Abstract
Nationally, it was estimated that 11.4 million people misused opioids in 2017 with more than 47,000 opioid-related deaths. Although medication-assisted treatment (MAT) has been effective in enhancing treatment retention and decreasing frequency of opioid use, relapse rates for opioids and other substances remain high, emphasizing the importance of investigating novel interventions to augment MAT. One potential treatment approach is repetitive transcranial magnetic stimulation (rTMS)—a noninvasive, electrophysiological method of neuromodulation. Recently published studies of rTMS in individuals with alcohol, nicotine, and cocaine use disorder have suggested that this treatment shows promise in reducing cravings and substance use. The literature specific to rTMS and opioid use disorder (OUD) is limited to a single published study in heroin users, which showed that a single session of rTMS to the left dorsolateral prefrontal cortex (DLPFC) reduced cue-induced craving, with a further reduction following 5 consecutive days of rTMS. The following case report involved a 25-year-old Caucasian male diagnosed with OUD and cocaine use disorder. This subject continued to demonstrate ongoing substance use despite participating in comprehensive MAT with buprenorphine/naloxone in combination with psychosocial interventions. He was administered 7 separate sessions of rTMS targeting the left DLPFC. Substance-related cues were presented prior to, during, and following these rTMS administration sessions and the subject rated his substance cravings via a 100-point Visual Analog Scale. When compared with his cue-induced craving ratings, there was a mean reduction in craving for heroin and cocaine by ~60% to 82% following the 7 administration sessions. Although this is a single case, further investigation of rTMS as an augmentation strategy for OUD and polysubstance use is warranted. (PsyclNFO Database Record (c) 2019 APA, all rights reserved)
Keywords: heroin, cocaine, craving, neuromodulation, transcranial magnetic stimulation, opioid
Impact Statement:
Public Health Significance—Given the current opioid epidemic that our nation is facing, along with the additive impact caused by polysubstance use, investigating novel treatments, such as repetitive transcranial magnetic stimulation (rTMS), is of critical importance. In this case report involving an individual with opioid and cocaine use disorder, self-reported craving ratings for heroin and cocaine decreased by an average of 60% to 82% following seven rTMS administration sessions. Although this is a single case, further investigation of rTMS as an adjunctive treatment for opioid and polysubstance use disorder is warranted.
Nationally, it was estimated that 11.4 million people have misused opioids since 2000, and opioid-related overdoses have quadrupled with more than 47,000 opioid-related deaths in 2017, representing a 12% increase from 2016 (Center for Behavioral Health Statistics & Quality, 2018; National Institute on Drug Abuse, 2018; Scholl, Seth, Kariisa, Wilson, & Baldwin, 2018). West Virginia (WV) continues to be the epicenter with the highest drug overdose mortality in the nation with 49.6 opioid-related deaths per 100,000 population, well ahead of all other states (Scholl et al., 2018). The toll of this opioid epidemic goes well beyond overdose survivals and deaths; for example, there has been an upsurge of intravenous drug use resulting in significant increases in infectious diseases. Rates of hepatitis C (HCV) have steadily increased over the past decade with West Virginia having the second highest rate of HCV in the nation (7.1 per every 100,000 residents) and far above the national average (0.8 per 100,000; West Virginia Department of Health and Human Resources Bureau for Public Health, 2017). Future generations will also be affected as the number of pregnant women with HCV has doubled in recent years and this virus may be transmitted by a pregnant woman to her infant (Centers for Disease Control and Prevention, 2017). The morbidity and mortality secondary to the opioid epidemic is clearly one of the greatest public health problem that the United States currently faces.
