Highlights
-
•
This study investigated the potency of a xanthone derivative when used incombination with doxorubicin on a cancer cell line.
-
•
The xanthone-doxorubicin combination showed promising in vitro activity againstlymphoma cells.
-
•
Potential mechanisms of action responsible for the ability of this xanthone derivative to reduce doxorubicin resistance were investigated.
Keywords: c-Jun N-terminal kinase, Doxorubicin sensitivity, Lymphoma, Raf-1, Synergistic, Xanthone synthetic
Abstract
Background
The increasing rate of cancer chemoresistance and adverse side effects of therapy have led to the wide use of various chemotherapeutic combinations in cancer management, including lymphoid malignancy.
Objective
We investigated the effects of a combination of 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX) and doxorubicin on the Raji lymphoma cell line.
Methods
Raji cells were treated with different concentrations of TTX, doxorubicin, or combinations thereof. Cancer cell growth inhibition was evaluated using 3-(4,5-dimethyltiazol-2-yl)-2,5- diphenyltetrazolium bromide/MTT assay to determine the half-maximal inhibitory concentration. Combination index values were calculated using CompuSyn (ComboSyn, Inc, Paramus, NJ). Molecular docking was conducted using a Protein-Ligand ANT System.
Results
The mean (SD) half-maximal inhibitory concentration values of TTX and doxorubicin were 15.948 (3.101) µM and 25.432 (1.417) µM, respectively. The combination index values of the different combinations ranged from 0.057 to 0.285, indicating strong to very strong synergistic effects. The docking study results reveal that TTX docks at the active site of Raf-1 and c-Jun N-kinase receptors with predicted free energies of binding of −79.37 and −75.42 kcal/mol, respectively.
Conclusions
The xanthone-doxorubicin combination showed promising in vitro activity against lymphoma cells. The results also indicate that the TTX and doxorubicin combination's effect was due to the interaction between TTX with Raf-1 and c-Jun N-kinase receptors, 2 determinants of doxorubicin resistance progression.
Introduction
Malignant tumors result from complex structural and quantitative alterations at the molecular level, leading to altered cell behavior and uncontrolled cell proliferation. B-cell lymphoma is a lymphoid malignancy and type of cancer of the immune system. Worldwide data from Global Burden of Cancer (GLOBOCAN) in 2008 indicate that approximately half of all newly diagnosed hematologic neoplasms are lymphomas.1 Lymphoid neoplasms are the fourth most common cancer and the sixth leading cause of death in the United States. Approximately 136,000 Americans were diagnosed with lymphoma in 2016,2 and its incidence has been increasing. The prevalence of lymphoid malignancies is lower among Asians compared with other ethnic groups in the United States and among foreign-born Asians compared with US-born Asians.3 Developing treatment strategies for lymphoid malignancies is an ongoing effort, but relapses and treatment-related complications remain major obstacles. Thus, the development of new therapeutic strategies is a significant unmet need to improve the prognosis and quality of life of patients with malignant lymphomas.4
Doxorubicin is a broad-spectrum antitumor antibiotic isolated from Streptomyces species and is widely used as an anticancer agent to treat different types of cancer, including lymphoid malignancies.5 It is also widely accepted that using a single chemotherapeutic regimen is ineffective in producing the desired therapeutic effect in many cancers and, instead, causes the emergence of side effects and resistance. Half of the patients with B-cell lymphoma treated with anthracycline-based chemotherapy develop chemoresistance.6 A new approach is, therefore, required to improve doxorubicin sensitivity and prevent chemoresistance; a possible approach is using a combination of doxorubicin and another compound, leading to a synergistic effect that inhibits the proliferation of lymphoma cells. Using such chemotherapeutic combinations is a rational strategy to improve response and tolerability and decrease resistance.7 Drug combinations have been shown to be capable of lowering drug resistance (due to nonoverlapping mechanisms of action) and side effects (due to lower doses).8 Utilizing multiple drugs with different mechanisms of action may involve either single or multiple targets and result in a synergistic effect,9 thereby increasing the effectiveness of treatment.
Proteins dysregulated in B-cell lymphoma and involved in the development of doxorubicin resistance include Raf isoforms, nuclear factor kappa B (NFκB)-inducing kinase (NIK), c-Jun N-terminal kinase (c-JNK), survivin, Bruton tyrosine kinase (Btk), Ak mouse thymoma (Akt), and cyclin-dependent kinases. Activated Raf isoforms have been reported to increase p21 and c-Myelocytoma (c-Myc) levels, and this signal transduction pathway may be involved in doxorubicin resistance. Raf-1 expression increases Michigan Cancer Foundation-7 (MCF-7) cancer cells’ resistance to doxorubicin10 through the activation of p-glycoprotein, a member of the adenosine triphosphate-binding cassette transporter family that facilitates the efflux of a wide variety of anticancer drugs, including anthracyclines.11 NIK is a major enzyme involved in the activation of NFκB, a transcription factor with tumor-promoting properties.12 NFκB is constitutively activated in various lymphoid malignancies and plays a dominant role in the neoplastic transformation of B-lymphoid cells. Some key components of the NFκB pathway are affected in B-cell lymphoid malignancies, leading to uncontrollable cell behavior.13
Survivin is a member of the inhibitor of apoptosis protein family, which is highly expressed in various hematologic malignancies,14 including B-cell lymphoma.15 Survivin TmSm inhibitors increase apoptosis, suppress cell proliferation, and increase the sensitivity of cancer cells to doxorubicin when used in combination.16 Btk is a cytoplasmic tyrosine kinase; it plays an important role in B-cell maturation and is overexpressed in various B-cell malignancies.15 The activation of this protein catalyzes phosphorylation and activates phospholipase C2, resulting in the activation of Ras/Raf/MEK/ERK and NFκB pathways.17 Akt is an intracellular kinase that plays an important role in cell survival and proliferation and is highly expressed in B-cell lymphoma.15 Increased Akt expression is associated with breast cancer cells’ resistance to doxorubicin.18
Doxorubicin is an anticancer agent with apoptosis-inducing activity. c-JNK plays an important role in caspase activation and apoptosis induction by doxorubicin. c-JNK is also involved in the degradation of Myeloid cell leukemia-1 (Mcl-1) through phosphorylation and ubiquitination, which is an important process in sensitizing breast cancer cells to antimicrotubular agents. Inhibiting c-JNK activation leads to high concentrations of Mcl-1, which protects cancer cells from apoptosis, causing cancer cells’ resistance to doxorubicin.19 Suppressing Mcl-1 expression can induce matrix metalloproteinase degradation and caspase activation.20
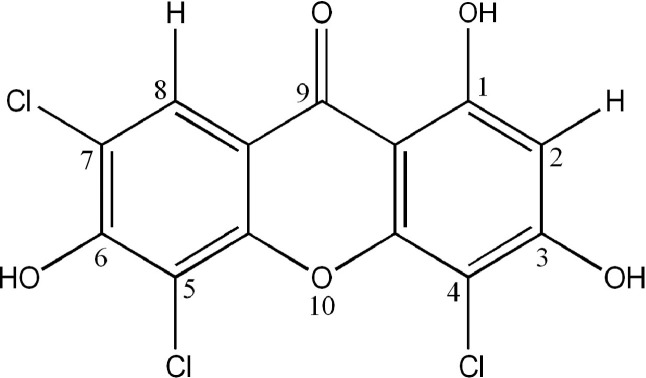
A xanthone-derivative compound, 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX), is a novel synthetic xanthone synthesized based on quantitative structure-activity relationship analyses of many xanthone compounds.21 This compound contains 3 hydroxyl groups and 3 chloro atoms, as shown in Figure 1; its synthesis and characterization have been recently reported.22 A previous study showed that hydroxyl group substitutions on the xanthone scaffold increase cytotoxic activity compared with the parent compound. Increasing the number of hydroxyl groups did not linearly increase cytotoxic activity, suggesting that the position of hydroxyl groups might also determine xanthones’ cytotoxic activity.20
Figure 1.
Structure of 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX).
Xanthone compounds, both natural and synthetic, have been widely studied as anticancer agents, but mostly for single-agent treatment and not in combination. The present study provides novel information regarding the potential role of TTX as a co-chemotherapeutic agent with doxorubicin; such a combination may be an effective strategy resulting in synergistic efficacy while minimizing resistance and side effects of doxorubicin. In addition, molecular docking of TTX on potential protein receptors involved in doxorubicin resistance was also performed to predict the mechanism(s) underlying the synergy.
