Graphical Abstract
Triphenylphosphonium-modified paclitaxel nanocrystals were prepared for mitochondrial targeting strategy. Cellular uptake of NCs was through endocytosis pathway for avoiding efflux of P-glycoprotein (P-gp). After escaping from endosome, targeting NCs were apt to deliver PTX to mitochondria causing the decrease of mitochondrial membrane potential and then induced cancer cell apoptosis.
Keywords: Monoacyl phosphatidylcholine, Self-nanoemulsifying drug delivery systems, D-optimal design, Polyoxyl 40 hydrogenated castor oil (Kolliphor® RH40), Droplet size, Cryogenic transmission electron microscopy
Abstract
The development of self-nanoemulsifying drug delivery systems (SNEDDS) to enhance the oral bioavailability of lipophilic drugs is usually based on traditional one-factor-at-a-time approaches. These approaches may be inadequate to analyse the effect of each excipient and their potential interactions on the emulsion droplet size formed when dispersing the SNEDDS in an aqueous environment. The current study investigates the emulsion droplet sizes formed from SNEDDS containing different levels of the natural surfactant monoacyl phosphatidylcholine to reduce the concentration of the synthetic surfactant polyoxyl 40 hydrogenated castor oil (Kolliphor® RH40). Monoacyl phosphatidylcholine was used in the form of Lipoid S LPC 80 (LPC, containing approximately 80% monoacyl phosphatidylcholine, 13% phosphatidylcholine and 4% concomitant components). The investigated SNEDDS comprised of long-chain or medium-chain glycerides (40% to 75%), Kolliphor® RH40 (5% to 55%), LPC (0 to 40%) and ethanol (0 to 10%). D-optimal design, multiple linear regression, and partial least square regression were used to screen different SNEDDS within the investigated excipient ranges and to analyse the effect of each excipient on the resulting droplet size of the dispersed SNEDDS measured by dynamic light scattering. All investigated formulations formed nano-emulsions with droplet sizes from about 20 to 200 nm. The use of medium-chain glycerides was more likely to result in smaller and more monodisperse droplet sizes compared to the use of long-chain glycerides. Kolliphor® RH40 exhibited the most significant effect on reducing the emulsion droplet sizes. Increasing LPC concentration increased the emulsion droplet sizes, possibly because of the reduction of Kolliphor® RH40 concentration. A higher concentration of ethanol resulted in an insignificant reduction of the emulsion droplet size. The study provides different ternary diagrams of SNEDDS containing LPC and Kolliphor® RH40 as a reference for formulation developers.
1. Introduction
The use of modern drug discovery programs has increased the number of new active pharmaceutical ingredients (API) with high lipophilicity and poor oral absorption [1]. Lipid-based drug delivery systems (LbDDS) have been used as one of the most effective strategies to enhance the oral bioavailability of these API [2]. The main rationale behind the utility of LbDDS is that they usually present the drug in solution, thus bypassing the dissolution step prior to absorption. At the same time the lipid excipients may enhance drug solubilisation and intestinal permeability and stimulate lymphatic transport in the intestine [2], [3], [4]. Among the large variety of LbDDS, the self-nanoemulsifying drug delivery systems (SNEDDS) are frequently used for oral delivery of lipophilic drugs. SNEDDS are mixture of glycerides, surfactants and co-solvent and spontaneously form nanoemulsions upon dispersion in aqueous media. Due to the small droplet size of the dispersed SNEDDS, lipid digestion and drug release from SNEDDS are less affected by inter- and intra-personal variations, including food effect [5].
The droplet size of the emulsion formed upon SNEDDS dispersion in aqueous environment depends on the type and amount of surfactants and co-solvent used. Monoacyl phosphatidylcholine (Lipoid S LPC 80 (LPC)) has been recently used as a natural lipophilic surfactant to significantly reduce the emulsion droplet size of SNEDDS containing medium-chain (MC) glycerides, caprylocaproyl polyoxyl-8 glycerides (Labrasol®), and ethanol [6]. However, using long-chain (LC) glycerides instead of MC glycerides in these formulations resulted in polydisperse emulsions with droplet sizes in the micrometer range upon dispersion in a simulated intestinal medium [6]. In an effort to formulate SNEDDS containing both LC glycerides and LPC, other hydrophilic surfactants need to be considered to replace Labrasol®. Kolliphor® surfactants, in particular polyoxyl 40 hydrogenated castor oil (Kolliphor® RH40 (KOL)), have been used in SNEDDS containing LC glycerides to obtain nanoemulsions. Formulations containing sesame oil, glyceryl monooleate (Peceol™), and KOL (at a ratio of 25:27:48) or soybean oil, glyceryl monolinoleate (Maisine™ 35-1), KOL, and ethanol (at a ratio of 25:25:40:10) formed emulsion droplets of 30 ± 1 and 41 ± 1 nm, respectively, upon dispersion in water (at a ratio of 1:250) [7], [8]. Besides their emulsification capacity, Kolliphor® surfactants are permeation enhancers with P-glycoprotein inhibition activity [3] making these excipients an important surfactant family to investigate. Therefore, KOL may be a good candidate to combine with both LC and MC glycerides and LPC to formulate SNEDDS.
Many SNEDDS have been developed with consideration on the resulting emulsion droplet sizes after dispersion using traditional one-factor-at-a-time approaches to construct ternary diagrams [8], [9], [10]. This approach often provides inadequate data to analyse the effect of each excipient and their potential interactions on the formulation performance. In addition, it does not always allow prediction outside or even within the investigated ranges of excipients. Experimental design has been recently applied in formulation development [11]. With this approach, an optimal amount of information can be obtained from a limited number of experiments [11], [12], [13]. Moreover, using an experimental design approach during initial screening can provide more insight on excipient effects and interactions on the selected response variables [14], [15]. Therefore, the objective of this study is to investigate the emulsion droplet size of different SNEDDS containing LPC, KOL and ethanol, using an experimental design approach with focus on maximising LPC level and minimising KOL level whilst maintaining a small droplet size of the dispersed systems.
