Abstract
The proteasome is the central component of the main cellular protein degradation pathway. During the past four decades, the critical function of the proteasome in numerous physiological processes has been revealed, and proteasome activity has been linked to various human diseases. The proteasome prevents the accumulation of misfolded proteins, controls the cell cycle, and regulates the immune response, to name a few important roles for this macromolecular “machine.” As a therapeutic target, proteasome inhibitors have been approved for the treatment of multiple myeloma and mantle cell lymphoma. However, inability to sufficiently inhibit proteasome activity at tolerated doses has hampered efforts to expand the scope of proteasome inhibitor-based therapies. With emerging new modalities in myeloma, it might seem challenging to develop additional proteasome-based therapies. However, the constant development of new applications for proteasome inhibitors and deeper insights into the intricacies of protein homeostasis suggest that proteasome inhibitors might have novel therapeutic applications. Herein, we summarize the latest advances in proteasome inhibitor development and discuss the future of proteasome inhibitors and other proteasome-based therapies in combating human diseases.
Keywords: proteostasis, proteasome, ubiquitin, immunoproteasome, unfolded protein response, Rpn11, Rpn13, p97, bortezomib, carfilzomib, ixazomib, oprozomib, marizomib, KZR-616, capzimin, RA190, RA183, KDT-11, RIP-1, NFE2L1/Nrf1
1. Introduction
Cells have evolved intricate regulatory mechanisms to adjust their proteomes in response to intracellular and environmental conditions, allowing for healthy growth and survival [1]. Aberrancies in protein synthesis, folding, trafficking and degradation can lead to many human illnesses and diseases, including neurodegenerative disorders, cancers, and autoimmune diseases [2,3,4]. The link between protein homeostasis, or proteostasis, and human health have motivated expansive efforts over the past three decades to discover and develop modulators of the major mammalian protein degradation pathway: the ubiquitin-proteasome system (UPS) [5,6]. The UPS is involved in a range of essential cellular processes, including cell-cycle progression, antigen presentation, inflammation, apoptosis, DNA repair, signal transduction, and protein quality control [7,8]. For example, the UPS is central to the unfolded protein response (UPR), which is activated when unfolded or misassembled proteins accumulate in the endoplasmic reticulum (ER). The UPR leads to apoptosis if ER stress is not mitigated [9,10]. The UPS is also critical for regulating the transcription factor NF-κB, thereby affecting the inflammatory response in addition to apoptosis and oncogenesis [11,12]. Neurons rely heavily on the UPS for maintaining proteostasis because of their long lifespans and specialized architectures, and deficiencies in the UPS are hallmarks of neurodegeneration [13,14,15].
While the fundamental mechanistic and structural details of the proteasome are still active areas of investigation [16], the use of proteasome inhibitors in clinic has proven to be successful in treating cancer, specifically multiple myeloma (MM) and mantle cell lymphoma (MCL), with the number and types of disease indications growing [17,18,19,20]. However, there are considerable challenges with using proteasome inhibitors because of the critical role that the proteasome plays in fundamental cellular processes. Additionally, while proteasome inhibitors have been successfully employed to treat cancer, where inhibiting uncontrolled cell growth is important, there are other circumstances in which activating the proteasome could be advantageous, including in the treatment of neurodegeneration. Proteasome activation has also been found to delay retinal degeneration in mice [21]. There is a fine line between having too much and too little proteasome activity; in some circumstances, one might want more proteasome activity, while in others, it is better to have less. There also must be a balance between preserving enough proteasome activity for survival of most cells in the body and eliminating unwanted activity in specific cells types or organs. With the number of clinical trials involving proteasome inhibitors alone or in combination with other therapies growing, and diseases treated with proteasome inhibitors expanding, it is an exciting time to think about how to improve current proteasome inhibitors and novel strategies for developing new ones. In addition to highlighting the current state of proteasome inhibitors in laboratory and clinic, this review will offer perspectives on how to improve the efficacy of current proteasome inhibitors (alone or as combination therapies), directions for developing new UPS inhibitors (including for targets other than the proteasome itself), and fundamental research directions that will enhance proteasome inhibitor development and validation.
1.1. Proteasome: Structure and Function
1.1.1. 26S Proteasome
The presence of a soluble ATP-dependent “proteolytic machine” was discovered in the 1970′s [22], and the identification and characterization of the proteasome and the role of ubiquitin in protein degradation emerged over the following 20 years [5,8,23,24]. The proteasome is a 2.4 megadalton multi-subunit enzyme complex of the conserved AAA+ (ATPase associated with various cellular activities) family [25]. The 26S proteasome degrades the majority (at least 80%) of proteins in the cytoplasm and nucleus of mammalian cells [26]. Proteasome substrates include misfolded or damaged proteins in addition to regulatory proteins that need to be degraded for proper cellular function. The central component of the 26S proteasome is the 28-subunit “core particle” (CP) comprising four stacked heptameric rings that form a donut-shape (referred to as the 20S proteasome). The middle of the donut creates an axial pore through which unfolded polypeptides are translocated (Figure 1A). The two outermost rings, formed by seven α-subunits, mediate interactions between the central pore and the cellular environment. The innermost rings are formed by seven β-subunits, and three of these subunits contain the proteasome’s enzymatic active sites. The N-terminal threonine of each enzymatic subunit serves as both the nucleophile and primary proton acceptor in the peptide bond cleavage reaction. Each active site has similar cleavage specificity to a known protease: β1 has caspase-like specificity, β2 has trypsin-like specificity, and β5 has chymotrypsin-like specificity. Despite the proclivity of each active site to cleave after certain amino acid types, the close proximity of the six sites facing the narrow axial pore allows for the processive degradation of a broad range of primary amino acid sequences into small peptides (~10 amino acids). These peptides can be further processed and used for antigen generation, or the amino acids can be recycled for new protein synthesis [27].
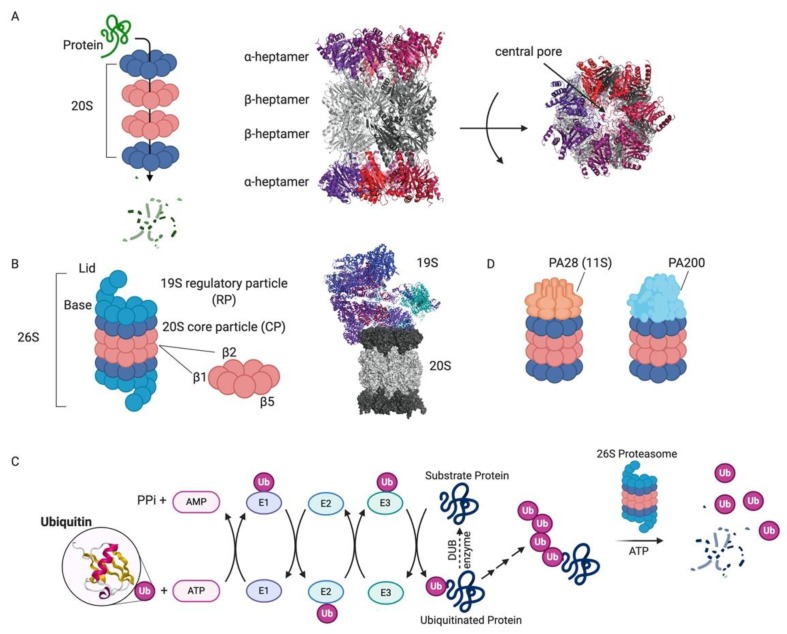
Figure 1.
Proteasome and ubiquitin-mediated degradation [85]. (A) Schematic of substrate protein degradation by passage through the central pore of the four stacked heptameric rings that comprise the 20S proteasome (left). The X-ray crystal structure of the yeast 20S proteasome (PDB 1RYP) shows the two capping α-heptameric rings (red/violet) and the middle two β-heptamers (grey; middle). The central pore through which substrates are threaded is indicated in the top view (right). (B) The 26S proteasome is the central component of the UPS. A cartoon depiction of the components of the 26S proteasome highlights the lid and base of the 19S RP in addition to the DUB Rpn11 and the catalytic β-rings (left). The cryo-EM structure of the human 26S proteasome (PDB 6MSB) shows the structure of the 26S proteasome in a state competent for ubiquitinated substrate engagement. (C) Schematic of the ubiquitin-proteasome system (ubiquitin PDB 1UBQ). (D) Schematic of alternative 20S proteasome complexes with a heptameric PA28 cap or a monomeric PA200 cap.
What prevents the proteasome from nonspecifically degrading cellular proteins? First, the active sites are sequestered within a deep pore, topologically preventing nonspecific proteolysis. The narrow radius of the pore prevents folded proteins from entering. Second, the CP itself is auto-inhibited by the N-terminal tails of the α-subunits [28], requiring regulatory factors to open the gates of the channel [29,30]. This allows for multiple regulatory checkpoints before a substrate is degraded. The main regulatory component of the 26S proteasome is the 19S regulatory particles (RP), also known as PA700, that caps either or both ends of the 20S CP (Figure 1B). The 19S RP is remarkably complex and comprises 19 stoichiometric subunits and many sub-stoichiometric interacting proteins [8,31]. UPS substrates must pass through this complex in order to be degraded, as the 19S RP opens the gates of the central channel of the 20S CP.
Prior to proteasomal degradation, proteins must be properly “tagged” and targeted to the proteasome. A cascade of enzymes, known as E1, E2, and E3 enzymes, are responsible for this “tagging” (Figure 1C). These enzymes catalyze isopeptide bond formation between the C-terminus of an 8 kDa protein called ubiquitin and the lysine side chain of the substrate (“acceptor”) protein. Ubiquitin chains can be elaborated by attaching additional ubiquitin moieties to any of its seven lysine residues, or the N-terminal amino group. At least four ubiquitin molecules are required to tag a substrate for proteasomal degradation [32], although there are some reported exceptions to this rule [19]. The large diversity of E3 enzymes in mammalian cells confers substrate specificity for ubiquitin conjugation. Ubiquitin chains are often expanded to form chains of different sizes and with branches at different lysine residues within the ubiquitin protein. Poly-ubiquitin chains, especially with K48 linkages, selectively mark proteins for degradation by the 26S proteasome [8]. Therefore, this complex pathway diversifies the range of substrates for the proteasome and enhances the regulation of proteins destined for degradation [33].
The 19S RP is divided into two biochemically separable sub-complexes: the base and the lid [34]. The distal lid contains at least nine proteins that are responsible for ubiquitin binding and removal. One core component of the lid is a Jab1/MPN domain-associated metalloisopeptidase (JAMM) domain-containing protein Rpn11 (POH1/PSMD14) that couples deubiquitination with degradation (Figure 1B) [35,36]. The development of Rpn11 inhibitors might have the ability to expand effective UPS inhibitors in the clinic, as discussed below [37,38,39,40]. The base of the 19S RP includes a ring-shaped heterohexamer of AAA+ ATPases that engage and unfold substrate polypeptides to allow for their translocation into the proteolytic pore of the 20S CP [8,16,31]. The ATPases couple ATP hydrolysis with the mechanical opening of the gates in the α-subunits of the 20S core.
1.1.2. Alternative Proteasome Complexes
In addition to the 26S proteasome, there are several other 20S complexes that catalyze ubiquitin- and ATP-independent degradation and serve a variety of roles in cells. These complexes include proteasome “activators” that open the gates of the central pore of the 20S CP, as the CP itself has very limited basal activity because of its auto-inhibition. Notably, the alternative 20S caps do not have polypeptide unfoldase activities. One such complex contains the 11S activator (PA28) in place of the 19S RP (Figure 1D). The 11S activator is mostly found in higher eukaryotes and is induced by interferon gamma, although there is basal expression in all tissues [19,41]. There are three different PA28 subunits (α, β and γ) that can form two kinds of heptameric barrel-like structures (either with PA28αβ or PA28γ). C-terminal motifs in PA28 subunits bind the α-subunits of the 20S CP and contain an internal activation loop that is involved in pore opening [42,43,44]. PA28αβ plays a role in antigen processing and MHC Class I antigen presentation [45,46,47]. Additionally, PA28γ has been shown to promote the degradation of cell cycle regulatory proteins such as p21 and SRC-3 [48,49]. Blm10/PA200 is another ubiquitin- and ATP-independent proteasome activator that is mostly found in the nucleus and has been implicated in such processes as DNA repair, mitochondrial function, and spermatogenesis (Figure 1D) [50,51,52,53]. Unlike the 11S activator, Blm10/PA200 is a monomeric ~250 kDa protein, conserved from yeast to higher eukaryotes, that forms a dome around the entrance to the 20S pore and makes extensive contacts with the α-subunits of the 20S CP. Blm10/PA200 has been found to stimulate the hydrolysis of small peptides and the unstructured tau protein in vitro [54]. Cumulatively, label-free quantitative proteomic analysis indicates that less than 10% of total cellular proteasomes are bound to ubiquitin-independent activators [55]; however, recruitment of PA28γ and PA200 to proteasomes is an unexpected consequence of catalytic inhibition, suggesting that their roles in cell biology might have clinical significance, such as in resistance to proteasome inhibition [56]. Additionally, the biological roles and structural characterization of hybrid proteasome complexes containing one 19S RP and one alternative cap raise the possibility that alternative proteasome structures, which can be “tuned” to specific cellular and environmental contexts, might be important therapeutic targets [57,58,59,60].
The presence of ATP- and ubiquitin-independent roles for the proteasome highlights the fundamental question of how substrates are specifically and selectively targeted for degradation. To be degraded, there must be a proteasome targeting mechanism, and ubiquitin solves that problem for UPS substrates. What about substrates that are degraded in a ubiquitin-independent manner? This is largely an unanswered question, but it is clear that proteins degraded in a ubiquitin-independent manner must be intrinsically disordered or contain disordered regions in order to fit into the proteolytic core [13]. It is estimated that up to 41% of eukaryotic proteins have intrinsically disordered regions, suggesting, at least in principle, that a significant portion of the proteome can be degraded in a ubiquitin-independent manner [61]. Well-characterized proteins that are degraded in a ubiquitin-independent manner include the yeast transcription factor Rpn4, thymidylate synthase, and ornithine decarboxylase [62]. Interestingly, in the case of ornithine decarboxylase, proteasomal degradation requires ATP and is accelerated by antizyme I; additionally, ubiquitinated substrates or free polyubiquitin chains compete for its proteasomal recognition [63,64]. This suggests that the same machinery responsible for ubiquitin-dependent degradation can also degrade proteins in a ubiquitin-independent manner. Discovering new substrates of ubiquitin-independent proteasome degradation pathways, and mechanistic details of these pathways, will be informative for developing inhibitors that block degradation of particular proteins important in human health and disease. Conversely, activating proteasomal degradation of intrinsically disordered proteins could be useful for treating neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis [13].