Materials and Methods
Reagents and chemicals
Doxorubicin was purchased from Dankos, a Kalbe subsidiary (Jakarta, Indonesia). One of the authors (EY) TTX, 1 of the novel xanthone-derived compounds produced. Procedures for the preparation, composition, and purity of this compound were reported in a previous study.22 Iscove's modified Dulbecco's media and M199 media for cell cultures were purchased from Gibco BRL (Massachusets, USA). Fetal bovine serum, penicillin, streptomycin, fungizone, (3-[4,5-dimethyl tiazol-2-yl]-2,5-diphenyltetrazolium bromide)/MTT, sodium dodecyl sulfate (SDS), and dimethyl sulfoxide (DMSO) were purchased from Sigma (St Louis, Missouri). The other reagents were locally procured.
Cell lines and cultures
Cells from the Raji B-cell lymphoma line and a normal cell line (Vero) were obtained from the Parasitology Laboratory of the Faculty of Medicine, Gadjah Mada University. The cells were cultivated in Iscove's modified Dulbecco's medium (Raji) or M199 medium (Vero) supplemented with 100 U/mL penicillin, 100 µL streptomycin, and 1% fungizone in a humidified incubator at 37°C and 5% carbon dioxide. The study received ethics approval from the Medical and Health Research Committee of the Faculty of Medicine, Gadjah Mada University−Dr. Sardjito General Hospital, Yogyakarta, Indonesia.
Compounds
The compounds tested were doxorubicin and a chloro-substituted xanthone derivative, TTX. Doxorubicin was available as a stock solution at a concentration of 2000 µg/mL (equivalent to 3679.85 µM) and diluted using culture media as needed. A total of 10 mg TTX was dissolved in 100 µL 10% DMSO in water to form a stock solution with a concentration of 100,000 µg/mL (equivalent to 244,200 µM). The highest TTX concentration tested was 100 µg/mL (dilution of 1000 times using culture media, equivalent to 244.200 µM) so that the DMSO concentration in this solution was 0.01%. This solution was diluted to varying concentrations of 122.100, 61.050, 30.525, 15.263, 7.631, and 3.816 µM TTX using culture media, and the DMSO concentrations in subsequent dilutions were 0.005%, 0.0025%, 0.00125%, 0.00063%, 0.00031%, and 0.000156% in each TTX concentration tested. A previous study has shown that 10% DMSO is safe to use in some in vitro studies without causing cytotoxic effects in various types of cancer cell lines. When conducting a cytotoxicity test, the DMSO concentration in the highest concentration of the tested compound should not exceed 0.1%.23 Dilutions of doxorubicin and TTX were used with a suitable culture medium for each cell line. TTX was used in combination with doxorubicin to treat Raji cells, and its potential mechanism of action was investigated through molecular docking.
In vitro cytotoxicity assay
The in vitro cytotoxic activity of TTX and doxorubicin against Raji and Vero cell lines was measured using MTT assay as described in a previous study,24 with some modifications, to determine the half-maximal inhibitory concentration (IC50) values of doxorubicin and TTX and their selectivity against Raji cancer cells. The IC50 values thus obtained were used to determine the concentrations to be used in TTX–doxorubicin combinations, comprising ratios of one-half, three-eighths, one-fourth, and one-eighth of the IC50 values.
Raji or Vero cells were seeded at a density of 1 × 104 cells per well in 96-well microplates and incubated overnight. Raji cells were treated with 100 µL of 244.200, 122.100, 61.050, 30.525, 15.263, 7.631, or 3.816 µM TTX, and 50 µL 91.996, 45.998, 22.999, 11.499, 5.749, 2.875, or 1.437 µM doxorubicin as a positive control. A total of 50 µL medium was added to cells as an untreated control and to wells without cells as a medium control. Vero cells were treated with 50 µL medium (as an untreated control), 50 µL of varying TTX concentrations (1221.001, 610.501, 305.250, 152.625, 76.312, or 38.156 µM), 50 µL of varying doxorubicin concentrations (459.981, 229.991, 114.995, 57.498, 28.749, or 13.374 µM), or 50 µL culture medium without cells as a medium control. The plates were incubated for 24 hours at 37°C in a 5% carbon dioxide incubator, followed by the addition of 50 µL 10% MTT solution and reincubation for another 4 hours. Next, 100 µL 10% SDS in water was added to all the wells, and the plate was incubated overnight to dissolve the formazan crystals. The absorbance (ie, optical density) of the wells at a wavelength of 595 nm was determined using an ELISA microplate reader. Viable cells (%) were counted using the following formula:
IC50 values represent the drug concentration in micrometers required to inhibit cell viability by 50%, calculated using probit analysis. The selectivity index was calculated from the ratio of IC50 against Vero cells to that against Raji cells. Selectivity index values >3 were considered to indicate good selectivity.25
Drug combinations
The effects of different concentrations of TTX, doxorubicin, and their combinations on Raji cell growth were measured using MTT assay as described previously, with modifications. Raji cells were plated in a 96-well microplate at a density of 1 × 104 cells per well and incubated at 37°C in 5% carbon dioxide for 24 hours. The cells were treated with one-half IC50, three-eighths IC50, one-fourth IC50, or one-eighth IC50 of TTX, doxorubicin, or combinations thereof at a 50-µL total volume for 24 hours. The plate was incubated for another 4 hours at 37°C in 5% carbon dioxide after adding 50 µL of 10% MTT to each well. Next, 100 µL SDS was added to the plate and incubated overnight to solubilize the MTT formazan crystals completely. The optical density of the wells at a wavelength of 595 nm was determined using an ELISA microplate reader. The percentage of viable cells was determined using the in vitro cytotoxicity assay method described above.
Analysis of drug combinations
Drug interactions between TTX and doxorubicin were determined using isobologram analysis26 and denoted with the combination index. The combination index analysis was based on the median-effect principle and computed using the following formula. Combination index = D1/(Dx)1 + D2/(Dx)2, where D1 and (Dx)1 are concentrations of TTX and doxorubicin, respectively, that inhibit cell growth to 50% of control when used alone, and D2 and (Dx)2 are concentrations of TTX and doxorubicin, respectively, that produce the same effect when used in combination.8 Combination index values, calculated using CompuSyn software (ComboSyn Inc, Paramus, New Jersey) indicate the effects of drug combinations and are interpreted as shown in Table 1.
Table 1.
Interpretation of combination index values in drug combinations.8
| Combination index value | Interpretation |
|---|---|
| <0.1 | Very strong synergism |
| 0.1–0.3 | Strong synergism |
| 0.3–0.7 | Synergism |
| 0.7–0.85 | Moderate synergism |
| 0.85–09 | Slight synergism |
| 0.9–1.1 | Nearly additive |
| 1.1–1.45 | Slight to moderate antagonism |
| 1.45–3.3 | Antagonism |
| 3.3–10 | Strong antagonism |
| >10 | Very strong antagonism |
Preparation for docking
A molecular docking study of TTX on protein receptors was conducted based on a procedure described previously.27 Three-dimensional crystal structures of protein receptors involved in doxorubicin resistance were retrieved from the Protein Data Bank(PDB) database (Research Collaboratory for Structural Bioinformatics/RCSB, USA). The downloaded protein structures were then prepared with YASARA ver. 10.1.8 (YASARA Biosciences GmbH, Vienna, Austria) (www.yasara.org) in the standard setting, and hydrogen atoms were added to the structures. The results were saved in the mol2 format for docking. The downloaded native ligands of protein receptors were prepared with MarvinSketch version 16.5.2.0 (ChemAxon, Budapest, Hungary) (http://www.chemaxon.com) by configuring them in a 2-dimensional format. They were found to be protonated at pKa 7.4, and 10 ligand conformations were built. The 10 conformers generated were then saved in the mol2 format for docking. TTX was used as an experimental ligand, and its 2-dimensional structure was prepared with Marvinsketch in the same way as the native ligand; the conformers were then saved in the mol2 format for docking.
Molecular docking
Molecular docking simulation was carried out using Protein-Ant Ligand Systems.28 The root median square deviation (RMSD) and free energy of binding were used as docking parameters and measured using Yasara. The docking process was considered valid and suitable for being reproduced if the RMSD value of the copy ligand after redocking was <2 Å.29 The best predictive binding position was selected based on the most electronegative free energy of binding. Interactions between the protein structure and ligands were visualized with PyMOL ver 1.7.5.0 (Schrödinger, New York, New York) (http://www.pymol.org).
