Highlights
-
•
Intra-network functional connectivity (FC) in the right precuneus within the posterior default mode network was decreased in PPPD patients.
-
•
Intra-network FC between the right precuneus and the bilateral precuneus and left premotor cortex was decreased.
-
•
Intra-network FC between the right precuneus and bilateral corpus callosum was enhanced.
-
•
Inter-network FC was increased between the visual network and auditory, sensorimotor networks.
-
•
FC changes were negatively correlated with dizziness handicap inventory functional scores.
Keywords: Persistent postural-perceptual dizziness, Resting state functional magnetic resonance imaging, Independent component analysis, Visual network, Functional connectivity
Abstract
Background
Persistent postural-perceptual dizziness (PPPD) is a functional vestibular disorder characterized by persistent dizziness, unsteadiness, and non-spinning vertigo. It is the most common cause of chronic vestibular syndrome, but its pathogenesis is currently unclear. Recent studies have indicated that sensory integration may be altered in PPPD patients.
Objective
Using independent component analysis (ICA) combined with seed-based functional connectivity analysis, we aimed to analyze changes in brain network functional connectivity (FC) in PPPD patients during the resting state and to explore the underlying pathogenesis of PPPD, particularly the abnormal integration of multiple sensations.
Methods
Study subjects included 12 PPPD patients and 12 healthy controls and were recruited from January to August 2018. Detailed medical data were collected from all participants. Vestibular function, neurological and medical examinations were conducted to exclude other diseases associated with chronic dizziness. Functional MRI was performed on all subjects. ICA and seed-based functional connectivity analysis were performed to examine changes in intra- and inter-network FC in PPPD patients.
Results
In total, 13 independent components were identified using ICA. Compared with healthy controls, PPPD patients showed decreased intra-network FC in the right precuneus within the posterior default mode network. Moreover, seed-based functional connectivity analysis showed decreased intra-network FC between the right precuneus and the bilateral precuneus and left premotor cortex, and enhanced FC between the right precuneus and bilateral corpus callosum. With respect to the inter-network, FC in PPPD patients was increased between the occipital pole visual network and auditory, sensorimotor networks, as well as the lateral visual and auditory networks. Additional analyses showed that FC changes were negatively correlated with dizziness handicap inventory functional scores.
Conclusion
In PPPD patients, dysfunction in the precuneus may cause abnormalities in external environment monitoring and in posture and movement regulation. Compensatory strategies may then be adopted to maintain balance. At the local level, information exchange between the two cerebral hemispheres is enhanced via the corpus callosum. At the whole brain level, through enhancement of functional activities of the visual network, the integration of multiple sensations and the regulation of posture and movement are primarily driven by visual information.
1. Introduction
Persistent postural-perceptual dizziness (PPPD) is a functional vestibular disorder with clinical symptoms, such as persistent dizziness and unsteadiness, and is the most common cause of chronic vestibular syndrome (Bittar and Lins, 2015; Staab et al., 2017a). The criteria for diagnosis of PPPD were formally presented in 2017 and include phobic postural vertigo, space and motion discomfort, visual-induced dizziness (VID), and chronic subjective dizziness (CSD). The variation in these diagnoses may reflect different perspectives on the potential pathogenesis of PPPD, which to date, remains unclear.
Visual aggravation of dizziness and instability are characteristic manifestations of PPPD (Staab et al., 2017a), suggesting that integration of visual and vestibular sensations in PPPD patients may be abnormal. With greater research into this area, substantial evidence has accumulated that abnormalities in multi-sensory information processing and integration may be involved in the occurrence of PPPD (Indovina et al., 2015; Lee et al., 2018a; Staab et al., 2017b; Van Ombergen et al., 2017a). In recent years, investigators have explored abnormalities in sensory integration in patients with PPPD using functional connectivity (FC) analysis. Van Ombergen et al. (2017a) found that FC was decreased in the superior temporal gyrus and enhanced in the occipital lobe in VID patients by using intrinsic connectivity contrast analysis. Moreover, seed-based analysis revealed increased FC between the visual cortex and the middle frontal gyrus and precuneus. These changes in FC between the visual cortex and vestibular cortex could explain why dizziness occurs in VID patients following exposure to complex visual environments. Lee et al. (2018a) performed whole brain and region of interest (ROI)-based FC analysis in 38 PPPD patients, and the results showed that FC between the left superior occipital gyrus and the left middle frontal gyrus was increased. These data indicate that the interaction between the visual and vestibular cortices of PPPD patients was altered and suggests that this interaction was driven primarily by visual information. Indovina et al. (2015) performed a task-based functional MRI (fMRI) study in 18 patients with CSD using sound-evoked vestibular stimuli (a VEMP-type stimulus). These investigators found that FC between the anterior insula and superior temporal gyrus, the anterior insula and middle occipital gyrus, the hippocampus and superior temporal gyrus, the anterior cingulate cortex and superior temporal gyrus were all changed in CSD patients. FC changes between the anterior insula and the middle occipital gyrus may be associated with visual-dependence in CSD patients. In our previous study, we found that spontaneous functional activities of the right precuneus and cuneus of PPPD patients was weakened, which may lead to abnormal integration of visual-vestibular information. Further FC analysis revealed a weakened FC between the precuneus and left precentral gyrus. These results suggested that the ability to adjust posture and movement using vestibular and visual information is impaired and that the integration of multiple sensations and regulation of sensorimotor skills may be abnormal in PPPD patients (Li et al., 2019).
