Main Text
Voltage-induced protein conformational changes are a remarkable molecular mechanism by which protein function can be regulated (1). The two-domain architecture of membrane-embedded ion channels illustrates a basic principle of coupling the function to electric field: their central pore domain is a functional unit that conducts ions, whereas dedicated voltage-sensing domain (VSD) acts as a molecular voltmeter, which responds to transmembrane potential by moving within membranes (2). The motions of VSD are transmitted to structural changes in the pore domain, thus switching the ion conductance on and off.
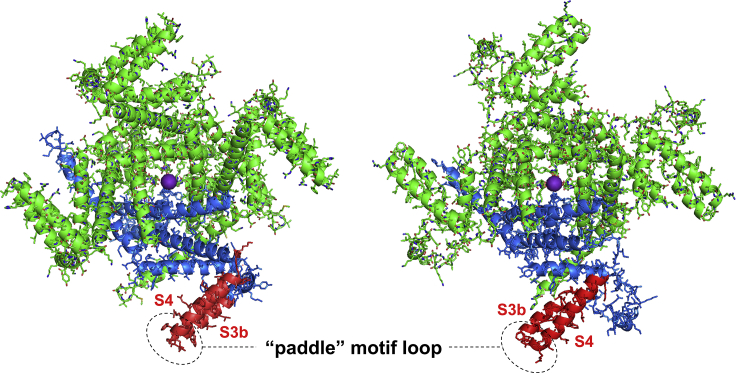
For the KvAP voltage-dependent K+ channel from archaebacterium Aeropyrum pernix, the dynamic nature of VSD was recognized early on (3, 4). The x-ray crystallographic structures of KvAP with and without antibodies, which were raised to specifically bind to VSD, provided the first glimpse of molecular details of the channel function and revealed significant conformational variability of VSD (3, 4). The KvAP is a tetramer, and each of its four subunits is composed of six hydrophobic helical segments: the last two (S5–S6) belong to the pore domain and line the central pore P fragment, whereas the first four segments (S1–S4) constitute the VSD. The highly mobile element of VSD is the helical hairpin (termed “paddle motif”) composed of S4 helix containing positively charged arginine residues and C-terminal part of S3 segment (helix S3b) (see Fig. 1).
Figure 1.
Top-down view of two structures of tetrameric KvAP channel obtained in the presence of monoclonal Fab (left; Protein Data Bank (PDB): 1ORQ) and Fv (right; PDB: 2A0L) antibody fragments (data not shown). Different antibodies and crystallization conditions resulted in distinct conformations of the channel. One of the subunits of tetramer is colored in blue with its “paddle” in red. The highly mobile helix(S3b)-loop-helix(S4) paddle motif is facing the membrane environment and undergoes major movements in response to changes of transmembrane potential. The structural dynamics of paddle motif loop (designated by the oval), which participates in protein-protein interactions (e.g., with gating modifier spider-venom peptide VSTx1), was characterized by Raghuraman and co-workers (7). To see this figure in color, go online.
To facilitate crystallization, these crystal structures were obtained in micelles in the presence of membrane-mimicking detergents with the pore domain remaining in open conformation (3, 4). The VSD domain was however in a nonnative conformation and it was suggested that membrane environment and protein-lipid interactions are critical for VSD to adopt the proper orientation relative to the pore domain. Importantly, the paddle motif loop serves as a specific binding site to natural toxins, for instance, the spider-venom peptide VSTx1 that modifies the function of the KvAP ion channel (5, 6). However, contradictory results were reported for binding the toxin to KvAP in micelles (typically used in structural studies by x-ray crystallography) and membrane-mimicking liposomes, such that the binding constants differed by four orders of magnitude (5).
To help in resolving this conundrum, Raghuraman and co-workers (7) characterized the conformational dynamics of the paddle motif loop connecting helices S3b and S4 in the isolated VSD of KvAP in detergent micelles versus more biomimetic liposomes. Eight different protein constructs labeled with environmentally sensitive dye were comprehensively studied using fluorescent spectroscopy techniques. Remarkably, the data revealed that whereas in micelles, the conformational dynamics of paddle motif loop is restricted resulting in a subset of distinct conformational states, in phospholipid membranes, the corresponding loop is exposed to a more nonpolar environment and undergoes high-frequency but smaller amplitude motions. Such differences can account for the dramatically different VSTx1 binding constants. Indeed, the membrane environment has to be chosen carefully to derive the physiologically relevant data.
These results nicely complement the previous characterization of conformational dynamics of voltage sensor paddle by NMR (8) and molecular dynamics simulations (9). The NMR studies are particularly relevant because they also revealed the structural changes in the VSD of KvAP in different membrane systems. The work by Raghuraman and co-workers (7) goes a step further delineating conformational dynamics for the functionally important voltage-sensing paddle loop.
It is clear that to fully understand the structural mechanism of voltage sensing in voltage-gated channels, it will be necessary to upgrade the current general picture of rigid-body motions of VSD reconstructed based on static x-ray structures. The next challenge is to provide a picture that includes dynamic reorganization of residues including the precise trajectories of gating arginine residues as they move in response to changes in voltage (10). Fluorescence-based approaches are likely to be the methodology that will make a major contribution in this direction, and the recent study by Raghuraman and co-workers (7) suggests that this is a feasible undertaking.
Editor: Sudha Chakrapani.
References
- 1.Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 2.Swartz K.J. Sensing voltage across lipid membranes. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.Y., Lee A., MacKinnon R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci. USA. 2005;102:15441–15446. doi: 10.1073/pnas.0507651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S.Y., MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 6.Lau C.H.Y., King G.F., Mobli M. Molecular basis of the interaction between gating modifier spider toxins and the voltage sensor of voltage-gated ion channels. Sci. Rep. 2016;6:34333. doi: 10.1038/srep34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A., Chatterjee S., Raghuraman H. Structural dynamics of the paddle motif loop in the activated conformation of KvAP voltage sensor. Biophys. J. 2020;118:873–884. doi: 10.1016/j.bpj.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shenkarev Z.O., Paramonov A.S., Arseniev A.S. NMR structural and dynamical investigation of the isolated voltage-sensing domain of the potassium channel KvAP: implications for voltage gating. J. Am. Chem. Soc. 2010;132:5630–5637. doi: 10.1021/ja909752r. [DOI] [PubMed] [Google Scholar]
- 9.Sands Z.A., Grottesi A., Sansom M.S. The intrinsic flexibility of the Kv voltage sensor and its implications for channel gating. Biophys. J. 2006;90:1598–1606. doi: 10.1529/biophysj.105.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezanilla F. Gating currents. J. Gen. Physiol. 2018;150:911–932. doi: 10.1085/jgp.201812090. [DOI] [PMC free article] [PubMed] [Google Scholar]