2. Materials and methods
2.1. Materials
Lipoid S LPC 80 (LPC) (containing 80.8% soybean monoacyl phosphatidylcholine (MAPC) and 13.2% phosphatidylcholine (PC)) and Lipoid S PC (containing 98.0% pure soybean PC) were provided by Lipoid GmbH (Ludwigshafen am Rhein, Germany). Sodium taurodeoxycholate hydrate (NaTDC) (>95% pure), 2-(N-morpholino)ethanesulfonic acid (MES) hydrate (>99.5% pure), MES sodium salt (>99% pure), Trizma® maleate, and soybean oil were purchased from Sigma-Aldrich (St Louis, MO, USA). Glyceryl monolinoleate (Maisine™ 35-1 (Maisine)) was a gift from Gattefossé (Saint-Priest, France). Polyoxyl 40 hydrogenated castor oil (KOL) was a gift from BASF (Ludwigshafen, Germany). Glyceryl tricaprylate/tricaprate (Captex 300 (Captex)) and glyceryl monocaprylate (Capmul MCM EP (Capmul)) were provided by Abitec (Columbus, OH, USA). Absolute ethanol (99.9%) and sodium chloride were obtained from VWR (Radnor, PA, USA). Water was purified using a SG Ultraclear water system (SG Water GmbH, Barsbüttel, Germany).
2.2. Methods
2.1.1. Design of experiments
Two formulation sets containing either LC glycerides (soybean oil: Maisine (1:1 w/w) or MC glycerides (Capmul: Captex (1:1 w/w)), LPC, KOL, and ethanol were studied. Experimental design was used to screen the effect of each excipient on the emulsion droplet size of the dispersed formulation. The concentration ranges of excipients are variable, therefore a D-optimal design was selected instead of a classical mixture design [11]. Details of independent variables and their levels in the investigated formulations are shown in Table 1.
Table 1.
Variables and levels used in the four D-optimal designs (DoE I – DoE IV).
| Formulation variables | Levels |
|||||||
|---|---|---|---|---|---|---|---|---|
| DoE I |
DoE II |
DoE III |
DoE IV |
|||||
| Low | High | Low | High | Low | High | Low | High | High |
| X1: Glyceride fraction | 0.40 | 0.40 | 0.40 | 0.40 | 0.60 | 0.60 | 0.75 | 0.75 |
| X2: KOL fraction | 0.30 | 0.55 | 0.15 | 0.30 | 0.05 | 0.25 | 0.05 | 0.25 |
| X3: LPC fraction | 0 | 0.25 | 0.25 | 0.40 | 0.05 | 0.25 | 0 | 0.2 |
| X4: Ethanol fraction | 0 | 0.10 | 0 | 0.10 | 0 | 0.10 | 0 | 0.10 |
A D-optimal design with 13 experiments, including 3 centre points, was generated for the two formulation sets containing 40% glycerides, 30%–55% KOL, 0–25% LPC, and 0–10% ethanol (design of experiment (DoE) I) (Table 1) by MODDE 11.0.2 software (Umetrics, Sweden). The LPC level was limited to 25% when combining LPC with LC glycerides and 40% when combining LPC with MC glycerides. These maximum levels were fixed because of the high viscosity of LPC-containing LbDDS [6] and the limited capacity of LPC to homogenously disperse in the lipid matrix. Table 2 shows the compositions of the DoE I formulations. The resulting emulsion droplet sizes of these formulations were evaluated by dispersing them in a medium simulating human fasted-state intestinal fluid (FastedM) [16] and measuring the droplet sizes of the formed emulsions using a dynamic light scattering technique (described below). Based on the feasibility of LPC incorporation and the resulting emulsion droplet sizes of DoE I formulations, further investigations with higher LPC and glyceride concentrations were then performed with DoE II (containing 25%–40% LPC), DoE III (containing 60% glycerides), and DoE IV (containing 75% glycerides). The objectives were to maximize LPC concentration and investigate the capacity of LPC and KOL to efficiently emulsify high glyceride levels. The levels of the formulation variables in DoE II, III and IV are shown in Table 1. Table 4, Table 5, Table 6 show the compositions of formulations in DoE II, III, and IV, respectively.
Table 2.
Composition and droplet size and PdI value of resulting emulsions of the LC formulation set prepared based on DoE I. The sizes and PdI values are presented as mean ± SD (n = 3).
| Formulation | Composition |
Appearance | Z-average (nm) | PdI | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||||
| LC1 | 0.40 | 0.55 | 0.05 | – | + | 26 ± 0 | 0.07 ± 0.01 |
| LC2 | 0.40 | 0.35 | 0.25 | – | +++ | 100 ± 0 | 0.37 ± 0.03 |
| LC3 | 0.40 | 0.50 | – | 0.10 | + | 34 ± 1 | 0.05 ± 0.01 |
| LC4 | 0.40 | 0.30 | 0.20 | 0.10 | ++ | 43 ± 1 | 0.24 ± 0.00 |
| LC5 | 0.40 | 0.533 | – | 0.067 | + | 28 ± 1 | 0.05 ± 0.02 |
| LC6 | 0.40 | 0.417 | 0.183 | – | +++ | 90 ± 5 | 0.38 ± 0.04 |
| LC7 | 0.40 | 0.30 | 0.217 | 0.083 | + | 59 ± 1 | 0.23 ± 0.00 |
| LC8 | 0.40 | 0.55 | 0.017 | 0.033 | ++ | 28 ± 1 | 0.05 ± 0.02 |
| LC9 | 0.40 | 0.317 | 0.25 | 0.033 | ++ | 75 ± 1 | 0.25 ± 0.01 |
| LC10 | 0.40 | 0.433 | 0.067 | 0.10 | + | 27 ± 1 | 0.07 ± 0.01 |
| LC11 | 0.40 | 0.425 | 0.125 | 0.05 | ++ | 46 ± 1 | 0.22 ± 0.00 |
| LC12 | 0.40 | 0.425 | 0.125 | 0.05 | ++ | 41 ± 0 | 0.23 ± 0.00 |
| LC13 | 0.40 | 0.425 | 0.125 | 0.05 | ++ | 42 ± 1 | 0.22 ± 0.00 |
X1: glyceride fraction, X2: KOL fraction, X3: LPC fraction, X4: ethanol fraction.
