Abstract
High fidelity and effective adaptive changes of the cell and tissue metabolism to changing environments requires strict coordination of numerous biological processes. Multicellular organisms developed sophisticated signaling systems of monitoring and responding to these different contexts. Among these systems, oxygenated lipids play a significant role realized via a variety of re-programming mechanisms. Some of them are enacted as a part of pro-survival pathways that eliminate harmful or unnecessary molecules or organelles by a variety of degradation/hydrolytic reactions or specialized autophageal processes. When these “partial” intracellular measures are insufficient, the programs of cells death are triggered with the aim to remove irreparably damaged members of the multicellular community. These regulated cell death mechanisms are believed to heavily rely on signaling by a highly diversified group of molecules, oxygenated phospholipids (PLox). Out of thousands of detectable individual LPox species, redox phospholipidomics deciphered several specific molecules that seem to be diagnostic of specialized death programs. Oxygenated cardiolipins (CLs) and phosphatidylethanolamines (PEs) have been identified as predictive biomarkers of apoptosis and ferroptosis, respectively. This has led to decoding of the enzymatic mechanisms of their formation involving mitochondrial oxidation of CLs by cytochrome c and endoplasmic reticulum-associated oxidation of PE by lipoxygenases. Understanding of the specific biochemical radical-mediated mechanisms of these oxidative reactions opens new avenues for the design and search of highly specific regulators of cell death programs. This review emphasizes the usefulness of such selective lipid peroxidation mechanisms in contrast to the concept of random poorly controlled free radical reactions as instruments of non-specific damage of cells and their membranes. Detailed analysis of two specific examples of phospholipid oxidative signaling in apoptosis and ferroptosis along with their molecular mechanisms and roles in reprogramming has been presented.
Graphical Abstract
The weariest and most loathed worldly life That age, ache, penury and imprisonment Can lay on nature, is a paradise To what we fear of death.”
William Shakespeare,
Measure for Measure (1603).
Programs of Regulated Cell Death or Re-programming Cell Populations?
Fidelity and quality control of biological systems depend, to a large extent, on the reprogramming or elimination of unnecessary or harmful cells and their components [1, 2]. Reprogramming, including trans-generational reprogramming [3, 4], is commonly engaged when the responses to changing environments are still reversible, while elimination is triggered usually when the effects of damaging or toxic materials exceed the repair capacities of cells. For a long time, cell death has been viewed as a catastrophic and mostly chaotic chain of events. Over the last 3-4 decades, this point of view has changed as experimental biology has discovered several organized and highly regulated cascades of cell death, thus “erasing” the differences between reprogramming and death [5, 6].
The emotional characterizations of the meaning of death have been transferred into the field of biology, particularly cell biology, where rationally designed sophisticated programs of elimination of unnecessary or harmful cells have earned the anthropocentric term “death.” One of the greatest Masters in describing the tragedy of human death and loss of the beloved people, W. Shakespeare, emphasized the “sweetness” of death that liberates from suffering, pain and loathed life. His words may sound like an appreciation of useful consequences of elimination or reprogramming mechanisms helping to rid of harmful attributes of life, yet are associated with the tragic symbolism of death. Interestingly, the emotional and philosophical categories of death are commonly applied to biological processes starting from cells.
The programs taking care of excessive or harmful cells and described as mechanisms of “regulated cell death” are important for the maintenance of healthy cell populations. The genetically-controlled mechanisms of individual death of organisms - phenoptosis - are widely spread at higher levels and have been described in prokaryotes, unicellular eukaryotes, and all kingdoms of multicellular eukaryotes (animals, plants, and fungi) [7]. Phenoptosis, although tragic at the individual level, serves an important evolutionary function. Two types of phenoptosis have been considered: i) acute phenoptosis - rapid deterioration of an organism induced by an essential biological function (eg, breeding), and ii) age-induced slow phenoptosis - slow deterioration and death of an organism due to accumulated stresses over long periods of time [8].
Independently of the “emotional flavors” of all these biochemical and biological processes and responses [9], understanding the molecular mechanisms of reprograming and death programs and pathways is essential for the very pragmatic goals of deciphering the etiology and pathogenesis of injury and disease conditions. Indeed, insufficient levels of elimination of harmful materials lead to the accumulation of antigenic/genotoxic materials - typical of cancer or auto-immune diseases; vice versa, exceedingly massive elimination leads to tissue and organ injury - eg, brain injury, sepsis, radiation injury, chronic degenerative diseases, etc (Fig. 1). In addition to the general biological, philosophical and even social aspects of the interpretation of death, this dualism of the elimination processes is remarkably important in biomedicine and will be discussed in the current review. Of particular importance to understanding the etiology and pathogenesis of disease is not only the philosophy behind, but also the effectiveness of controlling the fine balance between preserving and eliminating death signals. The balance that ultimately controls the transition of biological units from live thermodynamically open systems into dead material is fundamental to our understanding of the etiology and pathogenesis of disease.
Fig. 1.
Acute injuries and chronic diseases associated with programmed cell death pathways.
Oxidative Stress/Injury, Free Radicals and Antioxidants.
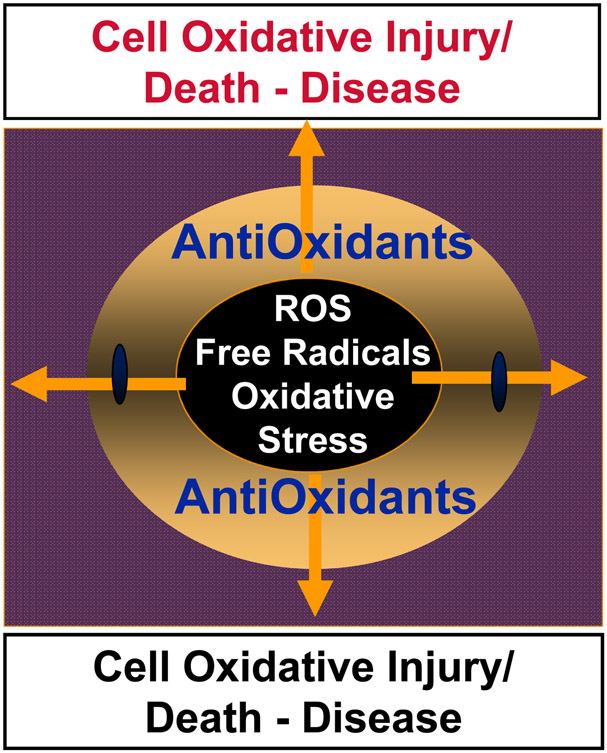
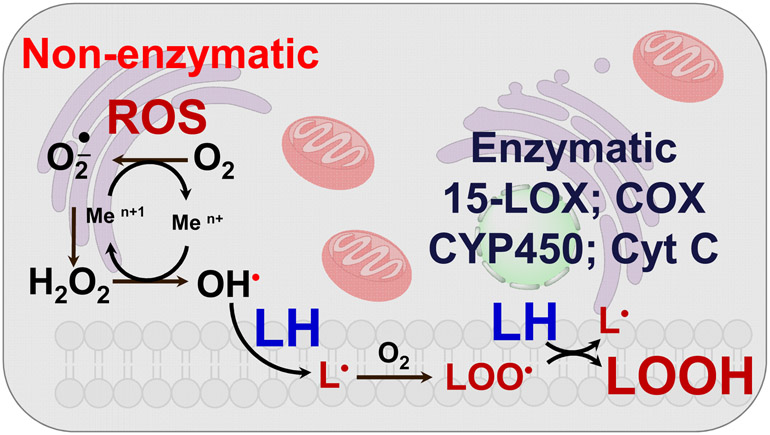
Paradoxically, among the instruments of elimination or cell death, one the most important is the molecule of life, oxygen, that is involved in many oxidative reactions. Over the last six decades, the concept of free radicals and antioxidants has been developed, propagated and subjected to experimental testing and clinical trials [10-13]. The simplicity of the concept - uncontrolled free radical chemical reactions that cause injury, disease and even death are counteracted by a network of antioxidant mechanisms maintaining health - has attracted remarkable attention of experimental researchers as well as clinicians [14, 15]. As a subclass of these processes, the term oxidative stress has been created to encompass multiple, in most cases unknown or poorly understood, events setting the stage for the disbalance between pro-oxidant free radical reactions and their antioxidant regulators [16, 17]. Essentially, a huge variety of redox signals with their effects on biological functions has been trivialized into a concept of free radicals <-> antioxidant balance or dysbalance as if only these two states were meaningful (Fig. 2). This over-simplified concept ignored the molecular mechanisms of action of oxygenated molecules and their functions. Instead, the war on toxic and injurious free radicals and triumph of beneficial antioxidants have been declared. The major endogenous antioxidant molecules - water-soluble and lipid-soluble and their cascades have been identified and the interactions thoroughly investigated [18]. Along with the endogenous radical scavenging molecules in mammalian cells, the myriads of natural molecules of plant, fungal and bacterial origin have been isolated and their antioxidant characteristics evaluated [19-21]. Synthetic efforts of chemists yielded a multitude of novel classes of radical scavenging molecules as well as an arsenal of chemically modified and perfected natural antioxidants - such as modified homologues of vitamins E and C [22-24]. The multitude of effective chain breaking radical scavenging tools with phenolic and aromatic amino-groups as well as sulfhydryls - have been well characterized in chemical systems and simple physical-chemical model systems [25, 26]. Unfortunately, specific regulatory functions of antioxidant molecules as a part of regulatory cascades controlling the production and degradation of specific signaling molecules has been neglected. Instead, a huge amount of work on perfection and optimization of antioxidants and their mixtures has been performed and raised the “plank” of expectations very high - to the level of a new revolution in health improvements, disease prevention and the stimulation of extraordinary new therapies. This has been followed by a series of multiple sobering clinical trials. Subsequent decades of these trials and analysis of their results were disappointing. A journey through the PubMed database clearly quantitates the results (Table 1): not a single one of the conducted trials has been was successful in producing a positive effect. The conclusion was clear: the concept of chemical free radical chain reactions and their correction by chain-breaking radical scavengers/antioxidants failed as a biomedical preventive or therapeutic endeavor [10-13]. New ideas and a better understanding of redox regulation and its enzymatic mechanisms had to be developed. While the general schemas of random chemical lipid peroxidation and enzymatic lipid peroxidation seem to be quite similar (Fig. 3), detailed analysis reveals dramatic differences in the selectivity and specificity of catalytic mechanisms and products. This is best illustrated in the case of lipids containing polyunsaturated fatty acids (PUFA)
Fig. 2.
