Abstract
High Density Lipoprotein (HDL) particles, beyond serving as lipid transporters and playing a key role in reverse cholesterol transport, carry a highly variable number of proteins, micro-RNAs, vitamins, and hormones, which endow them with the ability to mediate a plethora of cellular and molecular mechanisms that promote cardiovascular health. It is becoming increasingly evident, however, that the presence of cardiovascular risk factors and co-morbidities alters HDLs cargo and protective functions. This concept has led to the notion that metrics other than HDL-cholesterol levels, such as HDL functionality and composition, may better capture HDL cardiovascular protection. On the other hand, the potential of HDL as natural delivery carriers has also fostered the design of engineered HDL-mimetics aiming to improve HDL efficacy or as drug-delivery agents with therapeutic potential. In this paper, we first provide an overview of the molecules known to be transported by HDL particles and mainly discuss their functions in the cardiovascular system. Second, we describe the impact of cardiovascular risk factors and co-morbidities on HDL remodeling. Finally, we review the currently developed HDL-based approaches.
Keywords: HDL, cardiovascular risk factors, cardiovascular disease, engineered HDL-mimetics
1. Introduction
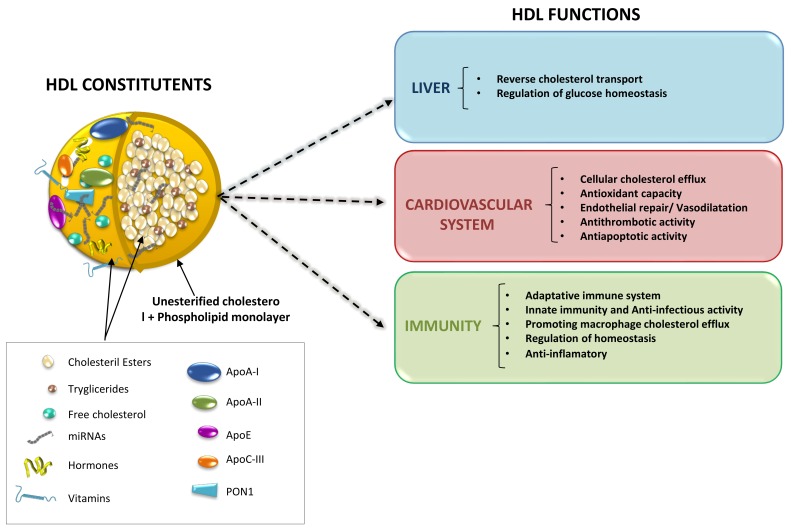
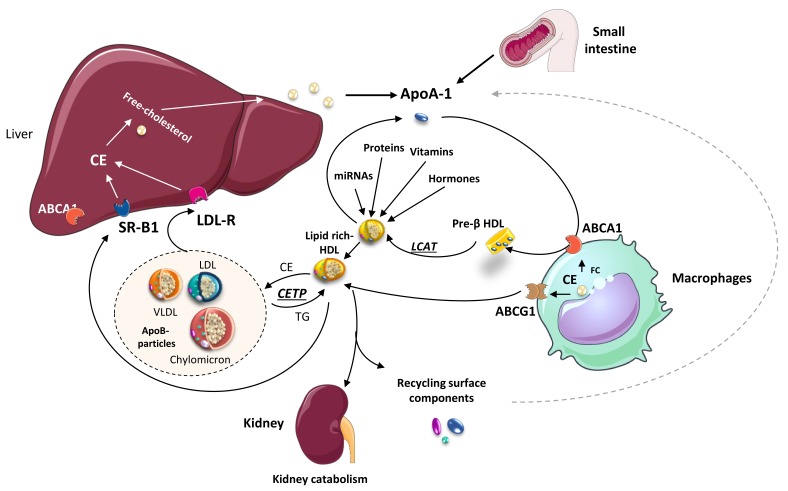
High-density lipoproteins (HDL) represent a variety of lipoproteins with mean size of 8–10 nm and density of 1.063–1.21 g/mL [1]. The fundamental structure of an HDL particle includes a central core of esterified cholesterol, surrounded by a monolayer of phospholipids, free cholesterol, and apolipoproteins (apo), predominantly apoA-I and apoA-II (Figure 1) [2,3]. HDL size spans the spectrum from small, lipid depleted, discoidal particles, to larger, cholesterol-rich, spherical particles. The biogenesis of HDL occurs in the liver and intestine, which both synthesize and secrete apoA-I. Shortly after secretion as a lipid-poor protein, apoA-I interacts with the cholesterol–phospholipid transporter ABCA1 (ATP Binding Cassette A1) expressed by many cell types (including hepatocytes, enterocytes, and macrophages) to exchange lipids generating a nascent HDL particle (pre-β-HDL) [4]. The enzyme lecithin cholesteryl acyltransferase (LCAT) present on HDL particles favors the generation of cholesteryl ester, which forms the core of mature HDL particles [5]. Conversely, there are two metabolic pathways involved in HDL-cholesteryl ester clearance, which include a direct uptake by the liver or by steroidogenic tissues via the scavenger receptor BI (SR-BI); and, the transfer to apoB-containing lipoproteins (usually in exchange for triglyceride) by the plasma protein cholesteryl ester transfer protein (CETP). Uptake via SR-BI is selective, and after the removal of cholesteryl ester, the smaller apoA-I containing HDL particle dissociates and recycles [6]. In contrast, CETP not only contributes to HDL cholesteryl esters depletion but favors HDL-triglyceride enrichment. Triglyceride-enriched HDL particles are, in turn, susceptible to lipolytic modification by hepatic and endothelial lipases. Upon change by these two enzymes, the consequent smaller HDL particle becomes more sensitive to undergo faster catabolism [7]. Finally, apoA-I is mostly catabolized in the kidneys via a process involving the proteins cubilin and megalin (Figure 2) [8]. In summary, HDL becomes the primary vehicle for the transport of cholesterol from peripheral cells to the liver for excretion and catabolism. Yet, during this complex metabolic process, molecules other than lipids (i.e., hormones, vitamins, proteins, and miRNAs) are known to be incorporated into HDL particles and transported to distal organs, likely contributing to maintaining cardiovascular health.
Figure 1.
HDL main constituents and HDL-related functions in different cellular systems.
Figure 2.
HDL biogenesis and its role in lipid metabolism. Nascent HDL particles (Pre-β HDL), formed from Apo-I secreted either by the liver or the thin intestine, uptake cholesterol from different cell types, including macrophages (via ATP binding cassette transporter A1; ABCA1). Concomitantly, other molecular constituents, including miRNAs, proteins, hormones, and vitamins, become incorporated into HDL-particles. Free cholesterol within the HDL is further transformed into cholesterol esters (CE) by the enzyme Lecithin: Cholesterol Acyltransferase (LCAT). Lipid-rich (mature) HDL particles exchange lipids with ApoB-containing particles [(chylomicrons, very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL)] through cholesteryl ester transfer protein (CETP) enzyme and with macrophages via ABCG1. Finally, the liver receptors scavenger receptor (SR)-B1 and LDL-R transfer HDL lipids to the liver, ApoA1 is catabolized in the kidneys, and the remaining surface constituents are recycled.
In the following paper, we will first provide an overview of the molecules known to be transported by HDL particles and discuss the functions they are considered to regulate, describe the impact of cardiovascular risk factors and co-morbidities on HDL remodeling, and eventually review the HDL-based approaches developed so far.
2. Molecules Carried by HDL Particles
The study of HDL-particle composition has become a matter of high interest, particularly in light of the disappointing clinical data for HDL-cholesterol (HDL-C), raising therapies in secondary prevention and the lack of association between HDL-C and the risk of cardiovascular events in coronary artery disease (CAD) patients [9,10,11]. HDLs are complex molecules that, beyond lipids, are known to transport proteins, hormones, vitamins, and miRNAs, which are delivered to target tissues/cells. Implementation of mass spectrometry-based proteomic studies has allowed identifying 85 proteins associated with HDL particles. These proteins have been mainly related to the following main biological processes or molecular functions: lipid metabolism and transport, hemostasis, immune response, metal binding, and vitamin transport [12]. The most frequently detected proteins are ApoA-I and ApoA-II. ApoA-I is synthesized in both the intestine and the liver and constitutes approximately 70% of HDL protein size. ApoA-II is only synthesized in the liver, represents approximately 20% of HDL protein, and is present on about two-thirds of HDL particles in humans [13,14]. Besides apoA -I and -II, HDLs express other apolipoproteins, which, in order of detection, include ApoC-III, ApoL1, ApoA-II, ApoE, ApoC-I, ApoC-II, and ApoM [15]. At a lipid level, more than 200 lipid species have been identified to constitute HDL particles [16]. These are generally classified as neutral hydrophobic lipids (cholesteryl esters and triglycerides present in the HDL core), and amphipathic lipids (free cholesterol or phospholipids mainly transported on the HDL surface) [13]. The HDL lipid composition is comprised of 35–50% of phospholipids, 5–10% of sphingolipids, 30–40% of cholesterol esters, 5–10% of free cholesterol, and 5–12% of triglyceride [16]. HDL also carries hormones that bind to the HDL-apolipoproteins, including thyroxine (T4), retinol, estradiol, pregnenolone, and dehydroepiandrosterone [17]. HDL transport of hormones and their intercellular transfer are crucial for the endocrine and reproduction systems, particularly during pregnancy [18,19]. Finally, HDL also incorporate vitamins, including carotenoids (lipophilic precursors for vitamin A) and vitamin E. As to the former, although HDLs transport a small percentage of nonpolar carotenoids (e.g., lycopene, 17%; α-carotene, 26%; and β-carotene, 22%), they carry a large proportion of polar carotenoids (lutein, 53%; cryptoxanthin 39%) [20,21]. Carotenoids are mainly delivered to the eye retina where they are accumulated in the macula, likely protecting against its degeneration, cataract development, and blindness. Moreover, carotenoid treatment has been associated with an increase in HDL reverse cholesterol transport capacity in mice [22] and has been shown to protect against early atherosclerosis development [23]. As per vitamin E, HDL has demonstrated to deliver this vitamin to epithelial cells, although its exact role remains uncertain [20].
