Abstract
An efficient nickel-catalyzed removal of alkene protection group under mild condition with high functional group tolerance through chain walking process has been established. Not only phenolic ethers, but also alcoholic ethers can be tolerated with the retention of stereocenter adjacent to hydroxyl group. The new reaction brings the homoallyl group into a start of new type of protecting group.
Keywords: deprotection, nickel, chain walking, phenol, alcohol
1. Introduction
Functional group protecting and deprotecting is a common strategy widely used in organic chemistry especially in total synthesis [1]. The most conventional masking of phenols and alcohols are allylethers derived from facile base-promoted substitution of allyl halides [2]. Meanwhile, allylethers are easily removed under acidic, basic, reductive, or oxidative conditions with or without the aid of transition metals (Scheme 1a) [3,4,5,6,7,8,9,10,11]. On the other hand, it means allylethers are not stable in these conditions which diminished its usage in application. Long-chain alkene ethers such as homoallylethers are significantly steadier than allylethers which have been serving as a protecting group for decades [12,13,14,15,16,17,18], but lack of utility in practice due to the few deprotection methods. Ozonolysis of homoallylic carboxylic esters was reported by Barrett [19]. Cossy [20] and Lipshutz [21] reported removal of homoallyl group by Grubbs-Hoveyda catalyst through isomerization or metathesis. These reports are encountered from harsh reaction conditions. Meanwhile, chain walking strategy in bond construction has been emerging as a powerful tool utilizing alkene as feedstock in synthetic chemistry [22,23,24,25]. Hu [26,27], Martin [28,29,30], Zhu [31,32,33,34,35,36], and Hartwig [37] recently demonstrated that nickel was a prominent catalyst in chain walking chemistry. Streuff reported a zirconium catalyzed deallylation through chain walking mechanism [38]. Inspired by these reports and our interest in group transfer reactions [39] and nickel-catalyzed reactions [40], we envisaged that the nickel-hydride complex I formed through en route nickel-boryl [41,42,43] water or methanol addiction intermediate σ-bond metathesis would trigger the chain walking process (III, Scheme 1c). Low valent Nickel(I) was reported to go through oxidative addition to allyl C-O bond [44,45,46], which made the formation of intermediate V possible. The alkene (VI) was released after reductive elimination and regenerated the catalyst. Free phenols or alcohols would be retrieved by simple acid workup of VIII. Herein, we report the nickel-catalyzed removal of alkene protection group of phenols and alcohols under mild condition with high functional group tolerance through chain walking process.
Scheme 1.
Different removal of alkene protecting group and new mechanistic rationale.
2. Results
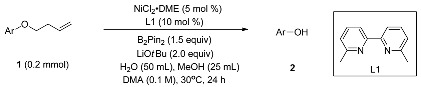
In order to testify our proposal, we began our study by optimizing the reaction conditions using 4-(but-3-enyloxy)biphenyl (1a) as the model substrate (Table 1). Investigation of a range of parameters, we found that the combination of nickel(II) chloride ethylene glycol dimethyl ether complex (NiCl2∙DME, 5 mol%), 6,6′-dimethyl-2,2′-bipyridine (L1) as ligand, Bis(pinacolato)diboron (B2pin2, 1.5 equiv) as the reductant, Lithium tert-butoxide as the base, in N,N-dimethylacetamide (DMA) (0.1M) with methanol and water as additives gave excellent isolated yield (92%) (entry 1, Table 1). Other nickel sources such as zero valent Bis(1,5-cyclooctadiene)nickel (Ni(COD)2, entry 2, Table 1) or less soluble NiCl2 (entry 3, Table 1) diminished the yield. Similar to the previous reports [31,32,33,34,35,36], more bulky ligand was critical for the reaction and probably facilitate the chain walking process (entry 4–5, Table 1). Other common phosphine ligands such as triphenylphosphine (entry 6, Table 1) and tricyclohexylphosphine (entry 7, Table 1) were all low efficient. Nickel(I) was proposed as the active spices in the chain walking reactions [31,32,33,34,35,36], so we tested some reductant to generate of nickel(I) spices. B2pin2 in combination of base was reported as efficient reductant in nickel-catalyzed reductive reactions [47], to our pleased moderated yield was observed (entry 8, Table 1). Other widely used metal reductants in nickel-catalyzed cross reductive reaction such as Zn and Mn gave very few product (entry 9–10, Table 1) [48,49,50,51,52]. Next, we surveyed some additives to accelerate the reaction. We found that a certain amount of methanol greatly prompted the yield (entry 11, Table 1). Water was added to further boost the efficiency of the reaction. Both water and methanol might serve as a hydride donor in σ-bond metathesis. Considering both Lithium tert-butoxide and water were added, in situ lithium hydroxide might be formed. Directly using lithium hydroxide as base did not have a similar result (entry 13, Table 1). Switching reaction solvent from DMA to less polar solvent such as tetrahydrofuran (THF) resulted in lower yields (entries 15, Table 1). Finally, control experiments showed that both nickel and B2pin2 were indispensable for the reaction. In order to demonstrate its possibility in large scale synthesis, a 20 mmol scale reaction was done (entry 18, Table 1). The yield was lower but still outstanding. The attempt to lower the loading of nickel catalyst and ligand greatly diminished the yield with substantial starting material left (entry 19, Table 1).
Table 1.
Optimization of removal homoallyl group.