The concept of free radical/antioxidant balance or predominance of uncontrolled free radical reactions leading to oxidative injury, disease and death
Table 1.
Diseases associated with generation of free radicals and antioxidant clinical trials
| The number of entries in Pubmed | |
|---|---|
| Disease | 6,526,878 |
| Disease + Free radicals | 45,972 |
| Antioxidant Clinical Trials | 28,983 |
| Antioxidant Clinical Trials + NIH Funded | 18 |
| Antioxidant Clinical Trials + NIH Funded + Successful | 0 |
| Antioxidant Clinical Trials + Successful + Positive results | 0 |
Fig. 3.
“Apparent similarities” in the general schemas of enzymatic and non-enzymatic lipid peroxidation.
Enzymatically regulated vs. free radical-mediated lipid peroxidation.
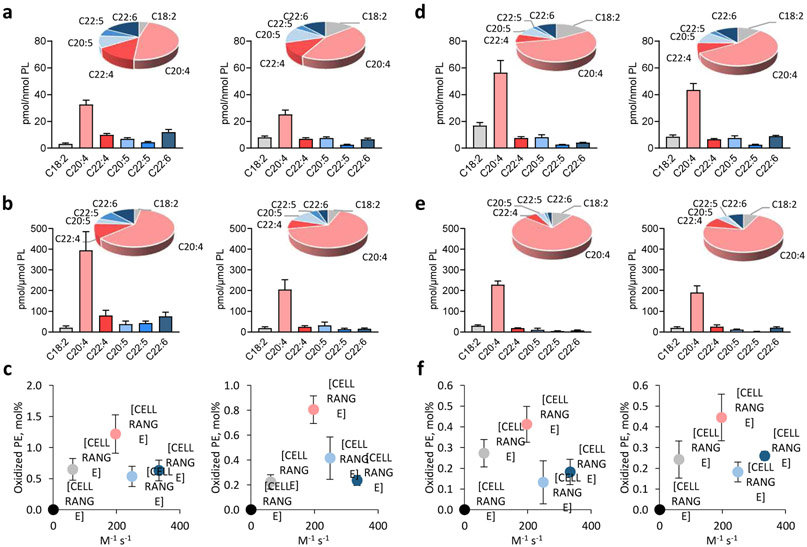
The susceptibility of polyunsaturated fatty acid (PUFA)-containing lipids to radical oxidation in chemical systems could be characterized by the rate constant of H-atom abstraction, which increases with the number of bis-allylic -CH2-centers in the molecule [27]. Based on this simple chemical rule, one can expect that highly polyunsaturated lipid substrates with multiple double bonds with several bis-allylic sites will be the preferred oxidation substrates. In contrast, the enzymatic process is directed towards oxidation substrates specifically positioned within the catalytic site of the enzyme, such that specificity of the reaction products may deviate significantly from this chemical rule. In other words, oxidation programs realized via enzymatic mechanisms may yield products inconsistent with the dominance of bis-allylic sites in the substrates. With this in mind, we performed this type of analysis using two types of genetic models with genetically manipulated levels of PUFA phospholipids. In particular, based on previously published results we compared the oxidizability of phospholipids, i.e. the total amount of major oxidizable PUFA-phosphatidylethanolamines (PEs) and their oxidation products in WT and ACSL4 (acyl-CoA synthase 4) KO mouse kidney and embryonic fibroblast (Pfa1) cells (Fig. 4 a-c) and WT and FATP2 (fatty acid transport protein 2) KO polymorphonuclear myeloid-derived suppressor (PMN-MDSCs) cells (Fig 4. d-f) in the presence and absence of RSL3 – a pro-ferroptotic agent. Both ACSL4 and FATP2 control delivery and activation of PUFA, particularly AA, into phospholipids [28-32]. We focused our analysis on AA-PEs with three bis-allylic centers in the sn-2 position of the phospholipid - accounting for the majority of oxidizable PUFA-PEs (44-56%, Fig. 4 a, d) and oxidation products (66-76%, Fig. 4 b, e). DHA-PEs, another major group of oxidizable PUFA-PEs with five bis-allylic centers in the sn-2 position, contribute a significant amount of oxidation substrates (10-17%, Fig. 4 a, d) but displayed a relatively lower oxidizability (lower levels of oxidation products (3-12%, Fig. 4 b, e). Notably, no significant correlation was observed between oxidation levels (mole%) of PUFA-Pes and the rate constants of hydrogen abstraction for their corresponding PUFAs in the sn-2 position predicted by chemical reactivity (Fig. 4 c, f). Based on these calculations other assessments of this kind may be performed using the published data on PUFA content in specific classes of phospholipids and their oxidation preferences in cells and tissues where enzymatic machineries may be involved in the catalytic process. These data indicate that cellular lipid peroxidation is not a simple chemically-driven free radical-mediated oxidation process but it rather represents a complex of biochemical reactions each of which dictates its selectivity with toward the substrates and specificity with regards to the oxidation products generated [27, 33]. Another support for the strictly selective and controlled lipid peroxidation can be provided by the results showing that the oxidation levels of PUFA-PEs (mole%) are significantly decreased in accordance with the lack of one of the oxidation substrates, AA-containing species of PE, in ACSL4-deficient Pfa1 cells compared to WT cells during execution of ferroptotic program in these cells. (Fig. 4 c).
Fig. 4. Relationship between contents of phosphatidylethanolamine (PE) oxidation products in cells and rate constants of H-atom abstraction for polyunsaturated fatty acids (PUFAs).
a) Contents of PUFA-PEs in Pfa1 WT (left) and ACSL4 KO (right) cells treated with RSL3 (100 nM, 6 h). Data are means ± SD, n = 4. b) Contents of PUFA-PEs oxidation products in Pfa1 WT (left) and ACSL4 KO (right) cells treated with RSL3 (100 nM, 6 h). Data are means ± SD, n = 4. c) Relationship between mole percentages of PUFA-PEs oxidation products in Pfa1 WT (left) and ACSL4 KO (right) cells treated with RSL3 (100 nM, 6 h) and rate constants of H-atom abstraction for their corresponding PUFAs. Data are means ± SD, n = 4. d) Contents of PUFA-PEs in PMN WT (left) and FATP2 KO (right) cells. Data are means ± SD, n = 4. e) Contents of PUFA-PEs oxidation products in PMN WT (left) and FATP2 KO (right) cells. Data are means ± SD, n = 4. f) Relationship between mole percentages of PUFA-PEs oxidation products in PMN WT (left) and FATP2 KO (right) cells and rate constants of H-atom abstraction for PUFAs. Data are means ± SD, n = 4. PEs and their oxidation products are classified by polyunsaturated fatty acyl chain on sn-2. Rate constants of H-atom abstraction for PUFAs were assayed in liposomes [21].
Analytical Techniques for Detection of Lipid Peroxidation Products.
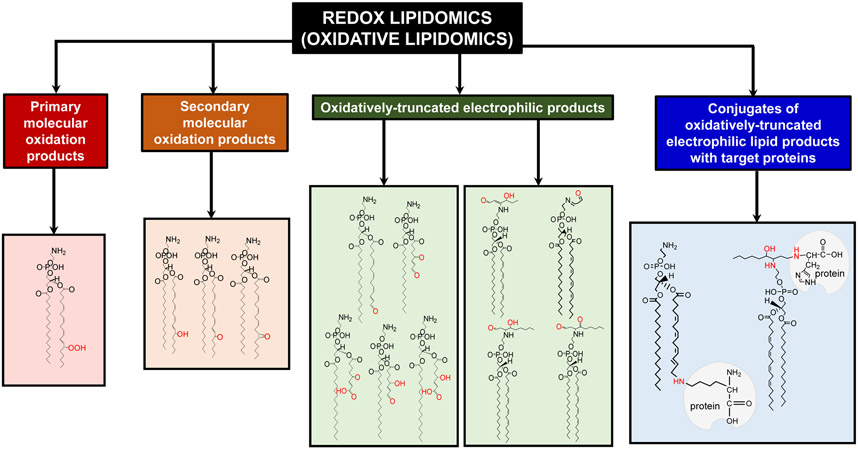
Over the last three decades, several distinctive genetically pre-determined cell death programs have been discovered and their molecular mechanisms described. Notably, redox disbalance and lipid peroxidation have been claimed as inherent features of essentially each of them [33-36]. These claims, however, have been made on the basis of experimentally indiscriminative and mostly non-specific analytical protocols that were not able to provide information on the molecular identity of the oxidation reactions and their targets. Among the most common characterizations were assessments of protein carbonyls [37, 38], 4-hydroxynonenal [39], malondialdehyde [40, 41], antioxidant depletion [42, 43] and similar features incapable of identifying of the specific oxidations products with the predictive characteristics of biomarkers [44]. Deficiencies of these molecular protocols have been revealed even in in vitro experiments with cells but became particularly obvious when in vivo assessments of cell death in tissues of animals or available samples from human subjects have been attempted [41, 43, 44]. This ambiguity of analytical protocols has been overcome with the advent of new LC-MS based approaches with a variety of different specific protocols [45-49]. Given that polyunsaturated lipids represent the most vulnerable substrates in pro-oxidant environments, many of the current LC-MS based techniques were designed to detect lipid peroxidation products. Among the first findings in the new field of research - redox lipidomics or oxidative lipidomics [48, 50-58] - was the documentation of thousands of individual molecular species of oxidatively modified phospholipids [48, 50-58]. These included several major categories of products: i) the primary molecular oxidation products - hydroperoxy-containing fatty acid residues with the same length of the hydrocarbon chain, ii) the secondary products with a variety of oxygen-containing functionalities (epoxy-, hydroxy, oxo-, etc) retaining the same chain length, iii) oxidatively-truncated electrophilic products with oxygen-containing functionalities, and iv) conjugates of oxidatively-truncated electrophilic lipid products with target proteins or “oxidatively lipidated proteins” (Fig. 5). The analytical power of redox lipidomics - high sensitivity and resolution - has resulted in the identification of numerous oxidatively modified lipid species and set the stage for several fields of research seeking to find specific features of lipid peroxidation products causatively related to particular types of physiological re-programming mechanisms or conditions associated with acute injuries or chronic diseases [48, 50, 51, 53, 55, 56, 59, 60].
