This review summarizes our current understanding of how specific cytokines produced by pathogenic CD4 T cells contribute to central nervous system autoimmune disease. Data obtained from animal models and patients with multiple sclerosis are discussed.
Abstract
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system that is believed to have an autoimmune etiology. As MS is the most common nontraumatic disease that causes disability in young adults, extensive research has been devoted to identifying therapeutic targets. In this review, we discuss the current understanding derived from studies of patients with MS and animal models of how specific cytokines produced by autoreactive CD4 T cells contribute to the pathogenesis of MS. Defining the roles of these cytokines will lead to a better understanding of the potential of cytokine-based therapies for patients with MS.
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating, neurodegenerative disease of the central nervous system (CNS) that affects ∼2.3 million people worldwide (Filippi et al., 2018). MS most commonly follows a two-stage disease course. The first stage is referred to as relapsing-remitting MS (RRMS) and is marked by alternating episodes of neurological disability and recovery. Hallmark features of this stage are the formation of inflammatory lesions and plaques of demyelination in the CNS. The second stage is referred to as secondary progressive MS and is marked by a decrease in inflammatory lesions detectable by magnetic resonance imaging while neurological decline and brain atrophy steadily progress. MS is believed to be an autoimmune disease initiated by CD4 T helper cells (Th cells) specific for antigens in the myelin sheath. This perspective is supported by the strong association of MS susceptibility with MHC class II alleles (Hafler et al., 2007) and the fact that experimental autoimmune encephalomyelitis (EAE), a widely used animal model of MS, is induced by activation of CD4 T cells specific for myelin antigens (Goverman, 2009). The predominance of CD8 T cells within MS lesions (Salou et al., 2015) and the therapeutic efficacy of eliminating B cells in patients with MS (Bar-Or et al., 2008; Hauser et al., 2008) indicate that other lymphocytes play important roles in this disease. However, the ability to initiate EAE by adoptive transfer of myelin-specific CD4 T cells alone into naive animals suggests that CD4 T cells may trigger both the initial inflammatory cascade and potentially subsequent relapses (Goverman, 2009). Because the major function of CD4 T cells is to orchestrate immune responses via production of cytokines and other soluble mediators, there has been intense effort using the EAE model to identify which cytokines produced by CD4 T cells account for their pathogenic activity. This is a critical area of investigation as these cytokines could be attractive therapeutic targets.
Research directed toward defining pathogenic cytokines in EAE has focused on determining which of the traditionally defined CD4 T cell effector subsets can induce EAE. CD4 T cell effector subsets have been defined by distinct patterns of cytokine production, requirements for specific cytokine growth factors, and expression of master transcription factors. CD4 T cell subsets with the potential to induce EAE include both Th1 and Th17 cells. Th1 cells produce IFNγ as their “signature” cytokine. Differentiation of naive CD4 T cells into the Th1 cell subset is promoted by exposure to IL-12 during the initial priming of CD4 T cells, and the transcription factor Tbet is considered a master regulator responsible for regulating expression of genes associated with the Th1 cell lineage. IL-17 is the signature cytokine for Th17 cells and RORγt is the master transcription factor controlling their differentiation. IL-6 and TGFβ promote differentiation of naive CD4 T cells into Th17 cells; however, IL-23 has been identified as an important cytokine that stabilizes the encephalitogenic potential of Th17 cells (Langrish et al., 2005). Despite our increased understanding of the factors that control CD4 T cell lineage commitment and the array of cytokines produced by different T effector subsets, a clear picture of a single pathogenic T cell phenotype required to induce CNS autoimmunity has not emerged. One challenge in defining “the” pathogenic T cell phenotype is that CD4 T cells exhibit plasticity in vivo, and T cells can simultaneously express the signature cytokines associated with different effector subsets. Furthermore, the T cell effector subsets produce multiple cytokines in addition to their signature cytokines, and there is overlap between the T cell subsets in expression of these cytokines. In this review, we will discuss our current understanding of how the major cytokines produced by encephalitogenic CD4 T cells contribute to the pathogenesis and regulation of EAE, and how well these insights parallel what is observed in patients with RRMS.
Pathogenic cytokines in EAE
IFNγ
Based on the hypothesis that MS is caused by either a virus or by immunoregulatory defects, clinical trials were initiated in the 1980s in which IFNα, IFNβ, and IFNγ were administered to patients. While IFNα and IFNβ reduced the frequency and severity of exacerbations in RRMS, a pilot study administering IFNγ resulted in an increased exacerbation rate, suggesting that IFNγ exerts pathogenic activity in MS (Panitch et al., 1987). Subsequent studies in animal models were consistent with a pathogenic role as encephalitogenic T cells were observed to produce IFNγ (Baron et al., 1993), and direct injection of IFNγ into the CNS resulted in inflammatory pathology resembling EAE (Simmons and Willenborg, 1990; Vass and Lassmann, 1990; Sethna and Lampson, 1991). Furthermore, treatment of myelin-specific CD4 T cells in vitro with IL-12, which is critical for Th1 cell polarization, enhanced their encephalitogenicity (Leonard et al., 1995; Segal and Shevach, 1996). Mice deficient in IL-12p40, a component of IL-12, were also completely resistant to EAE (Segal et al., 1998). However, many studies point to a more complex role for IFNγ, reflecting its pleiotropic activities and ability to act on multiple cell types. Protective effects in EAE are suggested by the exacerbating effect of either administering IFNγ-blocking antibodies (Billiau et al., 1988) or introducing genetic deficiency in IFNγ or the IFNγ receptor (IFNγR; Ferber et al., 1996; Krakowski and Owens, 1996; Willenborg et al., 1996; Tran et al., 2000). IFNγ also exerts different outcomes at different stages of disease. Administering IFNγ during disease induction exacerbates EAE, while administration at disease onset reduces severity (Naves et al., 2013). IFNγ may exacerbate EAE during disease induction via its ability to disrupt tight junctions and induce adhesion molecule expression on endothelial cells of the blood–brain barrier (BBB), allowing transendothelial migration of CD4 T cells into the parenchyma (Sonar et al., 2017). IFNγ also triggers astrocyte chemokine production (Ding et al., 2015), enhancing the recruitment and activation of myeloid cells, and increases MHC class I and II expression on CNS resident and infiltrating cells (Miller et al., 2015; Ottum et al., 2015). The ameliorating effect of IFNγ following disease onset may reflect the ability of IFNγ to inhibit microglia proliferation (Ding et al., 2015) and promote phagocytosis of myelin debris, which would prevent accumulation of neurotoxic lipid peroxidation products (Sosa et al., 2015).
IFNγ also appears to influence the localization of lesions within the CNS. In most EAE models, the clinical signs correspond to ascending flaccid paralysis associated with inflammation localized in the spinal cord, referred to as “classic EAE.” However, mice deficient in IFNγ or IFNγR exhibit additional symptoms associated with brain inflammation, including body lean and axial rotation, referred to as “atypical EAE” (Abromson-Leeman et al., 2004; Wensky et al., 2005; Lees et al., 2008; Simmons et al., 2014; Stoolman et al., 2014), indicating that IFNγ inhibits brain inflammation. IFNγ- or IFNγR-deficient mice exhibit inflammatory infiltrates within the brain characterized by a high number of neutrophils and an increase in neutrophil-attracting chemokines, such as CXCL2 (Lees et al., 2008; Simmons et al., 2014; Stoolman et al., 2014). Neutrophils are essential for brain inflammation in these models as neutrophil depletion or inhibition of neutrophil migration to the CNS via administration of either antibodies against CXCR2 or inhibitors of CXCR2 activity reduced the incidence of brain inflammation (Simmons et al., 2014; Stoolman et al., 2014). Some of these studies showed that IFNγ signaling enhances spinal cord inflammation in the same mice in which it is inhibiting brain inflammation, suggesting that IFNγ may have opposite effects in the brain and spinal cord (Lees et al., 2008; Simmons et al., 2014). In these mice, IFNγ promoted CCL2-mediated infiltration of monocytes and macrophages into the spinal cord (Stoolman et al., 2014). Collectively, these studies demonstrate that IFNγ can play both a protective and a pathogenic role in CNS autoimmunity in part by the differential modulation of chemokine production in the brain versus spinal cord.
