immunotherapy.
The impact of Th17 cells on antitumor immunity is apparent across multiple tumor models. In immunocompetent mastocytoma and lymphoma murine models, IL-17 inhibits tumor growth in a T cell–dependent fashion mediated by the Th1 cytokines IL-6 and IL-12 (Benchetrit et al., 2002). In patients with human papillomavirus–positive oropharyngeal cancer, the presence of IFN-γ+Th17 cytotoxic T cells correlated with disease-free survival, whereas the presence of IL-17–producing non-T cells was associated with an unfavorable immune response and poor survival (Punt et al., 2016). Th17 cells have also been reported to eradicate melanoma to a greater extent than Th1 cells by recruiting DCs and CD8+ T cells to the tumor environment (Martin-Orozco et al., 2009). Finally, in a mouse model of pancreatic cancer, RIP1, a protein kinase important in inflammatory signaling and cell death pathways, suppresses STAT1 signaling in macrophages and potentiates a tolerogenic macrophage phenotype (Wang et al., 2018). Inhibition of RIP1 on macrophages polarized the immune environment such that CD4+ T cells acquired a highly immunogenic, mixed Th1/Th17 phenotype that increased expression of IFN-γ, IL-17, T-bet, and RORγT. Not only was RIP1 inhibition protective against pancreatic cancer, but the associated cytokine shift also made both CD4+ and CD8+ T cells receptive to concurrent immune-checkpoint blockade and ICOS-based immunotherapy, further enhancing tumor destruction and providing a preclinical rationale for targeting these cells in antitumor immunity (Wang et al., 2018).
Immunotherapy targets in the IL-17 immune axis
The modulatory potential of the IL-17 immune axis makes IL-17 an attractive target for cancer immunotherapy. However, the paradoxical roles of IL-17 in both tumor growth and elimination make a “one size fits all” approach unlikely to succeed. Instead, it is likely that IL-17–based immunotherapy will need to be individualized to cancer type, stage, and perhaps even the specific IL-17 isoform and receptor subunit, since different IL-17 isoforms signal via unique pathways and with different signaling strengths even within the same cancer type (Zhou et al., 2007). Consequently, it is essential to appreciate how IL-17 modulates cancer cell proliferation and immunity in the tumor microenvironment, and the specific function of IL-17 in the tumor environment is an important determinant in predicting therapeutic response to IL-17–based therapy. Similarly, current animal-based models explore the role of exogenous or germline-deleted IL-17, the findings of which may not be directly attributable to IL-17–producing immune cells. When testing the potential for IL-17–producing immune cell immunotherapy, therefore, conditional KO models may further validate and more accurately predict the therapeutic potential of IL-17–based immunotherapy.
While there is some evidence to suggest that anti–IL-17 antibodies potentiate anti-VEGF therapy in colorectal cancer and prevent the progression of pancreatic intraepithelial neoplasia and pancreatic cancer metastasis (Ibrahim et al., 2018; McAllister et al., 2014; Wu et al., 2015a), redundant signaling mechanisms in the MAPK, NF-κB, and STAT3 signaling pathways may reduce the therapeutic impact of direct, cytokine-based, anti–IL-17 immunotherapy in established tumors. The most potent antitumor properties of the IL-17 axis appear to be related to the induction of IFN-γ+IL-17+ mixed Th1/Th17 cells and IFN-γ+ γδ T cells, which hinges on the plasticity of Th17 and γδ T cells, making Th17 and γδ T cell immunomodulation a substantial target in the IL-17 axis. A thorough understanding of the cytokines dictating γδ and Th17 cell antitumoral polarity is critical (Fig. 3). TCR stimulation, IL-1, IL-6, and IL-12 enable Th17 cells to acquire a mixed Th1/Th17 phenotype and produce IFN-γ. While some data imply that the production of IFN-γ and IL-17 by γδ T cells is developmentally defined through epigenetic and transcriptional programming in the thymus (Ribot et al., 2009; Schmolka et al., 2013; Shibata et al., 2014), IL-1, IL-12, IL-15, microbial peptides, and ligation of specific γδ TCRs can also unleash IFN-γ production. Additional research should focus on optimizing the cytokine environment before administering γδ and Th17 cell immunotherapy.
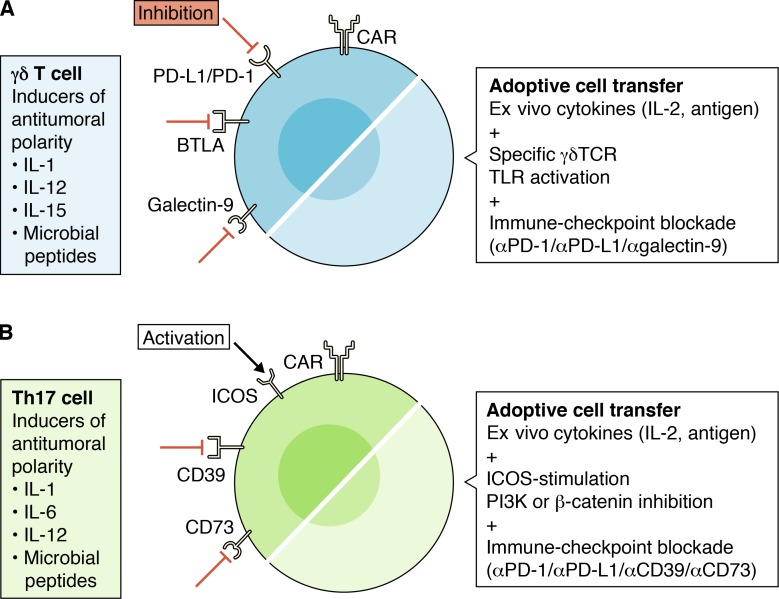
Figure 3.