| entry | Deviation from standard conditions | Yield of 2 (%) a |
| 1 | None | 96(92) b |
| 2 | Ni(COD)2 | 84 |
| 3 | NiCl2 instead of NiCl2(DME) | 53 |
| 4 | L2 instead of L1 | 37 |
| 5 | L3 | 9 |
| 6 | L4 | 42 |
| 7 | L5 | 45 |
| 8 | B2pin2 w/o additives | 61 |
| 9 | Zn instead of B2pin2 | 5< |
| 10 | Mn instead of B2pin2 | 5< |
| 11 | No CH3OH | 73 |
| 12 | No H2O | 78 |
| 13 | LiOH instead of LiOtBu | 71 |
| 14 | KOH instead of LiOtBu | 68 |
| 15 | THF instead of DMA | 56 |
| 16 | No Ni | 0 |
| 17 | No B2pin2 | 0 |
| 18 | 20 mmol scale | 86 |
| 19 | 1% loading of nickel, 2% L1 | 52(42) c |
a Conditions: 1a (0.2 mmol), Amine (1.5 equiv), Determined by dibromomethane as an internal standard by 1H NMR. b Isolated yield. c 1a remained in reaction.
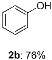
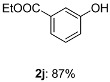
With the optimized condition in hand, the scope of substrate of the reaction was examined (Table 2). First, we tested the phenol scope. Ortho methyl group of phenol (1d) gave good yields, although ortho position was more hindered. Electron effect of the substitution groups did not play great roll on the yield. Strong electron-donating (benzyloxy, 1m) as well as strong electron-withdrawing (trifluoromethyl, 1p) groups were all tolerated. More strikingly, fragile groups in basic conditions such as aldehyde (1o), ester (1i), even free carboxylic acid (1l) were all tolerated in the reaction conditions, although the yield of aldehyde (1o) was low Furthermore, different halides (1e–1h) even iodide were untouched during the reactions which could be used in the further elaborating reactions, for instance, Suzuki reactions. It is worth highlighting that heteroaromatics (1q, 1r), which are prevalent structure motifs in medicinal molecular, did not corrupt the efficiency of the reaction.
Table 2.
Scope of phenol. Conditions: See Supplementary Materials for details.

|
|

|

|

|

|

|
|

|

|

|

|

|

|

|

|
Next, different alkene protecting groups of phenol were tested (Table 3). Interestingly, vinyl (3a–3c) was removed at the comparable yield without nickel catalyst indicated another mechanism might be involved. Except for the homoallyl group, the allyl group also could be easily removed under existing conditions (3d–3i). Interestingly, not only the acyclic alkene protecting groups, but also the cyclic alkene was smoothly detached in outstanding yield (3i). Longer alkene protecting group (pentenyl) afforded products albeit in relatively lower yields (3j–3m). Control experiments showed that nickel catalyst was indispensable for alkene protecting groups longer than vinyl.
Table 3.
Scope of alkene protecting groups. Conditions: a Without Ni catalyst and ligand. See Supplementary Materials for details.