Fig. 5.
Major types of lipid oxidation products detectable in cells and tissues revealed by LC-MS based redox lipidomics. These products include: hydroperoxy-, hydroxy-, oxo-, epoxy- and oxidatively truncated phospholipid molecular species as well as conjugates of electrophilic lipid products with proteins.
While conventional high mass resolution LC-MS/MS protocols can fully structurally characterize diversified lipids and their oxidation products, they provide no information on the distribution of lipid molecules of interest in cells and tissues. Given the obvious importance of this type of knowledge, much effort has been concentrated on the development of mass-spectrometric imaging (MSI) of lipids. Matrix-assisted laser desorption-ionization (MALDI) protocols have become the most commonly employed techniques which allowed to get substantial data on the localization of different types of lipid molecules in tissues, particularly brain, and their changes associated with injury or disease conditions [61-63]. Direct detection of oxidized lipids has not been yet achieved; however, much useful information on the redox reactions in tissues lipid have been obtained through the MSI analysis of the major substrates of lipid peroxidation, PUFA-phospholipids [64]. In spite of substantial progress in MALDI-technologies in the topographical identification of different lipids, including low abundance and higher mass classes of them, such as CLs [65], he major deficiency remained a relatively low spatial resolution incompatible with the subcellular mapping of their major lipid components of individual cells and their organelles. Revolutionizing breakthrough in this area has been associated with the introduction of gas-cluster ion beam time-of-flight secondary-ion MS which, with its spatial resolution ~1 micron, permits subcellular analysis of essential lipids in individual cells and their organelles (Fig. 6) [66-68]. Further improvements in this technology promise the opportunity to directly visualize the peroxidized lipids and their changes produced by physoiological and/or pathological conditions at the subcellular level.
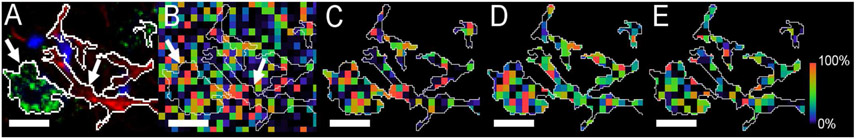
Fig. 6.
(A) NeuN (green, neurons) and GFAP (red, astroglia) signals marked with arrows and their thresholded overlays used to define cell body limits (bar=10 μm). (B) The PE(36:4p) SIMS signal (m/z 722.5) from the same region rendered as a pseudocolored heatmapped panel, intensity-scaled relative to the field of view. The thresholded NeuN and GFAP signals have been overlaid on the SIMS image. (C) PE(36:4p) cropped to the NeuN and GFAP thresholds. (D) PE(38:4) m/z 766.5, and, (E) PE(40:6) m/z790.5 also thresholded and cropped.
Reprogramming via Apoptosis.
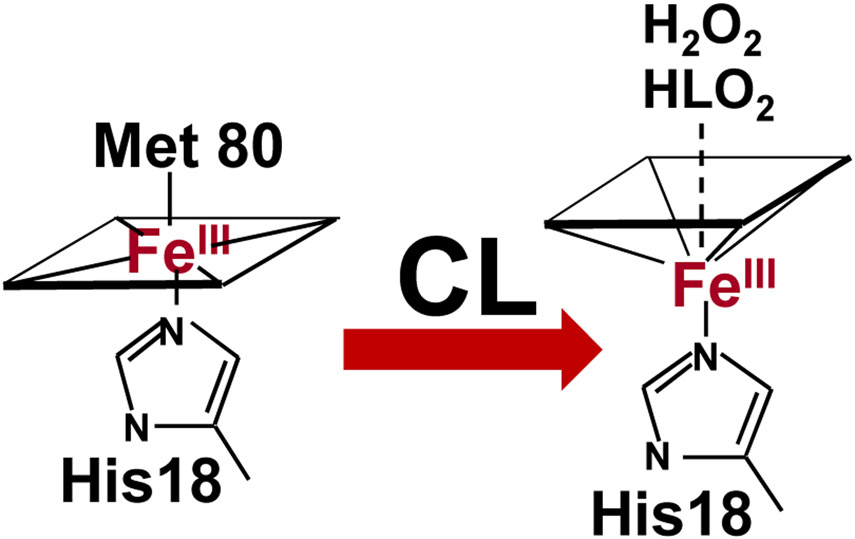
Among the first applications of redox lipidomics were the studies of cell death programs. It seemed tempting to examine the specific meaning of the common notion that execution of cell death programs is associated with lipid peroxidation (Fig. 7). While changes of lipid mediators have been related to cell death, none of them were found directly involved in the execution of death signaling [52]. The new opportunities offered by redox phospholipidomics turned out to be successful and revealed a highly selective engagement of two phospholipids in the course of intrinsic mitochondria-dependent apoptosis in a number of cell models as well as in tissues of animals (eg, via exposure to acute brain injury, lung challenge by inhalation of nanoparticles [49-51, 53, 69, 70]). Detailed studies revealed the mechanism of mitochondria-confined peroxidation of CL in a complex process of its trans-migration from the inner mitochondrial membrane through the intermembrane space to the outer mitochondrial membrane [71-74]. During this journey - initiated by the decreased membrane potential across the IMM - the physical encounter and binding of CL to a hemoprotein cytochrome c (cyt c) occurs. The significance of this interaction was quite unexpected: within the complex with CL, cyt c changes its catalytic competence in the process of conversion from a mobile electron carrier between respiratory complexes III and IV into a potent peroxidase capable of oxidizing many organic compounds, including PUFA-CLs (Fig. 6) [75]. In this process a hexa-coordinate Fe transitions into a penta-coordinate state as a result of weakening and rupture of the Fe-Met(80) bond (Fig. 8) [76]. This dramatic change is accompanied by a sharp drop of the cyt c/CL redox potential (by ~400mV) such that, in contrast to native cyt c, the complex cannot act as an acceptor of electrons from respiratory complex III [75]. As a result, the normal flow of electrons through the disarrayed electron transport chain is no longer possible, thus causing a massive production of superoxide-anion-radicals [77, 78]. As the latter can be converted - spontaneously or in the Mn-superoxide dismutase-catalyzed reaction - into H2O2 [79], pro-apoptotic mitochondria become a source of oxidizing equivalents feeding the peroxidase cycle of cyt c/CL complexes (Fig. 9) [80]. Typical of the peroxidase cycle is the sequential production of reactive intermediates [81] - cation-radicals of the porphyrins, compounds I and II with protein immobilized radicals [82] capable of effective H-abstraction from the bis-allylic positions and leading to the formation of the alkyl and, in the presence of oxygen, peroxyl radicals (Fig. 9). The exact nature of the changes in cyt c structure upon its interaction with CL and leading to the “weakening” and breach of the Fe-(Met80) bond and hexa-coordinate state of Fe is still a matter of active on-going studies. The results may be interpreted as supporting the significant unfolding of the protein [83, 84] or relatively small changes of the protein structure but highly increased dynamics of the specific portions of the protein (eg, a highly dynamic loop formed by the residues 70-85) [85-87]. In either structural rearrangement, the formation of the penta-coordinate state of Fe is essential for its peroxidase function.
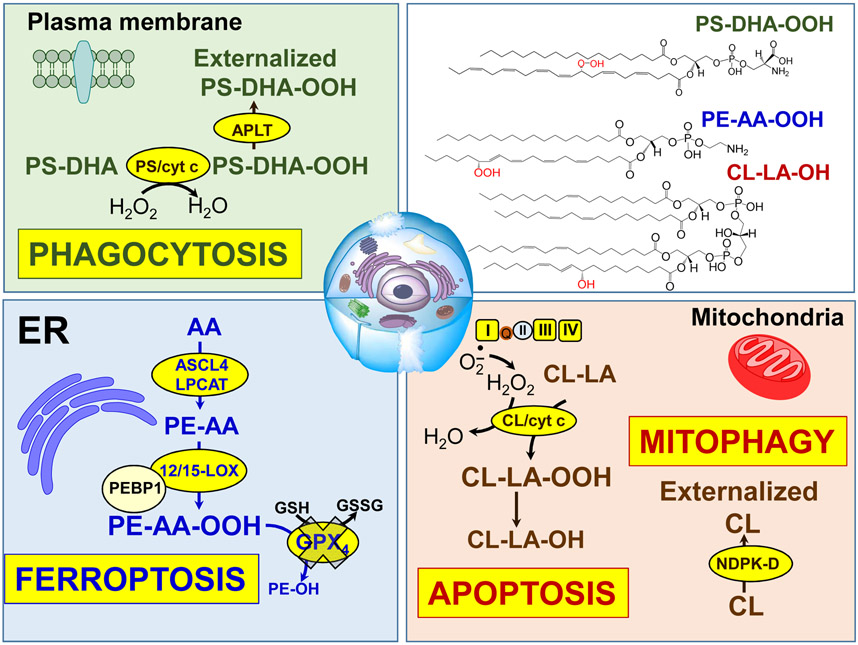
Fig. 7.
Oxygenated phospholipids associated with execution of programmed cell death processes and elimination of damaged mitochondria and cells. Formation of CL/cyt c complex with peroxidase activity in mitochondria results in the oxidation of CL, release of cyt c from mitochondria and triggering of the apoptotic cell death pathway. Released cyt c interacts with PS to form cyt c/PS complexes that cause PS oxidation and externalization on the cell surface. Externalized PS/PSox serve as an “Eat-me” signal for phagocytes. Damaged mitochondria externalize CL to be eliminated via the mitophagy pathway. Interaction of 15LOX with PEBP1 during ferroptosis results in the generation of the ferroptotic cell death signal, hydroperoxy-arachidonoyl-PE.