These important findings indicated that the interaction between the visual cortex and the vestibular cortex of PPPD patients has clearly changed. However, these previous studies focused mainly on seed-based and ROI-based FC analyses, which are limited as they represent FC changes in only a selected seed region or ROI. However, the human brain is a complex, dynamic system and relies on a network of multiple brain regions and synergy between multiple neural networks (Perlbarg and Marrelec, 2008). Therefore, FC analysis of brain networks using independent component analysis (ICA) may be more useful in exploring the changes in sensory integration and understanding the pathogenesis of PPPD.
We therefore performed ICA and seed-based functional connectivity analysis to investigate the changes in brain network FC in PPPD patients and to explore the underlying pathogenesis of PPPD, particularly the abnormal integration of multiple sensations.
2. Subjects
Twelve PPPD patients were enrolled in this study between January and August 2018. All subjects volunteered to participate in this study and signed informed consent. This study was approved by the Ethics Committee of Peking University Aerospace School of Clinical Medicine, China. Detailed medical information was collected for all subjects. Videonystagmography (VNG), vestibular caloric test, video head impulse test (vHIT), and VEMPs were performed to exclude peripheral vestibular lesions. MRI was further performed to exclude focal lesions and other neurological diseases. Other examinations, including blood pressure, blood routine test, and electrocardiogram were conducted to exclude other medical diseases associated with chronic dizziness. The 7-item Generalized Anxiety Disorder scale (GAD-7) and 9-item Patient Health Questionnaire depression scale (PHQ-9) were used to assess anxiety and depression levels in patients with PPPD. Meanwhile, the subjective vestibular disability score (SVDS), the clinical vestibular score (CVS), and the dizziness handicap inventory score (DHI) were applied to evaluate patients' symptoms. The diagnosis of PPPD was based on the diagnostic criteria of Bárány Society (the 2017 edition). Simultaneously, 12 age- and gender-matched healthy controls were included. All healthy controls had no history of headache or dizziness and no serious medical diseases. Then, all subjects were further scanned for fMRI.
3. fMRI data acquisition
All images were acquired using 3.0-Tesla MR (MAGNETOM Skyra syngo MR D13; Siemens, Germany) with a 32-channel head & neck coil. During image acquisition, the head of the subjects was fixed using foam padding to reduce head movement and earplugs were utilized to minimize scanner noise. Subjects were instructed to relax, keep eyes closed, but stay awake during scanning. The structural images were recorded using three-dimension magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.43 ms, flip angle = 8°, field of view (FOV) = 256 × 256 mm2, voxel size: 1.0 × 1.0 × 1.0 mm3, a total of 192 slices were acquired. The functional images were recorded using an echo-planar imaging (EPI) sequence with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 222 × 222 mm2, voxel size = 3.0 × 3.0 × 3.0 mm3. A total of 200 volumes were collected with a scanning time of 6 min and 48 s. All subjects remained awake during the scan and there was no significant discomfort during or after scan.
4. Pre-processing of fMRI data
4.1. Independent component analysis
The first 10 time points of the functional imaging were deleted because of the instability of the initial MRI signal and the need for participants to adapt to the scanning environment. Slice timing and realignment were then conducted to ensure the accuracy of the positional information. The subjects who had more than a 1.5 mm head movement in the x, y, or z direction and a 1.5˚ head rotation were removed from the analysis. Furthermore, fMRI images were spatially normalized to the Montreal Neurological Institute space using DARTEL, and resampled at a resolution of 3 × 3 × 3 mm3 cubic voxels. fMRI images were then smoothed with a Gaussian kernel of 8 × 8 × 8 mm3 using full width at half maximum to reduce registration errors and increase the normality of the data.
4.2. Seed-based functional connectivity analysis
The data processing was performed as follows: (1) deletion of the first 10 time points; (2) slice timing; (3) realignment; (4) spatial normalization; (5) regression: a linear regression model was used to remove the interference signal in the BOLD signal. In this study, the regression interference signal includes linear drift, head motion parameter signals, white matter signal, and spinal fluid signal; (6) band-pass filtering: the time series for each voxel was temporally band-pass filtered (0.01–0.1 Hz) to reduce low-frequency drift and physiological high-frequency noise.
5. Indicator calculation and analysis
5.1. Independent component analysis
ICA was performed using GIFT software (Version 3.0a, http://mialab.mrn.org/software/gift/index.html). (1) Data reduction was conducted using principle component analysis; (2) the minimum description length was used to estimate the number of independent components; (3) the infomax algorithm was utilized for group analysis of ICA. To ensure data reproducibility, we performed the ICASSO 100 times; (4) the independent component (IC) time courses and spatial map of individual subjects were reverse reconstructed using Spatial-temporal Regression. After the reverse reconstruction, the time course of each participant's IC and space IC was acquired, and the spatial map was converted into a Z-score.
5.2. Intra-network FC
For each IC of each subject, a one-sample t-test was performed in healthy controls and PPPD patients to create a sample-specific component map and a network mask. Multiple comparisons were corrected with a false discovery rate (FDR) method (P< 0.05). Each mask of the healthy controls and PPPD patients was further combined into a total MASK for each component. A two-sample t-test with regressing covariates, such as age and gender, was conducted to compare the differences in FC between PPPD patients and healthy controls of each component within the corresponding network mask. The results of multiple comparisons were corrected by family-wise error (FWE) (P < 0.05). And due to the small sample size of our study, we re-analyzed the comparison of patients and controls using threshold-free cluster enhancement (TFCE) with 5000 permutations via the TFCE toolbox for SPM.
5.3. Inter-network FC
For each subject, the time series of each network component was extracted to calculate the Pearson correlation coefficient (r) of each network component and other network time series, namely functional network connectivity (FNC). The resulting r values were normalized into Z values by Fisher-Z transformation (Liu et al., 2017). A 13 × 13 matrix was then obtained. A two-sample t-test was conducted to compare the differences in FNC between PPPD patients and healthy controls. The results of multiple comparisons were corrected for FDR (P < 0.05).
5.4. Seed-based functional connectivity analysis
Based on intra-network FC analysis, areas with altered intra-network FC were selected as seeds to further explore FC changes between brain regions. The average time series in the seeds were extracted. Pearson correlation coefficients between the time series of the seeds and all other voxels in the whole brain and each component were calculated to obtain FC (Liu et al., 2015). Then the images were smoothed with a Gaussian kernel of 8 × 8 × 8 mm3 to reduce registration errors and increase the normality of the data. The resulting r values were normalized into Z values by Fisher-Z transformation for further comparison between groups (Liu et al., 2017).
5.5. Correlation analysis
Correlation analysis of intra- and inter-network FC, and seed-based FC with duration of medical history, DHI, SVDS, CVS, GAD7, PHQ9 scores were further performed to examine the correlation between FC changes and clinical manifestations in PPPD patients.
6. Results
6.1. Clinical data
Eight males and four females were included. All participants were right-handed. The mean age was 44.25 ± 10.73 years, mean SVDS was 9.09 ± 4.93, mean CVS was 4.58 ± 2.84, mean GAD7 score was 9.09 ± 4.93, mean PHQ9 score was 4.58 ± 2.84, mean DHI score was 53.92 ± 8.87, mean DHI-P score was 15.50 ± 3.09, mean DHI-E score was 16.17 ± 5.62, and mean DHI-F score was 16.17 ± 5.62 (Tables 1, 2).
Table 1.
Clinical baseline data of patients with persistent postural-perceptual dizziness.
| NO. | Age (year) | Duration (month) | DHI | DHI-P | DHI-E | DHI-F | SVDS | CVS | GAD7 | PHQ9 |
|---|---|---|---|---|---|---|---|---|---|---|
| P01 | 35 | 6 | 40 | 12 | 10 | 18 | 5 | 2 | 8 | 1 |
| P02 | 34 | 3 | 58 | 18 | 14 | 26 | 5 | 0 | 5 | 5 |
| P03 | 57 | 8 | 62 | 14 | 26 | 24 | 6 | 8 | 7 | 1 |
| P04 | 62 | 120 | 65 | 18 | 20 | 28 | 12 | 9 | 8 | 6 |
| P05 | 46 | 156 | 52 | 20 | 12 | 20 | 22 | 5 | 9 | 3 |
| P06 | 55 | 36 | 56 | 12 | 22 | 22 | 3 | 3 | 7 | 1 |
| P07 | 32 | 12 | 50 | 18 | 10 | 22 | 8 | 0 | 10 | 5 |
| P08 | 40 | 6 | 52 | 10 | 20 | 22 | 8 | 5 | 13 | 6 |
| P09 | 49 | 7 | 64 | 18 | 20 | 26 | 11 | 4 | 9 | 4 |
| P10 | 33 | 3 | 60 | 12 | 25 | 23 | 6 | 3 | 7 | 3 |
| P11 | 53 | 12 | 60 | 13 | 20 | 24 | 5 | 3 | 7 | 2 |
| P12 | 35 | 6 | 40 | 12 | 10 | 18 | 5 | 2 | 9 | 1 |
DHI, dizziness handicap inventory score; DHI-F; DHI-functional score; DHI-E, DHI-emotional score; DHI-P, DHI-physical score; SVDS, subjective vestibular disability score; CVS, clinical vestibular score, GAD-7, 7-item Generalized Anxiety Disorder scale, PHQ-9, 9-item Patient Health Questionnaire depression scale.
Table 2.
Clinical characteristics of patients with persistent postural-perceptual dizziness.
| NO. | Core vestibular symptoms | Increased as the day progressed | Exacerbating factors | ||||
|---|---|---|---|---|---|---|---|
| Dizziness | Unsteadiness | Non-spinning vertigo | Upright posture | Active or passive motion | Complex visual environment | ||
| P01 | √ | √ | √ | √ | √ | √ | |
| P02 | √ | √ | √ | √ | √ | √ | √ |
| P03 | √ | √ | √ | √ | √ | √ | |
| P04 | √ | √ | √ | √ | √ | ||
| P05 | √ | √ | √ | √ | √ | √ | |
| P06 | √ | √ | √ | √ | √ | √ | |
| P07 | √ | √ | √ | √ | √ | √ | |
| P08 | √ | √ | √ | √ | √ | ||
| P09 | √ | √ | √ | √ | √ | √ | |
| P10 | √ | √ | √ | √ | √ | √ | |
| P11 | √ | √ | √ | √ | √ | √ | |
| P12 | √ | √ | √ | √ | √ | √ | |
7. fMRI data
7.1. Resting-state network components
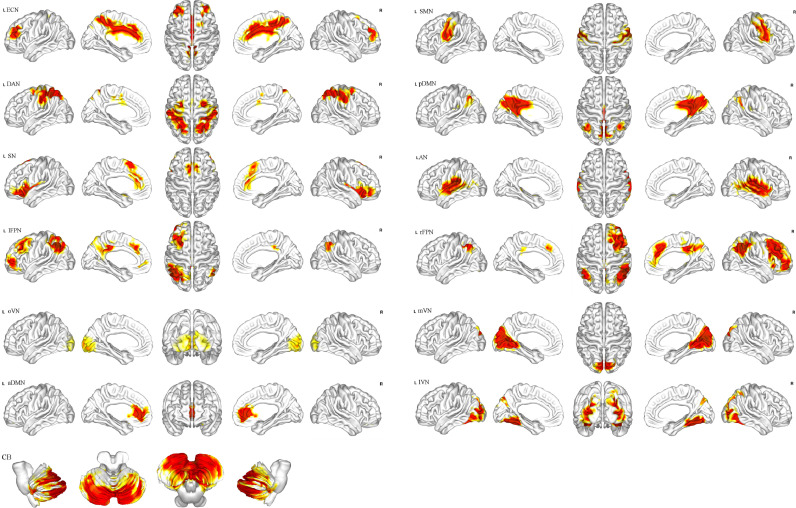
Network components were identified in accordance with a previous study (Smith et al., 2009). In total, 13 independent components were obtained (Fig. 1), including the anterior default mode network (aDMN), posterior default mode network (pDMN), occipital pole visual network (oVN), medial visual network (mVN), lateral visual network (lVN), auditory network (AN), sensorimotor network (SMN), executive control network (ECN), cerebellar network (CB), salience network (SN), dorsal attention network (DAN), right frontoparietal network (rFPN), and left frontoparietal network (lFPN).