Current Treatments for Opioid Use Disorder
Although medication-assisted treatment (MAT) has been effective in improving outcomes (abstinence and harm reduction), current opioid use disorder (OUD) treatment is far from ideal as approximately 50% of those seeking treatment relapse to opioids and/or other substances. In a multisite, randomized trial, Weiss et al. (2011) reported that the rate of unsuccessful outcomes following MAT (using buprenorphine-naloxone) exceeded 90% and, even when individuals were stabilized on MAT over 12 weeks, the rate of successful outcomes was less than 50% (Weiss et al., 2011). A recent review of extended-release injectable naltrexone revealed that many patients never even start the treatment because of withdrawal symptoms, and those who start often discontinue (Jarvis et al., 2018). In addition, a separate study found that treatment with extended release Naltrexone and buprenorphine have unacceptably high relapse rates following 24 weeks of treatment (65% vs. 57% respectively; Lee et al., 2018). Clearly, new modalities are urgently needed to treat OUD.
A Complicating Factor—Polysubstance Use
Although we are clearly in the midst of an opioid epidemic, we must not neglect the detrimental impact of other illicit substance use co-occurring with OUD. In a retrospective study utilizing the West Virginia University (WVU) Medicine electronic medical record data repository, de-identified data were extracted from the following health care encounters: inpatient psychiatric admissions, psychiatric outpatient visits, and emergency department visits between 2009 and 2018. Patients were extracted based on the following inclusion criteria: (a) diagnosis of OUD and (b) positive urine toxicology for opioids at the time of the initial encounter within the health care system. Of the 5,208 patients who met these criteria, ~70% were also positive for other addictive substances, ~31% of whom were positive for opioids and two or more additional substances. In addition to opioids, benzodiazepines and cannabis (~51%) were the most common co-occuring substances, and specific to the current case report, cocaine was the third most common co-occurring substance (~28%).
Novel Treatment Approaches—Transcranial Magnetic Stimulation
Given the current opioid epidemic, the high rate of relapse and overdose deaths, and the additive impact of polysubstance use, investigating novel treatment approaches is of vital importance. National Institute Drug Abuse (NIDA) Director Nora Volkow recently provided a statement on the NIDA website reiterating the commitment of NIDA and NIH in maintaining and putting forth an “all scientific hands on deck” effort to end the opioid crisis. Specifically, she noted that the goals of this initiative include investigating “innovative and ambitious new treatment approaches,” specifically referencing transcranial magnetic stimulation (TMS)—a noninvasive method of neuromodulation approved by the FDA for the treatment of major depressive disorder. Recently published studies of TMS targeting the left dorsolateral prefrontal cortex (DLPFC) have demonstrated reductions in alcohol, cocaine, methamphetamine, and nicotine use and craving, a risk factor for relapse (Barr et al., 2011; Gorelick, Zangen, & George, 2014; Hanlon et al., 2013; Hanlon, Dowdle, Moss, Canterberry, & George, 2016; Li et al., 2013; Liu, Li, Shen, Liu, & Yuan, 2019; Mishra, Praharaj, Katshu, Sarkar, & Nizamie, 2015). The one published study on the use of repetitive TMS (rTMS) for OUD found that a single session of rTMS to the left DLPFC reduced cue-induced craving in long-term heroin users, with further reduction following five consecutive days of rTMS (Shen et al., 2016).
The main brain circuitry target for rTMS in the treatment of substance use disorders (SUD) is the prefrontal cortical network, specifically the dorsolateral prefrontal cortex (DLPFC) and the orbitofrontal cortex (Gorelick et al., 2014; Mishra et al., 2015). These brain regions have important functions in inhibitory control, which is a neurobehavioral output often impaired in patients with SUDs and disinhibition is associated with relapse susceptibility (Herremans et al., 2015; Kravitz et al., 2015; Prikryl et al., 2014; Protasio et al., 2015). It is hypothesized that increased activity of the PFC may be a major mechanism of action of rTMS in patients with SUD (Feil & Zangen, 2010). Other possible mechanisms for the effectiveness of rTMS include the reduction of craving, modulation of the dopaminergic and hypothalamic pituitary adrenal axis, and modulation of executive and decision-making processes, the latter of which may lead to a reduction in risk-taking behavior (Knoch et al., 2006). Although evaluation of rTMS in the treatment of SUDs is promising, larger studies are necessary, especially related to OUD and polysubstance use, given the high prevalence along with the lack of literature related to rTMS as a potential treatment for OUD and polysubstance use disorder.