Fig. 8.
Cardiolipin induces restructuring and unfolding of cyt c accompanied by the loss or exchange of the distal heme iron ligand Met80 and facilitates heme interaction with small molecules.
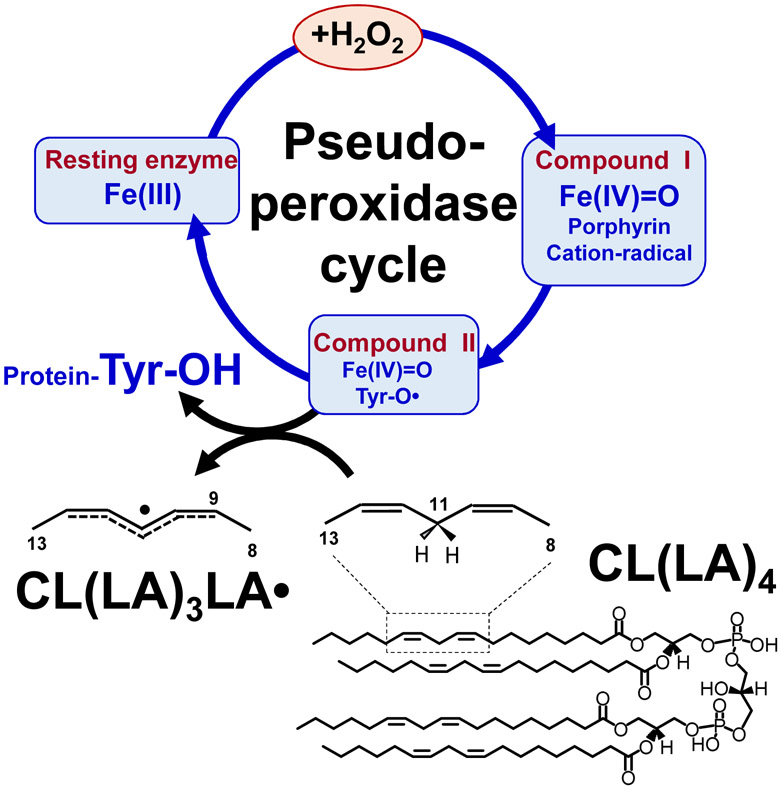
Fig. 9.
Catalytic cycle of pseudo-peroxidases [74, 75]. Interaction between hydrogen peroxide and native ferric pseudo-peroxidase heme leads to the formation of Compound I which is most likely oxoferryl porphyrin-π-cationic radical. Compound I oxidizes amino acid residues (Tyr, Trp, His) located near the heme with the formation of protein based radicals (most likely, Tyr67 of cyt c) and oxoferryl heme (Compound II) [76]. Oxoferryl heme iron can oxidize protein amino acids and peroxidase substrates. The protein-based tyrosyl radicals are the alternative reactive intermediates of pseudo-peroxidases, which oxidize CLH in the mitochondrial membrane as exemplified by TLCL oxidation.
While CL is the major intramitochondrial substrate of attack on phospholipids, redox lipidomics studies revealed that another anionic phospholipid, phosphatidylserine (PS) was also involved in the oxidation process during apoptosis [88, 89]. As PS is an extramitochondrial phospholipid, the role of cyt c in its oxidation is less clear although complexes of cyt c/PS reveal significant, albeit lower than cyt c/CL complexes, peroxidase activity (Fig. 6) [90]. It is likely, although not unequivocally proven, that released from mitochondria into the cytosol cyt c may form complexes with abundant extra-mitochondrial PS. In apoptosis the significance of this event has been interpreted in terms of facilitated trans-migration of PS from the inner to the outer surface of the plasma membrane and the appearance of PSox on the cell surface [57, 91]. The biological significance of this has been associated with a much higher phagocytosing activity of macrophages and microglia [91-93]. Given the well-established anti-inflammatory (pro-resolving) effects of phagocytosing macrophages with predominantly expressing the M2 phenotype, the role of enhanced phagocytosis of apoptotic cells bearing PSox by macrophages may be quite significant, yet insufficiently studied [93].
The function and identity of CLox products in apoptosis have been the subject of detailed studies [48-51, 53-56, 58, 69, 94-96]. Selectivity of CL oxidation is caused by the high affinity of cyt c towards CL binding. It has been estimated that dissociation constants for cyt c/CL complexes are on the order of 2.0-4.2 × 10−5 M [97]. Thus, it is likely that tightly bound PUFA-CL species represent the oxidation substrates for the cyt c/CL complexes. It has been established that oxidized CL species (CLox) bind less avidly with cyt c [98]. This suggests that after oxidation, CLox is liberated from the complex. One can speculate that during apoptosis cyt c is released into the cytosol in complexes with CL and/or CLox. The latter, however, are not stable and their dissociation leaves cytosolic cyt c available for interactions with alternative substrates. Given that the extra-mitochondrial concentration of CL is very low, PS may get involved in the interactions with cyt c. The cyt c/PS complex can also act as a peroxidase resulting in oxidation of PUFA-PS species [90].
In terms of the mechanisms of oxidation, cyt c/CL complexes are engaged in typical peroxidase cycle [82, 99]. The peroxide bond -O-O- in hydroperoxy-phospholipids is weak and can be readily cleaved to yield secondary CL oxidation products, frequently with oxidatively truncated, shortened chains [100, 101]. Common among those oxidiatively-truncated products, are electrophilic moieties that can react with the nucleophilic sites in proteins and produce covalent-adducts. If their formation is non-random and follows a specific pattern, these protein aggregates may form oligomeric structures in plasma membranes thus affecting its integrity and contribute to the execution of death. In lieu of this, it is noteworthy that apoptosis-associated CLox products are enriched with electrophilic epoxy- and oxo-derivatives [94, 102]. However, their role in plasma membrane disturbances and formation of apoptotic membrane “pores” and “blebs” has not been directly established.
Translocations of CL and CLox to the surface of mitochondria and its release into the cytosol may be followed by the appearance of CL/CLox on cell surface. These signals have been associated with two major effects on professional phagocytes - activation of phagocytosis realized via CD36 and, independently of this, on bonding with the TLR4/Md2 complex leading to the strong suppression of LPS-induced cytokine production, ie, immune-paralysis [103]. The suggested mechanisms for this may be similar to the effects of immature lipid A that prevents the hetero-oligomerizaton of TLR4 with Md2, which is necessary for the stimulation of the pro-inflammatory cytokine response [104-106]. In spite of the obvious importance, the significance of these effects as regulators of the inflammatory responses has not been sufficiently studied in vivo.
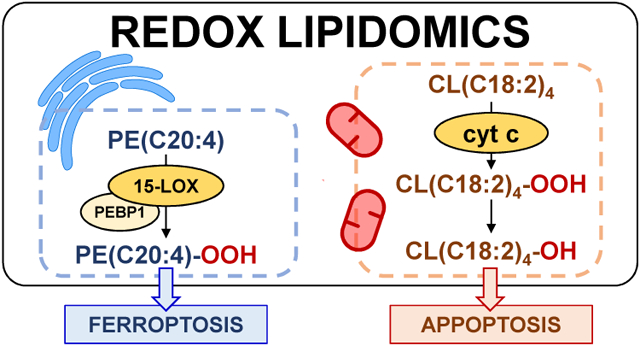
Reprogramming via Ferroptosis: mechanisms, significance and applications.
Ferroptosis is the type of cellular response to the changing redox environments associated with disturbed iron homeostasis, the accumulation of lipid peroxidation products, and deficiency of the thiols system, particularly of GPX4 (a seleno-enzyme catalyzing the reduction of phospholipid hydroperoxides to their respective alcohols) (Fig. 6) [107-110]. Lipid peroxides are believed to be primarily responsible for the cell demise but the direct evidence supporting this point of view is scarce because the majority of the available experimental material is based on the employment of surrogate measurements potentially correlating with lipid peroxidation [111, 112]. The most commonly utilized protocol is based on the fluorescent assessments of BODIPY 581/591 C11 that may be co-oxidized congruently with the development of the lipid peroxidation response [112, 113]. Direct LC-MS based redox lipidomics assessments showed remarkable specificity of the ferroptosis-associated changes in the levels of lipid oxidation products determined by the effects of pro-ferroptotic stimulation (eg, treatment with a GPX4 inhibitor, RSL3) and elimination of these signals by an anti-ferroptotic agent, Ferrostatin-1 [52]. In spite of hundreds of oxidatively-modified individual phospholipids detected in cells undergoing ferroptosis, only four of them “survived” the scrutiny of intensive filtering and sieving through several criteria: i) significantly increased content (≥3-fold) in ferroptotic vs control cells; ii) correlation with cell death; iii) reduced contents of non-oxygenated oxidizable precursors in Acsl4 KO cells; iv) elevated levels in Gpx4 KO cells in vitro and Gpx4 KO mice in vivo [52]. These predictive ferroptotic signals were identified as hydroperoxy-derivatives of arachidonoyl- (C20:4) or adrenoyl- (C22:4, the product of AA elongation) phosphatidylethanolamines [52]. This high selectivity within the oxidation profile was also supported by independent findings demonstrating a strong suppressive effects of genetic or pharmacological (rosiglitazone) depletion of ACSL4 - an enzyme responsible for Co-A-activation of arachidonoyl residues [31, 52] as well as lyso-phospholipid acyltransferase (LPCAT3) facilitating arachidonoyl re-acylation of lysophospholipids (Fig. 4) [52]. In other words, the availability of sufficient amounts of arachidonoyl-PE was necessary for the successful completion of the ferroptotic program.
