Abstract
Antimicrobial peptides (AMPs) have a unique action mechanism that can help to solve global problems in antibiotic resistance. However, their low therapeutic index and poor stability seriously hamper their development as therapeutic agents. In order to overcome these problems, we designed peptides based on the sequence template XXRXXRRzzRRXXRXX-NH2, where X represents a hydrophobic amino acid like Phe (F), Ile (I), and Leu (L), while zz represents Gly–Gly (GG) or d-Pro–Gly (pG). Showing effective antimicrobial activity against Gram-negative bacteria and low toxicity, designed peptides had a tendency to form an α-helical structure in membrane-mimetic environments. Among them, peptide LRpG (X: L, zz: pG) showed the highest geometric mean average treatment index (GMTI = 73.1), better salt, temperature and pH stability, and an additive effect with conventional antibiotics. Peptide LRpG played the role of anti-Gram-negative bacteria through destroying the cell membrane. In addition, peptide LRpG also exhibited an anti-inflammatory activity by effectively neutralizing endotoxin. Briefly, peptide LRpG has the potential to serve as a therapeutic agent to reduce antibiotic resistance owing to its high therapeutic index and great stability.
Keywords: antimicrobial peptides, cell selectivity, stability, mechanism, endotoxin neutralization
1. Introduction
With the widespread use of antibiotics in clinical practice in recent years, the resistance rate of bacteria to traditional antibiotics is increasing. The development of antibacterial reagents with new antibacterial mechanisms is imminent [1]. As an important part of innate immunity, antimicrobial peptides (AMPs) form the first line of defense against antimicrobial infections, as found in many microorganisms including insects, plants, and animals [2]. Furthermore, AMPs are known for their anti-inflammatory properties, anti-biofilm formation, promotion of tissue and injury repair, and anti-cancer effects [2,3,4]. Compared to traditional antibiotics, AMPs exert an antibacterial effect through non-receptor-mediated membrane permeability. AMPs are expected to be alternatives to antibiotics because of their unique action mechanism [5,6,7]. Although more than 3000 AMPs were found, their application as therapeutic agents is seriously hindered due to some limiting factors including manufacturing costs, cytotoxicity, and poor stability [8].
The heptad repeat sequence is a repeat sequence composed of seven amino acids, where the “a” and “d” positions are occupied by hydrophobic amino acids [9]. It was found that natural AMPs possessing heptad repeat sequences have great antibacterial and anti-inflammatory activity, but their application is hindered by high cytotoxicity [10,11]. Previous studies mostly focused on how different hydrophobic amino acids at the “a” and “d” positions influence antibacterial activity and cytotoxicity of AMPs, but few studies used this sequence element to design new AMPs [12,13]. Some papers showed that symmetric AMPs centered on Gly–Gly (GG) or d-Pro–Gly (pG) have good antibacterial activity and high cell selectivity [14,15,16,17]. Therefore, we wondered if the same effect could be achieved when GG or pG was introduced into two symmetric heptad repeat sequences.
In this study, two symmetric heptad repeat sequences were connected by a short loop, and the sequence was designed as XXRXXRRzzRRXXRXX-NH2, where X represents Phe (F), Ile (I), and Leu (L), while zz represents Gly–Gly (GG) or d-Pro–Gly (pG). The net charge was +6 and the hydrophobicity was 40%–60%, which is consistent with the statistical information on natural AMPs [2,18]. Hydrophobic amino acids F, I, and L were used to damage microbial cell membranes [19]. Flexible (GG) and rigid (pG) loops were selectively inserted into the middle of two symmetric heptad repeat sequences to enhance cell selectivity [17,20]. In addition, Arg (R) was selectively introduced into the sequence template. The guanidine side chain of R allows it to form strong bidentate H-bonds with the phosphate moiety of lipid head groups, thereby enhancing the electrostatic action between peptides and anionic bacterial membranes [20]. To determine the role of loops in the designed peptides, a control peptide was designed as a heptad repeat sequence as follows: the central pG loop of peptide LRpG with highest cell selectivity was removed, and Leu located at the N-terminus and C-terminus of the peptide was exchanged with Gly and d-Pro, respectively. The positive charge and hydrophobicity were consistent with LRpG. Finally, by amidating the C-terminus of the peptides, the charge of the AMPs was increased, and the stability of the structure was improved [21,22,23]. Using this method of designing and optimizing AMPs, we obtained LRpG (X: L, zz: pG) with high cell selectivity and good conditional stability. The antibacterial mechanism study demonstrated that LRpG played the role of an anti-Gram-negative bacterial agent through destroying the cell membrane.
2. Results
2.1. Peptide Design and Characteristics
In this study, two symmetric heptad repeat sequences were connected by a short loop, and the sequence was designed as XXRXXRRzzRRXXRXX-NH2 (X represents F, I, and L; zz represents GG or pG). The molecular mass of all peptides was consistent with theoretical values through measurement, demonstrating that the peptides were successfully synthesized. The hydrophobicity order of the designed peptides was as follows: IRpG > FRpG > IRGG > FRGG = LRpG = LRα > LRGG (Table 1). MALDI-TOF MS of the designed peptides is shown in Supplementary Figure S1, and HPLC spectra of the designed peptides is shown in Supplementary Figure S2.
Table 1.
Peptides and their key physicochemical parameters.
| Peptides | Sequence | Theoretical MW | Measured MW 1 | Net Charge | H 2 | µHre 3 |
|---|---|---|---|---|---|---|
| IRGG | IIRIIRRGGRRIIRII-NH2 | 1974.52 | 1973.5 | 6 | 0.521 | 0.770 |
| IRpG | IIRIIRRp 4 GRRIIRII-NH2 | 2014.58 | 2013.8 | 6 | 0.566 | 0.800 |
| FRGG | FFRFFRRGGRRFFRFF-NH2 | 2246.66 | 2245.68 | 6 | 0.516 | 0.767 |
| FRpG | FFRFFRRp 4 GRRFFRFF-NH2 | 2286.72 | 2285.74 | 6 | 0.561 | 0.797 |
| LRGG | LLRLLRRGGRRLLRLL-NH2 | 1974.52 | 1973.51 | 6 | 0.471 | 0.743 |
| LRpG | LLRLLRRp 4 GRRLLRLL-NH2 | 2014.58 | 2013.58 | 6 | 0.516 | 0.773 |
| LRα | GLRLLRRLLRRLLRLp 4 -NH2 | 2014.58 | 2013.58 | 6 | 0.516 | 0.735 |
1 Molecular weight (MW) was determined by mass spectrometry (MS). 2 The mean hydrophobicity (H) is the total hydrophobicity (sum of all residue hydrophobicity indices) divided by the number of residues. 3 The relative hydrophobic moment of a peptide is its hydrophobic moment relative to that of a perfectly amphiphilic peptide. 4 Lowercase letters indicate the d-enantiomer of the proline.
2.2. Circular Dichroism (CD) Spectroscopy
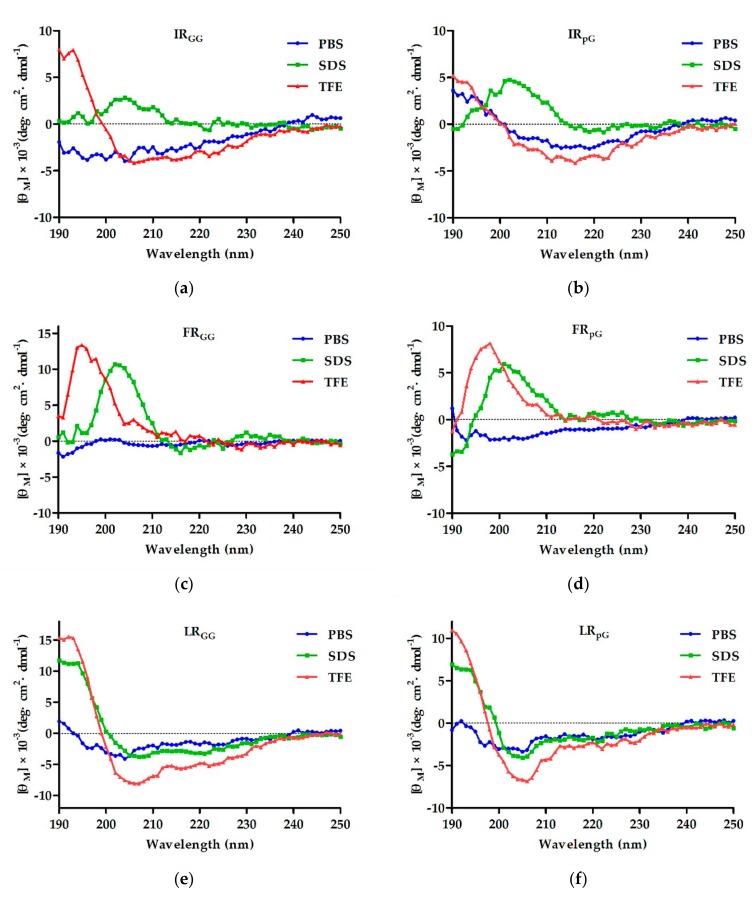
The secondary structure of the designed peptides was detected using CD spectra in 10 mM phosphate-buffered saline (PBS), 50% trifluoroethanol (TFE), and 30 mM sodium dodecyl sulfate (SDS). In 10 mM PBS, the six designed peptides containing a central loop exhibited disordered conformations, and the control LRα showed a more ordered conformation. In 50% TFE (mimicking the hydrophobic environment of the microbial membrane) and 30 mM SDS (environment comparable to a negatively charged prokaryotic membrane) [15], FRGG, FRpG, IRGG, and IRpG showed disordered conformations. LRpG and LRGG had a tendency to form an α-helical structure with negative peaks at approximately 205 nm and 220 nm. In contrast, although the hydrophobic and net charges of the control peptide LRα were consistent with LRpG, a typical α-helical structure with negative peaks at approximately 208 nm and 222 nm was observed (Figure 1). The results reveled that the central loop (pG or GG) disrupted the formation of the α-helical structure. The helical wheel projections of LRGG, LRpG, and LRα are shown in Supplementary Figure S3.
