STRUCTURED ABSTRACT
Aims.
Compare bladder cancer incidence between patients initiating pioglitazone and patients initiating dipeptidyl-peptidase-4 inhibitors [DPP-4s] or sulfonylureas.
Methods.
We identified Medicare beneficiaries aged >65 initiating pioglitazone (N=38,700), DPP-4s (N=82,552), or sulfonylureas (N=126,104) 2007–2014 after at least 6 months without prescriptions for these drug classes. Patients were followed from second prescription until bladder cancer outcome (2 claims within 60 days) using a 6-month induction/latency period, censoring for treatment change, death, or end of 2014. We used propensity score weighted Cox proportional-hazards models to obtain adjusted hazard ratios (aHR) and their 95% confidence intervals.
Results.
Overall mean age was 75 and 41% were men. Over a median of 1.2 treatment years, 727 beneficiaries developed bladder cancer. Pioglitazone initiators had an increased bladder cancer incidence (308 vs 204 (DPP-4s) or 231 (sulfonylureas) per 100,000 person-years; aHR=1.57 [1.23–2.00]) vs DPP-4s and 1.32 [1.02–1.70] vs sulfonylureas. The increased risk emerged within the first 2 years of treatment (aHR=1.63 [1.22–2.17] vs DPP-4s and 1.32 [0.98–1.78] vs sulfonylureas). If treatment was stopped within the first 2 years, the risk after 2 years post-initiation was attenuated (aHR=0.89 [0.61–1.28]) compared with patients treated for more than 2 years (aHR=1.45 [0.93–2.26], both vs DPP-4s). Findings were consistent across secondary and sensitivity analyses.
Conclusions.
Pioglitazone was associated with an elevated bladder cancer risk compared with DPP-4s and sulfonylureas. The elevated risk emerged within the first 2 years of treatment and was attenuated after stopping. Pioglitazone’s relative effectiveness should be weighed against a small absolute increase in bladder cancer risk.
Keywords: antidiabetic drug, pharmaco-epidemiology, database research, thiazolidinediones, DPP-IV inhibitor
INTRODUCTION
Pioglitazone’s safety has been greatly debated in the literature over the past decade. Before its Food and Drug Administration (FDA) approval in 1999, excess bladder tumors were reported in preclinical rat studies.1 This was thought to be a rat-specific phenomenon2 until the three-year PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) published excess bladder tumors in humans assigned pioglitazone versus placebo (0.5%[N=14] vs. 0.2%[N=6]) in 2005,3 behooving the FDA to request a 10-year safety study. When interim results of the 10-year study identified an increased risk of bladder cancer after 2 years (HR=1.44 [1.03–2.02]),4 the FDA issued a bladder cancer warning for pioglitazone exposure more than 2 years in 2011;5 a warning that was recently re-issued in December 2016.6
Multiple publications followed the initial safety warning that reported no evidence of an increased risk of bladder cancer for pioglitazone,4,7–14 including the final results from the 10-year study (HR=1.06 [0.89–1.26])4 and a PROactive follow-up study that observed patients for an additional period of 10 years after the trial (RR=1.05 [0.61–1.79]12. Far fewer publications reported an increased risk overall,15–18 including three more recent studies that compared pioglitazone users to non-users of pioglitazone’s thiazolidinediones drug class at the time of pioglitazone initiation (HR=1.63 [1.22–2.19]),16 never users of pioglitazone (RR=1.83 [1.10–3.05]),18 or placebo (0.6%[n=12] vs. 0.4%[n=8]) in the Insulin Resistance Intervention after Stroke (IRIS) trial.17
Although much has been published on the safety of pioglitazone, explanations for discrepancies in observed bladder cancer risk are not satisfactory, rendering the totality of evidence inconclusive.19 One potential explanation highlighted in this paper is comparator choice. Others include differing data sources, whether or not prevalent users were included, and how the risk window was defined. This is the first study to employ the incident user design and wide range of secondary and sensitivity analyses in a national sample of older US adults that compares the risk of incident bladder cancer in patients initiating pioglitazone to patients initiating clinically meaningful treatment alternatives, dipeptidyl-peptidase-4 inhibitors (DPP-4s) and sulfonylureas, as is recommended for comparative effectiveness research to present the least biased comparison.20,21
MATERIALS AND METHODS.