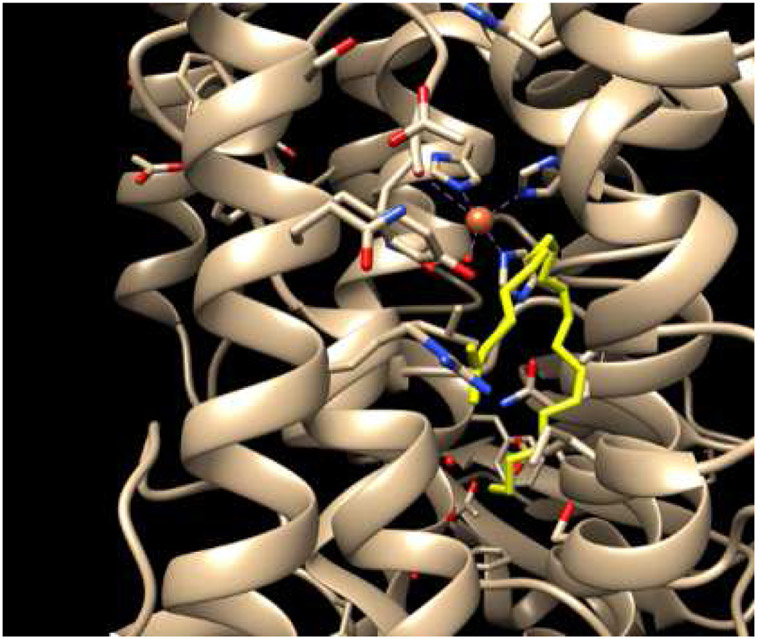
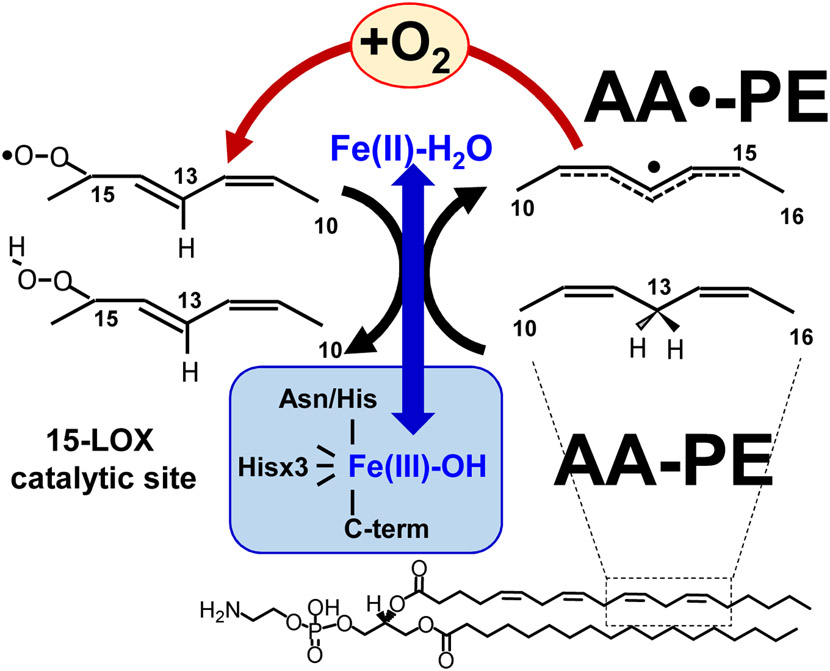
Establishment of the nature of this exclusive selectivity of PE oxidation in ferroptosis has uncovered the role of 15-lipoxygenase (15-LOX) [52], a typical dioxygenase capable of oxidizing not only free fatty acids but also membrane phospholipids [114]. Mammalian LOXes oxidize PUFA localized in their a U-shaped FA binding channel where distinct amino acids control FA orientation positioning the selected pentadiene structure opposite the non-heme iron in the catalytic site (Fig. 10, [115]) [116, 117]. Dependent on the depth of the channel relative to the site of the catalytic LOX-iron, arachidonic acid oxidation can occur at the 5th, 8th, 12th, or 15th carbon of the AA. These different LOXs are designated as 5-, 8-, 12-, and 15-LOX, respectively. The iron (III) at the LOX catalytic site has 5 coordination bonds with the protein’s amino acids with the hydroxy-group occupying the sixth coordination position [118]. Due to the very tight alignment of PUFA, Fe(III)-OH abstracts a hydrogen from substrates at the bis-allylic carbon in a highly site- and stereo-specific fashion. The intermediates of this reaction are the carbon-centered radical and Fe(II)-OH2. An oxygen molecule delivered through the special oxygen channel attaches to the rearranged PUFA radical causing the formation of an oxygen-centered peroxy-radical that, upon hydrogen transfer from Fe(II)-H2O, is converted into the molecular hydroperoxy-product [119]. As an oxidized PUFA is produced, the catalytic iron is converted back into Fe(III)-OH (Fig. 11). One can see that this mechanism is markedly different from the peroxidase mechanisms operated in CL oxidation by cyt c/CL complexes (see above).
Fig. 10.
X-ray structure showing the constrained substrate placement within the active site of 8R-lipoxygenase (PDB 4QWT) [101]. The arachidonic acid (yellow sticks) is bound in the active site in a specific orientation, such the protein directs the oxidation to occur in a site-specific fashion.
Fig. 11.
Mechanism of LOX catalyzed reaction of PUFA oxidation. The blue arrow shows changes in Fe-containing active site of LOX during FA oxidation. Black arrows follow the reactions catalyzed by redox-active Fe in the LOX catalytic site. Red arrows show the Fe-independent radical rearrangements with simultaneous reaction of oxygen insertion into oxidized FA. Details are in the text.
It has been established that the catalytic competence of the 15-LOX towards AA-PE is strongly facilitated by the formation of its complex with a scaffold-protein, phosphatidylethanolamine binding protein 1 (PEBP1), that allosterically adapts the enzyme towards accommodating a bulkier substrate and also limits access to the catalytic site by other phospholipids with larger polar heads (eg, phosphatidylcholine) [59]. In terms of oxidation specificity, the 15th carbon in AA-PE and the 17th carbon in adrenoyl-PE (AdA-PE) have been determined as preferred sites of oxygenation [120]. The essential role of 15LOX/PEBP1 complexes in ferroptosis has been established in vivo in models of acute traumatic brain injury and kidney failure by folic acid and sepsis as well as in airway epithelium in asthma [59]. Importantly, in the majority cases when ferroptosis has been detected, GPX4 degradation was a hallmark of the response [109, 121, 122]. Thus, it has become obvious that GPX4 represents the major check-point in regulation of ferroptosis. A more detailed study of the mechanisms of this degradation indicated the involvement of specific proteolytic mechanisms, chaperone-mediate degradation [123].
Central to understanding the intricacies of the ferroptotic program is the identification of the proximate death executing mechanism. While HOO-AA-PE is a predictive ferroptosis biomarker, the role of this oxygenated phospholipid as an immediate instrument of cell death has to be further explored. Because the hydroperoxy-group confers instability leading to oxidative truncation of HOO-AA-PE, the resulting electrophilic products may operate as modifiers of sensitive nucleophilic sites in one or more proteins that will ultimately form “pores” leading to the permeability of plasma membranes and cellular demise. Identification of HOO-AA-PE as a precursor of these electrophilic truncated products makes the search for the immediate executioners of cell death feasible via their isolation and combined redox lipidomics/proteomics analysis.
Concluding remarks:
Programmed cell death mechanisms are important adaptive factors of cell populations in response to changed environments caused by chemical and physical factors, pathogens etc. By eliminating the unnecessary or harmful (damaged beyond repair) individual cells, these programs are beneficial and they enhance the overall vitality and survival of the organism. When the scale of the demise exceeds the allowable limits they may become pathogenic and correcting/limiting the execution of the programs becomes a necessity. Understanding the molecular mechanisms of the programs is therefore critical to maintenance of optimal health. Given the central role of lipid peroxidation with regulated cell death pathways, redox lipidomics is one of the most powerful tools in achieving this goal. Here we presented two cases - apoptosis and ferroptosis - when understanding/deciphering the mechanisms has led to important and useful interventions. Clearly, these first experimental steps are only the beginning of the required conceptual developments and transforming new ideas and approaches into more effective therapies.
Lipid mediators have been known as signaling molecules for more than five decades. Their diversity defines their important roles in the regulation of many physiological functions [54, 124-126]. The production of these signals follows a typical temporal chain of events that begins with the phospholipase A2-catalyzed hydrolysis of phospholipids followed by the oxygenation step catalyzed by one of the isoforms of cyclooxygenases or LOXs [127]. Lately, it has become apparent that oxygenated lipid mediators esterified into phospholipids represent a very rich source of signaling molecules whose diversity may be remarkably high and reach hundreds of thousands of individual molecular species. The significance of this signaling pathway and deciphering of the meaning of the individual “words” in this language has become possible due to the development of redox phospholipidomics and this new research field started bringing its first significant results. Identification of specific oxygenated species of phospholipids as signals of apoptotic and ferroptotic death as predictive biomarkers of these programs and the re-programming of cell populations as a response to acute injury or chronic disease condition offers an entirely unique opportunity to monitor these responses in vivo [48, 53, 55, 56, 59, 69, 128, 129]. Given the important role that these types of cell death programs play in the pathogenesis of several types of injury and diseases, redox phospholpidomics can be utilized for the detection of cell death mechanisms in the overall disease process thus guiding the temporally optimized treatments representing new mechanism-based therapeutic approaches. It is becoming clear that a search for a single effective “silver bullet” for the treatment of diseases related to redox dis-homeostasis is based on the elusive simplistic concept of counterbalancing the exceedingly high production of free radicals by antioxidants. Understanding the specific enzymatic phospholipid oxidation mechanisms triggering cell death is a new approach that has to be tested as an alternative to antioxidant-based therapeutic strategies. Quantitative redox phospholipidomics assessments of biomarkers of cell death programs triggered and executed at specific stages of disease will dictate and guide the rational combinations of different specific inhibitors as well as the temporally harmonized regimens of their application.
Highlights:
Enzymatic phospholipid peroxidation generates death signals
Redox lipidomics establishes cardiolipin peroxidation in apoptosis
Cytochrome c/cardiolipin acts as a peroxidase complex
Hydroperoxy-phosphatidylethanolamines as biomarkers of ferroptosis
Lipoxygenases peroxidize phosphatidylethanolamines in ferroptosis
Acknowledgements:
This work was supported by the National Institute of Health (HL114453, U19AI068021, CA165065, GM113908 and NS076511).