| Substrate | Product | Substrate | Product |

|
2a: 92%, 88% a |

|
2s: 96% |

|
2c: 87%, 90% a; 2f: 92%,84% a |

|
2c: 93% |

|
2g: X = I, 63%; 2h: X = Br, 65% |

|
2a: 81% |

|
2i: 95% |

|
2d: 74% |

|
2o: 83% |

|
2e: 72% |

|
2r: 53% |

|
2p: 63% |
We tried to expand the scope from phenols to aliphatic alcohols (Scheme 2). To our delight, allyl protected alcohols were easily removed to give both aminoalcohol (5) and cholesterol (7) in moderate yield. More importantly, the stereocenter adjacent to hydroxyl of 7 was preserved after the reaction. For the homoallyl ether analogs the yield dropped to around 40% which was not practical in synthetic view.
Scheme 2.
Removal of alcohol protecting groups. Conditions: See Supplementary Materials for details.
Taking further advantage of our new reaction (Scheme 3, Method B), we compared it with the classic palladium deprotection protocol (Scheme 3, Method A). These two methods were about the same efficiency when the alkene protecting groups were closed to the oxygen atom (n < 1). In contrast, Method A drastically lost its activity when the interval between the alkene and oxygen is prolonged (n > 2), meanwhile, Method B still maintained medium yield even at n = 4. Through this comparison, it is possible to remove allyl and homoallyl protecting groups sequentially by utilizing these methods.
Scheme 3.
Comparison with previous method.
Although the clear panorama of the catalytic cycle was still under pursuing, the control experiments were designed to unveil the reaction mechanism (Scheme 4). First, butoxybenzene (8) was put into the optimized condition, no product was detected. It means the coordination of the nickel catalyst of the substrate was necessary. Second, ((2,2-dimethylbut-3-en-1-yl)oxy)benzene (9) was subjected to optimal condition, similarly no desire product was observed (3), which means the chain walking was intercepted with the quaternary carbon to stop the formation of key allyl ether. When we analyzed the byproduct of the reaction 3j by GC-MS, the borylated alkene 10 was not detected which means the reaction might not be initiated by Ni-Bpin. Due to the low boiling point of pentene (30 °C), we did not see pentene signal on the GC-MS. Substrate (11) was synthesized to get more information of the fate of deprotected group. Allylbenzene (12) was detected as equivalent to the product instead of the corresponding borylation product (13) indicating the alkene was formed after the deprotection. The combination of B2pin2 and water/methanol might serve as a hydride donor to form the Ni-H species. A preliminary survey with silane to generate Ni-H in situ [11] was done and a great amount of product was also found which supported the possibility of Ni-H as the true catalyst in the reaction.
Scheme 4.
Mechanistic studies. Conditions: See Supplementary Materials for details.
3. Discussion
To summarize, we reported the nickel-catalyzed removal of alkene protecting groups of phenols and alcohols through chain walking process. The facile and mild reaction condition as well as high functional tolerance highlight its utilization in the future. More importantly, the nonconventional homoallyl may rival as protecting group with this report that is complementary to existing protocols. Future efforts are directed toward expanding this type of new transformation in practice as well as understanding the mechanism of the reaction.
4. Experimental Section
4.1. Representative General Procedure for Nickel-Catalyzed Deprotecting Reaction
In glovebox, B2pin2 (76.17 mg, 0.3 mmol, 1.5 equiv), NiCl2(DME) (2.20 mg, 0.01 mmol, 0.05 equiv), 6,6-dimethyl-2,2-dipyridyl (3.68 mg, 0.02 mmol, 0.10 equiv), LiOtBu (32 mg, 0.4 mmol, 2.0 equiv), and protected phenol (if solid) (0.2 mmol) were added to an oven-dried tube. The reaction tube was equipped with a magnetic stir bar and sealed with Teflon-lined cap, refilled the system with argon and performed two more evacuation-backfill cycles, protected phenol was added by syringe under argon flow (if liquid). DMA (2.00 mL), H2O (50 µL), MeOH (25 µL) were added to the tube sequentially. The reaction mixture turned dark and was stirred at 30 °C for 24 h. When the reaction was finished, 10 mL aqueous HCl (0.1 M) was added to the mixture and extracted with CH2Cl2 (2 × 10 mL), dried over anhydrous MgSO4, and concentrated in vacuo. The resulting residue was purified by silica gel flash chromatography to give the product.