1.1.3. Immunoproteasome
During oxidative stress or in response to proinflammatory cytokines (e.g., interferon-gamma), cells produce specialized proteasome assemblies called immunoproteasomes [65]. Antigen-presenting cells have greater basal expression levels of immunoproteasome subunits. Immunoproteasomes are large proteasome complexes that are nearly identical to the constitutive proteasome except for different catalytic β-subunits (β1i, β2i, and β5i) that share high sequence homology with the associated constitutive proteasome β-subunits [65,66] (Figure 1). Interestingly, the immunoproteasome has a different substrate specificity than the constitutive proteasome and favors production of peptides that terminate in basic or hydrophobic amino acids [67,68,69,70,71]. Therefore, immunoproteasomes enhance ligand generation for MHC Class I molecules, allowing for surveillance by CD8+ T lymphocytes. Immunoproteasome formation following cytokine induction is rapid, cooperative, and can be transient [72,73,74]. Originally thought to be strictly involved in antigen presentation, immunoproteasomes have also been implicated in other cellular processes, including T cell polarization and cytokine production by macrophages [66]. Immunoproteasomes generate different peptides than constitutive proteasomes because they contain slightly different substrate binding pockets [75]. There is considerable effort to generate immunoproteasome-specific inhibitors, as immunoproteasome complexes appear to be present at low levels normally but higher levels in certain cancers, inflammatory diseases, and autoimmune diseases. Therefore, immunoproteasome inhibitors can be more selective and have fewer toxic side effects than constitutive proteasome inhibitors [76,77]. KZR-616, the only immunoproteasome inhibitor that has advanced to clinical trials, is described below. Based on the reversible epoxyketone β5i inhibitor ONX0914, the compound shows minimal cross-reactivity with the constitutive β5 subunit [17]. Advances in understanding the structural differences between the constitutive proteasome and the immunoproteasome will lead to the development of better, more specific and potent immunoproteasome inhibitors [66,75,78,79]. In addition to the immunoproteasome, it should be noted that there is a thymus-specific proteasome (thymoproteasome) that is involved in thymic positive selection and the development of CD8+ T lymphocytes [72,80]. The thymoproteasome incorporates a different β5 subunit, denoted β5t, and thymoproteasome-expressing cells display a unique set of antigenic peptides [72,80,81]. Finally, the testes-specific spermatoproteasome contains unique 20S proteasomal subunits in addition to some immunoproteasome subunits [82]. The PA200 activator is also abundant in testes and plays a role in spermatogenesis [83,84].
2. Proteasome Inhibitors
Scientists have been using proteasome inhibitors for many years to interrogate the biological function of the proteasome and its involvement in various cellular processes. The essential role of the proteasome in cell biology makes proteasome inhibition challenging to manage: one wants to achieve enough inhibition to kill diseased cells that rely heavily on the proteasome but not healthy cells. Complete proteasome inhibition is toxic. This was the early rationale for developing proteasome inhibitors to treat neoplastic cells that proliferate rapidly and have aberrant cell cycle checkpoint regulation [86]. Proteasome inhibitors are especially effective at treating hematological cancers because of the high dependence of these cancer cells on proteostasis mechanisms (i.e., they have a high secretory load) [17,87,88,89]. These cancer cells rely heavily on several mechanisms collectively called ER-associated degradation (ERAD), which dislocate aberrantly folded secretory and membrane proteins from the ER to the cytosol, where they are degraded by the proteasome [90]. We discuss the range of proteasome inhibitors used in the clinic for treating hematological cancers, and those under investigation in the lab for treating other forms of cancer and diseases.
2.1. Structural Characteristics of Proteasome Inhibitors
The proteasome inhibitors discussed in this review are largely covalent electrophilic inhibitors that react with the active-site threonine residues of the proteasome. The first proteasome inhibitors were analogs of serine protease inhibitors: hydrophobic peptide aldehydes that mimicked substrates of the β5 active site. Peptide aldehydes react with the nucleophilic hydroxyl group of threonine to form reversible hemiacetal adducts. One of the early aldehyde inhibitors, MG132, is a tool compound that is still used by many researchers today. However, MG132 has off-target effects in cells, including the inhibition of cathepsin B and calpains [7]. As a consequence of its structure, it is also quickly oxidized. In the years following the discovery of MG132, there were major advances in the development, optimization, and applications of new proteasome inhibitor structural scaffolds. Other structural classes, such as peptide boronates (e.g., bortezomib and ixazomib) and peptide epoxyketones (e.g., carfilzomib, oprozomib, and immunoproteasome-specific inhibitors ONX0914 and KZR-616), have had great success in the clinic, in part because the active-site adducts that they form are stabilized by specific interactions with the threonine amino group. For example, peptide boronates have an additional stabilizing hydrogen bonding interaction, and epoxyketones form stable morpholine adducts [18]. These additional interactions greatly reduce the off-rates of these inhibitors, making some of them essentially irreversible under physiological-treatment conditions. While most clinical proteasome inhibitors are covalent peptidic molecules, we also describe a nonpeptidic inhibitor, marizomib, which contains a reactive β-lactone that forms a tetrahydrofuran ring with the active-site threonine [18]. While more specific than peptide aldehydes, β-lactone inhibitors are less specific than epoxyketones. Nonpeptidic inhibitors were also developed for the immunoproteasome with the aim of improving metabolic stability and bioavailability, which is a general liability of peptidic backbones [91,92,93]. The lead compound from a virtual-screening effort showed excellent selectivity for β5i compared to the constitutive ®5 subunit [91]. However, the cell-based activity of this compound is much weaker than that of ONX0914 [91]. More efforts are needed to improve the compound’s potency. Efforts to screen for novel nonpeptidic inhibitors of the constitutive proteasome and immunoproteasome could result in molecules with improved pharmacokinetics and broader therapeutic potential. However, with significant interest in improving peptidic therapeutics [94], it is unclear which class of molecule is ultimately most efficacious in the proteasome inhibitor space.
The enzymatic mechanism of the 20S proteasome has prompted certain electrophilic structural classes of inhibitors to stand out, and the peptidic nature of many 20S inhibitors resembles natural proteasome substrates. In addition to inhibiting the 20S proteasome, there are multiple new inhibitors that target other UPS components, conferring unique phenotypes and toxicity profiles when used in preclinical studies. These inhibitors also represent more diverse structural classes than 20S inhibitors because of their mechanisms, opening the door to other therapeutic indications. Although proteasome inhibitors have seen the most success to date in treating MM and MCL, the list of inhibitors being tested, either alone or in combination with other drugs, to treat solid tumors and other diseases is long and growing.
2.2. Proteasome Inhibitors in Clinic
2.2.1. Bortezomib (Velcade)
Bortezomib was the first clinically approved proteasome inhibitor and is now indicated for the treatment of newly diagnosed multiple myeloma (NDMM) and relapsed MCL (Figure 2). The introduction of bortezomib into the clinic was a milestone in the treatment of MM, prolonging progression-free survival, and improving overall survival in patients with NDMM [95,96,97]. Bortezomib is a slowly reversible peptide boronate inhibitor that binds the catalytic site of the 20S proteasome, enabling the potent inhibition of the chymotrypsin-like activity of the β5 subunits (IC50, 7 nM; Table 1) [98]. Bortezomib also inhibits the trypsin-like activity of the β2 subunits and caspase-like activity of the β1 subunits at high concentrations, but these inhibitory activities are much less potent compared to its inhibition of the β5 sites [98]. Since its FDA approval in 2003 for refractory disease, bortezomib has become the frontline therapy for MM patients [99]. At therapeutic doses, bortezomib inhibits approximately 65% of β5 activity in patient whole blood lysate, which effectively induces apoptosis in myeloma cells without causing toxicity to nontransformed cells [89,100]. Given the essential role of the proteasome in all cells, it is critical to understand the mechanism of bortezomib specificity for MM cells. Physiologically, MM cells have elevated NF-κB activity, which is vital for their survival and proliferation [101,102]. Proteasome inhibitors block the NF-κB pathway by inhibiting the proteasomal degradation of the NF-κB inhibitor IκB, therefore enhancing the therapeutic efficacy of conventional treatments such as dexamethasone and lenalidomide [103]. This partially explains the reason why bortezomib specifically targets transformed MM. However, it is unlikely to be bortezomib’s critical mechanism of action because other NF-κB pathway inhibitors, like IκB kinase inhibitors, cannot kill MM cells as effectively as bortezomib [104]. An additional explanation for the sensitivity of MM cells to bortezomib is the cells’ low tolerance for proteotoxic stress. Bortezomib inhibits the primary protein-recycling machinery in cells, leading to the accumulation of misfolded proteins and UPR induction. A sustained UPR signal eventually triggers irreversible cell death [89]. MM cells produce large quantities of antibodies and rely heavily on the protective UPR. A brief exposure to the proteasome inhibitor can tip the balance to a terminal UPR, which commits MM cells to an apoptotic fate [88]. Therefore, MM cells appear to be prone to a cytotoxic bortezomib-induced terminal UPR.
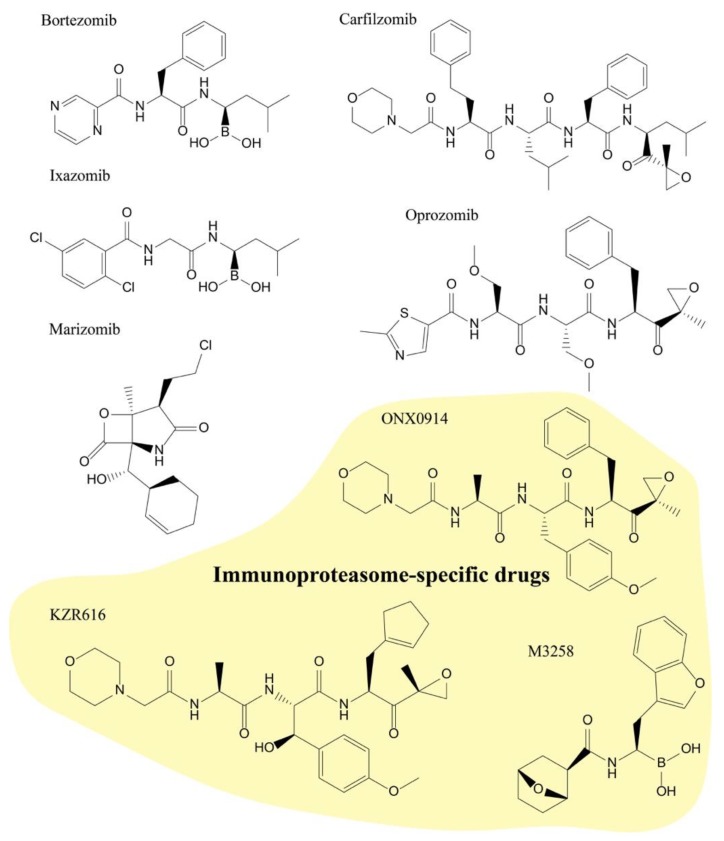
Figure 2.
Clinical proteasome and immunoproteasome inhibitors. Chemical structures of clinically used proteasome and immunoproteasome inhibitors. For more information, see Table 1 and Table 2.
Table 1.
Proteasome inhibitors in the clinic and in clinical trials.
| Name | Type | IC50 (nM) | β5 Dissoc. T1/2 (min) |
Administration | ||
|---|---|---|---|---|---|---|
| β5 Chymotrypsin-Like |
β1 Caspase- Like |
β2 Trypsin- Like |
||||
| Bortezomib (PS-341) |
Boronate | 2.4–7 *,# | 24–74 *,# | 1200–4200 *,# | 110 # | Intravenous |
| Carfilzomib (PR-171) |
Epoxyketone | 6 * | 2400 * | 3600 * | Irreversible | Intravenous |
| Ixazomib (MLN9708) |
Boronate | 3.4# | 31 # | 3500 # | 18 # | Oral |
| Marizomib (Salinosporamide A) |
β-lactone | 3.5 * | 430 * | 28 * | Irreversible | Intravenous |
| Oprozomib (ONX0912) |
Epoxyketone | 36 ^ | NA | NA | Irreversible | Oral |
Despite the fact that bortezomib is a successful therapy compared to conventional chemotherapeutic agents, it is not a panacea. It has several limitations. First, it is not uncommon for resistance and relapse to occur in patients who initially respond well to it. Several factors were found to contribute to bortezomib resistance. For example, mutations in the β5 subunits were identified in bortezomib-resistant MM cell lines [107,108]. These mutations, such as A108T and G322A, change the bortezomib binding pocket and affect β5–bortezomib interaction. Second, there are changes in cellular proteasome subunit composition and expression upon inhibition [109]. In addition to the changes in the proteasome itself, global changes in the transcriptome, such as increased expression of antiapoptotic proteins and decreased expression of proapoptotic proteins, also affect the sensitivity of MM cells to bortezomib [110,111]. Environmental factors, such as the bone-marrow microenvironment, also play a role in supporting bortezomib resistance [112]. In addition to acquired resistance, a significant issue limiting the usage of bortezomib is its toxicity towards the peripheral nervous system [113]. Patients treated with bortezomib can develop peripheral neuropathy (PN), which results in a painful burning sensation, numbness, and tingling in the extremities. PN is the primary cause of dose reduction or discontinuation among patients. Lastly, despite its efficacy in treating hematologic malignancies such as MM and MCL, bortezomib has had little success in clinical trials for treating solid tumors because of its rapid clearance from the blood and poor tissue penetration [114].
2.2.2. Carfilzomib (Kyprolis)
The success of bortezomib inspired researchers to hunt for a new generation of proteasome inhibitors to overcome its shortcomings. In 2012, the FDA approved the first second-generation proteasome inhibitor, carfilzomib (Kyprolis). Carfilzomib is an epoxyketone with a structure based on the natural product epoxomicin (Figure 2) [115]. It inhibits the chymotryptic activity of the β5 subunit with high potency and specificity, with an IC50 value of ~6 nM (Table 1) [98]. Unlike bortezomib, carfilzomib is an irreversible inhibitor that forms a dual covalent bond with active site Thr1 in the β5 subunits [116]. Compared to bortezomib, carfilzomib significantly prolonged the progression-free survival in patients with refractory and relapsed MM [117,118]. Combination therapy with carfilzomib and immunomodulatory drugs for NDMM was also evaluated in multiple clinical trials. This treatment regimen was shown to be efficacious with a favorable safety profile in NDMM [119]. The major adverse event of bortezomib, peripheral neuropathy, was found to be less severe in patients receiving carfilzomib treatment [118]. Across several clinical trials, 18.1% of phase 1–3 MM patients treated with carfilzomib experienced adverse cardiovascular events of all grades [120]. These complications can be managed by a combination of basic risk assessments, hydration, cardiovascular monitoring, intervention (such as reducing the carfilzomib dose), and patient education to notice cardiovascular symptoms and regularly monitor blood pressure [121]. Clinical guidelines were developed in collaboration with cardiologists to minimize the risk of cardiovascular events in patients treated with carfilzomib [122]. Despite the improvements of carfilzomib over first-generation proteasome inhibitors, it still has to be administered intravenously, and has demonstrated limited efficacy in patients with solid tumors [123]. The lack of efficacy is potentially due to the different physiology in solid tumors, and the pharmacokinetics of the “omib” drugs [89].