Fig. 1.
Totally 13 independent components identified via independent component analysis.
7.2. Intra-network FC
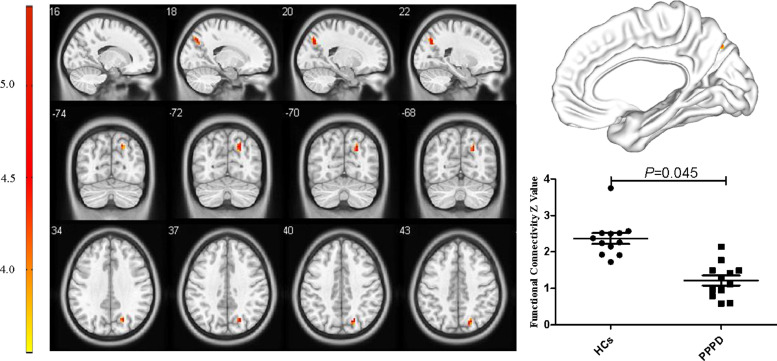
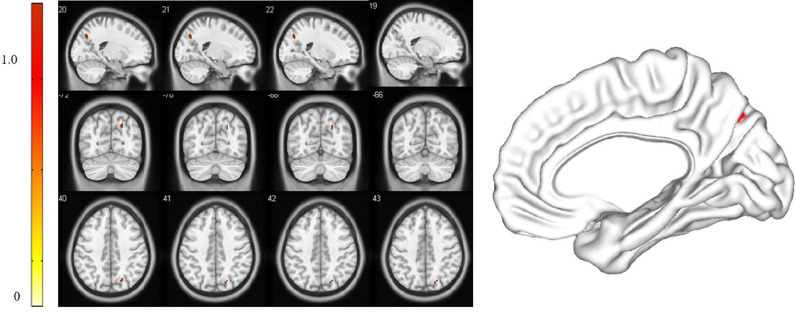
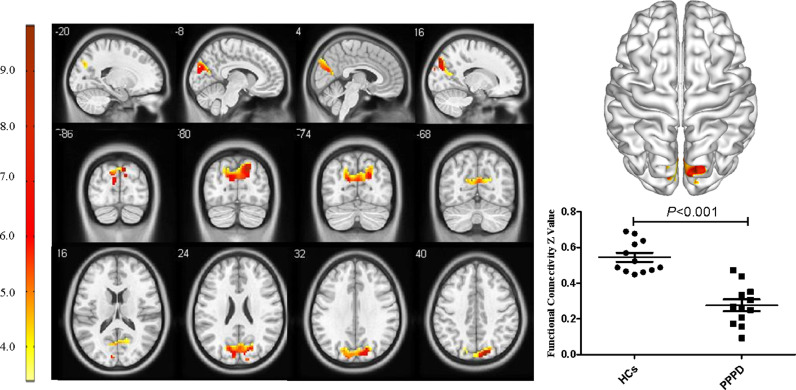
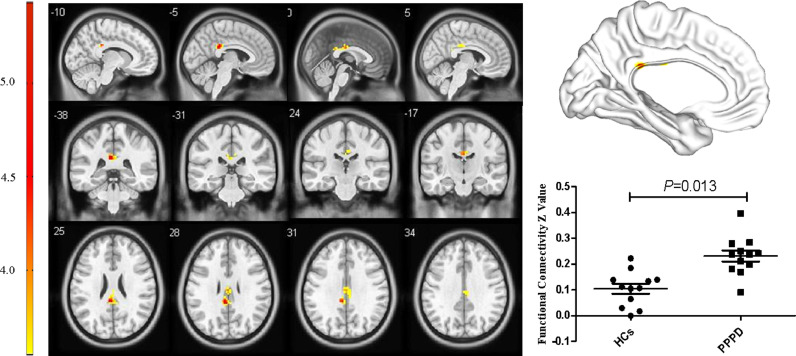
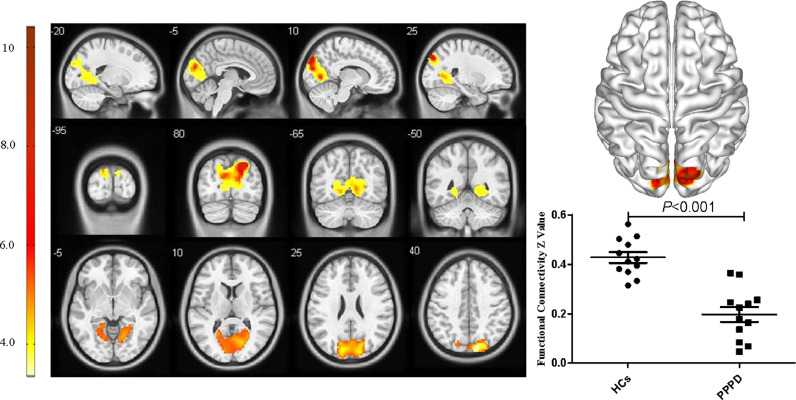
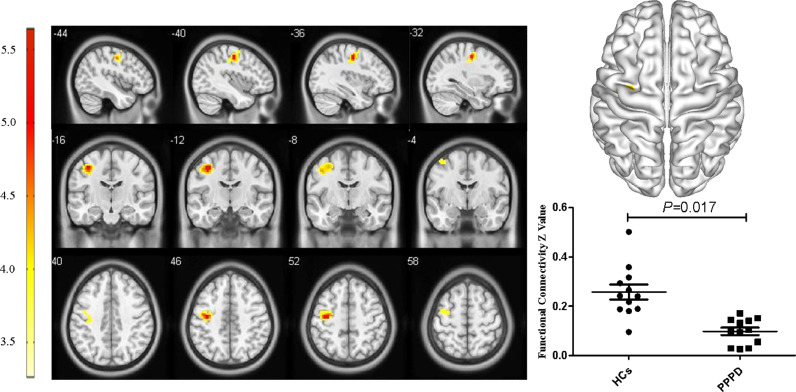
For the pDMN, FC in the right precuneus in PPPD patients was significantly decreased (X = 21, Y=−69, Z = 36, k = 23, P = 0.045, FWE corrected, Fig. 2). Results from TFCE also revealed decreased FC in the right precuneus of the pDMN in PPPD patients (X = 21, Y=−69, Z = 36, k = 8, P < 0.05, TFCE-FWE corrected, Fig. 3). Further seed-based functional connectivity analysis revealed decreased FC between right precuneus and bilateral precuneus (X = 12, Y=−84, Z = 39, k = 484, P < 0.001, FWE corrected), and enhanced FC between right precuneus and bilateral corpus callosum (X=−6, Y=−36, Z = 27, k = 76, P = 0.013, FWE corrected, Fig. 4, 5). In the whole brain, decreased FC was observed between the right precuneus and the bilateral precuneus, cuneus (X = 12, Y=−84, Z = 3, k = 2037, P<0.001, FWE corrected), and left premotor cortex (X=−36, Y=−12, Z = 5, k = 159, P = 0.017, FWE corrected, Fig. 6,7). There was no significant difference in FC within other network components between PPPD patients and healthy controls.
Fig. 2.