Case Report
Participant Characteristics
JD is a 25-year-old Caucasian male who has been diagnosed with opioid use disorder and cocaine use disorder. Age of first use of heroin use was 14 years of age, and first use of cocaine was 22 years of age. He has been using heroin regularly (defined as >3 days per week) for the past 9 years and using cocaine regularly for the last 2 years. Although his weekly use of heroin and cocaine would fluctuate based on access and availability, he reported typically using both heroin and cocaine three to four times per week over the past month. He has been receiving treatment intermittently since 2014 in the Intensive Outpatient Program and in the MAT program referred to as the Comprehensive Opioid Addiction Treatment (COAT) program at the WVU School of Medicine’s Chestnut Ridge Center. At the time of study participation, the subject was enrolled in the COAT program, which utilizes a multidisciplinary and multimodal approach including behavioral intervention (both group and individual therapy) and buprenorphine/naloxone maintenance. He also received treatment in 28-day residential treatment programs twice over the past 4 years. During all of these treatments, he has had frequent relapses, being unable to remain drug abstinent for longer than approximately 50 days, and being unable to remain abstinent for more than a few days over the past six months. This individual’s substance use has led to several physical, psychosocial, and legal complications, including infectious disease-related abscesses, drug overdose, car accidents, depression, anxiety, impaired interpersonal relationships, job loss, and multiple arrests (with one episode of incarceration).
Procedures
The procedures conducted as part of this study were approved by the WVU Institutional Review Board (WVU06HSC17 Protocol Number: 703528163; Study Title: Repetitive Transcranial Magnetic Stimulation in Recently Abstinent Individuals with Opioid Use Disorder). The subject participated in a screening session after providing written informed consent, and he met the approved inclusion/exclusion criteria to participate in this study. Inclusion criteria included the following: (1) actively enrolled in the COAT Program; (b) meet Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) criteria for a primary OUD and comorbid SUD assessed via structured clinical interview; (c) 18–60 years of age; (d) abstinent from opioids (other than prescribed buprenorphine/naloxone) and illicit substances other than marijuana at the time of the enrollment (confirmed via urine drug screen); (e) willing to practice contraception to avoid pregnancy the duration of the study; (f) able to provide written informed consent and to comply with study procedures. Exclusion criteria included the following: (a) Medical conditions that preclude rTMS; (b) DSM-5 criteria for major psychiatric illness; (c) major cognitive disorder; (d) pregnancy; (e) positive responses to the Transcranial Magnetic Stimulation Adult Safety Screen; (f) taking any medications that are a strong potential hazard for rTMS; (g) intracranial metallic objects; (h) uncorrected visual acuity problems; (i) clinically significant electrocardiogram abnormalities; (j) unwilling to abstain from non-prescribed drugs; (k) suicidal ideation; (l) prior rTMS treatment; and (m) other mental or physical conditions that, in the PI’s opinion, would be inappropriate for study participation.