2.2.3. Ixazomib (Ninlaro)
In 2015, a third proteasome inhibitor, ixazomib (Ninlaro), was approved by the FDA to treat relapsed/refractory MM [124]. It was developed as a second-generation peptide boronic acid-based proteasome inhibitor and, notably, was the first orally available proteasome inhibitor (Figure 2) [105]. It is administered as a boronic ester prodrug that rapidly hydrolyzes to form the active inhibitor [105]. Although both bortezomib and ixazomib are reversible inhibitors from the same class with similar potencies, ixazomib has a much faster off-rate for proteasome binding than bortezomib (18 vs. 110 min t1/2; Table 1) [105,125]. When bortezomib is bound to the proteasome in red blood cells, its slow dissociation rate limits the drug distribution. Ixazomib was developed to overcome this limitation. Ixazomib’s fast off-rate allows for it to associate and dissociate with multiple proteasomes, allowing for its accessibility to more cells and larger tissue distribution [105].
2.3. Proteasome Inhibitors in Clinical Trials
2.3.1. Marizomib
Marizomib is a natural product isolated from the marine actinomycete Salinispora tropica, and the only nonpeptidic proteasome inhibitor currently in clinical trials (Figure 2) [126,127]. The combination of marizomib, pomalidomide, and low-dose dexamethasone has demonstrated promising activity in heavily pretreated, high-risk relapsed/refractory multiple-myeloma patients [126]. Marizomib is an orally available drug that belongs to the β-lactone chemical family and irreversibly inhibits the activity of all catalytic β subunits [128,129]. It is one of the most potent inhibitors against the proteasome’s chymotryptic activity, with an IC50 value of ~3 nM (Table 1) [98,130]. Marizomib was demonstrated to treat patients with relapsed and/or refractory MM, either alone or in combination with other drugs, without some of the side effects (e.g., peripheral neuropathy) of other proteasome inhibitors [126,131]. It is also the smallest proteasome inhibitor identified to date and, likely because of its more lipophilic structure, the only proteasome inhibitor in clinical trials that can cross the blood–brain barrier [132]. These unique properties make marizomib an attractive candidate for the treatment of central-nervous-system (CNS) malignancies. Marizomib was found to induce caspase 9-dependent cell death in glioblastoma (GBM) cells and mouse models [133]. Currently, it is in clinical trials for treating newly diagnosed GBM [134].
2.3.2. Oprozomib
Oprozomib is the result of efforts to develop orally available epoxyketone proteasome inhibitors (Figure 2) [106,135]. It belongs to the same molecular family as carfilzomib and irreversibly inhibits the β5 subunit (Table 1) [106]. It is in clinical trials for treating newly diagnosed and relapsed/refractory MM. The trials indicate that oprozomib shows encouraging activity in both relapsed/refractory and newly diagnosed MM. It could become an important MM therapy if the concerns regarding its gastrointestinal tolerability are addressed [136,137,138,139]. Oprozomib has also been evaluated for treating solid tumors, but unfortunately no valid treatment efficacy has been observed so far [140]. However, considering that the patients in the ongoing clinical study were pretreated with other therapies and have advanced cancers, the efficacy of oprozomib for treating earlier stages of cancer is yet to be determined [140].
2.3.3. KZR-616
KZR-616 is the only immunoproteasome inhibitor that has advanced to the stage of clinical trials [141]. It was developed on the basis of ONX0914, an epoxyketone that selectively inhibits the β5i subunit with an IC50 of ~39 nM (Figure 2 and Table 2) [142]. Unlike constitutive β5 inhibitors, such as bortezomib and carfilzomib, it reduces the production of proinflammatory cytokines and cell-surface MHC Class I expression without apoptotic effects [142]. ONX0914 has shown efficacy in animal models of autoimmune disorders without affecting normal immune function [143,144]. Compared to ONX0914, KZR-616 has improved solubility and shows better efficacy in mouse models of antibody-induced arthritis [141]. It is in clinical testing to treat autoimmune diseases [141], expanding the indications of proteasome inhibitors beyond cancer. Notably, a second immunoproteasome inhibitor, M3258, targeting β5i was disclosed and will be tested in combination with dexamethasone in an upcoming phase I clinical trial for treating MM [145].
Table 2.
Immunoproteasome inhibitors.
| Name | Type | IC50 (nM) Against Immuno- and Constitutive-Proteasome [141] |
|||||
|---|---|---|---|---|---|---|---|
| LMP7 (β5i) | β5 | LMP2 (β1i) |
β1 | MECL-1 (β2i) |
β2 | ||
| ONX0914 | Epoxyketone | 39 | 422 | 287 | >12700 | 902 | 927 |
| KZR-616 | Epoxyketone | 39 | 688 | 131 | >10600 | 623 | 604 |
2.4. Proteasome Inhibitors in Lab
The history of proteasome inhibitor development teaches us two critical lessons: (i) MM is a starting point but not an end point for proteasome inhibitor-based therapies, and there are more therapeutic areas worth exploring; and (ii) despite the tremendous patient benefits conferred by proteasome inhibitors over the last decade, there are opportunities to expand their utility. There is sustained enthusiasm for identifying other mechanisms to inhibit the proteasome because: (i) inhibition of the 20S core particle by ‘omibs’ may not be the best route to inhibit the proteasome; (ii) other mechanisms of proteasome inhibition may provide benefits to patients who are refractory to bortezomib and carfilzomib; (iii) other mechanisms of proteasome inhibition, when combined with carfilzomib, may yield synergistic effects that provide even more dramatic progression-free survival; and (iv) the improved potency and selectivity of carfilzomib and potentially other classes of proteasome inhibitors may allow for expansion into other hematological malignancies and solid tumors. Therefore, we describe alternative strategies to develop non-conventional proteasome inhibitors in the lab with the potential for use as therapeutics in the future.
2.4.1. Block the Removal of Ubiquitin Chains from Substrates: Rpn11 Inhibitors
Rpn11 is an essential subunit of the 19S RP, which removes the polyubiquitin (polyUb) chains from substrates prior to degradation (Figure 1B) [35,146]. The active site is located within the JAMM domain of Rpn11, which contains a catalytic zinc ion coordinated by two histidine residues and an aspartate [147,148]. Importantly, substrate deubiquitination by Rpn11 is essential for degradation, to which it is tightly coupled [35,146]. Inactivation of the Rpn11 enzymatic active site by mutations of the zinc-coordinating histidine residues results in a severe decrease in substrate degradation in cells [33,149]. An HTS campaign with more than 300,000 compounds was conducted to discover Rpn11 inhibitors and led to the discovery of a metal-binding pharmacophore with a moderate IC50 of 2.5 µM [40,150]. Further optimization of this hit compound yielded capzimin, a more specific inhibitor with a biochemical IC50 of ~300 nM against Rpn11 (Table 3 and Figure 3) [40]. Capzimin blocks proteasome function by inhibiting the deubiquitinase activity of Rpn11, which causes accumulation of polyubiquitinated proteins, triggers the UPR, and blocks cell proliferation [40]. Capzimin is active against multiple cancer cell lines, including bortezomib-resistant cells [40]. In addition to capzimin, natural products such as thiolutin and gliotoxin were also identified as active inhibitors of JAMM proteases, including Rpn11. These compounds are active in cell culture and inhibit proteasome function by chelating the zinc ion in the Rpn11 active site [38,39]. However, these compounds are less specific towards Rpn11 than capzimin. Capzimin and other natural compounds need to be further optimized to improve the potency and specificity and to gain more drug-like properties.
Table 3.
Inhibitors of non-20S UPS components.
| Name | Target | IC50/Kd | Mechanism |
|---|---|---|---|
| Capzimin | Rpn11 | 0.3 µM (In vitro Rpn11 assay); 0.6 µM (UbG76V-GFP degradation assay in cells) |
Chelating the zinc ion in Rpn11, inactivating its DUB activity [40] |
| Ubistatin B | polyUb | 10 µM (CFTR ubiquitination assay in cells) |
Binding to the polyUb chain and blocking the substrate/Ub receptor interaction [151,152] |
| RA190 | Rpn13 | Not Applicable | Covalently binding to cysteine residue 88 (Cys88) of Rpn13 when it is not bound to the proteasome and Uch37 at the proteasome [158,159,160] |
| RA183 | |||
| KDT-11 | Rpn13 | 1.7 µM (Kd) | Peptoid ligand binding to Rpn13 [161] |
| RIP-1 | Rpt4 | 3.0 µM (proteasome-mediated stripping of the Gal4-VP16 protein from DNA in vitro) |
Inhibiting the protein unfolding activity of the 19S RP [167,168] |
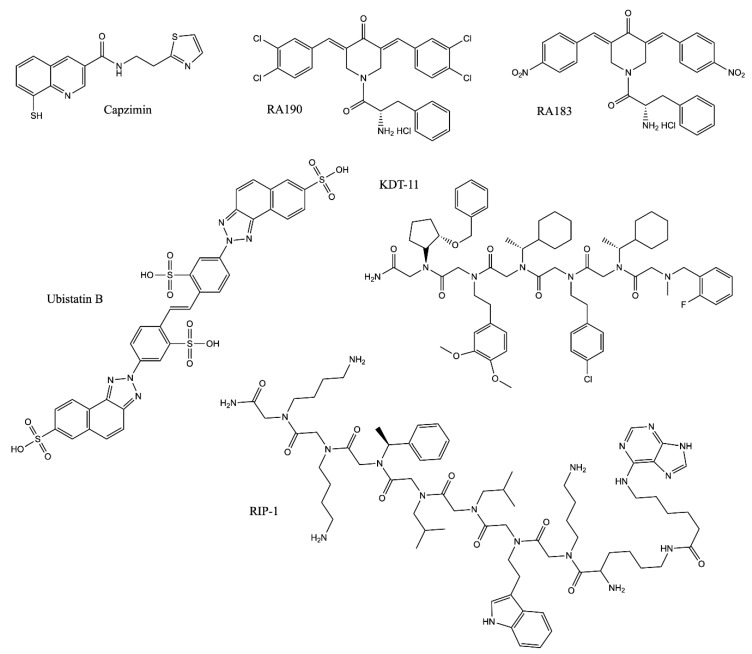
Figure 3.
Structures of non-20S UPS inhibitors. For more information, see Table 3.
2.4.2. Block the Interaction between Substrate and the Proteasome
Rpn11 is not the only potential drug target in the 19S RP. The first reported inhibitor of the 19S RP was ubistatin, which blocks the binding of ubiquitin chains to their receptors [151]. Ubistatins A and B compete for the polyUb receptor Rpn10 and the shuttle protein Rad23 for their interactions with polyUb chains (Table 3 and Figure 3) [151]. As a result, ubistatins inhibit protein degradation in vitro [151]. More recently, a structural study revealed an interaction between ubistatin and ubiquitin. Ubistatin directly interacts with a hydrophobic patch and the surrounding basic/polar residues on the ubiquitin surface. Ubistatin shows a strong preference for K48 linkages over K11 and K63 linkages due to differences in the conformation and distribution of the hydrophobic patches on the surfaces [152]. The major challenge to further develop ubistatins as therapeutics is their negative charge, which precludes efficient membrane penetration, thereby limiting their potency in cells.
Another polyUb receptor in the 19S RP, Rpn13, is an attractive target for the development of alternative proteasome inhibitors [39]. Rpn13 and the deubiquitinating enzyme that it activates, Uch37, have been found to be important for cell cycle progression in cell culture [153], and increased expression of the gene encoding Rpn13 (ADRM1) has been identified in several forms of cancer [154,155,156,157]. Multiple efforts have been put forth to look for Rpn13 inhibitors. RA190, a bis-benzylidine piperidone, was the first identified inhibitor of Rpn13. It was believed to covalently interact with Cys88 of Rpn13 and block the binding of polyUb substrates (Table 3) [158]. RA190 induces the accumulation of polyUb proteins, causes ER stress, and stabilizes proteasome substrates, including p53 [158]. It showed anti-tumor effects in mouse models for MM and ovarian cancer [158]. A subsequent study suggests that RA190 has two synergistic mechanisms of action: targeting Rpn13 when it is not bound to the proteasome (at Cys88), and inactivating Uch37 at the proteasome [159]. Recently, an improved compound with higher potency, RA183, was developed and tested in mice [160]. Similar to RA190, RA183 is an irreversible inhibitor targeting Cys88 of Rpn13 and inhibits tumor growth in a xenograft mouse model of ovarian cancer (Table 3) [160]. In addition to RA190/RA183, a peptoid inhibitor, KDT-11, was identified as a Rpn13 inhibitor that binds with a modest Kd of 1.7 µM (Table 3) [161]. KDT-11 causes ubiquitin accumulation and acts synergistically with bortezomib in treating MM cells. KDT-11 is a reversible inhibitor that does not compete with RA190, indicating a different binding mechanism [161]. It should be noted that “enones” like RA190 have been described as “pan-assay interference compounds,” or PAINS, which raises concern about their specificity [162]. However, demonstrated examples of ways to circumvent the specificity problem with thiol-reactive compounds are described below (Section 3.2.2). Irrespective of the type of inhibitor, further support for inhibiting Rpn13 comes from the recent development of a proteolysis-targeting chimeric molecule (PROTAC), WL40, that fuses RA190 with thalidomide, targeting Rpn13 for degradation [163]. This molecule successfully inhibits MM cell growth in a mouse xenograft model. In fact, the molecule shows improved MM cell death compared to Rpn13 inhibitors. This presents a conundrum, though, because the PROTAC works by targeting Rpn13 for proteasomal degradation, but the effect of the drug is to hamper proteasomal degradation. Nonetheless, this proof-of-principle study shows that there could be some benefit to degrading a proteasomal or proteasome-associated target rather than functionally hindering it. There is a lot of excitement surrounding PROTACS like WL40 and their uses in selectively degrading proteins by linking a ubiquitin ligase to a particular target; the advantages and disadvantages of this strategy are reviewed elsewhere [164,165,166].
2.4.3. Block Substrate Translocation into the 20S CP: Proteasome ATPase inhibitors
The third potential target in the 19S RP is the proteasomal ATPase, which provides the energy to power substrate translocation into the 20S CP and therefore facilitates protein degradation. RIP-1 was the first identified peptoid inhibitor targeting Rpt4 (PSMC6), a subunit of the proteasome ATPase [167,168]. RIP-1 biochemically blocks the protein unfolding activity of the 19S RP and inhibits turnover of the proteasome substrate p27 in cells (Table 3 and Figure 3) [167]. However, since the discovery of RIP-1 in 2007, there have been no follow-up studies to further understand the biological effects of this inhibitor. It will be interesting to understand its selectivity towards the proteasomal ATPase, given the abundance and similarity of ATPases in cells.