Immunotherapy opportunities in IL-17+ Th17 and γδ T cells. (A and B) Immune cell receptors (left) found to be expressed on (A) γδ T cells and (B) Th17 cells, which may ultimately be used for personalized Th17 and γδ T cell cancer immunotherapy. Considerations and environments conducive for (A) γδ T cell and (B) Th17 cell ACT therapy (right). CAR, chimeric antigen receptor.
Examination of the immune receptors contributing to the antitumoral polarity of γδ and Th17 T cells is also important in order to optimize immunotherapy and ACT (Fig. 3). γδ T cells express PD-L1, PD-1, BTLA, galectin-9, TLRs, and NK immunoglobulin receptors including NKG2A, KIR2DL1, and KIR3DL1 (D’Ombrain et al., 2007; Papotto et al., 2017). Anti–PD-1 and anti–PD-L1 therapy has already been shown to enhance γδ T cell–dependent tumor destruction (Daley et al., 2016; Hoeres et al., 2018). B- and T-lymphocyte attenuator (BTLA) negatively regulates γδ T cell cytotoxicity and IL-17 production (Bekiaris et al., 2013; Gertner-Dardenne et al., 2013a; Gertner-Dardenne et al., 2013b), but the clinical applications of BTLA antagonism in γδ T cells have yet to be explored. TLR ligation enhances the cytotoxic response of γδ T cells directly through costimulation with antigen-specific TCRs and should continue to be explored (Wesch et al., 2011). Galectin-9 has both immune-suppressive and immune-enhancing functions (Chou et al., 2018). In EBV+ nasopharyngeal carcinoma, tumor cell expression of galectin-9 ligates TIM-3 on Th1 helper cells to promote cell apoptosis and induce immune escape (Chen et al., 2017b). Similarly, pancreatic cancer cell expression of galectin-9 ligates dectin-1 on macrophages to promote a tolerogenic immune environment (Daley et al., 2017). Our group has recently shown that galectin-9 is also highly expressed on γδ T cells, and galectin-9 inhibition synergized with checkpoint-blockade immunotherapy to induce tumor regression (Daley et al., 2017; Daley et al., 2016). Concordantly, antibodies targeting γδ T cell immune receptors are a novel approach to cancer immunotherapy, and production of an anti-human galectin-9 antibody is underway (Puretech Health).
Like γδ T cells, Th17 cells also have a characteristic immune receptor profile, generally exhibiting low levels of PD-1, GRZB, and perforin (Kryczek et al., 2009a). They also appear to highly express the costimulatory markers ICOS and CD28 (Paulos et al., 2010), which become an important consideration for ACT. Th17 cells mediate and promote long-term antitumor immunity in vivo due to their capacity for self-renewal, durable persistence, and resistance to apoptosis (Kryczek et al., 2011). Ex vivo stimulation of Th17 cells in polarizing conditions (with IL-2 and antigen; Fig. 3) doubled the duration of antitumor activity and increased the number of Th17 cells persisting in vivo nearly 16-fold (Hinrichs et al., 2009). ICOS stimulation also induces PI3K and Wnt/β-catenin survival pathways that promote the self-renewal and generation of memory Th17 cells (Majchrzak et al., 2017; Nelson, M., et al. 2012. Immunology 2012 Meeting. Abstr. 46.29). In a mouse model of melanoma, PI3K and β-catenin inhibition unleash a potent antitumor Th17 response via conversion to an effector phenotype. ICOS agonism also optimally expands Th17 cells for persistence in vivo (Guedan et al., 2014). As a result, generation of an ICOS-stimulatory CAR T cell for ACT showed improvement in tumor elimination with enhanced IFN-γ and T-bet expression when compared with traditional CAR T cells (Guedan et al., 2014). ICOS stimulation, PI3K inhibition, and β-catenin blockade should therefore be considered in future Th17 ACT therapy.
γδ T cells are similarly being used for ACT immunotherapy. Vγ9Vδ2 T cells are the most abundant γδ T cells in human blood (Poupot and Fournié, 2004) and have been the major focus of ACT immunotherapy. Polarization and ACT of γδ T cells using IL-2 and zalendronate (which mimics TLR ligation) has shown some efficacy in prostate cancer (Dieli et al., 2007), advanced renal cell carcinoma (Bennouna et al., 2008; Fisher et al., 2014b), non-small cell lung cancer (Nakajima et al., 2010), and neuroblastoma (Capsomidis et al., 2018). Notably, γδ TCRs do not require an antigen to be complexed to an MHC receptor in order to generate an immune response, enabling them to rapidly respond to foreign peptides in concert with the innate immune system. The monoclonal γδ T cell receptor is also antigen specific, which limits an uncontrolled immune response in the absence of MHC presentation. As a result, different δ and γ chains have specific activating ligands, including MICA/MICB for Vδ1, ULBP4 for Vδ2, and phosphoantigens for Vγ9Vδ2 (Vantourout and Hayday, 2013). Some TCRs have already proven to be targetable in pancreatic and colon cancer (Devaud et al., 2013; Oberg et al., 2014; Ramutton et al., 2014), and the cytotoxic features of each subset of γδ T cell are currently being studied and evaluated for additional ACT applications (Hannani et al., 2012; Wu et al., 2017).