+: transparent; ++: bluish; +++: turbid.
Table 4.
Composition and droplet size and PdI value of resulting emulsions of the MC formulation set prepared based on DoE I (MC1 to MC13) and DoE II (MC14 to MC22). The sizes and PdI values are presented as mean ± SD (n = 3). Absence of an SD value for z-average signifies SD < 0.5.
| Formulation | Composition |
Appearance | Z-average (nm) | PdI | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||||
| MC1 | 0.40 | 0.55 | 0.05 | – | + | 18 | 0.07 ± 0.01 |
| MC2 | 0.40 | 0.35 | 0.25 | – | + | 19 | 0.09 ± 0.01 |
| MC3 | 0.40 | 0.50 | – | 0.10 | + | 21 | 0.08 ± 0.00 |
| MC4 | 0.40 | 0.30 | 0.20 | 0.10 | + | 18 | 0.11 ± 0.01 |
| MC5 | 0.40 | 0.533 | – | 0.067 | + | 20 | 0.05 ± 0.01 |
| MC6 | 0.40 | 0.417 | 0.183 | – | + | 19 | 0.09 ± 0.00 |
| MC7 | 0.40 | 0.30 | 0.217 | 0.083 | + | 18 | 0.11 ± 0.01 |
| MC8 | 0.40 | 0.55 | 0.017 | 0.033 | + | 20 | 0.10 ± 0.05 |
| MC9 | 0.40 | 0.317 | 0.25 | 0.033 | + | 21 | 0.21 ± 0.01 |
| MC10 | 0.40 | 0.433 | 0.067 | 0.10 | + | 18 | 0.06 ± 0.01 |
| MC11 | 0.40 | 0.425 | 0.125 | 0.05 | + | 17 | 0.08 ± 0.02 |
| MC12 | 0.40 | 0.425 | 0.125 | 0.05 | + | 17 | 0.09 ± 0.01 |
| MC13 | 0.40 | 0.425 | 0.125 | 0.05 | + | 17 | 0.17 ± 0.02 |
| MC14 | 0.40 | 0.30 | 0.30 | – | + | 23 | 0.10 ± 0.01 |
| MC15 | 0.40 | 0.30 | 0.25 | 0.05 | + | 19 | 0.13 ± 0.00 |
| MC16 | 0.40 | 0.20 | 0.40 | – | + | 32 | 0.11 ± 0.01 |
| MC17 | 0.40 | 0.15 | 0.40 | 0.05 | ++ | 40 | 0.25 ± 0.01 |
| MC18 | 0.40 | 0.25 | 0.20 | 0.10 | + | 19 | 0.13 ± 0.01 |
| MC19 | 0.40 | 0.15 | 0.35 | 0.10 | + | 26 | 0.20 ± 0.00 |
| MC20 | 0.40 | 0.225 | 0.325 | 0.05 | + | 23 | 0.17 ± 0.00 |
| MC21 | 0.40 | 0.225 | 0.325 | 0.05 | + | 23 | 0.17 ± 0.00 |
| MC22 | 0.40 | 0.225 | 0.325 | 0.05 | + | 23 | 0.17 ± 0.00 |
X1: glyceride fraction, X2: KOL fraction, X3: LPC fraction, X4: ethanol fraction.
+: transparent; ++: bluish; +++: turbid.
Table 5.
Composition and droplet size and PdI value of resulting emulsions of the MC formulation set prepared based on DoE III. The sizes and PdI values are presented as mean ± SD (n = 3).
| Formulation | Composition |
Appearance | Z-average (nm) | PdI | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||||
| MC23 | 0.60 | 0.25 | 0.15 | – | + | 23 ± 1 | 0.06 ± 0.01 |
| MC24 | 0.60 | 0.25 | 0.05 | 0.10 | + | 27 ± 1 | 0.05 ± 0.01 |
| MC25 | 0.60 | 0.15 | 0.25 | – | ++ | 70 ± 2 | 0.44 ± 0.00 |
| MC26 | 0.60 | 0.05 | 0.25 | 0.10 | +++ | 169 ± 5 | 0.32 ± 0.02 |
| MC27 | 0.60 | 0.25 | 0.10 | 0.05 | + | 23 ± 1 | 0.04 ± 0.01 |
| MC28 | 0.60 | 0.10 | 0.25 | 0.05 | ++ | 53 ± 1 | 0.25 ± 0.00 |
| MC29 | 0.60 | 0.15 | 0.15 | 0.10 | + | 27 ± 1 | 0.09 ± 0.01 |
| MC30 | 0.60 | 0.175 | 0.175 | 0.05 | + | 26 ± 1 | 0.09 ± 0.01 |
| MC31 | 0.60 | 0.175 | 0.175 | 0.05 | + | 26 ± 1 | 0.11 ± 0.01 |
| MC32 | 0.60 | 0.175 | 0.175 | 0.05 | + | 25 ± 0 | 0.11 ± 0.01 |
X1: glyceride fraction, X2: KOL fraction, X3: LPC fraction, X4: ethanol fraction.
+: transparent; ++: bluish; +++: turbid.
Table 6.