4.2. Characterization Data for Products 2a–2s, 5 and 7 (Tables 2, 3 and Scheme 2)
4-Phenylphenol (2a): White solid. IR (cm−1, KBr): 3434, 3367, 3130, 1662, 1611, 1522, 1477, 1459, 1401, 1257, 1139, 1069, 998, 954, 862, 833, 758, 686, 555, 517. 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 7.4 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 7.45 (t, J = 7.3 Hz, 2H), 7.35 (d, J = 7.1 Hz, 1H), 6.94 (d, J = 8.1 Hz, 2H), 4.95 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.0, 140.7, 134.0, 128.7, 128.4, 126.7, 115.6. m.p.: 163–164 °C.
Phenol (2b): White solid. IR (cm−1, KBr): 3440, 3134, 1659, 1624, 1401, 1075, 989, 951, 858, 538. 1H NMR (400 MHz, CDCl3) δ 7.31 (t, J = 7.7 Hz, 2H), 7.02 (t, J = 7.3 Hz, 1H), 6.93 (d, J = 8.2 Hz, 2H), 6.04 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.2, 129.9, 121.2, 115.6. m.p.: 103–104 °C.
3-Methoxyphenol (2c): Brown liquid. IR (cm−1, KBr): 3439, 3133, 1671, 1609, 1401, 1163, 1073, 991, 946, 856, 769, 689, 541. 1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 8.0 Hz, 1H), 6.54 (d, J = 7.8 Hz, 1H), 6.47 (d, J = 7.9 Hz, 2H), 5.65 (s, 1H), 3.80 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.8, 156.7, 130.3, 108.1, 106.6, 101.7, 55.3.
o-Cresol (2d): Brown liquid. IR (cm−1, KBr): 3439, 3134, 1661, 1401, 1075, 993, 952, 858, 536. 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 7.2 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.02 (t, J = 7.4 Hz, 1H), 6.90 (d, J = 8.0 Hz, 1H), 5.70 (s, 1H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 153.7, 131.3, 127.3, 124.4, 121.1, 115.3, 15.9.
3-Fluorophenol (2e): Light yellow liquid. IR (cm−1, KBr): 3441, 3134, 1666, 1627, 1131, 1074, 993, 952, 857, 774, 531. 1H NMR (400 MHz, CDCl3) δ 7.22 (dd, J = 15.2, 7.9 Hz, 1H), 6.74–6.57 (m, 3H), 5.76 (s, 1H). 13C NMR (101 MHz, Chloroform-d) δ 163.7 (d, J = 245.6 Hz), 156.3 (d, J = 11.3 Hz), 130.7 (d, J = 10.0 Hz), 111.3 (d, J = 2.9 Hz), 108.2 (d, J = 21.3 Hz), 103.3 (d, J = 24.7 Hz).
3-Chlorophenol (2f): Yellow liquid. IR (cm−1, KBr): 3439, 3134, 1664, 1623, 1401, 1250, 1138, 1074, 996, 952, 857, 771, 682, 530. 1H NMR (400 MHz, CDCl3) δ 7.19 (t, J = 8.1 Hz, 1H), 6.96 (d, J = 8.0 Hz, 1H), 6.89 (s, 1H), 6.75 (dd, J = 7.2, 0.9 Hz, 1H), 5.35 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.9, 135.0, 130.6, 121.3, 116.0, 113.8.
2-Iodophenol (2g): Brown solid. IR (cm−1, KBr): 3440, 3133, 1658, 1624, 1401, 1050, 1075, 991, 951, 858, 535. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 7.9 Hz, 1H), 7.27 (t, J = 6.4 Hz, 1H), 7.03 (d, J = 8.1 Hz, 1H), 6.75–6.63 (m, 1H), 5.36 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 154.8, 138.3, 130.2, 122.5, 115.2, 85.7. m.p.: 41–42 °C.
2-Bromophenol (2h): Brown liquid. IR (cm−1, KBr): 3439, 3132, 1663. 1619, 1401, 1160, 1077, 993, 946, 858, 744, 539. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.0 Hz, 1H), 7.25 (t, J = 7.7 Hz, 1H), 7.06 (d, J = 8.1 Hz, 1H), 6.84 (t, J = 7.7 Hz, 1H), 5.59 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 152.3, 132.1, 129.2, 121.8, 116.2, 110.3.
Methyl 4-hydroxybenzoate (2i): White solid. IR (cm−1, KBr): 3305, 2963, 2846, 2806, 1919, 1684, 1372, 1114, 954, 851, 691. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.5 Hz, 2H), 6.91 (d, J = 8.6 Hz, 2H), 6.33 (s, 1H), 3.92 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 167.3, 160.2, 131.9, 122.3, 115.2, 52.0. m.p.: 130–131 °C.
Ethyl 3-hydroxybenzoate (2j): Colorless liquid. IR (cm−1, KBr): 3350, 2385, 2581, 1692, 1597, 1512, 1459, 1415, 1311, 1232, 1163, 1108, 1074, 926, 881, 760, 670, 565, 522. 1H NMR (400 MHz, DMSO) δ 9.83 (s, 1H), 7.43–7.35 (m, 2H), 7.31 (t, J = 7.8 Hz, 2H), 7.03 (d, J = 7.9 Hz, 1H), 4.28 (q, J = 7.0 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.6, 156.3, 131.3, 129.7, 121.6, 120.5, 116.4, 61.6, 14.1.