Finally, within the last years, it has become evident that HDLs also transport miRNAs, short (~22 nucleotides), non-coding, single-stranded RNA molecules that regulate the expression of target genes by blocking its translation or promoting its degradation. So far, miR-33a, miR-30c, miR-92a, miR-122, miR-125a, miR-126, miR-145, miR-146a, miR-150, miR-155, miR-223, miR-378, miR-486, and miR-17/92 cluster have [24,25] been detected in HDL particles and have been suggested to regulate crucial pathways for maintaining cardiovascular homeostasis [26]. Particularly, miRNA-33a and miRNA-223 have been described to protect against atherosclerosis by modulating HDL cholesterol efflux capacity and lipid metabolism [27,28].
3. Protective Effects of HDL Particles beyond Lipid Removal
Since the Framingham Heart Study in the 1960s–1980s [29], many epidemiological and experimental studies have supported a beneficial role of HDL particles in the cardiovascular system. The critical benefit attributed to HDL particles relies on their ability to induce reverse cholesterol transport (RCT) [30,31,32]. HDL loss of cholesterol efflux capacity is associated with cardiovascular diseases [33,34,35]. However, HDLs exert several other benefits that protect the cardiovascular system, including antioxidant, anti-inflammatory, vasodilatory, antithrombotic, immunomodulatory, and endothelial repair properties, including the recruitment of endothelial progenitor cells [3,36,37,38]. Many clinical data, however, support the fact that HDL particles isolated from CVD patients have their benefits altered, including lower cholesterol efflux capacity and less anti-oxidant and anti-inflammatory properties, compared with healthy subjects [39,40,41,42]. Furthermore, HDL particles have been reported to even be deleterious [43,44]. As such, HDL isolated from CAD patients and antiphospholipid syndrome have been shown to promote apoptosis by stimulating p38-mitogen-activated protein kinase-mediated activation of the proapoptotic Bcl-2 protein tBid and to reduce nitric oxide (NO) bioavailability, impairing HDL anti-inflammatory and antioxidant properties, respectively.
3.1. Antioxidant and Anti-Inflammatory Effects
HDLs have experimentally been shown to protect against LDL oxidation, thereby preventing the generation of proinflammatory oxidized lipids, mainly lipid hydroperoxides and short-chain oxidized phospholipids [36,45,46,47]. The main antioxidant enzyme carried by HDL particles is paraoxonase 1 (PON1). PON1 is a member of a family of proteins that also includes PON2 and PON3. PON1 and PON3 are part of the HDL particles, while PON2 is found in a wide variety of tissues, such as endothelial cells, smooth muscle cells, and macrophages [48]. Interestingly, besides its antioxidant potential, PON1 has also been suggested to play a crucial role in HDL-endothelial nitric oxide synthase (eNOS) activation. PON1-deficient mice have failed to stimulate endothelial nitric oxide (NO) production, leading to impaired endothelium protection and repair [37]. HDL has also demonstrated, however, to activate eNOS through an SR-B1-associated intracellular kinase cascade activation [49].
Other mechanisms by which HDL may modulate inflammation partly involve miR-223. In this regard, in vitro studies have demonstrated that macrophages (J774) can transfer miR-223 to HDL [26], which, in turn, exert anti-inflammatory effects through HDL-miR-223 delivery and translational repression of intercellular adhesion molecule (ICAM)-1 in endothelial cells [50].
3.2. Protection against Ischemia/Reperfusion
Ischaemic heart disease, mainly presented as myocardial infarction (MI) due to occlusion of an epicardial coronary artery, remains the leading cause of death and disability worldwide. If timely reperfusion is not achieved and no collateral circulation is present, cardiomyocytes will be irreversibly damaged and die and substituted by fibrous scar tissue with impairment of myocardial contractile function [51]. However, reperfusion, although mandatory to salvage ischemic myocardium from cell death, contributes per se to enhance cardiac injury by affecting myocytes not yet severely damaged by the ischemic insult, the so-called ischemia/reperfusion (IR) injury [52]. Theilmeier et al. were the first to demonstrate in an in vivo mouse model of MI that HDL markedly reduces the size of infarction [53]. Previous findings further demonstrated, in a translational animal model with clinical impact and by magnetic resonance imaging analysis, that HDL infusion not only limited cardiac damage, but also improved cardiac contractility as compared to infusion with vehicle [54,55,56]. Besides HDL particles, its component sphingosine-1-phosphate (S1P), which is mainly bound to ApoM, has also been shown to protect against IR injury [57,58], and administration of fingolimod, an S1P receptor agonist, has demonstrated to limit the size of infarction and improve cardiac performance in a pig model of MI [59].
miRNAs transported by HDL have also been shown to protect against IR. For instance, reduction of miR-92a levels has been shown to reduce infarct size and post-ischemic loos of cardiac function by displaying cell-protective, pro-angiogenic, and anti-inflammatory effects, whereas miR-486 has demonstrated to prevent cardiomyocyte apoptosis execution [60,61,62,63]. These promising experimental data have encouraged the development of synthetic HDL-like formulations containing, within the structural HDL components, locked nucleic acid-modified antisense against a given miRNA or different proportion of these candidate miRNAs [64].
3.3. Endothelial Progenitor Cell Recruitment
Considerable experimental evidence supports the ability of HDL particles to display a plethora of beneficial effects on the endothelium [32,46,65]. Besides inducing eNOS activation and NO production, HDL has been shown to inhibit endothelial expression of adhesion molecules, reduce apoptosis execution, and promote EPCs-mediated endothelial cell repair [39]. Particularly, ApoA-I has been shown to enhance re-endothelialization post-percutaneous coronary intervention and post-stent aortic implantation in ApoA-I infused animals [66,67,68] by enhancing EPCs recruitment, reducing neointimal hyperplasia, and inhibiting the inflammatory response [69]. Moreover, engineered-HDL formulations have also been demonstrated to increase EPC mobilization in diabetic patients [70]. Although the mechanisms behind EPC mobilization remain poorly understood, cell culture studies have suggested that it involves HDL-SR-BI interaction and [49,71] activation of the CD36- mitogen-activated protein kinase (MAPK)-thrombospondin-1 pathway [72].
3.4. Antithrombotic Effects
HDL antithrombotic properties have been reported within both the arterial and venous beds [3,73]. The ability of HDLs to lessen the thrombotic risk is thought to be mediated by several mechanisms involving a reduction in platelet susceptibility to aggregation and attenuation of the activation of the coagulation cascade. HDL has been shown to increase endothelial NO and prostacyclin synthesis and bioavailability [74], thereby preventing platelet activation from occurring, to attenuate vascular expression of tissue factor and exert anticoagulant cofactor activity by enhancing activated protein C (APC): protein S. This leads to a diminishment in thrombin generation, a potent platelet inducer, and precursor of fibrin formation [49].
3.5. Immunomodulatory Properties
Several clinical trials have supported a link between HDL and immunity in the setting of autoimmune diseases (e.g., systemic lupus erythematosus ankylosing spondylitis, and rheumatoid arthritis) [75,76]; yet, the immunomodulatory properties of HDL within the cardiovascular system remains far less understood. Accumulating data have suggested that HDLs are involved in host defense as part of both the innate and adaptive immune system [38,77,78]. In this latter regard, HDL has demonstrated to affect T cell activation and signaling by altering cholesterol content within the lipid rafts [79,80]. Furthermore, HDL has also been shown to inhibit the ability of antigen-presenting cells (APCs) to stimulate T cells, regulate complement activation, and block the conversion of monocytes into migratory dendritic cells (DCs) through the HDL-associated platelet-activating factor-acetylhydrolase [81].