IL-17
Observations that the p40 subunit of IL-12, but not IFNγ itself, was essential for EAE shifted attention to the cytokine IL-23, which shares the p40 subunit with IL-12 (Oppmann et al., 2000). Mice deficient in p19, the subunit specific to IL-23, were completely resistant to active EAE (Cua et al., 2003), while mice deficient in p35, the subunit specific to IL-12, were similar to mice lacking IFNγ in that they developed more severe EAE (Becher et al., 2002; Gran et al., 2002). These observations have been interpreted to indicate that IL-23 and not IL-12 is essential to generate pathogenic T cells. However, adoptive transfer of Th1 cells that were not exposed to IL-23 during priming or following transfer into recipient mice still induced EAE, indicating that IL-23 is also not essential to generate encephalitogenic T cells (Carbajal et al., 2015; Grifka-Walk et al., 2015). Nevertheless, IL-23 is clearly influential in EAE, as it was shown to promote an encephalitogenic T cell population that expresses a pattern of proinflammatory cytokines distinct from T cells differentiated with IL-12, including IL-17A and IL-17F (Langrish et al., 2005), referred to as Th17 cells. While both Th1 and Th17 cells can induce EAE, they promote different patterns of lesion localization. Notably, while IFNγ inhibits brain inflammation, IL-17 promotes inflammation in the brain versus the spinal cord in both C3Heb/FeJ and B6 models of EAE (Stromnes et al., 2008; Kroenke and Segal, 2011; Simmons et al., 2014). Several mechanisms have been proposed to account for how IL-17 contributes to CNS autoimmunity. IL-17 increases BBB permeability in vitro, thus enhancing the transmigration of CD4 T cells and monocytes to the CNS parenchyma (Kebir et al., 2007; Huppert et al., 2010). IL-17 also induces chemokine production by CNS resident cells (Kang et al., 2010; Simmons et al., 2014). In C3Heb/FeJ mice, IL-17–stimulated production of CXCL2 by brain astrocytes promoted neutrophil recruitment, which was essential for development of atypical but not classic EAE (Simmons et al., 2014). Additionally, adoptive transfer of Th17 cells induces tertiary lymphoid tissue–like structures within the meninges, and IL-17 directly contributes to the remodeling of meningeal fibroblasts and formation of fibrous networks in vitro and in vivo (Peters et al., 2011; Pikor et al., 2015).
While IL-17 deficiency clearly affects the manifestation of EAE, multiple studies have shown that, like IFNγ, IL-17 is dispensable for EAE. EAE was only partially ameliorated by administrating IL-17A–blocking antibodies (Hofstetter et al., 2005; Langrish et al., 2005; Park et al., 2005; Chen et al., 2006), and EAE could be actively induced in IL-17A−/− mice and by adoptive transfer of IL-17A−/− CD4 T cells (Komiyama et al., 2006; Haak et al., 2009). EAE could also be induced in IL-17F–deficient mice that received IL-17A–neutralizing antibodies, although the disease course was relatively mild (Haak et al., 2009). Consistent with the notion that IL-17 is not required for encephalitogenicity, Th17 cells differentiated in the presence of TGFβ and IL-6 but not IL-23 are not pathogenic, even though they exhibit a high expression of IL-17 (McGeachy et al., 2007). These data suggest that IL-23 confers encephalitogenic potential that is distinct from its ability to promote IL-17 production.
Collectively, these studies demonstrate that IFNγ and IL-17 both contribute to EAE pathogenesis. Highly polarized Th1 and Th17 cells that had been exposed to IL-12 or IL-23, respectively, only in vitro can independently induce EAE (Carbajal et al., 2015; Grifka-Walk et al., 2015), and the pathogenic mechanisms are different for Th1- versus Th17-initiated EAE (Kroenke et al., 2008). However, as neither cytokine is required for EAE, and 22% of C3Heb/Fej mice deficient in both IL-17R and IFNγR still exhibited EAE (Simmons et al., 2014), other cytokines have been evaluated for their role in CNS autoimmunity.
GM-CSF
Multiple strains of mice deficient in GM-CSF or its receptor demonstrate strong resistance to EAE induction (McQualter et al., 2001; Duncker et al., 2018); therefore, this cytokine has been considered essential in EAE. It remains controversial whether there is a distinct lineage of GM-CSF–producing T cells (Sheng et al., 2014; Komuczki et al., 2019); however, both Th1 and Th17 cells produce GM-CSF (Codarri et al., 2011; El-Behi et al., 2011). Adoptive transfer of Th1- and Th17-polarized cells deficient in GM-CSF strongly reduced the manifestation of EAE (Ponomarev et al., 2007; El-Behi et al., 2011; Duncker et al., 2018) and in some cases completely prevented disease induction (Codarri et al., 2011), demonstrating that GM-CSF contributes to the pathogenicity of both Th1- and Th17-skewed cells. However, the requirement for GM-CSF is not universal across all strains of mice. In SJL/J mice, anti–GM-CSF treatment before disease onset ameliorated clinical signs in Th17- but not Th1-initiated EAE (Kroenke et al., 2008). In C3Heb/FeJ mice, GM-CSF synergized with IL-17 to induce atypical EAE but was completely redundant with IL-17 in the induction of classic EAE (Pierson and Goverman, 2017). Collectively, these studies point to an important role of GM-CSF in EAE pathogenesis, especially in promoting brain inflammation, but the relative importance for this cytokine appears model dependent.
Effector T cell priming is compromised in the absence of GM-CSF (McQualter et al., 2001; Sonderegger et al., 2008; King et al., 2009; Pierson and Goverman, 2017; Duncker et al., 2018), which may contribute to the complete resistance of mice deficient in GM-CSF and its receptor in some strains. GM-CSF receptors are not expressed on T cells (Morrissey et al., 1987), suggesting that the effects of GM-CSF on T cell priming is indirect. Specifically, GM-CSF−/− mice lack a subset of CD103+ dendritic cells (DCs) that are important for Th1 cell differentiation (King et al., 2010), although the importance of GM-CSF in the differentiation of Th1 cells is somewhat controversial (Ponomarev et al., 2007; Sonderegger et al., 2008). In addition, DCs isolated from GM-CSF−/− mice produce less IL-6 and IL-23, which are required for the generation and maintenance of Th17 cells (Sonderegger et al., 2008). GM-CSF also contributes to the effector phase of disease as anti–GM-CSF antibodies administered at disease onset ameliorated disease (Codarri et al., 2011). The GM-CSF receptor was shown to be required on hematopoietic cells in both Th1- and Th17-intiated EAE (Codarri et al., 2011; Duncker et al., 2018). In B10.PL mice, adoptive transfer of CD4 T cells deficient in GM-CSF failed to induce EAE because of impaired GM-CSF–dependent activation of microglia (Ponomarev et al., 2007). However, this finding is somewhat controversial as a study in B6 mice found that GM-CSF signaling was not required on microglia (Croxford et al., 2015). Instead, EAE was dependent on GM-CSF signaling on CCR2+Ly6Chi monocytes, which induces phagocytic and proinflammatory responses in their progeny (Croxford et al., 2015). Another study in B6 mice found that, in the absence of IFNγ and IL-17, GM-CSF was required for disease induction, which was inhibited in the absence of neutrophils (Kroenke et al., 2010). In C3Heb/FeJ mice, GM-CSF independently promoted neutrophil recruitment to the brain, which is required to induce atypical EAE in this model (Pierson and Goverman, 2017).