Finally, consideration for the microbiome in the IL-17–immune axis warrants attention, since the IL-17 axis itself plays an integral role in the host immune response to pathogenic and commensal organisms. In pancreatic cancer, the intestinal microbiome induces Th17 IL-17 expression and promotes pancreatic oncogenesis (McAllister et al., 2014; Sethi et al., 2018). Ablation of the microbiome with antibiotics reduces cancer growth in an IL-17–dependent fashion, which supports the protumoral role of bacteria and IL-17 in the early stages of oncogenesis (Sethi et al., 2019). Moreover, microbial stimuli have been shown to program the immunosuppressive phenotype of many innate and adaptive immune cells, including γδ T cells, Th17 T cells, and the recently described IL-17–producing innate αβ T cells (Hundeyin et al., 2019). Consequently, modulation of the microbiome with concurrent immune-checkpoint blockade may alter IL-17 production and open further opportunities for anti–IL-17–based therapy (Onishi and Gaffen, 2010).
Concluding remarks
IL-17 function is both tumor promoting and tumor protective. Published data support the hypothesis that IL-17 is protumoral early in inflammation and cancer initiation, while antitumoral activity develops in coordination with changes in the cytokine and immune cell microenvironment in established tumors. Immune-checkpoint blockade, activation of costimulatory receptors, and ACT of γδ T cells and Th17 should consider the unique ontogenies, TCRs, and immune receptor profiles of these cells. Further characterization of the immune profiles and factors affecting functional plasticity of Th17 and γδ T cells, including the role of the microbiome, may open opportunities for additional IL-17–based immunotherapy.
Acknowledgments
Work in the S. Arthur Localio Laboratory of G. Miller is supported by National Institutes of Health grants CA168611, CA203105, CA215471, CA19311, and DK106025 (all to G. Miller). G. Vitiello is supported by National Institutes of Health Division of Loan Repayment grant L30 TR002111.
G. Miller has Research Contract Agreements with Puretech Health, Pfizer, and GlaxoSmithKline. The authors have no additional competing financial interests.
Author contributions:The manuscript was written and edited by G. Vitiello and G. Miller.
References
- Al Omar S., Flanagan B.F., Almehmadi M., and Christmas S.E.. 2013. The effects of IL-17 upon human natural killer cells. Cytokine. 62:123–130. 10.1016/j.cyto.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Al-Samadi A., Moossavi S., Salem A., Sotoudeh M., Tuovinen S.M., Konttinen Y.T., Salo T., and Bishehsari F.. 2016. Distinctive expression pattern of interleukin-17 cytokine family members in colorectal cancer. Tumour Biol. 37:1609–1615. 10.1007/s13277-015-3941-x [DOI] [PubMed] [Google Scholar]
- Alexander A.A., Maniar A., Cummings J.S., Hebbeler A.M., Schulze D.H., Gastman B.R., Pauza C.D., Strome S.E., and Chapoval A.I.. 2008. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 14:4232–4240. 10.1158/1078-0432.CCR-07-4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadzadeh Z., Mohammadi H., Safarzadeh E., Hemmatzadeh M., Mahdian-Shakib A., Jadidi-Niaragh F., Azizi G., and Baradaran B.. 2017. The paradox of Th17 cell functions in tumor immunity. Cell. Immunol. 322:15–25. 10.1016/j.cellimm.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Bailey S.R., Nelson M.H., Himes R.A., Li Z., Mehrotra S., and Paulos C.M.. 2014. Th17 cells in cancer: the ultimate identity crisis. Front. Immunol. 5:276 10.3389/fimmu.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran V.P., Beatty G.L., and Dougan S.K.. 2019. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 156:2056–2072. 10.1053/j.gastro.2018.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekiaris V., Šedý J.R., Macauley M.G., Rhode-Kurnow A., and Ware C.F.. 2013. The inhibitory receptor BTLA controls γδ T cell homeostasis and inflammatory responses. Immunity. 39:1082–1094. 10.1016/j.immuni.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit F., Ciree A., Vives V., Warnier G., Gey A., Sautès-Fridman C., Fossiez F., Haicheur N., Fridman W.H., and Tartour E.. 2002. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 99:2114–2121. 10.1182/blood.V99.6.2114 [DOI] [PubMed] [Google Scholar]
- Bennouna J., Bompas E., Neidhardt E.M., Rolland F., Philip I., Galéa C., Salot S., Saiagh S., Audrain M., Rimbert M., et al. . 2008. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 57:1599–1609. 10.1007/s00262-008-0491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. . 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373:1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. . 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455–2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. . 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373:123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsomidis A., Benthall G., Van Acker H.H., Fisher J., Kramer A.M., Abeln Z., Majani Y., Gileadi T., Wallace R., Gustafsson K., et al. . 2018. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 26:354–365. 10.1016/j.ymthe.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casetti R., Agrati C., Wallace M., Sacchi A., Martini F., Martino A., Rinaldi A., and Malkovsky M.. 2009. Cutting edge: TGF-β1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J. Immunol. 183:3574–3577. 10.4049/jimmunol.0901334 [DOI] [PubMed] [Google Scholar]