Composition and droplet size and PdI value of resulting emulsions of the MC formulation set prepared based on DoE IV. The sizes and PdI values are presented as mean ± SD (n = 3).
| Formulation | Composition |
Appearance | Z-average (nm) | PdI | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||||
| MC33 | 0.75 | 0.25 | – | – | + | 36 ± 0 | 0.05 ± 0.00 |
| MC34 | 0.75 | 0.05 | 0.2 | – | +++ | 175 ± 1 | 0.19 ± 0.01 |
| MC35 | 0.75 | 0.15 | – | 0.1 | ++ | 57 ± 0 | 0.16 ± 0.01 |
| MC36 | 0.75 | 0.05 | 0.15 | 0.05 | +++ | 187 ± 2 | 0.18 ± 0.01 |
| MC37 | 0.75 | 0.2 | – | 0.05 | ++ | 46 ± 0 | 0.13 ± 0.01 |
| MC38 | 0.75 | 0.125 | 0.125 | – | ++ | 51 ± 1 | 0.16 ± 0.00 |
| MC39 | 0.75 | 0.1 | 0.1 | 0.05 | ++ | 58 ± 1 | 0.09 ± 0.01 |
| MC40 | 0.75 | 0.1 | 0.1 | 0.05 | ++ | 56 ± 1 | 0.09 ± 0.01 |
| MC41 | 0.75 | 0.1 | 0.1 | 0.05 | ++ | 62 ± 1 | 0.09 ± 0.01 |
X1: glyceride fraction, X2: KOL fraction, X3: LPC fraction, X4: ethanol fraction.
+: transparent; ++: bluish; +++: turbid.
2.1.2. Cryogenic transmission electron microscopy studies
The droplets formed by dispersing the formulation in FastedM were investigated by cryogenic transmission electron microscopy (Cryo-TEM). Three microlitre of the samples were carefully injected on a Lacey carbon film grid (Ted Pella Inc., Redding, CA, US). The grids were blotted in a Vitrobot automated vitrification device (FEI, Eindhoven, The Netherlands) under controlled environmental conditions (25 °C, 100% relative humidity), automatically plunged into liquid ethane to freeze the samples and then transferred to liquid nitrogen. The frozen samples were then transferred to a Gatan 626 cryoholder (Gatan Inc., Pleasanton, CA, USA) coupled to a FEI Tecnai G2 transmission electron microscope (FEI, Eindhoven, The Netherlands). The samples were observed under low-dose condition at −174 °C. Images were recorded by a FEI Eager 4k CCD camera (FEI, Eindhoven, The Netherlands).
2.1.3. Droplet size measurements
The droplet sizes of the emulsions formed when dispersing the formulations in FastedM was measured to evaluate the emulsification capacity of the formulations. FastedM contains 2.63 mM NaTDC, 0.23 mM PC, 3.25 mM MES hydrate, 11.50 mM MES sodium salt, and 109.75 mM sodium chloride, at pH 6.6 ± 0.1 [16]. The formulations were dispersed in FastedM at a ratio of 1:200 (v/v) and gently mixed at 20 rpm and 37 °C for 5 min using an Intelli-Mixer RM-2M rotator (ELMI, Riga, Latvia). The droplet size of the formed emulsion was measured by dynamic light scattering (DLS) at 37 °C using a Zetasizer Nano ZS (Malvern, Worcestershire, UK) (173 o backscattering angle, 0.686 cP sample viscosity). Three independent samples of each formulation were investigated for each measurement. The particle sizes are reported as the mean z-average values (i.e. particle sizes calculated based on the signal intensity) and the polydispersity is expressed as polydispersity index (PdI) values.
2.1.4. Data analysis
The effect of each excipient concentration on the emulsification capacity and the interaction between excipients were investigated by correlating the matrix of excipient concentrations to the matrix of measured emulsion droplet sizes using the MODDE 11.0.2 software. These data were fitted to a quadratic equation:
| (Eq. 1) |
where the response Y is the real or the transformed value of the emulsion droplet size, the variables X2, X3, X4 are the fractions of KOL, LPC and ethanol, respectively, expressed in a 0–1 range, are equation coefficients. The model is reliable when the goodness of fit (R2) is close to 1, and the goodness of prediction (Q2) is larger than 0.5 [11].
3. Results and discussion
The emulsification capacity of formulations with different excipient concentrations was evaluated to investigate the utility of KOL, LPC, and ethanol in combination with glycerides to formulate SNEDDS. The emulsification capacity was compared between LC and MC formulations. Based on the models representing the relationship between excipient concentrations and emulsion droplet sizes, the effects of each excipient on the emulsion droplet size were analysed.
3.1. Emulsification capacity of LC formulations containing LPC and KOL
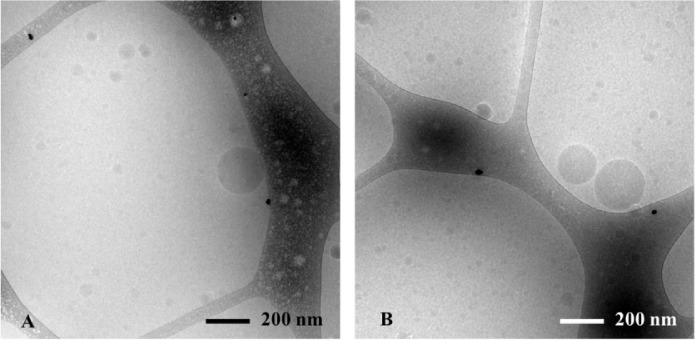
The structures formed upon dispersion of LC formulations of DoE I in FastedM at a ratio 1:200 (v/v) were observed by Cryo-TEM (Fig. 1A) and measured the size by DLS (Table 2). Nanoemulsion droplets were the only particle species found in the obtained colloidal systems confirming the self-nanoemulsification capacity of the formulations. The obtained droplet sizes of 13 emulsions were fitted to Eq. 1 to analyse the effect of each excipient on the emulsion droplet size formed. The coefficients of the fitted quadratic model are shown in Table 3.
Fig. 1.
Cryo-TEM images of emulsions obtained from dispersing (A) LC7 (see Table 2) and (B) MC34 (see Table 6) in FastedM (at a ratio of 1:200).
Table 3.