Figure 1.
The circular dichroism (CD) spectra of (a) IRGG, (b) IRpG, (c) FRGG, (d) FRpG, (e) LRGG, (f) LRpG, and (g) LRα were dissolved in 10 mM phosphate-buffered saline (PBS) (blue), 30 mM sodium dodecyl sulfate (SDS) (green), and 50% trifluoroethanol (TFE) (red). The average value after three scans of every sample is shown. The CD spectrum of the buffer was subtracted.
2.3. Antimicrobial Activity
The antimicrobial activity of designed peptides is summarized in Table 2. The designed peptides showed better antimicrobial activity against Gram-negative bacteria than Gram-positive bacteria. For Gram-negative bacteria, LRpG showed the best antimicrobial activity among the six designed peptides containing a central loop (LRpG > LRGG > FRpG > FRGG > IRpG > IRGG), and the lowest geometric mean minimal inhibitory concentration (GMMIC) value of 3.5 μM. The control peptide LRα exhibited antimicrobial activity that was a little better than LRpG with a GMMIC value of 3 μM. For Gram-positive bacteria, peptides LRZZ and FRZZ had a relatively weaker antimicrobial effect compared to the control peptide LRα (Table 2).
Table 2.
Minimum inhibitory concentrations (MICs) (µM) and therapeutic index (TI) of the designed peptides.
| Bacterial Species | IRGG | IRpG | FRGG | FRpG | LRGG | LRpG | LRα | Melittin | |
|---|---|---|---|---|---|---|---|---|---|
| MIC (µM) | |||||||||
| Gram (−) | |||||||||
| Escherichia coli ATCC25922 | 8 | 4 | 8 | 8 | 2 | 2 | 2 | 1 | |
| E. coli K88 | 16 | 16 | 16 | 8 | 4 | 2 | 4 | 2 | |
| Salmonella Pullorum NCTC5776 | 32 | 16 | 16 | 16 | 4 | 4 | 2 | 2 | |
| Klebsiella pneumonia CMCC46117 | 32 | 32 | 16 | 16 | 8 | 4 | 4 | 4 | |
| Pseudomonas aeruginosa ATCC27853 | 32 | 32 | 32 | 32 | 8 | 8 | 4 | 2 | |
| Gram (+) | |||||||||
| Staphylococcus aureus ATCC25923 | >128 | >128 | 32 | 16 | 16 | 16 | 4 | 2 | |
| S. aureus ATCC29213 | >128 | >128 | 16 | 16 | 16 | 16 | 8 | 1 | |
| S. aureus ATCC43300 | >128 | >128 | 32 | 32 | 32 | 16 | 8 | 2 | |
| Enterococcus faecalis ATCC29212 | >128 | >128 | 32 | 32 | 32 | 32 | 16 | 1 | |
| GM 1 (µM) | |||||||||
| Gram (−) | 21.1 | 16 | 16 | 13.9 | 4.6 | 3.5 | 3 | 2 | |
| Gram (+) | 256 | 256 | 26.9 | 22.6 | 22.6 | 19.0 | 8 | 1.4 | |
| MHC10 2 (µM) | 512 | 512 | 128 | 128 | 256 | 256 | 4 | 0.25 | |
| TI 3 | |||||||||
| TI (−) | 24.2 | 32 | 8 | 9.2 | 55.6 | 73.1 | 1.3 | 0.125 | |
| TI (+) | 2 | 2 | 4.8 | 5.7 | 11.2 | 13.5 | 0.5 | 0.178 | |
1 Geometric mean (GM) of MICs for the nine strains was measured. A value of 256 μM was used for the TI when no antimicrobial activity was examined at 128 μM. 2 Minimum hemolysis concentration 10% (MHC10) was characterized as the lowest concentration inducing 10% hemolysis. 3 TI was calculated as MHC10/GM.
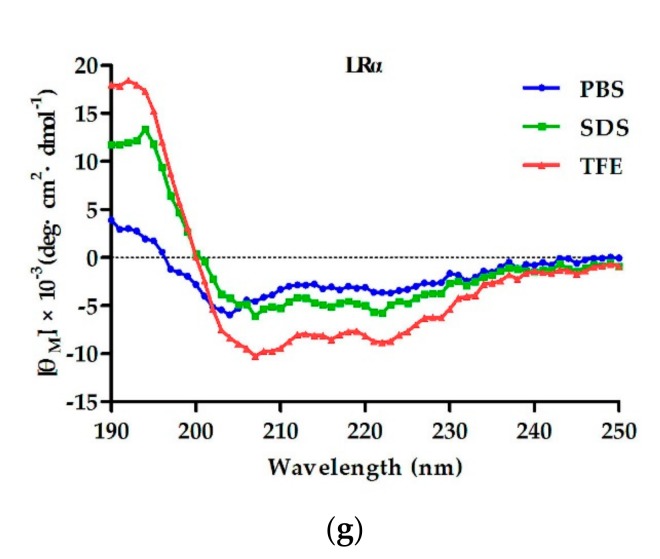
2.4. Biocompatibility Assays
The hemolytic activity of designed peptides against human red blood cells (hRBCs) was determined in a range of 1–512 μM. The MHC10 values of IRGG, IRpG, FRGG, FRpG, LRGG, LRpG, and LRα were 512 μM, 512 μM, 128 μM, 128 μM, 256 μM, 256 μM, and 4 μM, respectively (Figure 2a). In general, the cytotoxicity of the designed peptides was consistent with the hemolytic activity. Later on, the murine macrophage cell line (RAW 264.7) and human embryonic kidney cells (HEK293T) were treated with 64 μM peptides, and the cell survival rate of RAW 264.7 cells was as follows: IRpG (98%) > IRGG (95%) > LRpG (92%) > LRGG (84%) > FRpG (78%) > FRGG (66%) (Figure 2b). Similarly, the cell survival rate of HEK293T cells was as follows: IRpG (98%) > IRGG (96%) > LRpG (93%) > LRGG (91%) > FRpG (85%) > FRGG (76%) (Supplementary Figure S4). However, the cell survival rate of both cells treated with LRα was less than 10%. To determine the cell selectivity of the designed peptide, the therapeutic index (TI) (MHC10/GMMIC) of each peptide was calculated. Among all tested peptides, LRpG showed the highest GMTI (73.1) against Gram-negative bacteria (Table 2).
Figure 2.
(a) Hemolytic activity of designed peptides against human red blood cells (hRBCs); (b) cytotoxicity of the designed peptides against RAW 264.7 cells. The diagrams are based on at least three independent experiments.
2.5. Condition Sensitivity Assays
These results demonstrated that LRpG could maintain antimicrobial activity in different conditions. Therefore, LRpG may have good potential in clinical application (Table 3 and Table 4).
Table 3.
