Abstract
To ensure the safety of the commercially available chenpi, a convenient and fast analytical method was developed for the determination of 133 pesticide residues in chenpi using gas chromatography-tandem mass spectrometry (GC-MS/MS). In this study, different extraction solvents, redissolution solvents and adsorbents were tested according to the recovery and purification effect to obtain a modified QuEChERS method. The samples were extracted with acetonitrile. During the clean-up step, octadecyl-modified silica (C18) and graphitized carbon black (GCB) were selected, and aminopropyl (NH2) was used instead of primary secondary amine (PSA) because of its weaker ion exchange capacity which had little effect on the recovery of ditalimfos. Samples were quantified by matrix-matched calibration with internal standards. All pesticides showed good linearity in the respective range, both with values of r2 > 0.99. The average recoveries of the pesticides spiked samples ranged from 70.0% to 112.2% with the RSDs of 0.2%–14.4%. The modified QuEChERS method was validated and applied to twenty real samples. Five pesticides were found in eight batches, but no pesticide exceeded the maximum residue limits (MRL, MRL reference to European commission).
Keywords: Pesticide residues, Chenpi, GC-MS/MS, QuEChERS
1. Introduction
Pericarpium citri Reticulatae (chenpi), the dried pericarp of the fruit of Citrus reliculate Blanco or its cultivars, is used in medicine and food [1]. As a food, chenpi has the effect of strengthening the spleen. As a traditional herb, chenpi is widely used to treat indigestion and inflammatory respiratory tract conditions [2].
Pesticide residues are detected frequently in commercially available chenpi. Relevant literature shows that pesticides pollution in chenpi is serious [3]. Pesticides are very toxic to humans and research has shown that some pesticides have teratogenic, carcinogenic and mutagenic effects [4], [5]. In 2016, Peng et al. [6] used gas chromatography to determine organophosphorus pesticides in chenpi and only 11 kinds of organophosphorus pesticides were determined by this method. Therefore, it is extremely important to establish a set of convenient and fast detection techniques for the determination of multiple pesticide residues in chenpi.
Sample preparation is a crucial step in all analytical methods and an appropriate clean-up method was developed for the extraction of pesticide residues with high selectivity, and low co-extraction. The most common sample preparation methods include solid-phase extraction (SPE) [7], [8], QuEChERS [9], solid-phase micro extraction (SPME) [10], [11] and gel permeation chromatography (GPC) [12], [13], [14].
The QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) method was developed by Anastassiades et al. in 2003 [15] and it has become one of the most commonly used methods for the determination of pesticides. A typical QuEChERS method involves an extraction with acetonitrile (and water in dry commodities), followed by a phase partitioning assisted by salting out and further clean up by d-SPE. [16], [17], [18], [19]. The method can be used to analyze many compounds, including highly polar pesticides and highly acidic compounds. It is suitable for the detection of samples with a low fat content and a high water content. Furthermore, the method has been applied to pesticide determination of many different matrices like vegetables, fruits and tea [20], [21], [22].
In the clean-up step, sorbent C18 and primary secondary amine (PSA) are used in most published methods, and florisil is used in some, but NH2 is seldom used. NH2 has a similar adsorption performance to PSA, while PSA contains two amino groups giving it a higher ion exchange capacity than NH2. However, PSA sorbents result in the pH value of the final extract solutions being more than 8, which affects the stability of base-sensitive pesticides [23] and NH2 can be used when PSA affects the determination of analytes.
In order to ensure food safety, in this study, a method was developed for multi-residue determination of pesticides in chenpi by GC-MS/MS and NH2 sorbent was used in a modified QuEChERS method because of the pesticides influenced by PSA.
2. Experimental
2.1. Chemicals and other materials
Pesticide standards and the internal standard (IS), chlorpyrifos-d10 with a purity >98%, were provided by Dr. Ehrenstorfer (Augsburg, Germany). HPLC grade acetonitrile was obtained from Omni Chem (Schaumburg, IL, USA). HPLC grade acetone, ethyl acetate and n-hexane were obtained from Merck (Darmstadt, Germany). Anhydrous magnesium sulfate (MgSO4) was obtained from Sigma-Aldrich (St. Louis, USA). Analytical reagent grade anhydrous sodium chloride (NaCl) was obtained from Weichen Chemical Reagent Co., Ltd (Tianjin, China). PSA, octadecyl-modified silica (C18), Florisil, graphitized carbon black (GCB) and aminopropyl (NH2) were supplied by DIKMA Technologies (Beijing, China).
2.2. Instruments
GC-MS/MS analyses were carried out with a Shimadzu GCMS-TQ8030 (Japan). A Hitachi CF 16RN centrifuge (Japan) and an Eppendorf centrifuge 5804 (Germany) were used for the 50 mL and 10 mL centrifuge tubes, respectively, along with a BUCHI rotary evaporator (Switzerland), KQ-500DE numerical control ultrasonic cleaner (China) and Eppendorf tube (EP, China).
2.3. Preparation of pesticide standards and internal standard solutions
Individual stock standard solutions of each pesticide (1 mg/mL) were prepared by weighing pesticides and dissolving them in n-hexane. Mixed solutions of multiple pesticides (2.5 μg/mL) were prepared by combining appropriate volume of each stock standard solution and stored in a freezer (−20 °C). A suitable amount of mixed standard reserve solution was transferred into 10 mL volumetric flask, and was diluted into matrix-matched standard working solutions with concentrations of 1, 2, 5, 10, 20, 50, 100 and 200 ng/mL respectively by blank (pesticide-free) chenpi extract. The internal standard solution, chlorpyrifos-d10, was prepared at a concentration of 1 mg/mL, and then diluted to 2 μg/mL.
2.4. Sample treatment and preparation
Each batch of chenpi was obtained from different markets in China. Pesticide residues can be easily extracted from small particle samples, so before use, all samples were ground to a powder mechanically, and passed through a no. 24 mesh sieve. (The particles retained in the sieve are not included for the analysis.)
2 g samples and 100 μL internal standard (2 μg/mL) were added to 50 mL polypropylene (PP) centrifuge tubes and then 10 mL acetonitrile was used for extraction. The ultrasonic extraction was carried out for 5 min and 0.8 g anhydrous MgSO4 and 0.2 g NaCl were added. Each mixture was shaken by hand for 1 min and centrifuged at 11,180 g (rcf) for 5 min. Then, 7 mL of the upper acetonitrile layer was transferred to a 10 mL EP tube containing 200 mg C18, 200 mg NH2, 200 mg anhydrous MgSO4 and 30 mg GCB. The solution was subjected to vortex mixing for 1 min, and then centrifuged at 7155 g (rcf) for 5 min, and 5 mL of the upper layer was transferred to a 50 mL round-bottom flask and evaporated to near dryness on a rotary vacuum evaporator at 40 °C. The dry residue was redissolved in 2 mL acetone for analysis by GC-MS/MS.
2.5. GC-MS/MS conditions
GC separation was performed on a DB-5MS IU capillary column (30 m × 0.25 mm × 0.25 µm; Agilent, America) and helium (purity ≥ 99.996%) was used as a carrier gas at a constant flow of 1.5 mL/min. The inlet temperature was set at 250 °C; the mode of inlet was splitless; the injection volume was 1 μL. The column temperature program is as follows: the initial temperature was maintained at 50 °C for 1 min, increased to 125 °C at a rate of 25 °C/min, raised to 230 °C at 4 °C/min, and then at 8 °C/min up to 310 °C, and held there for 3 min.
The mass spectrometer was operated with an electron impact (EI) source in multiple reaction monitoring (MRM) mode. The electron energy was 70 eV, and the ion and transfer line temperatures were set at 200 °C and 250 °C, respectively. In order to prevent instrument damage, the solvent delay was set at 3.5 min. Table 1 shows the optimized parameters of ion transition for 133 pesticide residues in chenpi.
Table 1.