Additionally, the hydrogen bonds formed between protein receptors and ligands were compared with those of the native ligand.30
Results
Cytotoxicity assay
The cytotoxicity of TTX and doxorubicin individually and in combination against Raji or Vero cells was determined using the MTT method. The results are shown in Table 2. The Council of Scientific and Industrial Research classifies cytotoxic activities into inactive (mean IC50, >50 µg/mL), weak (15 µg/mL< mean IC50, <50 µg/mL), moderate (6.25 µg/mL< mean IC50, <15 µg/mL), or potent (mean IC50, <6.25 µg/mL).31 According to these criteria, the cytotoxic activities of TTX 15.948 µM (equivalent to 6.53 µg/mL) and doxorubicin 25.432 µM (equivalent to 13.82 µg/mL) found in the present study are classified as moderate. The sensitivity index values of TTX and doxorubicin >3 indicate their selectivity against Raji cells.25
Table 2.
The half-maximal inhibitory concentration (IC50) and selectivity index (SI) values of 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX) and doxorubicin against Raji or Vero cell lines.*
| Compound | IC50 value (µM) |
SI value† | |
|---|---|---|---|
| Raji | Vero | ||
| TTX | 15.948 (3.101) | 256.288 (25.617) | >76.57 |
| Doxorubicin | 25.432 (1.417) | 149.917 (28.277) | 5.89 |
Values are the mean (SD) of 3 experiments. Viability of Raji or Vero cells was evaluated after 24 hours of TTX or doxorubicin individual treatment and evaluated using the MTT method.
Selective if >3.23
Combined effect of TTX and doxorubicin on Raji cells
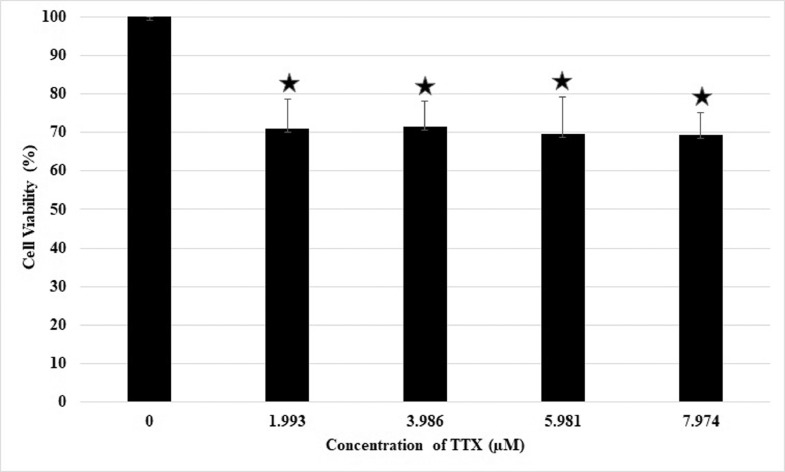
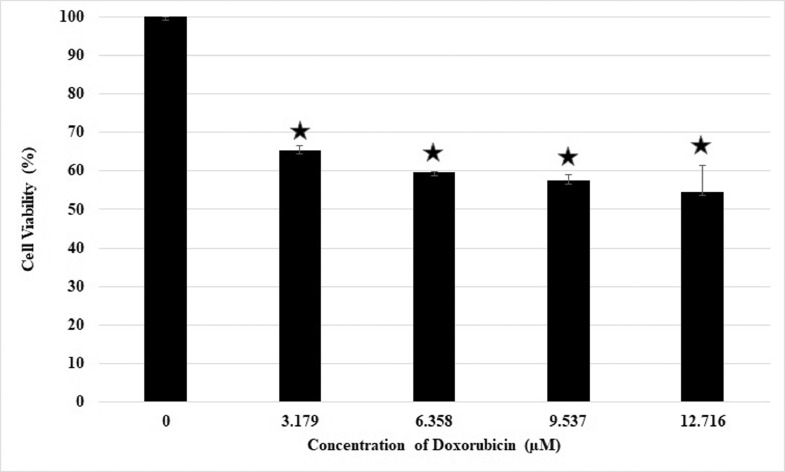
Cells were treated with various concentrations of TTX and doxorubicin for 24 hours to investigate the combined effect of TTX and doxorubicin on the viability of Raji cells. The IC50 values obtained after single treatments of TTX or doxorubicin were used to determine their concentrations in TTX–doxorubicin combinations. The concentrations used were calculated as one-half, three-eighths, three-fourths, or one-eighth IC50 of TTX or doxorubicin single treatments; thus, the TTX concentrations used were 7.974, 5.981, 3.986, and 1.993 µM, and the doxorubicin concentrations used were equivalent to 12.716, 9.537, 6.358, and 3.179 µM. The experiments consisted of 4 groups: a control group treated with medium alone, a group treated with TTX alone, a group treated with doxorubicin alone, and a group treated with a combination of TTX and doxorubicin. Cell viability was measured using MTT assay, and the data were converted into percentages of viable cells. The data confirmed that in individual treatments, all concentrations of either TTX (Figure 2) or doxorubicin (Figure 3) alone inhibited cell proliferation by <50%.
Figure 2.
Inhibitory effect of TTX alone on Raji cell proliferation. ⁎P < 0.05 compared to control (concentration 0) Values are the mean of two independent experiments. Raji cell viability was measured after 24 h of TTX treatment using the MTT method.
Figure 3.
Inhibitory effect of doxorubicin alone on Raji cell proliferation. ⁎P < 0.05 compared to control (concentration 0) Values are mean of two independent experiments. Raji cell viability was measured after 24 h of doxorubicin treatment using the MTT method.
The cytotoxic activity of TTX or doxorubicin against Raji cells did not increase in direct proportion to the concentration. The percentage of viable cells at a TTX concentration of 1.993, 3.986, 5.981, and 7.974 µM was 71.04%, 71.57%, 69.65%, and 69.30%, respectively. In the doxorubicin-treated cells, the percentage of viable cells was 65.23%, 59.77%, 57.53%, and 54.65% at a concentration of 3.179, 6.358, 9.537, and 12.716 µM, respectively. There were no significant differences among the effects of the different drug concentrations (P > 0.05), except in comparison with untreated cells (P < 0.05).
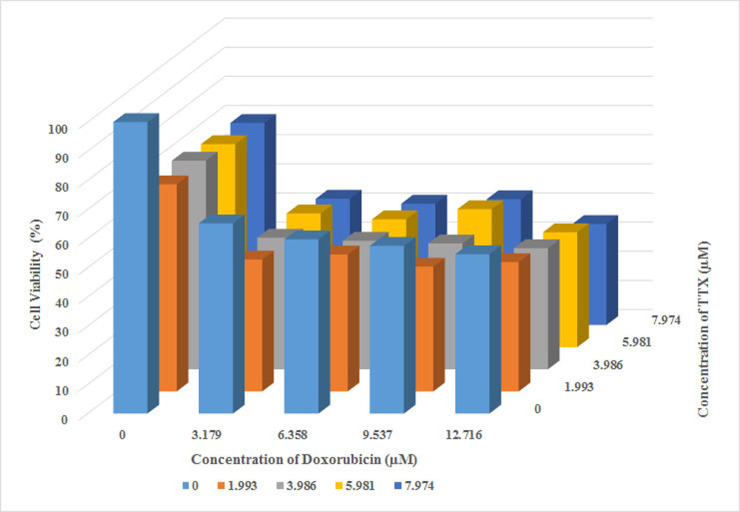
In contrast to the treatments with TTX or doxorubicin alone, their combinations at the 4 concentrations above inhibited cell proliferation significantly, as shown in Figure 4. The percentage of viable cells at all concentrations was <50%, ranging from 34.64% to 45.24%. The smallest number of viable cells was seen with the combination containing the highest concentrations of TTX and doxorubicin (7.974 and 12.716 µM, respectively). Furthermore, this combination suppressed Raji cell proliferation in a concentration-dependent manner.
Figure 4.
Combined effect of TTX and doxorubicin on Raji cell proliferation. Values are the mean of two independent experiments. Raji cell viability was measured after 24 h of TTX and doxorubicin combination treatments using the MTT method.
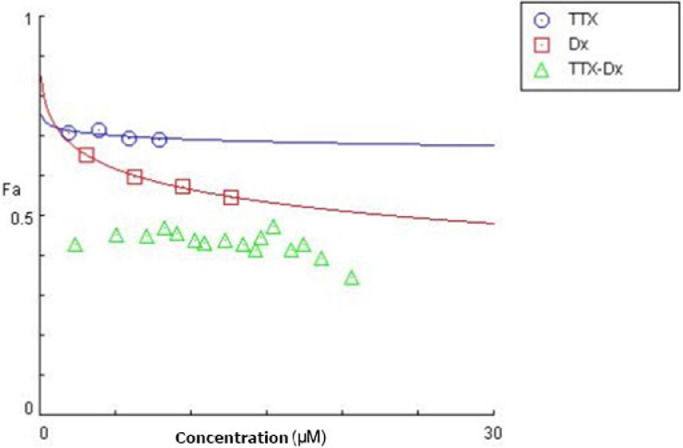
Combining TTX and doxorubicin enhanced their anticancer effect against Raji cells. In comparison with TTX or doxorubicin alone, this combination resulted in greater efficacy in inhibiting the growth of Raji cells (Figure 5). This suggested a possible role of TTX in the doxorubicin-induced growth inhibition of B lymphoma cancer cells. Figure 5 shows the concentration-effect curves of TTX, doxorubicin, and the TTX–doxorubicin combination, and the combination index values of the TTX–doxorubicin combination obtained using CompuSyn calculations (Table 3). These data indicated that all the tested concentrations had strong or very strong synergistic effects (combination index, 0.1−0.3 and <0.1, respectively).8
Figure 5.