Craving was assessed at baseline, at which time the subject was asked to rate his cravings for heroin, cocaine, and other substances, via a 100-point visual analog scale (VAS) where 100 represented maximum craving and 0 represented no craving. After this initial craving assessment, the patient was then exposed to heroin and other substance-related cues (e.g., images of drugs, paraphernalia, people using drugs), which were presented on a laptop for 10 min, followed by an assessment of craving (VAS) to determine changes in craving following cue exposure. Using the same parameters in the only published study of rTMS in heroin users (Shen et al., 2016; 10 Hz, strength at 100% resting motor threshold, 5 seconds on and 10 seconds off for 10 min; 2000 total pulses), rTMS was then applied unilaterally over the left DLPFC during seven sessions across a 3-week time course using the Neurostar TMS device (Neuronetics, Inc.). The DLPFC was identified using the “5-cm method”, which involves stimulating the motor cortex, observing motor evoked potentials in the contralateral hand, and then measuring 5 cm anterior from this position along a parasaggital line (George et al., 1995; Pascual-Leone, Rubio, Pallardó, & Catala, 1996). Cue exposure continued throughout the rTMS administration and for 10 min following the completion of the rTMS sessions. Craving was assessed immediately after rTMS administration and again at 5 and 10 min following the completion of the rTMS sessions.
Results
Over the course of the study, all procedures were well-tolerated with no adverse events reported by the subject (assessed before, during, and following TMS sessions). Across the seven sessions, exposure to the drug-related cues resulted in an increase in craving for heroin (average: 41.4 pre-cue exposure vs. 68.6 post-cue exposure) and cocaine (41.4 vs. 71.4). Following the 10-min rTMS administration, craving ratings for heroin decreased from 68.6 to 27.1 immediately following the completion of rTMS with an even further decrease 5 min (18.6) and 10 min (14.3) post-rTMS completion on average across the seven sessions (Figure 1a). Craving ratings for cocaine decreased from 71.4 to 25.7 immediately following the completion of rTMS sessions with an even further decrease 5 min (18.6) and 10 min (12.9) post-rTMS completion on average across the seven sessions (Figure 1b).
Figure 1.
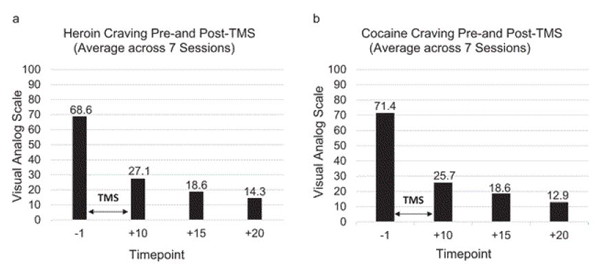
Average heroin and cocaine craving ratings prior to and following seven sessions of rTMS.
Conclusions
Although this is a single case, these findings demonstrate that further investigation of rTMS as an augmentation strategy with comprehensive MAT for OUD and polysubstance use disorder is warranted. There are two primary findings which became apparent from this case. First, cue-induced craving could be elicited via the presentation of substance related cues with increases of heroin and cocaine craving by approximately 57% to 59% in comparison to his baseline, pre-cue exposure craving ratings. Following 10 min of rTMS applied unilaterally over the left DLPFC, there was on average an approximate 60% to 80% decrease in heroin craving and 63% to 82% decrease in cocaine craving across the seven sessions. The decreases in craving following rTMS administration, at which time the participant had continued exposure to the drug related cues, were approximately 36% to 68% below his initial pre-cue exposure craving rating. This demonstrates that cue-induced craving, at least in this particular case, could be extinguished by rTMS. This is of importance given that cues (such as images of drugs, situations when drugs are used, people who are affiliated with an individual’s drug use, etc.) are associated with continued drug taking and relapse. By extinguishing cue induced craving, the probability of an individual relapsing secondary to increased craving elicited by drug related cues will hopefully then be reduced. While the cues presented to the subject clearly elicited a craving response, this must be interpreted in the context of the environment (clinic setting) and potentially reflect a response less robust than that which would be elicited in a naturalistic setting. As such, tailoring the cue presentation to each individual is one possible method for maximizing the craving response in an attempt to approximate the response which is elicited outside of the clinic. For example, selecting cues as proximal to the subject’s characteristics (e.g., age, gender, ethnicity, preferred substances and route of administration, etc.) proved to be beneficial in eliciting a craving response which this subject reported as being relatively comparable with his typical level of craving outside the clinic in a more naturalistic setting.