GC-MS/MS acquisition parameters for 133 pesticide residues in chenpi.
| No. | Pesticides | Ion ratio (%) | tR(min) | Quantitative transition |
Qualitative transition |
||
|---|---|---|---|---|---|---|---|
| Precursor>product | CE/V | Precursor>product | CE/V | ||||
| IS | Chlorpyrifos-D10 | 28.37 | 22.122 | 324.0 > 260.0 | 15 | 324.0 > 195.0 | 30 |
| 1 | Dichlorvos | 29.96 | 6.585 | 185.0 > 93.0 | 14 | 185.0 > 109.0 | 14 |
| 2 | Mevinphos | 25.09 | 9.585 | 127.0 > 109.1 | 9 | 192.0 > 127.0 | 9 |
| 3 | Methacrifos | 26.34 | 11.112 | 240.0 > 208.0 | 4 | 240.0 > 180.0 | 10 |
| 4 | Isoprocarb | 20.26 | 13.571 | 136.0 > 121.0 | 9 | 121.0 > 103.1 | 12 |
| 5 | Propoxur | 12.34 | 13.571 | 152.1 > 110.1 | 8 | 152.1 > 64.0 | 28 |
| 6 | Ethoprophos | 20.32 | 14.228 | 158.0 > 97.0 | 18 | 158.0 > 114.0 | 6 |
| 7 | Dicrotophos | 28.07 | 14.885 | 127.1 > 109.0 | 12 | 127.1 > 95.0 | 18 |
| 8 | Phorate | 28.61 | 15.591 | 260.0 > 75.0 | 8 | 260.0 > 231.0 | 18 |
| 9 | α-hexachlorocyclohexane | 21.02 | 15.640 | 218.9 > 182.9 | 8 | 218.9 > 144.9 | 20 |
| 10 | Hexachlorobenzene | 21.31 | 15.762 | 283.8 > 248.8 | 24 | 283.8 > 213.8 | 28 |
| 11 | 2,6-dichloro-4-nitroaniline | 18.89 | 16.227 | 206.0 > 176.0 | 10 | 206.0 > 160.0 | 16 |
| 12 | Dimethoate | 29.65 | 16.276 | 125.0 > 47.0 | 14 | 125.0 > 79.0 | 8 |
| 13 | β-hexachlorocyclohexane | 29.41 | 16.794 | 218.9 > 182.9 | 8 | 218.9 > 144.9 | 20 |
| 14 | Pentachloroanisole | 30.00 | 16.917 | 265.0 > 237.0 | 15 | 280.0 > 265.0 | 10 |
| 15 | Quintozene | 22.09 | 16.917 | 294.8 > 236.8 | 16 | 294.8 > 264.8 | 12 |
| 16 | γ-hexachlorocyclohexane | 24.30 | 17.188 | 218.9 > 182.9 | 8 | 218.9 > 144.9 | 20 |
| 17 | Fonofos | 23.98 | 17.656 | 137.0 > 109.0 | 6 | 246.0 > 137.0 | 6 |
| 18 | Pyrimethanil | 23.67 | 18.050 | 198.0 > 183.0 | 12 | 198.0 > 156.1 | 24 |
| 19 | Diazinon | 23.34 | 18.075 | 304.1 > 179.1 | 10 | 304.1 > 162.1 | 8 |
| 20 | δ-hexachlorocyclohexane | 26.83 | 18.542 | 218.9 > 182.9 | 10 | 218.9 > 144.9 | 20 |
| 21 | Isazofos | 25.67 | 18.542 | 161.0 > 119.0 | 9 | 162.0 > 120.0 | 9 |
| 22 | Etrimfos | 23.67 | 18.786 | 292.1 > 181.1 | 8 | 292.1 > 153.1 | 20 |
| 23 | Disulfoton | 10.60 | 18.786 | 153.0 > 97.0 | 10 | 153.0 > 125.0 | 6 |
| 24 | Tefluthrin | 24.13 | 18.859 | 177.0 > 127.1 | 16 | 177.0 > 137.1 | 16 |
| 25 | Iprobenfos | 24.06 | 19.103 | 204.0 > 91.0 | 8 | 204.0 > 122.0 | 12 |
| 26 | Pirimicarb | 16.69 | 19.128 | 238.1 > 166.1 | 12 | 238.1 > 72.0 | 24 |
| 27 | Pentachloroaniline | 23.80 | 19.128 | 265.0 > 194.0 | 25 | 263.0 > 192.0 | 25 |
| 28 | Fenchlorphos-oxon | 23.63 | 19.372 | 269.0 > 254.0 | 20 | 269.0 > 224.0 | 25 |
| 29 | Dichlofenthion | 26.07 | 19.811 | 279.0 > 222.9 | 14 | 279.0 > 250.9 | 8 |
| 30 | Chlorpyrifos-methyl | 27.07 | 20.031 | 285.9 > 93.0 | 22 | 285.9 > 270.9 | 14 |
| 31 | Acetochlor | 20.12 | 20.031 | 223.1 > 132.1 | 22 | 223.1 > 147.1 | 10 |
| 32 | Vinclozolin | 29.39 | 20.275 | 285.0 > 212.0 | 12 | 285.0 > 178.0 | 14 |
| 33 | Parathion-methyl | 15.15 | 20.299 | 263.0 > 109.0 | 14 | 263.0 > 136.0 | 8 |
| 34 | Tolclofos-methyl | 20.41 | 20.348 | 264.9 > 249.9 | 14 | 264.9 > 93.0 | 24 |
| 35 | Alachlor | 29.58 | 20.421 | 188.1 > 160.1 | 10 | 188.1 > 132.1 | 18 |
| 36 | Heptachlor | 7.54 | 20.586 | 271.8 > 236.9 | 20 | 271.8 > 117.0 | 32 |
| 37 | N-desethyl-pirimiphos-methyl | 28.92 | 20.788 | 277.0 > 135.0 | 10 | 277.0 > 168.0 | 10 |
| 38 | Metalaxyl | 22.46 | 20.788 | 249.2 > 190.1 | 8 | 249.2 > 146.1 | 22 |
| 39 | Fenchlorphos | 22.29 | 20.788 | 284.9 > 269.9 | 16 | 284.9 > 93.0 | 24 |
| 40 | Paraoxon | 28.72 | 21.028 | 139.0 > 109.0 | 10 | 149.0 > 102.0 | 25 |
| 41 | Prometryn | 22.61 | 21.028 | 241.2 > 199.1 | 6 | 241.2 > 58.0 | 14 |
| 42 | Pentachlorothioanisole | 30.00 | 21.504 | 296.0 > 263.0 | 15 | 246.0 > 211.0 | 20 |
| 43 | Fenitrothion | 27.21 | 21.552 | 277.0 > 260.0 | 6 | 277.0 > 109.1 | 14 |
| 44 | Pirimiphos-methyl | 20.84 | 21.576 | 305.1 > 180.1 | 8 | 305.1 > 290.1 | 12 |
| 45 | Bromacil | 28.20 | 21.647 | 204.9 > 187.9 | 14 | 204.9 > 162.0 | 14 |
| 46 | Metolachlor | 23.38 | 22.172 | 238.1 > 162.1 | 12 | 238.1 > 133.1 | 26 |
| 47 | Aldrin | 13.34 | 2.195 | 262.9 > 193.0 | 28 | 262.9 > 203.0 | 26 |
| 48 | Clorpyrifos | 20.16 | 22.338 | 313.9 > 257.9 | 14 | 313.9 > 285.9 | 8 |
| 49 | Dacthal | 23.42 | 22.481 | 300.9 > 222.9 | 26 | 300.9 > 272.9 | 14 |
| 50 | Fenthion | 24.47 | 22.505 | 278.0 > 109.0 | 20 | 278.0 > 125.0 | 20 |
| 51 | Parathion | 25.33 | 22.672 | 291.1 > 109.0 | 14 | 291.1 > 137.0 | 6 |
| 52 | Isocarbophos | 26.63 | 22.863 | 289.1 > 136.0 | 10 | 289.1 > 113.0 | 14 |
| 53 | Bromophos | 29.87 | 23.244 | 330.9 > 315.9 | 14 | 330.9 > 285.9 | 28 |
| 54 | Pirimiphos ethyl | 23.99 | 23.544 | 304.0 > 168.0 | 10 | 318.0 > 166.0 | 15 |
| 55 | Cyprodinil | 26.30 | 23.891 | 224.1 > 208.1 | 16 | 224.1 > 197.1 | 22 |
| 56 | E-chlorfenvinphos | 29.22 | 23.960 | 323.0 > 267.0 | 16 | 323.0 > 295.0 | 6 |
| 57 | trans-heptachlorepoxide | 24.21 | 24.237 | 352.8 > 253.0 | 26 | 352.8 > 289.0 | 6 |
| 58 | Fipronil | 21.44 | 24.237 | 366.9 > 212.9 | 30 | 366.9 > 254.9 | 22 |
| 59 | Z-chlorfenvinphos | 27.49 | 24.422 | 323.0 > 267.0 | 16 | 323.0 > 295.0 | 6 |
| 60 | Mecarbam | 21.06 | 24.584 | 329.0 > 159.1 | 4 | 329.0 > 131.1 | 18 |
| 61 | Quinalphos | 25.62 | 24.630 | 298.0 > 156.0 | 5 | 298.0 > 190.0 | 10 |
| 62 | Phenthoate | 27.03 | 24.630 | 273.9 > 125.0 | 20 | 273.9 > 246.0 | 6 |
| 63 | Procymidone | 17.47 | 24.722 | 283.0 > 96.0 | 10 | 283.0 > 255.0 | 12 |
| 64 | Triadimenol | 9.37 | 24.791 | 168.1 > 70.0 | 10 | 168.1 > 112.1 | 4 |
| 12.67 | 25.161 | ||||||
| 65 | Methidathion | 22.20 | 25.161 | 145.0 > 85.0 | 8 | 145.0 > 58.0 | 14 |
| 66 | Bromophos-ethyl | 28.69 | 25.276 | 358.9 > 302.9 | 16 | 358.9 > 330.9 | 10 |