Dose-effect curves of TTX, doxorubicin, and the TTXdoxorubicin combination. Dose-effect curves were generated from the CompuSyn calculations, and the values are the mean of three experiments. Abbreviations; Fa, fraction affected (the effects resulting from the intervention); TTX, 1,3,6-trihydroxy-4,5,7-trichloroxanthone; Dx, doxorubicin; TTX-Dx, combination of TTX and doxorubicin.
Table 3.
Combination index values of 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX)–doxorubicin calculated using CompuSyn (ComboSyn, Inc, Paramus, NJ).
| Concentration | Doxorubicin (µM) |
||||
|---|---|---|---|---|---|
| 3.179 | 6.358 | 9.537 | 12.716 | ||
| TTX (µM) | 1.993 | 0.073* | 0.181† | 0.159† | 0.258† |
| 3.986 | 0.072* | 0.124† | 0.166† | 0.175† | |
| 5.981 | 0.078* | 0.121† | 0.285† | 0.135† | |
| 7.974 | 0.057* | 0.091* | 0.166† | 0.069* | |
Combination index values <0.1 indicate very strong synergism.
Combination index values 0.1 to 0.3 indicate strong synergism.8
Molecular docking of TTX on protein receptors involved in doxorubicin resistance
Based on the synergistic effects of the TTX and doxorubicin combination against Raji cells, it is rational to explore the mechanism(s) underlying the effect of the TTX and doxorubicin combination in increasing cell sensitivity to doxorubicin or decreasing the risk of doxorubicin resistance progression. This was conducted using molecular docking with proteins involved in doxorubicin resistance. The ability of TTX to occupy the active site of these proteins would suggest its ability to decrease resistance to doxorubicin. The results of a molecular docking analysis of native ligand and TTX against some protein receptors involved in doxorubicin resistance are shown in Table 4.
Table 4.
Molecular docking of 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX) against some receptors involved in doxorubicin resistance.
| Protein signaling | Protein Data Bank ID | Root median square deviation | Free energy of binding |
Hydrogen bond |
|||
|---|---|---|---|---|---|---|---|
| Native ligand | TTX | Difference | Native ligand | TTX | |||
| Raf-1 | 3OMV | 1.3823 | −83.22 | −79.37 | 3.85 | Cys424 | Cys424, Lys431, Ser427, Gly426 |
| NIK | 5T8O | 1.6348 | −107.13 | −69.95 | 37.18 | Glu442, Glu472, Leu474, Phe537 | Leu474 (2) |
| c-JNK | 3PZE | 1.2523 | −75.91 | −75.42 | 0.49 | Met111 (2), Glu109 | Met111, Glu109, Ser34 (2) |
| CDK2 | 2UZO | 0.9929 | −80.93 | −68.47 | 12.46 | His84, Asp86, Asp145, Asn132 | Leu83, Asp145, Lys33 |
| Survivin | 2QFA | 1.7134 | −59.85 | −46.89 | 12.96 | Arg18 (3), Phe93, Glu40 | Arg18, Val89 |
| CDK6 | 5L2I | 1.6338 | −107.04 | −71.89 | 35.15 | Glu18, Val101 | Val101, Asp102, Asp104 |
| p38 MAPK | 4TYH | 1.6099 | −92.61 | −72.15 | 20.46 | Lys53, Met109 | Gly33, Lys53, Met109 |
| Btk | 3GEN | 0.9364 | −80.78 | −68.38 | 12.4 | Thr474, Glu475, Met477 | Leu408 |
| Akt | 3MV5 | 0.9403 | −83.18 | −56.66 | 26.52 | Glu434, Ala230 | Leu156 |
| MMP-1 | 966C | 1.0760 | −88.55 | −63.35 | 25.2 | Glu219, Ala182, Asn180, Leu181 | Gly179, His228, His218 |
| Bcl-2 | 4C5D | 1.7437 | −126.44 | −61.43 | 65.01 | – | – |
Protein Data Bank ID were obtained from PDB Database (RCSB, USA).
The boldface types in Raf-1 and c-JNK represented the smallest differences free energy of binding of TTX to that of the native ligands.
The number in parentheses represented the number of hydrogen bonds which were formed by each amino acid.
NIK = nuclear factor kappa B (NFκB)-inducing kinase, c-JNK = c-Jun N- 42 terminal kinase, Btk = Bruton tyrosine kinase, Akt = Ak mouse thymoma, CDK = cyclin-dependent kinases, MAPK = mitogen-activated protein kinase, MMP = matrix metalloproteinase, Bcl = B cell lymphoma.
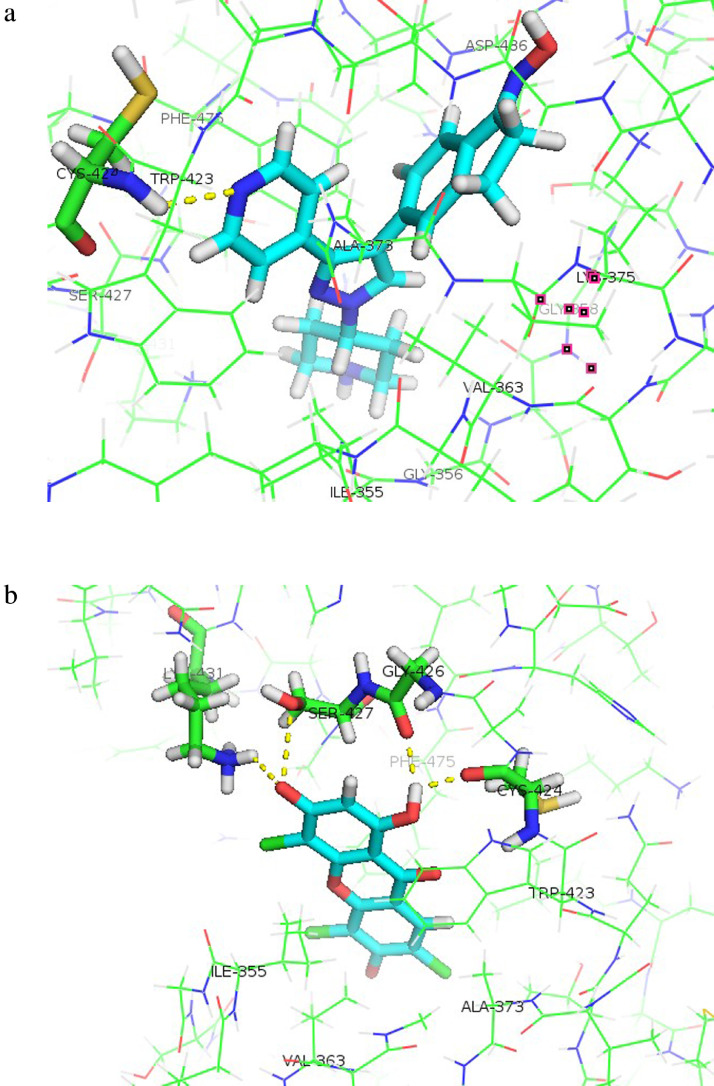
The ligand copies almost coincided with all the protein receptor binding sites, with all the RMSD values being less than Å, and thus, they met the validity criteria for molecular docking. Among the protein receptors investigated, Raf-1 and c-JNK showed minimal free energy of binding and differences compared with that of the native ligand, suggesting these are the most likely targets mediating the mechanism of synergy. The native ligand's free energy of binding to Raf-1 was −83.22 kcal/mol, with 1 hydrogen bond with Cys424 (Figure 6A). TTX occupied the binding site of Raf-1 with a free energy of binding of −79.37 kcal/mol and formed 4 hydrogen bonds with the amino acid residues Cys424, Lys431, Ser427, and Gly426 (Figure 6B). Amino acid residues involved in the hydrogen bonds are shown in Table 5.
Figure 6.
Hydrogen bonds formed between the amino acid residues of Raf-1 (PDB ID: 3OMV) and the ligands. (a) native ligand, (b) TTX. The hydrogen bonds are marked by yellow dashed lines. Orientation of ligands to Raf-1 is displayed with Pymol.