- Chae W.J., Gibson T.F., Zelterman D., Hao L., Henegariu O., and Bothwell A.L.. 2010. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA. 107:5540–5544. 10.1073/pnas.0912675107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmin F., Mignot G., Bruchard M., Chevriaux A., Végran F., Hichami A., Ladoire S., Derangère V., Vincent J., Masson D., et al. . 2012. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 36:362–373. 10.1016/j.immuni.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Chen J.G., Xia J.C., Liang X.T., Pan K., Wang W., Lv L., Zhao J.J., Wang Q.J., Li Y.Q., Chen S.P., et al. . 2011. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. Int. J. Biol. Sci. 7:53–60. 10.7150/ijbs.7.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Wang Y., Huang C.Y., Zhou Z.Q., Zhao J.J., Zhang X.F., Pan Q.Z., Wu J.X., Weng D.S., Tang Y., et al. . 2017a IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. OncoImmunology. 7:e1373234 10.1080/2162402X.2017.1373234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.C., Chen C.H., Wang C.P., Lin P.H., Yang T.L., Lou P.J., Ko J.Y., Wu C.T., and Chang Y.L.. 2017b The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci. Rep. 7:10349 10.1038/s41598-017-10386-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y.H., Zeng X., and Prinz I.. 2013. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. 34:151–154. 10.1016/j.it.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou F.C., Chen H.Y., Kuo C.C., and Sytwu H.K.. 2018. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 19:E430 10.3390/ijms19020430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D., Morris L.G.T., Grigg C.M., Weber J.K., Samstein R.M., Makarov V., Kuo F., Kendall S.M., Requena D., Riaz N., et al. . 2018. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 359:582–587. 10.1126/science.aao4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochaud S., Giustiniani J., Thomas C., Laprevotte E., Garbar C., Savoye A.M., Curé H., Mascaux C., Alberici G., Bonnefoy N., et al. . 2013. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 3:3456 10.1038/srep03456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., Verstegen N.J.M., Ciampricotti M., Hawinkels L.J.A.C., Jonkers J., and de Visser K.E.. 2015. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 522:345–348. 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpuz T.M., Stolp J., Kim H.O., Pinget G.V., Gray D.H., Cho J.H., Sprent J., and Webster K.E.. 2016. Differential responsiveness of innate-like IL-17- and IFN-γ-producing γδ T cells to homeostatic cytokines. J. Immunol. 196:645–654. 10.4049/jimmunol.1502082 [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. 2013. Breakthrough of the year 2013. Cancer immunotherapy. Science. 342:1432–1433. 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- Cui G., Yuan A., Goll R., and Florholmen J.. 2012. IL-17A in the tumor microenvironment of the human colorectal adenoma-carcinoma sequence. Scand. J. Gastroenterol. 47:1304–1312. 10.3109/00365521.2012.725089 [DOI] [PubMed] [Google Scholar]
- D’Ombrain M.C., Hansen D.S., Simpson K.M., and Schofield L.. 2007. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-γ response to Plasmodium falciparum malaria. Eur. J. Immunol. 37:1864–1873. 10.1002/eji.200636889 [DOI] [PubMed] [Google Scholar]
- Daley D., Zambirinis C.P., Seifert L., Akkad N., Mohan N., Werba G., Barilla R., Torres-Hernandez A., Hundeyin M., Mani V.R.K., et al. . 2016. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell. 166:1485–1499.e15. 10.1016/j.cell.2016.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D., Mani V.R., Mohan N., Akkad N., Ochi A., Heindel D.W., Lee K.B., Zambirinis C.P., Pandian G.S.B., Savadkar S., et al. . 2017. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 23:556–567. 10.1038/nm.4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud C., Rousseau B., Netzer S., Pitard V., Paroissin C., Khairallah C., Costet P., Moreau J.F., Couillaud F., Dechanet-Merville J., and Capone M.. 2013. Anti-metastatic potential of human Vδ1(+) γδ T cells in an orthotopic mouse xenograft model of colon carcinoma. Cancer Immunol. Immunother. 62:1199–1210. 10.1007/s00262-013-1402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S., and Chiplunkar S.V.. 2010. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9Vδ2 T cells. Cancer Immun. 10:10. [PMC free article] [PubMed] [Google Scholar]
- Dieli F., Vermijlen D., Fulfaro F., Caccamo N., Meraviglia S., Cicero G., Roberts A., Buccheri S., D’Asaro M., Gebbia N., et al. . 2007. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67:7450–7457. 10.1158/0008-5472.CAN-07-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Thi V.A., Park S.M., Lee H., and Kim Y.S.. 2016. The membrane-bound form of IL-17A promotes the growth and tumorigenicity of colon cancer cells. Mol. Cells. 39:536–542. 10.14348/molcells.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M., Nakasone E.S., and Werb Z.. 2010. Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell. 18:884–901. 10.1016/j.devcel.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A.M., Chiarion-Sileni V., Grob J.J., Dummer R., Wolchok J.D., Schmidt H., Hamid O., Robert C., Ascierto P.A., Richards J.M., et al. . 2016. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375:1845–1855. 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov E.B., Bhojnagarwala P.S., Quatromoni J.G., Stephen T.L., Ranganathan A., Deshpande C., Akimova T., Vachani A., Litzky L., Hancock W.W., et al. . 2014. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Invest. 124:5466–5480. 10.1172/JCI77053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre J.A.S., Giustinniani J., Garbar C., Merrouche Y., Antonicelli F., and Bensussan A.. 2018. The interleukin-17 family of cytokines in breast cancer. Int. J. Mol. Sci. 19:E3880 10.3390/ijms19123880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.T., Appenheimer M.M., and Evans S.S.. 2014a The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 26:38–47. 10.1016/j.smim.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.P., Heuijerjans J., Yan M., Gustafsson K., and Anderson J.. 2014b γδ T cells for cancer immunotherapy: A systematic review of clinical trials. OncoImmunology. 3:e27572 10.4161/onci.27572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.P., Yan M., Heuijerjans J., Carter L., Abolhassani A., Frosch J., Wallace R., Flutter B., Capsomidis A., Hubank M., et al. . 2014c Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 20:5720–5732. 10.1158/1078-0432.CCR-13-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta S., Jeng Y.M., Zhou L., Huang L., Kuhn I., Bissell M.J., and Lee W.H.. 2011. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 3:78ra31 10.1126/scitranslmed.3001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S.L. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556–567. 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S.L., Jain R., Garg A.V., and Cua D.J.. 2014. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14:585–600. 10.1038/nri3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yang W., Pan M., Scully E., Girardi M., Augenlicht L.H., Craft J., and Yin Z.. 2003. γ δ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 198:433–442. 10.1084/jem.20030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J., Fauriat C., and Olive D.. 2013a BTLA, a key regulator of Vγ9Vδ2 T-cell proliferation. OncoImmunology. 2:e25853 10.4161/onci.25853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J., Fauriat C., Orlanducci F., Thibult M.L., Pastor S., Fitzgibbon J., Bouabdallah R., Xerri L., and Olive D.. 2013b The co-receptor BTLA negatively regulates human Vγ9Vδ2 T-cell proliferation: a potential way of immune escape for lymphoma cells. Blood. 122:922–931. 10.1182/blood-2012-11-464685 [DOI] [PubMed] [Google Scholar]
- Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J., Lee J., Posey A.D. Jr., Scholler J., Scholler N., et al. . 2014. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 124:1070–1080. 10.1182/blood-2013-10-535245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéry L., and Hugues S.. 2015. Th17 cell plasticity and functions in cancer immunity. BioMed Res. Int. 2015:314620 10.1155/2015/314620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannani D., Ma Y., Yamazaki T., Déchanet-Merville J., Kroemer G., and Zitvogel L.. 2012. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 33:199–206. 10.1016/j.it.2012.01.006 [DOI] [PubMed] [Google Scholar]
- He D., Li H., Yusuf N., Elmets C.A., Li J., Mountz J.D., and Xu H.. 2010. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 184:2281–2288. 10.4049/jimmunol.0902574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S., Kaiser A., Paulos C.M., Cassard L., Sanchez-Perez L., Heemskerk B., Wrzesinski C., Borman Z.A., Muranski P., and Restifo N.P.. 2009. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 114:596–599. 10.1182/blood-2009-02-203935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. . 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363:711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeres T., Holzmann E., Smetak M., Birkmann J., and Wilhelm M.. 2018. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. OncoImmunology. 8:1550618 10.1080/2162402X.2018.1550618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundeyin M., Kurz E., Mishra A., Rossi J.A.K., Liudahl S.M., Leis K.R., Mehrotra H., Kim M., Torres L.E., Ogunsakin A., et al. . 2019. Innate αβ T cells mediate antitumor immunity by orchestrating immunogenic macrophage programming. Cancer Discov. 9:1288–1305. 10.1158/2159-8290.CD-19-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S., Girault A., Ohresser M., Lereclus E., Paintaud G., Lecomte T., and Raoul W.. 2018. Monoclonal antibodies targeting the IL-17/IL-17RA axis: an opportunity to improve the efficiency of anti-VEGF therapy in fighting metastatic colorectal cancer? Clin. Colorectal Cancer. 17:e109–e113. 10.1016/j.clcc.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., and Manel N.. 2010. [Induction of gut mucosal Th17 cells by segmented filamentous bacteria]. Med. Sci. (Paris). 26:352–355. 10.1051/medsci/2010264352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishigame H., Saijo S., and Nakae S.. 2011. Functional specialization of interleukin-17 family members. Immunity. 34:149–162. 10.1016/j.immuni.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Jain P., Javdan M., Feger F.K., Chiu P.Y., Sison C., Damle R.N., Bhuiya T.A., Sen F., Abruzzo L.V., Burger J.A., et al. . 2012. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica. 97:599–607. 10.3324/haematol.2011.047316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., and Pelletier J.P.. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 160:3513–3521. [PubMed] [Google Scholar]