Regression coefficients of the fitted quadratic models (Eq. 1) for the different experimental designs.
| Coefficient | Variable | Formulation set |
|||
|---|---|---|---|---|---|
| LC |
MC |
||||
| DoE I | DoE I & II | DoE III | DoE IV | ||
| ß0 | – | 42 | 0.48 | 0.49 | 1.34 |
| ß2 | X2 (KOL) | 125a | −0.08a | −0.37a | −0.31a |
| ß3 | X3 (LPC) | −159a | 0.16a | 0.34a | 0.31a |
| ß4 | X4 (Ethanol) | 85a | −0.02 | 0.05 | 0.22a |
| ß22 | X2*X2 | −120a | 0.02a | 0.05a | – |
| ß33 | X3*X3 | 147a | 0.10a | 0.10a | – |
| ß44 | X4*X4 | − | 0.01 | 0.09 | – |
| ß23 | X2*X3 | − | −0.10a | −0.13a | – |
| ß24 | X2*X4 | − | 0.01 | 0.04 | – |
| ß34 | X3*X4 | − | −0.03 | −0.14a | – |
| Transformation |
None |
Logarithm |
Logarithm |
Logarithm |
|
| Method | Multiple linear regression | Partial least square regression | Partial least square regression | Partial least square regression | |
| R2 | 0.93 | 0.92 | 0.98 | 0.89 | |
| Q2 | 0.82 | 0.86 | 0.67 | 0.70 | |
X1: glyceride fraction, X2: KOL fraction, X3: LPC fraction, X4: ethanol fraction.
Signifies significant coefficient.
All LC formulations of DoE I generated nanoemulsions with mean emulsion droplet sizes ranging from 26 to 100 nm and PdI values from 0.05 to 0.38 (Table 2). The appearance of the emulsions formed agreed with the size of the oil droplets: emulsions with droplet sizes from 26 to 34 nm were transparent, emulsions with droplet sizes from 43 to 59 nm were bluish and emulsions with droplet sizes from 100 to 124 nm were turbid. The results confirm that all investigated LC formulations from DoE I were SNEDDS.
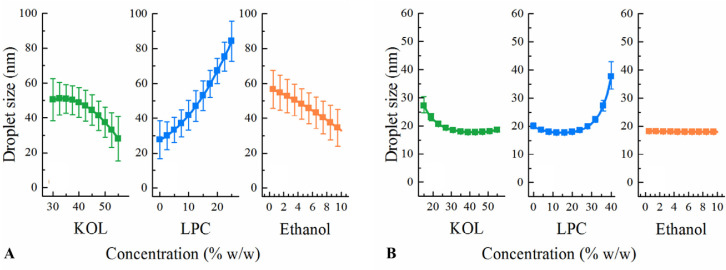
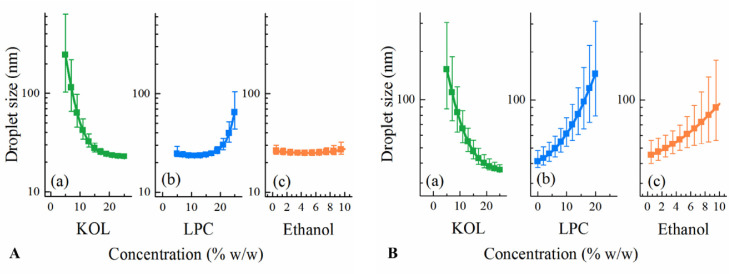
After fitting the droplet sizes of the thirteen LC SNEDDS of DoE I to Eq. 1 using multiple linear regression, the coefficients were calculated and are shown in Table 3. Statistically insignificant coefficients, i.e. ß44, ß23, ß24, and ß34 were eliminated. The R2 and Q2 values of the fitted model are 0.93 and 0.82, respectively, suggesting a satisfactory goodness of fit and predictivity of the model. The statistically significant coefficients of the fitted equation were ß2, ß3, ß4, ß22, and ß33, corresponding to the concentration of KOL, LPC, and ethanol and the square terms of KOL and LPC levels (Table 3). Based on the significance of these formulation factors, the effect of KOL, LPC and ethanol concentrations on the resulting emulsion droplet size was significant. The effect of each component on the resulting droplet size was analysed by predicting the droplet size from SNEDDS with concentration of each excipient (KOL or LPC or ethanol) varying from its lowest to highest level (30%–55% KOL, 0–25% LPC, and 0–10% ethanol) while maintaining the ratio of other two excipients as in a reference formulation. The reference formulation contains glycerides:KOL:LPC:ethanol (at a ratio of 40:42.5:12.5:5 w/w) as it was selected at the centre point of the constrained region of DoE I. The predicted droplet sizes are plotted in Fig. 2A to analyze the excipient effect on the resulting emulsion droplet sizes. The fitted model suggests that KOL and ethanol have a significant effect on reducing the nanoemulsion droplet sizes while a high LPC concentration results in increased droplet sizes of the resulting nanoemulsions.
Fig. 2.
Factor effect plots showing the effect of each component on the nanoemulsion droplet size from (A) LC SNEDDS of DoE I and (B) MC SNEDDS of DoE I & II. The displayed droplet sizes are the predicted values obtained when varying one excipient concentration (KOL, LPC or ethanol) and maintaining the ratio of the other excipients as in a reference formulation. The reference formulation is the centre point, containing KOL:LPC:ethanol (at a ratio of 42.5:12.5:5 w/w for (A) and 35:20:5 w/w for (B)). A 40% glyceride concentration is fixed for all formulations.
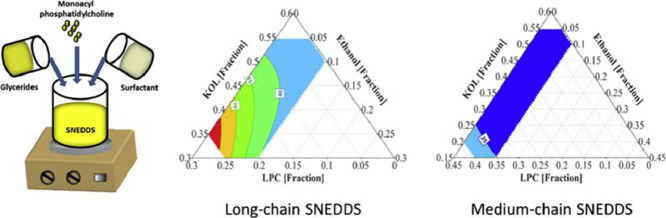
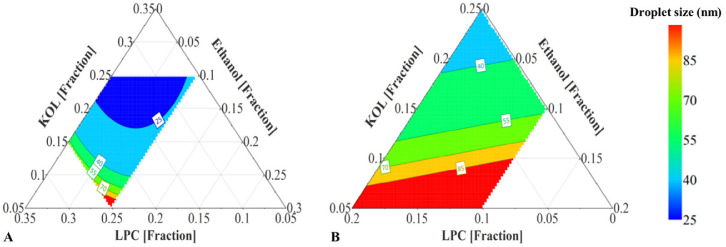
The fitted model provided a prediction plot for the droplet size of the emulsions from the LC formulation set (Fig. 3A). All LC formulations in the investigated range were predicted to generate nanoemulsions in FastedM. Increasing KOL and ethanol and reducing LPC concentration in LC formulations shifted the obtained nanoemulsions to the area of smaller droplet sizes in the predicted ternary diagram (Fig. 3A). With 20%–25% LPC, LC SNEDDS are semi-solid and formed emulsions with high polydispersity index (higher than 0.2); therefore LC SNEDDS with LPC level higher than 25% were not investigated.
Fig. 3.
(A) Prediction plot of LC SNEDDS containing 40% LC glycerides (soybean oil:Maisine (1:1 w/w)), 30–55% KOL, 0–25% LPC, and 0–10% ethanol (DoE I). (B) Prediction plot of MC SNEDDS containing 40% MC glycerides (Captex:Capmul (1:1 w/w)), 15–55% KOL, 0–40% LPC, and 0–10% ethanol (DoE I & II). Excipient concentrations are presented as fractions.
3.2. Emulsification capacity of MC formulations containing LPC and KOL
MC formulations containing LPC and KOL were prepared according to the compositions suggested by DoE I to IV (Table 1). Oil-in-water emulsion droplets were the only species observed when dispersing MC SNEDDS in FastedM using cryo-TEM (Fig. 1B). For DoE I, MC formulations formed transparent emulsions with droplet sizes ranging from 17 to 21 nm and PdI values ranging from 0.06 to 0.17 (Table 4). The droplet sizes from MC SNEDDS were smaller and more homogeneous as seen from the lower PdI values compared to those from LC SNEDDS of the same composition ratios (Table 2). There were no significant differences between the emulsion droplet sizes of all MC formulations in DoE I. It can therefore be concluded that MC formulations containing 30%–55% KOL formed monodisperse nanoemulsions with droplet sizes of approximately 20 nm without a significant influence of LPC and ethanol levels. The small emulsion droplet sizes of MC formulations in DoE I suggests the possibility of formulating MC SNEDDS with lower KOL concentrations and higher LPC or glycerides concentrations. These excipient ranges were investigated in DoE II, DoE III and DoE IV (Table 1) to maximise LPC level and to investigate the capacity of LPC and KOL to emulsify high glyceride levels.
Compared to DoE I, MC formulations in DoE II contained lower KOL levels and higher LPC levels, while the glyceride level was kept constant. LPC was unable to be dispersed at higher concentrations than 40% in MC lipid matrix and high LPC levels resulted in high formulation viscosity [6]. The emulsions obtained from the MC formulations of DoE II were transparent or slightly bluish with droplet sizes varying in a narrow range from 19 to 40 nm and PdI values varying in a narrow range from 0.10 to 0.25, signifying monodisperse nanoemulsions. When fitting the emulsion droplet size and excipient concentration from both DoE I and DoE II to Eq. 1, the obtained R2 and Q2 of the fitted equation were 0.92 and 0.86, respectively. Based on the coefficient values (Table 3), increasing KOL concentrations resulted in reduced emulsion droplet sizes while increasing LPC concentration resulted in larger emulsion droplet sizes, as also shown in the factor effect plots (Fig. 2B) and ternary diagram (Fig. 3B), albeit in a narrow size range.
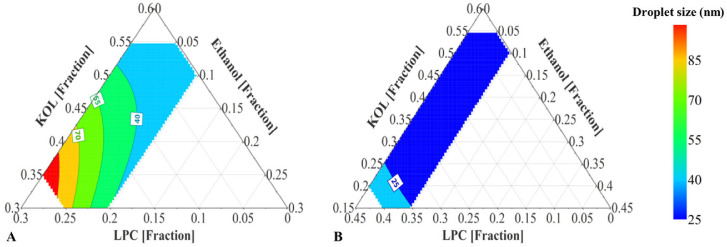
To study the capacity of LPC and KOL to emulsify higher MC glyceride concentrations than 40%, DoE III was designed with the extended glyceride range up to 60%. The resulting emulsion droplet sizes (from 23 to 169 nm) and PdI values (from 0.04 to 0.44) of MC SNEDDS of DoE III are shown in Table 5. For DoE III, MC formulations containing high glyceride and low KOL concentrations formed larger emulsion droplet sizes (e.g. MC25 and 26). The fitted model obtained for DoE III has R2 and Q2 values of 0.98 and 0.67, respectively. Fig. 4A presents the main effect of each excipient on the droplet sizes for DoE III. This effect was evaluated by predicting the droplet sizes from different SNEDDS with varied concentrations of one excipient (KOL or LPC or ethanol) and a constant ratio of other two excipients. The ratio of those two excipients was kept as in a reference formulation containing glycerides:KOL:LPC:ethanol (at a ratio of 60:17.5:17.5:5 w/w). Increasing the KOL concentration reduced the emulsion droplet size, whilst increasing LPC from 15% to 25% resulted in an opposite effect and ethanol exhibited an insignificant effect on the droplet size (Fig. 4A). Increasing LPC concentration between 5% to 15% did not result in a significant effect on droplet size, possibly because of the high KOL concentrations. Changing KOL concentrations resulted in more significant variation of emulsion droplet sizes compared to changing LPC and ethanol concentrations (Fig. 4A). The predicted droplet size of formulations within the investigated range is plotted in the ternary diagram in Fig. 5A, which shows that emulsion droplet sizes increased for SNEDDS with high LPC and low KOL concentrations.