Table 5.
The atom components and amino acid residues involved in the hydrogen bonds formed between Raf-1 and 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX).
| Ligand components* | Amino acid residues of Raf-1 protein |
|---|---|
| H of OH (C-1) | O atom (C=O) of Cys424 |
| H of OH (C-1) | O atom (C=O) of Gly426 |
| O of OH (C-3) | O atom (OH) of Ser427 |
| O of OH (C-3) | H atom (NH) of Lys431 |
C = Carbon atom, H = Hydrogen atom, NH = Amine group, O = Oxygen atom, OH = Hydroxyl group.
C-1 and C-3 show that hydrogen bonds are formed on OH groups of C atoms number 1 and number 3 (the order of C atoms refers to Figure 1).
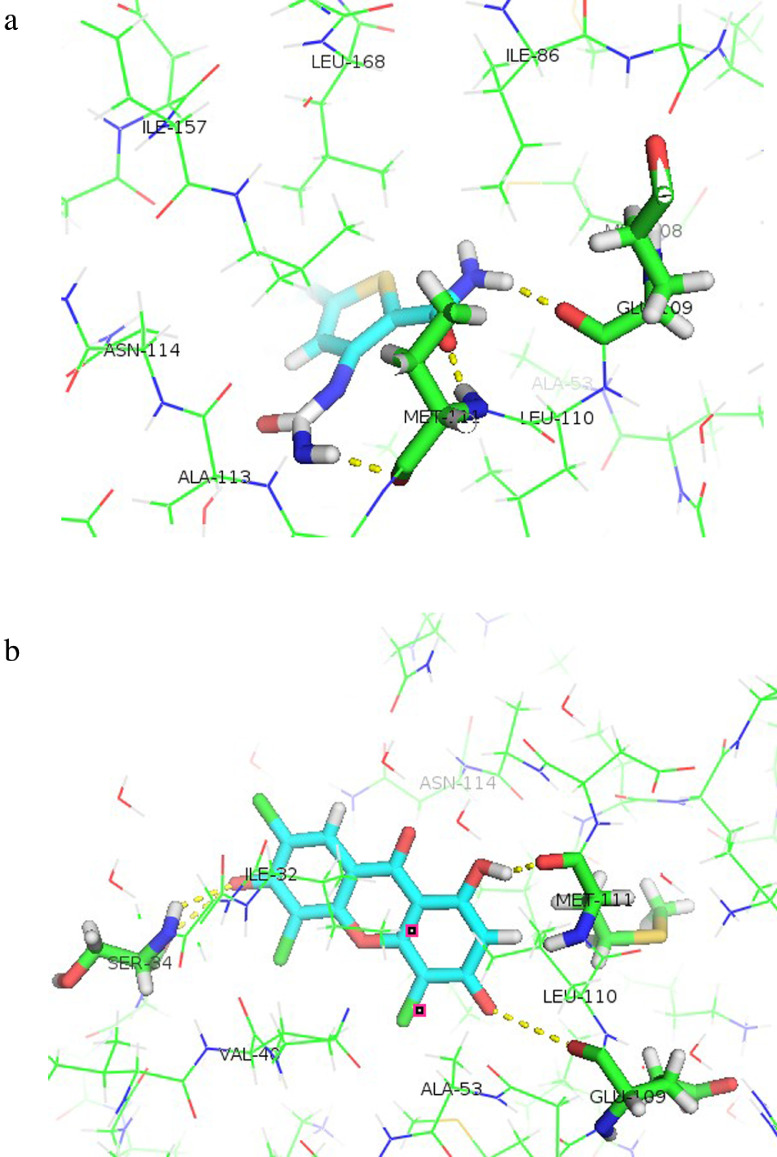
The native ligand's free energy of binding with c-JNK was –75.91 kcal/mol, with 3 hydrogen bonds formed with Met111 (2 bonds) and Glu109 (Figure 7A). The free energy of binding of TTX to the c-JNK receptor was −75.42 kcal/mol, and 4 hydrogen bonds were formed at Met111, Glu109, and Ser34 (2 bonds) (Figure 7B). Amino acid residues involved in the hydrogen bonds are shown in Table 6. The ability of TTX to occupy the active site of Raf-1 or c-JNK suggests the potential mechanism of action of its synergistic effect with doxorubicin.
Figure 7.
Hydrogen bonds formed between the amino acid residues of c-JNK (PDB ID: 3PZE) and the ligands. A. native ligand, B. TTX. The hydrogen bonds are marked by yellow dashed lines. Orientation of ligands to c-JNK is displayed with Pymol.
Table 6.
The atom components and amino acid residues involved in the hydrogen bonds formed between c-JNK protein and 1,3,6-trihydroxy-4,5,7-trichloroxanthone (TTX).
| Ligand components* | Amino acid residues of c-JNK protein |
|---|---|
| H of OH (C-1) | O atom (C=O) of Met111 |
| O of OH (C-3) | O atom (C=O) of Glu109 |
| O of OH (C-6) | H atom (NH) of Ser34 |
| O of OH (C-6) | O atom (C=O) of Ser34 |
C = Carbon atom, H = Hydrogen atom, NH = Amine group, O = Oxygen atom, OH = Hydroxyl group.
C-1, C-3, and C-6 show that hydrogen bonds are formed on OH groups of C atoms number 1, 3, and number 3 (the order of C atoms refers to Figure 1).
Discussion
TTX is a xanthone derivative containing 3 hydroxyl groups and 3 chloro atoms. Xanthone compounds have been reported to show anticancer activity against several cancer cell lines, with IC50 values varying from 1.81 µg/mL (P388 murine leukemia cells) to 91 µg/mL (HepG2 cells).22 Hydroxyl substitutions in the xanthone scaffold are known to increase its cytotoxic activity. Increasing the number of hydroxyl groups does not linearly increase the cytotoxic activity of xanthones, suggesting that the position of the hydroxyl groups may also play a role in their inhibitory effect.20 Modifying the hydroxyxanthone scaffold with halogen groups, especially chloro-substitutions, is predicted to increase cytotoxic activity.21
The present study resulted in an important finding relevant to the potential of xanthone as an anticancer agent, especially in terms of a possible combination with an established anticancer agent. Doxorubicin was used as a positive control drug because it is a chemotherapeutic agent used to treat B-cell lymphoma6 and has a nucleus structure similar to that of xanthone compounds.20 Doxorubicin is widely used as an anticancer agent to treat many cancer types, including B-cell lymphoma. This anticancer effect is due to its intercalation into DNA, leading to the inhibition of DNA synthesis and function. Doxorubicin is also a DNA topoisomerase II inhibitor by forming cleavable complexes with DNA and DNA topoisomerases II5 and generating the formation of reactive oxygen species.32 However, there is a significant concern with respect to resistance and adverse side effects. Combination anticancer therapy may decrease these risks due to nonoverlapping mechanisms of action and lower drug doses.8
The cytotoxicity assay results in the present study show that TTX is a selective, moderately cytotoxic agent against Raji cells. Natural xanthone compounds normally exhibit in vitro anticancer activities through a variety of mechanisms in many cancer cell types, including the induction of apoptosis and cell cycle arrest,33 stimulation of Bax proteins, and inhibition of Bcl-2 and NFκB,34 as well as the inhibition of many cyclin proteins.33 Apoptotic induction and cell cycle arrest by xanthone compounds are associated with an increase in reactive oxygen species levels,35 which subsequently induce caspase activity,36 leading to apoptosis. Increased Bax levels with the inhibition of Bcl-2 and NFκB cause the release of mitochondrial cytochrome c into the cytosol,34 which leads to apoptosis. The induction of cell cycle arrest by natural xanthones may also be stimulated by a decrease in many cyclin proteins33 and an increase in microtubule depolymerization, microtubule cytoskeleton disruption, and the phosphorylation of p38 and c-JNK.37 Further investigations are required to ascertain whether the mechanisms of action of TTX in cancer cell lines are similar to those of natural xanthones.
Previous studies have revealed that the cytotoxic activity of doxorubicin against many cancer cell lines varies among cancer cell types (between 0.190 and 0.892 µg/mL), and all are <6.25 µg/mL (smaller than 11.50 µM).38,39 In the present study, doxorubicin showed weaker cytotoxic activity against Raji cells (6.528 µg/mL or 25.432 µM) compared with those observed in previous studies (<11.5 µM), suggesting that Raji cells were less sensitive to doxorubicin in the present study. The study was continued using TTX–doxorubicin combinations to investigate xanthones’ ability to increase cells’ sensitivity to doxorubicin.