4-tert-Butylphenol (2k): White solid. IR (cm−1, KBr): 3144, 2962, 2868, 1608, 1513, 1447, 1399, 1269, 1175, 822, 543. 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.5 Hz, 2H), 6.85 (d, J = 8.5 Hz, 2H), 5.53 (s, 1H), 1.36 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 152.9, 143.7, 126.5, 115.0, 34.1, 31.6. m.p.: 97–99 °C.
4-Hydroxybenzoic acid (2l): White solid. IR (cm−1, KBr): 3382, 3130, 3013, 1676, 1600, 1402, 1321, 1286, 1238, 1160, 1096, 1000, 929, 848, 611, 541. 1H NMR (400 MHz, DMSO) δ 12.41 (s, 1H), 10.21 (s, 1H), 7.79 (d, J = 8.5 Hz, 2H), 6.82 (d, J = 8.5 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 167.6, 162.0, 132.0, 121.8, 115.5. m.p.: 213–215 °C.
4-Benzyloxyphenol (2m): White solid. IR (cm−1, KBr): 3429, 3132, 1659, 1614, 1510, 1401, 1236, 1164, 1086, 1012, 818, 734, 520. 1H NMR (400 MHz, CDCl3) δ 7.48–7.39 (m, 4H), 7.38–7.33 (m, 1H), 6.89 (d, J = 8.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 5.04 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 153.0, 149.7, 137.2, 128.5, 127.9, 127.5, 116.1, 116.1, 70.8. m.p.: 122–124 °C.
2-Naphthol (2n): White solid. IR (cm−1, KBr): 3501, 3190, 1628, 1590, 1510, 1459, 1401, 1277, 1215, 1168, 955, 845, 813, 741, 476. 1H NMR (400 MHz, CDCl3) δ 7.84–7.77 (m, 2H), 7.71 (d, J = 8.2 Hz, 1H), 7.48 (t, J = 7.5 Hz, 1H), 7.39 (t, J = 7.4 Hz, 1H), 7.21–7.11 (m, 2H), 5.52 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 153.3, 134.6, 129.9, 129.0, 127.8, 126.6, 126.4, 123.7, 117.8, 109.6. m.p.: 120–122 °C.
p-Hydroxybenzaldehyde (2o): White solid. IR (cm−1, KBr): 3144, 2962, 1608, 1513, 1399, 1239, 1176, 821, 543. 1H NMR (400 MHz, DMSO) δ 10.59 (s, 1H), 9.79 (s, 1H), 7.76 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.4 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 191.3, 163.7, 132.5, 128.9, 116.3. m.p.: 116–118 °C.
3-Trifluoromethylphenol (2p): Yellow liquid. IR (cm−1, KBr): 3440, 3132, 1662, 1622, 1401, 1160, 1076, 946, 858, 538. 1H NMR (400 MHz, CDCl3) δ 7.38 (t, J = 7.9 Hz, 1H), 7.23 (d, J = 7.7 Hz, 1H), 7.12 (s, 1H), 7.04 (d, J = 8.1 Hz, 1H), 5.36 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 155.3, 132.4 (q, J = 32.7 Hz), 130.3, 123.8 (q, J = 271.3 Hz), 117.9 (q, J = 4.1 Hz), 112.3 (q, J = 4.1 Hz).
tert-Butyl 4-hydroxy-1H-indole-1-carboxylate (2q): Corlorless liquid. IR (cm−1, KBr): 3449, 3135, 1664, 1626, 1400, 1136, 1074, 962, 855. 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.1 Hz, 1H), 7.55 (d, J = 3.5 Hz, 1H), 7.18 (t, J = 8.1 Hz, 1H), 6.68 (dd, J = 10.2, 5.8 Hz, 2H), 5.40 (s, 1H), 1.70 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 149.8, 148.7, 136.9, 125.1, 124.7, 119.6, 108.3, 107.7, 103.5, 83.8, 28.2.
6-Hydroxyflavone (2r): White solid. IR (cm−1, KBr): 3437, 3128, 3013, 1621, 1569, 1402, 1255, 1077, 835. 1H NMR (400 MHz, DMSO) δ 10.02 (s, 1H), 8.08 (d, J = 6.9 Hz, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.59 (d, J = 6.5 Hz, 3H), 7.34 (d, J = 2.2 Hz, 1H), 7.27 (dd, J = 9.1, 2.3 Hz, 1H), 6.95 (s, 1H). 13C NMR (101 MHz, DMSO) δ 177.4, 162.6, 155.3, 149.8, 132.0, 131.8, 129.5, 126.7, 124.7, 123.5, 120.3, 107.9, 106.4. m.p.: 238–240 °C.
4-Cyanophenol (2s): White solid. IR (cm−1, KBr): 3283, 2229, 1603, 1505, 1441, 1391, 1283, 1221, 1159, 834, 696, 534. 1H NMR (400 MHz, DMSO) δ 10.63 (s, 1H), 7.61 (s, 2H), 7.13–6.68 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 160.6, 134.3, 119.3, 116.5, 102.5. m.p.: 112–114 °C.
2-(N,N-Dibenzylamino)-2-phenylethan-1-ol (2t): White solid. IR (cm−1, KBr): 3440, 3134, 1661, 1624, 1401, 1070. 1H NMR (400 MHz, CDCl3) δ 7.53–7.19 (m, 15H), 4.17 (t, J = 10.6 Hz, 1H), 4.00–3.91 (m, 3H), 3.64 (dd, J = 10.6, 5.1 Hz, 1H), 3.18 (d, J = 13.4 Hz, 2H), 3.04 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 139.1, 135.1, 129.3, 129.0, 128.5, 128.4, 128.0, 127.2, 63.0, 60.4, 53.5. m.p.: decomposed.