Abbreviations:
- PLox
oxygenated phospholipids
- CL
cardiolipin
- CLox
oxidized cardiolipin species
- PS
phosphatidylserine
- PSox
oxidized phosaphatidylserine species
- PE
phosphatidylethanolamine
- AdA-PE
adrenoyl- phosphatidylethanolamine
- AA-PE
arachidonoyl-phosphotidylethanolamine
- Cyt c
cytochrome c
- APLT
aminophospholipid translocase
- FATP2
fatty acid transport protein 2
- ACSL4
acyl-CoA synthase 4
- LPCAT
lysophosphatidylcholine acyltransferase
- 15-LOX
15-lpoxygenase
- GPX4
glutathione peroxidase 4
- PEBP1
phosphatidylethanolamine binding protein
- NDPK-D
nucleoside diphosphate kinase D
- RSL3
(1S,3R)-methyl 2-(2-chloroacetyl)-1-(4-(methoxycarbonyl)phenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate
- PUFA
polyunsaturated fatty acid
- LA
linoleic acid
- AA
arachidonic acid
- AdA
adrenic acid
Footnotes
Declaration of competing interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Virag L, Jaen RI, Regdon Z, Bosca L, Prieto P, Self-defense of macrophages against oxidative injury: Fighting for their own survival, Redox Biol 26 (2019) 101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia M, Misso G, Mitochondria as playmakers of apoptosis, autophagy and senescence, Semin Cell Dev Biol (2019). [DOI] [PubMed] [Google Scholar]
- [3].de Vega D, Newton AC, Sadanandom A, Post-translational modifications in priming the plant immune system: ripe for exploitation?, FEBS Lett 592(12) (2018) 1929–1936. [DOI] [PubMed] [Google Scholar]
- [4].Skvortsova K, Iovino N, Bogdanovic O, Functions and mechanisms of epigenetic inheritance in animals, Nat Rev Mol Cell Biol 19(12) (2018) 774–790. [DOI] [PubMed] [Google Scholar]
- [5].Fan J, Dawson TM, Dawson VL, Cell Death Mechanisms of Neurodegeneration, Adv Neurobiol 15 (2017) 403–425. [DOI] [PubMed] [Google Scholar]
- [6].Humeau J, Bravo-San Pedro JM, Vitale I, Nunez L, Villalobos C, Kroemer G, Senovilla L, Calcium signaling and cell cycle: Progression or death, Cell Calcium 70 (2018) 3–15. [DOI] [PubMed] [Google Scholar]
- [7].Skulachev VP, Phenoptosis: programmed death of an organism, Biochemistry (Mosc) 64(12) (1999) 1418–26. [PubMed] [Google Scholar]
- [8].Skulachev VP, Aging as a particular case of phenoptosis, the programmed death of an organism (a response to Kirkwood and Melov "On the programmed/non-programmed nature of ageing within the life history"), Aging (Albany NY) 3(11) (2011) 1120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hui GH, Mi SS, Deng SP, Sweet and bitter tastants specific detection by the taste cell-based sensor, Biosens Bioelectron 35(1) (2012) 429–38. [DOI] [PubMed] [Google Scholar]
- [10].Ashor AW, Brown R, Keenan PD, Willis ND, Siervo M, Mathers JC, Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses, Nutr Res 61 (2019) 1–12. [DOI] [PubMed] [Google Scholar]
- [11].Celik T, Yuksel C, Iyisoy A, Alpha tocopherol use in the management of diabetic cardiomyopathy: lessons learned from randomized clinical trials, J Diabetes Complications 24(4) (2010) 286–8. [DOI] [PubMed] [Google Scholar]
- [12].Dotan Y, Lichtenberg D, Pinchuk I, No evidence supports vitamin E indiscriminate supplementation, Biofactors 35(6) (2009) 469–73. [DOI] [PubMed] [Google Scholar]
- [13].Dotan Y, Pinchuk I, Lichtenberg D, Leshno M, Decision analysis supports the paradigm that indiscriminate supplementation of vitamin E does more harm than good, Arterioscler Thromb Vasc Biol 29(9) (2009) 1304–9. [DOI] [PubMed] [Google Scholar]
- [14].Athreya K, Xavier MF, Antioxidants in the Treatment of Cancer, Nutr Cancer 69(8) (2017) 1099–1104. [DOI] [PubMed] [Google Scholar]
- [15].Waslo C, Bourdette D, Gray N, Wright K, Spain R, Lipoic Acid and Other Antioxidants as Therapies for Multiple Sclerosis, Curr Treat Options Neurol 21(6) (2019) 26. [DOI] [PubMed] [Google Scholar]
- [16].Sies H, Oxidative stress: a concept in redox biology and medicine, Redox Biol 4 (2015) 180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bouayed J, Bohn T, Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses, Oxid Med Cell Longev 3(4) (2010) 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Halliwell B, Free radicals and antioxidants: updating a personal view, Nutr Rev 70(5) (2012) 257–65. [DOI] [PubMed] [Google Scholar]
- [19].Pohl F, Kong Thoo Lin P, The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials, Molecules 23(12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bastianetto S, Quirion R, Natural antioxidants and neurodegenerative diseases, Front Biosci 9 (2004) 3447–52. [DOI] [PubMed] [Google Scholar]
- [21].Kozarski M, Klaus A, Jakovljevic D, Todorovic N, Vunduk J, Petrovic P, Niksic M, Vrvic MM, van Griensven L, Antioxidants of Edible Mushrooms, Molecules 20(10) (2015) 19489–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loyd DO, Lynch SM, Lipid-soluble vitamin C palmitate and protection of human high-density lipoprotein from hypochlorite-mediated oxidation, Int J Cardiol 152(2) (2011) 256–7. [DOI] [PubMed] [Google Scholar]
- [23].Neuzil J, Vitamin E succinate and cancer treatment: a vitamin E prototype for selective antitumour activity, Br J Cancer 89(10) (2003) 1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Panin G, Strumia R, Ursini F, Topical alpha-tocopherol acetate in the bulk phase: eight years of experience in skin treatment, Ann N Y Acad Sci 1031 (2004) 443–7. [DOI] [PubMed] [Google Scholar]
- [25].Kagan VE, Tyurina YY, Recycling and redox cycling of phenolic antioxidants, Ann N Y Acad Sci 854 (1998) 425–34. [DOI] [PubMed] [Google Scholar]
- [26].Foti MC, Antioxidant properties of phenols, J Pharm Pharmacol 59(12) (2007) 1673–85. [DOI] [PubMed] [Google Scholar]
- [27].Xu L, Davis TA, Porter NA, Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes, J Am Chem Soc 131(36) (2009) 13037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Black PN, DiRusso CC, Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation, Biochim Biophys Acta 1771(3) (2007) 286–98. [DOI] [PubMed] [Google Scholar]
- [29].Melton EM, Cerny RL, DiRusso CC, Black PN, Overexpression of human fatty acid transport protein 2/very long chain acyl-CoA synthetase 1 (FATP2/Acsvl1) reveals distinct patterns of trafficking of exogenous fatty acids, Biochem Biophys Res Commun 440(4) (2013) 743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Black PN, Ahowesso C, Montefusco D, Saini N, DiRusso CC, Fatty Acid Transport Proteins: Targeting FATP2 as a Gatekeeper Involved in the Transport of Exogenous Fatty Acids, Medchemcomm 7(4) (2016) 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M, ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition, Nat Chem Biol 13(1) (2017) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, Ricciotti E, DiRusso C, Murphy ME, Vonderheide RH, Lieberman PM, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Lin C, Nefedova Y, Black P, Kagan VE, Gabrilovich DI, Fatty acid transport protein 2 reprograms neutrophils in cancer, Nature 569(7754) (2019) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tyurina YY, St Croix CM, Watkins SC, Watson AM, Epperly MW, Anthonymuthu TS, Kisin ER, Vlasova II, Krysko O, Krysko DV, Kapralov AA, Dar HH, Tyurin VA, Amoscato AA, Popova EN, Bolevich SB, Timashev PS, Kellum JA, Wenzel SE, Mallampalli RK, Greenberger JS, Bayir H, Shvedova AA, Kagan VE, Redox (phospho)lipidomics of signaling in inflammation and programmed cell death, J Leukoc Biol 106(1) (2019) 57–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gaschler MM, Stockwell BR, Lipid peroxidation in cell death, Biochem Biophys Res Commun 482(3) (2017) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Basit F, van Oppen LM, Schockel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, Grefte S, Kopitz C, Heroult M, Hgm Willems P, Koopman WJ, Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells, Cell Death Dis 8(3) (2017) e2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stoyanovsky DA, Tyurina YY, Shrivastava I, Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV, Vladimirov YA, Shvedova AA, Philpott CC, Bayir H, Kagan VE, Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction?, Free Radic Biol Med 133 (2019) 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC, Protein carbonyl measurement by a sensitive ELISA method, Free Radic Biol Med 23(3) (1997) 361–6. [DOI] [PubMed] [Google Scholar]
- [38].Alamdari DH, Kostidou E, Paletas K, Sarigianni M, Konstas AG, Karapiperidou A, Koliakos G, High sensitivity enzyme-linked immunosorbent assay (ELISA) method for measuring protein carbonyl in samples with low amounts of protein, Free Radic Biol Med 39(10) (2005) 1362–7. [DOI] [PubMed] [Google Scholar]
- [39].Borovic S, Rabuzin F, Waeg G, Zarkovic N, Enzyme-linked immunosorbent assay for 4-hydroxynonenal-histidine conjugates, Free Radic Res 40(8) (2006) 809–20. [DOI] [PubMed] [Google Scholar]
- [40].Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P, Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors, Clin Chem 43(7) (1997) 1209–14. [PubMed] [Google Scholar]
- [41].Yepes-Calderon M, Sotomayor CG, Gomes-Neto AW, Gans ROB, Berger SP, Rimbach G, Esatbeyoglu T, Rodrigo R, Geleijnse JM, Navis GJ, Bakker SJL, Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study, J Clin Med 8(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Buico A, Cassino C, Ravera M, Betta PG, Osella D, Oxidative stress and total antioxidant capacity in human plasma, Redox Rep 14(3) (2009) 125–31. [DOI] [PubMed] [Google Scholar]
- [43].Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, Graham SH, Janesko K, Clark RS, Kochanek PM, Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children, Pediatr Res 51(5) (2002) 571–8. [DOI] [PubMed] [Google Scholar]
- [44].Romano A, Serviddio G, Calcagnini S, Villani R, Giudetti AM, Cassano T, Gaetani S, Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy-2-nonenal, Free Radic Biol Med 111 (2017) 281–293. [DOI] [PubMed] [Google Scholar]