Data Source.
We used a 20% random sample of all Medicare beneficiaries with concurrent fee-for-service enrollment in Parts A (inpatient), B (outpatient) and D (pharmacy) in at least one month between January 2007 and December 2014. Medicare provides public insurance to over 98% of older US adults, and contains information about demographic and enrollment characteristics, diagnoses, procedures, and dispensed prescriptions for enrollees.22
Study Population.
We included patients aged 66 or older who initiated pioglitazone or active comparator (DPP-4s [largely sitagliptin and saxagliptin] and sulfonylureas [largely glyburide, glipizide and glimepiride]) with continuous enrollment in Medicare Parts A and B during the year prior to the drug initiation.(e-Figure S1) Incident use required patients to have no prescription claims for the drug classes included in each comparison during the 180-day period prior to the initial claim and have a second claim for the same drug class within 90 days. The second claim date defined the cohort entry date. Patients who started both pioglitazone and the comparator on the same day were excluded due to the inability to disentangle the individual effects of either drug. Patients with a diagnostic claim for bladder cancer or procedure code for a common bladder cancer treatment (bacillus Calmette-Guérin [BCG] immunotherapy, transurethral resection of bladder tumor [TURBT], chemotherapeutic bladder instillation, or cystectomy) at any time prior to the cohort entry date were excluded. Because secondary malignancies account for only 1.5% of all bladder tumors,23 we did not exclude patients with a history of non-bladder malignancies to maximize power for this rare outcome. Treatment classification for each comparison was determined based on first qualifying treatment per patient during the study period.
Outcome.
Incident events were defined as at least two International Classification of Disease (ICD-9) diagnostic claims for bladder cancer within 60 days, an algorithm previously validated for other solid tumors.24 We included non-invasive (233.7) in addition to invasive (188.x) claims since the majority of bladder cancers are diagnosed at an early stage.25 The first claim date defined the event date, as thought to be closest to date of actual diagnosis.
Follow-up.
The primary approach for defining the follow-up period for outcome ascertainment, referred to here as the “as-treated” (AT) approach, started on the cohort entry date and continued until first occurrence of incident bladder cancer, disenrollment, study end (December 2014), or treatment discontinuation (no subsequent dispensing for initiated drug class within days-supply plus a 90-day grace period). We added an additional 6-month latent period after treatment discontinuation to allow time for disease manifestation and detection. Additional analyses, referred to here as the “initial-treatment” (IT) approach, did not censor on treatment discontinuation, similar to the intent-to-treat model used in randomized controlled trials. We present AT as primary approach since non-adherence in IT analyses can attenuate results towards the null, potentially masking drug effects on safety outcomes. The first 6 months of follow-up were excluded regardless of censoring approach to allow for time between exposure and development of disease (induction period) to reduce the potential for spurious associations attributable to increased medicalization after start of a therapy or the possibility of preclinical symptoms of bladder cancer influencing treatment choice (protopathic bias). Latency and induction periods were also varied from 0–12 and 0–18 months, respectively. (Table S1)
Because the original FDA warning was for exposure greater than 2 years, follow-up was analyzed overall and stratified at 2 years. We further evaluated risk of bladder cancer during the time period greater than 2 years after drug initiation when actual treatment duration was less than 2 years. Only a subset of patients not otherwise censored within 2 years were included in analyses evaluating associations 2 years after drug initiation.
Detection Procedures.
Urologic screening and diagnostic procedures (cytology, dipstick urinalysis, non-dipstick urinalysis, urine function test, and cystoscopy) were enumerated at 0–6 and 6–12 months pre- and post-drug initiation to evaluate whether or not an increased bladder cancer incidence could be attributed to earlier and more frequent detection due to an increased rate of urologic procedures.
Confounding Control and Statistical Analysis.
Available Parts A and B medical claims started January 2006, but Part D pharmacy claims did not start until January 2007. Therefore, the baseline covariate assessment period prior to drug initiation was 6 months for medications and 12 months for comorbidities and healthcare utilization, maximizing use of available data. Descriptive statistics summarized covariates. The crude bladder cancer incidence rates (first event per patient) were calculated based on the Poisson distribution overall and for each treatment category. We used propensity scores (PS) based on all covariates to control for remaining differences between the compared cohorts. The propensities of initiating pioglitazone versus DPP-4s and pioglitazone versus sulfonylureas were estimated for each patient using two separate logistic regression models (one for each comparison).26 Standardized morbidity ratio (SMR) weighting that assigned the pioglitazone group a weight of 1 and each comparator group a weight of [PS/(1-PS)]27 was used to standardize the DPP-4s and sulfonylureas comparator covariates to the covariate distribution observed in the pioglitazone group. We report weighted comparison columns that represent pseudo-populations of patients initiating DPP-4s and sulfonylureas with covariate distribution balanced to that of the pioglitazone treatment group, allowing for unconfounded treatment effect estimates.27,28 We used weighted Kaplan-Meier plots to evaluate the proportional hazards assumption. Weighted Cox proportional hazards models with treatment as the only independent variable were used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals of bladder cancer incidence for each comparison and then for each detection procedure during each time period.
Sensitivity Analyses.
We conducted multiple sensitivity analyses to quantify the robustness of our results. First, we assessed robustness of the outcome definition using a more conservative (any claim; e-Table S3) and a more stringent definition (require additional procedure claim for a bladder cancer treatment within 3 months of the initial diagnosis; e-Table S4) to increase sensitivity and specificity. Second, we evaluated the cohort selection processes, separately excluding patients with each of the following: any cancer diagnosis except non-melanoma skin cancer identified using all available data as prevalent cancer may also affect the outcome (e-Tables S5–S6), no metformin use during baseline as treated contrary to guidelines (e-Table S7), congestive heart failure (CHF) diagnosis as treated contrary to FDA warning issued for thiazolidinediones (e-Tables S8–S9), treatment initiation after the FDA bladder cancer warning as propensity to initiate pioglitazone was likely to change (e-Tables S10–11), and those in the upper 1% and 2% tails of the PS distribution of each drug group, as trimming those treated contrary to prediction can reduce unmeasured confounding29 (e-Tables S12–S15). Third, we re-estimated the PS excluding indicator variables for calendar time of drug initiation from the model, since time may be an instrumental variable for pioglitazone treatment rather than confounder following the bladder cancer warning (e-Tables S16–S17)30. Fourth we implemented a range of grace periods for defining treatment discontinuation in the AT analysis from 90 to 45 and 180 days (e-Table S18), as well as calculating individual grace periods based on the days-supply, double-days-supply, and triple-days-supply of the last dispensing (e-Table S19). Finally, given the poor sensitivity of claims for identification of smoking status,31 we conducted an external validation study using data from the 2007 to 2011 Medicare Current Beneficiary Survey (MCBS). We identified new users of all three drug classes based on Part D data and present data on smoking and BMI reported during the interview.32 (e-Table S20)
All data were analyzed in SAS, v9.4. The University of North Carolina at Chapel Hill institutional review board approved this study. This study started as a methodological comparison of various study design approaches comparing pioglitazone and DPP-4s. Upon the review of early findings suggesting a safety concern, a new study protocol was written and registered in the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance electronic register of studies (http://www.encepp.eu, EU PAS Register Number: EUPAS13279).
RESULTS.
Distribution of patient characteristics for the pioglitazone versus DPP-4s (38,700 vs. 82,552) and pioglitazone versus sulfonylureas (20,075 vs. 126,104) initiators are presented in Table 1. Overall, when compared to each respective comparator, pioglitazone initiators were more likely to be younger, non-white men, and less likely to have a smoking-related claim or have comorbid diagnoses of cancer, chronic obstructive pulmonary disorder (COPD) or CHF. Although differences were present for both comparisons, they were generally more pronounced in the sulfonylureas comparison. Relative to DPP-4s, pioglitazone users were less likely to have a history of metformin, insulin, or angiotensin receptor blocker use, which reversed when compared to sulfonylureas. After SMR weighting, the distribution of the variables presented in Table 1 for the weighted DPP-4s and sulfonylureas pseudo-populations became virtually identical to the pioglitazone group within each respective comparison, indicating no confounding by these variables.
Table 1.
Distribution of Patient Covariates of Patients who Initiated Pioglitazone, Dipeptidyl-peptidase-4, or Sulfonylureas
| PIO* (N=38,700) | DPP (N=82,552) | SMRW DPP† | PIO* (N=20,075) | SU (N=126,104) | SMRW SU† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | % | N | % | N | % | % | |||
| DEMOGRAPHIC CHARACTERISTICS AT DRUG INITIATION | |||||||||||||
| Age, Mean, Median (IQR) | 74.9, 74 (69,79) | 75.8, 75 (70,81) | 74.8, 73 (69,79) | 75.9, 75 (70,81) | |||||||||
| Age 66 to <70 | 10,050 | 26.0 | 18,838 | 22.8 | 26.1 | 5,316 | 26.5 | 29,020 | 23.0 | 26.4 | |||
| Age 70 to <75 | 11,195 | 28.9 | 22,311 | 27.0 | 29.0 | 5,832 | 29.1 | 33,267 | 26.4 | 29.1 | |||
| Age 75 to <80 | 8,018 | 20.7 | 17,356 | 21.0 | 20.6 | 4,144 | 20.6 | 25,689 | 20.4 | 20.7 | |||
| Age 80 to <85 | 5,495 | 14.2 | 12,925 | 15.7 | 14.2 | 2,733 | 13.6 | 19,616 | 15.6 | 13.6 | |||
| Age >=85 | 3,942 | 10.2 | 11,122 | 13.5 | 10.0 | 2,050 | 10.2 | 18,512 | 14.7 | 10.2 | |||
| Male | 16,280 | 42.1 | 31,683 | 38.4 | 42.3 | 8,350 | 41.6 | 51,459 | 40.8 | 41.7 | |||
| White | 27,849 | 72.0 | 62,274 | 75.4 | 72.6 | 14,332 | 71.4 | 98,793 | 78.3 | 71.3 | |||
| Black | 4,676 | 12.1 | 9,165 | 11.1 | 11.7 | 2,411 | 12.0 | 14,999 | 11.9 | 12.0 | |||
| Other Race‡ | 6,175 | 16.0 | 11,113 | 13.5 | 15.7 | 3,332 | 16.6 | 12,312 | 9.8 | 16.7 | |||
| Drug Initiation Year: 2007 | 2,384 | 6.2 | 1,915 | 2.3 | 6.1 | 1,138 | 5.7 | 4,278 | 3.4 | 5.7 | |||
| Drug Initiation Year: 2008 | 9,515 | 24.6 | 7,459 | 9.0 | 24.5 | 4,511 | 22.5 | 17,222 | 13.7 | 22.5 | |||
| Drug Initiation Year: 2009 | 8,362 | 21.6 | 7,546 | 9.1 | 21.6 | 3,910 | 19.5 | 17,293 | 13.7 | 19.4 | |||
| Drug Initiation Year: 2010 | 7,146 | 18.5 | 9,084 | 11.0 | 18.5 | 3,610 | 18.0 | 16,869 | 13.4 | 18.0 | |||
| