Abstract
Acute kidney injury (AKI) causes over 1 million deaths worldwide every year. AKI is now recognized as a major risk factor in the development and progression of chronic kidney disease (CKD). Diabetes is the main cause of CKD as well. Renal fibrosis and inflammation are hallmarks in kidney diseases. Various cytokines contribute to the progression of renal diseases; thus, many drugs that specifically block cytokine function are designed for disease amelioration. Numerous studies showed IL-20 functions as a pro-inflammatory mediator to regulate cytokine expression in several inflammation-mediated diseases. In this review, we will outline the effects of pro-inflammatory cytokines in the pathogenesis of AKI and CKD. We also discuss the role of IL-20 in kidney diseases and provide a potential therapeutic approach of IL-20 blockade for treating renal diseases.
Keywords: IL-20, acute kidney injury, chronic kidney disease
1. Introduction
The kidney functions as a filter to remove metabolic waste products and toxic substances as well as excess water from the body and thus maintains the balance of the body fluids, electrolytes, and blood pressure. The nephron is the functional unit of the kidney and is composed of the glomerulus and renal tubular. Renal injury results in nephron loss and then causes tubular atrophy and interstitial fibrosis. Renal dysfunction causes nitrogenous wastes accumulation in the body, resulting in poisoning (e.g., uremia). Kidney failure can be divided into acute kidney injury (AKI) and chronic kidney disease (CKD) based on its duration. The former may be cured as long as it is properly treated, while the latter is usually irreversible. Tissue damage can be repaired and regenerated to restore the normal structure and function. However, if the injury is very severe or prolonged, repair may be incomplete (maladaptive repair), which leads to tissue dysfunction and fibrosis. Persistent inflammation, increased numbers of myofibroblasts, and extracellular matrix (ECM) accumulation are usually observed in many kinds of kidney diseases [1].
ECM are produced from several cells, such as interstitial fibroblasts, mesangial cells, epithelial cells, and endothelial cells and composes by fibrous proteins and glycosaminoglycans [2]. Moreover, these cells contribute to renal inflammation by producing different kinds of cytokines and chemokines. In diabetes-induced CKD, also called diabetic nephropathy (DN), both metabolic stimuli and reactive oxygen species (ROS) production regulate gene expression and transcription factor activation, leading to impaired renal function and structure. The alterations of renal structure include thickening of the glomerular basement membrane (GBM), capillary, and tubular basement membrane and reduction of glomerular endothelium fenestration, expansion of the mesangium and loss of podocytes [3,4,5].
IL-20 is a member of IL-10 family, which includes IL-10, IL-19, IL-22, IL-24, IL-26, IL-28, and IL-29. Many studies have demonstrated that IL-20 is associated with several inflammatory diseases, such as rheumatoid arthritis, atherosclerosis, cancer, and liver fibrosis via regulating cytokines and chemokines. In vivo experiments also showed that blocking IL-20 with specific antibodies can reduce inflammation and improve disease progression. Previous studies indicated that IL-20 and its receptors are increased in the kidneys of mice with acute renal failure (ARF), which might cause apoptosis and necrosis in tubular epithelial cells by activating caspase-9. Besides, the serum level of IL-20 is elevated in patients with CKD and DN. IL-20 upregulates TGF-β1 expression in proximal tubule epithelial cells and promotes IL-1β expression under hypoxic conditions. IL-20 also regulates TGF-β1 production in interstitial fibroblasts. The expression of MCP-1, TGF-β1, MMP-9, and VEGF is upregulated in IL-20-treated podocytes [6]. In this review, we will discuss the role of cytokines in the progression of kidney diseases AKI, CKD, and DN. In the end, we will summarize the impact of IL-20 in the renal lesion and discuss the effect of IL-20 blockade in kidney disease therapy.
2. Acute Kidney Injury (AKI)
Acute kidney injury (AKI), previously known as ARF, is characterized by a rapid loss of kidney function within a few hours or days. AKI is common in hospitalized patients with kidney transplantation, trauma, and sepsis and is associated with poor prognosis, high morbidity, and mortality. The mortality rate of AKI in intensive care unit (ICU) patients ranges from 20% to 50% [7,8]. The etiologies of AKI can be classified as pre-renal, intrinsic, and post-renal. Pre-renal AKI is the most common cause and occurs when blood flowing to the kidney is reduced. Intrinsic AKI refers to damage to renal structures, including vascular, glomerular, interstitial, and tubular. Post-renal AKI is usually caused by urinary tract obstruction. Pre-renal and post-renal AKI may eventually develop into intrinsic renal disease. Diagnostic criteria for AKI are based on serum creatinine level (SCr) and urine output. Kidney Disease: Improving Global Outcomes (KDIGO) defined AKI as the following: increase in SCr by ≥ 0.3 mg/dL (≥ 26.5 µmoL/L) within 48 h or increase in SCr to ≥ 1.5 times baseline which has occurred within the prior 7 days; or urine volume < 0.5 mL/kg/h for 6 h [9].
AKI usually occurs if insufficient blood is flowing through the kidney (ischemia) or toxins are overloaded in the kidney (nephrotoxins). The injury site of AKI is mainly in the proximal tubule. Tubular necrosis and inflammatory cell infiltration are observed in the early progression of AKI [10,11]. Necrotic tubular endothelial cells initiate early inflammatory responses and promote the infiltration of immune cells. In the early stage of AKI, neutrophils first rapidly accumulate and adhere to endothelium by adhesion molecules such as P-selectin and intracellular adhesion molecule-1 (ICAM-1) and release cytokines [12]. ICAM-1, IL-1, and TNF-α mRNA levels are increased within 1 h after ischemia/reperfusion. Monocytes and macrophages, following neutrophils, migrate into injury sites and remove debris of dying renal cells and neutrophils. Forbes et al. observed that monocyte/macrophage significantly increases at 4 days after ischemic injury [13]. Cellular debris is also cleared by dendritic cells and dedifferentiated epithelial cells. Surviving epithelial cells dedifferentiate and proliferate to replace the injury cells for restoring the integrity of the tubular epithelial cell layer. Several pieces of evidence display that inhibition of leukocyte (neutrophil, monocyte, and macrophage) reduces tubular necrosis and inflammation [14,15,16].
3. Chronic Kidney Disease (CKD)
CKD is defined as kidney damage or glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 lasting for more than 3 months with implications for health. It is estimated that more than 700 million people have CKD [17]. The effect of AKI and CKD is bidirectional [18]. Individuals who survive after AKI have a high risk of developing CKD and hastened development of end-stage renal disease (ESRD) because of incomplete repair (maladaptive repair), which leads to vascular rarefaction, tissue fibrosis, and chronic inflammation [19,20,21,22]. Maladaptive repair is characterized by interstitial fibrosis and persistent inflammation. Severe or recurrent injury leads to cell-cycle arrest in tubular epithelial cells, which fail to restore damaged renal structures. Yang et al. investigated the cell-cycle of tubular epithelial cells arrested in the G2/M phase and upregulated fibrogenic factors TGF-β1, collagen-1, and collagen-4 via c-Jun N-terminal kinase (JNK) signaling in AKI models [1]. The macrophage phenotype is important for repair progression in kidney diseases. Macrophage phenotype is traditionally divided into M1 (pro-inflammatory) and M2 (anti-inflammatory). In the early stage of the repair process, M1 macrophages are dominant to clear cellular debris. Macrophages switch from M1 to M2 to help tissue regeneration by enhancing tubular cell proliferation in the late stage. However, arrested cells cause macrophage to stay as the M1 phenotype through releasing pro-inflammatory cytokines. Besides, glomerular mesangial cells also acquire a myofibroblast phenotype and play a role in renal fibrosis. CKD is one of the risk factors for AKI development. Patients with a baseline GFR of 45–59, 30–44, and < 30 mL/min had a relative risk for AKI of 2.9, 6.2, and 18.3, respectively [23,24,25].
Some chronic diseases that cause renal dysfunction include systemic lupus erythematosus (SLE), diabetes mellitus, and hypertension, which lead to lupus nephritis, diabetic nephropathy, and hypertensive nephropathy, respectively. Additionally, some analgesic medications such as aspirin, phenacetin, and nonsteroidal anti-inflammatory drugs lead to renal papillary necrosis and chronic interstitial nephritis [26]. Lupus nephritis is a frequent complication of SLE, an autoimmune disease, and is characterized by autoantibodies and complement deposition in the kidneys, which lead to inflammation and loss of kidney function. The morbidity and mortality rates of lupus nephritis account for approximately 60% of SLE patients [27,28,29]. Hypertensive nephropathy is a kidney disorder that is associated with hypertension. Hypertension results in arteriolosclerosis and damage of blood vessel lining, including thickening of blood vessel walls and narrowing of preglomerular arteries and arterioles openings, thereby reducing glomerular blood flow. Insufficient blood flow can cause glomerular ischemia and hyperfiltration, which can lead to tubular atrophy, interstitial fibrosis, and changes in glomerular structure [30]. IgA nephropathy (IgAN), also known as Berger’s disease, is the most common glomerulonephritis worldwide and is caused by the accumulation of the IgA in the glomerulus [31]. IgA predominantly deposits in the mesangium of the kidney, which triggers mesangial cell proliferation and production of pro-inflammatory cytokines and pro-fibrotic mediators to mediate tubulointerstitial injury and fibrosis. Up to 30% of patients with IgAN will develop ESRD within 20 years [32,33].
4. Diabetic Nephropathy
Diabetic nephropathy (DN), also known as diabetic kidney disease (DKD), is a complication of diabetes mellitus and is the most frequent cause of ESRD. Diabetes leads to alterations in metabolism and hemodynamics and reactive oxygen species (ROS) generation. Hyperglycemia activates protein kinase C (PKC) pathway and increases advanced glycated proteins (AEG) generation, mitochondrial oxidative phosphorylation, and ROS production [34]. PKC activation is associated with impairment of renal function and structure, including glomerular filtration, ECM accumulation, and cell apoptosis [35]. ROS disrupts the cellular structure by destroying lipid, protein, and DNA. ROS also causes podocyte apoptosis or detachment [36]. Podocytes are highly specialized epithelial cells with complex cellular structures called foot processes and constitute the epithelial layer of Bowman’s capsule and wrap around capillaries. The foot processes of podocytes form filtration slits to regulate GFR. Thus, podocyte loss resulting in albuminuria increases [37,38]. Hyperglycemia also increases oxygen consumption to cause renal hypoxia. Several studies show that high glucose increases inflammatory cytokine production, including that of TNF-α, MCP-1, IL-1, IL-6, and IL-8 in renal tubular cells, podocytes, and mesangial cells, which synergistically aggravate local and systemic inflammation in kidney.
5. Inflammation and Kidney Disease
Inflammation response is mediated by several types of immune cells and essential for pathogen elimination, tissue repair, and regeneration. However, the excessive inflammatory response may result in tissue damage, fibrosis, and functional loss in many diseases such as cancer and kidney diseases. Immune cells release soluble mediators, cytokines, and chemokines, which impaire biological function and cellular structure of renal cells. In addition to immune cells, renal cells such as podocytes, mesangial cells, and tubular cells also release many cytokines to promote kidney injury. It has been determined that pro-inflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-6, IL-8, and MCP-1 are involved in the development of kidney diseases.
6. MCP-1(CCL2)/CCR2
Serum and urinary MCP-1 levels are significantly elevated in patients with kidney diseases [39,40]. MCP-1 and its receptor CCR2 are important for recruiting monocyte/macrophage into the kidney in patients with AKI and CKD. Several cytokines induce renal tubular epithelial cells and mesangial cells to secrete MCP-1, which in turn stimulates ICAM-1 expression to promote leukocyte retention [41,42,43,44,45,46]. High glucose and AEG stimulation also induce MCP-1 expression to enhance fibrosis-associated factors production through activation of nuclear factor-κB (NF-κB) [47,48,49,50,51,52]. Podocytes’ exposure to high glucose activates TGF-β-dependent MCP-1 expression, which in turn induces apoptosis and increases permeability [53,54]. Blockade of MCP-1/CCR2 signaling ameliorates glomeruli hypertrophy, interstitial fibrosis, and tubular atrophy [55,56,57,58,59]. MCP-1-deficient mice with renovascular hypertension have reduced renal damage and inflammation [55]. In the DN animal model, the numbers of macrophages in the kidney are decreased in MCP-1-deficient mice. MCP-1 inhibition decreases the production of TNF-α, IL-6, and TGF-β and ameliorates podocyte function and albuminuria. CCR2 antagonist also showed an improvement of mesangial expansion and GBM thickening [56]. However, a previous study indicated that MCP-1 plays a protective role in the early phase of ischemia/reperfusion injury (IRI)-induced AKI progression. Stroo et al. determined that MCP-1 deficiency reduces survival and increases renal damage after IRI [60]. Furthermore, in MCP-1 knockout mice with IRI-AKI, macrophage type moves towards more M1 (pro-inflammatory) phenotype. It is found that MCP-1 activates M2-type macrophage polarization, which promotes tubule epithelial cell proliferation in the repair phase of AKI. Therefore, there may be a need to consider whether the target MCP-1 is suitable for the treatment of IRI-AKI.
7. IL-8/CXCL8
IL-8 acts as a chemoattractant, which predominantly drives neutrophils to inflammation sites through CXCR1 and CXCR2. Pro-inflammatory cytokines IL-1 and TNF-α stimulate IL-8 expression in human mesangial cells and proximal tubule cells [45,61,62,63]. IL-8 stimulates ICAM-1 production in human proximal tubule cells through the p38 pathway [64,65]. IL-8 is increased in the podocytes and endothelial cells of kidneys in patients with glomerulonephritis. Administration of IL-8 antibody ameliorates renal function in rabbits with glomerulonephritis [66]. G31P, a CXCR1/CXCR2 inhibitor, ameliorates sepsis-induced renal damage through reducing renal cell apoptosis and improves inflammation via reducing IL-1β, IL-6, and TNF-α expression, and neutrophil infiltration. Similar results are also confirmed in cisplatin-induced AKI model [67,68]. G31P also showed the reduction of renal structure change, including GBM thickening, mesangial expansion, collagen deposition, and podocyte loss in diabetic mice [69]. In addition, G31P downregulates TGF-β, CTGF, and fibronectin expression as well as upregulates MMP-2 and MMP-9. G31P inhibits renal inflammation by reducing IL-1β, IL-6, and TNF-α expression and decreasing macrophage infiltration. G31P also inhibited high-glucose-induced TNF-α and TGF-β expression in mesangial cells by reducing the phosphorylation of JAK2, STAT3, and ERK1/2 [69].
8. TNF-α
The role of TNF-α in kidney disease was first discovered in 1989 by Bertani et al. [70] TNF-α causes renal damage through inducing apoptosis in epithelial cells, tubular cells, and mesangial cells, which can be inhibited by blockade of TNF-α [71,72,73]. TNF-α stimulates MCP-1 and IL-8 expression as well as adhesion molecular ICAM-1 expression in mesangial cells, tubular epithelial cells, and podocytes to promote neutrophil and monocyte infiltration. TNF-α also increases ROS production in mesangial cells. In addition, TNF-α is correlated with urinary albumin excretion [74,75,76]. TNF-α induces the loss of glomerular endothelial cell fenestration and then causes GFR decrement and albumin leakage. Inhibition of TNF-α decreases macrophage recruitment and inhibits G-MCSF, keratinocyte-derived cytokine (KC), and MCP-1 levels and reduces albuminuria in DN animal model [77]. Several studies demonstrated that systemic TNF-α inhibition attenuates renal function and inflammation in many kidney disease animal models [78,79,80]. However, Wen et al. found that TNF-α deletion in T lymphocytes increases IL-17A expression and the numbers of CD4+ and CD8+ T cells in the kidney with nephrotoxic nephritis. T cell-derived TNF-α protects against renal injury and fibrosis in mice with nephrotoxic nephritis [81].
9. IL-1β
IL-1β is mainly secreted from infiltrating leukocytes (dendritic cells, macrophages, and neutrophils) [82]. Podocytes are the only renal intrinsic cells in the kidney from CKD patients that secrete IL-1β, but numerous in vitro experiments indicate that other renal cells can release IL-1β under some stimulus [83,84,85,86]. Macrophage-derived IL-1β promotes cell proliferation and induces fibronectin production in fibroblasts and mesangial cells [87,88,89]. IL-1β increases the expression of IL-6 and IL-8 in primary human renal fibroblasts and mesangial cells [89,90,91]. IL-1β induces ROS production and fibrotic factor expression, such as that of TGF-β, collagen I, and fibronectin in tubular epithelial cells [85,92,93]. Additionally, IL-1β promotes tubular epithelial cells to transdifferentiate into fibroblast-like phenotype, which is critical in the progression of renal fibrosis [94,95]. Faubel et al. observed that the level of IL-1β is increased in cisplatin-induced AKI mice. Shahzad et al. identified that IL-1β gradually increases in DN progression of db/db mice, which implies that IL-1β is involved in the development of DN [96]. IL-1β deficiency ameliorated renal function and inflammation in mice with nephrotoxic nephritis [97,98]. IL-1β activation is mediated by caspase-1. NLRP3 inflammasome, a multiple protein complex composed of the sensor molecule NLRP3, the adaptor protein ASC, and pro-caspase-1, regulates caspase-1 activation. Caspase-1 is associated with pyroptosis, a programmed lytic cell death that is different from apoptosis and necrosis, which accelerates tubular epithelial cell death and is the most common cause of AKI [99,100,101,102]. NLRP3 inflammasome can be activated by glucose in glomerular endothelial cells and podocytes [96]. A few in vivo studies have shown that NLRP3 deficiency reduces renal inflammation, ECM accumulation, and fibrosis [86,92,103,104,105]. In addition to IL-1β, NLRP3 inflammasome can also trigger IL-18 maturation, which is associated with the pathogenesis of various kidney diseases [105,106,107,108].
10. IL-6
IL-6 signals through two distinct pathways to exert its cellular effects. (1) IL-6 acts on membrane-bound IL-6R (mbIL-6R) and then triggers gp130 to activate subsequent downstream signaling (classical signaling). (2) IL-6 forms dimer with soluble IL-6R (sIL-6R) and then binds to gp130 to initiate signal transduction (trans-signaling). IL-6 can be produced by renal resident cells, including tubular epithelial cells, podocytes, and mesangial cells under some stimuli such as TNF-α and IL-1β [109,110,111,112]. However, the expression of IL-6R in renal cells is limited. Except for podocytes, other renal resident cells do not express mbIL-6R, which implies that IL-6 stimulates these cells via trans-signaling [113]. IL-6 induces collagen I expression in mouse proximal tubular epithelial cells via STAT3 phosphorylation. IL-6 plays a role in mesangial cell proliferation, which is associated with glomeruli hypertrophy and stimulates MCP-1 expression [112,114,115]. In addition, IL-6 is involved in high-glucose-induced podocyte apoptosis through regulating caspase-3 and caspase-9 activation. Furthermore, IL-6 increases p21 and p27 production, which results in cell cycle arrest in podocytes [116]. IL-6 enhances the fibrotic response via TGF-β, collagen I, and collagen IV in IRI-AKI model. IL-6 deficiency ameliorates renal function and decreases neutrophil infiltration in IRI- and HgCl2-induced AKI models [117,118]. IL-6 blockade improves renal inflammation in IRI mice by reducing TNF-α and IL-1β production and decreasing ICAM-1 and P-selectin expression, which contribute to neutrophil infiltration. Tocilizumab (TCZ), a humanized IL-6R antibody, attenuates albuminuria and glomerular hypertrophy as well as suppresses NLRP3 inflammasome activation in diabetic mice and mice with lupus nephritis. Inhibition of IL-6 trans-signaling downstream transcription factor protects against renal fibrosis and attenuates inflammation [119]. These findings reflect the fact that IL-6 contributes to renal inflammation and declining renal function as well as disrupts glomerular structure. However, some studies indicate that IL-6 has anti-inflammatory effects in kidney diseases [117,120]. IL-6 administration protects against nephrotoxic nephritis. Enhanced IL-6/sIL-6 axis protects against HgCl2-induced AKI via reducing oxidative stress. Therefore, IL-6 might have both pro-inflammatory and anti-inflammatory functions in different kidney diseases.
11. Transforming Growth Factor-β (TGF-β)
TGF-β exerts biological functions, including cell proliferation, differentiation, and apoptosis in different types of cells. There are three isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3). Numerous studies demonstrate that TGF-β1 is involved in renal fibrosis and causes the excessive accumulation of ECM components in renal cells through downregulating MMPs and upregulating TIMPs. TGF-β1 induces fibroblast proliferation via basic fibroblast growth factor (bFGF) and increases osteopontin and collagen I expression in fibroblasts in unilateral ureter obstruction (UUO) mice [121]. Moreover, TGF-β1 is involved in tubular epithelial-to-myofibroblast transition (EMT), which contributes to more ECM accumulation and regulates macrophage infiltration to mediate tubulointerstitial fibrosis [122,123]. Furthermore, elevated glucose levels stimulate TGF-β1 production through the PKC pathway to upregulate Glut4 expression, which results in increasing cellular glucose uptake and accelerating the progression of diabetic kidney. In addition to promoting fibrosis, TGF-β also induces caspase-3-dependent podocyte apoptosis by activating the mTOR pathway [124,125]. Based on the role of TGF-β1 in fibrogenesis, several strategies for inhibiting TGF-β1 have proven to alleviate renal fibrosis effectively. Ziyadeh et al. demonstrated that treatment with TGF-β1 antibody prevents renal fibrosis in DN mice [126]. Gewin et al. showed that HgCl2-induced apoptosis of proximal tubule epithelial cells is mitigated in TGF-β1 receptor deficiency mice [127]. Several clinical trials using TGF-β antibodies for treating focal segmental glomerulosclerosis and DN have been explored. However, completely blocking TGF-β signaling may cause severe side effects according to the anti-inflammatory effect and anti-tumor role of TGF-β1 [128]. Hence, other groups target the downstream of the TGF-β1 signaling pathway. TGF-β1 binds to receptor complexes, TGF-β type I receptor (TβRI), and TGF-β receptor type II (TβRII) and then phosphorylates Smad2 and Smad3 to regulate fibrogenic genes. Smad3, but not Smad2, is recognized as an important factor in the EMT process. It is confirmed that Smad3 deficiency inhibits fibrosis and improves renal function in different CKD models [129,130]. Smad7, an inhibitory Smad, negatively regulates TGF-β1 signaling by TGF-β receptor competition and degradation; therefore, Smad7 overexpression protects against TGF-β1-induced fibrosis in kidney [131,132,133,134].
12. Hypoxia in Kidney Disease
Several research groups postulated that hypoxia is critical in the advancement of AKI to CKD and ESRD [135,136,137]. Healthy kidneys receive over 20% of the cardiac output and comprise roughly 10% of total oxygen consumption. In healthy kidneys, renal blood flow and oxygen level in the medulla are lower compared to those in the cortex [138]. Thus, the medulla is susceptible to oxygen change. Hyperglycemia increases oxygen consumption in DN [139]. Basile et al. demonstrated that capillary density around the tubules is reduced by about 30–50% in ischemic AKI rats. Reduction of renal vascular density activates the hypoxia-dependent pathway to exacerbate inflammation and tissue fibrosis [140,141]. Mazzali and colleagues place rats in a hypobaric chamber for 24 days to mimic chronic hypoxia. They found that chronic hypoxia increases blood pressure and serum uric acid due to changes in the kidney, including arteriolopathy, glomerular hypertrophy, mild inflammation, and fibrosis [142]. A previous study indicated that hypoxia promotes fibrotic progression by inducing collagen and TGF-β1expression as well as myofibroblast differentiation [143].
Hypoxia-inducible factor-1 (HIF-1), composed of HIF-1α with HIF-1β, is thought to play a role in kidney disease. HIF-1α is activated in response to hypoxia stimulation and is unstable under normal oxygen concentration. Prolyl hydroxylase domain (PHD) proteins hydroxylate HIF-1α, which triggers ubiquitination and proteasomal degradation. Pretreatment of cobalt chloride, PHD inhibitors, reduces model [144]. Furthermore, cobalt markedly reduces renal AEG contents and TGF-β in the DN model [145]. Since cobalt chloride is harmful in the human body, several PHD inhibitors are synthesized and used in the clinical trials [146,147,148,149].
HIF-1α activation is time-dependent. HIF-1α is rapidly induced at the beginning of hypoxia and then disappears within 72 h. This is because HIF-1α mRNA is unstable under prolonged hypoxic conditions [150,151]. Rosenberger et al. identified that HIF-1α expression is undetectable at 48 h after AKI [152]. In addition, chronic hypoxia may activate PHD, which in turn promotes HIF-1α degradation [153,154].
13. IL-20
Blumberg et al. identified sequences of IL-20 by EST databases in 2001. They found that overexpression of IL-20 leads to abnormal differentiation in epidermal keratinocytes and causes the death of newborn mice. IL-20 activates JAK/STAT signal pathway through two heterodimeric receptors IL-20R1/IL20R2 and IL-222R1/IL-20R2. IL-20 is mainly secreted by immune cells such as monocytes, macrophages, dendritic cells, and lymphocytes. Under the pathological condition, IL-20 is also expressed in various types of cells, including endothelial cells, synovial fibroblasts, chondrocytes, and osteoclasts. Previous studies reported that IL-20 is involved in several inflammatory diseases like psoriasis, rheumatoid arthritis (RA), atherosclerosis, osteoarthritis (OA), and stroke (Table 1). IL-20, as a pro-inflammatory mediator, regulates cytokine and chemokine expression in different types of cells. In RA, IL-20 activates the ERK-1/2 pathway to stimulate MCP-1, IL-6, and IL-8 in synovial fibroblasts as well as promotes neutrophil migration [155]. In OA, IL-20 induces TNF-α and IL-1β expression in synovial fibroblasts and increases IL-6 and MCP-1 in chondrocytes [156]. IL-20 promotes TNF-α, IL-1β, and MCP-1 expression and increases ROS production in oral cancer cells (OC-3 and OEC-M1) [157].
Table 1.
IL-20: biological effects and target cells.
| Organ | Diseases | Target Cells | Role | Reference |
|---|---|---|---|---|
| Brain | Ischemic Stroke | Glia-like cells | ↑ Inflammation ↑ Ischemic infarction |
[162] |
| Mouth | Oral Cancer | Oral carcinoma cells | ↑ Tumor progression ↑ Inflammation |
[157] |
| Airway | Asthma | Lung epithelial cells | ↑ Lung fibrosis | [160] |
| Arterial | Atherosclerosis | Endothelial cells | ↑ Inflammation ↑ Angiogenesis ↑ Atherosclerosis |
[158] |
| Liver | Hepatocellular Carcinoma (HCC) | Liver cancer cells | ↑ Tumor progression | [163] |
| Liver Injury | Hepatocytes | ↑ Liver fibrosis ↑ Inflammation |
[164] | |
| Pancreas | Type 2 Diabetes | Pancreatic islets | ↑ Inflammation | [165] |
| Kidney | Hgcl2-Induced AKI | Proximal tubular epithelial cells | ↑ Inflammation ↑ Renal fibrosis ↑ Cell death |
[161] |
| 5/6 nephrectomy-Induced CKD | Tubular epithelial cells Interstitial fibroblasts |
↑ Renal fibrosis | [166] | |
| STZ-induced DN | Podocytes | ↑ Inflammation ↑ Fibrosis ↓ Renal function |
[167] | |
| Lupus Nephritis | Mesangial cells | ↑ Inflammation | [168] | |
| Skin | Psoriasis | Keratinocytes | ↑ Cell proliferation | [169,170] |
| Bone | RA | Synovial fibroblasts Osteoclasts Osteoblasts Chondrocytes |
↑ Inflammation | [155] |
| Spondyloarthritis | Synovial fluid monocytes Synovial fibroblasts Osteoblasts |
↑ Inflammation ↑ Osteoblastogenesis |
[171] | |
| Osteoporosis | Osteoclasts Osteoblasts |
↑ Osteoclastogenesis ↓ Osteoblastogenesis |
[172] | |
| Osteoarthritis | Synovial fibroblasts Chondrocytes |
↑ Inflammation ↓ Chondrogenesis ↑ Osteoblastogenesis |
[156] | |
| Intervertebral Disc (IVD) Herniation | Disc Cells | ↑ Inflammation | [173] |
↑ = increased; ↓ = decrease.
In addition to the effects on pro-inflammatory responses, IL-20 is also involved in angiogenesis and fibrosis. IL-20 promotes angiogenesis by upregulating angiogenesis factors bFGF, VEGF, and MMP-2 to enhance proliferation, migration, and vascular tube formation of endothelial cells (human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HMECs)) [158,159]. IL-20 is expressed in liver tissues of patients with liver cirrhosis. IL-20 is increased in mice with CCl4-induced liver fibrosis. IL-20 induces TGF-β1 expression and arrests the cell cycle in the G0/G1-phase by upregulating p21 production in hepatocyte Clone-9 cells. IL-20 upregulates TNF-α, TGF-β1, and Col-I mRNA transcripts in hepatic stellate cells (HSCs) [158]. Moreover, IL-20 acts on lung epithelial cells (MLE-12) and promotes fibronectin-1 and α-SMA protein levels [160]. Hypoxia, a critical factor in the pathogenesis of kidney disease, also stimulates IL-20 expression in different type cells (HaCaT cells, HEK293 cells, chondrocytes, glioblastoma cells, and HUVECs).
14. IL-20 in AKI
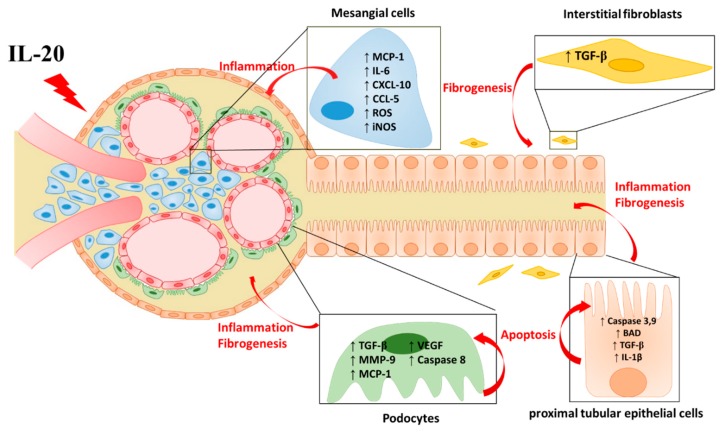
The leading causes of AKI are ischemia and nephrotoxicity. We previously showed that IL-20 and its receptors were upregulated in the kidneys of IRI- and HgCl2-induced AKI models, which implies that IL-20 may play a role in AKI. IL-20 not only upregulates TGF-β1 expression but also promotes cell death by activating caspase-9 in human proximal tubular epithelial cells (HK-2). Under hypoxic conditions, IL-1β transcript is increased by IL-20 in HK-2 cells. These data suggest that IL-20 may be associated with tubular cell death, tubulointerstitial fibrosis, and renal inflammation in the progression of AKI. In addition, the expression of IL-20 showed a similar trend to serum creatinine and BUN levels, which suggests that IL-20 contributes to the severity of AKI [161]. Renal cell death, fibrosis, and inflammation are important in AKI-to-CKD transition. IL-20 may also contribute to the progression from AKI to CKD.
15. IL-20 in CKD
We found that the serum levels of IL-20 were increased in patients with stage 5 CKD compared with healthy controls. Additionally, the cellular sources of IL-20 are mesangial cells and macrophages in the kidney of patients with lupus nephritis. IL-20 expression is positively correlated with the severity of lupus nephritis, which suggests that IL-20 may participate in the development of lupus nephritis. In 5/6 nephrectomy-induced CKD rats, IL-20 is elevated in the kidney. The tubular epithelial cells, interstitial immune cells, and glomerular mesangial cells are the major cellular sources of IL-20 [166,168]. IL-20 promotes cell arrest at G0/G1-phase and induces cell apoptosis via caspase-3 and BAD in mouse tubular epithelial cells (TKPTS and M-1). Pro-fibrotic factor TGF-β is induced by IL-20 in interstitial fibroblast (NRK-49F) cells [166]. IL-20 increases the mRNA transcripts of MCP-1, CXCL10, CCL5, and IL-6, as well as the ROS and iNOS generation in mesangial cells through ERK 1/2 activation [168]. Previous studies reported that MCP-1, CXCL10, and CCL5 promote leukocyte recruitment. Oxidative stress plays an essential role in the pathogenesis of various kidney diseases [174,175]. ROS and iNOS inhibit cell growth and induce cell death. Based on the roles of IL-20 in CKD, we speculate that the infiltrating inflammatory cells, mesangial cells, or renal epithelial cells produce IL-20 and then trigger fibroblasts to produce more fibrogenic factors. IL-20 induces cell apoptosis in epithelial cells. TGF-β1 secreted by IL-20-stimulated fibroblasts further induces mesangial cells or fibroblasts to synthesize various matrix proteins and promote ECM synthesis, which eventually leads to ESRD. All of these events promote CKD progression.
16. IL-20 in DN
We previously reported that IL-20 was upregulated in the serum of patients with diabetes mellitus. The level of IL-20 is significantly increased in diabetic patients with kidney dysfunction. These data suggest that IL-20 participates in the pathogenesis of DN. In addition, the expression of IL-20 and IL-20R1 was increased in the kidneys of streptozotocin (STZ)-induced diabetic mice, which indicates that IL-20R1 signal might be critical for IL-20-mediated biological function in this model. Interestingly, we observed that IL-20 was not detected in conditionally immortalized murine podocytes; however, IL-20 was highly expressed in podocytes of STZ-induced diabetes mice, which raises the possibility that IL-20 might be upregulated under pathological conditions. We further found that hydrogen peroxide, high glucose, and TGF-β1 stimulate podocytes to secrete IL-20, which supports our hypothesis. We discovered that IL-20R1, IL-20R2, and IL-22R1 were expressed in conditionally immortalized murine podocytes, which indicates that podocyte is a target cell for IL-20. IL-20 enhances MCP-1, TGF-β1, MMP-9, and VEGF expression in podocytes through ERK, JNK, and p38 pathway. IL-20 promotes podocyte apoptosis through activating caspase-8 [167]. These data support the notion that IL-20 is involved in the progression of DN and may also contribute to the cascade of inflammation and diabetic glomerulopathy.
17. IL-20 Antibody Therapy in Kidney Disease
In 1975, Kohler and Milstein successfully manufactured B lymphocyte and myeloma cell fusion cells (hybridoma), which opened the application of monoclonal antibodies. Ten years later, the FDA approved the first monoclonal antibody drug, murine IgG2a CD3 (also called muromonab), to be used in organ transplantation. Currently, there are more than 100 FDA-approved antibodies for treatment. A lot of monoclonal antibodies are used in clinical trials of kidney diseases such as Adalimumab, Fresolimumab, and Rituximab [176]. Several animal experiments showed that blockade of IL-20 can effectively attenuate inflammation and ameliorate the severity of liver fibrosis [164]. Inhibition of IL-20 with specific antibody reduces renal tubular damage and decreases TGF-β1 and IL-1β production in the kidney of HgCl2-induced AKI rats [161]. In the DN model, neutralizing IL-20 decreases urine albumin/creatinine ratio and improves STZ-induced renal structure damages, including glomerular hypertrophy and mesangial cell expansion. Moreover, anti-IL-20 mAb ameliorates renal inflammation through reducing iNOS, TNF-α, and MCP-1 expression in STZ-induced DN mice. Our previous study showed that only IL-20R1 was increased in the kidneys of diabetic mice, which suggests that IL-20R1 may be necessary for IL-20-mediated DN. Blood urea nitrogen (BUN) and glomerular hypertrophy were improved in IL-20R1-deficienct mice [177]. Furthermore, anti-IL-20R1 mAb inhibits ROS production in mesangial cells and reduces the protein level of TGF-β1 in fibroblasts. Together, these data indicate that anti-IL-20 mAb might have a therapeutic potential to ameliorate kidney disease, including renal hypertrophy, inflammation, and fibrosis.
Fletikumab, a recombinant human anti–IL-20 mAb, has been tested in two clinical trials, psoriasis and RA [178]. Under the dose range from 0.05 to 3.0 mg/kg, Fletikumab was tolerable and non-toxic in patients with psoriasis. However, the clinical study was terminated because no apparent efficacy was observed. In the phase 2a trial, Fletikumab significantly reduced tender joint counts and swollen joint counts in seropositive RA patients. However, this clinical study ended at phase 2b because it failed to meet the primary endpoint [178]. We expect to see new clinical trials to validate the efficacy of using anti–IL-20 mAb for treating kidney diseases in the near future.
IL-20 shares receptors with IL-19 and IL-24; thus, they might have similar biological functions. The expression of IL-19 is upregulated in human renal proximal tubule cells (RPTEC/TERT1) treated with nephrotoxic agents. The mRNA level of IL-19 and its receptors IL-20R1/IL-20R2 is upregulated in the kidney, lung, and liver of mice that underwent IRI. IL-19 upregulates TGF-β1 and MCP-1 expression in renal cortical collecting duct cells (M-1). IL-19 also activates caspase-3 and caspase-9 to promote cell apoptosis through the p38 MAPK pathway in M-1 cells [179]. IL-20R1 deficiency ameliorates IRI-induced AKI. The role of IL-19 in DN is still unclear. The level of IL-19 is increased in uremia and DM patients. Moreover, the extent of IL-19 is positively correlated with the severity of urinary albumin excretion [180,181]. However, the mechanism of IL-19 in DN awaits further investigation. IL-24 is known as an anti-tumor cytokine for inhibiting cancer cell growth. The role of IL-24 in kidney disease is little known. Pap et al. found that renal IL-19 and IL-24 are increased in UUO newborn rats. TGF-β treatment inhibits IL-24 expression in HK-2 cells [182]. Besides, IL-24 reduces H2O2-induced-cell apoptosis by inhibiting caspase-3 activity and ROS generation in HUVECs. In hypertensive rats, IL-24 mRNA and protein levels are significantly decreased in kidney and increased after administration of anti-hypertensive drugs. According to these findings, IL-24 may be a protective cytokine in hypertension. Hypertension is an important risk factor for the development of CKD. However, the effect of IL-24 in kidney disease needs to be identified in the future [183].
18. Conclusions
In patients with kidney disease, renal inflammation and fibrosis commonly occur in tubulointerstitium with tubular atrophy, ECM accumulation, and loss of peritubular capillaries. Several factors participate in the development of the progression of renal diseases AKI, CKD, and DN. IL-20 acts on renal cells and contributes to inflammation, fibrosis, and apoptosis through its receptors IL-20R1, IL-20R2, and IL-22R1. We summarized the renal target cells of IL-20 and the possible regulating role of IL-20 in the pathogenesis of kidney diseases (Figure 1). In animal studies, we observed that blockade of IL-20 could improve renal function and structure. Therefore, we expected that therapeutic targeting of IL-20 might be beneficial to those patients in the future.
Figure 1.
The effect of IL-20 in renal cells. IL-20 acts on interstitial fibroblasts, renal epithelial cells, mesangial cells, and podocytes and contributes to the progression of kidney disease, including inflammatory response, renal fibrosis, and cell apoptosis. ↑ = increased; ↓ = decrease.
Acknowledgments
We are thankful to professor Ming-Shi Chang, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan for providing many valuable opinions during the study.
Author Contributions
Y.-H.H. conceptualized the manuscript; T.-Y.L. and Y.-H.H. drafted the manuscript. Y.-H.H. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Science and Technology, Taiwan: 108-2320-B-006-052.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng X.-M., Tang P.M.-K., Li J., Lan H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper M.E. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z., Cooper M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011;2:243–247. doi: 10.1111/j.2040-1124.2011.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper M.E. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213–219. doi: 10.1016/S0140-6736(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 6.Chang M.-S., Hsu Y.-H. The role of IL-20 in chronic kidney disease and diabetic nephropathy: Pathogenic and therapeutic implications. J. Leukoc. Biol. 2018;104:919–923. doi: 10.1002/JLB.MR1217-489R. [DOI] [PubMed] [Google Scholar]
- 7.Kes P., Basić Jukić N. Acute kidney injury in the intensive care unit. Bosn. J. Basic Med. Sci. 2010;10:S8–S12. doi: 10.17305/bjbms.2010.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid S.-A., Adnan W.-N.-A., Naing N., Adnan A. Acute kidney injury in intensive care unit, hospital Universiti Sains Malaysia: A descriptive study. Saudi J. Kidney Dis. Transpl. 2018;29:1109–1114. doi: 10.4103/1319-2442.243961. [DOI] [PubMed] [Google Scholar]
- 9.Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ysebaert D.K., De Greef K.E., Vercauteren S.R., Ghielli M., Verpooten G.A., Eyskens E.J., De Broe M.E. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol. Dial. Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 11.Berger K., Moeller M.J. Mechanisms of Epithelial Repair and Regeneration After Acute Kidney Injury. Semin. Nephrol. 2014;34:394–403. doi: 10.1016/j.semnephrol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Awad A.S., Rouse M., Huang L., Vergis A.L., Reutershan J., Cathro H.P., Linden J., Okusa M.D. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes J.M., Hewitson T.D., Becker G.J., Jones C.L. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–2385. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Jo S.-K., Sung S.-A., Cho W.-Y., Go K.-J., Kim H.-K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 15.Kelly K.J., Williams W.W., Jr., Colvin R.B., Meehan S.M., Springer T.A., Gutierrez-Ramos J.C., Bonventre J.V. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Investig. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi S., Yuen D.A., Bajwa A., Huang Y.-W., Sokollik C., Huang L., Lam G.Y., Tole S., Liu G.-Y., Pan J., et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2013;24:1274–1287. doi: 10.1681/ASN.2012090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikbov B., Perico N., Remuzzi G. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139:313–318. doi: 10.1159/000489897. [DOI] [PubMed] [Google Scholar]
- 18.Kline J., Rachoin J.-S. Acute Kidney Injury and Chronic Kidney Disease: It’s a Two-Way Street. Ren. Fail. 2013;35:452–455. doi: 10.3109/0886022X.2013.766572. [DOI] [PubMed] [Google Scholar]
- 19.Chawla L.S., Kimmel P.L. Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 20.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishani A., Xue J.L., Himmelfarb J., Eggers P.W., Kimmel P.L., Molitoris B.A., Collins A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grgic I., Campanholle G., Bijol V., Wang C., Sabbisetti V.S., Ichimura T., Humphreys B.D., Bonventre J.V. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannu N., James M., Hemmelgarn B.R., Dong J., Tonelli M., Klarenbach S. Modification of Outcomes After Acute Kidney Injury by the Presence of CKD. Am. J. Kidney Dis. 2011;58:206–213. doi: 10.1053/j.ajkd.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Grams M.E., Astor B.C., Bash L.D., Matsushita K., Wang Y., Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J. Am. Soc. Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James M., Hemmelgarn B., Wiebe N., Pannu N., Manns B., Klarenbach S., Tonelli M. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 26.Vadivel N., Trikudanathan S., Singh A.K. Analgesic nephropathy. Kidney Int. 2007;72:517–520. doi: 10.1038/sj.ki.5002251. [DOI] [PubMed] [Google Scholar]
- 27.Bao L., Cunningham P.N., Quigg R.J. Complement in Lupus Nephritis: New Perspectives. Kidney Dis. 2015;1:91–99. doi: 10.1159/000431278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faurschou M., Starklint H., Halberg P., Jacobsen S. Prognostic factors in lupus nephritis: Diagnostic and therapeutic delay increases the risk of terminal renal failure. J. Rheumatol. 2006;33:1563. [PubMed] [Google Scholar]
- 29.Cheng Y., Yang X., Zhang X., An Z. Analysis of expression levels of IL-17 and IL-34 and influencing factors for prognosis in patients with lupus nephritis. Exp. Ther. Med. 2019;17:2279–2283. doi: 10.3892/etm.2019.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tylicki L., Rutkowski B. Hypertensive nephropathy: Pathogenesis, diagnosis and treatment. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2003;14:168–173. [PubMed] [Google Scholar]
- 31.D’ Amico G. The Commonest Glomerulonephritis in the World: IgA Nephropathy. QJM: An. Int. J. Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 32.Shu D., Xu F., Su Z., Zhang J., Chen C., Zhang J., Ding X., Lv Y., Lin H., Huang P. Risk factors of progressive IgA nephropathy which progress to end stage renal disease within ten years: A case-control study. BMC Nephrol. 2017;18:11. doi: 10.1186/s12882-016-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012;8:275–283. doi: 10.1038/nrneph.2012.58. [DOI] [PubMed] [Google Scholar]
- 34.Reidy K., Kang H.M., Hostetter T., Susztak K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noh H., King G.L. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. 2007;72:S49–S53. doi: 10.1038/sj.ki.5002386. [DOI] [PubMed] [Google Scholar]
- 36.Susztak K., Raff A.C., Schiffer M., Böttinger E.P. Glucose-Induced Reactive Oxygen Species Cause Apoptosis of Podocytes and Podocyte Depletion at the Onset of Diabetic Nephropathy. Diabetes. 2006;55:225. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 37.Weil E.J., Lemley K.V., Mason C.C., Yee B., Jones L.I., Blouch K., Lovato T., Richardson M., Myers B.D., Nelson R.G. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–1017. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J.S., Susztak K. Podocytes: The Weakest Link in Diabetic Kidney Disease? Curr. Diabetes Rep. 2016;16:45. doi: 10.1007/s11892-016-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tashiro K., Koyanagi I., Saitoh A., Shimizu A., Shike T., Ishiguro C., Koizumi M., Funabiki K., Horikoshi S., Shirato I., et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rovin B.H., Doe N., Tan L.C. Monocyte chemoattractant protein-1 levels in patients with glomerular disease. Am. J. Kidney Dis. 1996;27:640–646. doi: 10.1016/S0272-6386(96)90097-9. [DOI] [PubMed] [Google Scholar]
- 41.Viedt C., Dechend R., Fei J., Hänsch G.M., Kreuzer J., Orth S.R. MCP-1 Induces Inflammatory Activation of Human Tubular Epithelial Cells: Involvement of the Transcription Factors, Nuclear Factor-κB and Activating Protein-1. J. Am. Soc. Nephrol. 2002;13:1534. doi: 10.1097/01.ASN.0000015609.31253.7F. [DOI] [PubMed] [Google Scholar]
- 42.Giunti S., Pinach S., Arnaldi L., Viberti G., Perin P.C., Camussi G., Gruden G. The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 2006;69:856–863. doi: 10.1038/sj.ki.5000197. [DOI] [PubMed] [Google Scholar]
- 43.Rovin B.H., Yoshiumura T., Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J. Immunol. 1992;148:2148. [PubMed] [Google Scholar]
- 44.Prodjosudjadi W., Gerritsma J.S.J., Klar-Mohamad N., Gerritsen A.F., Bruijn J.A., Daha M.R., van Es L.A. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 45.Zoja C., Wang J.M., Bettoni S., Sironi M., Renzi D., Chiaffarino F., Abboud H.E., Van Damme J., Mantovani A., Remuzzi G. Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am. J. Pathol. 1991;138:991–1003. [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S.-H., Lu C., Dong L., Chen Z.-Q. Signal transduction involved in CTGF-induced production of chemokines in mesangial cells. Growth Factors. 2008;26:192–200. doi: 10.1080/08977190802227828. [DOI] [PubMed] [Google Scholar]
- 47.Ihm C.G., Park J.K., Hong S.P., Lee T.W., Cho B.S., Kim M.J., Cha D.R., Ha H. A High Glucose Concentration Stimulates the Expression of Monocyte Chemotactic Peptide 1 in Human Mesangial Cells. Nephron. 1998;79:33–37. doi: 10.1159/000044988. [DOI] [PubMed] [Google Scholar]
- 48.Ha H., Yu M.R., Choi Y.J., Kitamura M., Lee H.B. Role of High Glucose-Induced Nuclear Factor-κB Activation in Monocyte Chemoattractant Protein-1 Expression by Mesangial Cells. J. Am. Soc. Nephrol. 2002;13:894. doi: 10.1681/ASN.V134894. [DOI] [PubMed] [Google Scholar]
- 49.Banba N., Nakamura T., Matsumura M., Kuroda H., Hattori Y., Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–690. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 50.Chow F.Y., Nikolic-Paterson D.J., Ozols E., Atkins R.C., Rollin B.J., Tesch G.H. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 51.Wei M., Li Z., Xiao L., Yang Z. Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol. Immunol. 2015;68:261–271. doi: 10.1016/j.molimm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Park J., Ryu D.-R., Li J.J., Jung D.-S., Kwak S.-J., Lee S.H., Yoo T.-H., Han S.H., Lee J.E., Kim D.K., et al. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am. J. Physiol. Ren. Physiol. 2008;295:F749–F757. doi: 10.1152/ajprenal.00547.2007. [DOI] [PubMed] [Google Scholar]
- 53.Nam B.Y., Paeng J., Kim S.H., Lee S.H., Kim D.H., Kang H.-Y., Li J.J., Kwak S.-J., Park J.T., Yoo T.-H., et al. The MCP-1/CCR2 axis in podocytes is involved in apoptosis induced by diabetic conditions. Apoptosis. 2012;17:1–13. doi: 10.1007/s10495-011-0661-6. [DOI] [PubMed] [Google Scholar]
- 54.Lee E.Y., Chung C.H., Khoury C.C., Yeo T.K., Pyagay P.E., Wang A., Chen S. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am. J. Physiol. Ren. Physiol. 2009;297:F85–F94. doi: 10.1152/ajprenal.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashyap S., Osman M., Ferguson C.M., Nath M.C., Roy B., Lien K.R., Nath K.A., Garovic V.D., Lerman L.O., Grande J.P. Ccl2 deficiency protects against chronic renal injury in murine renovascular hypertension. Sci. Rep. 2018;8:8598. doi: 10.1038/s41598-018-26870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitagawa K., Wada T., Furuichi K., Hashimoto H., Ishiwata Y., Asano M., Takeya M., Kuziel W.A., Matsushima K., Mukaida N., et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanamori H., Matsubara T., Mima A., Sumi E., Nagai K., Takahashi T., Abe H., Iehara N., Fukatsu A., Okamoto H., et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem. Biophys. Res. Commun. 2007;360:772–777. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- 58.Furuichi K., Wada T., Iwata Y., Kitagawa K., Kobayashi K.-I., Hashimoto H., Ishiwata Y., Asano M., Wang H., Matsushima K., et al. CCR2 Signaling Contributes to Ischemia-Reperfusion Injury in Kidney. J. Am. Soc. Nephrol. 2003;14:2503. doi: 10.1097/01.ASN.0000089563.63641.A8. [DOI] [PubMed] [Google Scholar]
- 59.Kashyap S., Warner G.M., Hartono S.P., Boyilla R., Knudsen B.E., Zubair A.S., Lien K., Nath K.A., Textor S.C., Lerman L.O., et al. Blockade of CCR2 reduces macrophage influx and development of chronic renal damage in murine renovascular hypertension. Am. J. Physiol. Ren. Physiol. 2015;310:F372–F384. doi: 10.1152/ajprenal.00131.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stroo I., Claessen N., Teske G.J.D., Butter L.M., Florquin S., Leemans J.C. Deficiency for the chemokine monocyte chemoattractant protein-1 aggravates tubular damage after renal ischemia/reperfusion injury. PLoS ONE. 2015;10:e0123203. doi: 10.1371/journal.pone.0123203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown Z., Strieter R.M., Chensue S.W., Ceska M., Lindley I., Neild G.H., Kunkel S.L., Westwick J. Cytokine-activated human mesangial cells generate the neutrophil chemoattractant, interleukin 8. Kidney Int. 1991;40:86–90. doi: 10.1038/ki.1991.184. [DOI] [PubMed] [Google Scholar]
- 62.Abbott F., Ryan J.J., Ceska M., Matsushima K., Sarraf C.E., Rees A.J. Interleukin-1β stimulates human mesangial cells to synthesize and release interleukins-6 and-8. Kidney Int. 1991;40:597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- 63.Kusner D.J., Luebbers E.L., Nowinski R.J., Konieczkowski M., King C.H., Sedor J.R. Cytokine- and LPS-induced synthesis of interleukin-8 from human mesangial cells. Kidney Int. 1991;39:1240–1248. doi: 10.1038/ki.1991.157. [DOI] [PubMed] [Google Scholar]
- 64.Li H., Nord E.P. IL-8 amplifies CD40/CD154-mediated ICAM-1 production via the CXCR-1 receptor and p38-MAPK pathway in human renal proximal tubule cells. Am. J. Physiol.Ren. Physiol. 2009;296:F438–F445. doi: 10.1152/ajprenal.90214.2008. [DOI] [PubMed] [Google Scholar]
- 65.Gerritsma J.S., Hiemstra P.S., Gerritsen A.F., Prodjosudjadi W., Verweij C.L., Van Es L.A., Daha M.R. Regulation and production of IL-8 by human proximal tubular epithelial cells in vitro. Clin. Exp. Immunol. 1996;103:289–294. doi: 10.1046/j.1365-2249.1996.d01-617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wada T., Tomosugi N., Naito T., Yokoyama H., Kobayashi K., Harada A., Mukaida N., Matsushima K. Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J. Exp. Med. 1994;180:1135–1140. doi: 10.1084/jem.180.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L., Khan N., Li Q., Chen X., Wei J., Wang B., Cheng J.-W., Gordon J., Li F. G31P, CXCR1/2 inhibitor, with cisplatin inhibits the growth of mice hepatocellular carcinoma and mitigates high-dose cisplatin-induced nephrotoxicity. Oncol. Rep. 2014;33:751–757. doi: 10.3892/or.2014.3659. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y., Xu W., Zhu H. CXCL8(3–72) K11R/G31P protects against sepsis-induced acute kidney injury via NF-κB and JAK2/STAT3 pathway. Biol. Res. 2019;52:29. doi: 10.1186/s40659-019-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui S., Zhu Y., Du J., Khan M.N., Wang B., Wei J., Cheng J.-W., Gordon J.R., Mu Y., Li F. CXCL8 Antagonist Improves Diabetic Nephropathy in Male Mice With Diabetes and Attenuates High Glucose–Induced Mesangial Injury. Endocrinology. 2017;158:1671–1684. doi: 10.1210/en.2016-1781. [DOI] [PubMed] [Google Scholar]
- 70.Bertani T., Abbate M., Zoja C., Corna D., Perico N., Ghezzi P., Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am. J. Pathol. 1989;134:419–430. [PMC free article] [PubMed] [Google Scholar]
- 71.Peralta Soler A., Mullin J.M., Knudsen K.A., Marano C.W. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am. J. Physiol. Ren. Physiol. 1996;270:F869–F879. doi: 10.1152/ajprenal.1996.270.5.F869. [DOI] [PubMed] [Google Scholar]
- 72.Meldrum K.K., Meldrum D.R., Hile K.L., Yerkes E.B., Ayala A., Cain M.P., Rink R.C., Casale A.J., Kaefer M.A. p38 MAPK mediates renal tubular cell TNF-α production and TNF-α-dependent apoptosis during simulated ischemia. Am. J. Physiol. Cell Physiol. 2001;281:C563–C570. doi: 10.1152/ajpcell.2001.281.2.C563. [DOI] [PubMed] [Google Scholar]
- 73.Guo Y.-L., Baysal K., Kang B., Yang L.-J., Williamson J.R. Correlation between Sustained c-Jun N-terminal Protein Kinase Activation and Apoptosis Induced by Tumor Necrosis Factor-α in Rat Mesangial Cells. J. Biol. Chem. 1998;273:4027–4034. doi: 10.1074/jbc.273.7.4027. [DOI] [PubMed] [Google Scholar]
- 74.Lampropoulou I.-T., Stangou M., Papagianni A., Didangelos T., Iliadis F., Efstratiadis G. TNF-α and Microalbuminuria in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014;2014:7. doi: 10.1155/2014/394206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Navarro J.F., Mora C., Muros M., García J. Urinary tumour necrosis factor-α excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol. Dial. Transplant. 2006;21:3428–3434. doi: 10.1093/ndt/gfl469. [DOI] [PubMed] [Google Scholar]
- 76.McCarthy E.T., Sharma R., Sharma M., Li J.Z., Ge X.L., Dileepan K.N., Savin V.J. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J. Am. Soc. Nephrol. 1998;9:433. doi: 10.1681/ASN.V93433. [DOI] [PubMed] [Google Scholar]
- 77.Awad A.S., You H., Gao T., Cooper T.K., Nedospasov S.A., Vacher J., Wilkinson P.F., Farrell F.X., Brian Reeves W. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88:722–733. doi: 10.1038/ki.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao G., Zhang B., Ramesh G., Betterly D., Tadagavadi R.K., Wang W., Reeves W.B. TNF-α mediates increased susceptibility to ischemic AKI in diabetes. Am. J. Physiol. Ren. Physiol. 2013;304:F515–F521. doi: 10.1152/ajprenal.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H., Li J., Gai Z., Kullak-Ublick G.A., Liu Z. TNF-α Deficiency Prevents Renal Inflammation and Oxidative Stress in Obese Mice. Kidney Blood Press. Res. 2017;42:416–427. doi: 10.1159/000478869. [DOI] [PubMed] [Google Scholar]
- 80.Karkar A.M., Smith J., Pusey C.D. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-α. Nephrol. Dial. Transplant. 2001;16:518–524. doi: 10.1093/ndt/16.3.518. [DOI] [PubMed] [Google Scholar]
- 81.Wen Y., Rudemiller N.P., Zhang J., Robinette T., Lu X., Ren J., Privratsky J.R., Nedospasov S.A., Crowley S.D. TNF-α in T lymphocytes attenuates renal injury and fibrosis during nephrotoxic nephritis. Am. J. Physiol. Ren. Physiol. 2019;318:F107–F116. doi: 10.1152/ajprenal.00347.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Timoshanko J.R., Kitching A.R., Iwakura Y., Holdsworth S.R., Tipping P.G. Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am. J. Pathol. 2004;164:1967–1977. doi: 10.1016/S0002-9440(10)63757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niemir Z.I., Stein H., Dworacki G., Mundel P., Koehl N., Koch B., Autschbach F., Andrassy K., Ritz E., Waldherr R., et al. Podocytes are the major source of IL-1α and IL-1β in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- 84.Anders H.-J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016;27:2564. doi: 10.1681/ASN.2016020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miao N.-j., Xie H.-y., Xu D., Yin J.-y., Wang Y.-z., Wang B., Yin F., Zhou Z.-l., Cheng Q., Chen P.-P., et al. Caspase-11 promotes renal fibrosis by stimulating IL-1β maturation via activating caspase-1. Acta Pharmacol. Sin. 2019;40:790–800. doi: 10.1038/s41401-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai Y.-L., Hua K.-F., Chen A., Wei C.-W., Chen W.-S., Wu C.-Y., Chu C.-L., Yu Y.-L., Lo C.-W., Ka S.-M. NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy. Sci. Rep. 2017;7:41123. doi: 10.1038/srep41123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chow F.Y., Nikolic-Paterson D.J., Atkins R.C., Tesch G.H. Macrophages in streptozotocin-induced diabetic nephropathy: Potential role in renal fibrosis. Nephrol. Dial. Transplant. 2004;19:2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 88.Vesey D.A., Cheung C., Cuttle L., Endre Z., Gobe G., Johnson D.W. Interleukin-1β stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-β-dependent mechanism. J. Lab. Clin. Med. 2002;140:342–350. doi: 10.1067/mlc.2002.128468. [DOI] [PubMed] [Google Scholar]
- 89.Lovett D.H., Ryan J.L., Sterzel R.B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J. Immunol. 1983;131:2830. [PubMed] [Google Scholar]
- 90.Lonnemann G., Engler-Blum G., Müller G.A., Koch K.M., Dubarello C.A. Cytokines in human renal interstitial fibrosis. II. Intrinsic interleukin (IL)-1 synthesis and IL-1-dependent production of IL-6 and IL-8 by cultured kidney fibroblasts. Kidney Int. 1995;47:845–854. doi: 10.1038/ki.1995.127. [DOI] [PubMed] [Google Scholar]
- 91.Robson R.L., Westwick J., Brown Z. Interleukin-1-induced IL-8 and IL-6 gene expression and production in human mesangial cells is differentially regulated by cAMP. Kidney Int. 1995;48:1767–1777. doi: 10.1038/ki.1995.475. [DOI] [PubMed] [Google Scholar]
- 92.Wu M., Han W., Song S., Du Y., Liu C., Chen N., Wu H., Shi Y., Duan H. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell. Endocrinol. 2018;478:115–125. doi: 10.1016/j.mce.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Torbohm I., Berger B., Schönermark M., von Kempis J., Rother K., Hänsch G.M. Modulation of collagen synthesis in human glomerular epithelial cells by interleukin 1. Clin. Exp. Immunol. 1989;75:427–431. [PMC free article] [PubMed] [Google Scholar]
- 94.Fan J.-M., Huang X.-R., Ng Y.-Y., Nikolic-Paterson D.J., Mu W., Atkins R.C., Lan H.Y. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-β1-dependent mechanism in vitro. Am. J. Kidney Dis. 2001;37:820–831. doi: 10.1016/S0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- 95.Zhang M., Tang J., Li X. Interleukin-1β-Induced Transdifferentiation of Renal Proximal Tubular Cells Is Mediated by Activation of JNK and p38 MAPK. Nephron Exp. Nephrol. 2005;99:e68–e76. doi: 10.1159/000083414. [DOI] [PubMed] [Google Scholar]
- 96.Shahzad K., Bock F., Dong W., Wang H., Kopf S., Kohli S., Al-Dabet M.D.M., Ranjan S., Wolter J., Wacker C., et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Timoshanko J.R., Kitching A.R., Iwakura Y., Holdsworth S.R., Tipping P.G. Contributions of IL-1β and IL-1α to Crescentic Glomerulonephritis in Mice. J. Am. Soc. Nephrol. 2004;15:910. doi: 10.1097/01.ASN.0000115704.86897.F4. [DOI] [PubMed] [Google Scholar]
- 98.Lei Y., Devarapu S.K., Motrapu M., Cohen C.D., Lindenmeyer M.T., Moll S., Kumar S.V., Anders H.-J. Interleukin-1β Inhibition for Chronic Kidney Disease in Obese Mice With Type 2 Diabetes. Front. Immunol. 2019;10:1223. doi: 10.3389/fimmu.2019.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Z., Shao X., Jiang N., Mou S., Gu L., Li S., Lin Q., He Y., Zhang M., Zhou W., et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 2018;9:983. doi: 10.1038/s41419-018-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lorenz G., Darisipudi M.N., Anders H.-J. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol. Dial. Transplant. 2013;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 101.Chung S.D., Lai T.Y., Chien C.T., Yu H.J. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS ONE. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang J.-R., Yao F.-H., Zhang J.-G., Ji Z.-Y., Li K.-L., Zhan J., Tong Y.-N., Lin L.-R., He Y.-N. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am. J. Physiol. Ren. Physiol. 2013;306:F75–F84. doi: 10.1152/ajprenal.00117.2013. [DOI] [PubMed] [Google Scholar]
- 103.Kim H.-J., Lee D.W., Ravichandran K., O Keys D., Akcay A., Nguyen Q., He Z., Jani A., Ljubanovic D., Edelstein C.L. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J. Pharm. Exp. Ther. 2013;346:465–472. doi: 10.1124/jpet.113.205732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vilaysane A., Chun J., Seamone M.E., Wang W., Chin R., Hirota S., Li Y., Clark S.A., Tschopp J., Trpkov K., et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hutton H.L., Ooi J.D., Holdsworth S.R., Kitching A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology. 2016;21:736–744. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 106.Wu H., Craft M.L., Wang P., Wyburn K.R., Chen G., Ma J., Hambly B., Chadban S.J. IL-18 contributes to renal damage after ischemia-reperfusion. J. Am. Soc. Nephrol. 2008;19:2331–2341. doi: 10.1681/ASN.2008020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bani-Hani A.H., Leslie J.A., Asanuma H., Dinarello C.A., Campbell M.T., Meldrum D.R., Zhang H., Hile K., Meldrum K.K. IL-18 neutralization ameliorates obstruction-induced epithelial–mesenchymal transition and renal fibrosis. Kidney Int. 2009;76:500–511. doi: 10.1038/ki.2009.216. [DOI] [PubMed] [Google Scholar]
- 108.Kitching A.R., Turner A.L., Wilson G.R.A., Semple T., Odobasic D., Timoshanko J.R., O’Sullivan K.M., Tipping P.G., Takeda K., Akira S., et al. IL-12p40 and IL-18 in Crescentic Glomerulonephritis: IL-12p40 is the Key Th1-Defining Cytokine Chain, Whereas IL-18 Promotes Local Inflammation and Leukocyte Recruitment. J. Am. Soc. Nephrol. 2005;16:2023. doi: 10.1681/ASN.2004121075. [DOI] [PubMed] [Google Scholar]
- 109.Moutabarrik A., Nakanishi I., Ishibashi M. Interleukin-6 and Interleukin-6 Receptor are Expressed by Cultured Glomerular Epithelial Cells. Scand. J. Immunol. 1994;40:181–186. doi: 10.1111/j.1365-3083.1994.tb03448.x. [DOI] [PubMed] [Google Scholar]
- 110.Boswell R.N., Yard B.A., Schrama E., van Es L.A., Daha M.R., van der Woude F.J. Interleukin 6 production by human proximal tubular epithelial cells in vitro: Analysis of the effects of interleukin-1α (IL-1α) and other cytokines. Nephrol. Dial. Transplant. 1994;9:599–606. doi: 10.1093/ndt/9.6.599. [DOI] [PubMed] [Google Scholar]
- 111.van den Dobbelsteen M.E.A., van der Woude F.J., Schroeijers W.E.M., van Es L.A., Daha M.R. Soluble aggregates of IgG and immune complexes enhance IL-6 production by renal mesangial cells. Kidney Int. 1993;43:544–553. doi: 10.1038/ki.1993.81. [DOI] [PubMed] [Google Scholar]
- 112.Ruef C., Budde K., Lacy J., Northemann W., Baumann M., Sterzel R.B., Coleman D.L. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int. 1990;38:249–257. doi: 10.1038/ki.1990.193. [DOI] [PubMed] [Google Scholar]
- 113.Su H., Lei C.-T., Zhang C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017;8:405. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. USA. 1989;86:7547–7751. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coletta I., Soldo L., Polentarutti N., Mancini F., Guglielmotti A., Pinza M., Mantovani A., Milanese C. Selective Induction of MCP-1 in Human Mesangial Cells by the IL-6/sIL-6R Complex. Nephron Exp. Nephrol. 2000;8:37–43. doi: 10.1159/000059327. [DOI] [PubMed] [Google Scholar]
- 116.Kim D.I., Park S.H. Sequential signaling cascade of IL-6 and PGC-1α is involved in high glucose-induced podocyte loss and growth arrest. Biochem. Biophys. Res. Commun. 2013;435:702–707. doi: 10.1016/j.bbrc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 117.Nechemia-Arbely Y., Barkan D., Pizov G., Shriki A., Rose-John S., Galun E., Axelrod J.H. IL-6/IL-6R Axis Plays a Critical Role in Acute Kidney Injury. J. Am. Soc. Nephrol. 2008;19:1106. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel N.S.A., Chatterjee P.K., Di Paola R., Mazzon E., Britti D., De Sarro A., Cuzzocrea S., Thiemermann C. Endogenous Interleukin-6 Enhances the Renal Injury, Dysfunction, and Inflammation Caused by Ischemia/Reperfusion. J. Pharmacol. Exp. Ther. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 119.Chen W., Yuan H., Cao W., Wang T., Chen W., Yu H., Fu Y., Jiang B., Zhou H., Guo H., et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics. 2019;9:3980–3991. doi: 10.7150/thno.32352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karkar A.M., Smith J., Tam F.W.K., Pusey C.D., Rees A.J. Abrogation of glomerular injury in nephrotoxic nephritis by continuous infusion of interleukin-6. Kidney Int. 1997;52:1313–1320. doi: 10.1038/ki.1997.456. [DOI] [PubMed] [Google Scholar]
- 121.Strutz F., Zeisberg M., Renziehausen A., Raschke B., Becker V., Van Kooten C., Müller G.A. TGF-β1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2) Kidney Int. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 122.Yang J., Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chung S., Overstreet J.M., Li Y., Wang Y., Niu A., Wang S., Fan X., Sasaki K., Jin G.-N., Khodo S.N., et al. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 2018;3:e123563. doi: 10.1172/jci.insight.123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schiffer M., Bitzer M., Roberts I.S., Kopp J.B., ten Dijke P., Mundel P., Böttinger E.P. Apoptosis in podocytes induced by TGF-beta and Smad7. J. Clin. Investig. 2001;108:807–816. doi: 10.1172/JCI200112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Das R., Xu S., Nguyen T.T., Quan X., Choi S.-K., Kim S.-J., Lee E.Y., Cha S.-K., Park K.-S. Transforming Growth Factor β1-induced Apoptosis in Podocytes via the Extracellular Signal-regulated Kinase-Mammalian Target of Rapamycin Complex 1-NADPH Oxidase 4 Axis. J. Biol. Chem. 2015;290:30830–30842. doi: 10.1074/jbc.M115.703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ziyadeh F.N., Hoffman B.B., Han D.C., Iglesias-De La Cruz M.C., Hong S.W., Isono M., Chen S., McGowan T.A., Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gewin L., Vadivelu S., Neelisetty S., Srichai M.B., Paueksakon P., Pozzi A., Harris R.C., Zent R. Deleting the TGF-β receptor attenuates acute proximal tubule injury. J. Am. Soc. Nephrol. 2012;23:2001–2011. doi: 10.1681/ASN.2012020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulkarni A.B., Ward J.M., Yaswen L., Mackall C.L., Bauer S.R., Huh C.G., Gress R.E., Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am. J. Pathol. 1995;146:264–275. [PMC free article] [PubMed] [Google Scholar]
- 129.Fujimoto M., Maezawa Y., Yokote K., Joh K., Kobayashi K., Kawamura H., Nishimura M., Roberts A.B., Saito Y., Mori S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem. Biophys. Res. Commun. 2003;305:1002–1007. doi: 10.1016/S0006-291X(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 130.Sato M., Muragaki Y., Saika S., Roberts A.B., Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003;112:1486–1494. doi: 10.1172/JCI200319270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lan H.Y., Mu W., Tomita N., Huang X.R., Li J.H., Zhu H.-J., Morishita R., Johnson R.J. Inhibition of Renal Fibrosis by Gene Transfer of Inducible Smad7 Using Ultrasound-Microbubble System in Rat UUO Model. J. Am. Soc. Nephrol. 2003;14:1535. doi: 10.1097/01.ASN.0000067632.04658.B8. [DOI] [PubMed] [Google Scholar]
- 132.Hou C.-C., Wang W., Huang X.R., Fu P., Chen T.-H., Sheikh-Hamad D., Lan H.Y. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am. J. Pathol. 2005;166:761–771. doi: 10.1016/S0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ka S.M., Yeh Y.C., Huang X.R., Chao T.K., Hung Y.J., Yu C.P., Lin T.J., Wu C.C., Lan H.Y., Chen A. Kidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia. 2012;55:509–519. doi: 10.1007/s00125-011-2364-5. [DOI] [PubMed] [Google Scholar]
- 134.Chen H.Y., Huang X.R., Wang W., Li J.H., Heuchel R.L., Chung A.C.K., Lan H.Y. The protective role of Smad7 in diabetic kidney disease: Mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nangaku M. Chronic Hypoxia and Tubulointerstitial Injury: A Final Common Pathway to End-Stage Renal Failure. J. Am. Soc. Nephrol. 2006;17:17. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 136.Tanaka S., Tanaka T., Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 137.Fine L.G., Bandyopadhay D., Norman J.T. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. 2000;57:S22–S26. doi: 10.1046/j.1523-1755.2000.07512.x. [DOI] [PubMed] [Google Scholar]
- 138.Singh P., Ricksten S.-E., Bragadottir G., Redfors B., Nordquist L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin. Exp. Pharm. Physiol. 2013;40:138–147. doi: 10.1111/1440-1681.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sada K., Nishikawa T., Kukidome D., Yoshinaga T., Kajihara N., Sonoda K., Senokuchi T., Motoshima H., Matsumura T., Araki E. Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1. PLoS ONE. 2016;11:e0158619. doi: 10.1371/journal.pone.0158619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Basile D.P., Friedrich J.L., Spahic J., Knipe N., Mang H., Leonard E.C., Changizi-Ashtiyani S., Bacallao R.L., Molitoris B.A., Sutton T.A. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Ren. Physiol. 2010;300:F721–F733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Basile D.P., Donohoe D., Roethe K., Osborn J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Ren. Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.00050.2001. [DOI] [PubMed] [Google Scholar]
- 142.Mazzali M., Jefferson J.A., Ni Z., Vaziri N.D., Johnson R.J. Microvascular and tubulointerstitial injury associated with chronic hypoxia-induced hypertension. Kidney Int. 2003;63:2088–2093. doi: 10.1046/j.1523-1755.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 143.Norman J.T., Clark I.M., Garcia P.L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 144.Matsumoto M., Makino Y., Tanaka T., Tanaka H., Ishizaka N., Noiri E., Fujita T., Nangaku M. Induction of Renoprotective Gene Expression by Cobalt Ameliorates Ischemic Injury of the Kidney in Rats. J. Am. Soc. Nephrol. 2003;14:1825. doi: 10.1097/01.ASN.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 145.Ohtomo S., Nangaku M., Izuhara Y., Takizawa S., Strihou C.V.Y.D., Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol. Dial. Transplant. 2007;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 146.Brigandi R.A., Johnson B., Oei C., Westerman M., Olbina G., de Zoysa J., Roger S.D., Sahay M., Cross N., McMahon L., et al. A Novel Hypoxia-Inducible Factor−Prolyl Hydroxylase Inhibitor (GSK1278863) for Anemia in CKD: A 28-Day, Phase 2A Randomized Trial. Am. J. Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 147.Pergola P.E., Spinowitz B.S., Hartman C.S., Maroni B.J., Haase V.H. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 148.Provenzano R., Besarab A., Sun C.H., Diamond S.A., Durham J.H., Cangiano J.L., Aiello J.R., Novak J.E., Lee T., Leong R., et al. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2016;11:982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Holdstock L., Meadowcroft A.M., Maier R., Johnson B.M., Jones D., Rastogi A., Zeig S., Lepore J.J., Cobitz A.R. Four-Week Studies of Oral Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor GSK1278863 for Treatment of Anemia. J. Am. Soc. Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Uchida T., Rossignol F., Matthay M.A., Mounier R., Couette S., Clottes E., Clerici C. Prolonged Hypoxia Differentially Regulates Hypoxia-inducible Factor (HIF)-1α and HIF-2α Expression in Lung Epithelial Cells: IMPLICATION OF NATURAL ANTISENSE HIF-1α. J. Biol. Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 151.Koh M.Y., Powis G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosenberger C., Heyman S.N., Rosen S., Shina A., Goldfarb M., Griethe W., Frei U., Reinke P., Bachmann S., Eckardt K.-U. Up-regulation of HIF in experimental acute renal failure: Evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005;67:531–542. doi: 10.1111/j.1523-1755.2005.67110.x. [DOI] [PubMed] [Google Scholar]
- 153.Ginouvès A., Ilc K., Macías N., Pouysségur J., Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFα and protects cells from necrosis. Proc. Natl. Acad. Sci. USA. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marxsen J.H., Stengel P., Doege K., Heikkinen P., Jokilehto T., Wagner T., Jelkmann W., Jaakkola P., Metzen E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem. J. 2004;381:761–767. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hsu Y.-H., Li H.-H., Hsieh M.-Y., Liu M.-F., Huang K.-Y., Chin L.-S., Chen P.-C., Cheng H.-H., Chang M.-S. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54:2722–2733. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- 156.Hsu Y.-H., Yang Y.-Y., Huwang M.-H., Weng Y.-H., Jou I.M., Wu P.-T., Lin T.-Y., Wu L.-W., Chang M.-S. Anti-IL-20 monoclonal antibody inhibited inflammation and protected against cartilage destruction in murine models of osteoarthritis. PLoS ONE. 2017;12:e0175802. doi: 10.1371/journal.pone.0175802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hsu Y.-H., Wei C.-C., Shieh D.-B., Chan C.-H., Chang M.-S. Anti–IL-20 Monoclonal Antibody Alleviates Inflammation in Oral Cancer and Suppresses Tumor Growth. Mol. Cancer Res. 2012;10:1430. doi: 10.1158/1541-7786.MCR-12-0276. [DOI] [PubMed] [Google Scholar]
- 158.Chen W.-Y., Cheng B.-C., Jiang M.-J., Hsieh M.-Y., Chang M.-S. IL-20 Is Expressed in Atherosclerosis Plaques and Promotes Atherosclerosis in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2090–2095. doi: 10.1161/01.ATV.0000232502.88144.6f. [DOI] [PubMed] [Google Scholar]
- 159.Hsieh M.Y., Chen W.Y., Jiang M.J., Cheng B.C., Huang T.Y., Chang M.S. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–242. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- 160.Gong W., Wang X., Zhang Y., Hao J., Xing C., Chu Q., Wang G., Zhao J., Wang J., Dong Q., et al. Interleukin-20 Promotes Airway Remodeling in Asthma. Inflammation. 2014;37:2099–2105. doi: 10.1007/s10753-014-9944-8. [DOI] [PubMed] [Google Scholar]
- 161.Li H.H., Hsu Y.H., Wei C.C., Lee P.T., Chen W.C., Chang M.S. Interleukin-20 induced cell death in renal epithelial cells and was associated with acute renal failure. Genes Immun. 2008;9:395–404. doi: 10.1038/gene.2008.28. [DOI] [PubMed] [Google Scholar]
- 162.Chen W.-Y., Chang M.-S. IL-20 Is Regulated by Hypoxia-Inducible Factor and Up-Regulated after Experimental Ischemic Stroke. J. Immunol. 2009;182:5003. doi: 10.4049/jimmunol.0803653. [DOI] [PubMed] [Google Scholar]
- 163.Chiu Y.-S., Hsing C.-H., Li C.-F., Lee C.-Y., Hsu Y.-H., Chang M.-S. Anti-IL-20 monoclonal antibody inhibited tumor growth in hepatocellular carcinoma. Sci. Rep. 2017;7:17609. doi: 10.1038/s41598-017-17054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiu Y.-S., Wei C.-C., Lin Y.-J., Hsu Y.-H., Chang M.-S. IL-20 and IL-20R1 antibodies protect against liver fibrosis. Hepatology. 2014;60:1003–1014. doi: 10.1002/hep.27189. [DOI] [PubMed] [Google Scholar]
- 165.Mayer C., Bergholdt R., Cucak H., Rolin B.C., Sams A., Rosendahl A. Neutralizing Anti-IL20 Antibody Treatment Significantly Modulates Low Grade Inflammation without Affecting HbA1c in Type 2 Diabetic db/db Mice. PLoS ONE. 2015;10:e0131306. doi: 10.1371/journal.pone.0131306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei C.-C., Li H.-H., Hsu Y.-H., Hsing C.-H., Sung J.-M., Chang M.-S. Interleukin-20 targets renal cells and is associated with chronic kidney disease. Biochem. Biophys. Res. Commun. 2008;374:448–453. doi: 10.1016/j.bbrc.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 167.Hsu Y.-H., Li H.-H., Sung J.-M., Chen W.-Y., Hou Y.-C., Weng Y.-H., Lai W.-T., Wu C.-H., Chang M.-S. Interleukin-20 targets podocytes and is upregulated in experimental murine diabetic nephropathy. Exp. Mol. Med. 2017;49:e310. doi: 10.1038/emm.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li H.-H., Cheng H.-H., Sun K.-H., Wei C.-C., Li C.-F., Chen W.-C., Wu W.-M., Chang M.-S. Interleukin-20 targets renal mesangial cells and is associated with lupus nephritis. Clin. Immunol. 2008;129:277–285. doi: 10.1016/j.clim.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 169.Wei C.-C., Chen W.-Y., Wang Y.-C., Chen P.-J., Lee J.Y.-Y., Wong T.-W., Chen W.C., Wu J.-C., Chen G.-y., Chang M.-S., et al. Detection of IL-20 and its receptors on psoriatic skin. Clin. Immunol. 2005;117:65–72. doi: 10.1016/j.clim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 170.Stenderup K., Rosada C., Worsaae A., Clausen J.T., Norman Dam T. Interleukin-20 as a Target in Psoriasis Treatment. Ann. N.Y. Acad. Sci. 2007;1110:368–381. doi: 10.1196/annals.1423.039. [DOI] [PubMed] [Google Scholar]
- 171.Kragstrup T.W., Andersen M.N., Schiøttz-Christensen B., Jurik A.G., Hvid M., Deleuran B. Increased interleukin (IL)-20 and IL-24 target osteoblasts and synovial monocytes in spondyloarthritis. Clin. Exp. Immunol. 2017;189:342–351. doi: 10.1111/cei.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsu Y.-H., Chen W.-Y., Chan C.-H., Wu C.-H., Sun Z.-J., Chang M.-S. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J. Exp. Med. 2011;208:1849–1861. doi: 10.1084/jem.20102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang K.-Y., Lin R.M., Chen W.-Y., Lee C.-L., Yan J., Chang M.-S. IL20 May Contribute to the Pathogenesis of Human Intervertebral Disc Herniation. Spine. 2008;33:2034–2040. doi: 10.1097/BRS.0b013e31817eb872. [DOI] [PubMed] [Google Scholar]
- 174.Jha J.C., Banal C., Chow B.S.M., Cooper M.E., Jandeleit-Dahm K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Daenen K., Andries A., Mekahli D., Van Schepdael A., Jouret F., Bammens B. Oxidative stress in chronic kidney disease. Pediatric Nephrol. 2019;34:975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- 176.Manrique J., Cravedi P. Role of monoclonal antibodies in the treatment of immune-mediated glomerular diseases. Nefrol. Publ. Of. de La Soc. Esp. Nefrol. 2014;34:388–397. doi: 10.3265/Nefrologia.pre2014.Feb.12506. [DOI] [PubMed] [Google Scholar]
- 177.Hsu Y.-H., Chang M.-S. Interleukin-20 antibody is a potential therapeutic agent for experimental arthritis. Arthritis Rheum. 2010;62:3311–3321. doi: 10.1002/art.27689. [DOI] [PubMed] [Google Scholar]
- 178.Kragstrup T.W., Andersen T., Heftdal L.D., Hvid M., Gerwien J., Sivakumar P., Taylor P.C., Senolt L., Deleuran B. The IL-20 Cytokine Family in Rheumatoid Arthritis and Spondyloarthritis. Front. Immunol. 2018;9:2226. doi: 10.3389/fimmu.2018.02226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hsu Y.-H., Li H.-H., Sung J.-M., Chen W.-T., Hou Y.-C., Chang M.-S. Interleukin-19 mediates tissue damage in murine ischemic acute kidney injury. PLoS ONE. 2013;8:e56028. doi: 10.1371/journal.pone.0056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jennings P., Crean D., Aschauer L., Limonciel A., Moenks K., Kern G., Hewitt P., Lhotta K., Lukas A., Wilmes A., et al. Interleukin-19 as a translational indicator of renal injury. Arch. Toxicol. 2015;89:101–106. doi: 10.1007/s00204-014-1237-3. [DOI] [PubMed] [Google Scholar]
- 181.Li L., Jiang X.-G., Hu J.-Y., Yu Z.-Q., Xu J.-Y., Liu F., Zhao G.-C., Zhang L., Gu H.-M., Zhang S.-J., et al. The association between interleukin-19 concentration and diabetic nephropathy. BMC Nephrol. 2017;18:65. doi: 10.1186/s12882-017-0488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pap D., Sziksz E., Kiss Z., Rokonay R., Veres-Székely A., Lippai R., Takács I.M., Kis E., Fekete A., Reusz G., et al. Microarray Analysis Reveals Increased Expression of Matrix Metalloproteases and Cytokines of Interleukin-20 Subfamily in the Kidneys of Neonate Rats Underwent Unilateral Ureteral Obstruction: A Potential Role of IL-24 in the Regulation of Inflammation and Tissue Remodeling. Kidney Blood Press. Res. 2017;42:16–32. doi: 10.1159/000464317. [DOI] [PubMed] [Google Scholar]
- 183.Wang Z., Wang Y., Chen Y., Lv J. The IL-24 gene protects human umbilical vein endothelial cells against H2O2-induced injury and may be useful as a treatment for cardiovascular disease. Int. J. Mol. Med. 2016;37:581–592. doi: 10.3892/ijmm.2016.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]