| 67 | Methoprene | 22.56 | 25.392 | 111.0 > 55.1 | 15 | 109.0 > 67.1 | 9 |
| 68 | o,p'-DDE | 15.41 | 25.415 | 246.0 > 176.0 | 30 | 246.0 > 211.0 | 22 |
| 69 | Paclobutrazol | 29.19 | 25.554 | 236.1 > 125.0 | 14 | 236.1 > 167.0 | 10 |
| 70 | Trans-chlordane | 19.56 | 25.754 | 374.8 > 263.9 | 28 | 372.8 > 336.8 | 10 |
| 71 | Fenothiocarb KCO-3001 Panocon | 7.55 | 25.843 | 160.1 > 72.0 | 10 | 160.1 > 106.1 | 12 |
| 72 | Ditalimfos | 20.77 | 25.977 | 130.0 > 102.1 | 12 | 148.0 > 130.1 | 12 |
| 73 | N,N-Diethyl-2-(1-naphthyloxy)propanamide | 22.18 | 26.266 | 128.0 > 72.1 | 6 | 271.0 > 128.1 | 6 |
| 74 | Prothiofos | 25.48 | 26.600 | 309.0 > 238.9 | 14 | 309.0 > 280.9 | 10 |
| 75 | Cis-chlordane | 27.66 | 26.823 | 374.8 > 338.8 | 8 | 372.8 > 265.9 | 22 |
| 76 | Profenofos | 24.54 | 26.823 | 336.9 > 266.9 | 14 | 336.9 > 308.9 | 6 |
| 77 | Myclobutanil | 21.15 | 27.179 | 179.0 > 125.0 | 18 | 179.0 > 90.1 | 27 |
| 78 | Carboxin | 29.42 | 27.290 | 235.0 > 143.1 | 12 | 235.0 > 87.0 | 21 |
| 79 | Flusilazole | 29.81 | 27.335 | 233.1 > 165.1 | 14 | 233.1 > 152.1 | 14 |
| 80 | Buprofezin | 21.48 | 27.380 | 172.1 > 57.0 | 14 | 172.1 > 131.1 | 6 |
| 81 | p,p'-DDE | 16.07 | 27.888 | 246.0 > 211.0 | 22 | 246.0 > 176.0 | 30 |
| 82 | Dieldrin | 22.21 | 27.888 | 262.9 > 193.0 | 34 | 262.9 > 228.0 | 24 |
| 83 | Endrin | 21.22 | 27.888 | 262.9 > 191.0 | 30 | 262.9 > 193.0 | 28 |
| 84 | Nitrofen | 27.28 | 27.995 | 282.9 > 162.0 | 24 | 282.9 > 253.0 | 12 |
| 85 | Fenthionsulfoxide | 20.42 | 28.509 | 279.0 > 109.0 | 20 | 294.0 > 279.0 | 10 |
| 86 | Fensulfothion | 29.20 | 28.595 | 293.0 > 125.0 | 14 | 293.0 > 153.0 | 8 |
| 87 | Diniconazole | 27.40 | 28.638 | 268.0 > 232.0 | 12 | 268.0 > 149.0 | 24 |
| 88 | Fenthion-sulfone | 24.24 | 28.766 | 310.0 > 105.0 | 15 | 310.0 > 109.0 | 20 |
| 89 | o,p'-DDD | 23.54 | 28.852 | 235.0 > 165.0 | 24 | 235.0 > 199.0 | 14 |
| 90 | o,p'-DDT | 23.54 | 28.766 | 235.0 > 165.0 | 24 | 235.0 > 199.0 | 16 |
| 91 | Ethion | 29.55 | 29.024 | 230.9 > 174.9 | 14 | 230.9 > 184.9 | 12 |
| 92 | Fensulfothion sulfone | 22.99 | 29.324 | 188.0 > 109.1 | 18 | 324.0 > 109.1 | 18 |
| 93 | Famphur | 18.34 | 29.902 | 218.0 > 109.0 | 16 | 218.0 > 79.0 | 24 |
| 94 | Benalaxyl | 24.19 | 29.981 | 148.0 > 77.1 | 27 | 148.0 > 105.1 | 18 |
| 95 | Endosulfan sulfate | 22.75 | 30.191 | 386.8 > 252.9 | 16 | 386.8 > 288.8 | 10 |
| 96 | Propiconazole | 26.54 | 30.325 | 259.0 > 69.0 | 14 | 259.0 > 191.0 | 8 |
| 29.43 | 30.593 | ||||||
| 97 | p,p'-DDT | 30.00 | 30.554 | 235.0 > 165.0 | 24 | 235.0 > 199.0 | 16 |
| 98 | Tebuconazole | 30.00 | 31.147 | 250.1 > 125.1 | 22 | 250.1 > 153.1 | 12 |
| 99 | Piperonyl butoxide | 27.48 | 31.739 | 176.1 > 131.1 | 12 | 176.1 > 117.1 | 20 |
| 100 | Pyridaphenthion | 26.92 | 32.223 | 340.0 > 199.2 | 9 | 199.0 > 77.1 | 30 |
| 101 | Phosmet | 25.23 | 32.332 | 160.0 > 77.0 | 24 | 160.0 > 133.0 | 14 |
| 102 | Bromopropylate | 23.50 | 32.612 | 340.9 > 182.9 | 18 | 340.9 > 184.9 | 20 |
| 103 | Bifenthrin | 5.87 | 32.736 | 181.1 > 166.1 | 12 | 183.1 > 153.1 | 8 |
| 104 | Bifenazate | 26.83 | 32.829 | 300.1 > 258.1 | 8 | 300.1 > 199.1 | 20 |
| 105 | Methoxychlor | 26.37 | 32.845 | 227.1 > 169.1 | 24 | 227.1 > 212.1 | 14 |
| 106 | Fenpropathrin | 12.20 | 33.016 | 265.1 > 210.1 | 12 | 265.1 > 172.1 | 14 |
| 107 | Tebufenpyrad | 24.61 | 33.187 | 333.0 > 171.1 | 21 | 318.0 > 131.2 | 21 |
| 108 | Tetradifon | 29.03 | 33.455 | 355.9 > 228.9 | 12 | 355.9 > 159.0 | 18 |
| 109 | Phenothrin | 24.35 | 33.522 | 183.1 > 153.1 | 14 | 183.1 > 168.1 | 14 |
| 29.11 | 33.736 | ||||||
| 110 | Phosalone | 21.89 | 33.682 | 182.0 > 111.0 | 14 | 182.0 > 138.0 | 8 |
| 111 | Azinphos-methyl | 21.74 | 33.736 | 160.1 > 132.1 | 6 | 160.1 > 77.0 | 20 |
| 112 | Mefenacet | 25.43 | 34.069 | 192.0 > 136.0 | 12 | 192.0 > 109.0 | 27 |
| 113 | Mirex | 13.20 | 34.216 | 272.0 > 237.0 | 15 | 270.0 > 235.0 | 5 |
| 114 | Cyhalothrin | 27.81 | 34.510 | 197.0 > 141.0 | 8 | 197.0 > 161.0 | 22 |
| 28.81 | 34.900 | ||||||
| 115 | λ-Cyhalothrin | 21.73 | 34.510 | 181.0 > 152.1 | 21 | 197.0 > 141.1 | 9 |
| 116 | Fenarimol | 28.56 | 34.550 | 251.0 > 139.0 | 14 | 251.0 > 111.0 | 26 |
| 117 | Pyrazophos | 223.60 | 34.710 | 221.1 > 193.1 | 12 | 221.1 > 149.1 | 14 |
| 118 | Acrinathrin | 24.36 | 34.900 | 289.1 > 93.0 | 14 | 289.1 > 77.0 | 26 |
| 24.67 | 35.206 | ||||||
| 119 | Bitertanol | 29.26 | 35.548 | 170.0 > 141.1 | 18 | 170.0 > 115.2 | 27 |
| 120 | Permethrin | 22.70 | 35.713 | 183.1 > 168.1 | 14 | 183.1 > 165.1 | 14 |
| 24.90 | 35.912 | ||||||
| 121 | Coumaphos | 23.63 | 35.759 | 362.0 > 109.0 | 16 | 362.0 > 226.0 | 14 |
| 122 | Flusilazole | 25.80 | 35.807 | 340.0 > 298.0 | 14 | 340.0 > 313.0 | 14 |
| 123 | Pyridaben | 20.10 | 35.889 | 147.1 > 117.1 | 22 | 147.1 > 132.1 | 14 |
| 124 | Cyfluthrin | 27.68 | 36.556 | 226.1 > 206.1 | 6 | 226.1 > 199.1 | 14 |
| 24.88 | 36.717 | ||||||
| 24.11 | 36.792 | ||||||
| 23.14 | 36.867 | ||||||
| 125 | Boscalid | 26.41 | 37.004 | 342.1 > 140.1 | 14 | 342.1 > 112.1 | 28 |
| 126 | Cypermethrin | 15.11 | 37.024 | 163.1 > 127.1 | 6 | 163.1 > 109.1 | 22 |
| 17.62 | 37.182 | ||||||
| 19.05 | 37.252 | ||||||
| 11.96 | 37.321 | ||||||
| 127 | Quizalofop-ethyl | 29.31 | 37.242 | 372.0 > 299.2 | 12 | 299.0 > 91.2 | 24 |
| 128 | Flucythrinate | 22.11 | 37.281 | 199.1 > 157.1 | 10 | 199.1 > 107.1 | 22 |
| 21.41 | 37.568 | ||||||
| 129 | Ethofenprox | 25.46 | 37.479 | 163.0 > 135.1 | 10 | 163.0 > 107.1 | 18 |
| 130 | Phenvalerate | 29.21 | 38.261 | 419.1 > 225.1 | 6 | 419.1 > 125.1 | 26 |
| 29.01 | 38.558 | ||||||
| 131 | Tua-Fluvalinate | 17.50 | 38.465 | 250.1 > 55.0 | 20 | 250.1 > 208.0 | 20 |
| 16.53 | 38.567 | ||||||
| 132 | Difenoconazole | 15.30 | 38.846 | 323.0 > 265.0 | 14 | 323.0 > 202.0 | 28 |
| 19.56 | 38.927 | ||||||