The MICs (µM) of the designed peptides against E. coli ATCC25922 in the presence of physiological salts.
| Peptides | Control 1 | Physiological Salts 2 | |||||
|---|---|---|---|---|---|---|---|
| NaCl | KCl | NH4Cl | MgCl2 | ZnCl2 | FeCl3 | ||
| IRGG | 8 | 64 | 8 | 8 | 16 | 16 | 8 |
| IRpG | 4 | 32 | 4 | 4 | 8 | 4 | 4 |
| FRGG | 8 | 64 | 8 | 8 | 16 | 16 | 8 |
| FRpG | 8 | 32 | 8 | 8 | 8 | 8 | 8 |
| LRGG | 2 | 8 | 2 | 2 | 4 | 2 | 2 |
| LRpG | 2 | 8 | 2 | 2 | 2 | 2 | 2 |
| LRα | 2 | 8 | 2 | 2 | 2 | 2 | 2 |
| Melittin | 1 | 4 | 1 | 1 | 1 | 1 | 1 |
1 The control MICs were determined in Mueller–Hinton broth MHB medium without physiological salts. 2 The final physiological concentrations of NaCl, KCl, NH4Cl, MgCl2, ZnCl2, and FeCl3 were 150 mM, 4.5 mM, 6 μM, 1 mM, 8 μM, and 4 μM, respectively. The data are based on at least three independent experiments.
Table 4.
The MICs (µM) of the designed peptides against E. coli ATCC25922 after temperature and pH treatment.
| Peptides | Control (pH 7) | Temperature | pH | |||||
|---|---|---|---|---|---|---|---|---|
| 0 °C | 37 °C | 100 °C | pH 4 | pH 6 | pH 8 | pH 10 | ||
| IRGG | 8 | 8 | 8 | 8 | 16 | 8 | 8 | 16 |
| IRpG | 4 | 4 | 4 | 4 | 16 | 4 | 8 | 16 |
| FRGG | 8 | 8 | 8 | 8 | 32 | 8 | 8 | 32 |
| FRpG | 8 | 8 | 8 | 8 | 16 | 8 | 8 | 32 |
| LRGG | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| LRpG | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| LRα | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 |
| Melittin | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 |
2.6. Additive Effect of AMPs and Conventional Antibiotics
Based on the MICs of the designed peptides and antibiotics, the antimicrobial interactions of peptides and antibiotics were determined using a checkerboard assay. The combination of LRpG and streptomycin exerted a synergistic effect against Escherichia coli ATCC25922. The fractional inhibitory concentration index (FICI) value was 0.5, while the combination of LRpG with other conventional antibiotics produced an additive effect with FICI values from 0.625 to 1 (Table 5).
Table 5.
The fractional inhibitory concentration index (FICI) 1 for LRpG in combination with conventional antibiotics against E. coli ATCC25922.
| Peptide | Streptomycin 2 | Ciprofloxacin 2 | Chloramphenicol 2 | Cefotaxime 2 |
|---|---|---|---|---|
| LRpG | 0.5 | 0.625 | 0.75 | 1 |
1 FICI ≤ 0.5 denotes synergy, 0.5 < FICI ≤ 1.0 denotes additive, and 1.0 < FICI ≤ 4.0 denotes indifferent. 2 The MICs of streptomycin, ciprofloxacin, chloramphenicol, and cefotaxime against E. coli ATCC25922 were 2 μM, 8 μM, 8 μM, and 2 μM, respectively.
2.7. Antimicrobial Mechanism Study
2.7.1. Outer Membrane Permeability Assay
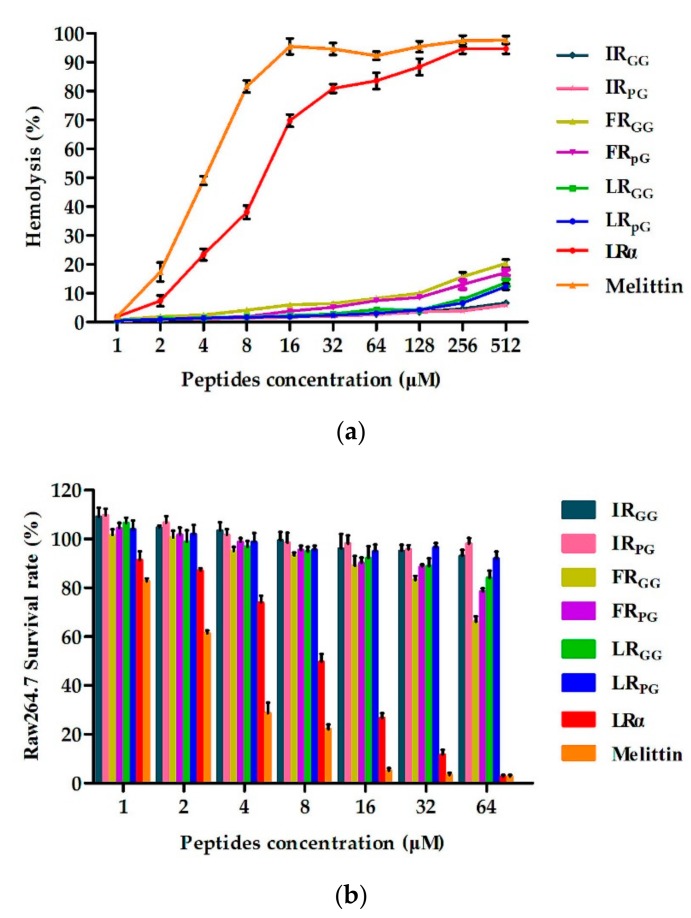
Most AMPs kill bacteria by destroying the bacterial cell membrane. Since the outer membrane of the cell is negatively charged at physiological pH, the permeability of the cationic AMPs to the outer membrane is increased [21]. The permeability of peptides to the Gram-negative bacterial outer membrane can be reflected by N-phenyl-1-naphthylamine (NPN) uptake [15,17,21]. NPN is normally excluded from the outer membrane; however, once the outer membrane is permeabilized, NPN is taken up intracellularly and shows increased fluorescence. As shown in Figure 3, LRpG and LRα were able to permeabilize the outer membrane of E. coli ATCC25922 at concentrations from 1 to 32 μM in a concentration-dependent manner. At peptide concentrations greater than 8 µM, the outer membrane permeability induced by LRpG and LRα was over 75%. Moreover, the outer membrane permeability induced by LRα was slightly stronger than that induced by LRpG at the same concentration.
Figure 3.
Outer membrane permeability of LRpG and LRα at the concentrations from 1 to 32 μM. The diagram is based on at least three independent experiments.
2.7.2. Inner Membrane Permeability Assay
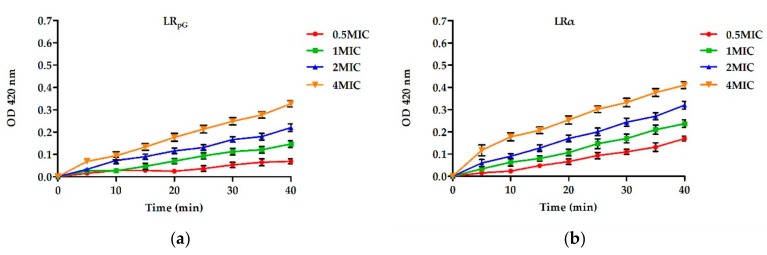
The LRpG permeability to the Gram-negative bacterial inner membrane was determined by measuring the cytoplasm β-galactosidase activity. LRpG and LRα were able to induce an increase in the inner membrane permeability from 0 to 40 min. However, LRα displayed an optical density value at 420 nm (OD420) much higher than LRpG at the same point in time. This phenomenon was different from the results of outer membrane permeability (Figure 4).
Figure 4.
Inner membrane permeability of (a) LRpG and (b) LRα at different concentrations. The diagrams are based on at least three independent experiments.
2.7.3. Cytoplasmic Membrane Depolarization
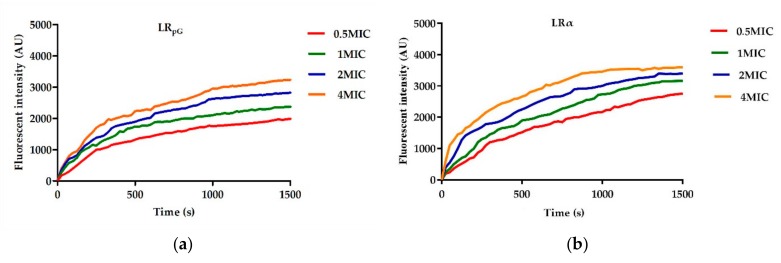
In addition to inner and outer membrane permeability, changes in the plasma membrane potential energy of E. coli ATCC25922 were determined using 3,3’-dipropylthiadicarbocyanine (diSC3-5). The depolarization ability of cell membranes induced by LRpG and LRα was dose- and time-dependent over 1500 s of measured time. This experiment showed that LRα had a faster and stronger depolarization ability than LRpG (Figure 5).