Decreased functional connectivity in right precuneus within posterior default mode network in patients with persistent postural-perceptual dizziness.
Fig. 3.
Decreased functional connectivity in right precuneus within posterior default mode network in patients with persistent postural-perceptual dizziness (TFCE-FWE corrected).
Fig. 4.
Decreased functional connectivity between precuneus and bilateral precuneus within posterior default mode network in patients with persistent postural–perceptual dizziness.
Fig. 5.
Enhanced functional connectivity between precuneus and the bilateral corpus callosum in patients with persistent postural–perceptual dizziness.
Fig. 6.
Decreased functional connectivity between right precuneus, bilateral precuneus and cuneus in patients with persistent postural–perceptual dizziness.
Fig. 7.
Decreased functional connectivity between precuneus were and left premotor cortex.
7.3. Inter-network FC
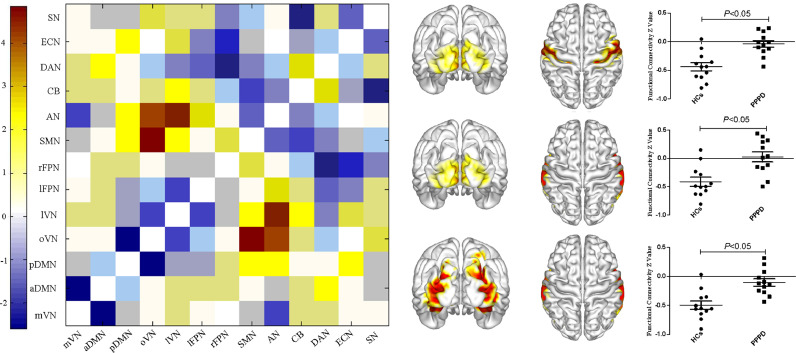
Compared with healthy controls, FC in PPPD patients was enhanced between the oVN and AN, the oVN and SMN, as well as the lVN and AN (P < 0.05, FDR corrected, Fig. 8).
Fig. 8.
Altered inter-network functional connectivity in patients with persistent postural–perceptual dizziness. Functional connectivity was increased between occipital pole visual network and auditory, sensorimotor networks, as well as lateral visual and auditory networks in patients with persistent postural-perceptual dizziness.