4. Impact of Cardiovascular Risk Factor and Co-Morbidities on HDL Composition and Function
Accumulating evidence suggests that HDL function and composition are found to be altered in the presence of overt cardiovascular disease. As such, the presence of CAD, MI [82], heart failure [83], and stroke [84] have been associated with impaired HDL anti-atherogenic properties (i.e., stimulation of vascular NO production, anti-oxidative and anti-apoptotic potential, and cholesterol efflux capacity) [43,85]. In addition, we cannot forget that clinical trials (particularly phase III) include elderly patients under multiple drug treatments. In this regard, the use of statins has been suggested to abolish HDL-related benefits. [86]. Moreover, the presence of cardiovascular risk factors, including hypercholesterolemia [13,60,87], obesity [88], diabetes type 1 and 2 [89,90], and even frequent exposure to pollution [91,92,93] or ethnicity have demonstrated to impact HDL properties and induce structural changes leading to variations in their subtypes profile. Altogether, these conditions may help explain the disappointing results observed in clinical trials aiming to increase HDL-c levels in secondary prevention. The venue of new technology systems, like genome-wide association studies (GWAS), has allowed assessing genetic predisposition to display improved or diminished HDL-related benefits. For instance, recent studies have reported that the cholesterol efflux capacity is a heritable trait and that certain genetic polymorphism in genes involved in HDL and triglycerides metabolism are associated with less HDLs RCT and anti-oxidative capacity [94,95,96]. We have recently demonstrated that HDL remodeling is affected by low-density lipoprotein (LDL)-cholesterol levels. As such, we have shown in pigs that the presence of hypercholesterolemia modifies HDL lipidomic and proteomic profile and renders HDL particles dysfunctional [56]. Particularly, HDL particles formed under hypercholesterolemic conditions, as compared to HDL particles formed in a normocholesterolemic milieu, have lower levels of phosphatidylcholine-lipid species and higher levels of cholesteryl esters (i.e., mature HDL particles with lower membrane fluidity) and a lower cargo of several proteins with known cardioprotective functions, including ApoM and lipocalin retinol-binding protein-4. Interestingly, such adverse structural remodeling is associated with a marked reduction in HDL antioxidant and cholesterol efflux capacity and complete loss of HDL-related cardioprotection [13].
At an epigenetic level, the presence of co-morbidities has also been shown to induce changes to the HDL-miRNA signature [60]. As such, an association between HDL-miRNAs profile and CAD stability has been established [97], and the content of HDL-miR-223 is found to be altered in the presence of insulin resistance and familial hypercholesterolemia subjects [26,98,99,100,101,102]. Extending these previous findings, we have recently evidenced that hypercholesterolemic HDL particles transport higher levels of miR-126 as compared to native-HDL, which in turn is delivered to endothelial cells through an SRB1-dependent mechanism likely modulating HIF-1α expression [103].
Finally, HDL size, composition, and functionality are being studied as potential biomarkers to predict microangiopathy, coronary artery disease [104,105], aortic aneurysm [106], and atherosclerosis [107]. In this regard, we have detected alterations in ApoJ proteomic profile due to differential glycosylation patterns in acute MI-patients within the first six h of onset of the event, becoming a potential early marker of acute MI [108]. Noteworthy, changes in lifestyle behavior, like implementing exercise and a healthy diet daily, have demonstrated to restore and enhance HDL protective properties. As such, regular intake of Mediterranean diet-related functional foods, such as fermented beverages or tomato, have experimentally shown, not only to enhance HDL levels but, most importantly, to improve HDL functionality (i.e., antioxidant potential) [109,110,111,112].
5. HDL-Based Approaches
The venue of nanotechnology has provided a platform for the development of reconstituted HDL (rHDL) or synthetic HDL (sHDL). These HDL-mimicking particles are derived from cholesterol-free HDL particles (prepared from purified plasma or recombinantly expressed) and ApoA-I or short synthetic ApoA-I mimetic peptides [like ApoAI Milano (ApoA-IM)] complexed with phospholipids (Table 1). Of note, the number of ApoA-I chains incorporated into the particle, and its overall size is the primary mechanism by which HDL-mimetic particles are classified and labeled [113]. Such inorganic nanoparticles that mirror HDL composition provide a scaffold to create synthetic structures that mimic mature, spherical, or discoidal forms of HDL. The resultant nanoparticle retains both the physicochemical properties as well as the biological functions of the native HDL. As such, these “empty” particles have demonstrated to be highly effective in cholesterol efflux from lipid-laden cells both in vitro and in vivo and to target SR-BI-expressing cells [114,115]. These engineered nanoparticles can act as versatile drug delivery carriers of miRNAs, specific proteins, and drug-delivery systems. As such, the ability of rHDL/sHDL to deplete cellular cholesterol and target SR-BI along with the biocompatibility of the individual components and proven clinical safety has enhanced the interest of developing rHDL/sHDL constructs in different medical areas, such as cancer, where HDL-like particles are being used for anti-cancer therapeutics and tumor imaging chemotherapy [116].
Table 1.
HDL synthetic-based approaches.
| HDL-Mimetic | Composition | Mechanism of Action | Experimental Design | Added Capacity (Compared to Native HDLs) | Reference |
|---|---|---|---|---|---|
| ETC-216 | Human recombinant Apolipoprotein A-I Milano | ABCA1 | Stopped ClinicalTrials.gov Identifier: NCT02678923 | __ | [129] |
| MDCO-216 | Human recombinant Apolipoprotein A-I Milano | ABCA1 | Phase 2 clinical trial: ClinicalTrials.gov Identifier: NCT02678923 | Increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL. | [130] |
| CSL112 | Human plasma-derived apolipoprotein A-I (apoA-I) | ABCA1 | Phase 2a in stable atherothrombotic patients and Phase 2b for patients with acute MI: AEGIS-I trial ClinicalTrials.gov NCT02108262 | Increases cholesterol efflux capacity | [131] |
| rHDL-apoA-I | Human plasma-derived apolipoprotein A-I (apoA-I) | ABCA1 | Phase 2 on atherosclerosis ClinicalTrials.gov Identifier: NCT00225719 | Increases cholesterol efflux capacity | [132,133] |
| CER-001 | Lipoprotein complex mimicking discoidal pre-β HDL, consisting of recombinant human apoA-I | ABCA1 | Phase 2 studies: CHI-SQUARE and CARAT trials clinicaltrials.gov Identifier: NCT01201837 and NCT02484378 respectively | Can rapidly mobilise large amounts of cholesterol into the HDL fraction | [134,135] |
| Nanolipoprotein Particles (NLPs) | Phospholipid bilayer stabilized by an apolipoprotein scaffold protein | ABCA1 | Initial in vitro state | Enhanced particle stability | [114] |
| sHDL-T1317 | sHDL apoA-I peptide+A synthetic LXR agonist, T0901317 (T1317) | ABCA1 | Preclinical studies | Upregulates the expression of ATP-binding cassette transporters and increases cholesterol efflux in macrophages in vitro and in vivo. | [136] |
| ApoE-Based rHDL | rHDL particles containing ApoE3 | ABCA1 and LCAT | Phase 1 in China, preclinical studies in Europe | Enhances endosomal/lysosomal escape capacity | [137,138,139] |
| rHDL-DiR-BOA | Mimicking peptide phospholipid scaffold (HPPS) | SR-B1 | Initial in vitro state | Endosomal/lysosomal avoidance capacity which makes a highly biocompatible, exhibited long circulation half-life in serum nanocarrier | [140] |
| cp-rHDL | rHDL+cell penetrating peptides | __ | Initial in vitro state | Easily overcome the cellular plasma membrane | [141,142] |
| Receptor-Mediated rHDL Cellular Internalization | rHDL+cell receptor signalling structures | SR-B1 | Initial in vitro state | Enhances the accumulation of nanoparticles and increased uptaking | [143,144] |
| rHDL-siRNA | rHDL+siRNAs | SR-B1 | Initial in vitro state | Allows directed siRNA delivery | [142] |
| AT-DXS-LP-rHDL | Atorvastatin calcium (AT)-loaded dextran sulfate (DXS)-coated core-shell reconstituted high-density lipoprotein (rHDL) | SR-AI | Initial in vitro state | High-affinity SR-AI as well as depletion of intracellular cholesterol by apoA-I mediated cholesterol efflux | [145] |
| rHDL-AuNP | AuNPs+rHDL+ApoE | Receptor-mediated endocytosis in glioblastoma cells | Initial in vitro state | A platform for transport and delivery of hydrophobic gold nanoparticles | [146] |
| rHDL-rApoJ | phospholipids with recombinant human ApoJ (rApoJ) | Amyloid beta (Aβ) interaction | Preclinical studies | Maintains the ability to prevent the Aβ fibrillation and mediated higher cholesterol efflux from cultured macrophages | [147] |
| sHDL-EL | sHDL+Substrate for plasma endothelial lipase (EL) with useful specificity | Endothelial lipase | Initial in vitro state | Specificity for EL | [148] |
5.1. HDL-Mimetics: Composition and Characteristics
Despite all these advances, there are specific nanoarchitecture requirements that cannot be overlooked [117,118]. One of the most important is to prolong HDLs’ mimetics life-span to retain the molecule on the organism long enough to enhance its beneficial properties and reduce the maintaining dosage. This feature is mainly determined by the type, proportion, and interaction between lipid and protein cargo [119]. Many of the early structural studies on rHDL/sHDL employed particles reconstituted with dimyristoylphosphatidylcholine (DMPC, a shorter saturated acyl chain PL, 14:0), due to its ability to spontaneously form rHDL/sHDL particles when DMPC vesicles were mixed with apoA-I [120,121,122,123,124,125]. Later, the cholate dialysis method [126] was reported to allow reconstitution of rHDL/sHDL with physiologically relevant phospholipids that have longer and unsaturated acyl chains like palmitoyloleoylphosphatidylcoline (POPC, 16:0–18:1). Schwendeman A et al. reported that rHDL-POPC displayed enhanced anti-inflammatory properties compared to rHDL rich in sphingomyelin, and consequently, had higher atheroprotective effects [127]. Overall, HDL formulations should aim to increase the proportion of protein and reduce the percentage of lipid since this approach confers HDL-mimetics with a longer lifespan and solid structure at the same time, retaining their flexibility enough for cell and tissue interactions. Moreover, HDL-engineered particles can then be further stabilized by protecting them from degradation with the conjugation of small RNAs (siRNAs or miRNAs) [128].