TNFα
TNFα is also expressed by both Th1 and Th17 cells (Langrish et al., 2005). TNFα overexpression in the CNS resulted in demyelination (Probert et al., 1995; Akassoglou et al., 1998; Dal Canto et al., 1999), and blocking TNFα before EAE onset attenuated disease (Ruddle et al., 1990; Baker et al., 1994; Selmaj et al., 1995). However, it is increasingly understood that TNFα signaling is complex due to the fact that TNFα signals through two distinct receptors, TNFR1 and TNFR2, which trigger pathogenic or protective outcomes, respectively (Probert, 2015). In EAE, mice deficient in TNFR1 are either completely resistant (Suvannavejh et al., 2000; Kassiotis and Kollias, 2001) or develop milder disease (Eugster et al., 1999; Steeland et al., 2017). TNFR1 signaling promotes leukocyte migration from the perivascular space into the parenchyma during EAE by promoting adhesion molecules and chemokine expression by CNS-resident cells, including astrocytes (Gimenez et al., 2004, 2006). Signaling via TNFR1 also triggers oligodendrocyte death (Akassoglou et al., 1998; Hövelmeyer et al., 2005). In contrast, TNFR2-deficient mice exhibit more severe disease compared with WT mice (Eugster et al., 1999; Suvannavejh et al., 2000), demonstrating its importance in neuroprotection. TNFR2 signaling contributes to the proliferation and differentiation of oligodendrocyte progenitor cells and subsequent remyelination in the cuprizone model of demyelination (Arnett et al., 2001). TNFR2 signaling may act directly on oligodendrocytes, which express high levels of TNFR2 (Brambilla et al., 2011), as specific deletion of TNFR2 on oligodendrocytes exacerbated EAE and impaired remyelination (Madsen et al., 2016). TNFR2 is also expressed on a subset of activated regulatory T cells (T reg cells; Chen et al., 2008), and specific deletion of TNFR2 on T reg cells resulted in loss of suppressive functions and exacerbated EAE (Atretkhany et al., 2018). Together, these data demonstrate that TNFα signaling is complex and exerts opposing effects in CNS autoimmunity.
Pathogenic cytokines in MS
IFNγ
The demonstration that administration of recombinant IFNγ exacerbated MS launched many studies analyzing the expression of IFNγ in patients with MS, which collectively have shown that IFNγ is increased in the blood, cerebrospinal fluid, and brain lesions of MS patients compared with healthy controls (HCs). Elevated serum levels of IFNγ have been reported in MS patients relative to HCs (Beck et al., 1988; Arellano et al., 2017), and a longitudinal study showed that increases in IFNγ occurred just before a relapse (Beck et al., 1988). Studies have reported an increased frequency of T cells producing IFNγ in response to myelin antigen stimulation of peripheral blood monocytes (PBMCs) from patients with RRMS versus HCs (Arbour et al., 2003; Hedegaard et al., 2008). Increases in myelin-specific IFNγ protein–secreting and IFNγ mRNA–expressing mononuclear cells have also been detected in the blood (Olsson et al., 1990; Sun et al., 1991; Link et al., 1994; Pelfrey et al., 2000; Wallström et al., 2000; Huber et al., 2014) and cerebrospinal fluid (Olsson et al., 1990; Sun et al., 1991; Wallström et al., 2000) of MS patients relative to HCs. IFNγ-expressing cells are also enriched in the cerebrospinal fluid compared with blood within individual MS patients (Olsson et al., 1990; Sun et al., 1991; Wallström et al., 2000). IFNγ protein and IFNγ mRNA have also been detected in active areas of MS lesions (Woodroofe and Cuzner, 1993; Cannella and Raine, 1995; Mycko et al., 2003). Furthermore, increases in IFNγ levels or IFNγ+ myelin-specific T cells derived from blood have been correlated with the clinically active phase of disease compared with remission (Correale et al., 1995; Arbour et al., 2003) and with increasing disability (Link et al., 1994; Arbour et al., 2003; Moldovan et al., 2003).
IFNγ may also contribute to MS via its expression in T reg cells. A higher frequency of IFNγ-secreting Foxp3+ T reg cells are observed in untreated MS patients relative to HCs, and these T reg cells exhibit impaired suppressive activity in vitro compared with T reg cells that are not IFNγ+ (Dominguez-Villar et al., 2011). The defect in suppressive activity of these Th1-like T reg cells could be partially reversed by IFNγ-specific antibodies. Interestingly, IFNβ treatment of patients with RRMS restored the levels of these Th1-like T reg cells to that seen in HCs, suggesting that modulation of Th1-like T reg cells may be a mechanism underlying the therapeutic benefit of IFNβ (Dominguez-Villar et al., 2011). In sum, these studies support a pathogenic role for IFNγ in MS.
IL-17
Following the discovery that IL-17 is an influential cytokine in the pathogenesis of EAE, substantial evidence has accumulated implicating a pathogenic role for IL-17 in MS. Expression of IL-17 transcripts and protein was enhanced in MS lesions compared with normal-appearing white matter and brain tissue of those with nonneurological diseases and HCs (Lock et al., 2002; Tzartos et al., 2008). IL-17+ T cells primarily localized to active areas of lesions and active borders of chronic/active lesions, and, surprisingly, similar frequencies of IL-17–producing T cells in active lesions expressed CD8 as CD4 (Tzartos et al., 2008). Additionally, as was the case for IFNγ, the frequency of PBMCs expressing IL-17 mRNA was reported to be higher in the blood and cerebrospinal fluid of patients with MS relative to HCs, and was enriched in the cerebrospinal fluid compared with the blood within individual patients with MS (Matusevicius et al., 1999). Importantly, the frequency of PBMCs expressing IL-17 transcripts increased during clinical exacerbation compared with remission (Matusevicius et al., 1999), and a higher frequency of Th17 cells was also observed in the cerebrospinal fluid of RRMS patients in relapse compared with remission (Brucklacher-Waldert et al., 2009). The frequency of PBMCs producing IL-17 in response to MBP stimulation also correlated with the number of gadolidium-enhancing lesions in a cohort of patients (Hedegaard et al., 2008). Consistent with this observation, a clinical trial assessing the efficacy of secukinumab, an IL-17A–neutralizing antibody, in patients with RRMS demonstrated a significant reduction in the number of cumulative new gadolinium-enhancing T1 lesions, although the reduction in the cumulative number of combined unique active lesions did not quite achieve significance (Havrdová et al., 2016).