Figure 5.
Cytoplasmic membrane potential variation of E. coli ATCC25922 treated with (a) LRpG and (b) LRα at different levels of concentration. The diagram is based on at least three independent experiments.
2.7.4. Scanning Electron Microscopy (SEM)
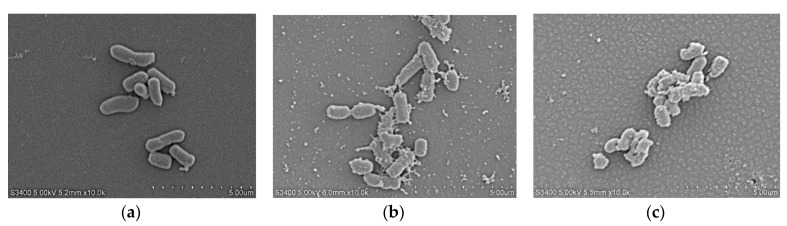
The cell morphology and membrane damage of E. coli ATCC25922 after LRpG and melittin treatment were observed by SEM. In the control group, E. coli cells had a smooth and bright surface (Figure 6a), while those treated with peptides showed a rough surface with blebbing, as well as shrunken and destroyed shapes. The effect observed in the SEM micrographs was stronger in the cells treated with LRpG than with the control (Figure 6b,c).
Figure 6.
SEM micrographs of E. coli ATCC25922: (a) control; (b) LRpG-treated; (c) LRα-treated.
2.7.5. DNA Binding Assay
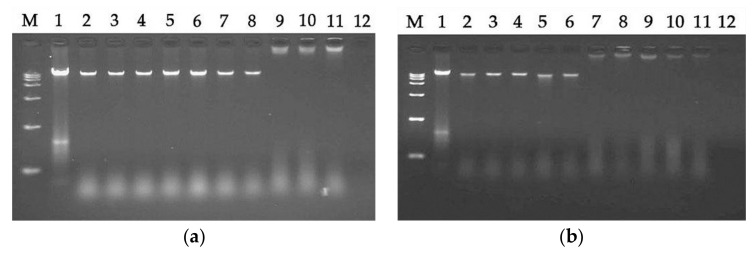
The antibacterial effects of AMPs are exerted not only by acting on cell membranes, but also by binding to intracellular substances, such as DNA. Therefore, the ability to induce intracellular effects was measured using a DNA binding analysis. As shown in Figure 7, LRpG and LRα exhibited binding ability at 64 μM and 16 μM, respectively. This experiment showed that LRα had a stronger DNA binding ability than LRpG.
Figure 7.
A gel retardation experiment was used to measure the DNA binding assay: (a) LRpG-treated; (b) LRα-treated. M: DNA marker alone; 1: genomic DNA alone; 2: with 0.5 μM peptide; 3: with 1 μM peptide; 4: with 2 μM peptide; 5: with 4 μM peptide; 6: with 8 μM peptide; 7: with 16 μM peptide; 8: with 32 μM peptide; 9: with 64 μM peptide; 10: with 128 μM peptide; 11: with 256 μM peptide; 12: 256 μM peptide alone.
2.7.6. Lipopolysaccharide (LPS) Binding Assay
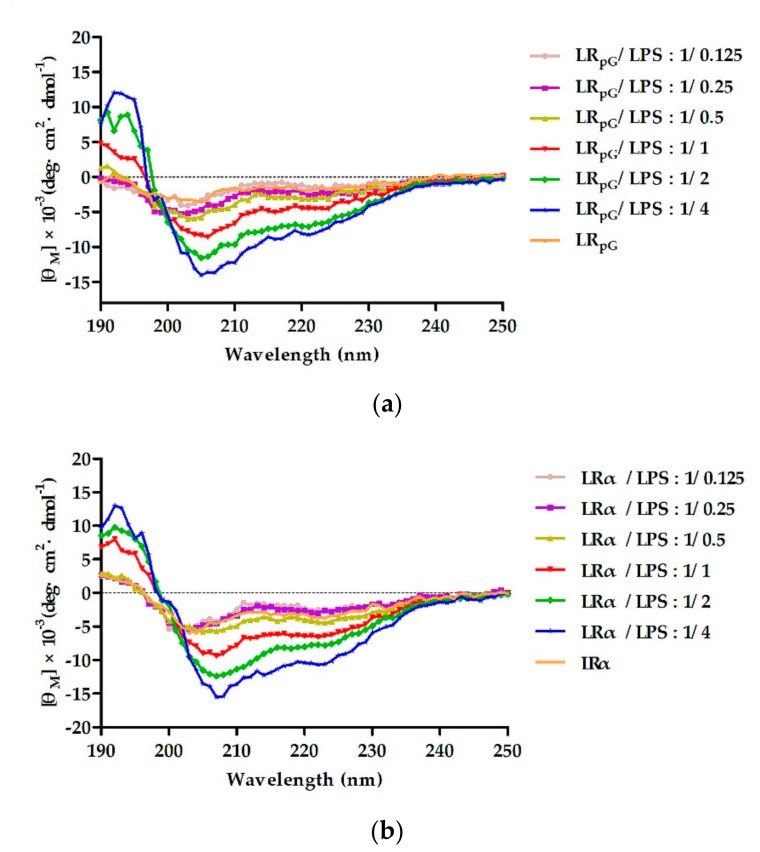
To detect the binding ability between peptides and LPS, the secondary structure of the peptides was measured using the CD spectrum in different concentrations of LPS. As shown in Figure 8, in 10 mM PBS without LPS solution, LRpG exhibited disordered conformations and LRα exhibited a more ordered conformation. However, with the increase in LPS concentration, LRpG and LRα tended to gradually form an α-helical structure. At the molar ratio peptide/LPS of 1:1, both LRpG and LRα began forming an α-helical structure, indicating a similar binding ability to LPS.
Figure 8.
The circular dichroism (CD) spectra of (a) LRpG and (b) LRα were dissolved in different concentrations of lipopolysaccharide (LPS), and the peptide/LPS molar ratio ranged from 1/0.125 to 1/4. The average value after three scans of every sample is shown. The CD spectrum of LPS was subtracted.
2.7.7. Limulus Amoebocyte Lysate (LAL) Assay
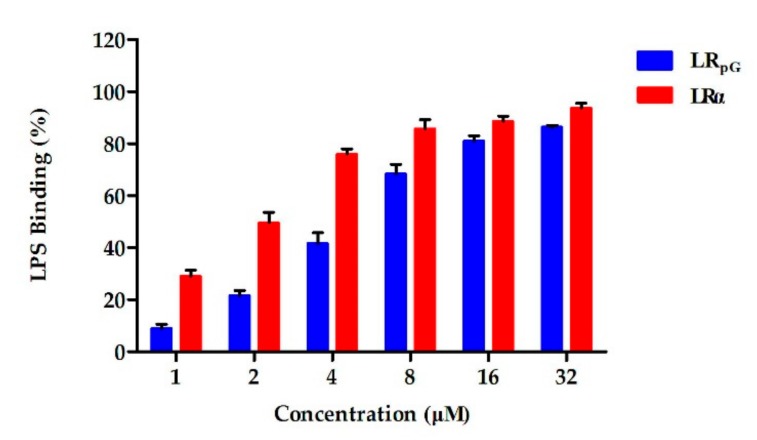
The chromogenic limulus amoebocyte lysate (LAL) assay was used to analyze the binding ability of the peptide to lipopolysaccharide (LPS). As shown in Figure 9, the activation of the LPS-mediated LAL coagulase was effectively inhibited by both LRpG and LRα in a concentration-dependent manner. At low concentrations, the binding ability of LRpG to LPS was much stronger than LRα, but the difference tended to be diminished with the increase in peptide concentration.
Figure 9.
The binding ability of LRpG and LRα to lipopolysaccharide (LPS), determined by limulus amoebocyte lysate (LAL) assay. The diagram is based on at least three independent experiments.