- Kehlen A., Thiele K., Riemann D., Rainov N., and Langner J.. 1999. Interleukin-17 stimulates the expression of IkappaB α mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. J. Neuroimmunol. 101:1–6. 10.1016/S0165-5728(99)00111-3 [DOI] [PubMed] [Google Scholar]
- Kolls J.K., and Lindén A.. 2004. Interleukin-17 family members and inflammation. Immunity. 21:467–476. 10.1016/j.immuni.2004.08.018 [DOI] [PubMed] [Google Scholar]
- Kryczek I., Banerjee M., Cheng P., Vatan L., Szeliga W., Wei S., Huang E., Finlayson E., Simeone D., Welling T.H., et al. . 2009a Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 114:1141–1149. 10.1182/blood-2009-03-208249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Wei S., Szeliga W., Vatan L., and Zou W.. 2009b Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 114:357–359. 10.1182/blood-2008-09-177360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Zhao E., Liu Y., Wang Y., Vatan L., Szeliga W., Moyer J., Klimczak A., Lange A., and Zou W.. 2011. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 3:104ra100 10.1126/scitranslmed.3002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M., Cui Z.H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D.C., Skoogh B.E., and Lindén A.. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347–2352. [PubMed] [Google Scholar]
- Lafont V., Sanchez F., Laprevotte E., Michaud H.A., Gros L., Eliaou J.F., and Bonnefoy N.. 2014. Plasticity of γδ T cells: impact on the anti-tumor response. Front. Immunol. 5:622 10.3389/fimmu.2014.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Wang P., Kattah M.G., Youssef S., Steinman L., DeFea K., and Straus D.S.. 2008. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J. Immunol. 181:6536–6545. 10.4049/jimmunol.181.9.6536 [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., and Weaver C.T.. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity. 30:92–107. 10.1016/j.immuni.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lau G.K., Chen L., Dong S.S., Lan H.Y., Huang X.R., Li Y., Luk J.M., Yuan Y.F., and Guan X.Y.. 2011a Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 6:e21816 10.1371/journal.pone.0021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xu Q., Peng H., Cheng R., Sun Z., and Ye Z.. 2011b IFN-γ enhances HOS and U2OS cell lines susceptibility to γδ T cell-mediated killing through the Fas/Fas ligand pathway. Int. Immunopharmacol. 11:496–503. 10.1016/j.intimp.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Lin Y., Xu J., Su H., Zhong W., Yuan Y., Yu Z., Fang Y., Zhou H., Li C., and Huang K.. 2015. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin. Transl. Oncol. 17:50–56. 10.1007/s12094-014-1197-3 [DOI] [PubMed] [Google Scholar]
- Liu S., Zhang Q., Chen C., Ge D., Qu Y., Chen R., Fan Y.M., Li N., Tang W.W., Zhang W., et al. . 2016. Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor. Oncotarget. 7:13651–13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Presti E., Pizzolato G., Corsale A.M., Caccamo N., Sireci G., Dieli F., and Meraviglia S.. 2018. γδ T cells and tumor microenvironment: from immunosurveillance to tumor evasion. Front. Immunol. 9:1395 10.3389/fimmu.2018.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Pan K., Zheng H.X., Li J.J., Qiu H.J., Zhao J.J., Weng D.S., Pan Q.Z., Wang D.D., Jiang S.S., et al. . 2013. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J. Immunother. 36:451–458. 10.1097/CJI.0b013e3182a802cf [DOI] [PubMed] [Google Scholar]
- Majchrzak K., Nelson M.H., Bailey S.R., Bowers J.S., Yu X.Z., Rubinstein M.P., Himes R.A., and Paulos C.M.. 2016. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol. Immunother. 65:247–259. 10.1007/s00262-016-1797-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrzak K., Nelson M.H., Bowers J.S., Bailey S.R., Wyatt M.M., Wrangle J.M., Rubinstein M.P., Varela J.C., Li Z., Himes R.A., et al. . 2017. β-catenin and PI3Kδ inhibition expands precursor Th17 cells with heightened stemness and antitumor activity. JCI Insight. 2:90547 10.1172/jci.insight.90547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N., Muranski P., Chung Y., Yang X.O., Yamazaki T., Lu S., Hwu P., Restifo N.P., Overwijk W.W., and Dong C.. 2009. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 31:787–798. 10.1016/j.immuni.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G.J., Nurieva R.I., Yang X.O., and Dong C.. 2008. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 1143:188–211. 10.1196/annals.1443.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F., Bailey J.M., Alsina J., Nirschl C.J., Sharma R., Fan H., Rattigan Y., Roeser J.C., Lankapalli R.H., Zhang H., et al. . 2014. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 25:621–637. 