Adding complexity to our understanding of the role of individual cytokines in MS is the fact that human T cells producing IL-17 can also express IFNγ and other cytokines. T cells expanded from PBMCs of RRMS patients preferentially adopted an IFNγ+IL-17+ phenotype and were readily detected in brain tissue of MS patients, consistent with the observation that these dual cytokine-producing T cells preferentially crossed a human BBB in vitro (Kebir et al., 2009). One study of T cell libraries expanded from patients with MS showed that myelin-reactive, CCR6+ T cells have enhanced production of IFNγ, IL-17, and GM-CSF, as well as reduced production of IL-10 when compared with healthy individuals (Cao et al., 2015). A later study supported the importance of the relative expression of IL-10 versus pathogenic cytokines. These investigators identified different transcriptional signatures for human IFNγ+IL-17+ versus IFNγ−IL-17+ T cells. The IFNγ+IL-17+ T cell signature resembled that of pathogenic murine Th17 cells, and, in patients with MS, IFNγ+IL-17+ exhibited reduced expression of IL-10 while IFNγ−IL-17+ T cells exhibited a higher IL-10 expression in clinically stable versus active patients (Hu et al., 2017). Together, these studies implicate both IL-17 and IFNγ as playing a pathogenic role in MS. However, in a phase II clinical trial to test the efficacy of blocking the p40 subunit shared by IL-23 and IL-12, no beneficial effect was observed (Segal et al., 2008). While there are many potential explanations for why this trial failed, this finding encourages further examination of other cytokines that may play a pathogenic role in MS.
GM-CSF
The observation that levels of GM-CSF are elevated in the cerebrospinal fluid of relapsing compared with stable MS patients suggested a potential role for GM-CSF in MS pathogenesis before studies of this cytokine in EAE (Carrieri et al., 1998). Since this early observation, evidence supporting a role for GM-CSF in MS has steadily accumulated. Numerous studies have shown that the number and frequency of GM-CSF–producing T cells in peripheral blood and cerebrospinal fluid is increased in MS patients relative to those with other neurological diseases or HCs (Hartmann et al., 2014; Noster et al., 2014; Cao et al., 2015; Rasouli et al., 2015; van Langelaar et al., 2018; Ghezzi et al., 2019). Additional studies have shown that GM-CSF production by T cells is reduced in patients on immunomodulatory therapies (Hartmann et al., 2014; Rasouli et al., 2015). GM-CSF–producing T cells have also been identified in active MS lesions (Rasouli et al., 2015). Among patients with MS, those with higher frequencies of GM-CSF–producing T cells exhibited increased levels of biomarkers of MS progression in their cerebrospinal fluid and a greater number of T2 lesions (Hartmann et al., 2014). Finally, IL-2 is a strong inducer of T cell GM-CSF production (Hartmann et al., 2014), and a genetic polymorphism in the IL-2 receptor α-chain is a known MS risk allele (Hafler et al., 2007; Sawcer et al., 2011; Beecham et al., 2013). Naive T cells from healthy individuals expressing this risk allele exhibited increased IL-2 receptor α-chain expression on T cells and increased secretion of GM-CSF after IL-2 stimulation compared with T cells with the protective allele. Higher frequencies of GM-CSF–producing memory T cells are also found in PBMCs of healthy individuals with the risk allele compared with those with the protective allele. These findings suggest that some of the risk conferred by this allele may be associated with increased T cell production of GM-CSF. Together, these findings support the notion that GM-CSF produced by T cells exerts pathogenic activity in MS and point to GM-CSF as a potential therapeutic target that is currently being tested in clinical trials (Constantinescu et al., 2015).
TNFα
Early observations indicating that TNFα levels in the blood and cerebrospinal fluid of patients with MS correlated with disease activity (Beck et al., 1988; Hauser et al., 1990; Sharief and Hentges, 1991; Rieckmann et al., 1995) suggested that TNFα antagonists may be an effective therapy in MS. However, infliximab, an anti-TNFα monoclonal antibody, increased gadolinium-enhancing lesions in two patients with MS during a phase 1 safety trial (van Oosten et al., 1996), and lenercept, a nonselective TNFα inhibitor, exacerbated disease in an MS phase II clinical trial (The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group, 1999). TNFα inhibitors have also induced demyelinating lesions in patients with rheumatoid arthritis (Mohan et al., 2001; Kaltsonoudis et al., 2014) and irritable bowel disease (Colombel et al., 2004; Thomas et al., 2004; Freeman and Flak, 2005; Hare et al., 2014). Additionally, a single-nucleotide polymorphism in the gene that encodes TNFR1 is associated with MS (De Jager et al., 2009; Sawcer et al., 2011) and directs the expression of a soluble form of TNFR1 that blocks TNFα signaling (Gregory et al., 2012), further confirming a protective role of TNFα in MS. However, EAE studies demonstrated that signaling through TNFR1 and TNFR2 have opposing outcomes in CNS autoimmunity, with TNFR1 signaling promoting pathogenic activity and TNFR2 signaling enhancing remyelination via effects on oligodendrocyte precursors. Thus, the adverse effects seen with administration of infliximab or lenercept may be a result of targeting the beneficial as well as detrimental signaling pathways. Specifically inhibiting TNFR1 and/or enhancing TNFR2 signaling rather than broadly targeting TNFα in patients with MS may be a beneficial treatment strategy.
Concluding remarks
The cytokines discussed above clearly play a key role in CNS autoimmunity. However, MS and EAE studies demonstrate that disease is not governed by any one particular cytokine but instead involves a complex interplay between pro- and anti-inflammatory cytokines (Fig. 1). MS is a heterogeneous disease that involves variations in interactions between genetic and environmental factors in individual patients. Depending on which interactions are at play within an individual, different cytokines may assume a greater or lesser role in the pathogenesis of human disease, and the roles may vary over time. This notion is consistent with EAE studies that have demonstrated that myelin-specific CD4 T cells with diverse cytokine profiles have encephalitogenic potential, suggesting that one cytokine may predominate in some MS patients, or multiple cytokines may contribute in an additive or synergistic manner. Due to this heterogeneity, targeting individual cytokines may have limited efficacy in patients with MS. Instead, targeting multiple cytokines or tailoring therapies to individual patients may be attractive avenues for future MS therapies.
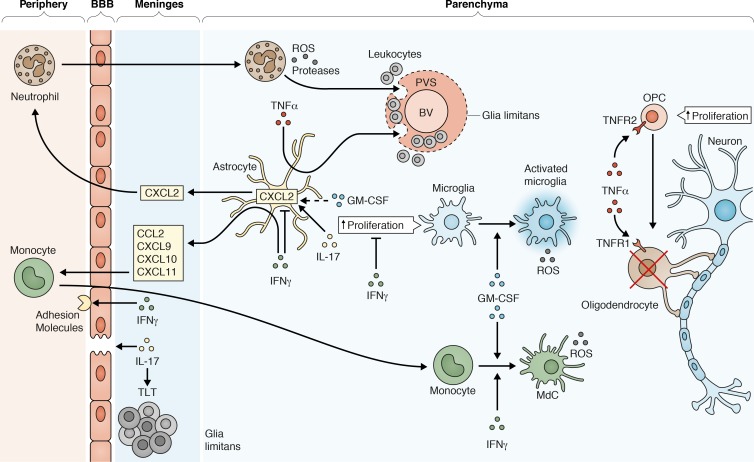
Figure 1.