- [45].Hayakawa J, Okabayashi Y, Simultaneous analysis of phospholipid in rabbit bronchoalveolar lavage fluid by liquid chromatography/mass spectrometry, J Pharm Biomed Anal 35(3) (2004) 583–92. [DOI] [PubMed] [Google Scholar]
- [46].Holcapek M, Liebisch G, Ekroos K, Lipidomic Analysis, Anal Chem 90(7) (2018) 4249–4257. [DOI] [PubMed] [Google Scholar]
- [47].Ikeda K, Oike Y, Shimizu T, Taguchi R, Global analysis of triacylglycerols including oxidized molecular species by reverse-phase high resolution LC/ESI-QTOF MS/MS, J Chromatogr B Analyt Technol Biomed Life Sci 877(25) (2009) 2639–47. [DOI] [PubMed] [Google Scholar]
- [48].Tyurina YY, Polimova AM, Maciel E, Tyurin VA, Kapralova VI, Winnica DE, Vikulina AS, Domingues MR, McCoy J, Sanders LH, Bayir H, Greenamyre JT, Kagan VE, LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: Implication for mitochondrial dysfunction in Parkinson's disease, Free Radic Res 49(5) (2015) 681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Samhan-Arias AK, Ji J, Demidova OM, Sparvero LJ, Feng W, Tyurin V, Tyurina YY, Epperly MW, Shvedova AA, Greenberger JS, Bayir H, Kagan VE, Amoscato AA, Oxidized phospholipids as biomarkers of tissue and cell damage with a focus on cardiolipin, Biochim Biophys Acta 1818(10) (2012) 2413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, Zhao Q, Zhang XJ, Janesko-Feldman KL, Alexander H, Basova LV, Clark RS, Kochanek PM, Kagan VE, Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis, Ann Neurol 62(2) (2007) 154–69. [DOI] [PubMed] [Google Scholar]
- [51].Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kagan VE, Bayir H, Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury, Nat Neurosci 15(10) (2012) 1407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H, Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis, Nat Chem Biol 13(1) (2017) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tyurina YY, Kisin ER, Murray A, Tyurin VA, Kapralova VI, Sparvero LJ, Amoscato AA, Samhan-Arias AK, Swedin L, Lahesmaa R, Fadeel B, Shvedova AA, Kagan VE, Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes, ACS Nano 5(9) (2011) 7342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthu TS, Kapralova VI, Vikulina AS, Jung MY, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Kochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, Kagan VE, A mitochondrial pathway for biosynthesis of lipid mediators, Nat Chem 6(6) (2014) 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, Epperly MW, Greenberger JS, Pitt BR, Kagan VE, Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation, Radiat Res 175(5) (2011) 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, Li J, Mosher M, Wright L, Wipf P, Watkins S, Pitt BR, Kagan VE, Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation, Am J Physiol Lung Cell Mol Physiol 299(1) (2010) L73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, Kagan VE, Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis?, Biochem Biophys Res Commun 324(3) (2004) 1059–64. [DOI] [PubMed] [Google Scholar]
- [58].Tyurina YY, Winnica DE, Kapralova VI, Kapralov AA, Tyurin VA, Kagan VE, LC/MS characterization of rotenone induced cardiolipin oxidation in human lymphocytes: implications for mitochondrial dysfunction associated with Parkinson's disease, Mol Nutr Food Res 57(8) (2013) 1410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, Mikulska-Ruminska K, Shrivastava IH, Kenny EM, Yang Q, Rosenbaum JC, Sparvero LJ, Emlet DR, Wen X, Minami Y, Qu F, Watkins SC, Holman TR, VanDemark AP, Kellum JA, Bahar I, Bayir H, Kagan VE, PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals, Cell 171(3) (2017) 628–641 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, Hashimoto A, Kapralov A, Amoscato A, Angelini R, Patel S, Alicea-Torres K, Weiner D, Murphy ME, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI, Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer, Nat Commun 8(1) (2017) 2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Passarelli MK, Ewing AG, Single-cell imaging mass spectrometry, Curr Opin Chem Biol 17(5) (2013) 854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sparvero LJ, Amoscato AA, Dixon CE, Long JB, Kochanek PM, Pitt BR, Bayir H, Kagan VE, Mapping of phospholipids by MALDI imaging (MALDI-MSI): realities and expectations, Chem Phys Lipids 165(5) (2012) 545–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sparvero LJ, Amoscato AA, Kochanek PM, Pitt BR, Kagan VE, Bayir H, Mass-spectrometry based oxidative lipidomics and lipid imaging: applications in traumatic brain injury, J Neurochem 115(6) (2010) 1322–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sparvero LJ, Amoscato AA, Fink AB, Anthonymuthu T, New LA, Kochanek PM, Watkins S, Kagan VE, Bayir H, Imaging mass spectrometry reveals loss of polyunsaturated cardiolipins in the cortical contusion, hippocampus, and thalamus after traumatic brain injury, J Neurochem 139(4) (2016) 659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Amoscato AA, Sparvero LJ, He RR, Watkins S, Bayir H, Kagan VE, Imaging mass spectrometry of diversified cardiolipin molecular species in the brain, Anal Chem 86(13) (2014) 6587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tian H, Sparvero LJ, Blenkinsopp P, Amoscato AA, Watkins SC, Bayir H, Kagan VE, Winograd N, Secondary-Ion Mass Spectrometry Images Cardiolipins and Phosphatidylethanolamines at the Subcellular Level, Angew Chem Int Ed Engl 58(10) (2019) 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Passarelli MK, Pirkl A, Moellers R, Grinfeld D, Kollmer F, Havelund R, Newman CF, Marshall PS, Arlinghaus H, Alexander MR, West A, Horning S, Niehuis E, Makarov A, Dollery CT, Gilmore IS, The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power, Nat Methods 14(12) (2017) 1175–1183. [DOI] [PubMed] [Google Scholar]
- [68].Angerer TB, Magnusson Y, Landberg G, Fletcher JS, Lipid Heterogeneity Resulting from Fatty Acid Processing in the Human Breast Cancer Microenvironment Identified by GCIB-ToF-SIMS Imaging, Anal Chem 88(23) (2016) 11946–11954. [DOI] [PubMed] [Google Scholar]
- [69].Chao H, Anthonymuthu TS, Kenny EM, Amoscato AA, Cole LK, Hatch GM, Ji J, Kagan VE, Bayir H, Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury, JCI Insight 3(21) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kapralov AA, Feng WH, Amoscato AA, Yanamala N, Balasubramanian K, Winnica DE, Kisin ER, Kotchey GP, Gou P, Sparvero LJ, Ray P, Mallampalli RK, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA, Kagan VE, Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration, ACS Nano 6(5) (2012) 4147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE, Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells, Nat Cell Biol 15(10) (2013) 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kagan VE, Chu CT, Tyurina YY, Cheikhi A, Bayir H, Cardiolipin asymmetry, oxidation and signaling, Chem Phys Lipids 179 (2014) 64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U, NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy, Cell Death Differ 23(7) (2016) 1140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schlattner U, Tokarska-Schlattner M, Epand RM, Boissan M, Lacombe ML, Kagan VE, NME4/nucleoside diphosphate kinase D in cardiolipin signaling and mitophagy, Lab Invest 98(2) (2018) 228–232. [DOI] [PubMed] [Google Scholar]
- [75].Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE, Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity, Biochemistry 46(11) (2007) 3423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE, Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes, Biochemistry 45(15) (2006) 4998–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brand MD, Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling, Free Radic Biol Med 100 (2016) 14–31. [DOI] [PubMed] [Google Scholar]
- [78].Murphy MP, How mitochondria produce reactive oxygen species, Biochem J 417(1) (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zou X, Ratti BA, O'Brien JG, Lautenschlager SO, Gius DR, Bonini MG, Zhu Y, Manganese superoxide dismutase (SOD2): is there a center in the universe of mitochondrial redox signaling?, J Bioenerg Biomembr 49(4) (2017) 325–333. [DOI] [PubMed] [Google Scholar]
- [80].Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG, Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors, Nat Chem Biol 1(4) (2005) 223–32. [DOI] [PubMed] [Google Scholar]
- [81].Reeder BJ, The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms, Antioxid Redox Signal 13(7) (2010) 1087–123. [DOI] [PubMed] [Google Scholar]
- [82].Kapralov AA, Yanamala N, Tyurina YY, Castro L, Samhan-Arias A, Vladimirov YA, Maeda A, Weitz AA, Peterson J, Mylnikov D, Demicheli V, Tortora V, Klein-Seetharaman J, Radi R, Kagan VE, Topography of tyrosine residues and their involvement in peroxidation of polyunsaturated cardiolipin in cytochrome c/cardiolipin peroxidase complexes, Biochim Biophys Acta 1808(9) (2011) 2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mandal A, Hoop CL, DeLucia M, Kodali R, Kagan VE, Ahn J, van der Wel PC, Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c, Biophys J 109(9) (2015) 1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pandiscia LA, Schweitzer-Stenner R, Coexistence of native-like and non-native partially unfolded ferricytochrome c on the surface of cardiolipin-containing liposomes, J Phys Chem B 119(4) (2015) 1334–49. [DOI] [PubMed] [Google Scholar]