3. Perspective on the Use of Proteasome Inhibitors in Clinic
The initial development and clinical success of 20S proteasome inhibitors validated the maintenance of proteostasis as a therapeutic approach for treating human diseases. Proteasome inhibitors have revolutionized the treatment of MM, greatly extending patients’ lives. However, part of the success of proteasome inhibitors thus far is attributed to unique characteristics of transformed plasma cells. Additionally, the development of resistance and the toxicity profiles of currently used inhibitors cast doubts over the future utility of proteasome inhibition. The essential role of the proteasome in cell biology makes it difficult to envisage proteasome inhibition as a single therapeutic strategy for treating a range of human illnesses. To use proteasome inhibition to its full potential, it is critical to develop deeper understandings of the biological pathways affected by proteasome inhibition and strategies to confer inhibitor specificity to cell and proteasome types. This includes targeting other components of the UPS than the 20S proteasome itself. It also includes understanding mechanisms of proteasome resistance and trying to develop ways to stop resistance from developing.
3.1. Combatting Proteasome Inhibitor Resistance
Cancer cells are often described as resilient cells that continue growing despite efforts to stop them. True to the description, there have been multiple observed mechanisms of resistance to proteasome inhibitors in the lab and the clinic. Combatting these resistance mechanisms can serve as a potentially useful strategy for making proteasome inhibitors more effective for treating blood-based cancers and for expanding their profiles to treat solid tumors. Combination therapies comprised of a proteasome inhibitor and an inhibitor targeting a resistance mechanism is one example of a strategy that can be employed, much like amoxicillin and clavulanate are often used together to treat bacterial infections. Mechanisms of resistance to proteasome inhibitors (especially bortezomib) seen in the lab or clinic include, but are not limited to mutations in PSMB5 (see Section 2.2.1), aberrant expression of UPS pathway components [169,170,171], induction of drug efflux from cells, and activation of signaling cascades that promote cell survival [172]. An early rationale for administering combination therapies to treat cancer was that drugs with nonoverlapping mechanisms would reduce the chances of developing therapeutic resistance [173]. The combination-therapy strategy showed promise for treating BRAFV600E colorectal cancers [174]. BRAFV600E melanomas, which have low expression of EGFR, are treatable with RAF inhibitors (e.g., vemurafenib); however, BRAFV600E colorectal cancers adapt to RAF inhibitors through compensatory activation of the EGFR and MAPK signaling pathways [175,176]. An understanding of this biology paved the way for the encouraging results of a clinical trial for combination BRAF–EGFR–MEK inhibitor therapy for treating BRAFV600E colorectal-cancer patients [174]. Although more has to be done to optimize the treatment regimen, this example highlights the power of understanding compensatory-resistance mechanisms for improving cancer therapies.
An interesting mechanism of resistance to sublethal proteasome inhibition involves activation of ER resident transcription factor Nrf1/NFE2L1. Nrf1 is constitutively retrotranslocated from the ER lumen to the cytoplasm, where it is degraded by the proteasome under homeostatic conditions [177,178,179]. However, if proteasome activity is inhibited or hindered, then Nrf1 is instead cleaved by the DDI2 protease in the cytoplasm to form the active transcription factor that translocates to the nucleus and enhances transcription of UPS genes, among others [180,181]. This allows for a “bounce-back” response in which cells react to proteasome inhibition by producing more proteasome subunits. As an aspartyl protease with a substrate binding site, DDI2 might be a promising, potentially “druggable” candidate for combination therapies with proteasome inhibitors. However, for this to be a viable strategy, demonstration is critical of the requirement of DDI2 and Nrf1 for this compensatory mechanism in actual human-cancer-patient samples. Additionally, more thorough characterization of the DDI2 cleavage mechanism, including reconstitution of the activity of the human protein and characterization of its other roles in the cell, are critical. In addition to DDI2, Nrf1 deglycosylation by the NGLY1 enzyme was demonstrated to be important for Nrf1 activity [182,183]. Although loss of NGLY1 is responsible for a rare human genetic disorder, and the range of its substrates necessitate thorough characterization [184], inhibiting NGLY1 might serve as another druggable target in this pathway.
An additional metabolic mechanism of resistance was recently described and can potentially be exploited therapeutically. The mechanism involves a surprising relationship between proteasome inhibition and mitochondrial metabolism [185]. Resistance to proteasome inhibitors is related to a shift from glycolysis to oxidative phosphorylation, and such a shift renders cells especially sensitive to the small molecule elesclomol, whose target is the mitochondrial reductase FDX1. This finding raises the possibility that perhaps there are other resistance pathways that have not yet been discovered that can be exploited for therapeutic intervention.
Cumulatively, understanding mechanisms of resistance to proteasome inhibitors, and how to target them using combination therapies can help improve the efficacy of treatments for MM and MCL, and can potentially broaden the scope of proteasome inhibitors to the treatment of solid tumors—a holy grail. By increasing the extent and duration of proteasome inhibition by concomitantly inhibiting a resistance mechanism, cancer cells on the brink of apoptosis might be pushed over the edge [89].
3.2. Other UPS Inhibitors
3.2.1. VCP/p97 Inhibition
There are many UPS components other than the 20S CP that can serve as viable targets for therapeutic intervention. One very promising oncological target is vasolisin-containing protein (VCP)/p97, an essential and conserved homohexameric AAA+ ATPase that has been demonstrated in recent years to be involved in numerous cellular pathways [186]. p97 is central to proteostasis by serving as a “segragase” that extracts challenging UPS substrates from cellular structures (downstream of E3 ubiquitin ligases) [187,188]. For example, p97 is essential for extracting ER-associated substrates during ERAD and tRNA-nascent chains from ribosomes that stall during translation [189]; p97 also separates protein aggregates so that the proteasome can degrade them. There are also nondegradative roles for p97, such as in endosomal trafficking and chromatin remodeling [190]. There have been substantial efforts to understand the structure and mechanism of p97, and the nature of its interaction with various adapter proteins that are required for its function in different pathways [191,192,193,194]. Because p97 is essential, it cannot be completely eliminated from cells without inducing the UPR and apoptosis. However, cancer cells appear to have a heightened dependence on p97 than noncancerous cells do, and hence more rapid apoptosis upon p97 depletion [89].
There have been substantial efforts to find effective and potent p97 inhibitors, and despite many tool compounds that can be used in the lab, there has been only one that made it to phase 1 clinical trials: CB-5083 developed by Cleave Biosciences. CB-5083 is an orally available, potent inhibitor that shows efficacy in tumor models that are insensitive to proteasome inhibitors. Additionally, CB-5083 inhibition is more efficacious in solid tumor models than proteasome inhibitors. Transcriptomics studies with MM cell lines indicate that the cellular response to CB-5083 is distinct from that of proteasome inhibitors, supporting the notion that targeting different components of the UPS can be beneficial for treating different diseases or disease states [195]. Interestingly, the transcription factor Nrf1 is an ERAD substrate and depends on p97 for its retrotranslocation [179]. CB-5083 inhibits Nrf1-mediated proteasome gene upregulation, thereby inhibiting a potentially important form of resistance to proteasome inhibition. While the pre-clinical data were promising for this first-in-class compound, clinical trials for its efficacy in treating MM were terminated in 2018.
P97 inhibition can be effective in treating diseases other than cancer. Inclusion body myopathy with early-onset Paget disease and frontotemporal dementia (IBMPFD) is caused by mutations in VCP [196,197]. Studies from a biochemical reconstitution of p97 demonstrate that IBMPFD mutants show enhanced p97 unfoldase activity [198,199]. Therefore, it is possible that inhibiting p97 activity can be efficacious for treating IBMPFD.
3.2.2. Inhibiting other UPS Components
Despite the diversity and the number of E3 ubiquitin ligases, which confer substrate specificity to the ubiquitination reaction, there are only two mammalian E1 ubiquitin-activating enzymes. Theoretically, inhibiting the E1 reaction would inhibit all ubiquitin-dependent pathways in cells, both degradative and non-degradative. The first E1 inhibitor, PYR-41, was shown to attenuate NF-kB activation and proteasomal degradation of IkB. Proof-of-principle studies also demonstrated that it can differentially kill cancer cells [200]. A more potent and selective inhibitor, TAK-243, was recently reported to cause complete loss of cellular ubiquitylation, impaired cell cycle progression, and impaired DNA damage repair [201]. TAK-243 shows efficacy in tumor models and is under investigation as a clinical candidate for treating myelodysplastic syndrome and leukemia.
Targeting E3 ubiquitin ligases could be efficacious for finding more specific and less toxic inhibitors of the UPS. There are over 600 E3 ligases in mammalian cells that catalyze a range of ubiquitin linkages and chain types. Ubiquitinated proteins can have both degradative and nondegradative fates. The three main families of ligases, RING, U-box, and HECT-containing E3s, have different characteristics and properties. Most efforts to find E3 inhibitors have focused on MDM2, IAP, and SCF [202]. The IAP (antiapoptotic proteins) family inhibits proapoptotic caspases and Smac proteins. One target of IAP is RIP1, which activates NF-kB. Therefore, inhibiting RIP1 ubiquitylation inhibits NF-kB activation, and this has been exploited as a potential therapeutic strategy [203,204,205].
An indirect approach for inhibiting cullin-RING ubiquitin ligases, the largest class of E3 ligases, is to inhibit their activation by neddylation, post-translational modification similar to ubiquitylation. Pevonedistat (MLN4924/TAK-924) is a potent inhibitor of the Nedd8-activating enzyme (similar to the ubiquitin-activating E1 enzyme) that has shown efficacy in killing a variety of cancer-cell types, and has been under clinical investigation for treating leukemia in addition to solid tumors [206,207,208].
As described above, inhibiting Rpn11, the de-ubiquitinating (DUB) enzyme in the lid of the 19S RP, has proven efficacious in the lab. DUBs in general are promising targets for cancer therapies because they are aberrantly activated or expressed in certain cancer types. A challenge with identifying potent and selective DUB inhibitors is that DUBs contain an active-site cysteine, and many DUB inhibitors that have been discovered and investigated are thiol-reactive molecules. Therefore, they have the potential problem of reacting nonspecifically with exposed cysteine residues in various proteins. One way to circumvent this problem and to confer specificity is to have multiple binding modes or binding sites distal to the catalytic cysteine. This has been exploited for finding inhibitors of the DUB Usp7, for example, which show efficacy in tumor models [209,210,211,212]. There is precedent for bringing thiol-reactive drugs to the clinic [213,214,215,216,217]. The recent development of AMG 510 by Amgen for treating solid tumors with a particular KRAS-G12C mutation further demonstrates that cysteine-reactive drugs can advance to clinical trials [218].
Besides Rpn11, there are two proteasomal non-JAMM-domain-containing DUBs, Usp14 and Uch37 [8,219]. Interestingly, the activities of these DUBs appear to reduce degradation of their substrates, presumably because they are not coupled to the ATPase activity of the 19S RP. Consistent with this activity, a well-characterized small-molecular inhibitor of Usp14, IU1, showed increased degradation of model substrates, biochemically and in cells [219,220]. Recent structural evidence demonstrated that IU1 is specific for Usp14 by binding to an allosteric site that blocks access of ubiquitin to the active site [221]. This class of inhibitor could be useful for treating diseases in which proteasome activity needs to be activated, such as neurodegenerative diseases. Interestingly, b-AP15, a dual inhibitor of Usp14 and Uch37, was shown to prevent protein degradation and inhibit tumor progression in acute myeloid leukemia models [222].
3.3. Other Indications for Proteasome Inhibitors
Although clinical proteasome inhibitors were originally developed to treat cancers, the rich biology of the proteasome and its essentiality in many processes make it a promising target for other indications, too. For example, there is a straightforward connection between proteasome inhibition and immune disorders. The proteasome is involved in generating antigens for presentation by MHC Class I, and proteasome inhibitors are effective at targeting antibody-producing B cells. As such, bortezomib and carfilzomib continue to be explored as therapies for antibody-mediated allograft rejection (AMR) [223,224,225]. Proteasome inhibitors might be useful for treating AMR because they reduce the number of human leukocyte antigen (HLA) antibodies [226]. Bortezomib showed early efficacy when used alone according to the MM treatment regimen to treat rejection following renal transplantation [227]. Bortezomib has also been used in combination with other AMR treatments [228]. Additionally, proteasome inhibitors have been efficacious in treating heart, pancreas, and lung allograft rejection [229,230,231]. There are also clinical trials that were completed or are underway to evaluate the use of proteasome inhibitors to treat graft-versus-host disease and other transplantation-related immune disorders (clinicaltrials.gov).
Another interesting clinical application of proteasome inhibition is in the treatment of malaria [232,233,234,235]. Malaria parasites contain their own form of the 26S proteasome in the cytoplasm and nucleus. Additionally, in silico predictions and biochemical analyses suggest that a significant portion of the Plasmodium falciparum proteome can be ubiquitinated during asexual reproduction, and ubiquitination is associated with resistance to antimalarial agents [236]. Selective inhibitors of the protozoal proteasome have proven effective in killing P. falciparum while sparing human cells [237,238]. Because the proteasome is vital in all stages of the P. falciparum life cycle in human hosts, it remains a promising antimalarial drug target that merits further investigation.
While the protozoan proteasome is an unusual drug target, even more surprising is the use of proteasome inhibitors as antibiotics. Although the proteasome is essential in eukaryotes, there are conditionally essential proteasomal genes in many bacterial Actinomycetales and Nitrospirales lineages [239]. This includes highly pathogenic species Mycobacterium tuberculosis, a major global threat affecting at least 10 million people in 2017 [240,241,242,243,244]. Researchers have turned to the M. tuberculosis proteasome as a potential drug target because of its importance for resistance to oxidative and nitrosative stresses in a human host. As with malarial proteasome inhibitors, molecules targeting the M. tuberculosis proteasome must be selective over human proteasomes. Significant efforts were made to identify selective M. tuberculosis proteasome inhibitors [245,246], and with growing interest in using proteasome inhibitors to treat infectious diseases amid the threat of drug resistance, this is an exciting time to investigate fundamental properties of bacterial proteasomes and novel ways to distinguish them from human proteasome complexes [246].
4. Conclusions
Proteasome inhibitors have proven efficacious in the clinic for treating hematological malignancies. Their efficacy for treating these cancer types and for expanding their indications to solid tumors has been challenged by the development of resistance and toxicity, in addition to past limitations in crossing the blood-brain barrier and therefore treating glioblastomas. However, innovative applications of proteasome inhibitors that can have clinical relevance continue to be uncovered. Considering the importance of the proteasome for antigen presentation by immune cells, uses for proteasome inhibitors (especially at low doses) in modulating antigen presentation and immunoproteasome-specific inhibitors will clearly be active areas of basic and applied research in the coming years. Exploring specific degradation trajectories of target proteins can also prove to be a viable approach for finding specific inhibitors, as there is tremendous diversity in the players involved in the UPS. Finding the right balance between inhibiting the proteasome “just enough” and maintaining proteasome activity where it is needed will continue to be a challenge that will need to be addressed. However, fundamental and applied research pertaining to the proteasome and proteasome inhibitors, and ongoing and new clinical trials that make use of proteasome inhibitors for treating a growing number of diseases, will inform future drug discovery efforts. The proteotoxic crisis exists in many cell types and is central to a vast number of human diseases, and as long as this is the case, there will be a need for more specific and selective proteasome inhibitors (Figure 4).