5.2. Potential Clinical Applicability of HDL Mimetics
So far, several “HDL mimetics” have been successfully tested in experimental animal models for their ability to reduce the burden of atherosclerotic cardiovascular disease [149,150]. In contrast, however, they have failed to continually prove beneficial effects in the clinical setting [151] (Table 1), and most importantly, none have demonstrated to reduce mortality, which likely reflects the complexity of the association between HDL and atherosclerotic cardiovascular disease. Moreover, it also suggests that HDL particles may undergo remodeling when infused to patients that suffer from many cardiovascular risk factors or are under drug treatment.
Serial infusion of the ApoA-I Milano phospholipid complex ETC-216 demonstrated in Phase I clinical trials to exert profound lipid changes to an extent, comparable to that found in ApoA-I Milano mutation carriers. Furthermore, the infusion of ETC-216 resulted in a reduction in atherosclerotic plaque burden [152] in line with our previous observations in atherosclerotic rabbits [153]. However, ETC-216 development was halted because of the presence of side effects, including a dose-dependent increase in neutrophil and a concomitant decline in lymphocyte levels, leading to multi-organ failure. Interestingly, a careful evaluation of the entire manufacturing process revealed that ETC-216 contained small quantities of residual host cell proteins that elicited an immune response. Consequent removal of the contaminant proteins from the nanoconstruct and essential improvements in the downstream manufacturing process led to the development of a recombinant ApoA-I Milano formulation named MDCO-216. MDCO-216 has been tested in healthy volunteers and patients with stable CAD, showing modulation of lipid parameters and an increase of cholesterol efflux with no adverse effects [129,130]. More recently, MDCO-216 has also reported, at an experimental level, to revert heart failure progression in Type 2 diabetic mice as well as restore cardiac function [154], lower myocardial acetyl-coenzyme A carboxylase levels, and decrease myocardial transforming growth factor-β1 [155]. CSL112, a reconstituted, infusible, plasma-derived ApoA-I structure, is another promising HDL-mimetic. CSL112 administration has been shown to enhance the quantity and functionality of plasma ApoA-I as well as promote ABCA1 dependent cholesterol efflux capacity, becoming an attractive option for rapid cholesterol plaque removal [131]. Finally, CER001 is a lipoprotein complex mimicking discoidal pre-β HDL consisting of recombinant human ApoA-I. Yet, so far, CER001 has demonstrated no benefits in reducing coronary atherosclerosis assessed by intravascular ultrasonography and by coronary angiography when compared with the administration of placebo [152].
6. Concluding Remarks
In summary, HDLs are complex particles constituted by a vast array of lipids, proteins, hormones, vitamins, and miRNAs, which confer HDL particles with multiple cardiovascular protective properties mainly related to atherosclerosis development. Advances in our outstanding on the structural components of HDL particles in different physiological and pathological conditions (HDL-remodeling) have allowed us to better understand the potential changes that may account for the loss of HDL function. Moreover, it has provided us with potential targets amenable for therapeutical modulation to restore HDL functionality or enhance specific protective features. In this regard, the implementation of new platforms aimed at designing and developing different reconstituted HDL formulations has offered a unique opportunity to overcome HDL-related structural alterations in the presence of co-morbid conditions or risk factors as well as to deliver drugs to target cells accurately. There is, however, much work that needs to be done in the arena of HDL-nano synthesis to obtain HDL constructs that remains stable and effective over time in the body and more closely resemble the healthy and native HDL particles endowed with multiple cardiovascular benefits (Table 1) [114].
Author Contributions
S.B.-A., writing—original draft preparation; L.B. and G.V., supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Plan Nacional de Salud (PNS) [PGC2018-094025-B-I00 to G.V. and SAF2016-76819-R to L.B.] from the Spanish Ministry of Science and Innovation and funds FEDER “Una Manera de Hacer Europa”; a grant from the Spanish Society of Cardiology (Beca FEC Investigación Básica/2016; to G.V.) and CIBERCV (to L.B.). We thank the Generalitat of Catalunya (Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat, 2017 SGR 1480) and the Fundación Investigación Cardiovascular-Fundación Jesus Serra for their continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Havel R.J., Eder H.A., Bragdon J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinecke J.W. The HDL proteome: A marker—And perhaps mediator—Of coronary artery disease. J. Lipid Res. 2009;50:S167–S171. doi: 10.1194/jlr.R800097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badimon L., Vilahur G. HDL particles—More complex than we thought. Thromb. Haemost. 2014;112:857. doi: 10.1160/TH14-10-0831. [DOI] [PubMed] [Google Scholar]
- 4.Parks J.S., Chung S., Shelness G.S. Hepatic ABC transporters and triglyceride metabolism. Curr. Opin. Lipidol. 2012;23:196–200. doi: 10.1097/MOL.0b013e328352dd1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousset X., Vaisman B., Amar M., Sethi A.A., Remaley A.T. Lecithin: Cholesterol acyltransferase—From biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009;16:163–171. doi: 10.1097/MED.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoekstra M., Van Berkel T.-J., Van Eck M. Scavenger receptor BI: A multi-purpose player in cholesterol and steroid metabolism. World J. Gastroenterol. 2010;16:5916–5924. doi: 10.3748/wjg.v16.i47.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamarche B., Uffelman K.D., Steiner G., Barrett P.H., Lewis G.F. Analysis of particle size and lipid composition as determinants of the metabolic clearance of human high density lipoproteins in a rabbit model. J. Lipid Res. 1998;39:1162–1172. [PubMed] [Google Scholar]
- 8.Barth J.L., Argraves W.S. Cubilin and Megalin: Partners in Lipoprotein and Vitamin Metabolism. Trends Cardiovasc. Med. 2001;11:26–31. doi: 10.1016/S1050-1738(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 9.Angeloni E., Paneni F., Landmesser U., Benedetto U., Melina G., Lüscher T.F., Volpe M., Sinatra R., Cosentino F. Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur. Heart J. 2013;34:3557–3562. doi: 10.1093/eurheartj/eht163. [DOI] [PubMed] [Google Scholar]
- 10.Keene D., Price C., Shun-Shin M.J., Francis D.P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. doi: 10.1136/bmj.g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armitage J., Holmes M.V., Preiss D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019;73:477–487. doi: 10.1016/j.jacc.2018.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah A.S., Tan L., Long J.L., Davidson W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padró T., Cubedo J., Camino S., Béjar M.T., Ben-Aicha S., Mendieta G., Escolà-Gil J.C., Escate R., Gutiérrez M., Casani L., et al. Detrimental Effect of Hypercholesterolemia on High-Density Lipoprotein Particle Remodeling in Pigs. J. Am. Coll. Cardiol. 2017;70:165–178. doi: 10.1016/j.jacc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Rader D.J. Molecular regulation of HDL metabolism and function: Implications for novel therapies. J. Clin. Investig. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada T., Ohama T., Takafuji K., Kanno K., Matsuda H., Sairyo M., Zhu Y., Saga A., Kobayashi T., Masuda D., et al. Shotgun proteomic analysis reveals proteome alterations in HDL of patients with cholesteryl ester transfer protein deficiency. J. Clin. Lipidol. 2019;13:317–325. doi: 10.1016/j.jacl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Kontush A., Lhomme M., Chapman M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers K.C., Remaley A.T. HDL and cholesterol: Life after the divorce? J. Lipid Res. 2014;55:4–12. doi: 10.1194/jlr.R035964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulaiman W.N.W., Caslake M.J., Delles C., Karlsson H., Mulder M.T., Graham D., Freeman D.J. Does high-density lipoprotein protect vascular function in healthy pregnancy? Clin. Sci. 2016;130:491–497. doi: 10.1042/CS20150475. [DOI] [PubMed] [Google Scholar]
- 19.van Tienhoven-Wind L.J.N., Dullaart R.P.F. Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur. J. Clin. Investig. 2015;45:494–503. doi: 10.1111/eci.12423. [DOI] [PubMed] [Google Scholar]
- 20.Harrison E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019;63:1801046. doi: 10.1002/mnfr.201801046. [DOI] [PubMed] [Google Scholar]
- 21.Thomas S.E., Harrison E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016;57:1865–1878. doi: 10.1194/jlr.M070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou T.B., Zhu S.S., Luo F., Li W.Q., Sun X.R., Wu H.F. Effects of Astaxanthin on Reverse Cholesterol Transport and Atherosclerosis in Mice. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/4625932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwyer J.H., Navab M., Dwyer K.M., Hassan K., Sun P., Shircore A., Hama-Levy S., Hough G., Wang X., Drake T., et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation. 2001;103:2922–2927. doi: 10.1161/01.CIR.103.24.2922. [DOI] [PubMed] [Google Scholar]
- 24.Ren J., Zhang J., Xu N., Han G., Geng Q., Song J., Li S., Zhao J., Chen H. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS ONE. 2013;8:e80738. doi: 10.1371/journal.pone.0080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner J., Riwanto M., Besler C., Knau A., Fichtlscherer S., Roxe T., Zeiher A.M., Landmesser U., Dimmeler S. Characterization of Levels and Cellular Transfer of Circulating Lipoprotein-Bound MicroRNAs. Arterioscler. Thromb. Vasc. Biol. 2013;33:1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 26.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers K.C., Landstreet S.R., Levin M.G., Shoucri B.M., Toth C.L., Taylor R.C., Palmisano B.T., Tabet F., Cui H.L., Rye K.-A., et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. USA. 2014;111:14518–14523. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono K. Functions of microRNA-33a/b and microRNA therapeutics. J. Cardiol. 2016;67:28–33. doi: 10.1016/j.jjcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Wilson P.W., Garrison R.J., Castelli W.P., Feinleib M., McNamara P.M., Kannel W.B. Prevalence of coronary heart disease in the Framingham Offspring Study: Role of lipoprotein cholesterols. Am. J. Cardiol. 1980;46:649–654. doi: 10.1016/0002-9149(80)90516-0. [DOI] [PubMed] [Google Scholar]
- 30.Hernáez Á., Castañer O., Elosua R., Pintó X., Estruch R., Salas-Salvadó J., Corella D., Arós F., Serra-Majem L., Fiol M., et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation. 2017;135:633–643. doi: 10.1161/CIRCULATIONAHA.116.023712. [DOI] [PubMed] [Google Scholar]
- 31.Tuteja S., Rader D.J. High-Density Lipoproteins in the Prevention of Cardiovascular Disease: Changing the Paradigm. Clin. Pharmacol. Ther. 2014;96:48–56. doi: 10.1038/clpt.2014.79. [DOI] [PubMed] [Google Scholar]
- 32.Rye K.-A., Barter P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rader D.J., Hovingh G.K. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 34.Feingold K.R., Grunfeld C. Introduction to Lipids and Lipoproteins. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 35.Allard-Ratick M.P., Kindya B.R., Khambhati J., Engels M.C., Sandesara P.B., Rosenson R.S., Sperling L.S. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur. J. Prev. Cardiol. 2019 doi: 10.1177/2047487319848214. [DOI] [PubMed] [Google Scholar]
- 36.Kontush A., Chapman M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010;21:312–318. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- 37.Barter P.J., Nicholls S., Rye K.-A., Anantharamaiah G.M., Navab M., Fogelman A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 38.Vilahur G. High-density lipoprotein benefits beyond the cardiovascular system: A potential key role for modulating acquired immunity through cholesterol efflux. Cardiovasc. Res. 2017;113:e51–e53. doi: 10.1093/cvr/cvx193. [DOI] [PubMed] [Google Scholar]
- 39.Riwanto M., Landmesser U. High density lipoproteins and endothelial functions: Mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013;54:3227–3243. doi: 10.1194/jlr.R037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D.M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kontush A., Chapman M.J. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 42.Rohatgi A., Khera A., Berry J.D., Givens E.G., Ayers C.R., Wedin K.E., Neeland I.J., Yuhanna I.S., Rader D.R., De Lemos J.A., et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., von Eckardstein A., et al. Altered Activation of Endothelial Anti- and Proapoptotic Pathways by High-Density Lipoprotein from Patients with Coronary Artery Disease: Role of High-Density Lipoprotein-Proteome Remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 44.Charakida M., Besler C., Batuca J.R., Sangle S., Marques S., Sousa M., Wang G., Tousoulis D., Delgado Alves J., Loukogeorgakis S.P., et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA. 2009;302:1210–1217. doi: 10.1001/jama.2009.1346. [DOI] [PubMed] [Google Scholar]
- 45.Afonso C.B., Spickett C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2018;23:101066. doi: 10.1016/j.redox.2018.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorrentino S.A., Besler C., Rohrer L., Meyer M., Heinrich K., Bahlmann F.H., Mueller M., Horváth T., Doerries C., Heinemann M., et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 47.Cimmino G., Ibanez B., Vilahur G., Speidl W.S., Fuster V., Badimon L., Badimon J.J. Up-regulation of reverse cholesterol transport key players and rescue from global inflammation by ApoA-IMilano. J. Cell. Mol. Med. 2009;13:3226–3235. doi: 10.1111/j.1582-4934.2008.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackness M., Mackness B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene. 2015;567:12–21. doi: 10.1016/j.gene.2015.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mineo C., Deguchi H., Griffin J.H., Shaul P.W. Endothelial and Antithrombotic Actions of HDL. Circ. Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 50.Tabet F., Vickers K.C., Cuesta Torres L.F., Wiese C.B., Shoucri B.M., Lambert G., Catherinet C., Prado-Lourenco L., Levin M.G., Thacker S., et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilahur G., Juan-Babot O., Peña E., Oñate B., Casaní L., Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011;50:522–533. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Theilmeier G., Schmidt C., Herrmann J., Keul P., Schäfers M., Herrgott I., Mersmann J., Larmann J., Hermann S., Stypmann J., et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 54.Gomaraschi M., Calabresi L., Franceschini G. Protective Effects of HDL Against Ischemia/Reperfusion Injury. Front. Pharmacol. 2016;7:2. doi: 10.3389/fphar.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James R.W., Frias M.A. High density lipoproteins and ischemia reperfusion injury: The therapeutic potential of HDL to modulate cell survival pathways. Adv. Exp. Med. Biol. 2014;824:19–26. doi: 10.1007/978-3-319-07320-0_3. [DOI] [PubMed] [Google Scholar]
- 56.Vilahur G., Gutiérrez M., Casaní L., Cubedo J., Capdevila A., Pons-Llado G., Carreras F., Hidalgo A., Badimon L. Hypercholesterolemia Abolishes High-Density Lipoprotein–Related Cardioprotective Effects in the Setting of Myocardial Infarction. J. Am. Coll. Cardiol. 2015;66:2469–2470. doi: 10.1016/j.jacc.2015.08.901. [DOI] [PubMed] [Google Scholar]
- 57.Wang X., Wang F. Vascular protection by high-density lipoprotein-associated sphingosine-1-phosphate. J. Geriatr. Cardiol. 2017;14:696–702. doi: 10.11909/j.issn.1671-5411.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swendeman S.L., Xiong Y., Cantalupo A., Yuan H., Burg N., Hisano Y., Cartier A., Liu C.H., Engelbrecht E., Blaho V., et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal. 2017;10:eaal2722. doi: 10.1126/scisignal.aal2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos-Gallego C.G., Vahl T.P., Goliasch G., Picatoste B., Arias T., Ishikawa K., Njerve I.U., Sanz J., Narula J., Sengupta P.P., et al. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation. 2016;133:954–966. doi: 10.1161/CIRCULATIONAHA.115.012427. [DOI] [PubMed] [Google Scholar]
- 60.Michell D.L., Vickers K.C. Lipoprotein carriers of microRNAs. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2016;1861:2069–2074. doi: 10.1016/j.bbalip.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogg E.M., Abplanalp W.T., Bischof C., John D., Schulz M.H., Krishnan J., Fischer A., Poluzzi C., Schaefer L., Bonauer A., et al. Analysis of Cell Type-Specific Effects of MicroRNA-92a Provides Novel Insights into Target Regulation and Mechanism of Action. Circulation. 2018;138:2545–2558. doi: 10.1161/CIRCULATIONAHA.118.034598. [DOI] [PubMed] [Google Scholar]
- 62.Niculescu L.S., Simionescu N., Sanda G.M., Carnuta M.G., Stancu C.S., Popescu A.C., Popescu M.R., Vlad A., Dimulescu D.R., Simionescu M., et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE. 2015;10:e0140958. doi: 10.1371/journal.pone.0140958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun X.H., Wang X., Zhang Y., Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Rink J.S., Sun W., Misener S., Wang J.-J., Zhang Z.J., Kibbe M.R., Dravid V.P., Venkatraman S., Thaxton C.S. Nitric Oxide-Delivering High-Density Lipoprotein-like Nanoparticles as a Biomimetic Nanotherapy for Vascular Diseases. ACS Appl. Mater. Interfaces. 2018;10:6904–6916. doi: 10.1021/acsami.7b18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Besler C., Heinrich K., Riwanto M., Lüscher T.F., Landmesser U. High-density lipoprotein-mediated anti-atherosclerotic and endothelial-protective effects: A potential novel therapeutic target in cardiovascular disease. Curr. Pharm. Des. 2010;16:1480–1493. doi: 10.2174/138161210791051013. [DOI] [PubMed] [Google Scholar]
- 66.Tso C., Martinic G., Fan W.-H., Rogers C., Rye K.-A., Barter P.J. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:1144–1149. doi: 10.1161/01.ATV.0000216600.37436.cf. [DOI] [PubMed] [Google Scholar]
- 67.Noor R., Shuaib U., Wang C.X., Todd K., Ghani U., Schwindt B., Shuaib A. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis. 2007;192:92–99. doi: 10.1016/j.atherosclerosis.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Vanags L.Z., Tan J.T.M., Galougahi K.K., Schaefer A., Wise S.G., Murphy A., Ali Z.A., Bursill C.A. Apolipoprotein A-I Reduces In-Stent Restenosis and Platelet Activation and Alters Neointimal Cellular Phenotype. JACC Basic Transl. Sci. 2018;3:200–209. doi: 10.1016/j.jacbts.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Der Vorst E.P.C., Vanags L.Z., Dunn L.L., Prosser H.C., Rye K.A., Bursill C.A. High-density lipoproteins suppress chemokine expression and proliferation in human vascular smooth muscle cells. FASEB J. 2013;27:1413–1425. doi: 10.1096/fj.12-212753. [DOI] [PubMed] [Google Scholar]
- 70.Vanags L.Z., Wong N.K.P., Nicholls S.J., Bursill C.A. High-Density Lipoproteins and Apolipoprotein A-I Improve Stent Biocompatibility. Arterioscler. Thromb. Vasc. Biol. 2018;38:1691–1701. doi: 10.1161/ATVBAHA.118.310788. [DOI] [PubMed] [Google Scholar]
- 71.Yuhanna I.S., Zhu Y., Cox B.E., Hahner L.D., Osborne-Lawrence S., Lu P., Marcel Y.L., Anderson R.G., Mendelsohn M.E., Hobbs H.H., et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 72.Wu J., He Z., Gao X., Wu F., Ding R., Ren Y., Jiang Q., Fan M., Liang C., Wu Z. Oxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathways. Antioxid. Redox Signal. 2015;22:308–324. doi: 10.1089/ars.2013.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holy E.W., Besler C., Reiner M.F., Camici G.G., Manz J., Beer J.H., Lüscher T.F., Landmesser U., Tanner F.C. High-density lipoprotein from patients with coronary heart disease loses anti-thrombotic effects on endothelial cells: Impact on arterial thrombus formation. Thromb. Haemost. 2014;112:1024–1035. doi: 10.1160/th13-09-0775. [DOI] [PubMed] [Google Scholar]
- 74.van der Stoep M., Korporaal S.J.A., Van Eck M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res. 2014;103:362–371. doi: 10.1093/cvr/cvu137. [DOI] [PubMed] [Google Scholar]
- 75.Szabó M.Z., Szodoray P., Kiss E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017;65:543–550. doi: 10.1007/s12026-016-8892-9. [DOI] [PubMed] [Google Scholar]
- 76.van der Valk F.M., Bernelot Moens S.J., Verweij S.L., Strang A.C., Nederveen A.J., Verberne H.J., Nurmohamed M.T., Baeten D.L., Stroes E.S.G. Increased arterial wall inflammation in patients with ankylosing spondylitis is reduced by statin therapy. Ann. Rheum. Dis. 2016;75:1848–1851. doi: 10.1136/annrheumdis-2016-209176. [DOI] [PubMed] [Google Scholar]
- 77.Connelly M.A., Shalaurova I., Otvos Raleigh J.D. High-density lipoprotein and inflammation in cardiovascular disease. Transl. Res. 2016;173:7–18. doi: 10.1016/j.trsl.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Hu J., Xi D., Zhao J., Luo T., Liu J., Lu H., Li M., Xiong H., Guo Z. High-density Lipoprotein and Inflammation and Its Significance to Atherosclerosis. Am. J. Med. Sci. 2016;352:408–415. doi: 10.1016/j.amjms.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 79.Larbi A., Fortin C., Dupuis G., Berrougui H., Khalil A., Fulop T. Immunomodulatory role of high-density lipoproteins: Impact on immunosenescence. Age. 2014;36:9712. doi: 10.1007/s11357-014-9712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catapano A.L., Pirillo A., Bonacina F., Norata G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014;103:372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 81.Yu B., Wang S., Peng D., Zhao S. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010;88:285–290. doi: 10.1038/icb.2009.112. [DOI] [PubMed] [Google Scholar]
- 82.Annema W., Willemsen H.M., de Boer J.F., Dikkers A., van der Giet M., Nieuwland W., Muller Kobold A.C., van Pelt L.J., Slart R.H.J.A., van der Horst I.C.C., et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J. Clin. Lipidol. 2016;10:1318–1328. doi: 10.1016/j.jacl.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Oberbach A., Adams V., Schlichting N., Heinrich M., Kullnick Y., Lehmann S., Lehmann S., Feder S., Correia J.C., Mohr F.-W., et al. Proteome profiles of HDL particles of patients with chronic heart failure are associated with immune response and also include bacteria proteins. Clin. Chim. Acta. 2016;453:114–122. doi: 10.1016/j.cca.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Ortiz-Munoz G., Couret D., Lapergue B., Bruckert E., Meseguer E., Amarenco P., Meilhac O. Dysfunctional HDL in acute stroke. Atherosclerosis. 2016;253:75–80. doi: 10.1016/j.atherosclerosis.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 85.Zewinger S., Kleber M.E., Rohrer L., Lehmann M., Triem S., Jennings R.T., Petrakis I., Dressel A., Lepper P.M., Scharnagl H., et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 2017;38:1597–1607. doi: 10.1093/eurheartj/ehx118. [DOI] [PubMed] [Google Scholar]
- 86.Niesor E.J., Schwartz G.G., Perez A., Stauffer A., Durrwell A., Bucklar-Suchankova G., Benghozi R., Abt M., Kallend D. Statin-induced decrease in ATP-binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc. Drugs Ther. 2015;29:7–14. doi: 10.1007/s10557-015-6570-0. [DOI] [PubMed] [Google Scholar]
- 87.Versmissen J., Vongpromek R., Yahya R., van der Net J.B., van Vark-van der Zee L., Blommesteijn-Touw J., Wattimena D., Rietveld T., Pullinger C.R., Christoffersen C., et al. Familial hypercholesterolaemia: Cholesterol efflux and coronary disease. Eur. J. Clin. Investig. 2016;46:643–650. doi: 10.1111/eci.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woudberg N.J., Goedecke J.H., Blackhurst D., Frias M., James R., Opie L.H., Lecour S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016;15:92. doi: 10.1186/s12944-016-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davidson W.S., Shah A.S. High-Density Lipoprotein Subspecies in Health and Human Disease: Focus on Type 2 Diabetes. Methodist Debakey Cardiovasc. J. 2019;15:55–61. doi: 10.14797/mdcj-15-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermans M.P., Ahn S.A., Rousseau M.F. Crossing family histories of diabetes and cardiovascular disease leads to unexpected outcomes in diabetic offspring. J. Diabetes. 2019;11:301–308. doi: 10.1111/1753-0407.12840. [DOI] [PubMed] [Google Scholar]
- 91.Ruiz-Ramie J.J., Barber J.L., Sarzynski M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019;30:16–23. doi: 10.1097/MOL.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pedret A., Fernández-Castillejo S., Valls R.-M., Catalán Ú., Rubió L., Romeu M., Macià A., López de las Hazas M.C., Farràs M., Giralt M., et al. Cardiovascular Benefits of Phenol-Enriched Virgin Olive Oils: New Insights from the Virgin Olive Oil and HDL Functionality (VOHF) Study. Mol. Nutr. Food Res. 2018;62:1800456. doi: 10.1002/mnfr.201800456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathew A.V., Yu J., Guo Y., Byun J., Chen Y.E., Wang L., Liu M., Bard R.L., Morishita M., Huang W., et al. Effect of Ambient Fine Particulate Matter Air Pollution and Colder Outdoor Temperatures on High-Density Lipoprotein Function. Am. J. Cardiol. 2018;122:565–570. doi: 10.1016/j.amjcard.2018.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khera A.V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M.F., Jafri K., French B.C., Phillips J.A., Mucksavage M.L., Wilensky R.L., et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenson R.S., Brewer H.B., Barter P.J., Björkegren J.L.M., Chapman M.J., Gaudet D., Kim D.S., Niesor E., Rye K.-A., Sacks F.M., et al. HDL and atherosclerotic cardiovascular disease: Genetic insights into complex biology. Nat. Rev. Cardiol. 2018;15:9–19. doi: 10.1038/nrcardio.2017.115. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer E.J., Tsunoda F., Diffenderfer M., Polisecki E., Thai N., Asztalos B. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. The Measurement of Lipids, Lipoproteins, Apolipoproteins, Fatty Acids, and Sterols, and Next Generation Sequencing for the Diagnosis and Treatment of Lipid Disorders. [PubMed] [Google Scholar]
- 97.Kratzer A., Jakob P. Catch miR if you can—Transcoronary gradients of HDL-bound microRNAs. Int. J. Cardiol. 2018;253:145–147. doi: 10.1016/j.ijcard.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 98.Tabet F., Cuesta Torres L.F., Ong K.L., Shrestha S., Choteau S.A., Barter P.J., Clifton P., Rye K.-A. High-Density Lipoprotein-Associated miR-223 Is Altered after Diet-Induced Weight Loss in Overweight and Obese Males. PLoS ONE. 2016;11:e0151061. doi: 10.1371/journal.pone.0151061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choteau S.A., Cuesta Torres L.F., Barraclough J.Y., Elder A.M.M., Martínez G.J., Chen Fan W.Y., Shrestha S., Ong K.L., Barter P.J., Celermajer D.S., et al. Transcoronary gradients of HDL-associated MicroRNAs in unstable coronary artery disease. Int. J. Cardiol. 2018;253:138–144. doi: 10.1016/j.ijcard.2017.09.190. [DOI] [PubMed] [Google Scholar]
- 100.Mangat R., Borthwick F., Haase T., Jacome M., Nelson R., Kontush A., Vine D.F., Proctor S.D. Intestinal lymphatic HDL miR-223 and ApoA-I are reduced during insulin resistance and restored with niacin. FASEB J. 2018;32:1602–1612. doi: 10.1096/fj.201600298RR. [DOI] [PubMed] [Google Scholar]
- 101.Li K., Wong D.K., Luk F.S., Kim R.Y., Raffai R.L. Extracellular RNA. Volume 1740. Humana Press; New York, NY, USA: 2018. Isolation of Plasma Lipoproteins as a Source of Extracellular RNA; pp. 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Axmann M., Meier S.M., Karner A., Strobl W., Stangl H., Plochberger B. Serum and Lipoprotein Particle miRNA Profile in Uremia Patients. Genes. 2018;9:533. doi: 10.3390/genes9110533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben-Aicha S., Escate R., Casaní L., Padró T., Peña E., Arderiu G., Mendieta G., Badimón L., Vilahur G. HDL remodelled in hypercholesterolemic blood induce epigenetically driven downregulation of endothelial HIF-1α expression in a preclinical animal model. Cardiovasc. Res. 2019 doi: 10.1093/cvr/cvz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hermans M.P., Amoussou-Guenou K.D., Bouenizabila E., Sadikot S.S., Ahn S.A., Rousseau M.F. Size, density and cholesterol load of HDL predict microangiopathy, coronary artery disease and β-cell function in men with T2DM. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11:125–131. doi: 10.1016/j.dsx.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 105.Asztalos B.F., Horvath K.V., Mehan M., Yokota Y., Schaefer E.J. Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J. Lipid Res. 2017;58:1238–1246. doi: 10.1194/jlr.M075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martínez-López D., Camafeita E., Cedó L., Roldan-Montero R., Jorge I., García-Marqués F., Gómez-Serrano M., Bonzon-Kulichenko E., Blanco-Vaca F., Blanco-Colio L.M., et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. EBioMedicine. 2019;43:43–53. doi: 10.1016/j.ebiom.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papageorgiou N., Zacharia E., Androulakis E., Briasoulis A., Charakida M., Tousoulis D. HDL as a prognostic biomarker for coronary atherosclerosis: The role of inflammation. Expert Opin. Ther. Targets. 2016;20:907–921. doi: 10.1517/14728222.2016.1152264. [DOI] [PubMed] [Google Scholar]
- 108.Cubedo J., Padró T., García-Moll X., Pintó X., Cinca J., Badimon L. Proteomic signature of Apolipoprotein J in the early phase of new-onset myocardial infarction. J. Proteome Res. 2011;10:211–220. doi: 10.1021/pr100805h. [DOI] [PubMed] [Google Scholar]
- 109.Vilahur G., Cubedo J., Padró T., Casaní L., Mendieta G., González A., Badimon L. Intake of cooked tomato sauce preserves coronary endothelial function and improves apolipoprotein A-I and apolipoprotein J protein profile in high-density lipoproteins. Transl. Res. 2015;166:44–56. doi: 10.1016/j.trsl.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Padro T., Muñoz-García N., Vilahur G., Chagas P., Deyà A., Antonijoan R.M., Badimon L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients. 2018;10:1237. doi: 10.3390/nu10091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vilahur G., Casani L., Mendieta G., Lamuela-Raventos R.M., Estruch R., Badimon L. Beer elicits vasculoprotective effects through Akt/eNOS activation. Eur. J. Clin. Investig. 2014;44:1177–1188. doi: 10.1111/eci.12352. [DOI] [PubMed] [Google Scholar]
- 112.Vilahur G., Casani L., Guerra J.M., Badimon L. Intake of fermented beverages protect against acute myocardial injury: Target organ cardiac effects and vasculoprotective effects. Basic Res. Cardiol. 2012;107:291. doi: 10.1007/s00395-012-0291-3. [DOI] [PubMed] [Google Scholar]
- 113.Liu L., Bortnick A.E., Nickel M., Dhanasekaran P., Subbaiah P.V., Lund-Katz S., Rothblat G.H., Phillips M.C. Effects of Apolipoprotein A-I on ATP-binding Cassette Transporter A1-mediated Efflux of Macrophage Phospholipid and Cholesterol. J. Biol. Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 114.Gilmore S.F., Carpenter T.S., Ingólfsson H.I., Peters S.K.G., Henderson P.T., Blanchette C.D., Fischer N.O. Lipid composition dictates serum stability of reconstituted high-density lipoproteins: Implications for in vivo applications. Nanoscale. 2018;10:7420–7430. doi: 10.1039/C7NR09690A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Linthout S., Frias M., Singh N., De Geest B. Handbook of Experimental Pharmacology. Volume 224. Springer; Cham, Germany: 2015. Therapeutic Potential of HDL in Cardioprotection and Tissue Repair; pp. 527–565. [DOI] [PubMed] [Google Scholar]
- 116.Shen W.-J., Asthana S., Kraemer F.B., Azhar S. Scavenger receptor B type 1: Expression, Molecular Regulation, and Cholesterol Transport Function. J. Lipid Res. 2018;59:1114–1131. doi: 10.1194/jlr.R083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bricarello D.A., Smilowitz J.T., Zivkovic A.M., German J.B., Parikh A.N. Reconstituted Lipoprotein: A Versatile Class of Biologically-Inspired Nanostructures. ACS Nano. 2011;5:42–57. doi: 10.1021/nn103098m. [DOI] [PubMed] [Google Scholar]
- 118.Vedhachalam C., Duong P.T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G.H., Lund-Katz S., Phillips M.C. Mechanism of ATP-binding Cassette Transporter A1-mediated Cellular Lipid Efflux to Apolipoprotein A-I and Formation of High Density Lipoprotein Particles. J. Biol. Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 119.Auton M., Bassett G.R., Gillard B.K., Pownall H.J. Free Cholesterol Determines Reassembled High-Density Lipoprotein Phospholipid Phase Structure and Stability. Biochemistry. 2013;52:4324–4330. doi: 10.1021/bi4006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bassett G.R., Gillard B.K., Pownall H.J. Cholesterol Determines and Limits rHDL Formation from Human Plasma Apolipoprotein A-II and Phospholipid Membranes. Biochemistry. 2012;51:8627–8635. doi: 10.1021/bi3011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pownall H.J., Massey J.B., Kusserow S.K., Gotto A.M. Kinetics of lipid-protein interactions: Effect of cholesterol on the association of human plasma high-density apolipoprotein A-I with L-.alpha.-dimyristoylphosphatidylcholine. Biochemistry. 1979;18:574–579. doi: 10.1021/bi00571a004. [DOI] [PubMed] [Google Scholar]
- 122.Gilman T., Kauffman J.W., Pownall H.J. Raman spectroscopy of the thermal properties of reassembled high-density lipoprotein: Apolipoprotein A-I complexes of dimyristoylphosphatidylcholine. Biochemistry. 1981;20:656–661. doi: 10.1021/bi00506a032. [DOI] [PubMed] [Google Scholar]
- 123.Atkinson D., Smith H.M., Dickson J., Austin J.P. Interaction of apoprotein from porcine high-density lipoprotein with dimyristoyl lecithin: 1. The structure of the complexes. Eur. J. Biochem. 1976;64:541–547. doi: 10.1111/j.1432-1033.1976.tb10334.x. [DOI] [PubMed] [Google Scholar]
- 124.Tall A.R., Small D.M., Deckelbaum R.J., Shipley G.G. Structure and thermodynamic properties of high density lipoprotein recombinants. J. Biol. Chem. 1977;252:4701–4711. [PubMed] [Google Scholar]
- 125.Jonas A., Krajnovich D.J. Interaction of human and bovine A-1 apolipoproteins with L-alpha-dimyristoyl phosphadicylcholine and L-alpha-myristoyl lysophosphatidylcholine. J. Biol. Chem. 1977;252:2194–2199. [PubMed] [Google Scholar]
- 126.Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 127.Schwendeman A., Sviridov D.O., Yuan W., Guo Y., Morin E.E., Yuan Y., Stonik J., Freeman L., Ossoli A., Thacker S., et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015;56:1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolfrum C., Shi S., Jayaprakash K.N., Jayaraman M., Wang G., Pandey R.K., Rajeev K.G., Nakayama T., Charrise K., Ndungo E.M., et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 129.Kallend D.G., Reijers J.A.A., Bellibas S.E., Bobillier A., Kempen H., Burggraaf J., Moerland M., Wijngaard P.L.J. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2016;2:23–29. doi: 10.1093/ehjcvp/pvv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reijers J.A.A., Kallend D.G., Malone K.E., Jukema J.W., Wijngaard P.L.J., Burggraaf J., Moerland M. MDCO-216 Does Not Induce Adverse Immunostimulation, in Contrast to Its Predecessor ETC-216. Cardiovasc. Drugs Ther. 2017;31:381–389. doi: 10.1007/s10557-017-6746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Capodanno D., Mehran R., Gibson C.M., Angiolillo D.J. CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I: Safety and tolerability profiles and implications for management in patients with myocardial infarction. Expert Opin. Investig. Drugs. 2018;27:997–1005. doi: 10.1080/13543784.2018.1543399. [DOI] [PubMed] [Google Scholar]
- 132.Theofilatos D., Fotakis P., Valanti E., Sanoudou D., Zannis V., Kardassis D. HDL-apoA-I induces the expression of angiopoietin like 4 (ANGPTL4) in endothelial cells via a PI3K/AKT/FOXO1 signaling pathway. Metabolism. 2018;87:36–47. doi: 10.1016/j.metabol.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 133.Tardif J.-C., Grégoire J., L’Allier P.L., Ibrahim R., Lespérance J., Heinonen T.M., Kouz S., Berry C., Basser R., Lavoie M.-A., et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 134.Tardif J.-C., Ballantyne C.M., Barter P., Dasseux J.-L., Fayad Z.A., Guertin M.-C., Kastelein J.J.P., Keyserling C., Klepp H., Koenig W., et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J. 2014;35:3277–3286. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andrews J., Janssan A., Nguyen T., Pisaniello A.D., Scherer D.J., Kastelein J.J.P., Merkely B., Nissen S.E., Ray K., Schwartz G.G., et al. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: Rationale and design of the CARAT study. Cardiovasc. Diagn. Ther. 2017;7:45–51. doi: 10.21037/cdt.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo Y., Yuan W., Yu B., Kuai R., Hu W., Morin E.E., Garcia-Barrio M.T., Zhang J., Moon J.J., Schwendeman A., et al. Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression. EBioMedicine. 2018;28:225–233. doi: 10.1016/j.ebiom.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang J.-L., Jiang G., Song Q.-X., Gu X., Hu M., Wang X.-L., Song H.-H., Chen L.-P., Lin Y.-Y., Jiang D., et al. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat. Commun. 2017;8:15144. doi: 10.1038/ncomms15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qi Y., Liu J., Wang W., Wang M., Zhao F., Sun J., Liu J., Zhao D. Apolipoprotein E-containing high-density lipoprotein (HDL) modifies the impact of cholesterol-overloaded HDL on incident coronary heart disease risk: A community-based cohort study. J. Clin. Lipidol. 2018;12:89–98.e2. doi: 10.1016/j.jacl.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 139.Kypreos K.E., Zannis V.I. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 2007;403:359–367. doi: 10.1042/BJ20061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Z., Cao W., Jin H., Lovell J.F., Yang M., Ding L., Chen J., Corbin I., Luo Q., Zheng G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem. Int. Ed. Engl. 2009;48:9171–9175. doi: 10.1002/anie.200903112. [DOI] [PubMed] [Google Scholar]
- 141.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ding Y., Wang Y., Opoku-Damoah Y., Wang C., Shen L., Yin L., Zhou J. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi: 10.1016/j.biomaterials.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 143.Rui M., Xin Y., Li R., Ge Y., Feng C., Xu X. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol. Pharm. 2017;14:107–123. doi: 10.1021/acs.molpharmaceut.6b00732. [DOI] [PubMed] [Google Scholar]
- 144.Brulhart-Meynet M.-C., Braunersreuther V., Brinck J., Montecucco F., Prost J.-C., Thomas A., Galan K., Pelli G., Pedretti S., Vuilleumier N., et al. Improving reconstituted HDL composition for efficient post-ischemic reduction of ischemia reperfusion injury. PLoS ONE. 2015;10:e0119664. doi: 10.1371/journal.pone.0119664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao Y., Jiang C., He J., Guo Q., Lu J., Yang Y., Zhang W., Liu J. Multifunctional Dextran Sulfate-Coated Reconstituted High Density Lipoproteins Target Macrophages and Promote Beneficial Antiatherosclerotic Mechanisms. Bioconj. Chem. 2017;28:438–448. doi: 10.1021/acs.bioconjchem.6b00600. [DOI] [PubMed] [Google Scholar]
- 146.Chuang S.T., Shon Y.-S., Narayanaswami V. Apolipoprotein E3-mediated cellular uptake of reconstituted high-density lipoprotein bearing core 3, 10, or 17 nm hydrophobic gold nanoparticles. Int. J. Nanomed. 2017;12:8495–8510. doi: 10.2147/IJN.S145326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fernández-de-Retana S., Cano-Sarabia M., Marazuela P., Sánchez-Quesada J.L., Garcia-Leon A., Montañola A., Montaner J., Maspoch D., Hernández-Guillamon M. Characterization of ApoJ-reconstituted high-density lipoprotein (rHDL) nanodisc for the potential treatment of cerebral β-amyloidosis. Sci. Rep. 2017;7:14637. doi: 10.1038/s41598-017-15215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Papillon J.P.N., Pan M., Brousseau M.E., Gilchrist M.A., Lou C., Singh A.K., Stawicki T., Thompson J.E. Synthetic phospholipids as specific substrates for plasma endothelial lipase. Bioorg. Med. Chem. Lett. 2016;26:3514–3517. doi: 10.1016/j.bmcl.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 149.Ikenaga M., Higaki Y., Saku K., Uehara Y. High-Density Lipoprotein Mimetics: A Therapeutic Tool for Atherosclerotic Diseases. J. Atheroscler. Thromb. 2016;23:385–394. doi: 10.5551/jat.33720. [DOI] [PubMed] [Google Scholar]
- 150.Badimon J.J., Badimon L., Galvez A., Dische R., Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Investig. 1989;60:455–461. [PubMed] [Google Scholar]
- 151.Karalis I., Jukema J.W. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr. Cardiol. Rep. 2018;20:66. doi: 10.1007/s11886-018-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nissen S.E., Tsunoda T., Tuzcu E.M., Schoenhagen P., Cooper C.J., Yasin M., Eaton G.M., Lauer M.A., Sheldon W.S., Grines C.L., et al. Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients with Acute Coronary Syndromes: A Randomized Controlled Trial. J. Am. Med. Assoc. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 153.Ibanez B., Vilahur G., Cimmino G., Speidl W.S., Pinero A., Choi B.G., Zafar M.U., Santos-Gallego C.G., Krause B., Badimon L., et al. Rapid Change in Plaque Size, Composition, and Molecular Footprint After Recombinant Apolipoprotein A-IMilano (ETC-216) Administration. Magnetic Resonance Imaging Study in an Experimental Model of Atherosclerosis. J. Am. Coll. Cardiol. 2008;51:1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 154.Aboumsallem J., Muthuramu I., Mishra M., Kempen H., De Geest B. Effective Treatment of Diabetic Cardiomyopathy and Heart Failure with Reconstituted HDL (Milano) in Mice. Int. J. Mol. Sci. 2019;20:1273. doi: 10.3390/ijms20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mishra M., Muthuramu I., Aboumsallem J., Kempen H., De Geest B. Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice. Int. J. Mol. Sci. 2018;19:3399. doi: 10.3390/ijms19113399. [DOI] [PMC free article] [PubMed] [Google Scholar]