7.4. Correlation analysis
Using baseline data from patients, correlation analysis of changes in intra- and inter-network FC and seed-based FC was performed. Since the abnormal areas revealed by seed-based functional connectivity analysis were large, a total of nine ROIs were selected in this area for further correlation analyses (Table 3). The results showed that decreased FC between the precuneus and ROI1 (r=−0.648, P = 0.023), ROI2 (r=−0.580, P = 0.048), ROI3 (r=−0.628, P = 0.029), ROI4 (r=−0.660, P = 0.019), ROI5 (r=−0.642, P = 0.025), ROI6 (r=−0.654, P = 0.021), and ROI7 (r=−0.627, P = 0.029) was negatively correlated with the DHI-F score. FC between the lVN and AN was positively correlated with DHI-F score in PPPD patients (r = 0.613, P = 0.034).
Table 3.
The location of nine region of interests (ROIs).
| ROI | X | Y | Z | Radius (mm) |
|---|---|---|---|---|
| ROI1 | 18 | −81 | 33 | 6 |
| ROI2 | 15 | −72 | 6 | 6 |
| ROI3 | 0 | −72 | 21 | 6 |
| ROI4 | 0 | −84 | 15 | 6 |
| ROI5 | 0 | −81 | 33 | 6 |
| ROI6 | −6 | −84 | 12 | 6 |
| ROI7 | −18 | −90 | 27 | 6 |
| ROI8 | −39 | −9 | 51 | 6 |
| ROI9 | −3 | −36 | 27 | 6 |
8. Discussion
8.1. Altered intra-network FC in PPPD patients
Previous studies have shown that the DMN can be classified into the anterior and posterior DMN (aDMN and pDMN, respectively) (Buckner et al., 2008; Raichle, 2015). The aDMN contains the medial prefrontal lobe and is mainly associated with integration, planning and control. The pDMN contains the posterior cingulate gyrus and precuneus, which are mainly involved in self-centered cognition and attention monitoring (Raichle, 2015). The precuneus is an important node of the pDMN and has the highest resting metabolic rate. It participates in the integration of visual and vestibular information and plays an important role in spatial positioning and spatial perception (Cavanna and Trimble, 2006; Fransson and Marrelec, 2008; Margulies et al., 2009; Utevsky et al., 2014). In our previous studies, we found that amplitude of low frequency fluctuation and regional homogeneity values in the right precuneus were significantly lower in PPPD patients than in controls, indicating a decrease in spontaneous functional activity of the precuneus (Li et al., 2019). In the present study, the intra-network FC analysis showed decreased FC in the precuneus within the pDMN in PPPD patients. These results confirm our previous data and suggest that PPPD patients can no longer monitor the external environment, aggravating their symptoms of dizziness. Furthermore, seed-based functional connectivity analysis showed decreased FC between the precuneus and bilateral precuneus, which is also consistent with our previous studies. We speculate that decreased FC in the precuneus within the pDMN is possibly induced by weakened FC between the precuneus and bilateral precuneus. It is worth noting that the brain regions with abnormal FC were larger and more obvious than those found in previous studies, indicating that brain regions with abnormal spontaneous functional activity may be more extensive in PPPD patients than previously thought.
Seed-based functional connectivity analysis also showed weakened FC between the precuneus and premotor cortex, and this may be associated with abnormal posture control in PPPD patients. The posterior parietal cortex, including the precuneus, integrates sensory information to form an internal estimation model. It then transmits sensory information to the supplementary motor and premotor areas, thereby participating in motor and posture control (Hoshi and Tanji, 2007; Shadmehr, 2017; Takakusaki, 2017a). In PPPD patients, decreased FC between the precuneus and premotor cortex may lead to abnormal information transmission from the posterior parietal cortex to the premotor area. This might result in insufficient ability to adjust posture and movement using the integrated information, leading to postural instability. When patients are upright and in active/passive motion, their inability to adjust movement and posture is exacerbated, and symptoms of dizziness and unsteadiness are then aggravated. Moreover, the spontaneous functional activities of the precuneus and cuneus are weakened, and FC between the precuneus, cuneus, and premotor cortex are decreased. We speculate that functional changes in the brain of patients with PPPD may involve abnormalities of the cuneus-precuneus-premotor cortex loop.
Seed-based functional connectivity analysis also revealed increased FC between the precuneus and bilateral corpus callosum, which may be a result of compensation. Normal activity of the brain is based on the interaction of the bilateral cerebral hemispheres, and the corpus callosum allows the two hemispheres to communicate with one another, which facilitates coordination and integration (Hoptman and Davidson, 1994; Stark et al., 2008). In PPPD patients, FC in the right precuneus is weakened and the balance of activity between the bilateral precuneus is altered, resulting in a decreased synergistic effect of the bilateral precuneus. Correspondingly, the body also adopts a series of compensatory strategies. Enhanced FC between the precuneus and corpus callosum increases exchange of information between the bilateral hemispheres, thereby compensating for the imbalance of functional activity of the bilateral precuneus.
8.2. Altered inter-network FC in PPPD patients
8.2.1. Enhanced FC between the VN and AN in PPPD patients
Human perception of ourselves and of external space is a complex process, involving the integration of different sensory information, such as vision, hearing, vestibular sense, and proprioception (Bronstein, 2016; Takakusaki, 2017b). Studies have shown that hearing plays an important role in spatial perception and spatial orientation (Adams, 2016; Jaekl et al., 2014; Kaposvari et al., 2015; Odegaard et al., 2015; Oh et al., 2018; Wada et al., 2003). Humans can locate the position of a sound by comparing auditory information arriving at each ear, forming auditory spatial perception centered around the head (Wada et al., 2003). Moreover, through the integration of visual and vestibular information and the integration of different spatial references, the accuracy of spatial perception is improved, and a clear spatial map is formed (Bronstein, 2016; Jaekl et al., 2014; Kaposvari et al., 2015; Odegaard et al., 2015; Oh et al., 2018; Wada et al., 2003). In our study, the enhanced FC between the VN and AN indicates that integration of visual and auditory information is altered and is driven more by visual rather than auditory information.