| 133 | Deltamethrin | 26.41 | 39.015 | 252.9 > 93.0 | 20 | 251.0 > 172.0 | 10 |
| 27.14 | 39.278 | ||||||
3. Results and discussion
3.1. Optimization of extraction solvent
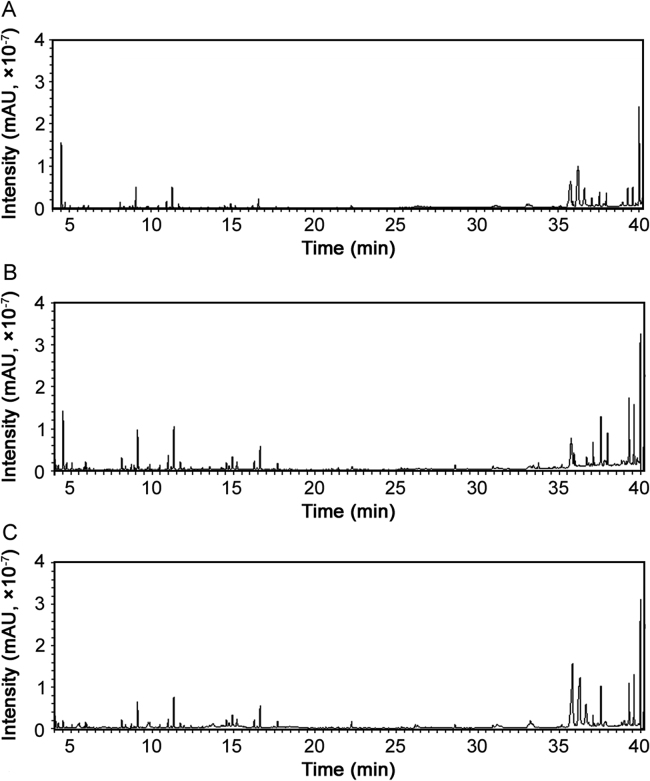
Acetonitrile, ethyl acetate and n-hexane are commonly used for extraction of multi-pesticide residues [24], [25], [26], [27]. Fig. 1 shows the TIC chromatograms of negative samples extracted by different solvents. Those extracted with n-hexane had the lowest matrix; however, recoveries of dimethoate, metalaxyl, paraoxon, bromacil, isocarbophos, E-chlorfenvinfos, fipronil, triadimenol, trans-chlordane, cis-chlordane, fenthionsulfoxide, fensulfothion, fenthion-sulfone, and azinphos-methyl were close to zero. When extracted with ethyl acetate, the recovery of trans-chlordane and pyridaben was less than 60%. Compared with n-hexane and ethyl acetate, acetonitrile had the strong dissolving capability for the analytes, meeting the recovery requirement. Therefore, in this study acetonitrile was used as the extraction solvent.
Fig. 1.
Full scan chromatograms of negative samples extracted with (A) n-hexane, (B) ethyl acetate and (C) acetonitrile.
3.2. Selection of solvent for redissolution
Acetonitrile possesses many advantages for extraction, but the polarity of acetonitrile is high, which can damage the gas chromatography column. For the protection of chromatographic columns, before analysis, acetonitrile should be replaced. In this study, n-hexane and acetone were selected as solvents for redissolution. The recoveries of pesticides obtained with these two solvents are shown in Fig. 2.
Fig. 2.
Recoveries of pesticides obtained using (A) n-hexane and (B) acetone for redissolution.
Compared with that of acetone, the recoveries of some pesticides were lower when redissolved with n-hexane, and approximately 42% of pesticides were outside the range of 60%–120%. Dimethoate and cis-chlordane were close to zero. When dissolved with acetone, most of analytical pesticides satisfied the recovery requirement. So acetone was chosen as the solvent for redissolution.
3.3. Optimization of adsorbents
The use of co-extraction leads to an unsatisfactory peak shape, and an increased or inhibited response, which adversely affects the quantification. The adsorbents PSA, C18, Florisil, GCB and NH2 were investigated to choose the most appropriate purification method.
If the extraction solution was injected into the GC-MS/MS without adding adsorbents, there was clear interference of the matrix for the pesticides bitertanol, cypermethrin, flucythrinate, and difenoconazole.
The addition of Florisil for purification seemed to have no effect. C18 can be used for the reduction of lipids and non-polar interference. Because of the addition of C18, the matrices that interfered with the determination of pesticides, such as bitertanol, flucythrinate and difenoconazole, were removed.
PSA can adsorb fatty acids and pigments extracted from chenpi to improve the chromatographic peak shape of cypermethrin (Fig. 3). When the amount of PSA was 200 mg, the chromatographic peaks of cypermethrin isomers was free of interference from impurities. However, PSA clearly had an effect on the recovery of ditalimfos. This study compared the effect of the addition of 50, 100, 150, 200 mg PSA on the recovery of ditalimfos, and the results obtained are shown in Fig. 4. When the adsorbent amount was 50 mg, the recovery of ditalimfos was about 73.1%, while the recovery of ditalimfos decreased to 23.9% when the amount of PSA reached 200 mg. The main reason for this may be that PSA may increase the pH value of the final extract solutions to more than 8. According to the structure of ditalimfos (Fig. 5), ditalimfos is unstable and decomposes easily in an alkaline environment, so as the amount of adsorbent is increased, the recovery of ditalimfos is reduced.
Fig. 3.
Chromatogram of cypermethrin with (A) PSA adsorbent and (B) without adsorbent.
Fig. 4.
Recoveries of ditalimfos with different amounts of PSA.
Fig. 5.

The structure of (A) ditalimfos, (B) PSA and (C) NH2.
Fig. 5 shows the structures of NH2 and PSA. They had a similar adsorption, while PSA contains two amino groups, which resulted in a higher ion exchange capacity than NH2. Therefore, NH2 could not only improve the chromatographic peak shape of cypermethrin, but also have small effect on ditalimfos. When the dose of NH2 reached 200 mg, the recoveries of pesticides were all between 72.4% and 118.6%.
The solution extracted with acetonitrile contained more pigments. GCB is widely used in the adsorption of pigments. The color of the extraction solution changed with an increase in the amount of GCB. In addition, GCB is well known for adsorbing pesticides with a planar structure, leading to unsatisfactory recoveries and poor precision. This study compared the effect of the addition of 10, 20, 30 and 40 mg GCB on the recovery of pesticides, and the results obtained are shown in Fig. 6. As the amount of GCB increased, the recoveries of some pesticides, such as hexachlorobenzene with a planar structure, decreased but were still in an acceptable range. Also, the extraction was improved when 30 mg GCB was added. In summary, 30 mg GCB was used as the adsorbent.
Fig. 6.
Recoveries of pesticides with different amounts of GCB.
3.4. Validation study
Under the modified QuEChERS method conditions, a validation study was carried out to evaluate the performance characteristics of the method for multiple pesticides in chenpi by estimating the linearity, limit of quantification (LOQ), accuracy (expressed by recovery), precision and matrix effects. Validation was performed following the European Union SANTE/11945/ 2015 guideline [28].