- [85].Li M, Mandal A, Tyurin VA, DeLucia M, Ahn J, Kagan VE, van der Wel PCA, Surface-Binding to Cardiolipin Nanodomains Triggers Cytochrome c Pro-apoptotic Peroxidase Activity via Localized Dynamics, Structure 27(5) (2019) 806–815 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV, Conformational properties of cardiolipin-bound cytochrome c, Proc Natl Acad Sci U S A 109(1) (2012) 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McClelland LJ, Mou TC, Jeakins-Cooley ME, Sprang SR, Bowler BE, Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity, Proc Natl Acad Sci U S A 111(18) (2014) 6648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tyurin VA, Tyurina YY, Jung MY, Tungekar MA, Wasserloos KJ, Bayir H, Greenberger JS, Kochanek PM, Shvedova AA, Pitt B, Kagan VE, Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli, J Chromatogr B Analyt Technol Biomed Life Sci 877(26) (2009) 2863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE, Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis, J Neurochem 107(6) (2008) 1614–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y, Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine, Free Radic Biol Med 37(12) (2004) 1963–85. [DOI] [PubMed] [Google Scholar]
- [91].Tyurina YY, Basova LV, Konduru NV, Tyurin VA, Potapovich AI, Cai P, Bayir H, Stoyanovsky D, Pitt BR, Shvedova AA, Fadeel B, Kagan VE, Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: implications for the resolution of inflammation, J Biol Chem 282(11) (2007) 8498–509. [DOI] [PubMed] [Google Scholar]
- [92].Tyurin VA, Yanamala N, Tyurina YY, Klein-Seetharaman J, Macphee CH, Kagan VE, Specificity of lipoprotein-associated phospholipase A(2) toward oxidized phosphatidylserines: liquid chromatography-electrospray ionization mass spectrometry characterization of products and computer modeling of interactions, Biochemistry 51(48) (2012) 9736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tyurin VA, Balasubramanian K, Winnica D, Tyurina YY, Vikulina AS, He RR, Kapralov AA, Macphee CH, Kagan VE, Oxidatively modified phosphatidylserines on the surface of apoptotic cells are essential phagocytic 'eat-me' signals: cleavage and inhibition of phagocytosis by Lp-PLA2, Cell Death Differ 21(5) (2014) 825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mao G, Qu F, St Croix CM, Tyurina YY, Planas-Iglesias J, Jiang J, Huang Z, Amoscato AA, Tyurin VA, Kapralov AA, Cheikhi A, Maguire J, Klein-Seetharaman J, Bayir H, Kagan VE, Mitochondrial Redox Opto-Lipidomics Reveals Mono-Oxygenated Cardiolipins as Pro-Apoptotic Death Signals, ACS Chem Biol 11(2) (2016) 530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ji J, Baart S, Vikulina AS, Clark RS, Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J, Skoda EM, Kline AE, Kochanek PM, Wipf P, Kagan VE, Bayir H, Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion, J Cereb Blood Flow Metab 35(2) (2015) 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kagan VE, Tyurina YY, Tyurin VA, Mohammadyani D, Angeli JP, Baranov SV, Klein-Seetharaman J, Friedlander RM, Mallampalli RK, Conrad M, Bayir H, Cardiolipin signaling mechanisms: collapse of asymmetry and oxidation, Antioxid Redox Signal 22(18) (2015) 1667–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ascenzi P, Coletta M, Wilson MT, Fiorucci L, Marino M, Polticelli F, Sinibaldi F, Santucci R, Cardiolipin-cytochrome c complex: Switching cytochrome c from an electron-transfer shuttle to a myoglobin- and a peroxidase-like heme-protein, IUBMB Life 67(2) (2015) 98–109. [DOI] [PubMed] [Google Scholar]
- [98].Yanamala N, Kapralov AA, Djukic M, Peterson J, Mao G, Klein-Seetharaman J, Stoyanovsky DA, Stursa J, Neuzil J, Kagan VE, Structural re-arrangement and peroxidase activation of cytochrome c by anionic analogues of vitamin E, tocopherol succinate and tocopherol phosphate, J Biol Chem 289(47) (2014) 32488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Araujo JC, Prieto T, Prado FM, Trindade FJ, Nunes GL, dos Santos JG, Di Mascio P, Castro FL, Fernandes GJ, Fernandes VJ Jr., Araujo AS, Politi MJ, Brochsztain S, Nascimento OR, Nantes IL, Peroxidase catalytic cycle of MCM-41-entrapped microperoxidase-11 as a mechanism for phenol oxidation, J Nanosci Nanotechnol 7(10) (2007) 3643–52. [DOI] [PubMed] [Google Scholar]
- [100].Liu W, Porter NA, Schneider C, Brash AR, Yin H, Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition, Free Radic Biol Med 50(1) (2011) 166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhong H, Yin H, Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria, Redox Biol 4 (2015) 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhong H, Xiao M, Zarkovic K, Zhu M, Sa R, Lu J, Tao Y, Chen Q, Xia L, Cheng S, Waeg G, Zarkovic N, Yin H, Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: A novel link between oxidative stress and cancer, Free Radic Biol Med 102 (2017) 67–76. [DOI] [PubMed] [Google Scholar]
- [103].Balasubramanian K, Maeda A, Lee JS, Mohammadyani D, Dar HH, Jiang JF, St Croix CM, Watkins S, Tyurin VA, Tyurina YY, Kloditz K, Polimova A, Kapralova VI, Xiong Z, Ray P, Klein-Seetharaman J, Mallampalli RK, Bayir H, Fadeel B, Kagan VE, Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity, Sci Signal 8(395) (2015) ra95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ohto U, Fukase K, Miyake K, Shimizu T, Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2, Proc Natl Acad Sci U S A 109(19) (2012) 7421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO, The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex, Nature 458(7242) (2009) 1191–5. [DOI] [PubMed] [Google Scholar]
- [106].Chilton PM, Embry CA, Mitchell TC, Effects of Differences in Lipid A Structure on TLR4 Pro-Inflammatory Signaling and Inflammasome Activation, Front Immunol 3 (2012) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M, Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice, Nat Cell Biol 16(12) (2014) 1180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Forcina GC, Dixon SJ, GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis, Proteomics 19(18) (2019) e1800311. [DOI] [PubMed] [Google Scholar]
- [109].Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR, Regulation of ferroptotic cancer cell death by GPX4, Cell 156(1-2) (2014) 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR, FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation, Nat Chem Biol 14(5) (2018) 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Garcia YJ, Rodriguez-Malaver AJ, Penaloza N, Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices, J Neurosci Methods 144(1) (2005) 127–35. [DOI] [PubMed] [Google Scholar]
- [112].Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA, C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology, Free Radic Biol Med 33(4) (2002) 473–90. [DOI] [PubMed] [Google Scholar]
- [113].Pap EH, Drummen GP, Winter VJ, Kooij TW, Rijken P, Wirtz KW, Op den Kamp JA, Hage WJ, Post JA, Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY(581/591), FEBS Lett 453(3) (1999) 278–82. [DOI] [PubMed] [Google Scholar]
- [114].Banthiya S, Kalms J, Galemou Yoga E, Ivanov I, Carpena X, Hamberg M, Kuhn H, Scheerer P, Structural and functional basis of phospholipid oxygenase activity of bacterial lipoxygenase from Pseudomonas aeruginosa, Biochim Biophys Acta 1861(11) (2016) 1681–1692. [DOI] [PubMed] [Google Scholar]
- [115].Neau DB, Bender G, Boeglin WE, Bartlett SG, Brash AR, Newcomer ME, Crystal structure of a lipoxygenase in complex with substrate: the arachidonic acid-binding site of 8R-lipoxygenase, J Biol Chem 289(46) (2014) 31905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Xu S, Mueser TC, Marnett LJ, Funk MO Jr., Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis, Structure 20(9) (2012) 1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Newcomer ME, Brash AR, The structural basis for specificity in lipoxygenase catalysis, Protein Sci 24(3) (2015) 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gaffney BJ, Connecting lipoxygenase function to structure by electron paramagnetic resonance, Acc Chem Res 47(12) (2014) 3588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Saam J, Ivanov I, Walther M, Holzhutter HG, Kuhn H, Molecular dioxygen enters the active site of 12/15-lipoxygenase via dynamic oxygen access channels, Proc Natl Acad Sci U S A 104(33) (2007) 13319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Anthonymuthu TS, Kenny EM, Shrivastava I, Tyurina YY, Hier ZE, Ting HC, Dar HH, Tyurin VA, Nesterova A, Amoscato AA, Mikulska-Ruminska K, Rosenbaum JC, Mao G, Zhao J, Conrad M, Kellum JA, Wenzel SE, VanDemark AP, Bahar I, Kagan VE, Bayir H, Empowerment of 15-Lipoxygenase Catalytic Competence in Selective Oxidation of Membrane ETE-PE to Ferroptotic Death Signals, HpETE-PE, J Am Chem Soc 140(51) (2018) 17835–17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Seibt TM, Proneth B, Conrad M, Role of GPX4 in ferroptosis and its pharmacological implication, Free Radic Biol Med 133 (2019) 144–152. [DOI] [PubMed] [Google Scholar]
- [122].Liu H, Schreiber SL, Stockwell BR, Targeting Dependency on the GPX4 Lipid Peroxide Repair Pathway for Cancer Therapy, Biochemistry 57(14) (2018) 2059–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J, Chaperone-mediated autophagy is involved in the execution of ferroptosis, Proc Natl Acad Sci U S A 116(8) (2019) 2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation, Nat Rev Immunol 15(8) (2015) 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J Clin Invest 128(7) (2018) 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sulciner ML, Gartung A, Gilligan MM, Serhan CN, Panigrahy D, Targeting lipid mediators in cancer biology, Cancer Metastasis Rev 37(2-3) (2018) 557–572. [DOI] [PubMed] [Google Scholar]
- [127].Mouchlis VD, Dennis EA, Phospholipase A2 catalysis and lipid mediator lipidomics, Biochim Biophys Acta Mol Cell Biol Lipids 1864(6) (2019) 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Anthonymuthu TS, Kenny EM, Lamade AM, Kagan VE, Bayir H, Oxidized phospholipid signaling in traumatic brain injury, Free Radic Biol Med 124 (2018) 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lamade AM, Kenny EM, Anthonymuthu TS, Soysal E, Clark RSB, Kagan VE, Bayir H, Aiming for the target: Mitochondrial drug delivery in traumatic brain injury, Neuropharmacology 145(Pt B) (2019) 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]