It is worth noting that the temporal and parietal cortices around the Sylvian fissure are an integrated region of the auditory-vestibular system and are the core of the multisensory vestibular cortex (Klingner et al., 2013). In a previous fMRI study of PPPD, the vestibular cortex showed an enhanced FC to the occipital lobe and was also located in this area (Indovina et al., 2015; Lee et al., 2018a; Van Ombergen et al., 2017a). We speculate that the enhanced FC between the VN and AN not only affects the integration of visual and auditory information, but also affects the integration of visual and vestibular information. Previous studies have performed brain functional imaging in patients with PPPD and found that FC between the visual and vestibular cortices is increased, indicating that visual-vestibular integration is driven primarily by visual information(Lee et al., 2018b; Van Ombergen et al., 2017b).
Our results are consistent with those of previous studies. We observed direct changes in FC between the visual and vestibular cortices at the network level and enhanced FC between the VN and AN. These results reflect altered visual-vestibular-auditory integration in PPPD patients, which appear to be driven more by visual than auditory information. Dysfunction in the precuneus causes abnormalities in spatial perception and abnormal vestibular information integration. As a result, the body appears to initiate a series of pathological compensation strategies, which then alters sensory system integration.
8.2.2. Enhanced FC between the SMN and oVN in PPPD patients
The SMN mainly consists of the postcentral gyrus, precentral gyrus and premotor area, and its main function is to execute and coordinate movement (Smith et al., 2009). In the present study, we speculated that the enhanced FC between the SMN and oVN is a result of compensation. The role of the SMN in the regulation of body and posture may be driven primarily by vision, which can compensate for the inability of the body to use vestibular information to regulate movement and posture caused by decreased FC between the precuneus and premotor cortex. However, this compensation also has limits. Previous studies have found that body sway in PPPD patients is more obvious than other symptoms because of its visual impact. Patients showed increased visual dependence, which aggravates the patient's instability in complex visual environments (Sohsten et al., 2016).
Changes in inter-network FC in PPPD patients may reflect sensory reconstruction of visual-vestibular-proprioceptive senses, with PPPD patients showing increased visual dependence. With vision as the primary cue, multiple brain networks work together to maintain body balance and posture regulation. This study is the first to analyze alterations in brain network FC in PPPD patients using ICA and seed-based functional connectivity analysis. It is vital to understand abnormalities in sensory integration of PPPD. Altered network FC in PPPD patients also confirmed abnormal information integration in PPPD patients.
However, there are still some limitations in this study. The sample size of this study is small, which might have affected the results, and hence, in the current study, we used TFCE method with 5000 permutations to correct for multiple comparisons, the results also demonstrated altered FC in precuneus in PPPD patients. Therefore, we speculate that the dysfunction of precuneus may play an important role in the occurrence and development of PPPD. These findings will require replication in a larger study. Furthermore, we enrolled PPPD patients without peripheral vestibular lesions in the current study. PPPD is a highly heterogeneous disease with a variety of different triggering factors and comorbidities, classified and stratified studies in future are also needed to investigate the changes of brain FC in PPPD patients who had different triggering factors and comorbid conditions.
9. Conclusions
In PPPD patients, dysfunction of the precuneus may impair the body's ability to monitor the external environment and to regulate posture and movement. Then, the body has to adopt a series of compensatory strategies. At the local level, the information exchanged between the two cerebral hemispheres is enhanced via the corpus callosum. At the whole brain level, by enhancing functional activities in the visual network, the integration of multiple sensations and regulation of posture and movement are driven primarily by visual information.
Funding
The study was supported by Aerospace Center Hospital (Grant No. YN201912).
Ethical approval
This study was approved by the Ethics Committee of Peking University Aerospace School of Clinical Medicine, China. All subjects volunteered to participate in this study and signed informed consent.