When asked qualitatively about why he felt his craving decreased during and after the rTMS sessions, the subject reported that the images had more of an “aversive effect” as opposed to when he viewed the cues in the absence of the rTMS. He reported that during and after the rTMS administration sessions, he reflected more about the negative impact his substance use has had on his life, specifically how it has led to issues within the legal system, caused friction within his family, and has interfered with his daily functioning (e.g., maintaining employment). Although the reasoning behind this cannot be stated with certainty, this may reflect changes in the targeted region where the rTMS was administered. Given that the left DLPFC is implicated in inhibition and decision-making, the subject’s report that the cues made him consider the negative impact substance use has had on his life may suggest increased activity in these decision-making networks. Of importance, this subject remained entirely abstinent for approximately one month following the completion of the final rTMS session, a considerable improvement given that his previous lengths of abstinence over the prior six months were typically no longer than a few days.
Future directions include conducting a randomized, sham-controlled trial investigating active versus sham rTMS on polysubstance use and craving. Based on the findings from this trial, once safety and feasibility is confirmed, modifying the duration, frequency, and intensity of treatment parameters (e.g., increasing treatment sessions to 5 days per week over 6 weeks) will be considered. The rationale for doing so is based on the additive impact and further reduction in depressive symptoms in individuals with major depressive disorder following 6 weeks of daily rTMS treatment (O’Reardon et al., 2007). In addition to increasing the frequency and duration, the intensity of the stimulation parameters in the current case was identical to the only previously published study investigating rTMS in heroin users; however, the intensity parameters used for other indications, such as depression, are generally higher (Perera et al., 2016). Future studies may also explore other forms of TMS, such as intermittent and continuous theta burst stimulation, to determine the most optimal and efficient form of TMS with this population. In addition, other areas of the brain which are also involved in reward neurocircuitry, such as the medial prefrontal cortex, can be targeted which has previously been done in other substance using populations (Hanlon et al., 2017).
Acknowledgement:
James J. Mahoney III receives support from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award U54GM104942–03. The funding source had no role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declares no conflicts of interest with the research reported in this article.
Grant Sponsorship: Sponsor: National Institutes of Health, National Institute of General Medical Sciences, US
Grant Number: U54GM104942–03 Recipients: Mahoney, James J. Ill
Footnotes
Note: William W. Stoops served as the action editor for this article.—WWS
Contributor Information
James J. Mahoney, III, Department of Behavioral Medicine and Psychiatry, and Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University School of Medicine.
Patrick J. Marshalek, Department of Behavioral Medicine and Psychiatry, and Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University School of Medicine
Ali R. Rezai, Departments of Behavioral Medicine and Psychiatry, Neuroscience, Neurosurgery, Neurology, and Radiology, Rockefeller Neuroscience Institute, West Virginia University School of Medicine
Laura R. Lander, Department of Behavioral Medicine and Psychiatry, and Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University School of Medicine
James H. Berry, Department of Behavioral Medicine and Psychiatry, and Department of Neuroscience, Rockefeller Neuroscience Institute, West Virginia University School of Medicine
Marc W. Haut, Departments of Behavioral Medicine and Psychiatry, Neuroscience, Neurology, and Radiology, Rockefeller Neuroscience Institute, West Virginia University School of Medicine
References
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, & Daskalakis ZJ (2011). Repetitive transcranial magnetic stimulation and drug addiction. International Review of Psychiatry, 23, 454–466. 10.3109/09540261.2011.618827 [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2018). 2017 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Morbidity and mortality weekly reports (MMWR): Incidence of neonatal abstinence syndrome - 28 states, 1999–2013. Atlanta, GA: Author; Retrieved from https://www.cdc.gov/mmwr/volumes/65/wr/mm6531a2.htm [Google Scholar]