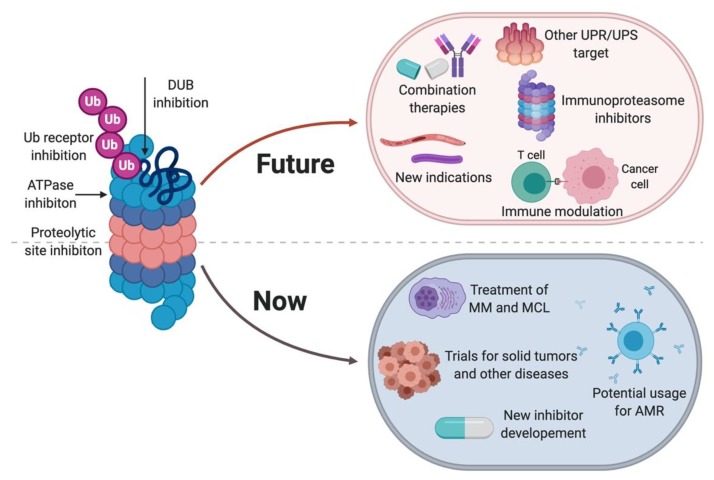
Figure 4.
State of proteasome inhibitors, including summary of current state and future of proteasome inhibitors [85].
Acknowledgments
We thank Rusty Lipford, Rati Verma, Weiru Wang, and Ingrid Wertz for their critical reading and comments on the manuscript. We thank Ray Deshaies for introducing us to the field of ubiquitin biology.
Author Contributions
D.J.S. and J.L. conceptualized, drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
D.J.S. is an employee of Amgen Inc and may hold Amgen stock.; J.L. is an employee Genentech, a member of the Roche group, and may hold Roche stock or stock options.
References
- 1.Harper J.W., Bennett E.J. Proteome complexity and the forces that drive proteome imbalance. Nature. 2016;537:328–338. doi: 10.1038/nature19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershey J.W., Sonenberg N., Mathews M.B. Principles of translational control: An overview. Cold Spring Harb. Perspect. Biol. 2012 doi: 10.1101/cshperspect.a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hetz C., Glimcher L.H. Protein homeostasis networks in physiology and disease. Curr. Opin. Cell Biol. 2011;23:123–125. doi: 10.1016/j.ceb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labbadia J., Morimoto R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Glickman M.H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 7.Kisselev A.F., Goldberg A.L. Proteasome inhibitors: From research tools to drug candidates. Chem. Biol. 2001;8:739–758. doi: 10.1016/S1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 8.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai Y.C., Weissman A.M. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1:764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piva R., Belardo G., Santoro M.G. NF-kappaB: A stress-regulated switch for cell survival. Antioxid. Redox Signal. 2006;8:478–486. doi: 10.1089/ars.2006.8.478. [DOI] [PubMed] [Google Scholar]
- 13.Opoku-Nsiah K.A., Gestwicki J.E. Aim for the core: Suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration. Transl. Res. 2018;198:48–57. doi: 10.1016/j.trsl.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai H.C., Schuman E.M. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat. Rev. Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran K.V., Margolis S.S. A mammalian nervous-system-specific plasma membrane proteasome complex that modulates neuronal function. Nat. Struct. Mol. Biol. 2017;24:419–430. doi: 10.1038/nsmb.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bard J.A.M., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibaudeau T.A., Smith D.M. A Practical Review of Proteasome Pharmacology. Pharm. Rev. 2019;71:170–197. doi: 10.1124/pr.117.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisselev A.F., van der Linden W.A., Overkleeft H.S. Proteasome inhibitors: An expanding army attacking a unique target. Chem. Biol. 2012;19:99–115. doi: 10.1016/j.chembiol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M., Finley D. Regulation of proteasome activity in health and disease. Biochim. Biophys. Acta. 2014;1843:13–25. doi: 10.1016/j.bbamcr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cromm P.M., Crews C.M. The Proteasome in Modern Drug Discovery: Second Life of a Highly Valuable Drug Target. ACS Cent. Sci. 2017;3:830–838. doi: 10.1021/acscentsci.7b00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobanova E.S., Finkelstein S., Li J., Travis A.M., Hao Y., Klingeborn M., Skiba N.P., Deshaies R.J., Arshavsky V.Y. Increased proteasomal activity supports photoreceptor survival in inherited retinal degeneration. Nat. Commun. 2018 doi: 10.1038/s41467-018-04117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etlinger J.D., Goldberg A.L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc. Natl. Acad. Sci. USA. 1977;74:54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K., Yoshimura T., Kumatori A., Ichihara A., Ikai A., Nishigai M., Kameyama K., Takagi T. Proteasomes (multi-protease complexes) as 20 S ring-shaped particles in a variety of eukaryotic cells. J. Biol. Chem. 1988;263:16209–16217. [PubMed] [Google Scholar]
- 24.Arrigo A.P., Tanaka K., Goldberg A.L., Welch W.J. Identity of the 19S “prosome” particle with the large multifunctional protease complex of mammalian cells (the proteasome) Nature. 1988;331:192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- 25.Sauer R.T., Baker T.A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 26.Collins G.A., Goldberg A.L. The Logic of the 26S Proteasome. Cell. 2017;169:792–806. doi: 10.1016/j.cell.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogyo M., Gaczynska M., Ploegh H.L. Proteasome inhibitors and antigen presentation. Biopolymers. 1997;43:269–280. doi: 10.1002/(SICI)1097-0282(1997)43:4<269::AID-BIP2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Groll M., Bajorek M., Kohler A., Moroder L., Rubin D.M., Huber R., Glickman M.H., Finley D. A gated channel into the proteasome core particle. Nat. Struct. Biol. 2000;7:1062–1067. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- 29.Whitby F.G., Masters E.I., Kramer L., Knowlton J.R., Yao Y., Wang C.C., Hill C.P. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature. 2000;408:115–120. doi: 10.1038/35040607. [DOI] [PubMed] [Google Scholar]
- 30.Finley D., Chen X., Walters K.J. Gates, Channels, and Switches: Elements of the Proteasome Machine. Trends Biochem. Sci. 2016;41:77–93. doi: 10.1016/j.tibs.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kish-Trier E., Hill C.P. Structural biology of the proteasome. Annu. Rev. Biophys. 2013;42:29–49. doi: 10.1146/annurev-biophys-083012-130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thrower J.S., Hoffman L., Rechsteiner M., Pickart C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma R., Deshaies R.J. A proteasome howdunit: The case of the missing signal. Cell. 2000;101:341–344. doi: 10.1016/S0092-8674(00)80843-0. [DOI] [PubMed] [Google Scholar]
- 34.Glickman M.H., Rubin D.M., Fried V.A., Finley D. The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol. Cell Biol. 1998;18:3149–3362. doi: 10.1128/MCB.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma R., Aravind L., Oania R., McDonald W.H., Yates J.R., Koonin E.V., Deshaies R.J. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 36.Worden E.J., Dong K.C., Martin A. An AAA Motor-Driven Mechanical Switch in Rpn11 Controls Deubiquitination at the 26S Proteasome. Mol. Cell. 2017;67:799–811. doi: 10.1016/j.molcel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Nalepa G., Wade Harper J. Therapeutic anti-cancer targets upstream of the proteasome. Cancer Treat. Rev. 2003;29(Suppl. 1):49–57. doi: 10.1016/S0305-7372(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Zhang Y., Da Silva Sil Dos Santos B., Wang F., Ma Y., Perez C., Yang Y., Peng J., Cohen S.M., Chou T.F., et al. Epidithiodiketopiperazines Inhibit Protein Degradation by Targeting Proteasome Deubiquitinase Rpn11. Cell Chem. Biol. 2018;25:1350–1358. doi: 10.1016/j.chembiol.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauinger L., Li J., Shostak A., Cemel I.A., Ha N., Zhang Y., Merkl P.E., Obermeyer S., Stankovic-Valentin N., Schafmeier T., et al. Thiolutin is a zinc chelator that inhibits the Rpn11 and other JAMM metalloproteases. Nat. Chem. Biol. 2017;13:709–714. doi: 10.1038/nchembio.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Yakushi T., Parlati F., Mackinnon A.L., Perez C., Ma Y., Carter K.P., Colayco S., Magnuson G., Brown B., et al. Capzimin is a potent and specific inhibitor of proteasome isopeptidase Rpn11. Nat. Chem. Biol. 2017;13:486–493. doi: 10.1038/nchembio.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noda C., Tanahashi N., Shimbara N., Hendil K.B., Tanaka K. Tissue distribution of constitutive proteasomes, immunoproteasomes, and PA28 in rats. Biochem. Biophys. Res. Commun. 2000;277:348–354. doi: 10.1006/bbrc.2000.3676. [DOI] [PubMed] [Google Scholar]
- 42.Ma C.P., Slaughter C.A., DeMartino G.N. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J. Biol. Chem. 1992;267:10515–10523. [PubMed] [Google Scholar]
- 43.Dubiel W., Pratt G., Ferrell K., Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J. Biol. Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- 44.Knowlton J.R., Johnston S.C., Whitby F.G., Realini C., Zhang Z., Rechsteiner M., Hill C.P. Structure of the proteasome activator REGalpha (PA28alpha) Nature. 1997;390:639–643. doi: 10.1038/37670. [DOI] [PubMed] [Google Scholar]
- 45.Realini C., Jensen C.C., Zhang Z., Johnston S.C., Knowlton J.R., Hill C.P., Rechsteiner M. Characterization of recombinant REGalpha, REGbeta, and REGgamma proteasome activators. J. Biol. Chem. 1997;272:25483–25492. doi: 10.1074/jbc.272.41.25483. [DOI] [PubMed] [Google Scholar]
- 46.Sijts A., Sun Y., Janek K., Kral S., Paschen A., Schadendorf D., Kloetzel P.M. The role of the proteasome activator PA28 in MHC class I antigen processing. Mol. Immunol. 2002;39:165–169. doi: 10.1016/S0161-5890(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 47.Groettrup M., Soza A., Eggers M., Kuehn L., Dick T.P., Schild H., Rammensee H.G., Koszinowski U.H., Kloetzel P.M. A role for the proteasome regulator PA28alpha in antigen presentation. Nature. 1996;381:166–168. doi: 10.1038/381166a0. [DOI] [PubMed] [Google Scholar]
- 48.Li X., Amazit L., Long W., Lonard D.M., Monaco J.J., O’Malley B.W. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol. Cell. 2007;26:831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Chen X., Barton L.F., Chi Y., Clurman B.E., Roberts J.M. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol. Cell. 2007;26:843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadre-Bazzaz K., Whitby F.G., Robinson H., Formosa T., Hill C.P. Structure of a Blm10 complex reveals common mechanisms for proteasome binding and gate opening. Mol. Cell. 2010;37:728–735. doi: 10.1016/j.molcel.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fehlker M., Wendler P., Lehmann A., Enenkel C. Blm3 is part of nascent proteasomes and is involved in a late stage of nuclear proteasome assembly. EMBO Rep. 2003;4:959–963. doi: 10.1038/sj.embor.embor938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doherty K., Pramanik A., Pride L., Lukose J., Moore C.W. Expression of the expanded YFL007w ORF and assignment of the gene name BLM10. Yeast. 2004;21:1021–1023. doi: 10.1002/yea.1146. [DOI] [PubMed] [Google Scholar]
- 53.Ustrell V., Hoffman L., Pratt G., Rechsteiner M. PA200, a nuclear proteasome activator involved in DNA repair. EMBO J. 2002;21:3516–3525. doi: 10.1093/emboj/cdf333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dange T., Smith D., Noy T., Rommel P.C., Jurzitza L., Cordero R.J., Legendre A., Finley D., Goldberg A.L., Schmidt M. Blm10 protein promotes proteasomal substrate turnover by an active gating mechanism. J. Biol. Chem. 2011;286:42830–42839. doi: 10.1074/jbc.M111.300178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fabre B., Lambour T., Garrigues L., Ducoux-Petit M., Amalric F., Monsarrat B., Burlet-Schiltz O., Bousquet-Dubouch M.P. Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J. Proteome Res. 2014;13:3027–3037. doi: 10.1021/pr500193k. [DOI] [PubMed] [Google Scholar]
- 56.Welk V., Coux O., Kleene V., Abeza C., Trumbach D., Eickelberg O., Meiners S. Inhibition of Proteasome Activity Induces Formation of Alternative Proteasome Complexes. J. Biol. Chem. 2016;291:13147–13159. doi: 10.1074/jbc.M116.717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toste Rêgo A., da Fonseca P.C.A. Characterization of Fully Recombinant Human 20S and 20S-PA200 Proteasome Complexes. Mol. Cell. 2019;76:138–147. doi: 10.1016/j.molcel.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopp F., Dahlmann B., Kuehn L. Reconstitution of hybrid proteasomes from purified PA700-20 S complexes and PA28alphabeta activator: Ultrastructure and peptidase activities. J. Mol. Biol. 2001;313:465–471. doi: 10.1006/jmbi.2001.5063. [DOI] [PubMed] [Google Scholar]
- 59.Dahlmann B. Mammalian proteasome subtypes: Their diversity in structure and function. Arch. Biochem. Biophys. 2016;591:132–140. doi: 10.1016/j.abb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Cascio P., Call M., Petre B.M., Walz T., Goldberg A.L. Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J. 2002;21:2636–2645. doi: 10.1093/emboj/21.11.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baugh J.M., Viktorova E.G., Pilipenko E.V. Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J. Mol. Biol. 2009;386:814–827. doi: 10.1016/j.jmb.2008.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erales J., Coffino P. Ubiquitin-independent proteasomal degradation. Biochim. Biophys. Acta. 2014;1843:216–221. doi: 10.1016/j.bbamcr.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M., Pickart C.M., Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22:1488–1496. doi: 10.1093/emboj/cdg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakami Y., Matsufuji S., Kameji T., Hayashi S., Igarashi K., Tamura T., Tanaka K., Ichihara A. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- 65.Ferrington D.A., Gregerson D.S. Immunoproteasomes: Structure, function, and antigen presentation. Prog. Mol. Biol. Transl. Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura H., Caturegli P., Takahashi M., Suzuki K. New Insights into the Function of the Immunoproteasome in Immune and Nonimmune Cells. J. Immunol. Res. 2015 doi: 10.1155/2015/541984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rock K.L., York I.A., Saric T., Goldberg A.L. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 2002;80:1–70. doi: 10.1016/s0065-2776(02)80012-8. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka K. Role of proteasomes modified by interferon-gamma in antigen processing. J. Leukoc. Biol. 1994;56:571–575. doi: 10.1002/jlb.56.5.571. [DOI] [PubMed] [Google Scholar]
- 69.Van Kaer L., Ashton-Rickardt P.G., Eichelberger M., Gaczynska M., Nagashima K., Rock K.L., Goldberg A.L., Doherty P.C., Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 70.Gaczynska M., Rock K.L., Goldberg A.L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- 71.Eskandari S.K., Seelen M.A.J., Lin G., Azzi J.R. The immunoproteasome: An old player with a novel and emerging role in alloimmunity. Am. J. Transpl. 2017;17:3033–3039. doi: 10.1111/ajt.14435. [DOI] [PubMed] [Google Scholar]