2.7.8. Endotoxin Neutralization Assay
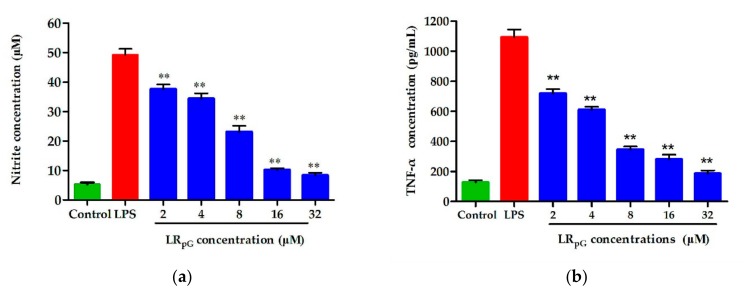
To detect the inhibitory effect of LRpG on the inflammatory response induced by LPS, the expression levels of two major inflammatory cytokines, tumor necrosis factor α (TNF-α) and nitric oxide (NO), in the macrophage supernatant were measured. Cells without stimulation and cells stimulated with only LPS (100 ng·mL−1) were used as negative and positive controls, respectively. As can be seen from Figure 10, LRpG restrained the production of NO and TNF-α in a concentration-dependent manner and exhibited a significant inhibitory effect at 16 μM. The results showed that LRpG had a good neutralization ability in LPS.
Figure 10.
Effects of LRpG on the production of (a) nitric oxide (NO) and (b) tumor necrosis factor α (TNF-α) in LPS-stimulated RAW 264.7 cells. The diagrams are based on the average of three independent experiments. ** p < 0.01, compared to the positive group (only LPS-stimulated).
3. Discussion
Most AMPs have unique mechanisms of killing bacteria, mainly involving membrane breaking, which endows them with the potential to be alternatives to antibiotics [2]. However, the progress of natural AMPs for therapeutic application is seriously hindered by their inherent defects (such as manufacturing costs, cytotoxicity, poor stability etc.) [2,8]. To overcome these inherent shortcomings, in this study, six symmetric heptad repeat sequences were connected by a short loop, and the sequence was designed as XXRXXRRzzRRXXRXX-NH2 (X represents F, I, and L; zz represents GG or pG).
The results of MICs indicated that the designed peptides had good antibacterial activity toward Gram-negative bacteria (Table 2). Compared to the single membrane and thick peptidoglycan layer of Gram-positive bacteria, the double-membrane structure of Gram-negative bacteria represents a weaker barrier; thus, Gram-negative bacteria are more sensitive to AMPs [17,21,24]. The antibacterial activity of AMPs can be affected by many factors. Previous studies demonstrated that a proper positive charge (+6 to +7) was essential for antibacterial activity, but antibacterial activity was no longer increased when the positive charge of AMPs was beyond the threshold [17,20]. In this study, the positive charge of the designed peptides was set to +6. Based on their hydrophobicity, the theoretical antibacterial activity of the designed peptides would be IRZZ > FRZZ > LRZZ = LRα; however, the result was LRα > LRZZ > FRZZ > IRZZ. Combined with the results of CD, although both LRZZ and LRα had the tendency to form an α-helical structure in membrane-mimetic environments, LRα’s tendency was stronger. One possible explanation is that the rigid loop (pG) of LRZZ disrupted the α-helical structure. Furthermore, we found that both FRZZ and IRZZ exhibited disordered conformations in membrane-mimetic environments. The result further confirmed the previous perspectives that the antibacterial activity of designed peptides is not only connected to the hydrophobicity of the peptides but also to the spatial stability of the secondary structure [25]. In addition, the antibacterial activity of XRpG was higher than that of XRGG. Some papers confirmed that the rigid loop (pG), which promotes tightness in the hydrophobic center, has a better effect than the flexible loop (GG) [20].
However, the antimicrobial activity of AMPs is not the only criterion when evaluating their potential for clinical application. The hemolytic activity and cytotoxicity to mammalian cells are also major limiting factors [26,27]. Therefore, the cytotoxicity and hemolytic activity of the designed peptides were tested. At all examined concentrations, the designed peptides had much lower hemolysis and cytotoxicity (Figure 2), showing better cell selectivity compared to LRα. This result may be due to the insertion of the central loop as previous papers reported. The cell selectivity of XRpG was better than that of XRGG, which may be due to the rigid pG having a better effect on promoting cell selectivity than flexible GG [20]. As shown in Table 2, LRpG had a great balance between antibacterial activity and cytotoxicity, and it had higher cell selectivity.
In addition to the cell selectivity assays of AMPs, stability is also a major reason impeding AMPs from becoming therapeutic agents [2,28,29]. In this study, the effects of the physiological concentrations of salts, temperature, and pH on the antibacterial activity of the designed peptides were investigated. As shown in Table 3, in salt ions, the antimicrobial activity of LRpG against E. coli ATCC25922 was negligibly affected by all tested cations except for Na+. Previous reports suggested that a stable α-helical structure helps salt tolerance [30]. However, the insertion of the loop (pG) structure into the sequence template still resulted in good salt stability in this study. The result was due to the loop (pG) promoting hydrogen bonding and hydrophobic interactions, thereby achieving the effect of salt tolerance [31]. The antibacterial activity of LRpG against E. coli ATCC25922 decreased because of the disruption of electrostatic interaction between LRpG and the cell membrane in the presence of 150 mM Na+ [32]. For the clinical application of AMPs, thermal stability is very important since many foods and feed products need to be heated during processing [33]. LRpG exerted good thermal stability (Table 4) and could maintain antibacterial activity in different pH conditions.
Furthermore, LRpG exhibited a synergistic effect with streptomycin and additive effects with other antibiotics against E. coli ATCC25922 (Table 5). Some studies reported that the antibacterial effect of streptomycin and chloramphenicol involved acting on ribosomes, while ciprofloxacin acted on DNA gyrase, and cefotaxime acted on the cell membrane. In this study, LRpG acted on cell membranes to increase membrane permeability and promote antibiotic entry into cells [34,35]. The combination of LRpG and antibiotics improved the binding efficiency and ultimately enhanced the therapeutic efficacy. Therefore, LRpG can serve as an additive to decrease the dose of antibiotics.
In this study, we also compared a natural AMP (melittin) to LRpG in the GMMIC, MHC10, GMSI, and stability results. Although it exhibited a slightly better antibacterial activity than LRpG, melittin had a strong hemolysis effect. By calculating the GMSI values (MHC10/GMMIC), we found that the GMSI of LRpG was 584 times higher than that of melittin. Furthermore, LRpG exerted a good conditional stability and additive effects with conventional antibiotics. Based on the results above, LRpG overcomes the main limiting factors of natural AMPs (such as melittin) and has the potential to develop into a therapeutic agent. Therefore, we further studied the antibacterial mechanism of LRpG. Previous studies demonstrated that cationic AMPs combine with anions on the surface of bacteria via electrostatic action, and their hydrophobic amino acids are inserted into the bacterial cell membrane. Once the critical concentration is exceeded, the membrane breaks, and this ultimately leads to death of the bacteria [21,22]. LRpG firstly bound to the LPS of the outer membrane of E. coli ATCC25922 through electrostatic action (Figure 8), and then destroyed the cell membrane. Compared to the outer membrane, LRpG had a much weaker permeability to the inner membrane than LRα (Figure 3 and Figure 4). This result might be due to the decreased helical tendency of the LRpG as a result of the loop (pG), which was consistent with the previous papers showing that the helical tendency of AMPs was closely related to the permeability of bacterial inner membrane [20]. After breaking the impermeable barrier, LRpG interacted with the cytoplasmic membrane, resulting in pore and ion channel formation (Figure 5). SEM results directly confirmed that LRpG killed E. coli (Figure 6) by destroying the bacterial cell membranes and causing intracellular lysate leakage. In addition to the membrane mechanisms mentioned above, LRpG can bind to intracellular material DNA at a concentration much higher than the MIC (Figure 7), suggesting that LRpG cannot kill bacteria by DNA binding at the MIC. Furthermore, we found that the DNA binding ability of LRpG was relatively weaker than that of LRα. One possible reason is that the α-helical forming ability of LRpG was weaker than that of LRα. In short, LRpG exerted its antibacterial effect by destroying the integrity of the bacterial cell membrane, causing leakage of the cellular content and binding to DNA through the cell membrane.
LPS is a crucial component of the outer membrane in Gram-negative bacteria [36]. Following bacterial death under the action of antibacterial agents, a large amount of LPS detaches from the cells and enters into the blood circulation of the body, activates the inflammatory signaling pathway, and releases inflammatory mediators such as NO and TNF-α [37,38]. Previous studies showed that positively charged amphipathic AMPs had a strong LPS neutralization ability and were ideal candidates for anti-inflammatory drugs [39,40]. The LPS-induced inflammatory mediators NO and TNF-α were remarkably inhibited by LRpG in a concentration-dependent manner (Figure 10). Based on this study, LRpG has the potential to develop into an anti-inflammatory agent by blocking LPS-mediated inflammatory mediators.