Cholesterol (7): White solid. IR (cm−1, KBr): 3435, 3132, 2943, 1666, 1624, 1459, 1399, 1057. 1H NMR (400 MHz, CDCl3) δ 5.37 (d, J = 4.3 Hz, 1H), 3.69–3.35 (m, 1H), 2.35–2.19 (m, 2H), 2.01 (t, J = 14.8 Hz, 2H), 1.86 (d, J = 9.9 Hz, 3H), 1.60–0.85 (m, 34H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 140.7, 121.7, 71.7, 56.7, 56.1, 50.1, 42.3, 42.3, 39.8, 39.5, 37.2, 36.5, 36.2, 35.8, 31.9, 31.6, 28.2, 28.0, 24.3, 23.8, 22.8, 22.5, 21.1, 19.4, 18.7, 11.8. m.p.: 146–148 °C.
Supplementary Materials
The followings are available online.
Author Contributions
J.Y. conceived and designed the experiments; C.M., H.N., J.N., W.W. performed the experiments; W.W., J.Y. checked and analyzed the data; J.Y. wrote and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Financial Support from National Natural Science Foundation of China (Grant 21602017), Natural Science Foundation of Jiangsu Province (Grant No. BK20160405) and Research Start-up Fund of Changshu Institute of Technology (No. XZ1508).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2a–2p are available from the authors.
References and Notes
- 1.Wuts P.G.M., Green T.W. Protection for Phenols and Catechols. John Wiley Sons Inc.; Hoboken, NJ, USA: 2006. pp. 367–430. [Google Scholar]
- 2.Wuts P.G.M., Green T.W. Greene’s Protective Groups in Organic Synthesis. 4th ed. Wiley; Hoboken, NJ, USA: 2007. [Google Scholar]
- 3.Tsukamoto H., Kondo Y. Facile removal strategy for allyl and allyloxycarbonyl protecting groups using solid-supported barbituric acid under palladium catalysis. Synlett. 2003;2003:1061. doi: 10.1055/s-2003-39320. [DOI] [Google Scholar]
- 4.Bailey W.F., England M.D., Mealy M.J., Thongsornkleeb C., Teng L. Facile O-Deallylation of Allyl Ethers via SN2‘ Reaction with tert-Butyllithium. Org. Lett. 2000;2:489–491. doi: 10.1021/ol991342g. [DOI] [PubMed] [Google Scholar]
- 5.Beugelmans R., Bourdet S., Bigot A., Zhu J. Reductive deprotection of aryl allyl ethers with Pd(Ph3)4/NaBH4. Tetrahedron Lett. 1994;35:4349–4350. doi: 10.1016/S0040-4039(00)73351-X. [DOI] [Google Scholar]
- 6.Kitov P.I., Bundle D.R. Mild oxidative one-pot allyl group cleavage. Org. Lett. 2001;3:2835–2838. doi: 10.1021/ol016278t. [DOI] [PubMed] [Google Scholar]
- 7.Vutukuri D.R., Bharathi P., Yu Z., Rajasekaran K., Tran M.-H., Thayumanavan S. A Mild Deprotection Strategy for Allyl-Protecting Groups and Its Implications in Sequence Specific Dendrimer Synthesis. J. Org. Chem. 2003;68:1146–1149. doi: 10.1021/jo026469p. [DOI] [PubMed] [Google Scholar]
- 8.Mao Y., Liu Y., Hu Y., Wang L., Zhang S., Wang W. Pd-Catalyzed Debenzylation and Deallylation of Ethers and Esters with Sodium Hydride. ACS Catal. 2018;8:3016–3020. doi: 10.1021/acscatal.8b00185. [DOI] [Google Scholar]
- 9.Hemming D.S., Talbot E.P., Steel P.G. A mild copper catalyzed method for the selective deprotection of aryl allyl ethers. Tetrahedron Lett. 2017;58:17–20. doi: 10.1016/j.tetlet.2016.11.084. [DOI] [Google Scholar]
- 10.Martínez-Calvo M., Couceiro J.R., Destito P., Rodriguez J., Mosquera J., Mascareñas J.L. Intracellular deprotection reactions mediated by palladium complexes equipped with designed phosphine ligands. ACS Catal. 2018;8:6055–6061. doi: 10.1021/acscatal.8b01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gärtner D., Konnerth H., von Wangelin A.J. Highly practical iron-catalyzed C–O cleavage reactions. Catal. Sci. Technol. 2013;3:2541–2545. doi: 10.1039/c3cy00266g. [DOI] [Google Scholar]
- 12.Kaleta Z., Makowski B.T., Soo’s T., Dembinski R. Thionation using fluorous Lawesson’s reagent. Org. Lett. 2006;8:1625–1628. doi: 10.1021/ol060208a. [DOI] [PubMed] [Google Scholar]
- 13.Youn S.W., Pastine S.J., Sames D. Ru (III)-catalyzed cyclization of arene-alkene substrates via intramolecular electrophilic hydroarylation. Org. Lett. 2004;6:581–584. doi: 10.1021/ol036385i. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X.-Z., Chen C.-F. A highly efficient approach to [4] pseudocatenanes by threefold metathesis reactions of a triptycene-based tris [2] pseudorotaxane. J. Am. Chem. Soc. 2005;127:13158–13159. doi: 10.1021/ja0546020. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou K.C., Cho S.Y., Hughes R., Winssinger N., Smethurst C., Labischinski H., Endermann R. Solid-and Solution-Phase Synthesis of Vancomycin and Vancomycin Analogues with Activity against Vancomycin-Resistant Bacteria. Chem. Eur. J. 2001;7:3798–3823. doi: 10.1002/1521-3765(20010903)7:17<3798::AID-CHEM3798>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Wipf P., Aslan D.C. Synthesis of bicyclic ortho esters by epoxy ester rearrangements and study of their ring-opening reactions. J. Org. Chem. 2001;66:337–343. doi: 10.1021/jo005665y. [DOI] [PubMed] [Google Scholar]