3.4.1. Linearity
The linearity for each pesticide was assessed in matrix-matched standard solution. The calibration curves of the compounds were obtained by plotting the pesticide/IS peak area ratios against the concentration of the corresponding calibration standards at eight different levels (1, 2, 5, 10, 20, 50, 100, 200 ng/mL). The linearity results are shown in Table 2. The linearity of the method for all the pesticides was satisfactory, with correlation coefficients (r2) higher than 0.99.
Table 2.
Validation results of the developed method for determination of multiple pesticides in chenpi.
| No. | Pesticide | Linear range (ng/mL) | r2 | Recovery (%RSD)( %, n = 6) |
LOQ (mg/kg) | ME(%) | ||
|---|---|---|---|---|---|---|---|---|
| 0.05 mg/kg | 0.1 mg/kg | 0.2 mg/kg | ||||||
| 1 | Dichlorvos | 1–200 | 0.9992 | 84.0(1.4) | 90.3(2.3) | 90.5(3.6) | 0.005 | 4.3 |
| 2 | Mevinphos | 1–200 | 0.9988 | 85.6(2.3) | 94.1(2.9) | 97.2(2.3) | 0.005 | 56.2 |
| 3 | Methacrifos | 1–200 | 0.9989 | 88.1(1.0) | 98.9(3.0) | 103.5(2.9) | 0.005 | 21.7 |
| 4 | Isoprocarb | 1–200 | 0.9988 | 92.8(3.9) | 92.6(1.8) | 93.9(2.0) | 0.005 | 2.5 |
| 5 | Propoxur | 1–200 | 0.9989 | 90.7(2.0) | 93.3(2.2) | 93.5(1.7) | 0.005 | 18.8 |
| 6 | Ethoprophos | 1–200 | 0.9987 | 92.1(3.7) | 86.5(2.0) | 85.4(1.7) | 0.005 | 14.5 |
| 7 | Dicrotophos | 1–200 | 0.9986 | 93.9(4.0) | 87.6(3.3) | 92.1(2.3) | 0.005 | 44.0 |
| 8 | Phorate | 1–200 | 0.9981 | 86.6(1.2) | 93.4(2.2) | 93.0(1.7) | 0.005 | 18.6 |
| 9 | α-hexachlorocyclohexane | 1–200 | 0.9989 | 93.4(6.4) | 91.8(2.2) | 94.1(2.3) | 0.005 | −15.5 |
| 10 | Hexachlorobenzene | 1–200 | 0.9990 | 71.9(2.9) | 73.0(2.2) | 72.0(2.5) | 0.005 | −19.9 |
| 11 | 2,6-dichloro-4-nitroaniline | 2–200 | 0.9970 | 84.2(2.4) | 93.0(3.8) | 97.7(2.4) | 0.01 | 62.1 |
| 12 | Dimethoate | 1–200 | 0.9984 | 90.7(1.6) | 91.4(2.7) | 91.9(1.4) | 0.01 | 46.9 |
| 13 | β-hexachlorocyclohexane | 1–200 | 0.9988 | 95.5(5.2) | 94.1(2.4) | 95.3(2.0) | 0.005 | −13.2 |
| 14 | Pentachloroanisole | 2–200 | 0.9974 | 85.4(4.9) | 78.0(2.9) | 69.8(3.2) | 0.005 | 17.5 |
| 15 | Quintozene | 2–200 | 0.9977 | 79.9(1.3) | 84.5(1.6) | 85.5(2.0) | 0.005 | 32.7 |
| 16 | γ-hexachlorocyclohexane | 1–200 | 0.9995 | 92.3(7.6) | 90.0(5.3) | 92.0(1.7) | 0.01 | −7.8 |
| 17 | Fonofos | 1–200 | 0.9988 | 88.0(1.5) | 95.5(2.8) | 98.4(2.0) | 0.005 | 11.0 |
| 18 | Pyrimethanil | 2–200 | 0.9988 | 77.6(1.5) | 78.1(3.2) | 80.7(1.8) | 0.005 | 9.1 |
| 19 | Diazinon | 1–200 | 0.9986 | 92.1(1.7) | 89.7(1.4) | 87.9(1.7) | 0.01 | −0.8 |
| 20 | δ-hexachlorocyclohexane | 1–200 | 0.9986 | 92.8(5.9) | 92.2(2.3) | 94.7(2.0) | 0.005 | −9.3 |
| 21 | Isazofos | 2–200 | 0.9982 | 89.8(0.4) | 96.6(3.1) | 99.8(2.0) | 0.005 | 1.3 |
| 22 | Etrimfos | 1–200 | 0.9980 | 93.7(5.2) | 89.9(2.0) | 93.4(2.2) | 0.01 | −18.3 |
| 23 | Disulfoton | 1–200 | 0.9976 | 88.1(2.4) | 92.5(2.5) | 95.0(2.1) | 0.01 | −6.9 |
| 24 | Tefluthrin | 1–200 | 0.9982 | 87.3(0.8) | 94.1(2.5) | 97.1(1.8) | 0.01 | −4.2 |
| 25 | Iprobenfos | 1–200 | 0.9973 | 85.5(3.9) | 98.9(3.2) | 103.0(1.9) | 0.01 | 26.9 |
| 26 | Pirimicarb | 1–200 | 0.9980 | 90.0(2.3) | 94.4(2.8) | 97.2(2.2) | 0.01 | −10.5 |
| 27 | Pentachloroaniline | 1–200 | 0.9982 | 87.0(7.0) | 71.4(3.2) | 70.9(2.3) | 0.005 | −19.9 |
| 28 | Fenchlorphos-oxon | 1–200 | 0.9978 | 93.4(3.5) | 91.3(3.2) | 96.4(2.6) | 0.005 | 2.2 |
| 29 | Dichlofenthion | 1–200 | 0.9983 | 91.3(1.1) | 88.1(1.5) | 87.3(2.0) | 0.005 | 0.9 |
| 30 | Chlorpyrifos-methyl | 1–200 | 0.9981 | 86.6(0.6) | 90.3(2.0) | 90.1(2.2) | 0.005 | 2.0 |
| 31 | Acetochlor | 5–200 | 0.9980 | 89.6(1.6) | 98.3(3.2) | 102.0(1.8) | 0.01 | 5.4 |
| 32 | Vinclozolin | 1–200 | 0.9984 | 93.8(4.0) | 89.5(1.2) | 88.4(1.7) | 0.005 | −4.9 |
| 33 | Parathion-methyl | 1–200 | 0.9953 | 85.8(5.5) | 85.5(1.2) | 86.8(1.6) | 0.01 | 70.7 |
| 34 | Tolclofos-methyl | 1–200 | 0.9984 | 90.0(1.9) | 98.5(3.0) | 103.0(2.2) | 0.005 | −11.3 |
| 35 | Alachlor | 2–200 | 0.9980 | 88.4(1.0) | 97.7(2.6) | 101.5(1.9) | 0.01 | 2.2 |
| 36 | Heptachlor | 1–200 | 0.9980 | 83.2(1.5) | 76.2(2.3) | 74.5(1.8) | 0.005 | −10.3 |
| 37 | N-desethyl-pirimiphos-methyl | 2–200 | 0.9981 | 86.9(1.9) | 99.7(3.7) | 104.4(1.9) | 0.005 | 9.6 |
| 38 | Metalaxyl | 2–200 | 0.9978 | 93.5(5.4) | 89.1(1.7) | 89.2(1.3) | 0.005 | −12.1 |
| 39 | Fenchlorphos | 1–200 | 0.9984 | 87.4(1.6) | 92.5(2.0) | 91.7(1.2) | 0.005 | −4.0 |
| 40 | Paraoxon | 5–200 | 0.9934 | 91.1(3.8) | 89.7(4.2) | 92.9(3.2) | 0.01 | 79.7 |
| 41 | Prometryn | 1–200 | 0.9984 | 91.3(1.6) | 88.4(1.4) | 86.7(1.9) | 0.005 | 0.2 |
| 42 | Pentachlorothioanisole | 2–200 | 0.9988 | 77.5(7.8) | 74.0(3.3) | 76.4(4.4) | 0.005 | −20.1 |
| 43 | Fenitrothion | 1–200 | 0.9917 | 82.4(4.4) | 84.2(0.9) | 84.4(1.5) | 0.01 | 67.3 |
| 44 | Pirimiphos-methyl | 2–200 | 0.9980 | 91.6(2.2) | 86.9(1.7) | 86.5(1.9) | 0.01 | −6.5 |
| 45 | Bromacil | 2–200 | 0.9990 | 89.2(2.0) | 93.7(2.3) | 94.4(2.6) | 0.01 | 28.7 |
| 46 | Metolachlor | 1–200 | 0.9986 | 89.4(1.1) | 100.1(3.3) | 104.6(2.1) | 0.005 | 3.3 |
| 47 | Aldrin | 2–200 | 0.9984 | 92.7(6.1) | 87.5(2.4) | 89.7(3.0) | 0.005 | −20.7 |
| 48 | Chlorpyrifos | 1–200 | 0.9985 | 88.3(3.5) | 105.5(4.9) | 107.3(2.4) | 0.005 | 1.8 |