4. Materials and Methods
4.1. Materials
Escherichia coli (E. coli) ATCC25922, E. coli K88, Pseudomonas aeruginosa (P. aeruginosa) ATCC27853, Salmonella Pullorum (S. Pullorum) NCTC5776, Klebsiella pneumoniae (K. pneumoniae) CMCC46117, Staphylococcus aureus (S. aureus) ATCC25923, S. aureus ATCC29213, methicillin-resistant S. aureus ATCC43300, and Enterococcus faecalis (E. faecalis) ATCC29212 were obtained from the pharmacology and toxicology laboratory of the College of Animal Science and Technology, Jilin Agricultural University (Changchun, China). HEK293T and RAW 264.7 cells were obtained from the College of Animal Science and Technology, Jilin Agricultural University (Changchun, China). The hRBCs were obtained from the Jilin Agricultural University Hospital.
Mueller–Hinton broth (MHB) powder and Mueller–Hinton agar (MHA) were purchased from GL Biochem (Shanghai, China). Bovine serum albumin (BSA), Triton X-100, and phosphate-buffered saline (PBS) solution were purchased from Solarbio (Beijing, China). O-nitrophenyl-β-d-galactopyranoside (ONPG), LPS from E. coli 0111 B4, diSC3-5, polymyxin B, 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), and NPN were all purchased from Sigma-Aldrich (Shanghai, China). TransDetect Cell Counting Kit-8 (CCK-8) and Dulbecco’s modified Eagle’s medium with high glucose (DMEM) were purchased from TransGen Biotech (Beijing, China). Fetal bovine serum (FBS) was purchased from Gibco (Shanghai, China).
4.2. Synthesis and Sequence Analysis of Peptides
The designed peptides were sent to GL Biochem (Shanghai, China) for synthesis and purification. The purity of the peptide was determined by reverse-phase high-performance liquid chromatography on an analytical Kromasil C18 column (Beijing, China). The purity of the peptide was over 95% (RP-HPLC). ESI-MS analysis was used to measure the molecular weight of designed peptides, and values were very close to the calculated values. The primary physical and chemical parameters of the designed peptides were measured online at http://web.expasy.org/compute_pi/. The hydrophobicity and relative hydrophobic moments were measured online at http://heliquest.ipmc.cnrs.fr/.
4.3. CD Spectroscopy
The CD Spectroscopy of the designed peptides was detected on a J-810 spectropolarimeter (Jasco, Tokyo, Japan) at 25 °C. The final concentration of peptides in 10 mM PBS (pH 7.4), 50% TFE, and 30 mM SDS was 150 µM. The experimental parameters were as follows: 10 nm·min−1 scanning rate, 1 mm path length, and 190–250 nm wavelength range. Each peptide was scanned an average of three times. The collected data were transformed to mean residue ellipticity as follows:
| (1) |
where θM represents the mean residue ellipticity (deg·cm2·dmol−1), θobs represents the ellipticity correction of that measurement buffer at a given wavelength (mdeg), l represents the path length (cm), c represents the peptide concentration (mM), and n represents the number of amino residues.
4.4. MIC Measurements
The antimicrobial activity of the designed peptides was examined using the micro-dilution method described previously [29]. The bacteria were cultured for 16 h in MHB at 37 °C, and the microbial suspension was diluted to a final concentration of 105 colony-forming units (CFU)/mL. Then, 50 µL of MHB containing different concentrations peptides (0.25–256 µM) was mixed with 50 µL of bacterial solution, and the mixture was added to a 96-well plate. After incubation for 16 h, MIC was examined by measuring the OD at 492 nm (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria). The assays were performed three times.
4.5. Biocompatibility Assays
The hemolytic activity was examined based on the method mentioned previously [22]. Briefly, the collected hRBCs were washed three times and diluted at 2% (v/v) with PBS (pH 7.4). Then, 100 µL of hRBCs were put into each tube including 100 µL of two-fold diluted peptides. The mixtures were centrifuged at 1000× g for 3 min. The OD value at 570 nm of the supernatant was measured, describing hemoglobin release (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria). For this assay, 1% Triton X-100-treated hRBCs were used as a positive control and untreated hRBCs were used as a negative control. MHC10 was defined as the peptide concentration that caused 10% hemolysis. The assays were performed three times.
The cytotoxicity was detected according to a CCK-8 assay as described previously. Briefly, RAW 264.7 and HEK293T cells were placed into 96-well plates at a density of 1.0–2.0 × 105, and then cultured for 16 h under conditions of 5% CO2. Then, two-fold diluted peptides in DMEM were added to the plates. After 16 h of culturing, CCK-8 (10%, v/v) was put into each well containing cell culture, and mixtures were cultured for 2 h. The cytotoxicity assay was examined by measuring the OD of the mixtures at 450 nm (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria). The assays were performed three times.
4.6. Condition Sensitivity Assays
The salt, temperature, and pH sensitivities of designed peptides were determined by MIC assay as described previously [40]. For the salt sensitivity assay, peptides were diluted in deionized water containing different salts, and mixed with E. coli ATCC25922 (105 CFU/mL). The final physiological concentrations of different salts were as follows: 150 mM NaCl, 4.5 mM KCl, 6 μM NH4Cl, 1 mM MgCl2, 8 μM ZnCl2, and 4 μM FeCl3. For temperature sensitivity, peptides were cooled on ice for 10 min, and incubated at 37 °C and 100 °C for 30 min. For pH sensitivity, peptides were treated with different pH for 1 h. Briefly, peptides were diluted in deionized water, and the pH of the solution was adjusted to 4.0, 6.0, 8.0, and 10.0 with HCl or NaOH. The subsequent steps were the same as for the MIC measurements. These tests were performed three times.
4.7. Synergy with Conventional Antibiotics
The combined antibacterial effect of peptides with other antibiotics was evaluated by checkerboard assay as described previously [31]. Briefly, peptides and antibiotics diluted two-fold were prepared at concentrations ranging from 1/8× MIC to 2× MIC. Subsequently, each peptide at the same concentration was added to the longitudinal column of wells, and each antibiotic at the same concentration was added to the horizontal row of wells. A suspension at 105 CFU/mL was put into each well for 16 h at 37 °C. Each assay was performed three times. The fractional inhibitory concentration (FIC) index was calculated as follows:
| (2) |
where FIC ≤ 0.5 denotes synergy, 0.5 < FIC ≤ 1.0 denotes additive, and 1.0 < FIC ≤ 4.0 denotes indifferent.
4.8. Outer Membrane Permeability Assay
The outer membrane permeability was examined using fluorescent dye NPN according to the method mentioned previously [17,21,41]. Briefly, E. coli ATCC25922 cells were washed three times and diluted to 105 CFU/mL in 5 mM HEPES buffer (pH 7.4, containing 5 mM glucose). Then, the final concentration of 10 μM NPN was added to the suspension, and incubated at room temperature in the dark for 30 min. An equal volume of suspension and peptides at various concentrations were mixed in a black 96-well plate. The fluorescence intensities of the samples were measured (emission λ = 420 nm, excitation λ = 350 nm) with an F4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). Polymyxin B (10 µg/mL) with strong outer membrane permeability was used as a positive control. Values were converted to percentage NPN uptake using the following equation:
| (3) |
where Fobs is the observed fluorescence of NPN with E. coli ATCC25922 cells at a given peptide concentration, F0 is the initial fluorescence of NPN with E. coli ATCC25922 cells, and F100 is the fluorescence of NPN with E. coli ATCC25922 cells with the addition of 10 µg/mL polymyxin B.
4.9. Inner Membrane Permeability Assay
The inner membrane permeabilization was determined by measuring the cytoplasm β-galactosidase activity according to the method mentioned previously [21]. In short, an equal volume of the suspension of E. coli ATCC25922 cells was washed three times and diluted to an OD600 of 0.05 with 10 mM PBS (containing 1.5 mM ONPG); then, the suspension was put into each well of a 96-well plate and cultured with 0.5× MIC to 4× MIC peptides at 37 °C. The OD at 420 nm (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria) was recorded for 40 min every 5 min eight times. The method was used to detect the permeabilization of the inner membrane through the reflection of the ONPG influx into the cells.