- Feil J, & Zangen A (2010). Brain stimulation in the study and treatment of addiction. Neuroscience and Biobehavioral Reviews, 34, 559–574. 10.1016/j.neubiorev.2009.11.006 [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, …Post RM (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport, 6, 1853–1856. 10.1097/00001756-199510020-00008 [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, & George MS (2014). Transcranial magnetic stimulation in the treatment of substance addiction. Annals of the New York Academy of Sciences, 1327, 79–93. 10.1111/nyas.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Canterberry M, Taylor JJ, DeVries W, Li X, Brown TR, & George MS (2013). Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: A pilot study. PLoS ONE, 8, e67917. 10.1371/journal.pone.0067917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Moss H, Canterberry M, & George MS (2016). Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: An interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology, 41, 3032–3041. 10.1038/npp.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Kearney-Ramos T, Dowdle LT, Hamilton S, DeVries W, Mithoefer O, …George MS (2017). Developing repetitive transcranial magnetic stimulation (rTMS) as a treatment tool for cocaine use disorder: A series of six translational studies. Current Behavioral Neuroscience Reports, 4, 341–352. 10.1007/s40473-017-0135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Van Schuerbeek P, De Raedt R, Matthys F, Buyl R, De Mey J, & Baeken C (2015). The impact of accelerated right prefrontal high-frequency repetitive transcranial magnetic stimulation (rTMS) on Cue-Reactivity: An fMRI study on craving in recently detoxified alcohol-dependent patients. PLoS ONE, 10, e0136182. 10.1371/journal.pone.0136182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, & Silverman K (2018). Extended-release injectable naltrexone for opioid use disorder: A systematic review. Addiction. 113 1188–1209. 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, & Brugger P (2006). Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. The Journal of Neuroscience. 26 6469–6472. 10.1523/JNEUR0SCI.0804-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tomasi D, LeBlanc KH, Baler R, Volkow ND, Bonci A, & Ferre S (2015). Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Research. 1628(Pt A), 186–198. 10.1016/j.brainres.2015.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, …Rotrosen J (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet. 391 309–318. 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, …George MS (2013). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biological Psychiatry, 73, 714–720. 10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Li Y, Shen Y, Liu X, & Yuan TF (2019). Gender does not matter: Add-on repetitive transcranial magnetic stimulation treatment for female methamphetamine dependents. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 92 70–75. 10.1016/j.pnpbp.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, & Nizamie SH (2015). Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: A randomized double-blind study. The Journal of Neuropsychiatry and Clinical Neurosciences. 27, e54–e59. 10.1176/appi.neuropsych.13010013 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2018). Overdose death rates. Bethesda, MA: Author; Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, …Sackeim HA (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological psychiatry. 62 1208–1216. PubMed PMID: 17573044. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardó F, & Catalá MD (1996). Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 348 233–237. 10.1016/S0140-6736(96)01219-6 [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, & Wirecki TS (2016). The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation. 9 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl R, Ustohal L, Kucerova HP, Kasparek T, Jarkovsky J, Hublova V, …Ceskova E (2014). Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 49 30–35. 10.1016/j.pnpbp.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Protasio MI, da Silva JP, Arias-Carrión O, Nardi AE, Machado S, & Cruz MS (2015). Repetitive transcranial magnetic stimulation to treat substance use disorders and compulsive behavior. CNS & Neurological Disorders - Drug Targets. 14 331–340. 10.2174/1871527314666150318114043 [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2018). Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. Morbidity and Mortality Weekly Report (MMWR). 67 1419–1427. 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, …Yuan TF (2016). 10-Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biological Psychiatry, 80 e13–e14. 10.1016/j.biopsych.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, …Ling W (2011). Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 68 1238–1246. 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West Virginia Department of Health and Human Resources Bureau for Public Health. (2017). West Virginia viral hepatitis epidemologic profile. Charleston, WV: Author; Retrieved from https://dhhr.wv.gov/oeps/disease/ob/documents/viral-hep-profile-2017.pdf [Google Scholar]