| 49 | Dacthal | 1–200 | 0.9989 | 97.7(6.9) | 92.0(2.1) | 94.3(1.7) | 0.005 | −21.2 |
| 50 | Fenthion | 1–200 | 0.9986 | 89.8(0.8) | 94.1(1.5) | 92.6(1.5) | 0.01 | 1.2 |
| 51 | Parathion | 2–200 | 0.9926 | 88.6(2.1) | 96.2(5.0) | 103.6(2.2) | 0.01 | 69.3 |
| 52 | Isocarbophos | 2–200 | 0.9979 | 92.5(2.2) | 96.6(3.8) | 102.0(2.4) | 0.005 | 18.5 |
| 53 | Bromophos | 1–200 | 0.9986 | 92.2(2.9) | 90.6(1.6) | 94.0(2.2) | 0.005 | 0.3 |
| 54 | Pirimiphos ethyl | 1–200 | 0.9986 | 86.4(1.8) | 99.4(3.8) | 104.9(2.6) | 0.005 | 6.1 |
| 55 | Cyprodinil | 1–200 | 0.9978 | 73.3(2.0) | 70.0(5.6) | 73.4(2.0) | 0.005 | −2.1 |
| 56 | E-chlorfenvinphos | 5–200 | 0.9980 | 95.8(5.8) | 92.8(3.1) | 90.6(3.0) | 0.01 | 24.8 |
| 57 | trans-heptachlorepoxide | 5–200 | 0.9974 | 99.2(12.0) | 101.1(5.0) | 97.2(3.1) | 0.01 | −8.7 |
| 58 | Fipronil | 2–200 | 0.9970 | 86.5(4.1) | 96.1(2.1) | 95.5(1.8) | 0.01 | 49.1 |
| 59 | Z-chlorfenvinphos | 1–200 | 0.9988 | 90.1(1.9) | 88.5(1.3) | 87.9(1.9) | 0.01 | 17.3 |
| 60 | Mecarbam | 2–200 | 0.9966 | 86.7(6.3) | 102.4(3.3) | 103.2(2.4) | 0.01 | 24.8 |
| 61 | Quinalphos | 2–200 | 0.9966 | 86.4(3.8) | 98.5(2.7) | 101.5(1.3) | 0.01 | 4.9 |
| 62 | Phenthoate | 1–200 | 0.9976 | 86.4(1.0) | 97.1(3.0) | 102.0(2.4) | 0.01 | −4.4 |
| 63 | Procymidone | 1–200 | 0.9983 | 91.5(1.5) | 92.0(1.2) | 90.4(1.3) | 0.01 | −8.4 |
| 64 | Triadimenol | 1–200 | 0.9975 | 86.4(3.5) | 95.3(3.5) | 99.5(2.4) | 0.01 | 29.0 |
| 65 | Methidathion | 1–200 | 0.9977 | 91.3(2.4) | 100.4(3.4) | 102.6(1.6) | 0.01 | 29.2 |
| 66 | Bromophos-ethyl | 1–200 | 0.9984 | 86.9(1.4) | 85.3(1.9) | 84.6(1.4) | 0.01 | −10.1 |
| 67 | Methoprene | 1–200 | 0.9924 | 74.1(2.5) | 96.5(4.2) | 95.6(8.3) | 0.01 | 13.0 |
| 68 | o,p'-DDE | 1–200 | 0.9984 | 87.7(2.6) | 95.2(3.1) | 97.2(1.7) | 0.005 | −14.6 |
| 69 | Paclobutrazol | 1–200 | 0.9979 | 84.9(2.8) | 96.9(2.1) | 97.7(1.4) | 0.01 | 28.6 |
| 70 | Trans-chlordane | 5–200 | 0.9960 | 100.4(7.3) | 90.1(5.5) | 88.4(4.0) | 0.01 | −11.4 |
| 71 | Fenothiocarb | 1–200 | 0.9978 | 88.5(0.7) | 94.1(1.9) | 94.2(1.6) | 0.01 | 51.7 |
| 72 | Ditalimfos | 1–200 | 0.9981 | 72.7(1.0) | 83.5(4.0) | 84.5(1.4) | 0.005 | 2.5 |
| 73 | Napropamid | 1–200 | 0.9986 | 92.7(5.4) | 92.9(2.5) | 94.3(1.3) | 0.005 | 3.6 |
| 74 | Prothiofos | 1–200 | 0.9989 | 90.3(2.8) | 86.6(1.6) | 86.1(1.7) | 0.005 | 14.3 |
| 75 | Cis-chlordane | 5–200 | 0.9914 | 89.9(8.9) | 97.9(13.1) | 102.7(3.7) | 0.01 | 24.5 |
| 76 | Profenofos | 1–200 | 0.9991 | 88.9(2.5) | 102.1(3.9) | 105.3(2.1) | 0.005 | 34.6 |
| 77 | Myclobutanil | 1–200 | 0.9986 | 88.8(1.6) | 96.3(2.9) | 99.6(2.0) | 0.01 | 15.8 |
| 78 | Carboxin | 1–200 | 0.9984 | 92.0(3.5) | 93.9(2.3) | 95.4(1.6) | 0.01 | 25.4 |
| 79 | Flusilazole | 1–200 | 0.9982 | 88.7(1.9) | 97.4(3.0) | 97.8(0.9) | 0.01 | 7.6 |
| 80 | Buprofezin | 1–200 | 0.9985 | 89.0(2.0) | 96.8(3.5) | 98.5(1.9) | 0.005 | 1.4 |
| 81 | p,p'-DDE | 2–200 | 0.9992 | 100.0(12.3) | 83.9(3.4) | 79.0(2.5) | 0.01 | −6.7 |
| 82 | Dieldrin | 5–200 | 0.9980 | 92.6(6.3) | 86.0(1.7) | 88.2(2.8) | 0.01 | −9.3 |
| 83 | Endrin | 5–200 | 0.9983 | 94.0(7.6) | 84.6(4.4) | 87.0(1.3) | 0.01 | −8.0 |
| 84 | Nitrofen | 2–200 | 0.9933 | 84.6(3.2) | 91.1(4.4) | 97.7(2.7) | 0.01 | 44.3 |
| 85 | Fenthionsulfoxide | 2–200 | 0.9931 | 89.1(1.8) | 84.1(2.3) | 84.2(2.2) | 0.01 | 21.6 |
| 86 | Fensulfothion | 1–200 | 0.9997 | 79.2(3.6) | 79.4(1.5) | 81.1(2.1) | 0.01 | 79.8 |
| 87 | Diniconazole | 1–200 | 0.9981 | 84.2(1.1) | 83.7(2.0) | 83.5(1.5) | 0.005 | 50.0 |
| 88 | Fenthion-sulfone | 2–200 | 0.9973 | 90.9(2.2) | 88.4(2.1) | 86.3(1.4) | 0.01 | 54.7 |
| 89 | o,p'-DDD | 1–200 | 0.9993 | 96.7(8.4) | 110.8(5.1) | 112.2(3.8) | 0.01 | 69.8 |
| 90 | o,p'-DDT | 1–200 | 0.9964 | 78.8(1.1) | 72.6(2.5) | 77.7(1.8) | 0.005 | −17.8 |
| 91 | Ethion | 1–200 | 0.9971 | 88.9(1.6) | 91.0(2.7) | 92.2(1.6) | 0.01 | 16.9 |
| 92 | Fensulfothion sulfone | 1–200 | 0.9970 | 88.8(1.1) | 97.7(3.8) | 101.6(1.9) | 0.01 | 34.9 |
| 93 | Famphur | 1–200 | 0.9977 | 90.7(2.9) | 92.8(3.0) | 95.4(2.0) | 0.005 | 99.3 |
| 94 | Benalaxyl | 1–200 | 0.9978 | 90.4(3.5) | 90.6(2.2) | 91.3(1.9) | 0.005 | 8.7 |
| 95 | Endosulfan sulfate | 5–200 | 0.9979 | 93.4(4.9) | 91.7(2.0) | 87.0(1.6) | 0.01 | 10.3 |
| 96 | Propiconazole | 1–200 | 0.9975 | 84.3(2.6) | 96.2(3.9) | 98.0(1.9) | 0.005 | 39.9 |
| 97 | p,p'-DDT | 1–200 | 0.9948 | 75.3(4.7) | 71.6(2.3) | 74.6(1.7) | 0.01 | −9.6 |
| 98 | Tebuconazole | 1–200 | 0.9980 | 88.3(3.1) | 90.5(2.4) | 89.9(1.4) | 0.005 | 49.1 |
| 99 | Piperonyl butoxide | 1–200 | 0.9980 | 85.1(2.6) | 94.8(3.1) | 96.7(1.8) | 0.01 | 45.6 |
| 100 | Pyridaphenthion | 1–200 | 0.9964 | 83.9(4.7) | 99.6(3.3) | 102.7(2.0) | 0.01 | 49.2 |
| 101 | Phosmet | 1–200 | 0.9952 | 85.2(1.3) | 91.6(3.6) | 96.1(2.2) | 0.01 | −2.8 |
| 102 | Bromopropylate | 1–200 | 0.9983 | 89.5(1.7) | 90.8(1.8) | 91.7(1.6) | 0.005 | 27.1 |
| 103 | Bifenthrin | 1–200 | 0.9983 | 86.0(1.8) | 96.8(3.8) | 99.2(1.8) | 0.005 | 26.3 |
| 104 | Bifenazate | 1–200 | 0.9962 | 83.3(2.9) | 94.6(2.8) | 95.9(1.8) | 0.01 | 50.3 |
| 105 | Methoxychlor | 1–200 | 0.9941 | 78.0(3.3) | 72.4(2.5) | 75.2(1.7) | 0.01 | −10.9 |