4.10. Cytoplasmic Membrane Depolarization Assay
DiSC3-5, a membrane potential-sensitive fluorescent dye, was used to assess the ability of the peptides to disrupt the cytoplasmic membrane as previously described, [42]. Briefly, E. coli ATCC25922 in the mid-log phase was washed three times and diluted to an OD600 of 0.05 with 5 mM HEPES buffer (containing 20 mM glucose and 100 mM KCl); then, the mixture of E. coli ATCC25922 and 0.5× MIC to 4× MIC peptides was mixed with diSC3-5 (final concentration of 0.4 μM) at 37 °C for 1 h. The fluorescence changes from 0 to 1500 s were recorded at the emission wavelength (λ = 670 nm) and excitation wavelength (λ = 622 nm) (Fluorescence Spectrophotometer, F4500, Hitachi, Tokyo, Japan).
4.11. Scanning Electron Microscopy
E. coli ATCC25922 cells in the mid-log phase were washed three times and diluted to 107 CFU/mL in 10 mM PBS. The bacterial suspension and peptide (1× MIC) were added to 24-well plates containing polylysine-treated glass slides. The bacteria untreated by peptide were used as a control. After incubation, the bacterial suspension was removed, and the treatment method with polylysine-glass slides was repeated. Then, the polylysine-treated glass slides were fixed with glutaraldehyde (2.5%) at 4 °C for 12 h and dehydrated with a series of graded ethanol solutions for 20 min. The polylysine-treated glass slides were transferred into a mixture (v:v = 1:1) of pure alcohol and tertiary butanol for 15 min, and then placed into pure tertiary butanol for 15 min. Finally, the samples were treated using a critical point dryer with liquid CO2, and then scanned by a S-3400N SEM (Hitachi, Tokyo, Japan) after coating with gold-palladium.
4.12. DNA Binding Assay
The DNA binding assays were determined according to gel retardation experiments described previously [17]. Briefly, 400 ng of genomic DNA (E. coli ATCC25922) was mixed with 0.5 to 256 µM peptide in a binding buffer (50 µg/mL BSA, 1 mM ethylene diamine tetraacetic acid (EDTA), 20 mM KCl, 10 mM Tris-HCl (pH = 8.0), 5% glycerol, and 1 mM dithiothreitol) for 60 min at 37 °C. Subsequently, the samples were examined by 1% agarose gel electrophoresis.
4.13. LPS Binding Assay
The ability of peptides to bind LPS was detected on a J-810 spectropolarimeter at 25 °C. Briefly, concentration of LPS ranged from 37.5–600 µM, diluted using the double dilution method. The final concentration of peptides in the LPS solution was 150 µM. The experimental parameters were the same as in Section 4.3.
4.14. LAL Assay
Peptide neutralization was evaluated using a quantitative chromogenic limulus amoebocyte lysate (LAL) assay (Xiamen, China) [43]. Briefly, 1 endotoxin unit (EU) of LPS was incubated with two-fold serial dilutions of peptides at 37 °C for 60 min; then, 100 µL of LAL reagent was added to the mixtures. After additional incubation for 15 min at 37 °C, 100 µL of chromogenic substrate solution was put into the tubes. Subsequently, the mixtures were incubated for another 10 min at 37 °C before adding 500 µL of azo reagent solutions (1, 2, and 3) in turn. The data were examined by measuring the OD value at 545 nm (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria).
4.15. Endotoxin Neutralization Assay
RAW 264.7 cells were put into 24-well plates at a density of 1.0–2.0 × 105 cells/well and stimulated with LPS (100 ng/mL) with (1–64 μM) or without peptide. After incubating for 24 h at 37 °C in 5% CO2, the supernatants were collected for analysis of the NO production using Griess reagent and TNF-α levels using ELISA based on the manufacturer’s protocol. The cells treated with LPS only and untreated cells were set as the positive and negative controls, respectively.
4.16. Statistical Analysis
ANOVA was used to analyze the data through the SPSS 18.0 software (IBM, Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation. A p-value < 0.01 was considered to have statistical significance.
5. Conclusions
In this study, we designed peptides based on the sequence template XXRXXRRzzRRXXRXX-NH2, where X represents a hydrophobic amino acid (F, I, and L) and zz represents the loop (GG or pG). The designed peptides effectively enhanced cell selectivity because of the introduction of the loop (GG or pG). Among them, LRpG (X: L, zz: pG) showed the highest average therapeutic index (GMTI = 73.1), with better conditional stability, and it had an additive effect with conventional antibiotics. The antibacterial mechanism study demonstrated that LRpG first destroyed the integrity of the bacterial cell membrane, caused leakage of the cellular content, then entered the cell and bound to the DNA, and ultimately led to bacterial death. Furthermore, LRpG also had an obvious impact on anti-inflammatory ability. These results indicated that the design and optimization of natural AMPs in this study will be helpful in improving the clinical application of AMPs.
Abbreviations
| AMPs | antimicrobial peptides |
| CD | circular dichroism |
| MIC | minimal inhibitory concentration |
| TI | theoretical index |
| hRBCs | human red blood cells |
| E. coli | Escherichia coli |
| FICI | fractional inhibitory concentration index |
| SEM | scanning electron microscopy |
| LPS | lipopolysaccharide |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/3/1140/s1.
Author Contributions
Conceptualization, B.J.; funding acquisition, H.M.; supervision, H.M. and X.J.; project administration, H.M. and X.J.; investigation, Z.W., Y.W., Z.P., and L.K.; validation, Y.Z., X.W., and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number: 31572574, 31872519 and 31572555), the National Key Research and Development Plan (Grant number: 2017YFD0501401), the Theme Guidance Project of Development of Science and Technology of Jilin Province (Grant number: 20160204002NY), the Natural Science Foundation of Jilin Province (Grant number: 20180101003JC), the Jilin Programs for Technology Research and Development (Grant number: 20170101025JC and 20170101021JC), and the Jilin Department of Education Programs for Science and Technology Research (Grant number: 201441).
Conflicts of Interest
The authors declare that no conflict of interest.
References
- 1.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J.J., Dou X.J., Song J., Lyu Y.F., Zhu X., Xu L., Li W.Z., Shan A.S. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019;39:831–859. doi: 10.1002/med.21542. [DOI] [PubMed] [Google Scholar]
- 3.Tellez G.A., Zapata J.A., Toro L.J., Henao D.C., Bedoya J.P., Rivera J.D., Trujillo J.Y., Rivas-Santiago B., Hoyos R.O., Castano J.C. Identification, characterization, immunolocalization, and biological activity of lucilin peptide. Acta Trop. 2018;185:318–326. doi: 10.1016/j.actatropica.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen C.X., Chen Y.C., Yang C., Zeng P., Xu H., Pan F., Lu J.R. High selective performance of designed antibacterial and anticancer peptide amphiphiles. ACS Appl. Mater. Interfaces. 2015;7:17346–17355. doi: 10.1021/acsami.5b04547. [DOI] [PubMed] [Google Scholar]
- 5.Omardien S., Brul S., Zaat S.A. Antimicrobial activity of cationic antimicrobial peptides against gram-positives: Current progress made in understanding the mode of action and the response of bacteria. Front. Cell Dev. Biol. 2016;4:111. doi: 10.3389/fcell.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y.H., Chen T.W., Pan Z.M., Sun X.B., Yin X., He M., Xiao S.Y., Liang H.J. Theoretical insights into the interactions between star-shaped antimicrobial polypeptides and bacterial membranes. Langmuir. 2018;34:13438–13448. doi: 10.1021/acs.langmuir.8b02677. [DOI] [PubMed] [Google Scholar]
- 7.Melo M.N., Ferre R., Castanho M.A. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 8.Ong Z.Y., Wiradharma N., Yang Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014;78:28–45. doi: 10.1016/j.addr.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi A.K., Kumari T., Tandon A., Sayeed M., Afshan T., Kathuria M., Shukla P.K., Mitra K., Ghosh J.K. Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat. Acta Biomater. 2017;57:170–186. doi: 10.1016/j.actbio.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A., Azmi S., Srivastava R.M., Srivastava S., Pandey B.K., Saxena R., Bajpai V.K., Ghosh J.K. Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: The role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry. 2009;48:10905–10917. doi: 10.1021/bi9009874. [DOI] [PubMed] [Google Scholar]