- 17.Corey E.J., Guzman-Perez A., Noe M.C. Highly enantioselective and regioselective catalytic dihydroxylation of homoallylic alcohol derivatives. Tetrahedron Lett. 1995;36:3481–3484. doi: 10.1016/0040-4039(95)00570-3. [DOI] [Google Scholar]
- 18.Yamamoto Y., Asao N. Selective reactions using allylic metals. Chem. Rev. 1993;93:2207–2293. doi: 10.1021/cr00022a010. [DOI] [Google Scholar]
- 19.Barrett A.G.M., Lebold S.A., Zhang X. 3-Butenyl esters as convenient protecting groups for carboxylic acids. Tetrahedron Lett. 1989;30:7317–7320. doi: 10.1016/S0040-4039(00)70686-1. [DOI] [Google Scholar]
- 20.Cadot C., Dalko P.I., Cossy J. Olefin isomerization by a ruthenium carbenoid complex. Cleavage of allyl and homoallyl groups. Tetrahedron Lett. 2002;43:1839–1841. doi: 10.1016/S0040-4039(02)00141-7. [DOI] [Google Scholar]
- 21.Lipshutz B.H., Ghorai S., Leong W.W.Y. Deprotection of Homoallyl (hAllyl) Derivatives of Phenols, Alcohols, Acids, and Amines. J. Org. Chem. 2009;74:2854–2857. doi: 10.1021/jo900012z. [DOI] [PubMed] [Google Scholar]
- 22.Sommer H., Juliá-Hernández F., Martin R., Marek I. Walking Metals for Remote Functionalization Sommer. ACS Cent. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner E.W., Mei T.-S., Burckle A.J., Sigman M.S. Enantioselective Heck Arylations of Acyclic Alkenyl Alcohols Using a Redox-Relay Strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seayad A., Ahmed M., Klein H., Jackstell R., Gross T., Beller M. Internal Olefins to Linear Amines. Science. 2002;297:1676–1678. doi: 10.1126/science.1074801. [DOI] [PubMed] [Google Scholar]
- 25.Mei T.-S., Patel H.H., Sigman M.S. Enantioselective construction of remote quaternary stereocentres. Nature. 2014;508:340–344. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buslov I., Becouse J., Mazza S., Montandon-Clerc M., Hu X. Chemoselective Alkene Hydrosilylation Catalyzed by Nickel Pincer Complexes. Angew. Chem. Int. Ed. 2015;54:14523–14526. doi: 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- 27.Buslov I., Song F., Hu X. An Easily Accessed Nickel Nanoparticle Catalyst for Alkene Hydrosilylation with Tertiary Silanes. Angew. Chem. Int. Ed. 2016;55:12295–12299. doi: 10.1002/anie.201606832. [DOI] [PubMed] [Google Scholar]
- 28.Juliá-Hernández F., Moragas T., Cornella J., Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature. 2017;545:84–88. doi: 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- 29.Sun S.Z., Romano C., Martin R. Site-selective catalytic deaminative alkylation of unactivated olefins. J. Am. Chem. Soc. 2019;141:16197–16201. doi: 10.1021/jacs.9b07489. [DOI] [PubMed] [Google Scholar]
- 30.Sun S.Z., Borjesson M., Martin-Montero R., Martin R. Site-Selective Ni-Catalyzed Reductive Coupling of alpha-Haloboranes with Unactivated Olefins. J. Am. Chem. Soc. 2018;140:12765–12769. doi: 10.1021/jacs.8b09425. [DOI] [PubMed] [Google Scholar]
- 31.He Y., Cai Y., Zhu S. Mild and Regioselective Benzylic C−H Functionalization: Ni-Catalyzed Reductive Arylation of Remote and Proximal Olefins. J. Am. Chem. Soc. 2017;139:1061–1064. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 32.Chen F., Chen K., Zhang Y., He Y., Wang Y.-M., Zhu S. Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of NiH. J. Am. Chem. Soc. 2017;139:13929–13935. doi: 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]
- 33.He J., Song P., Xu X., Zhu S., Wang Y. Migratory Reductive Acylation between Alkyl Halides or Alkenes and Alkyl Carboxylic Acids by Nickel Catalysis. ACS Catal. 2019;9:3253–3259. doi: 10.1021/acscatal.9b00521. [DOI] [Google Scholar]