Combinations of TTX and doxorubicin showed that TTX augmented the inhibitory effect of doxorubicin on the growth of Raji cells in vitro. The antiproliferative effect of combination treatment with TTX and doxorubicin depends on their individual concentrations, suggesting a synergistic effect. Synergy is inferred when the use of drug combinations at specific doses produces greater efficacy compared with the sum of the anticancer effects achieved by using the individual drugs at the same dose.40 The combination index values computed using CompuSyn support this finding, in which the combination index values represent strong or very strong synergism (combination index, 0.1−0.3 and < 0.1). CompuSyn was used because it generates higher-quality graphics ready for publication, provides more options and flexibility, and is able to handle data from large-scale drug combination studies.9
Most studies of drug combinations are conducted in vitro because the experimental conditions can be easily defined, fixed, and standardized, and the concentrations can be maintained at relatively constant levels during experiments. It is also quick, accurate, and economical.9 When investigating synergy in drug combination studies, the only prerequisite is the dose–effect curve for each drug alone, comprising the potency and shape of the dose–effect curve of each drug. Both of these parameters can be easily obtained from the median-effect equation using computer software such as CompuSyn.41
The molecular docking study's findings indicate that the synergistic effect of combining TTX and doxorubicin might be due to the ability of TTX to inhibit Raf-1 and c-JNK, the 2 proteins involved in doxorubicin resistance. The orientation of the TTX ligand to Raf-1 and c-JNK proteins indicate that TTX was capable of occupying the active site of Raf-1 and NIK receptors. The orientation of TTX in Pymol reveals that TTX is positioned deep in the binding pocket of Raf-1 and surrounded by the amino acid residues Ile355, Val363, Ala373, Trp423, and Phe475 (Figure 6B). These hydrophobic residues are part of the active site of Raf-1 and play a role in its activity.42 TTX docked at the active site of Raf-1 with minimal free energy of binding (−79.37 kcal/mol), close to that of the native ligand (−83.21 kcal/mol). The interaction between TTX and Raf-1 formed a hydrogen bond similar to that at Cys424. The hydrogen bond at Cys424 residues plays a significant role in the inhibition of Raf-1 activity,43 which suggests TTX has the potential to inhibit Raf-1 activity.
Raf isoform is an enzyme serine/threonine kinase that acts as a transduction signal in a cascade initiated by growth factors or mitogens. Raf isoform activation is associated with the emergence of chemotherapy drug resistance in leukemia, and Raf-1 overexpression decreases B-cell lymphoma cells’ sensitivity to doxorubicin.10 Increased Raf-1 expression also plays a role in the low response of breast cancer cells to doxorubicin chemotherapy, along with increased Akt expression. However, under low Raf-1 expression conditions, the sensitivity of cancer cells to doxorubicin is higher. This indicates the role of Raf-1 in inhibiting the emergence of cancer cell resistance.44 The effectiveness of using a combination of Raf inhibitors with doxorubicin is based on the role of the Raf/MEK/ERK pathway in regulating multidrug-resistance-1 gene promoter activity. The Raf/MEK/ERK pathway also appears to synergize with Bcl-2 in some hematologic malignancies.45
The design and synthesis of pyrimidine derivative molecules as Pan-Raf inhibitors to overcome anticancer resistance has resulted in identifying several effective compounds. Among the structures that increase the effectiveness of compounds as Pan-Raf inhibitors are the addition of halogen elements, especially the chloride (–Cl) group. Adding the –Cl group produces more effective compounds than other halogen groups, such as fluoride (–F).46 This is in accordance with the structure of TTX, which has 3 –Cl groups next to 3 hydroxyl (–OH) groups as sites of substitution. As explained previously, adding the –OH group to the xanthone core increases the inhibitory activity of xanthone compounds on cancer cells. Incorporating 2 –Cl groups into the compound increased the effectiveness of TTX, especially in inhibiting Raf-1.
Amino acid residues of Met111, Glu109, and Ser34 are part of the active ligand side of the c-JNK-1 receptor and play roles receptor, which play roles in c-JNK activation.47 Met111 and Glu109 are also important in binding to 3,6-dihydroxiflavones, which have been shown to be c-JNK inhibitors.48 The TTX–c-JNK receptor complex forms 4 hydrogen bonds, more than the number of hydrogen bonds formed between the native ligand and the c-JNK receptor. These hydrogen bonds potentially explain why the free energy of binding of TTX is close to that of the native ligand.
c-JNK activation is known to play a role in the cytotoxic mechanism of several anticancer agents. Under normal conditions, c-JNK can be activated due to several stress stimuli. Research shows that the combined use of tetrathiomolybdate (a chopper-chelating agent) at subcytotoxic levels with doxorubicin is effective in inhibiting the growth of doxorubicin-resistant endometrial cancer cell lines. Further studies have shown that combining these 2 agents increases cancer cells’ sensitivity to doxorubicin, with 1 route being increasing or activating c-JNK.49 c-JNK is an important determinant in assessing tumor sensitivity to anthracycline anticancer agents, such as doxorubicin. c-JNK activation stimulates Bax translocation, an apoptotic inducer, into mitochondria, which causes apoptosis.50
The different numbers of hydrogen bonds formed in TTX–receptor binding and native ligand-receptor binding could explain the much higher free energy of binding of TTX because the amino acid residues involved in the interaction determine the inhibitory activity of a protein receptor.51 The hydrogen bond is the main interaction contributing to the free energy of binding of a drug-receptor complex. Furthermore, the hydrogen bonds in a ligand-receptor complex and their positions can predict the strength and catalytic activity of the complex.52 A ligand-receptor complex's interactions become more stable as the free energy of binding becomes more negative.
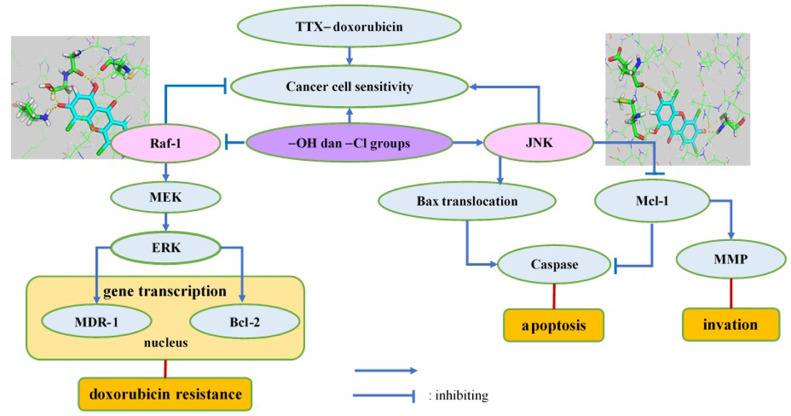
The proposed mechanism of how TTX increases the sensitivity of Raji cancer cells to doxorubicin is shown in Figure 8. Our findings indicate that TTX increases cancer cells’ sensitivity to doxorubicin by inhibiting Raf-1 and activating c-JNK. Raf-1, through its downstream targets, namely MEK and ERK, activates the transcription of multidrug-resistance-1 and Bcl-2 genes, which contribute to the resistance of cancer cells to doxorubicin; therefore, Raf-1 inhibition increases cancer cells’ sensitivity to doxorubicin. c-JNK activation causes the translocation of Bax, a proapoptotic protein, from mitochondria to the cytoplasm and inhibits Mcl-1, an anti-apoptotic protein, thereby stimulating apoptosis. Barriers to Mcl-1 also inhibit matrix metalloproteinase, so the invasion process can be inhibited. Therefore, verifying the docking results required further investigation of downstream proteins.
Figure 8.
The proposed mechanism of how TTX increases the sensitivity of Raji cancer cells toward doxorubicin. Abbreviations; TTX, 1,3,6-trihydroxy-4,5,7-trichloroxanthone; Raf-1, rapidly accelerated fibrosarcoma-1; MEK, mitogen activated protein kinase; ERK, extracellular signal regulated kinase; MDR-1, multidrug resistant gene-1; Bcl-2, B-cell lymphoma-2; c-JNK, Jun-N-terminal kinase; Mcl-1, myeloid cell leukemia-1; MMP, matrix metalloproteinase.
The synergistic effect of combining TTX and doxorubicin might also result from their different mechanisms of action in killing cancer cells (ie, their nonoverlapping mechanisms of action).8 Doxorubicin is known as a topoisomerase II inhibitor and acts as an anticancer agent by inducing cell death through DNA damage. It is also capable of inducing DNA injury and lipid peroxidation through free radical formation. Doxorubicin delays G-actin polymerization and inhibits small actin filament elongation during polymerization.40 Some natural xanthone compounds induce apoptosis and cell cycle arrest through a variety of mechanisms, such as microtubule depolymerization and microtubule cytoskeleton disruption.37 These facts indicate that the mechanisms of action of doxorubicin and xanthone compounds may be interrelated and are not entirely distinctive. The actin filament could be a potential inhibitory target of the combined use of TTX and doxorubicin.
Another possible effect of this synergism is the theory of doxorubicin toxicities, which is associated with the ability of xanthone compounds to inhibit the liver cytochrome (CYP) P450 enzyme. Xanthone-derivative compounds can inhibit the enzyme families CYP2C8, CYP2C9, CYP2B6, CYP2C19, CYP3A4, and CYP2D6.53 The CYP3A4 enzyme family contributes to the largest composition of CYP enzymes, and plays an important role in doxorubicin metabolism.32 The xanthone compounds are known to be CYP3A inhibitors, inhibiting doxorubicin metabolism and, consequently, increasing the concentration of doxorubicin in plasma. Increasing the doxorubicin concentration is believed to play a role in doxorubicin's cytotoxic activity against Raji cells, suggesting the synergistic effect of TTX and doxorubicin.
Altogether, these results indicate TTX and doxorubicin have a synergistic effect on Raji cells, which is likely induced by the inhibitory effect of TTX on Raf-1, stimulating sensitivity to doxorubicin, which is, presumably, also due to the nonoverlapping mechanisms of action of TTX and doxorubicin. The potential use of a combination of TTX and doxorubicin may enhance anthracycline-based therapy by overcoming anthracycline drug resistance and reducing their toxic side effects. The weakness of this study was the authors’ inability to mask observers, but this matter was minimized by using an objective measuring tool (ie, an ELISA reader).
Future Research
The present study demonstrated the synergistic effects of TTX and doxorubicin on Raji cells with unknown mutant forms of the target proteins. However, the status of doxorubicin resistance in these cells was unknown. It would be very interesting to evaluate the cytotoxic effects of this combination on Raji cells with mutant target proteins or a demonstrated resistance to doxorubicin. Furthermore, it would be of interest to also test combinations of these drugs at concentrations below one-eighth IC50, which was the lowest concentration tested in the present study.
Conclusions
Combining TTX and doxorubicin was found to enhance the antiproliferative effect of doxorubicin, a standard chemotherapy drug used to treat lymphoid malignancies. The combination index values at the concentrations used ranged from 0.057 to 0.258, suggesting the TTX and doxorubicin combination had strong and very strong synergistic effects. These synergistic effects were seen at much lower doses than the IC50 of doxorubicin used in monotherapy. The synergistic effects might be due to the inhibition of Raf-1 and c-JNK, as indicated by the molecular docking results. The free energy of binding and hydrogen bonding interactions between Raf-1 and c-JNK indicate that TTX is docked and positioned deeply in the binding sites of the above proteins. In short, combined treatment with TTX and doxorubicin is stronger than the respective single-agent treatments against B-cell lymphoma (Raji cell line), indicating this combination is a potentially promising antilymphoma treatment suitable for further development.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Author Contributions
IM is participated in conception and design, analysis and interpretation of the data, and drafting the article; EY is participated in drafting and revising it critically for important intellectual content; SN is participated in analysis and interpretation of the data; JJ, SMH, and MM are participated in revising it critically for important intellectual content and approval of the final version.
Acknowledgments
This research was supported financially by the Ministry of Research, Technology, and Higher Education's PhD scholarship program through Beasiswa Pendidikan Pascasarjana Dalam Negeri.
The authors thank Ms. Rumbiwati for providing helpful laboratory assistance and Mr. Hari Purnomo (Organic Pharmacy Department, Faculty of Pharmacy, Universitas Gadjah Mada) for supporting the molecular docking process.
References
- 1.Roman E., Smith A.G. Epidemiology of lymphomas. Histopathology. 2011;58:4–14. doi: 10.1111/j.1365-2559.2010.03696.x. [DOI] [PubMed] [Google Scholar]
- 2.Teras L.R., DeSantis C.E., Cerhan J.R., Morton L.M., Jemal A., Flowers C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi: 10.3322/caac.21357. [Internet] [DOI] [PubMed] [Google Scholar]
- 3.Clarke C.A., Glaser S.L., Gomez S.L., Wang S.S., Keegan T.H., Yang J. Lymphoid malignancies in U.S. Asians: Incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev. 2011;20:1064–1077. doi: 10.1158/1055-9965.EPI-11-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zha J., Chen F., Dong H., Shi P., Yao Y., Zhang Y. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J Transl Med. 2014;12:163. doi: 10.1186/1479-5876-12-163. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakharde A., Awad A., Bhagat A., Karuppayii S. Synergistic Activation of Doxorubicin against Cancer: A Review OPEN ACCESS. Am J Clin Mic Antimicrob. 2018;1(2):1–6. [Google Scholar]
- 6.Maxwell S.A., Li Z., Jaya D., Ballard S., Ferrell J., Fu H. 14-3-3ζ mediates resistance of diffuse large B cell lymphoma to an anthracycline-based chemotherapeutic regimen. J Biol Chem. 2009;284(33):22379–22389. doi: 10.1074/jbc.M109.022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto A.C., Moreira J.N., Simões S. 31) Combination Chemotherapy in Cancer : Principles, Evaluation and Drug Delivery Strategies. Curr Cancer Treat. 2010:695–714. [Google Scholar]
- 8.Kashif M., Andersson C., Hassan S., Karlsson H., Senkowski W., Fryknäs M. In vitro discovery of promising anti-cancer drug combinations using iterative maximisation of a therapeutic index. Sci Rep. 2015;5(August):14118. doi: 10.1038/srep14118. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou T. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [Internet] [DOI] [PubMed] [Google Scholar]
- 10.Davis J.M., Navolanic P.M., Weinstein-oppenheimer C.R., Steelman L.S., Hu W., Konopleva M. Raf-1 and Bcl-2 Induce Distinct and Common Pathways That Contribute to Breast Cancer Drug Resistance 1. Clin Cancer Res. 2003;9(March):1161–1170. [PubMed] [Google Scholar]
- 11.Katayama K., Noguchi K., Sugimoto Y. Regulations of P-Glycoprotein/ABSB1/MDR1 in Human Cancer Cells. New J Sci. 2014;2014 [Google Scholar]
- 12.Yeung K.C., Rose D.W., Dhillon A.S., Yaros D., Gustafsson M., Chatterjee D. Raf kinase inhibitor protein interacts with NFKB-inducing kinase and TAK1 and inhibits NF-K B activation. Mol Cell Biol. 2001;21(21):7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim K.H., Yang Y., Staudt L.M. Pathogenetic Importance and Therapeutic Implications of NF-KB in Lymphoid Malignancies. Immunol Rev. 2014;246(1):359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyner J.W., Jemal A.M., Thayer M., Druker B.J., Chang B.H. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia. 2012;26:623–632. doi: 10.1038/leu.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeder C.B., Ansell S.M. Novel therapeutic agents for B-cell lymphoma: Developing rational combinations. Blood. 2011;117(5):1453–1462. doi: 10.1182/blood-2010-06-255067. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Zheng W., Wang T., Wang P., Zhu L., Ma X. Genetic protein TmSm (T34A) enhances sensitivity of chemotherapy to breast cancer cell lines as a synergistic drug to doxorubicin. Biomed Pharmacother. 2012;66(5):368–372. doi: 10.1016/j.biopha.2011.12.004. [Internet] [DOI] [PubMed] [Google Scholar]
- 17.Roskoski R.J. Ibrutinib inhibition of Bruton protein-tyrosine kinase (BTK) in the treatment of B cell neoplasms. Pharmacol Res. 2016;113:395–408. doi: 10.1016/j.phrs.2016.09.011. [Internet] [DOI] [PubMed] [Google Scholar]
- 18.Taylor J.R., Lehmann B.D., Chappell W.H., Abrams S.L., Steelman L.S., McCubrey J.A. Cooperative Effects of Akt-1 and Raf-1 on the Induction of Cellular Senescence in Doxorubicin or Tamoxifen Treated Breast Cancer Cells. Oncotarget. 2015;2(8):610–626. doi: 10.18632/oncotarget.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naci D., El Azreq M.A., Chetoui N., Lauden L., Sigaux F., Charron D. α2β1 integrin promotes chemoresistance against doxorubicin in cancer cells through extracellular signal-regulated kinase (ERK) J Biol Chem. 2012;287(21):17065–17076. doi: 10.1074/jbc.M112.349365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Q., Liu Y., Cai Y., Sun Y., Wang B., Xian L. Anti-tumour effects of xanthone derivatives and the possible mechanisms of action. Invest New Drugs. 2011;29:1230–1240. doi: 10.1007/s10637-010-9468-5. [DOI] [PubMed] [Google Scholar]