| 106 | Fenpropathrin | 1–200 | 0.9973 | 91.3(2.4) | 88.1(3.4) | 89.8(2.0) | 0.01 | 6.7 |
| 107 | Tebufenpyrad | 1–200 | 0.9975 | 88.4(1.2) | 91.1(2.6) | 92.9(1.9) | 0.01 | 13.5 |
| 108 | Tetradifon | 2–200 | 0.9974 | 92.5(4.8) | 90.1(2.8) | 94.5(1.6) | 0.005 | −3.9 |
| 109 | Phenothrin | 1–200 | 0.9979 | 83.0(2.5) | 91.8(4.2) | 95.3(2.9) | 0.01 | 48.3 |
| 110 | Phosalone | 1–200 | 0.9959 | 84.9(2.2) | 94.3(3.5) | 98.7(2.1) | 0.01 | 51.8 |
| 111 | Azinphos-methyl | 1–200 | 0.9945 | 84.9(1.8) | 80.5(1.9) | 82.4(1.9) | 0.01 | −28.7 |
| 112 | Mefenacet | 1–200 | 0.9982 | 85.6(1.4) | 97.2(3.8) | 101(1.7) | 0.005 | 72.8 |
| 113 | Mirex | 1–200 | 0.9985 | 85.0(4.9) | 88.2(9.4) | 86.6(4.1) | 0.005 | −16.1 |
| 114 | Cyhalothrin | 1–200 | 0.9950 | 82.5(0.2) | 88.8(3.7) | 93.2(1.5) | 0.01 | 26.4 |
| 115 | λ-Cyhalothrin | 1–200 | 0.9943 | 81.8(2.3) | 86.8(3.6) | 91.2(2.3) | 0.01 | 35.6 |
| 116 | Fenarimol | 1–200 | 0.9979 | 84.7(0.8) | 94.0(2.5) | 94.6(1.8) | 0.01 | 23.8 |
| 117 | Pyrazophos | 1–200 | 0.9965 | 81.7(2.3) | 86.4(4.6) | 92.1(2.3) | 0.01 | 60.3 |
| 118 | Acrinathrin | 2–200 | 0.9922 | 83.0(2.7) | 83.0(2.4) | 88.0(1.3) | 0.01 | −50.9 |
| 119 | Bitertanol | 1–200 | 0.9977 | 85.6(1.7) | 90.0(3.3) | 93.1(1.8) | 0.01 | 88.7 |
| 120 | Permethrin | 1–200 | 0.9988 | 86.7(3.4) | 94.3(4.5) | 97.7(3.3) | 0.005 | −47.8 |
| 121 | Coumaphos | 1–200 | 0.9974 | 83.8(2.1) | 79.4(3.6) | 80.8(1.8) | 0.01 | 59.4 |
| 122 | Fluquinconazole | 1–200 | 0.9984 | 86.8(2.1) | 90.5(2.1) | 90.2(2.0) | 0.005 | 29.3 |
| 123 | Pyridaben | 1–200 | 0.9976 | 83.0(1.8) | 91.6(3.2) | 94.1(1.9) | 0.005 | 28.4 |
| 124 | Cyfluthrin | 2–200 | 0.9960 | 80.4(2.0) | 92.0(4.7) | 98.6(2.6) | 0.01 | −49.4 |
| 125 | Boscalid | 1–200 | 0.9973 | 82.9(1.3) | 92.3(2.2) | 89.0(1.0) | 0.005 | 64.4 |
| 126 | Cypermethrin | 1–200 | 0.9938 | 88.6(1.8) | 92.8(5.0) | 97.7(1.7) | 0.01 | −22.7 |
| 127 | Quizalofop-ethyl | 1–200 | 0.9964 | 82.7(0.9) | 91.3(4.9) | 95.4(2.6) | 0.01 | 56.4 |
| 128 | Flucythrinate | 1–200 | 0.9945 | 81.3(1.4) | 90.6(4.0) | 96.4(2.0) | 0.01 | −39.5 |
| 129 | Ethofenprox | 1–200 | 0.9948 | 74.4(14.4) | 85.2(10.8) | 93.9(3.4) | 0.01 | 1.3 |
| 130 | Phenvalerate | 2–200 | 0.9935 | 79.0(2.6) | 82.8(2.7) | 80.9(2.0) | 0.01 | −46.7 |
| 131 | Tua-Fluvalinate | 1–200 | 0.9911 | 79.5(0.9) | 78.8(2.2) | 80.1(1.4) | 0.01 | −36.4 |
| 132 | Difenoconazole | 1–200 | 0.9979 | 72.2(10.5) | 90.2(7.2) | 85.2(2.0) | 0.01 | −68.5 |
| 133 | Deltamethrin | 1–200 | 0.9905 | 75.5(3.8) | 82.6(2.8) | 86.6(2.2) | 0.01 | −34.3 |
3.4.2. LOQ
The LOQ for each pesticide was defined as the lowest validated spiked level satisfying the requirement of recovery ranging from 70% to 120% and a relative standard deviation (RSD) less than 20%. Samples were spiked at two different concentrations: 0.005 and 0.01 mg/kg (6 replicates per level). The LOQ values are presented in Table 2.
3.4.3. Accuracy and precision
Recovery was evaluated at three different spiked levels of 0.05, 0.1 and 0.2 mg/kg by spiking six blank samples at each level. Precision was expressed as the relative standard deviation (RSD) and was obtained from the six spiked samples at three spiking levels. Table 2 shows the recoveries and RSDs of all pesticides at all concentrations. The recovery of all the pesticides met the requirements of the pesticide residue determination.
3.4.4. Matrix effects (ME)
In this study, some pesticides, such as mevinphos, propoxur, dicrotophos and carboxine, have better chromatographic peak shapes in matrix-matched blank solutions than in pure solvent solutions because of the ME.
The ME was evaluated by the slope of the solvent calibration curve and the matrix-matched blank extract calibration curve according to the equation: ME (%) = [(slope in matrix/slope in solvent)−1] * 100 [29]. The ME results were grouped into 3 classes: a high ME (less than −50% or higher than +50%), a medium ME (between −50% and −20% or +20% and +50%) and a low ME (between +20% and −20%). Fig. 7 shows the ME of each pesticide. Among 133 pesticides, 53% showed low ME, 32% showed medium ME and 16% showed high ME. In order to avoid the ME, matrix-matched calibration standards were used for quantification to compensate for the ME.
Fig. 7.
Matrix effect of pesticides in chenpi.
3.5. Application to real samples
Once the analytical methodology was validated, it was used for monitoring pesticides in chenpi samples. The established method was used for the simultaneous determination of the pesticides in twenty real samples and the results are summarized in Table 3. Chlorpyrifos, isocarbophos, methidathion, profenofos and fenpropathrin were found in eight batches, most of which were insecticides and fungicides. Insecticide clorpyrifos and methidathion were frequently detected pesticides, but no pesticide exceeded the maximum residue limits (MRL, the values of MRL for orange were taken as reference) prescribed by Regulation (EC) no. 396/2005 [30]. Clorpyrifos and methidathion are low toxicity pesticides, but the others are moderately or highly toxic pesticides, which are harmful to human health. Therefore, it is important to pay attention to the appropriate use of pesticides.
Table 3.