- 34.Zhang Y., Xu X., Zhu S. Nickel-Catalysed Selective Migratory Hydrothiolation of Alkenes and Alkynes with Thiols. Nat. Commun. 2019;10:1752. doi: 10.1038/s41467-019-09783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao J., He Y., Ye F., Zhu S. Remote sp3 C–H Amination of Alkenes with Nitroarenes. Chem. 2018;4:1645–1657. doi: 10.1016/j.chempr.2018.04.008. [DOI] [Google Scholar]
- 36.Zhou F., Zhang Y., Xu X., Zhu S. NiH-Catalyzed Remote Asymmetric Hydroalkylation of Alkenes with Racemic α-Bromo Amides. Angew. Chem. Int. Ed. 2019;58:1754–1758. doi: 10.1002/anie.201813222. [DOI] [PubMed] [Google Scholar]
- 37.Bair J.S., Schramm Y., Sergeev A.G., Clot E., Eisenstein O., Hartwig J.F. Linear-Selective Hydroarylation of Unactivated Terminal and Internal Olefins with Trifluoromethyl-Substituted Arenes. J. Am. Chem. Soc. 2014;136:13098–13101. doi: 10.1021/ja505579f. [DOI] [PubMed] [Google Scholar]
- 38.Matt C., Kölblin F., Streuff J. Reductive C–O, C–N, and C–S Cleavage by a Zirconium Catalyzed Hydrometalation/β-Elimination Approach. Org. Lett. 2019;21:6983–6988. doi: 10.1021/acs.orglett.9b02572. [DOI] [PubMed] [Google Scholar]
- 39.Wu W., Yi J., Xu H., Li S., Yuan R. An Efficient, One-Pot Transamidation of 8-Aminoquinoline Amides Activated by Tertiary-Butyloxycarbonyl. Molecules. 2019;24:1234. doi: 10.3390/molecules24071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi J., Badir S.O., Kammer L.M., Ribagorda M., Molander G.A. Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis. Org. Lett. 2019;21:3346–3351. doi: 10.1021/acs.orglett.9b01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi J., Liu J.-H., Liang J., Dai J.-J., Yang C.-T., Fu Y., Liu L. Alkylboronic Esters from Palladium- and Nickel-Catalyzed Borylation of Primary and Secondary Alkyl Bromides. Adv. Synth. Catal. 2012;354:1685–1691. doi: 10.1002/adsc.201200136. [DOI] [Google Scholar]
- 42.Dudnik A.S., Fu G.C. Nickel-Catalyzed Coupling Reactions of Alkyl Electrophiles, Including Unactivated Tertiary Halides, To Generate Carbon–Boron Bonds. J. Am. Chem. Soc. 2012;134:10693–10697. doi: 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z., Bachman S., Dudnik A.S., Fu G.C. Nickel-Catalyzed Enantioconvergent Borylation of Racemic Secondary Benzylic Electrophiles. Angew. Chem. Int. Ed. 2018;57:14529–14532. doi: 10.1002/anie.201806015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Didiuk M.T., Morken J.P., Hoveyda A.H. Directed Regio- and Stereoselective Nickel-Catalyzed Addition of Alkyl Grignard Reagents to Allylic Ethers. J. Am. Chem. Soc. 1995;117:7273–7274. doi: 10.1021/ja00132a039. [DOI] [Google Scholar]
- 45.Butt N.A., Zhang W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015;44:7929–7967. doi: 10.1039/C5CS00144G. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Zheng S., Romero E., Matsui J.K., Molander G.A. Regioselective Single-Electron Tsuji–Trost Reaction of Allylic Alcohols: A Photoredox/Nickel Dual Catalytic Approach. Org. Lett. 2019;21:6543–6547. doi: 10.1021/acs.orglett.9b02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu H., Zhao C., Qian Q., Deng W., Gong H. Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci. 2013;4:4022–4029. doi: 10.1039/c3sc51098k. [DOI] [Google Scholar]
- 48.Ye Y., Chen H., Sessler J.L., Gong H. Zn-Mediated Fragmentation of Tertiary Alkyl Oxalates Enabling Formation of Alkylated and Arylated Quaternary Carbon Centers. J. Am. Chem. Soc. 2019;141:820–824. doi: 10.1021/jacs.8b12801. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Ren Q., Zhang Q., Gong H. Preparation of Vinyl Arenes by Nickel-Catalyzed Reductive Coupling of Aryl Halides with Vinyl Bromides. Angew. Chem. Chem. Int. 2016;55:15544–15548. doi: 10.1002/anie.201607959. [DOI] [PubMed] [Google Scholar]
- 50.Wang X., Wang S., Xue W., Gong H. Nickel-Catalyzed Reductive Coupling of Aryl Halides with Unactivated tert-Alkyl Halides. J. Am. Chem. Soc. 2015;137:11562–11565. doi: 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- 51.Everson D.A., Jones B.A., Weix D.J. Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2012;134:6146–6159. doi: 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biswas S., Weix D.J. Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