- 72.Murata S., Takahama Y., Kasahara M., Tanaka K. The immunoproteasome and thymoproteasome: Functions, evolution and human disease. Nat. Immunol. 2018;19:923–931. doi: 10.1038/s41590-018-0186-z. [DOI] [PubMed] [Google Scholar]
- 73.Heink S., Ludwig D., Kloetzel P.-M., Krüger E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. USA. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffin T.A., Nandi D., Cruz M., Fehling H.J., Kaer L.V., Monaco J.J., Colbert R.A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Groll M., Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: A structural approach. Biochim. Biophys. Acta. 2004;1695:33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 76.Kuhn D.J., Orlowski R.Z., Bjorklund C.C. Second generation proteasome inhibitors: Carfilzomib and immunoproteasome-specific inhibitors (IPSIs) Curr. Cancer Drug Targets. 2011;11:285–295. doi: 10.2174/156800911794519725. [DOI] [PubMed] [Google Scholar]
- 77.Basler M., Mundt S., Bitzer A., Schmidt C., Groettrup M. The immunoproteasome: A novel drug target for autoimmune diseases. Clin. Exp. Rheumatol. 2015;33:74–79. [PubMed] [Google Scholar]
- 78.Santos R.L.A., Bai L., Singh P.K., Murakami N., Fan H., Zhan W., Zhu Y., Jiang X., Zhang K., Assker J.P., et al. Structure of human immunoproteasome with a reversible and noncompetitive inhibitor that selectively inhibits activated lymphocytes. Nat. Commun. 2017 doi: 10.1038/s41467-017-01760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huber E.M., Basler M., Schwab R., Heinemeyer W., Kirk C.J., Groettrup M., Groll M. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell. 2012;148:727–738. doi: 10.1016/j.cell.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 80.Murata S., Sasaki K., Kishimoto T., Niwa S., Hayashi H., Takahama Y., Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 81.Sasaki K., Takada K., Ohte Y., Kondo H., Sorimachi H., Tanaka K., Takahama Y., Murata S. Thymoproteasomes produce unique peptide motifs for positive selection of CD8(+) T cells. Nat. Commun. 2015 doi: 10.1038/ncomms8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kniepert A., Groettrup M. The unique functions of tissue-specific proteasomes. Trends Biochem. Sci. 2014;39:17–24. doi: 10.1016/j.tibs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Khor B., Bredemeyer A.L., Huang C.-Y., Turnbull I.R., Evans R., Maggi L.B., White J.M., Walker L.M., Carnes K., Hess R.A., et al. Proteasome activator PA200 is required for normal spermatogenesis. Mol. Cell Biol. 2006;26:2999–3007. doi: 10.1128/MCB.26.8.2999-3007.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian M.-X., Pang Y., Liu C.H., Haratake K., Du B.-Y., Ji D.-Y., Wang G.-F., Zhu Q.-Q., Song W., Yu Y., et al. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 2013;153:1012–1024. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.BioRender.com, version 2017; M5V 2B7, Created Graphical Illustrations, BioRender 2017–2020. BioRender Customer Service; Toronto, ON, Canada: 2017. [Google Scholar]
- 86.Lee D.H., Goldberg A.L. Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/S0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 87.Wiest D.L., Burkhardt J.K., Hester S., Hortsch M., Meyer D.I., Argon Y. Membrane biogenesis during B cell differentiation: Most endoplasmic reticulum proteins are expressed coordinately. J. Cell Biol. 1990;110:1501–1511. doi: 10.1083/jcb.110.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meister S., Schubert U., Neubert K., Herrmann K., Burger R., Gramatzki M., Hahn S., Schreiber S., Wilhelm S., Herrmann M., et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 89.Deshaies R.J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014 doi: 10.1186/s12915-014-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith M.H., Ploegh H.L., Weissman J.S. Road to ruin: Targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sosič I., Gobec M., Brus B., Knez D., Živec M., Konc J., Lešnik S., Ogrizek M., Obreza A., Žigon D., et al. Nonpeptidic Selective Inhibitors of the Chymotrypsin-Like (β5 i) Subunit of the Immunoproteasome. Angew. Chem. Int. Ed Engl. 2016;55:5745–5748. doi: 10.1002/anie.201600190. [DOI] [PubMed] [Google Scholar]
- 92.Cui H., Baur R., Le Chapelain C., Dubiella C., Heinemeyer W., Huber E.M., Groll M. Structural Elucidation of a Nonpeptidic Inhibitor Specific for the Human Immunoproteasome. Chembiochem Eur. J. Chem. Biol. 2017;18:523–526. doi: 10.1002/cbic.201700021. [DOI] [PubMed] [Google Scholar]
- 93.Henninot A., Collins J.C., Nuss J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- 94.Lau J.L., Dunn M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 95.Richardson P.G., Sonneveld P., Schuster M.W., Irwin D., Stadtmauer E.A., Facon T., Harousseau J.-L., Ben-Yehuda D., Lonial S., Goldschmidt H., et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 96.Kane R.C., Farrell A.T., Sridhara R., Pazdur R. United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 97.Sonneveld P., Schmidt-Wolf I.G.H., van der Holt B., El Jarari L., Bertsch U., Salwender H., Zweegman S., Vellenga E., Broyl A., Blau I.W., et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: Results of the randomized phase III HOVON-65/GMMG-HD4 trial. J. Clin. Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 98.Demo S.D., Kirk C.J., Aujay M.A., Buchholz T.J., Dajee M., Ho M.N., Jiang J., Laidig G.J., Lewis E.R., Parlati F., et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 99.Kane R.C., Bross P.F., Farrell A.T., Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncology. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 100.Aghajanian C., Soignet S., Dizon D.S., Pien C.S., Adams J., Elliott P.J., Sabbatini P., Miller V., Hensley M.L., Pezzulli S., et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin. Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 101.Annunziata C.M., Davis R.E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keats J.J., Fonseca R., Chesi M., Schop R., Baker A., Chng W.-J., Van Wier S., Tiedemann R., Shi C.-X., Sebag M., et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hideshima T., Richardson P., Chauhan D., Palombella V.J., Elliott P.J., Adams J., Anderson K.C. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 104.Hideshima T., Chauhan D., Richardson P., Mitsiades C., Mitsiades N., Hayashi T., Munshi N., Dang L., Castro A., Palombella V., et al. NF-kappa B as a therapeutic target in multiple myeloma. J. Biol. Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 105.Kupperman E., Lee E.C., Cao Y., Bannerman B., Fitzgerald M., Berger A., Yu J., Yang Y., Hales P., Bruzzese F., et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 106.Zhou H.-J., Aujay M.A., Bennett M.K., Dajee M., Demo S.D., Fang Y., Ho M.N., Jiang J., Kirk C.J., Laidig G.J., et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J. Med. Chem. 2009;52:3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 107.Oerlemans R., Franke N.E., Assaraf Y.G., Cloos J., van Zantwijk I., Berkers C.R., Scheffer G.L., Debipersad K., Vojtekova K., Lemos C., et al. Molecular basis of bortezomib resistance: Proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112:2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- 108.Lü S., Yang J., Song X., Gong S., Zhou H., Guo L., Song N., Bao X., Chen P., Wang J. Point mutation of the proteasome beta5 subunit gene is an important mechanism of bortezomib resistance in bortezomib-selected variants of Jurkat T cell lymphoblastic lymphoma/leukemia line. J. Pharm. Exp. Ther. 2008;326:423–431. doi: 10.1124/jpet.108.138131. [DOI] [PubMed] [Google Scholar]
- 109.Rückrich T., Kraus M., Gogel J., Beck A., Ovaa H., Verdoes M., Overkleeft H.S., Kalbacher H., Driessen C. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia. 2009;23:1098–1105. doi: 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- 110.Kuhn D.J., Berkova Z., Jones R.J., Woessner R., Bjorklund C.C., Ma W., Davis R.E., Lin P., Wang H., Madden T.L., et al. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood. 2012;120:3260–3270. doi: 10.1182/blood-2011-10-386789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitsiades N., Mitsiades C.S., Poulaki V., Chauhan D., Fanourakis G., Gu X., Bailey C., Joseph M., Libermann T.A., Treon S.P., et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc. Natl. Acad. Sci. USA. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niewerth D., Jansen G., Assaraf Y.G., Zweegman S., Kaspers G.J.L., Cloos J. Molecular basis of resistance to proteasome inhibitors in hematological malignancies. Drug Resist. Updat. Rev. Comment Antimicrob. Anticancer Chemother. 2015;18:18–35. doi: 10.1016/j.drup.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 113.Richardson P.G., Weller E., Lonial S., Jakubowiak A.J., Jagannath S., Raje N.S., Avigan D.E., Xie W., Ghobrial I.M., Schlossman R.L., et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang Z., Wu Y., Zhou X., Xu J., Zhu W., Shu Y., Liu P. Efficacy of therapy with bortezomib in solid tumors: A review based on 32 clinical trials. Future Oncol. Lond. Engl. 2014;10:1795–1807. doi: 10.2217/fon.14.30. [DOI] [PubMed] [Google Scholar]
- 115.Kim K.B., Crews C.M. From epoxomicin to carfilzomib: Chemistry, biology, and medical outcomes. Nat. Prod. Rep. 2013;30:600–604. doi: 10.1039/c3np20126k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harshbarger W., Miller C., Diedrich C., Sacchettini J. Crystal structure of the human 20S proteasome in complex with carfilzomib. Struct. Lond. Engl. 1993. 2015;23:418–424. doi: 10.1016/j.str.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 117.Dimopoulos M.A., Moreau P., Palumbo A., Joshua D., Pour L., Hájek R., Facon T., Ludwig H., Oriol A., Goldschmidt H., et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 118.Dimopoulos M.A., Goldschmidt H., Niesvizky R., Joshua D., Chng W.-J., Oriol A., Orlowski R.Z., Ludwig H., Facon T., Hajek R., et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–1337. doi: 10.1016/S1470-2045(17)30578-8. [DOI] [PubMed] [Google Scholar]
- 119.Landgren O., Sonneveld P., Jakubowiak A., Mohty M., Iskander K.S., Mezzi K., Siegel D.S. Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma. Leukemia. 2019;33:2127–2143. doi: 10.1038/s41375-019-0517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waxman A.J., Clasen S., Hwang W.-T., Garfall A., Vogl D.T., Carver J., O’Quinn R., Cohen A.D., Stadtmauer E.A., Ky B., et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2017.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jakubowiak A.J., DeCara J.M., Mezzi K. Cardiovascular events during carfilzomib therapy for relapsed myeloma: Practical management aspects from two case studies. Hematol. Amst. Neth. 2017;22:585–591. doi: 10.1080/10245332.2017.1328165. [DOI] [PubMed] [Google Scholar]
- 122.Lendvai N., Tsakos I., Devlin S.M., Schaffer W.L., Hassoun H., Lesokhin A.M., Landau H., Korde N., Mailankody S., Smith E., et al. Predictive biomarkers and practical considerations in the management of carfilzomib-associated cardiotoxicity. Leuk. Lymphoma. 2018;59:1981–1985. doi: 10.1080/10428194.2017.1403020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Papadopoulos K.P., Burris H.A., Gordon M., Lee P., Sausville E.A., Rosen P.J., Patnaik A., Cutler R.E., Wang Z., Lee S., et al. A phase I/II study of carfilzomib 2-10-min infusion in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013;72:861–868. doi: 10.1007/s00280-013-2267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shirley M. Ixazomib: First Global Approval. Drugs. 2016;76:405–411. doi: 10.1007/s40265-016-0548-5. [DOI] [PubMed] [Google Scholar]
- 125.Dick L.R., Fleming P.E. Building on bortezomib: Second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov. Today. 2010;15:243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 126.Spencer A., Harrison S., Zonder J., Badros A., Laubach J., Bergin K., Khot A., Zimmerman T., Chauhan D., Levin N., et al. A phase 1 clinical trial evaluating marizomib, pomalidomide and low-dose dexamethasone in relapsed and refractory multiple myeloma (NPI-0052-107): Final study results. Br. J. Haematol. 2018;180:41–51. doi: 10.1111/bjh.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruiz S., Krupnik Y., Keating M., Chandra J., Palladino M., McConkey D. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Mol. Cancer Ther. 2006;5:1836–1843. doi: 10.1158/1535-7163.MCT-06-0066. [DOI] [PubMed] [Google Scholar]
- 128.Groll M., Huber R., Potts B.C.M. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 2006;128:5136–5141. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- 129.Potts B.C., Albitar M.X., Anderson K.C., Baritaki S., Berkers C., Bonavida B., Chandra J., Chauhan D., Cusack J.C., Fenical W., et al. Marizomib, a proteasome inhibitor for all seasons: Preclinical profile and a framework for clinical trials. Curr. Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Macherla V.R., Mitchell S.S., Manam R.R., Reed K.A., Chao T.-H., Nicholson B., Deyanat-Yazdi G., Mai B., Jensen P.R., Fenical W.F., et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J. Med. Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- 131.Harrison S.J., Mainwaring P., Price T., Millward M.J., Padrik P., Underhill C.R., Cannell P.K., Reich S.D., Trikha M., Spencer A. Phase I Clinical Trial of Marizomib (NPI-0052) in Patients with Advanced Malignancies Including Multiple Myeloma: Study NPI-0052-102 Final Results. Clin. Cancer Res. 2016;22:4559–4566. doi: 10.1158/1078-0432.CCR-15-2616. [DOI] [PubMed] [Google Scholar]