Myelin-specific CD4 T cells that cross the BBB and are reactivated within the meninges produce pathogenic cytokines that orchestrate the inflammatory response in EAE. IL-17 contributes to loss in the integrity of the BBB by breaking down tight junctions between endothelial cells, and acts on astrocytes in the brain to produce CXCL2, a chemokine that recruits neutrophils. Neutrophils produce ROS that can damage axons. They also produce proteases that help break down the glia limitans surrounding blood vessels (BV), allowing leukocytes to escape from the perivascular space (PVS). IL-17 also promotes stromal cell remodeling within the meninges, which results in the formation of tertiary lymphoid tissue (TLT). IFNγ promotes recruitment of leukocytes from the peripheral circulation by increasing the expression of adhesion molecules on the endothelial cells of the BBB. IFNγ also induces astrocytes to secrete chemokines that attract monocytes, e.g., CCL2, CXCL9, CXCL10, and CXCL11, and facilitates the maturation and activation of monocytes into inflammatory monocyte-derived cells (MdCs) that produce ROS. Additionally, IFNγ exerts inhibitory effects on CNS inflammation by decreasing microglial cell proliferation. In the brain, IFNγ inhibits expression of CXCL2 in astrocytes, which decreases recruitment of neutrophils. In contrast, IFNγ promotes neutrophil recruitment to the spinal cord (not shown). GM-CSF enhances neutrophil recruitment to the brain and spinal cord, promotes microglia activation, and activates monocytes to acquire an inflammatory phenotype. TNFα acts on astrocytes to produce factors that promote migration of leukocytes from the PVS into the parenchyma. TNFα also enhances oligodendrocyte cell death via signaling through TNFR1 but promotes oligodendrocyte progenitor cell (OPC) proliferation via TNFR2 signaling.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Grant R37 AI107494-01 (to J.M. Goverman), the National Institute of General Medicine Sciences Cell & Molecular Biology Training Grant 5T32GM007270, and the National Institute of Allergy and Infectious Diseases Immunology Training Grant 5T32AI106677 (both to C.A. Wagner).
The authors declare no competing financial interests.
References
- Abromson-Leeman S., Bronson R., Luo Y., Berman M., Leeman R., Leeman J., and Dorf M.. 2004. T-cell properties determine disease site, clinical presentation, and cellular pathology of experimental autoimmune encephalomyelitis. Am. J. Pathol. 165:1519–1533. 10.1016/S0002-9440(10)63410-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K., Bauer J., Kassiotis G., Pasparakis M., Lassmann H., Kollias G., and Probert L.. 1998. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am. J. Pathol. 153:801–813. 10.1016/S0002-9440(10)65622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Holz A., Sipe J.C., Naniche D., Romine J.S., Zyroff J., and Oldstone M.B.. 2003. A new approach for evaluating antigen-specific T cell responses to myelin antigens during the course of multiple sclerosis. J. Neuroimmunol. 137:197–209. 10.1016/S0165-5728(03)00080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano G., Acuña E., Reyes L.I., Ottum P.A., De Sarno P., Villarroel L., Ciampi E., Uribe-San Martín R., Cárcamo C., and Naves R.. 2017. Th1 and Th17 cells and associated cytokines discriminate among clinically isolated syndrome and multiple sclerosis phenotypes. Front. Immunol. 8:753 10.3389/fimmu.2017.00753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett H.A., Mason J., Marino M., Suzuki K., Matsushima G.K., and Ting J.P.. 2001. TNF α promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4:1116–1122. 10.1038/nn738 [DOI] [PubMed] [Google Scholar]
- Atretkhany K.N., Mufazalov I.A., Dunst J., Kuchmiy A., Gogoleva V.S., Andruszewski D., Drutskaya M.S., Faustman D.L., Schwabenland M., Prinz M., et al. 2018. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc. Natl. Acad. Sci. USA. 115:13051–13056. 10.1073/pnas.1807499115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Butler D., Scallon B.J., O’Neill J.K., Turk J.L., and Feldmann M.. 1994. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur. J. Immunol. 24:2040–2048. 10.1002/eji.1830240916 [DOI] [PubMed] [Google Scholar]
- Baron J.L., Madri J.A., Ruddle N.H., Hashim G., and Janeway C.A. Jr. 1993. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 177:57–68. 10.1084/jem.177.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L.H., Waubant E., Gazda S., Fox R.J., Panzara M., et al. 2008. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 63:395–400. 10.1002/ana.21363 [DOI] [PubMed] [Google Scholar]
- Becher B., Durell B.G., and Noelle R.J.. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497. 10.1172/JCI0215751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J., Rondot P., Catinot L., Falcoff E., Kirchner H., and Wietzerbin J.. 1988. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol. Scand. 78:318–323. 10.1111/j.1600-0404.1988.tb03663.x [DOI] [PubMed] [Google Scholar]
- Beecham A.H., Patsopoulos N.A., Xifara D.K., Davis M.F., Kemppinen A., Cotsapas C., Shah T.S., Spencer C., Booth D., Goris A., et al. International IBD Genetics Consortium (IIBDGC) . 2013. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45:1353–1360. 10.1038/ng.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Vandekerckhove F., Dijkmans R., Sobis H., Meulepas E., and Carton H.. 1988. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J. Immunol. 140:1506–1510. [PubMed] [Google Scholar]
- Brambilla R., Ashbaugh J.J., Magliozzi R., Dellarole A., Karmally S., Szymkowski D.E., and Bethea J.R.. 2011. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain. 134:2736–2754. 10.1093/brain/awr199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucklacher-Waldert V., Stuerner K., Kolster M., Wolthausen J., and Tolosa E.. 2009. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 132:3329–3341. 10.1093/brain/awp289 [DOI] [PubMed] [Google Scholar]
- Cannella B., and Raine C.S.. 1995. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 37:424–435. 10.1002/ana.410370404 [DOI] [PubMed] [Google Scholar]
- Cao Y., Goods B.A., Raddassi K., Nepom G.T., Kwok W.W., Love J.C., and Hafler D.A.. 2015. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 7:287ra74 10.1126/scitranslmed.aaa8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal K.S., Mironova Y., Ulrich-Lewis J.T., Kulkarni D., Grifka-Walk H.M., Huber A.K., Shrager P., Giger R.J., and Segal B.M.. 2015. Th cell diversity in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 195:2552–2559. 10.4049/jimmunol.1501097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri P.B., Provitera V., De Rosa T., Tartaglia G., Gorga F., and Perrella O.. 1998. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol. Immunotoxicol. 20:373–382. 10.3109/08923979809034820 [DOI] [PubMed] [Google Scholar]
- Chen X., Subleski J.J., Kopf H., Howard O.M., Männel D.N., and Oppenheim J.J.. 2008. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J. Immunol. 180:6467–6471. 10.4049/jimmunol.180.10.6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Langrish C.L., McKenzie B., Joyce-Shaikh B., Stumhofer J.S., McClanahan T., Blumenschein W., Churakovsa T., Low J., Presta L., et al. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116:1317–1326. 10.1172/JCI25308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., and Becher B.. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12:560–567. 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- Colombel J.F., Loftus E.V. Jr., Tremaine W.J., Egan L.J., Harmsen W.S., Schleck C.D., Zinsmeister A.R., and Sandborn W.J.. 2004. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 126:19–31. 10.1053/j.gastro.2003.10.047 [DOI] [PubMed] [Google Scholar]
- Constantinescu C.S., Asher A., Fryze W., Kozubski W., Wagner F., Aram J., Tanasescu R., Korolkiewicz R.P., Dirnberger-Hertweck M., Steidl S., et al. 2015. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2:e117 10.1212/NXI.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J., Gilmore W., McMillan M., Li S., McCarthy K., Le T., and Weiner L.P.. 1995. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J. Immunol. 154:2959–2968. [PubMed] [Google Scholar]