- 21.Yuanita E., Pranowo H.D., Jumina J., Mustofa M. Design of Hydroxy Xanthones Derivatives As Anticancer Using Quantitative Structure-Activity Relationship. Asian J Pharm Clin Res. 2016;9(2):3–8. [Google Scholar]
- 22.Yuanita E., Pranowo H.D., Mustofa M., Swasono R.T., Syahri J., Jumina J. Synthesis, Characterization and Molecular Docking of Chloro-substituted Hydroxyxanthone Derivatives. Chem J Mold. 2019;14(1):68–76. [Google Scholar]
- 23.Singh M., Mckenzie K., Ma X. Effect of dimethyl sulfoxide on in vitro proliferation of skin fibroblast cells. J Biotech Res. 2017;8:78–82. [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Prayong P., Barusrux S., Weerapreeyakul N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia. 2008;79:598–601. doi: 10.1016/j.fitote.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N., Fu J.N., Chou T.C. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: Experimental design and data analysis using the combination index method. Am J Cancer Res. 2016;6(1):97–104. [PMC free article] [PubMed] [Google Scholar]
- 27.Miladiyah I., Jumina J., Haryana S.M., Mustofa M. Biological activity, quantitative structure–activity relationship analysis, and molecular docking of xanthone derivatives as anticancer drugs. Drug Des Dev Ther. 2018;12:149–158. doi: 10.2147/DDDT.S149973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korb O., Stutzle T., Exner T.E. Empirical scoring functions for advanced Protein-Ligand docking with PLANTS. J Chem Inf Model. 2009;49:84–96. doi: 10.1021/ci800298z. [DOI] [PubMed] [Google Scholar]
- 29.Huang S., Zou X. Efficient molecular docking of NMR structures : Application to HIV-1 protease. Protein Sci. 2007;16:43–51. doi: 10.1110/ps.062501507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLano W.L. Review: The case for open-source software in drug discovery. Drug Discov Today. 2005;10(3):213–217. doi: 10.1016/S1359-6446(04)03363-X. [Internet] [DOI] [PubMed] [Google Scholar]
- 31.Fouche G., Cragg G.M., Pillay P., Kolesnikova N., Maharaj V.J., Senabe J. In vitro anticancer screening of South African plants. J Ethnopharm. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Miles J.S., Sojourner S.J., Whitmore A.M., Freeny D., Darling-Reed S., Flores-Rozas H. Synergistic Effect of Endogenous and Exogenous Aldehydes on Doxorubicin Toxicity in Yeast. Biomed Res Int. 2018;2018 doi: 10.1155/2018/4938189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuete V., Sandjo L.P., Nantchouang J.L., Fouotsa H., Wiench B., Efferth T. Cytotoxicity and modes of action of three naturally occurring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomed. 2014;21:315–322. doi: 10.1016/j.phymed.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Mohan S., Ibrahim S., Kamalidehghan B., Syam S., Sue K., Saad N. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomed. 2012;19:1007–1015. doi: 10.1016/j.phymed.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Cheng J.H., Huang A.-M., Hour T.C., Yang S.C., Pu Y.S., Lin C.N. Antioxidant xanthone derivatives induce cell cycle arrest and apoptosis and enhance cell death induced by cisplatin in NTUB1 cells associated with ROS. Eur J Med Chem. 2011;46:1222–1231. doi: 10.1016/j.ejmech.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 36.Liu L.L., He L.S., Xu Y., Han Z., Li Y.X., Zhong J.L. Caspase-3-dependent apoptosis of citreamicin ε-induced heLa iells Is associated with reactive oxygen species generation. Chem Res Toxicol. 2013;26:1055–1063. doi: 10.1021/tx4000304. [DOI] [PubMed] [Google Scholar]
- 37.Jia B., Li S., Hu X., Zhu G., Chen W. Recent Research on Bioactive Xanthones from Natural Medicine: Garcinia hanburyi. AAPS PharmSciTech. 2015;16(4):742–758. doi: 10.1208/s12249-015-0339-4. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Salahi R., Alswaidan I., Marzouk M. Cytotoxicity Evaluation of a New Set of 2-Aminobenzo[de]iso-quinoline-1,3-diones. Int J Mol Sci. 2014;15:22483–22491. doi: 10.3390/ijms151222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghanbari M., Asadi A. Study of the Cytotoxicity Effect of Doxorubicin-loaded/Folic acid-Targeted Super Paramagnetic Iron Oxide Nanoparticles on AGS Cancer Cell Line. J Nanomed Nanotechnol. 2016;7(2) [Internet] [Google Scholar]
- 40.Tsakalozou E., Eckman A.M., Bae Y. Combination effects of docetaxel and doxorubicin in hormone-refractory prostate cancer cells. Biochem Res Intern. 2012;2012 doi: 10.1155/2012/832059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou T.-C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 42.McDonald O., Lackey K., Davis-Ward R., Wood E., Samano V., Maloney P. Aza-stilbenes as potent and selective c-RAF inhibitors. Bioorg Med Chem Lett. 2006;16(April 2015):5378–5383. doi: 10.1016/j.bmcl.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 43.Thaimattam R., Daga P., Rajjak S.A., Banerjee R., Iqbal J. 3D-QSAR CoMFA, CoMSIA studies on substituted ureas as Raf-1 kinase inhibitors and its confirmation with structure-based studies. Bioorg Med Chem. 2004;12(January 2005):6415–6425. doi: 10.1016/j.bmc.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein-oppenheimer C.R., Henríquez-roldán C.F., Davis J.M., Henrı C.F., Navolanic P.M., Saleh O.A. Role of the Raf Signal Transduction Cascade in the in Vitro Resistance to the Anticancer Drug Doxorubicin Role of the Raf Signal Transduction Cascade in the in Vitro Resistance to the Anticancer Drug Doxorubicin 1. Clin Cancer Res. 2001;7(September):2898–2907. [PubMed] [Google Scholar]
- 45.Richly H., Henning B.F., Kupsch P., Passarge K., Grubert M., Hilger R.A. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol. 2006;17(February 2006):866–873. doi: 10.1093/annonc/mdl017. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Zhang Q., Zhu G., Zhang Z., Zhi Y., Zhang L. Design, synthesis and evaluation of derivatives based on pyrimidine scaffold as potent Pan-Raf inhibitors to overcome resistance. Eur J Med Chem. 2017;130:86–106. doi: 10.1016/j.ejmech.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 47.Schepetkin I.A., Kirpotina L.N., Hammaker D., Kochetkova I., Khlebnikov A.I., Lyakhov S.A. Anti-Inflammatory Effects and Joint Protection in Collagen-Induced Arthritis after Treatment with IQ-1S, a Selective c-Jun N-Terminal Kinase Inhibitor. J Pharmacol Exp Ther. 2015;353(June):505–516. doi: 10.1124/jpet.114.220251. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee E., Jeong K.-W., Jnawali H.N., Shin A., Heo Y.-S., Kim Y. Cytotoxic activity of 3,6-dihydroxyflavone in human cervical cancer cells and its therapeutic effect on c-Jun N-terminal kinase inhibition. Molecules. 2014;19:13200–13211. doi: 10.3390/molecules190913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim K.K., Kawar N.M., Singh R.K., Lange T.S., Brard L., Moore R.G. Tetrathiomolybdate induces doxorubicin sensitivity in resistant tumor cell lines. Gynecol Oncol. 2011;122:183–189. doi: 10.1016/j.ygyno.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 50.Ashenden M., van Weverwijk A., Murugaesu N., Fearns A., Campbell J., Gao Q. An In Vivo Functional Screen Identifies JNK Signaling As a Modulator of Chemotherapeutic Response in Breast Cancer. Mol Cancer Ther. 2017;16:1967–1978. doi: 10.1158/1535-7163.MCT-16-0731. [Internet] [DOI] [PubMed] [Google Scholar]
- 51.Kurumbail W., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y., Gildehaus D., Miyashiro J., Penning T.D., Seibert K., Isakson P.C. WCS. “Structural basis for selective inhibition of cyclooxigenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):400–402. doi: 10.1038/384644a0. (December) [DOI] [PubMed] [Google Scholar]
- 52.Meenambiga S.S., Rajagopal K., Durga R. In silico docking studies on the components of inonotus sp., a medicinal mushroom against cyclooxygenase-2 enzyme. Asian J Pharm Clin Res Res. 2015;8(3):142–145. [Google Scholar]
- 53.Foti R.S., Pearson J.T., Rock D.A., Wahlstrom J.L., Wienkers L.C. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab Dispos. 2009;37(9):1848–1855. doi: 10.1124/dmd.109.028043. [DOI] [PubMed] [Google Scholar]