- 132.Di K., Lloyd G.K., Abraham V., MacLaren A., Burrows F.J., Desjardins A., Trikha M., Bota D.A. Marizomib activity as a single agent in malignant gliomas: Ability to cross the blood-brain barrier. Neuro-Oncol. 2016;18:840–848. doi: 10.1093/neuonc/nov299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manton C.A., Johnson B., Singh M., Bailey C.P., Bouchier-Hayes L., Chandra J. Induction of cell death by the novel proteasome inhibitor marizomib in glioblastoma in vitro and in vivo. Sci. Rep. 2016 doi: 10.1038/srep18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weller M., Le Rhun E., Preusser M., Tonn J.-C., Roth P. How we treat glioblastoma. Esmo Open. 2019 doi: 10.1136/esmoopen-2019-000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chauhan D., Singh A.V., Aujay M., Kirk C.J., Bandi M., Ciccarelli B., Raje N., Richardson P., Anderson K.C. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 2010;116:4906–4915. doi: 10.1182/blood-2010-04-276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghobrial I.M., Vij R., Siegel D., Badros A., Kaufman J., Raje N., Jakubowiak A., Savona M.R., Obreja M., Berdeja J.G. A Phase Ib/II Study of Oprozomib in Patients with Advanced Multiple Myeloma and Waldenström Macroglobulinemia. Clin. Cancer Res. 2019;25:4907–4916. doi: 10.1158/1078-0432.CCR-18-3728. [DOI] [PubMed] [Google Scholar]
- 137.Hari P., Matous J.V., Voorhees P.M., Shain K.H., Obreja M., Frye J., Fujii H., Jakubowiak A.J., Rossi D., Sonneveld P. Oprozomib in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2019 doi: 10.1038/s41408-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hari P., Paba-Prada C.E., Voorhees P.M., Frye J., Chang Y.-L., Moreau P., Zonder J., Boccia R., Shain K.H. Efficacy and safety results from a phase 1b/2, multicenter, open-label study of oprozomib and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Leuk. Res. 2019 doi: 10.1016/j.leukres.2019.106172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shah J., Usmani S., Stadtmauer E.A., Rifkin R.M., Berenson J.R., Berdeja J.G., Lyons R.M., Klippel Z., Chang Y.-L., Niesvizky R. Oprozomib, pomalidomide, and Dexamethasone in Patients with Relapsed and/or Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019;19:570–578. doi: 10.1016/j.clml.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 140.Infante J.R., Mendelson D.S., Burris H.A., Bendell J.C., Tolcher A.W., Gordon M.S., Gillenwater H.H., Arastu-Kapur S., Wong H.L., Papadopoulos K.P. A first-in-human dose-escalation study of the oral proteasome inhibitor oprozomib in patients with advanced solid tumors. Investig. New Drugs. 2016;34:216–224. doi: 10.1007/s10637-016-0327-x. [DOI] [PubMed] [Google Scholar]
- 141.Johnson H.W.B., Lowe E., Anderl J.L., Fan A., Muchamuel T., Bowers S., Moebius D.C., Kirk C., McMinn D.L. Required Immunoproteasome Subunit Inhibition Profile for Anti-Inflammatory Efficacy and Clinical Candidate KZR-616 ((2 S,3 R)- N-((S)-3-(Cyclopent-1-en-1-yl)-1-((R)-2-methyloxiran-2-yl)-1-oxopropan-2-yl)-3-hydroxy-3-(4-methoxyphenyl)-2-((S)-2-(2-morpholinoacetamido)propanamido)propenamide) J. Med. Chem. 2018;61:11127–11143. doi: 10.1021/acs.jmedchem.8b01201. [DOI] [PubMed] [Google Scholar]
- 142.Muchamuel T., Basler M., Aujay M.A., Suzuki E., Kalim K.W., Lauer C., Sylvain C., Ring E.R., Shields J., Jiang J., et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 143.Liu H., Wan C., Ding Y., Han R., He Y., Xiao J., Hao J. PR-957, a selective inhibitor of immunoproteasome subunit low-MW polypeptide 7, attenuates experimental autoimmune neuritis by suppressing Th17-cell differentiation and regulating cytokine production. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017;31:1756–1766. doi: 10.1096/fj.201601147R. [DOI] [PubMed] [Google Scholar]
- 144.von Brzezinski L., Säring P., Landgraf P., Cammann C., Seifert U., Dieterich D.C. Low Neurotoxicity of ONX-0914 Supports the Idea of Specific Immunoproteasome Inhibition as a Side-Effect-Limiting, Therapeutic Strategy. Eur. J. Microbiol. Immunol. 2017;7:234–245. doi: 10.1556/1886.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanderson M., Busch M., Esdar C., Friese-Hamim M., Krier M., Ma J., Musil D., Rohdich F., Sloot W., Walter G., et al. Proceedings of the Molecular and Cellular Biology/Genetics. American Association for Cancer Research; Atlanta, GA, USA: 2019. Abstract DDT02-01: First-time disclosure of M3258: A selective inhibitor of the immunoproteasome subunit LMP7 with potential for improved therapeutic utility in multiple myeloma compared to pan-proteasome inhibitors; p. DDT02-01. [Google Scholar]
- 146.Yao T., Cohen R.E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 147.Altmann E., Erbel P., Renatus M., Schaefer M., Schlierf A., Druet A., Kieffer L., Sorge M., Pfister K., Hassiepen U., et al. Azaindoles as Zinc-Binding Small-Molecule Inhibitors of the JAMM Protease CSN5. Angew. Chem. Int. Ed. Engl. 2017;56:1294–1297. doi: 10.1002/anie.201608672. [DOI] [PubMed] [Google Scholar]
- 148.Tran H.J.T.T., Allen M.D., Löwe J., Bycroft M. Structure of the Jab1/MPN domain and its implications for proteasome function. Biochemistry. 2003;42:11460–11465. doi: 10.1021/bi035033g. [DOI] [PubMed] [Google Scholar]
- 149.Gallery M., Blank J.L., Lin Y., Gutierrez J.A., Pulido J.C., Rappoli D., Badola S., Rolfe M., Macbeth K.J. The JAMM motif of human deubiquitinase Poh1 is essential for cell viability. Mol. Cancer Ther. 2007;6:262–268. doi: 10.1158/1535-7163.MCT-06-0542. [DOI] [PubMed] [Google Scholar]
- 150.Perez C., Li J., Parlati F., Rouffet M., Ma Y., Mackinnon A.L., Chou T.-F., Deshaies R.J., Cohen S.M. Discovery of an Inhibitor of the Proteasome Subunit Rpn11. J. Med. Chem. 2017;60:1343–1361. doi: 10.1021/acs.jmedchem.6b01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Verma R., Peters N.R., D’Onofrio M., Tochtrop G.P., Sakamoto K.M., Varadan R., Zhang M., Coffino P., Fushman D., Deshaies R.J., et al. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science. 2004;306:117–120. doi: 10.1126/science.1100946. [DOI] [PubMed] [Google Scholar]
- 152.Nakasone M.A., Lewis T.A., Walker O., Thakur A., Mansour W., Castañeda C.A., Goeckeler-Fried J.L., Parlati F., Chou T.-F., Hayat O., et al. Structural Basis for the Inhibitory Effects of Ubistatins in the Ubiquitin-Proteasome Pathway. Structure. 2017;25:1839–1855. doi: 10.1016/j.str.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Randles L., Anchoori R.K., Roden R.B.S., Walters K.J. The Proteasome Ubiquitin Receptor hRpn13 and Its Interacting Deubiquitinating Enzyme Uch37 Are Required for Proper Cell Cycle Progression. J. Biol. Chem. 2016;291:8773–8783. doi: 10.1074/jbc.M115.694588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen W., Hu X.-T., Shi Q.-L., Zhang F.-B., He C. Knockdown of the novel proteasome subunit Adrm1 located on the 20q13 amplicon inhibits colorectal cancer cell migration, survival and tumorigenicity. Oncol. Rep. 2009;21:531–537. [PubMed] [Google Scholar]
- 155.Fejzo M.S., Dering J., Ginther C., Anderson L., Ramos L., Walsh C., Karlan B., Slamon D.J. Comprehensive analysis of 20q13 genes in ovarian cancer identifies ADRM1 as amplification target. Genes Chromosomes Cancer. 2008;47:873–883. doi: 10.1002/gcc.20592. [DOI] [PubMed] [Google Scholar]
- 156.Jang S.H., Park J.W., Kim H.R., Seong J.K., Kim H.K. ADRM1 gene amplification is a candidate driver for metastatic gastric cancers. Clin. Exp. Metastasis. 2014;31:727–733. doi: 10.1007/s10585-014-9663-4. [DOI] [PubMed] [Google Scholar]
- 157.Song Y., Ray A., Li S., Das D.S., Tai Y.T., Carrasco R.D., Chauhan D., Anderson K.C. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia. 2016;30:1877–1886. doi: 10.1038/leu.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anchoori R.K., Karanam B., Peng S., Wang J.W., Jiang R., Tanno T., Orlowski R.Z., Matsui W., Zhao M., Rudek M.A., et al. A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer. Cancer Cell. 2013;24:791–805. doi: 10.1016/j.ccr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu X., Nowicka U., Sridharan V., Liu F., Randles L., Hymel D., Dyba M., Tarasov S.G., Tarasova N.I., Zhao X.Z., et al. Structure of the Rpn13-Rpn2 complex provides insights for Rpn13 and Uch37 as anticancer targets. Nat. Commun. 2017 doi: 10.1038/ncomms15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anchoori R.K., Jiang R., Peng S., Soong R.-S., Algethami A., Rudek M.A., Anders N., Hung C.-F., Chen X., Lu X., et al. Covalent Rpn13-Binding Inhibitors for the Treatment of Ovarian Cancer. ACS Omega. 2018;3:11917–11929. doi: 10.1021/acsomega.8b01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trader D.J., Simanski S., Kodadek T. A reversible and highly selective inhibitor of the proteasomal ubiquitin receptor rpn13 is toxic to multiple myeloma cells. J. Am. Chem. Soc. 2015;137:6312–6319. doi: 10.1021/jacs.5b02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baell J., Walters M.A. Chemistry: Chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- 163.Song Y., Park P.M.C., Wu L., Ray A., Picaud S., Li D., Wimalasena V.K., Du T., Filippakopoulos P., Anderson K.C., et al. Development and preclinical validation of a novel covalent ubiquitin receptor Rpn13 degrader in multiple myeloma. Leukemia. 2019;33:2685–2694. doi: 10.1038/s41375-019-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paiva S.-L., Crews C.M. Targeted protein degradation: Elements of PROTAC design. Curr. Opin. Chem. Biol. 2019;50:111–119. doi: 10.1016/j.cbpa.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pettersson M., Crews C.M. PROteolysis TArgeting Chimeras (PROTACs) - Past, present and future. Drug Discov. Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schapira M., Calabrese M.F., Bullock A.N., Crews C.M. Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 2019;18:949–963. doi: 10.1038/s41573-019-0047-y. [DOI] [PubMed] [Google Scholar]
- 167.Lim H.-S., Cai D., Archer C.T., Kodadek T. Periodate-triggered cross-linking reveals Sug2/Rpt4 as the molecular target of a peptoid inhibitor of the 19S proteasome regulatory particle. J. Am. Chem. Soc. 2007;129:12936–12937. doi: 10.1021/ja075469+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lim H.-S., Archer C.T., Kodadek T. Identification of a peptoid inhibitor of the proteasome 19S regulatory particle. J. Am. Chem. Soc. 2007;129:7750–7751. doi: 10.1021/ja072027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsvetkov P., Sokol E., Jin D., Brune Z., Thiru P., Ghandi M., Garraway L.A., Gupta P.B., Santagata S., Whitesell L., et al. Suppression of 19S proteasome subunits marks emergence of an altered cell state in diverse cancers. Proc. Natl. Acad. Sci. USA. 2017;114:382–387. doi: 10.1073/pnas.1619067114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Acosta-Alvear D., Cho M.Y., Wild T., Buchholz T.J., Lerner A.G., Simakova O., Hahn J., Korde N., Landgren O., Maric I., et al. Paradoxical resistance of multiple myeloma to proteasome inhibitors by decreased levels of 19S proteasomal subunits. eLife. 2015 doi: 10.7554/eLife.08153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsvetkov P., Mendillo M.L., Zhao J., Carette J.E., Merrill P.H., Cikes D., Varadarajan M., van Diemen F.R., Penninger J.M., Goldberg A.L., et al. Compromising the 19S proteasome complex protects cells from reduced flux through the proteasome. eLife. 2015 doi: 10.7554/eLife.08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wallington-Beddoe C.T., Sobieraj-Teague M., Kuss B.J., Pitson S.M. Resistance to proteasome inhibitors and other targeted therapies in myeloma. Br. J. Haematol. 2018;182:11–28. doi: 10.1111/bjh.15210. [DOI] [PubMed] [Google Scholar]
- 173.Pritchard J.R., Lauffenburger D.A., Hemann M.T. Understanding resistance to combination chemotherapy. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2012;15:249–257. doi: 10.1016/j.drup.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Corcoran R.B., André T., Atreya C.E., Schellens J.H.M., Yoshino T., Bendell J.C., Hollebecque A., McRee A.J., Siena S., Middleton G., et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Corcoran R.B., Ebi H., Turke A.B., Coffee E.M., Nishino M., Cogdill A.P., Brown R.D., Della Pelle P., Dias-Santagata D., Hung K.E., et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prahallad A., Sun C., Huang S., Di Nicolantonio F., Salazar R., Zecchin D., Beijersbergen R.L., Bardelli A., Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 177.Steffen J., Seeger M., Koch A., Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 178.Radhakrishnan S.K., Lee C.S., Young P., Beskow A., Chan J.Y., Deshaies R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Radhakrishnan S.K., den Besten W., Deshaies R.J. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife. 2014 doi: 10.7554/eLife.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koizumi S., Irie T., Hirayama S., Sakurai Y., Yashiroda H., Naguro I., Ichijo H., Hamazaki J., Murata S. The aspartyl protease DDI2 activates Nrf1 to compensate for proteasome dysfunction. eLife. 2016 doi: 10.7554/eLife.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lehrbach N.J., Ruvkun G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife. 2016:5. doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tomlin F.M., Gerling-Driessen U.I.M., Liu Y.-C., Flynn R.A., Vangala J.R., Lentz C.S., Clauder-Muenster S., Jakob P., Mueller W.F., Ordoñez-Rueda D., et al. Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity. ACS Cent. Sci. 2017;3:1143–1155. doi: 10.1021/acscentsci.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lehrbach N.J., Breen P.C., Ruvkun G. Protein Sequence Editing of SKN-1A/Nrf1 by Peptide:N-Glycanase Controls Proteasome Gene Expression. Cell. 2019;177:737–750. doi: 10.1016/j.cell.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suzuki T., Huang C., Fujihira H. The cytoplasmic peptide:N-glycanase (NGLY1) - Structure, expression and cellular functions. Gene. 2016;577:1–7. doi: 10.1016/j.gene.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tsvetkov P., Detappe A., Cai K., Keys H.R., Brune Z., Ying W., Thiru P., Reidy M., Kugener G., Rossen J., et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019;15:681–689. doi: 10.1038/s41589-019-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xia D., Tang W.K., Ye Y. Structure and function of the AAA+ ATPase p97/Cdc48p. Gene. 2016;583:64–77. doi: 10.1016/j.gene.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ye Y., Tang W.K., Zhang T., Xia D. A Mighty “Protein Extractor” of the Cell: Structure and Function of the p97/CDC48 ATPase. Front. Mol. Biosci. 2017 doi: 10.3389/fmolb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van den Boom J., Meyer H. VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol. Cell. 2018;69:182–194. doi: 10.1016/j.molcel.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 189.Ye Y., Meyer H.H., Rapoport T.A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 190.Meyer H., Bug M., Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 191.Bodnar N.O., Kim K.H., Ji Z., Wales T.E., Svetlov V., Nudler E., Engen J.R., Walz T., Rapoport T.A. Structure of the Cdc48 ATPase with its ubiquitin-binding cofactor Ufd1-Npl4. Nat. Struct. Mol. Biol. 2018;25:616–622. doi: 10.1038/s41594-018-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bodnar N., Rapoport T. Toward an understanding of the Cdc48/p97 ATPase. F1000Research. 2017 doi: 10.12688/f1000research.11683.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Banerjee S., Bartesaghi A., Merk A., Rao P., Bulfer S.L., Yan Y., Green N., Mroczkowski B., Neitz R.J., Wipf P., et al. 2.3 Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Science. 2016;351:871–875. doi: 10.1126/science.aad7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bruderer R.M., Brasseur C., Meyer H.H. The AAA ATPase p97/VCP interacts with its alternative co-factors, Ufd1-Npl4 and p47, through a common bipartite binding mechanism. J. Biol. Chem. 2004;279:49609–49616. doi: 10.1074/jbc.M408695200. [DOI] [PubMed] [Google Scholar]