CRediT authorship contribution statement
Kangzhi Li: Formal analysis, Investigation, Software, Data curation, Validation, Writing - original draft. Lihong Si: Formal analysis, Investigation, Data curation, Resources. Bin Cui: Formal analysis, Investigation, Data curation, Resources. Xia Ling: Formal analysis, Investigation, Data curation, Resources. Bo Shen: Formal analysis, Investigation, Data curation, Resources. Xu Yang: Conceptualization, Methodology, Investigation, Supervision, Funding acquisition, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Adams W.J. The development of audio-visual integration for temporal judgements. PLoS Comput. Biol. 2016;12 doi: 10.1371/journal.pcbi.1004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar R.S., Lins E.M. Clinical characteristics of patients with persistent postural-perceptual dizziness. Braz. J. Otorhinolaryngol. 2015;81:276–282. doi: 10.1016/j.bjorl.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein A.M. Multisensory integration in balance control. Handb. Clin. Neurol. 2016;137:57–66. doi: 10.1016/B978-0-444-63437-5.00004-2. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroimageNeuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., Davidson R.J. How and why do the two cerebral hemispheres interact? Psychol. Bull. 1994;116:195–219. doi: 10.1037/0033-2909.116.2.195. [DOI] [PubMed] [Google Scholar]
- Hoshi E., Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Indovina I., Riccelli R., Chiarella G., Petrolo C., Augimeri A., Giofre L., Lacquaniti F., Staab J.P., Passamonti L. Role of the insula and vestibular system in patients with chronic subjective dizziness: an fMRI study using sound-evoked vestibular stimulation. Front. Behav. Neurosci. 2015;9:334. doi: 10.3389/fnbeh.2015.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekl P., Perez-Bellido A., Soto-Faraco S. On the 'visual' in 'audio-visual integration': a hypothesis concerning visual pathways. Exp. Brain Res. 2014;232:1631–1638. doi: 10.1007/s00221-014-3927-8. [DOI] [PubMed] [Google Scholar]
- Kaposvari P., Csete G., Bognar A., Csibri P., Toth E., Szabo N., Vecsei L., Sary G., Tamas K.Z. Audio-visual integration through the parallel visual pathways. Brain Res. 2015;1624:71–77. doi: 10.1016/j.brainres.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Klingner C.M., Volk G.F., Flatz C., Brodoehl S., Dieterich M., Witte O.W., Guntinas-Lichius O. Components of vestibular cortical function. Behav. Brain Res. 2013;236:194–199. doi: 10.1016/j.bbr.2012.08.049. [DOI] [PubMed] [Google Scholar]
- Lee J.O., Lee E.S., Kim J.S., Lee Y.B., Jeong Y., Choi B.S., Kim J.H., Staab J.P. Altered brain function in persistent postural perceptual dizziness: a study on resting state functional connectivity. Hum. Brain Mapp. 2018;39:3340–3353. doi: 10.1002/hbm.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.O., Lee E.S., Kim J.S., Lee Y.B., Jeong Y., Choi B.S., Kim J.H., Staab J.P. Altered brain function in persistent postural perceptual dizziness: a study on resting state functional connectivity. Hum. Brain Mapp. 2018;39:3340–3353. doi: 10.1002/hbm.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Si L., Cui B., Ling X., Shen B., Yang X. Altered spontaneous functional activity of the right precuneus and cuneus in patients with persistent postural-perceptual dizziness. Brain Imag. Behav. 2019 doi: 10.1007/s11682-019-00168-7. [DOI] [PubMed] [Google Scholar]
- Liu F., Guo W., Fouche J.P., Wang Y., Wang W., Ding J., Zeng L., Qiu C., Gong Q., Zhang W., Chen H. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct. Funct. 2015;220:101–115. doi: 10.1007/s00429-013-0641-4. [DOI] [PubMed] [Google Scholar]
- Liu F., Wang Y., Li M., Wang W., Li R., Zhang Z., Lu G., Chen H. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum. Brain Mapp. 2017;38:957–973. doi: 10.1002/hbm.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Vincent J.L., Kelly C., Lohmann G., Uddin L.Q., Biswal B.B., Villringer A., Castellanos F.X., Milham M.P., Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard B., Wozny D.R., Shams L. Biases in visual, auditory, and audiovisual perception of space. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.Y., Boegle R., Ertl M., Stephan T., Dieterich M. Multisensory vestibular, vestibular-auditory, and auditory network effects revealed by parametric sound pressure stimulation. NeuroimageNeuroimage. 2018;176:354–363. doi: 10.1016/j.neuroimage.2018.04.057. [DOI] [PubMed] [Google Scholar]
- Perlbarg V., Marrelec G. Contribution of exploratory methods to the investigation of extended large-scale brain networks in functional MRI: methodologies, results, and challenges. Int. J. Biomed. Imaging. 2008;2008 doi: 10.1155/2008/218519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Distinct neural circuits for control of movement vs. holding still. J. Neurophysiol. 2017;117:1431–1460. doi: 10.1152/jn.00840.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohsten E., Bittar R.S., Staab J.P. Posturographic profile of patients with persistent postural-perceptual dizziness on the sensory organization test. J. Vestib. Res. 2016;26:319–326. doi: 10.3233/VES-160583. [DOI] [PubMed] [Google Scholar]
- Staab J.P., Eckhardt-Henn A., Horii A., Jacob R., Strupp M., Brandt T., Bronstein A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the barany society. J. Vestib. Res. 2017;27:191–208. doi: 10.3233/VES-170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab J.P., Eckhardt-Henn A., Horii A., Jacob R., Strupp M., Brandt T., Bronstein A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the barany society. J. Vestib. Res. 2017;27:191–208. doi: 10.3233/VES-170622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D.E., Margulies D.S., Shehzad Z.E., Reiss P., Kelly A.M., Uddin L.Q., Gee D.G., Roy A.K., Banich M.T., Castellanos F.X., Milham M.P. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017;10:1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017;10:1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky A.V., Smith D.V., Huettel S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ombergen A., Heine L., Jillings S., Roberts R.E., Jeurissen B., Van Rompaey V., Mucci V., Vanhecke S., Sijbers J., Vanhevel F., Sunaert S., Bahri M.A., Parizel P.M., Van de Heyning P.H., Laureys S., Wuyts F.L. Altered functional brain connectivity in patients with visually induced dizziness. Neuroimage Clin. 2017;14:538–545. doi: 10.1016/j.nicl.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ombergen A., Heine L., Jillings S., Roberts R.E., Jeurissen B., Van Rompaey V., Mucci V., Vanhecke S., Sijbers J., Vanhevel F., Sunaert S., Bahri M.A., Parizel P.M., Van de Heyning P.H., Laureys S., Wuyts F.L. Altered functional brain connectivity in patients with visually induced dizziness. Neuroimage Clin. 2017;14:538–545. doi: 10.1016/j.nicl.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Kitagawa N., Noguchi K. Audio-visual integration in temporal perception. Int. J. Psychophysiol. 2003;50:117–124. doi: 10.1016/s0167-8760(03)00128-4. [DOI] [PubMed] [Google Scholar]