Fig. 4.
Factor effect plots showing the effect of each component on the nanoemulsion droplet size from MC SNEDDS – (A) DoE III and (B) DoE IV. The displayed droplet sizes are the predicted values obtained when varying one excipient concentration (KOL or LPC or ethanol) and maintaining the ratio of the other excipients as in a reference formulation. The reference formulation contains KOL:LPC:ethanol (at a ratio of 17.5:17.5:5 w/w for (A) and 13:8:4 w/w for (B)). A 60% glyceride concentration is fixed for all formulations of DoE III and a 75% glyceride concentration is fixed for all formulations of DoE IV.
Fig. 5.
(A) Prediction plot of MC SNEDDS containing 60% glycerides (Captex:Capmul (1:1 w/w)), 5%–25% KOL, 5%–25% LPC, and 0–10% ethanol (DoE III). (B) Prediction plot of MC SNEDDS containing 75% glycerides (Captex:Capmul (1:1 w/w)), 5%–25% KOL, 0–20% LPC, and 0–10% ethanol (DoE IV). Excipient concentrations are presented as fractions.
Based on small emulsion droplet size formed from MC SNEDDS of DoE III, it was possible to investigate the capacity of LPC and KOL to emulsify higher MC glycerides levels than 60%. The MC glyceride concentration was thus increased up to 75% in DoE IV (Table 1) for this purpose. Opaque emulsions with emulsion droplet sizes of approximately 200 nm were found in SNEDDS containing 5% KOL (i.e. MC34 and 36), while transparent emulsions with a small droplet size of about 20 nm were found in SNEDDS containing a high KOL concentration (e.g. MC33) (Table 6). All resulting emulsions were monodisperse with PdI < 0.2 [17]. A main effect plot of DoE IV was constructed to analyse the effect of each excipient on the resulting droplet sizes (Fig. 4B). The main effect plot was based on the droplet size prediction for SNEDDS with varied concentrations of one excipient (KOL or LPC or ethanol) while keeping the concentration of other two excipients at a constant ratio. The excipient ratio was from a reference formulation containing glycerides:KOL:LPC:ethanol (at a ratio of 75:13:8:4 w/w). The fitted model for DoE IV has an R2 = 0.89 and Q2 = 0.70. KOL is suggested to have a positive effect on reducing emulsion droplet size while adding LPC and ethanol had a negative effect on droplet size reduction. Increasing LPC and ethanol concentrations increased emulsion droplet sizes possibly because this reduced KOL concentration. In Fig. 5B, the ternary diagram presenting the predicted emulsion droplet sizes from SNEDDS of DoE IV shows that the droplet sizes were indeed reduced in the area with high KOL and low LPC and ethanol concentrations.
3.3. Effect of excipients on the emulsion droplet size
Lipid chain length had a substantial influence on the emulsion droplet sizes formed by dispersing SNEDDS in FastedM. SNEDDS containing 40% glycerides formed smaller emulsion droplet sizes when replacing LC by MC glycerides. A similar effect of fatty acid chain length on emulsion droplet size was observed by Thomas et al [8]. MC glycerides were more favourable to form smaller emulsion droplet sizes than the corresponding LC glycerides because shorter fatty acid chains relate to higher hydrophilicity [18]. Increasing MC glyceride concentrations from 40% to 75% resulted in larger emulsion droplet sizes. All emulsions formed by MC SNEDDS within the investigated ranges were predicted to be monodisperse with mean droplet sizes below 200 nm. The effect of increasing LC glyceride concentration on the emulsion droplet size was not investigated because LC6 with the lowest investigated LC glyceride concentration of (i.e. 40%) already formed a polydisperse emulsion.
According to Tran et al., combining LC glycerides with Labrasol®, LPC and ethanol resulted in polydisperse coarse emulsions when the formulations were dispersed in FastedM [6]. Replacing Labrasol® by KOL and keeping the other components at the same concentrations resulted in monodisperse nanoemulsions. Smaller emulsion droplet sizes were obtained from MC glycerides containing KOL instead of Labrasol®. KOL can reduce nanoemulsion droplet sizes better than Labrasol®, possibly because of the chemical structure of KOL with more polyethylene glycol groups and therefore higher hydrophilicity than Labrasol®. This is also expressed by the higher hydrophilic-lipophilic balance (HLB) value of KOL (HLB = 14–16) compared to Labrasol® (HLB = 12) [19], [20].
In the current study, to analyse the overall effect of different excipient concentrations on the resulting emulsion droplet sizes from different MC SNEDDS, all data from DoE I to IV were fitted together in one model. The obtained model had an R2 of 0.91 and a Q2 of 0.72. The ternary diagrams, corresponding to different excipient concentrations, are presented in Fig. S1 (Supporting Information) to facilitate the comparison. Varying the KOL and LPC ratio resulted in significantly different size distribution of the emulsions. Increasing KOL concentration caused smaller emulsion droplet sizes. The use of 20% KOL guarantees the formation of nanoemulsions with droplet sizes smaller than 40 nm when being dispersed in FastedM, while using only 5% KOL results in bluish or turbid nanoemulsions with droplet sizes larger than 70 nm. In general, increasing LPC concentrations led to emulsions of larger droplet sizes. But with MC SNEDDS containing 40%–60% glycerides, varying LPC level between 5% and 15% did not affect the emulsion droplet sizes because of high KOL concentration present. This effect is in contrast to the significant effect of LPC on reducing emulsion droplet sizes formed from MC SNEDDS containing Labrasol® instead of KOL. Combining these two findings, it is suggested that LPC reduces the emulsion droplet sizes less effectively than KOL but more effectively than Labrasol®. Since the effect of LPC on droplet sizes was governed by the KOL concentration, increased LPC concentration (i.e. reduced KOL concentration) might increase the resulting droplet sizes.