10.1016/j.ccr.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrouche Y., Fabre J., Cure H., Garbar C., Fuselier C., Bastid J., Antonicelli F., Al-Daccak R., Bensussan A., and Giustiniani J.. 2016. IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget. 7:53350–53361. 10.18632/oncotarget.10804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. . 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 112:362–373. 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Borman Z.A., Kerkar S.P., Klebanoff C.A., Ji Y., Sanchez-Perez L., Sukumar M., Reger R.N., Yu Z., Kern S.J., et al. . 2011. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 35:972–985. 10.1016/j.immuni.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G., and Saha B.. 2009. Protumor vs antitumor functions of IL-17. J. Immunol. 183:4169–4175. 10.4049/jimmunol.0901017 [DOI] [PubMed] [Google Scholar]
- Nakajima J., Murakawa T., Fukami T., Goto S., Kaneko T., Yoshida Y., Takamoto S., and Kakimi K.. 2010. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur. J. Cardiothorac. Surg. 37:1191–1197. 10.1016/j.ejcts.2009.11.051 [DOI] [PubMed] [Google Scholar]
- Novitskiy S.V., Pickup M.W., Gorska A.E., Owens P., Chytil A., Aakre M., Wu H., Shyr Y., and Moses H.L.. 2011. TGF-β receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 1:430–441. 10.1158/2159-8290.CD-11-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M., Watanabe M., Suzuki T., Takahashi H., Nakamura A., McAllister F., Hishinuma T., Goto J., Lotze M.T., Kolls J.K., and Sasaki H.. 2005. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J. Immunol. 175:6177–6189. 10.4049/jimmunol.175.9.6177 [DOI] [PubMed] [Google Scholar]
- Oberg H.H., Peipp M., Kellner C., Sebens S., Krause S., Petrick D., Adam-Klages S., Röcken C., Becker T., Vogel I., et al. . 2014. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74:1349–1360. 10.1158/0008-5472.CAN-13-0675 [DOI] [PubMed] [Google Scholar]
- Onishi R.M., and Gaffen S.L.. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129:311–321. 10.1111/j.1365-2567.2009.03240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papotto P.H., Ribot J.C., and Silva-Santos B.. 2017. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18:604–611. 10.1038/ni.3726 [DOI] [PubMed] [Google Scholar]
- Patil R.S., Shah S.U., Shrikhande S.V., Goel M., Dikshit R.P., and Chiplunkar S.V.. 2016. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer. 139:869–881. 10.1002/ijc.30134 [DOI] [PubMed] [Google Scholar]
- Paulos C.M., Carpenito C., Plesa G., Suhoski M.M., Varela-Rohena A., Golovina T.N., Carroll R.G., Riley J.L., and June C.H.. 2010. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci. Transl. Med. 2:55ra78 10.1126/scitranslmed.3000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Wang H.Y., Peng W., Kiniwa Y., Seo K.H., and Wang R.F.. 2007. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 27:334–348. 10.1016/j.immuni.2007.05.020 [DOI] [PubMed] [Google Scholar]
- Poupot M., and Fournié J.J.. 2004. Non-peptide antigens activating human Vgamma9/Vdelta2 T lymphocytes. Immunol. Lett. 95:129–138. 10.1016/j.imlet.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Prat A., Navarro A., Paré L., Reguart N., Galván P., Pascual T., Martínez A., Nuciforo P., Comerma L., Alos L., et al. . 2017. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 77:3540–3550. 10.1158/0008-5472.CAN-16-3556 [DOI] [PubMed] [Google Scholar]
- Punt S., van Vliet M.E., Spaans V.M., de Kroon C.D., Fleuren G.J., Gorter A., and Jordanova E.S.. 2015. FoxP3(+) and IL-17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol. Immunother. 64:745–753. 10.1007/s00262-015-1678-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt S., Dronkers E.A., Welters M.J., Goedemans R., Koljenović S., Bloemena E., Snijders P.J., Gorter A., van der Burg S.H., Baatenburg de Jong R.J., and Jordanova E.S.. 2016. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol. Immunother. 65:393–403. 10.1007/s00262-016-1805-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramutton T., Buccheri S., Dieli F., Todaro M., Stassi G., and Meraviglia S.. 2014. γδ T cells as a potential tool in colon cancer immunotherapy. Immunotherapy. 6:989–999. 10.2217/imt.14.59 [DOI] [PubMed] [Google Scholar]
- Rei M., Pennington D.J., and Silva-Santos B.. 2015. The emerging Protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 75:798–802. 10.1158/0008-5472.CAN-14-3228 [DOI] [PubMed] [Google Scholar]