- 195.Le Moigne R., Aftab B.T., Djakovic S., Dhimolea E., Valle E., Murnane M., King E.M., Soriano F., Menon M.-K., Wu Z.Y., et al. The p97 Inhibitor CB-5083 Is a Unique Disrupter of Protein Homeostasis in Models of Multiple Myeloma. Mol. Cancer Ther. 2017;16:2375–2386. doi: 10.1158/1535-7163.MCT-17-0233. [DOI] [PubMed] [Google Scholar]
- 196.Watts G.D.J., Wymer J., Kovach M.J., Mehta S.G., Mumm S., Darvish D., Pestronk A., Whyte M.P., Kimonis V.E. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 197.Tang W.K., Xia D. Mutations in the Human AAA+ Chaperone p97 and Related Diseases. Front. Mol. Biosci. 2016 doi: 10.3389/fmolb.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Blythe E.E., Olson K.C., Chau V., Deshaies R.J. Ubiquitin- and ATP-dependent unfoldase activity of P97/VCP•NPLOC4•UFD1L is enhanced by a mutation that causes multisystem proteinopathy. Proc. Natl. Acad. Sci. USA. 2017;114:4380–4388. doi: 10.1073/pnas.1706205114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blythe E.E., Gates S.N., Deshaies R.J., Martin A. Multisystem Proteinopathy Mutations in VCP/p97 Increase NPLOC4·UFD1L Binding and Substrate Processing. Structure. 2019;27:1820–1829. doi: 10.1016/j.str.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang Y., Kitagaki J., Dai R.-M., Tsai Y.C., Lorick K.L., Ludwig R.L., Pierre S.A., Jensen J.P., Davydov I.V., Oberoi P., et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 201.Hyer M.L., Milhollen M.A., Ciavarri J., Fleming P., Traore T., Sappal D., Huck J., Shi J., Gavin J., Brownell J., et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 2018;24:186–193. doi: 10.1038/nm.4474. [DOI] [PubMed] [Google Scholar]
- 202.Landré V., Rotblat B., Melino S., Bernassola F., Melino G. Screening for E3-ubiquitin ligase inhibitors: Challenges and opportunities. Oncotarget. 2014;5:7988–8013. doi: 10.18632/oncotarget.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.O’Donnell M.A., Legarda-Addison D., Skountzos P., Yeh W.C., Ting A.T. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr. Biol. CB. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.LaCasse E.C., Mahoney D.J., Cheung H.H., Plenchette S., Baird S., Korneluk R.G. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 205.Wang L., Du F., Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 206.Soucy T.A., Smith P.G., Milhollen M.A., Berger A.J., Gavin J.M., Adhikari S., Brownell J.E., Burke K.E., Cardin D.P., Critchley S., et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 207.Swords R.T., Coutre S., Maris M.B., Zeidner J.F., Foran J.M., Cruz J., Erba H.P., Berdeja J.G., Tam W., Vardhanabhuti S., et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018;131:1415–1424. doi: 10.1182/blood-2017-09-805895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarantopoulos J., Shapiro G.I., Cohen R.B., Clark J.W., Kauh J.S., Weiss G.J., Cleary J.M., Mahalingam D., Pickard M.D., Faessel H.M., et al. Phase I Study of the Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (TAK-924/MLN4924) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016;22:847–857. doi: 10.1158/1078-0432.CCR-15-1338. [DOI] [PubMed] [Google Scholar]
- 209.Kategaya L., Di Lello P., Rougé L., Pastor R., Clark K.R., Drummond J., Kleinheinz T., Lin E., Upton J.-P., Prakash S., et al. USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature. 2017;550:534–538. doi: 10.1038/nature24006. [DOI] [PubMed] [Google Scholar]
- 210.Turnbull A.P., Ioannidis S., Krajewski W.W., Pinto-Fernandez A., Heride C., Martin A.C.L., Tonkin L.M., Townsend E.C., Buker S.M., Lancia D.R., et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature. 2017;550:481–486. doi: 10.1038/nature24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lamberto I., Liu X., Seo H.-S., Schauer N.J., Iacob R.E., Hu W., Das D., Mikhailova T., Weisberg E.L., Engen J.R., et al. Structure-Guided Development of a Potent and Selective Non-covalent Active-Site Inhibitor of USP7. Cell Chem. Biol. 2017;24:1490–1500. doi: 10.1016/j.chembiol.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gavory G., O’Dowd C.R., Helm M.D., Flasz J., Arkoudis E., Dossang A., Hughes C., Cassidy E., McClelland K., Odrzywol E., et al. Discovery and characterization of highly potent and selective allosteric USP7 inhibitors. Nat. Chem. Biol. 2018;14:118–125. doi: 10.1038/nchembio.2528. [DOI] [PubMed] [Google Scholar]
- 213.Wang S., Song Y., Yan F., Liu D. Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors. Front. Med. 2016;10:383–388. doi: 10.1007/s11684-016-0488-1. [DOI] [PubMed] [Google Scholar]
- 214.Cross D.A.E., Ashton S.E., Ghiorghiu S., Eberlein C., Nebhan C.A., Spitzler P.J., Orme J.P., Finlay M.R.V., Ward R.A., Mellor M.J., et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hallenbeck K.K., Turner D.M., Renslo A.R., Arkin M.R. Targeting Non-Catalytic Cysteine Residues Through Structure-Guided Drug Discovery. Curr. Top. Med. Chem. 2017;17:4–15. doi: 10.2174/1568026616666160719163839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bond D.A., Woyach J.A. Targeting BTK in CLL: Beyond Ibrutinib. Curr. Hematol. Malig. Rep. 2019;14:197–205. doi: 10.1007/s11899-019-00512-0. [DOI] [PubMed] [Google Scholar]
- 217.Wu J., Zhang M., Liu D. Acalabrutinib (ACP-196): A selective second-generation BTK inhibitor. J. Hematol. Oncol. 2016 doi: 10.1186/s13045-016-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 219.Lee M.J., Lee B.-H., Hanna J., King R.W., Finley D. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol. Cell Proteom. MCP. 2011 doi: 10.1074/mcp.R110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee B.-H., Lee M.J., Park S., Oh D.-C., Elsasser S., Chen P.-C., Gartner C., Dimova N., Hanna J., Gygi S.P., et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang Y., Jiang Y., Ding S., Li J., Song N., Ren Y., Hong D., Wu C., Li B., Wang F., et al. Small molecule inhibitors reveal allosteric regulation of USP14 via steric blockade. Cell Res. 2018;28:1186–1194. doi: 10.1038/s41422-018-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.D’Arcy P., Brnjic S., Olofsson M.H., Fryknäs M., Lindsten K., De Cesare M., Perego P., Sadeghi B., Hassan M., Larsson R., et al. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat. Med. 2011;17:1636–1640. doi: 10.1038/nm.2536. [DOI] [PubMed] [Google Scholar]
- 223.Woodle E.S., Alloway R.R., Girnita A. Proteasome inhibitor treatment of antibody-mediated allograft rejection. Curr. Opin. Organ. Transpl. 2011;16:434–438. doi: 10.1097/MOT.0b013e328348c0e5. [DOI] [PubMed] [Google Scholar]
- 224.Ensor C.R., Yousem S.A., Marrari M., Morrell M.R., Mangiola M., Pilewski J.M., D’Cunha J., Wisniewski S.R., Venkataramanan R., Zeevi A., et al. Proteasome Inhibitor Carfilzomib-Based Therapy for Antibody-Mediated Rejection of the Pulmonary Allograft: Use and Short-Term Findings. Am. J. Transpl. Off. J. Am. Soc. Transpl. Am. Soc. Transpl. Surg. 2017;17:1380–1388. doi: 10.1111/ajt.14222. [DOI] [PubMed] [Google Scholar]
- 225.Valenzuela N.M., Reed E.F. Antibody-mediated rejection across solid organ transplants: Manifestations, mechanisms, and therapies. J. Clin. Investig. 2017;127:2492–2504. doi: 10.1172/JCI90597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Trivedi H.L., Terasaki P.I., Feroz A., Everly M.J., Vanikar A.V., Shankar V., Trivedi V.B., Kaneku H., Idica A.K., Modi P.R., et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation. 2009;87:1555–1561. doi: 10.1097/TP.0b013e3181a4b91b. [DOI] [PubMed] [Google Scholar]
- 227.Ejaz N.S., Alloway R.R., Halleck F., Dürr M., Budde K., Woodle E.S. Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid. Redox Signal. 2014;21:2401–2418. doi: 10.1089/ars.2014.5892. [DOI] [PubMed] [Google Scholar]
- 228.Ding Y., Francis J., Gautam A., Pelletier L., Sanchorawala V., Quillen K. Durable renal response after combination of bortezomib, corticosteroids, rituximab, and plasmapheresis for late antibody-mediated renal transplant rejection. Clin. Nephrol. 2018;89:252–259. doi: 10.5414/CN109278. [DOI] [PubMed] [Google Scholar]
- 229.Morrow W.R., Frazier E.A., Mahle W.T., Harville T.O., Pye S.E., Knecht K.R., Howard E.L., Smith R.N., Saylors R.L., Garcia X., et al. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation. 2012;93:319–324. doi: 10.1097/TP.0b013e31823f7eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Govil A., Walsh R.C., Tevar A., Alloway R., Roy-Chaudhury P., Mogilishetty G., Wall G.E., Brailey P., Girnita A., Woodle E.S. Bortezomib-based treatment of antibody mediated rejection in pancreas allograft recipients. Clin. Transpl. 2009;45:443–453. [PubMed] [Google Scholar]
- 231.Baum C., Reichenspurner H., Deuse T. Bortezomib rescue therapy in a patient with recurrent antibody-mediated rejection after lung transplantation. J. Heart Lung Transpl. Off. Publ. Int. Soc. Heart Transpl. 2013;32:1270–1271. doi: 10.1016/j.healun.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 232.Krishnan K.M., Williamson K.C. The proteasome as a target to combat malaria: Hits and misses. Transl. Res. J. Lab. Clin. Med. 2018;198:40–47. doi: 10.1016/j.trsl.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li H., O’Donoghue A.J., van der Linden W.A., Xie S.C., Yoo E., Foe I.T., Tilley L., Craik C.S., da Fonseca P.C.A., Bogyo M. Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature. 2016;530:233–236. doi: 10.1038/nature16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Le Chapelain C., Groll M. Rational Design of Proteasome Inhibitors as Antimalarial Drugs. Angew. Chem. Int. Ed. Engl. 2016;55:6370–6372. doi: 10.1002/anie.201602519. [DOI] [PubMed] [Google Scholar]
- 235.Li H., van der Linden W.A., Verdoes M., Florea B.I., McAllister F.E., Govindaswamy K., Elias J.E., Bhanot P., Overkleeft H.S., Bogyo M. Assessing subunit dependency of the Plasmodium proteasome using small molecule inhibitors and active site probes. ACS Chem. Biol. 2014;9:1869–1876. doi: 10.1021/cb5001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aminake M.N., Arndt H.-D., Pradel G. The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention? Int. J. Parasitol. Drugs Drug Resist. 2012;2:1–10. doi: 10.1016/j.ijpddr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Czesny B., Goshu S., Cook J.L., Williamson K.C. The proteasome inhibitor epoxomicin has potent Plasmodium falciparum gametocytocidal activity. Antimicrob. Agents Chemother. 2009;53:4080–4085. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li H., Ponder E.L., Verdoes M., Asbjornsdottir K.H., Deu E., Edgington L.E., Lee J.T., Kirk C.J., Demo S.D., Williamson K.C., et al. Validation of the proteasome as a therapeutic target in Plasmodium using an epoxyketone inhibitor with parasite-specific toxicity. Chem. Biol. 2012;19:1535–1545. doi: 10.1016/j.chembiol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Becker S.H., Darwin K.H. Bacterial Proteasomes: Mechanistic and Functional Insights. Microbiol. Mol. Biol. Rev. MMBR. 2017 doi: 10.1128/MMBR.00036-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lin G., Hu G., Tsu C., Kunes Y.Z., Li H., Dick L., Parsons T., Li P., Chen Z., Zwickl P., et al. Mycobacterium tuberculosis prcBA genes encode a gated proteasome with broad oligopeptide specificity. Mol. Microbiol. 2006;59:1405–1416. doi: 10.1111/j.1365-2958.2005.05035.x. [DOI] [PubMed] [Google Scholar]
- 241.Hu G., Lin G., Wang M., Dick L., Xu R.-M., Nathan C., Li H. Structure of the Mycobacterium tuberculosis proteasome and mechanism of inhibition by a peptidyl boronate. Mol. Microbiol. 2006;59:1417–1428. doi: 10.1111/j.1365-2958.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- 242.Darwin K.H., Lin G., Chen Z., Li H., Nathan C.F. Characterization of a Mycobacterium tuberculosis proteasomal ATPase homologue. Mol. Microbiol. 2005;55:561–571. doi: 10.1111/j.1365-2958.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- 243.Darwin K.H., Ehrt S., Gutierrez-Ramos J.-C., Weich N., Nathan C.F. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302:1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 244.Agins B.D., Ikeda D.J., Reid M.J.A., Goosby E., Pai M., Cattamanchi A. Improving the cascade of global tuberculosis care: Moving from the “what” to the “how” of quality improvement. Lancet Infect. Dis. 2019;19:437–443. doi: 10.1016/S1473-3099(19)30420-7. [DOI] [PubMed] [Google Scholar]
- 245.Zhan W., Hsu H.-C., Morgan T., Ouellette T., Burns-Huang K., Hara R., Wright A.G., Imaeda T., Okamoto R., Sato K., et al. Selective Phenylimidazole-Based Inhibitors of the Mycobacterium tuberculosis Proteasome. J. Med. Chem. 2019;62:9246–9253. doi: 10.1021/acs.jmedchem.9b01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Totaro K.A., Barthelme D., Simpson P.T., Jiang X., Lin G., Nathan C.F., Sauer R.T., Sello J.K. Rational Design of Selective and Bioactive Inhibitors of the Mycobacterium tuberculosis Proteasome. ACS Infect. Dis. 2017;3:176–181. doi: 10.1021/acsinfecdis.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]