No substantial effect of ethanol on the nanoemulsion droplet size was observed in both LC and MC SNEDDS (Fig. 3, Fig. 4), which agrees with the previously found insignificant effect of ethanol in a system containing soybean oil or rapeseed oil, KOL, Maisine and ethanol [12]. Comparing the ternary diagrams of SNEDDS containing 0 and 10% ethanol (Fig. S1), increased ethanol concentration resulted in an insignificant reduction of the nanoemulsion droplet size and the presence of ethanol was not essential to the formation of nanoemulsions from both LC and MC SNEDDS.
4. Conclusion
D-optimal design was used to facilitate the screening process of SNEDDS within wide ranges of glycerides, KOL, LPC and ethanol concentrations, based on the resulting emulsion droplet sizes when dispersing the formulations in a medium simulating human fasted-state intestinal fluid. All formulations formed nanoemulsions with droplet sizes from 20 to 200 nm. LPC can be incorporated at concentration of up to 25% in LC SNEDDS and 40% in MC SNEDDS. With LPC and KOL combined, LC glycerides can be incorporated at concentration of up to 40%, while MC glyceride concentration could be increased up to 75%. LC SNEDDS formed emulsions with larger and more polydisperse droplets than MC SNEDDS. KOL exhibited a strong impact on reducing nanoemulsion droplet sizes making emulsification enhancement effect of LPC and ethanol insignificant. The study provides a database of emulsion droplet sizes from SNEDDS containing LPC with insights into the emulsification effect of each excipient, which should be considered when selecting optimal formulation strategies.
Declaration of interest
The authors declare no conflict of interest.
Acknowledgements
Financial support from the University of Copenhagen and the Phospholipid Research Center (Heidelberg, Germany) is kindly acknowledged. We also thank Ramon Liebrechts from the Core Facility for Integrated Microscopy (University of Copenhagen) for the support with cryo-TEM imaging.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
Supplementary data to this article can be found online at doi:10.1016/j.ajps.2017.09.006.
Appendix. Supplementary material
The following is the supplementary data to this article:
Supplementary Information.
References
- 1.Stegemann S., Leveiller F., Franchi D. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31:249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 2.Müllertz A., Ogbonna A., Ren S. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J Pharm Pharmacol. 2010;62:1622–1636. doi: 10.1111/j.2042-7158.2010.01107.x. [DOI] [PubMed] [Google Scholar]
- 3.Aungst B.J. Intestinal permeation enhancers. J Pharm Sci. 2000;89:429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Yáñez J.A., Wang S.W.J., Knemeyer I.W. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63:923–942. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovarik J.M., Mueller E.A., Van Bree J.B. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci. 1994;83:444–446. doi: 10.1002/jps.2600830336. [DOI] [PubMed] [Google Scholar]
- 6.Tran T., Xi X., Rades T. Formulation and characterization of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine. Int J Pharm. 2016;502:151–160. doi: 10.1016/j.ijpharm.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Grove M., Müllertz A., Nielsen J.L. Bioavailability of seocalcitol: II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–242. doi: 10.1016/j.ejps.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Thomas N., Müllertz A., Graf A. Influence of lipid composition and drug load on the In Vitro performance of self-nanoemulsifying drug delivery systems. J Pharm Sci. 2012;101:1721–1731. doi: 10.1002/jps.23054. [DOI] [PubMed] [Google Scholar]
- 9.Yoo J.H., Shanmugam S., Thapa P. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch Pharm Res. 2010;33:417–426. doi: 10.1007/s12272-010-0311-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Chen Z.Q., Zhang X. An improved formulation screening and optimization method applied to the development of a self-microemulsifying drug delivery system. Chem Pharm Bull. 2010;58:16–22. doi: 10.1248/cpb.58.16. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson E.J.L., Kettaneh-Wold N., Wikström C. 2008. Design of experiments - principles and applications. Umetrics AB, Sweden; 2008. p. 7–26, 217–240. [Google Scholar]
- 12.Ren S., Mu H., Alchaer F. Optimization of self nanoemulsifying drug delivery system for poorly water-soluble drug using response surface methodology. Drug Dev Ind Pharm. 2013;39:799–806. doi: 10.3109/03639045.2012.710634. [DOI] [PubMed] [Google Scholar]
- 13.Badawi M.A., El-Khordagui L.K. A quality by design approach to optimization of emulsions for electrospinning using factorial and D-optimal designs. Eur J Pharm Sci. 2014;58:44–54. doi: 10.1016/j.ejps.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Dalvadi H., Patel N., Parmar K. Systematic development of design of experiments (DoE) optimised self-microemulsifying drug delivery system of zotepine. J Microencapsul. 2017;34:308–318. doi: 10.1080/02652048.2017.1324920. [DOI] [PubMed] [Google Scholar]
- 15.Pawar Y.B., Purohit H., Valicherla G.R. Novel lipid based oral formulation of curcumin: development and optimization by design of experiments approach. Int J Pharm. 2012;436:617–623. doi: 10.1016/j.ijpharm.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Madsen C.M., Boyd B., Rades T. Supersaturation of zafirlukast in fasted and fed state intestinal media with and without precipitation inhibitors. Eur J Pharm Sci. 2016;91:31–39. doi: 10.1016/j.ejps.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Bucak S. Springer Berlin Heidelberg; 2010. Trends in colloid and interface science XXIII; pp. 13–18. [Google Scholar]
- 18.Hauss D.J. CRC Press; 2007. Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs. p. 34–62, 107–128. [Google Scholar]
- 19.Gattefossé website. Labrasol information; 2017. Available from: http://www.gattefosse.com/en/applications/labrasol.html. [Accessed 15 June 2017].
- 20.BASF website. Kolliphor® RH40 - technical information; 2017. Available from: https://pharmaceutical.basf.com/en/Drug-Formulation/Kolliphor-RH40.html. [Accessed 15 June 2017].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information.