- 11.Asthana N., Yadav S.P., Ghosh J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004;279:55042–55050. doi: 10.1074/jbc.M408881200. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A., Tripathi A.K., Kathuria M., Shree S., Tripathi J.K., Purshottam R.K., Ramachandran R., Mitra K., Ghosh J.K. Single Amino acid substitutions at specific positions of the heptad repeat sequence of piscidin-1 yielded novel analogs that show low cytotoxicity and in vitro and in vivo antiendotoxin activity. Antimicrob. Agents Chemother. 2016;60:3687–3699. doi: 10.1128/AAC.02341-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava R.M., Srivastava S.M., Singh M., Bajpai V.K., Ghosh J.K. Consequences of alteration in leucine zipper sequence of melittin in its neutralization of lipopolysaccharide-induced proinflammatory response in macrophage cells and interaction with lipopolysaccharide. J. Biol. Chem. 2012;287:1980–1995. doi: 10.1074/jbc.M111.302893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong N., Ma Q.Q., Shan A.S., Lv Y.F., Hu W.N., Gu Y., Li Y.Z. Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob. Agents Chemother. 2012;56:2994–3003. doi: 10.1128/AAC.06327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou S.L., Shao C.X., Wang J.J., Shan A.S., Xu L., Dong N., Li Z.Y. Short, multiple-stranded β-hairpin peptides have antimicrobial potency with high selectivity and salt resistance. Acta Biomater. 2016;30:78–93. doi: 10.1016/j.actbio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Dong N., Chou S.L., Li J.W., Xue C.Y., Li X.R., Chen B.J., Shan A.S. Short symmetric-end antimicrobial peptides centered on β-turn amino acids unit improve selectivity and stability. Front. Microbiol. 2018;27:2832. doi: 10.3389/fmicb.2018.02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.J., Chou S.L., Xu L., Zhu X., Dong N., Shan A.S., Chen Z. High specific selectivity and membrane-active mechanism of the synthetic centrosymmetric α-helical peptides with gly-gly pairs. Sci. Rep. 2015;5:15963. doi: 10.1038/srep15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou S.L., Wang J.J., Shang L., Akhtar M.U., Wang Z.H., Shi B.M., Feng X.J., Shan A.S. Short, symmetric-helical peptides have narrow-spectrum activity with low resistance potential and high selectivity. Biomater. Sci. 2019;7:2394–2409. doi: 10.1039/C9BM00044E. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z., Yang J., Han J.Z., Gao L., Liu H.X., Lu Z.X., Zhao H.Z., Bie X.M. Insights into the antimicrobial activity and cytotoxicity of engineered α-helical peptide amphiphiles. J. Med. Chem. 2016;59:10946–10962. doi: 10.1021/acs.jmedchem.6b00922. [DOI] [PubMed] [Google Scholar]
- 20.Shao C.X., Tian H.T., Wang T.Y., Wang Z.H., Chou S.L., Shan A.S., Chen B.J. Central β-turn increases the cell selectivity of imperfectly amphipathic α-helical peptides. Acta Biomater. 2018;69:243–255. doi: 10.1016/j.actbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Dong N., Wang C.S., Zhang T.T., Zhang L., Xue C.Y., Feng X.J., Bi C.P., Shan A.S. Bioactivity and bactericidal mechanism of histidine-rich β-hairpin peptide against Gram-negative bacteria. Int. J. Mol. Sci. 2019;20:3954. doi: 10.3390/ijms20163954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao C.X., Li W.Z., Tan P., Shan A.S., Dou X.J., Ma D.Y., Liu C.Y. Symmetrical modification of minimized dermaseptins to extend the spectrum of antimicrobials with endotoxin neutralization potency. Int. J. Mol. Sci. 2019;20:1417. doi: 10.3390/ijms20061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelezetsky I., Tossi A. Alpha-helical antimicrobial peptides-using a sequence template to guide structure-activity relationship studies. Biochim. Biophys. Acta. 2006;1758:1436–1449. doi: 10.1016/j.bbamem.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi D., Shukla S.K., Prakash O., Zhang G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie. 2010;92:1236–1241. doi: 10.1016/j.biochi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A., Mahajan M., Awasthi B., Tandon A., Harioudh M.K., Shree S., Singh P., Shukla P.K., Ramachandran R., Mitra K., et al. Piscidin-1-analogs with double L- and D-lysine residues exhibited different conformations in lipopolysaccharide but comparable anti-endotoxin activities. Sci. Rep. 2017;7:39925. doi: 10.1038/srep39925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J.K., Park S.C., Hahm K.S., Park Y. A helix-PXXP-helix peptide with antibacterial activity without cytotoxicity against MDRPA-infected mice. Biomaterials. 2014;35:1025–1039. doi: 10.1016/j.biomaterials.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Zhang L.C., Wang J., Ma Z., Xu W., Li J.P., Shan A.S. Characterization of antimicrobial activity and mechanisms of low amphipathic peptides with different α-helical propensity. Acta Biomater. 2015;18:155–167. doi: 10.1016/j.actbio.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Wang J.J., Chou S.L., Yang Z.Y., Yang Y., Wang Z.H., Song J., Dou X.J., Shan A.S. Combating drug-resistant fungi with novel imperfectly amphipathic palindromic peptides. J. Med. Chem. 2018;61:3889–3907. doi: 10.1021/acs.jmedchem.7b01729. [DOI] [PubMed] [Google Scholar]
- 30.Park I.Y., Cho J.H., Kim K.S., Kim Y.B., Kim M.S., Kim S.C. Helix stability confers salt resistance upon helical antimicrobial peptides. J. Biol. Chem. 2004;279:13896–13901. doi: 10.1074/jbc.M311418200. [DOI] [PubMed] [Google Scholar]
- 31.Jin Y., Hammer J., Pate M., Zhang Y., Zhu F., Zmuda E., Blazy J. Antimicrobial activities and structures of two linear cationic peptide families with various amphipathic β-sheet and α-helical potentials. Antimicrob. Agents Chemother. 2005;49:4957–4964. doi: 10.1128/AAC.49.12.4957-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arouri A., Dathe M., Blume A. The helical propensity of KLA amphipathic peptides enhances their binding to gel-state lipid membranes. Biophys. Chem. 2013;180:10–21. doi: 10.1016/j.bpc.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Ma Q.Q., Dong N., Shan A.S., Lv Y.F., Li Y.Z., Chen Z.H., Cheng B.J., Li Z.Y. Biochemical property and membrane-peptide interactions of de novo antimicrobial peptides designed by helix-forming units. Amino Acids. 2012;43:2527–2536. doi: 10.1007/s00726-012-1334-7. [DOI] [PubMed] [Google Scholar]
- 34.Khara J.S., Wang Y., Ke X.Y., Liu S., Newton S.M., Langford P.R., Yang Y.Y., Ee P.L. Anti-mycobacterial activities of synthetic cationic α-helical peptides and their synergism with rifampicin. Biomaterials. 2014;35:2032–2038. doi: 10.1016/j.biomaterials.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.F., Xia X., Xu L., Wang Y.Z. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials. 2013;34:237–250. doi: 10.1016/j.biomaterials.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Hancock R.E., Haney E.F., Gill E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016;16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J., Vincent J.L., Adhikari N.K., Machado F.R., Angus D.C., Calandra T., Jaton K., Giulieri S., Delaloye J., Opal S., et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 38.Zong X., Song D., Wang T., Xia X., Hu W., Han F., Wang Y. LFP-20, a porcine lactoferrin peptide, ameliorates LPS-induced inflammation via the MyD88/NF-κB and MyD88/MAPK signaling pathways. Dev. Comp. Immunol. 2015;52:123–131. doi: 10.1016/j.dci.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Khara J.S., Obuobi S., Wang Y., Hamilton M.S., Robertson B.D., Newton S.M., Yang Y.Y., Langford P.R., Ee P.L.R. Disruption of drug-resistant biofilms using, de novo, designed short α-helical antimicrobial peptides with idealized facial amphiphilicity. Acta Biomater. 2017;57:103–114. doi: 10.1016/j.actbio.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.J., Song J., Yang Z.Y., He S.Q., Yang Y., Feng X.J., Dou X.J., Shan A.S. Antimicrobial peptides with high proteolytic resistance for combating gram-negative bacteria. J. Med. Chem. 2019;62:2286–2304. doi: 10.1021/acs.jmedchem.8b01348. [DOI] [PubMed] [Google Scholar]
- 41.Dong N., Zhu X., Chou S., Shan A.S., Li W.Z., Jiang J.G. Antimicrobial potency and selectivity of simplified symmetricend peptides. Biomaterials. 2014;27:8028–8039. doi: 10.1016/j.biomaterials.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Lv X.T., Ma Q.Q., Zhu D.D., Shao C.X., Lv Y.F., Shan A.S. The C-terminal sequences of porcine thrombin are active as antimicrobial peptides. Chem. Biol. Drug Des. 2016;88:905–914. doi: 10.1111/cbdd.12824. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y., Dong W.B., Sun L., Ma L.J., Shang D.J. Insights into the membrane interaction mechanism and antibacterial properties of chensinin-1b. Biomaterials. 2015;37:299–311. doi: 10.1016/j.biomaterials.2014.10.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.