- Revu S., Wu J., Henkel M., Rittenhouse N., Menk A., Delgoffe G.M., Poholek A.C., and McGeachy M.J.. 2018. IL-23 and IL-1β drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Reports. 22:2642–2653. 10.1016/j.celrep.2018.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot J.C., deBarros A., Pang D.J., Neves J.F., Peperzak V., Roberts S.J., Girardi M., Borst J., Hayday A.C., Pennington D.J., and Silva-Santos B.. 2009. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 10:427–436. 10.1038/ni.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. . 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., and Rosenberg S.A.. 2010. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33:828–833. 10.1097/CJI.0b013e3181eec14c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein R.M., Lee C.H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., Barron D.A., Zehir A., Jordan E.J., Omuro A., et al. . 2019. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51:202–206. 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolka N., Serre K., Grosso A.R., Rei M., Pennington D.J., Gomes A.Q., and Silva-Santos B.. 2013. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat. Immunol. 14:1093–1100. 10.1038/ni.2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi V., Kurtom S., Tarique M., Lavania S., Malchiodi Z., Hellmund L., Zhang L., Sharma U., Giri B., Garg B., et al. . 2018. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 155:33–37.e6. 10.1053/j.gastro.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi V., Vitiello G.A., Saxena D., Miller G., and Dudeja V.. 2019. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology. 156:2097–2115.e2. 10.1053/j.gastro.2018.12.045 [DOI] [PubMed] [Google Scholar]
- Shibata K., Yamada H., Nakamura M., Hatano S., Katsuragi Y., Kominami R., and Yoshikai Y.. 2014. IFN-γ-producing and IL-17-producing γδ T cells differentiate at distinct developmental stages in murine fetal thymus. J. Immunol. 192:2210–2218. 10.4049/jimmunol.1302145 [DOI] [PubMed] [Google Scholar]
- Skeen M.J., and Ziegler H.K.. 1995. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 154:5832–5841. [PubMed] [Google Scholar]
- Tartour E., Fossiez F., Joyeux I., Galinha A., Gey A., Claret E., Sastre-Garau X., Couturier J., Mosseri V., Vives V., et al. . 1999. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 59:3698–3704. [PubMed] [Google Scholar]
- Todaro M., D’Asaro M., Caccamo N., Iovino F., Francipane M.G., Meraviglia S., Orlando V., La Mendola C., Gulotta G., Salerno A., et al. . 2009. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J. Immunol. 182:7287–7296. 10.4049/jimmunol.0804288 [DOI] [PubMed] [Google Scholar]
- Vantourout P., and Hayday A.. 2013. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13:88–100. 10.1038/nri3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D., Ellis P., Langford C., Klein A., Engel R., Willimann K., Jomaa H., Hayday A.C., and Eberl M.. 2007. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J. Immunol. 178:4304–4314. 10.4049/jimmunol.178.7.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viey E., Fromont G., Escudier B., Morel Y., Da Rocha S., Chouaib S., and Caignard A.. 2005. Phosphostim-activated γ δ T cells kill autologous metastatic renal cell carcinoma. J. Immunol. 174:1338–1347. 10.4049/jimmunol.174.3.1338 [DOI] [PubMed] [Google Scholar]
- Wakita D., Sumida K., Iwakura Y., Nishikawa H., Ohkuri T., Chamoto K., Kitamura H., and Nishimura T.. 2010. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 40:1927–1937. 10.1002/eji.200940157 [DOI] [PubMed] [Google Scholar]
- Wang W., Marinis J.M., Beal A.M., Savadkar S., Wu Y., Khan M., Taunk P.S., Wu N., Su W., Wu J., et al. . 2018. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 34:757–774.e7. 10.1016/j.ccell.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch D., Glatzel A., and Kabelitz D.. 2001. Differentiation of resting human peripheral blood γ δ T cells toward Th1- or Th2-phenotype. Cell. Immunol. 212:110–117. 10.1006/cimm.2001.1850 [DOI] [PubMed] [Google Scholar]
- Wesch D., Peters C., Oberg H.H., Pietschmann K., and Kabelitz D.. 2011. Modulation of γδ T cell responses by TLR ligands. Cell. Mol. Life Sci. 68:2357–2370. 10.1007/s00018-011-0699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu P., Huang Q., Liu Y., Ye J., and Huang J.. 2013. Interleukin-17: a promoter in colorectal cancer progression. Clin. Dev. Immunol. 2013:436307 10.1155/2013/436307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.H., Hwang-Verslues W.W., Lee W.H., Huang C.K., Wei P.C., Chen C.L., Shew J.Y., Lee E.Y., Jeng Y.M., Tien Y.W., et al. . 2015a Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 212:333–349. 10.1084/jem.20141702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Chen X., Zhao J., Martin B., Zepp J.A., Ko J.S., Gu C., Cai G., Ouyang W., Sen G., et al. . 2015b A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212:1571–1587. 10.1084/jem.20150204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wu P., Qiu F., Wei Q., and Huang J.. 2017. Human γδT-cell subsets and their involvement in tumor immunity. Cell. Mol. Immunol. 14:245–253. 10.1038/cmi.2016.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Long H., He L., Han X., Lin K., Liang Z., Zhuo W., Xie R., and Zhu B.. 2015. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 34:165–176. 10.1038/onc.2013.537 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Niu C., and Cui J.. 2018. Gamma-delta (γδ) T cells: friend or foe in cancer development? J. Transl. Med. 16:3 10.1186/s12967-017-1378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Toh M.L., Zrioual S., and Miossec P.. 2007. IL-17A versus IL-17F induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in AGS gastric adenocarcinoma cells. Cytokine. 38:157–164. 10.1016/j.cyto.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Zou W., and Restifo N.P.. 2010. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10:248–256. 10.1038/nri2742 [DOI] [PMC free article] [PubMed] [Google Scholar]