Pesticide residues found in different batches of chenpi samples and their concentrations (mg/kg).
| No. | Pesticide found (mg/kg) |
||||
|---|---|---|---|---|---|
| Chlorpyrifos | Isocarbophos | Methidathion | Profenofos | Fenpropathrin | |
| 2 | <LOQ | n.d. | 0.016 | n.d. | n.d. |
| 5 | n.d. | n.d. | 0.014 | n.d. | n.d. |
| 10 | <LOQ | n.d. | 0.016 | n.d. | <LOQ |
| 14 | 0.007 | <LOQ | n.d. | n.d. | n.d. |
| 16 | 0.008 | <LOQ | <LOQ | 0.007 | 0.221 |
| 17 | 0.007 | 0.009 | 0.017 | 0.006 | 0.025 |
| 18 | <LOQ | <LOQ | 0.013 | <LOQ | 0.117 |
| 20 | <LOQ | <LOQ | <LOQ | n.d. | 0.042 |
| MRL | 0.01 | 0.01 | 0.02 | 0.01 | 2 |
Note: n.d.: no residues detected. MRL: maximum residue limit.
4. Conclusions
A modified QuEChERS method for the determination of multiple pesticides by GC-MS/MS was developed. Several sorbents were evaluated in order to reduce the ME as much as possible. C18 and PSA were mainly used in the Original QuEChERS method. In our study, PSA had an effect on the pesticide ditalimfos, which showed an unsatisfactory recovery on increasing the amount of PSA. NH2, with a similar adsorption performance to PSA, is a substitute product of PSA for base-sensitive pesticides or pesticides which could be affected by PSA. The addition of GCB can remove pigment extracted with acetonitrile. A validation procedure was performed, which showed good results for suitability, recovery and repeatability. The developed method was applied to the determination of real samples, and some pesticides were detected, which demonstrated that it is essential to constantly monitor pesticide residues in chenpi.
Acknowledgments
The authors wish to thank the management of Tasly Academy for supporting this research.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under the responsibility of Xi'an Jiaotong University.
References
- 1.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. China Medical Science Press; Beijing, China: 2015. [Google Scholar]
- 2.Yi Z.B., Yu Y., Liang Y. In vitro antioxidant and antimicrobial activities of the extract of pericarpium citri reticulatae of a new citrus cultivar and its main flavonoids. LWT-Food Sci. Technol. 2018;41:597–603. [Google Scholar]
- 3.Peng X.J., Liang W.H., Peng M. Determination of 8 organophosphorus pesticides in pericarpium citri Reticulatae and its products by solid phase extraction/gas chromatography. J. Instrum. Anal. 2016;10:1267–1272. [Google Scholar]
- 4.Ma M., Chen C., Yang G. Combined cytotoxic effects of pesticide mixtures present in the Chinese diet on human hepatocarcinoma cell line. Chemosphere. 2016;159:256–266. doi: 10.1016/j.chemosphere.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 5.Bapayeva G., Issayeva R., Zhumadilova A. Organochlorine pesticides and female puberty in South Kazakhstan. Reprod. Toxicol. 2016;65:67–75. doi: 10.1016/j.reprotox.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Peng X.J., Qin H., Wen Q.J. Application of homemade double solid phase extraction column for determination of 11 organophosphorus pesticides in Xinhui dried orange peel and its pdroducts. Chin. J. Chromatogr. 2016;34:817–822. [Google Scholar]
- 7.Pelajic M., Pecek G., Pavlovic D.M. Novel multiresidue method for determination of pesticides in red wine using gas chromatography–mass spectrometry and solid phase extraction. Food Chem. 2016;200:98–106. doi: 10.1016/j.foodchem.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Yang X., Zhang H., Liu Y. Multiresidue method for determination of 88 pesticides in berry fruits using solid-phase extraction and gas chromatography–mass spectrometry: determination of 88 pesticides in berries using SPE and GC–MS. Food Chem. 2011;127:855–865. doi: 10.1016/j.foodchem.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Machado I., Gerez N., Piston M. Determination of pesticide residues in globe artichoke leaves and fruits by GC-MS and LC-MS/MS using the same QuEChERS procedure. Food Chem. 2017;227:227–236. doi: 10.1016/j.foodchem.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Sajid M., Basheer C., Daud M. Evaluation of layered double hydroxide/graphene hybrid as a sorbent in membrane-protected stir-bar supported micro-solid-phase extraction for determination of organochlorine pesticides in urine samples. J. Chromatogr. A. 2017;1489:1–8. doi: 10.1016/j.chroma.2017.01.089. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N., Gao J., Huang C. In situ hydrothermal growth of ZnO/gC3N4 nanoflowers coated solid-phase microextraction fibers coupled with GC-MS for determination of pesticides residues. Anal. Chim. Acta. 2016;934:122–131. doi: 10.1016/j.aca.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 12.David F., Devos C., Dumont E. Determination of pesticides in fatty matrices using gel permeation clean-up followed by GC-MS/MS and LC-MS/MS analysis: a comparison of low-and high-pressure gel permeation columns. Talanta. 2017;165:201–210. doi: 10.1016/j.talanta.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Lian W., Ren F., Tang L. Analysis of polycyclic aromatic hydrocarbons in cigarette samples using gel permeation chromatography clean-up by gas chromatography–tandem mass spectrometry. Microchem. J. 2016;129:194–199. [Google Scholar]
- 14.Wu G., Bao X., Zhao S. Analysis of multi-pesticide residues in the foods of animal origin by GC–MS coupled with accelerated solvent extraction and gel permeation chromatography cleanup. Food Chem. 2011;126:646–654. [Google Scholar]
- 15.Anastassiades M., Lehotay S.J., Stajnbaher D. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘‘dispersive solid-phase extraction’’ for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- 16.He Z., Wang L., Peng Y. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography–tandem mass spectrometry-based method. Food Chem. 2015;169:372–380. doi: 10.1016/j.foodchem.2014.07.102. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Kong W., Zhao L. A multiresidue method for simultaneous determination of 44 organophosphorous pesticides in Pogostemon cablin and related products using modified QuEChERS sample preparation procedure and GC-FPD. J. Chromatogr. B. 2015;974:118–125. doi: 10.1016/j.jchromb.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Georgakopoulos P., Zachari R., Mataragas M. Optimisation of octadecyl (C18) sorbent amount in QuEChERS analytical method for the accurate organophosphorus pesticide residues determination in low-fatty baby foods with response surface methodology. Food Chem. 2011;128:536–542. doi: 10.1016/j.foodchem.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 19.Golge O., Kabak B. Evaluation of QuEChERS sample preparation and liquid chromatography-triple-quadrupole mass spectrometry method for the determination of 109 pesticide residues in tomatoes. Food Chem. 2015;176:319–332. doi: 10.1016/j.foodchem.2014.12.083. [DOI] [PubMed] [Google Scholar]
- 20.Boes E., Rosmalina R.T., Ridwan Y.S. Pesticide residues in vegetable. Procedia Chem. 2015;16:229–236. [Google Scholar]
- 21.Carneiro R.P., Oliveira F.A.S., Madureira Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC–MS/MS analysis. Food Control. 2013;33:413–423. [Google Scholar]
- 22.Martinez-Dominguez G., Nieto-García A.J., Romero-González R. Application of QuEChERS based method for the determination of pesticides in nutraceutical products (Camellia sinensis) by liquid chromatography coupled to triple quadrupole tandem mass spectrometry. Food Chem. 2015;177:182–190. doi: 10.1016/j.foodchem.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Lehotay S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: collaborative study. J. AOAC Int. 2007;90:485–520. [PubMed] [Google Scholar]
- 24.Tette P.S., da Silva Oliveira F.A., Pereira E.N. Multiclass method for pesticides quantification in honey by means of modified QuEChERS and UHPLC–MS/MS. Food Chem. 2016;211:130–139. doi: 10.1016/j.foodchem.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Palenikova A., Martinez-Dominguez G., Arrebola F.J. Multifamily determination of pesticide residues in soya-based nutraceutical products by GC/MS–MS. Food Chem. 2015;173:796–807. doi: 10.1016/j.foodchem.2014.10.100. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Li Y., Meng W. A multi-residue method for simultaneous determination of 74 pesticides in Chinese material medica using modified QuEChERS sample preparation procedure and gas chromatography tandem mass spectrometry. J. Chromatogr. B. 2016;1015:1–12. doi: 10.1016/j.jchromb.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Costa E.P., Caldas S.S., Primel E.G. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in canned and fresh peach. Food Chem. 2014;165:587–593. doi: 10.1016/j.foodchem.2014.05.099. [DOI] [PubMed] [Google Scholar]
- 28.EC-European Comission, Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. SANTE/11945/2015.
- 29.Lozowicka B., Ilyasova G., Kaczynski P. Multi-residue methods for the determination of over four hundred pesticides in solid and liquid high sucrose content matrices by tandem mass spectrometry coupled with gas and liquid chromatograph. Talanta. 2016;151:51–61. doi: 10.1016/j.talanta.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 30.EC-European Comission, Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC, Official Journal of the European Union, L70, 2005, 1–16.