- Croxford A.L., Lanzinger M., Hartmann F.J., Schreiner B., Mair F., Pelczar P., Clausen B.E., Jung S., Greter M., and Becher B.. 2015. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 43:502–514. 10.1016/j.immuni.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Dal Canto R.A., Shaw M.K., Nolan G.P., Steinman L., and Fathman C.G.. 1999. Local delivery of TNF by retrovirus-transduced T lymphocytes exacerbates experimental autoimmune encephalomyelitis. Clin. Immunol. 90:10–14. 10.1006/clim.1998.4653 [DOI] [PubMed] [Google Scholar]
- De Jager P.L., Jia X., Wang J., de Bakker P.I., Ottoboni L., Aggarwal N.T., Piccio L., Raychaudhuri S., Tran D., Aubin C., et al. International MS Genetics Consortium . 2009. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41:776–782. 10.1038/ng.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Yan Y., Li X., Li K., Ciric B., Yang J., Zhang Y., Wu S., Xu H., Chen W., et al. 2015. Silencing IFN-γ binding/signaling in astrocytes versus microglia leads to opposite effects on central nervous system autoimmunity. J. Immunol. 194:4251–4264. 10.4049/jimmunol.1303321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M., Baecher-Allan C.M., and Hafler D.A.. 2011. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17:673–675. 10.1038/nm.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker P.C., Stoolman J.S., Huber A.K., and Segal B.M.. 2018. GM-CSF promotes chronic disability in experimental autoimmune encephalomyelitis by altering the composition of central nervous system-infiltrating cells, but is dispensable for disease induction. J. Immunol. 200:966–973. 10.4049/jimmunol.1701484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., and Rostami A.. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575. 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster H.P., Frei K., Bachmann R., Bluethmann H., Lassmann H., and Fontana A.. 1999. Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur. J. Immunol. 29:626–632. [DOI] [PubMed] [Google Scholar]
- Ferber I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L., Dalton D., and Fathman C.G.. 1996. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5–7. [PubMed] [Google Scholar]
- Filippi M., Bar-Or A., Piehl F., Preziosa P., Solari A., Vukusic S., and Rocca M.A.. 2018. Multiple sclerosis. Nat. Rev. Dis. Primers. 4:43 10.1038/s41572-018-0041-4 [DOI] [PubMed] [Google Scholar]
- Freeman H.J., and Flak B.. 2005. Demyelination-like syndrome in Crohn’s disease after infliximab therapy. Can. J. Gastroenterol. 19:313–316. 10.1155/2005/358658 [DOI] [PubMed] [Google Scholar]
- Ghezzi L., Cantoni C., Cignarella F., Bollman B., Cross A.H., Salter A., Galimberti D., Cella M., and Piccio L.. 2019. T cells producing GM-CSF and IL-13 are enriched in the cerebrospinal fluid of relapsing MS patients. Mult. Scler.:1352458519852092 10.1177/1352458519852092 [DOI] [PubMed] [Google Scholar]
- Gimenez M.A., Sim J.E., and Russell J.H.. 2004. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. J. Neuroimmunol. 151:116–125. 10.1016/j.jneuroim.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Gimenez M.A., Sim J., Archambault A.S., Klein R.S., and Russell J.H.. 2006. A tumor necrosis factor receptor 1-dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. Am. J. Pathol. 168:1200–1209. 10.2353/ajpath.2006.050332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. 2009. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 9:393–407. 10.1038/nri2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran B., Zhang G.X., Yu S., Li J., Chen X.H., Ventura E.S., Kamoun M., and Rostami A.. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169:7104–7110. 10.4049/jimmunol.169.12.7104 [DOI] [PubMed] [Google Scholar]
- Gregory A.P., Dendrou C.A., Attfield K.E., Haghikia A., Xifara D.K., Butter F., Poschmann G., Kaur G., Lambert L., Leach O.A., et al. 2012. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature. 488:508–511. 10.1038/nature11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifka-Walk H.M., Giles D.A., and Segal B.M.. 2015. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur. J. Immunol. 45:2780–2786. 10.1002/eji.201545800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., and Waisman A.. 2009. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D.A., Compston A., Sawcer S., Lander E.S., Daly M.J., De Jager P.L., de Bakker P.I., Gabriel S.B., Mirel D.B., Ivinson A.J., et al. International Multiple Sclerosis Genetics Consortium . 2007. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357:851–862. 10.1056/NEJMoa073493 [DOI] [PubMed] [Google Scholar]
- Hare N.C., Hunt D.P., Venugopal K., Ho G.T., Beez T., Lees C.W., Gibson R., Weller B., and Satsangi J.. 2014. Multiple sclerosis in the context of TNF blockade and inflammatory bowel disease. QJM. 107:51–55. 10.1093/qjmed/hcr237 [DOI] [PubMed] [Google Scholar]
- Hartmann F.J., Khademi M., Aram J., Ammann S., Kockum I., Constantinescu C., Gran B., Piehl F., Olsson T., Codarri L., and Becher B.. 2014. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat. Commun. 5:5056 10.1038/ncomms6056 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Doolittle T.H., Lincoln R., Brown R.H., and Dinarello C.A.. 1990. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 40:1735–1739. 10.1212/WNL.40.11.1735 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., Bar-Or A., Panzara M., Sarkar N., Agarwal S., et al. HERMES Trial Group . 2008. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358:676–688. 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- Havrdová E., Belova A., Goloborodko A., Tisserant A., Wright A., Wallstroem E., Garren H., Maguire R.P., and Johns D.R.. 2016. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J. Neurol. 263:1287–1295. 10.1007/s00415-016-8128-x [DOI] [PubMed] [Google Scholar]
- Hedegaard C.J., Krakauer M., Bendtzen K., Lund H., Sellebjerg F., and Nielsen C.H.. 2008. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 125:161–169. 10.1111/j.1365-2567.2008.02837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H.H., Ibrahim S.M., Koczan D., Kruse N., Weishaupt A., Toyka K.V., and Gold R.. 2005. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237:123–130. 10.1016/j.cellimm.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Hövelmeyer N., Hao Z., Kranidioti K., Kassiotis G., Buch T., Frommer F., von Hoch L., Kramer D., Minichiello L., Kollias G., et al. 2005. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 175:5875–5884. 10.4049/jimmunol.175.9.5875 [DOI] [PubMed] [Google Scholar]
- Hu D., Notarbartolo S., Croonenborghs T., Patel B., Cialic R., Yang T.H., Aschenbrenner D., Andersson K.M., Gattorno M., Pham M., et al. 2017. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 8:1600 10.1038/s41467-017-01571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A.K., Wang L., Han P., Zhang X., Ekholm S., Srinivasan A., Irani D.N., and Segal B.M.. 2014. Dysregulation of the IL-23/IL-17 axis and myeloid factors in secondary progressive MS. Neurology. 83:1500–1507. 10.1212/WNL.0000000000000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert J., Closhen D., Croxford A., White R., Kulig P., Pietrowski E., Bechmann I., Becher B., Luhmann H.J., Waisman A., and Kuhlmann C.R.. 2010. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 24:1023–1034. 10.1096/fj.09-141978 [DOI] [PubMed] [Google Scholar]
- Kaltsonoudis E., Zikou A.K., Voulgari P.V., Konitsiotis S., Argyropoulou M.I., and Drosos A.A.. 2014. Neurological adverse events in patients receiving anti-TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res. Ther. 16:R125 10.1186/ar4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z., Altuntas C.Z., Gulen M.F., Liu C., Giltiay N., Qin H., Liu L., Qian W., Ransohoff R.M., Bergmann C., et al. 2010. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 32:414–425. 10.1016/j.immuni.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiotis G., and Kollias G.. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427–434. 10.1084/jem.193.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., and Prat A.. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 13:1173–1175. 10.1038/nm1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H., Ifergan I., Alvarez J.I., Bernard M., Poirier J., Arbour N., Duquette P., and Prat A.. 2009. Preferential recruitment of interferon-γ-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66:390–402. 10.1002/ana.21748 [DOI] [PubMed] [Google Scholar]
- King I.L., Dickendesher T.L., and Segal B.M.. 2009. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 113:3190–3197. 10.1182/blood-2008-07-168575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I.L., Kroenke M.A., and Segal B.M.. 2010. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 207:953–961. 10.1084/jem.20091844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., and Iwakura Y.. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573. 10.4049/jimmunol.177.1.566 [DOI] [PubMed] [Google Scholar]
- Komuczki J., Tuzlak S., Friebel E., Hartwig T., Spath S., Rosenstiel P., Waisman A., Opitz L., Oukka M., Schreiner B., et al. 2019. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1β. Immunity. 50:1289–1304.e6. 10.1016/j.immuni.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Krakowski M., and Owens T.. 1996. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26:1641–1646. 10.1002/eji.1830260735 [DOI] [PubMed] [Google Scholar]
- Kroenke M.A., and Segal B.M.. 2011. IL-23 modulated myelin-specific T cells induce EAE via an IFNγ driven, IL-17 independent pathway. Brain Behav. Immun. 25:932–937. 10.1016/j.bbi.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke M.A., Carlson T.J., Andjelkovic A.V., and Segal B.M.. 2008. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205:1535–1541. 10.1084/jem.20080159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke M.A., Chensue S.W., and Segal B.M.. 2010. EAE mediated by a non-IFN-γ/non-IL-17 pathway. Eur. J. Immunol. 40:2340–2348. 10.1002/eji.201040489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., and Cua D.J.. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J.R., Golumbek P.T., Sim J., Dorsey D., and Russell J.H.. 2008. Regional CNS responses to IFN-γ determine lesion localization patterns during EAE pathogenesis. J. Exp. Med. 205:2633–2642. 10.1084/jem.20080155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J.P., Waldburger K.E., and Goldman S.J.. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 181:381–386. 10.1084/jem.181.1.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link J., Söderström M., Olsson T., Höjeberg B., Ljungdahl A., and Link H.. 1994. Increased transforming growth factor-β, interleukin-4, and interferon-γ in multiple sclerosis. Ann. Neurol. 36:379–386. 10.1002/ana.410360309 [DOI] [PubMed] [Google Scholar]
- Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., Langer-Gould A., Strober S., Cannella B., Allard J., et al. 2002. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8:500–508. 10.1038/nm0502-500 [DOI] [PubMed] [Google Scholar]
- Madsen P.M., Motti D., Karmally S., Szymkowski D.E., Lambertsen K.L., Bethea J.R., and Brambilla R.. 2016. Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J. Neurosci. 36:5128–5143. 10.1523/JNEUROSCI.0211-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusevicius D., Kivisäkk P., He B., Kostulas N., Ozenci V., Fredrikson S., and Link H.. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5:101–104. 10.1177/135245859900500206 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., and Cua D.J.. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397. 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- McQualter J.L., Darwiche R., Ewing C., Onuki M., Kay T.W., Hamilton J.A., Reid H.H., and Bernard C.C.. 2001. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 194:873–882. 10.1084/jem.194.7.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.M., Wang J., Tan Y., and Dittel B.N.. 2015. Anti-inflammatory mechanisms of IFN-γ studied in experimental autoimmune encephalomyelitis reveal neutrophils as a potential target in multiple sclerosis. Front. Neurosci. 9:287 10.3389/fnins.2015.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Edwards E.T., Cupps T.R., Oliverio P.J., Sandberg G., Crayton H., Richert J.R., and Siegel J.N.. 2001. Demyelination occurring during anti-tumor necrosis factor α therapy for inflammatory arthritides. Arthritis Rheum. 44:2862–2869. [DOI] [PubMed] [Google Scholar]
- Moldovan I.R., Rudick R.A., Cotleur A.C., Born S.E., Lee J.C., Karafa M.T., and Pelfrey C.M.. 2003. Interferon gamma responses to myelin peptides in multiple sclerosis correlate with a new clinical measure of disease progression. J. Neuroimmunol. 141:132–140. 10.1016/S0165-5728(03)00221-2 [DOI] [PubMed] [Google Scholar]
- Morrissey P.J., Bressler L., Park L.S., Alpert A., and Gillis S.. 1987. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J. Immunol. 139:1113–1119. [PubMed] [Google Scholar]
- Mycko M.P., Papoian R., Boschert U., Raine C.S., and Selmaj K.W.. 2003. cDNA microarray analysis in multiple sclerosis lesions: detection of genes associated with disease activity. Brain. 126:1048–1057. 10.1093/brain/awg107 [DOI] [PubMed] [Google Scholar]
- Naves R., Singh S.P., Cashman K.S., Rowse A.L., Axtell R.C., Steinman L., Mountz J.D., Steele C., De Sarno P., and Raman C.. 2013. The interdependent, overlapping, and differential roles of type I and II IFNs in the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 191:2967–2977. 10.4049/jimmunol.1300419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noster R., Riedel R., Mashreghi M.F., Radbruch H., Harms L., Haftmann C., Chang H.D., Radbruch A., and Zielinski C.E.. 2014. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 6:241ra80 10.1126/scitranslmed.3008706 [DOI] [PubMed] [Google Scholar]
- Olsson T., Zhi W.W., Höjeberg B., Kostulas V., Jiang Y.P., Anderson G., Ekre H.P., and Link H.. 1990. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J. Clin. Invest. 86:981–985. 10.1172/JCI114800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B., Lesley R., Blom B., Timans J.C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. 10.1016/S1074-7613(00)00070-4 [DOI] [PubMed] [Google Scholar]
- Ottum P.A., Arellano G., Reyes L.I., Iruretagoyena M., and Naves R.. 2015. Opposing roles of interferon-gamma on cells of the central nervous system in autoimmune neuroinflammation. Front. Immunol. 6:539 10.3389/fimmu.2015.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch H.S., Hirsch R.L., Haley A.S., and Johnson K.P.. 1987. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1:893–895. 10.1016/S0140-6736(87)92863-7 [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., and Dong C.. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelfrey C.M., Rudick R.A., Cotleur A.C., Lee J.C., Tary-Lehmann M., and Lehmann P.V.. 2000. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J. Immunol. 165:1641–1651. 10.4049/jimmunol.165.3.1641 [DOI] [PubMed] [Google Scholar]
- Peters A., Pitcher L.A., Sullivan J.M., Mitsdoerffer M., Acton S.E., Franz B., Wucherpfennig K., Turley S., Carroll M.C., Sobel R.A., et al. 2011. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 35:986–996. 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson E.R., and Goverman J.M.. 2017. GM-CSF is not essential for experimental autoimmune encephalomyelitis but promotes brain-targeted disease. JCI Insight. 2:e92362 10.1172/jci.insight.92362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikor N.B., Astarita J.L., Summers-Deluca L., Galicia G., Qu J., Ward L.A., Armstrong S., Dominguez C.X., Malhotra D., Heiden B., et al. 2015. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity. 43:1160–1173. 10.1016/j.immuni.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Ponomarev E.D., Shriver L.P., Maresz K., Pedras-Vasconcelos J., Verthelyi D., and Dittel B.N.. 2007. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178:39–48. 10.4049/jimmunol.178.1.39 [DOI] [PubMed] [Google Scholar]
- Probert L. 2015. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. 302:2–22. 10.1016/j.neuroscience.2015.06.038 [DOI] [PubMed] [Google Scholar]
- Probert L., Akassoglou K., Pasparakis M., Kontogeorgos G., and Kollias G.. 1995. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA. 92:11294–11298. 10.1073/pnas.92.24.11294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli J., Ciric B., Imitola J., Gonnella P., Hwang D., Mahajan K., Mari E.R., Safavi F., Leist T.P., Zhang G.X., and Rostami A.. 2015. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β Therapy. J. Immunol. 194:5085–5093. 10.4049/jimmunol.1403243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P., Albrecht M., Kitze B., Weber T., Tumani H., Broocks A., Lüer W., Helwig A., and Poser S.. 1995. Tumor necrosis factor-α messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann. Neurol. 37:82–88. 10.1002/ana.410370115 [DOI] [PubMed] [Google Scholar]
- Ruddle N.H., Bergman C.M., McGrath K.M., Lingenheld E.G., Grunnet M.L., Padula S.J., and Clark R.B.. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172:1193–1200. 10.1084/jem.172.4.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salou M., Nicol B., Garcia A., and Laplaud D.A.. 2015. Involvement of CD8+ T cells in multiple sclerosis. Front. Immunol. 6:604 10.3389/fimmu.2015.00604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S., Hellenthal G., Pirinen M., Spencer C.C., Patsopoulos N.A., Moutsianas L., Dilthey A., Su Z., Freeman C., Hunt S.E., et al. Wellcome Trust Case Control Consortium 2 . 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 476:214–219. 10.1038/nature10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., and Shevach E.M.. 1996. IL-12 unmasks latent autoimmune disease in resistant mice. J. Exp. Med. 184:771–775. 10.1084/jem.184.2.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., Dwyer B.K., and Shevach E.M.. 1998. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 187:537–546. 10.1084/jem.187.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., Constantinescu C.S., Raychaudhuri A., Kim L., Fidelus-Gort R., and Kasper L.H.. Ustekinumab MS Investigators . 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7:796–804. 10.1016/S1474-4422(08)70173-X [DOI] [PubMed] [Google Scholar]
- Selmaj K., Papierz W., Glabiński A., and Kohno T.. 1995. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J. Neuroimmunol. 56:135–141. 10.1016/0165-5728(94)00139-F [DOI] [PubMed] [Google Scholar]
- Sethna M.P., and Lampson L.A.. 1991. Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-γ. J. Neuroimmunol. 34:121–132. 10.1016/0165-5728(91)90121-M [DOI] [PubMed] [Google Scholar]
- Sharief M.K., and Hentges R.. 1991. Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 325:467–472. 10.1056/NEJM199108153250704 [DOI] [PubMed] [Google Scholar]
- Sheng W., Yang F., Zhou Y., Yang H., Low P.Y., Kemeny D.M., Tan P., Moh A., Kaplan M.H., Zhang Y., and Fu X.Y.. 2014. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 24:1387–1402. 10.1038/cr.2014.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R.D., and Willenborg D.O.. 1990. Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J. Neurol. Sci. 100:37–42. 10.1016/0022-510X(90)90010-K [DOI] [PubMed] [Google Scholar]
- Simmons S.B., Liggitt D., and Goverman J.M.. 2014. Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis. J. Immunol. 193:555–563. 10.4049/jimmunol.1400807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonar S.A., Shaikh S., Joshi N., Atre A.N., and Lal G.. 2017. IFN-γ promotes transendothelial migration of CD4+ T cells across the blood-brain barrier. Immunol. Cell Biol. 95:843–853. 10.1038/icb.2017.56 [DOI] [PubMed] [Google Scholar]
- Sonderegger I., Iezzi G., Maier R., Schmitz N., Kurrer M., and Kopf M.. 2008. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J. Exp. Med. 205:2281–2294. 10.1084/jem.20071119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa R.A., Murphey C., Robinson R.R., and Forsthuber T.G.. 2015. IFN-γ ameliorates autoimmune encephalomyelitis by limiting myelin lipid peroxidation. Proc. Natl. Acad. Sci. USA. 112:E5038–E5047. 10.1073/pnas.1505955112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeland S., Van Ryckeghem S., Van Imschoot G., De Rycke R., Toussaint W., Vanhoutte L., Vanhove C., De Vos F., Vandenbroucke R.E., and Libert C.. 2017. TNFR1 inhibition with a Nanobody protects against EAE development in mice. Sci. Rep. 7:13646 10.1038/s41598-017-13984-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman J.S., Duncker P.C., Huber A.K., and Segal B.M.. 2014. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J. Immunol. 193:564–570. 10.4049/jimmunol.1400825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I.M., Cerretti L.M., Liggitt D., Harris R.A., and Goverman J.M.. 2008. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 14:337–342. 10.1038/nm1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Link H., Olsson T., Xiao B.G., Andersson G., Ekre H.P., Linington C., and Diener P.. 1991. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J. Immunol. 146:1490–1495. [PubMed] [Google Scholar]
- Suvannavejh G.C., Lee H.O., Padilla J., Dal Canto M.C., Barrett T.A., and Miller S.D.. 2000. Divergent roles for p55 and p75 tumor necrosis factor receptors in the pathogenesis of MOG(35-55)-induced experimental autoimmune encephalomyelitis. Cell. Immunol. 205:24–33. 10.1006/cimm.2000.1706 [DOI] [PubMed] [Google Scholar]
- The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 53:457–465. 10.1212/WNL.53.3.457 [DOI] [PubMed] [Google Scholar]
- Thomas C.W. Jr., Weinshenker B.G., and Sandborn W.J.. 2004. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm. Bowel Dis. 10:28–31. 10.1097/00054725-200401000-00004 [DOI] [PubMed] [Google Scholar]
- Tran E.H., Prince E.N., and Owens T.. 2000. IFN-γ shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 164:2759–2768. 10.4049/jimmunol.164.5.2759 [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., and Fugger L.. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172:146–155. 10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Langelaar J., van der Vuurst de Vries R.M., Janssen M., Wierenga-Wolf A.F., Spilt I.M., Siepman T.A., Dankers W., Verjans G.M.G.M., de Vries H.E., Lubberts E., et al. 2018. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. 141:1334–1349. 10.1093/brain/awy069 [DOI] [PubMed] [Google Scholar]
- van Oosten B.W., Barkhof F., Truyen L., Boringa J.B., Bertelsmann F.W., von Blomberg B.M., Woody J.N., Hartung H.P., and Polman C.H.. 1996. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 47:1531–1534. 10.1212/WNL.47.6.1531 [DOI] [PubMed] [Google Scholar]
- Vass K., and Lassmann H.. 1990. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am. J. Pathol. 137:789–800. [PMC free article] [PubMed] [Google Scholar]
- Wallström E., Khademi M., Andersson M., and Olsson T.. 2000. Increased numbers of mononuclear cells from blood and CSF expressing interferon-gamma mRNA in multiple sclerosis are from both the CD4+ and the CD8+ subsets. Eur. J. Neurol. 7:71–76. 10.1046/j.1468-1331.2000.00027.x [DOI] [PubMed] [Google Scholar]
- Wensky A.K., Furtado G.C., Marcondes M.C., Chen S., Manfra D., Lira S.A., Zagzag D., and Lafaille J.J.. 2005. IFN-γ determines distinct clinical outcomes in autoimmune encephalomyelitis. J. Immunol. 174:1416–1423. 10.4049/jimmunol.174.3.1416 [DOI] [PubMed] [Google Scholar]
- Willenborg D.O., Fordham S., Bernard C.C., Cowden W.B., and Ramshaw I.A.. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157:3223–3227. [PubMed] [Google Scholar]
- Woodroofe M.N., and Cuzner M.L.. 1993. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 5:583–588. 10.1016/S1043-4666(05)80008-0 [DOI] [PubMed] [Google Scholar]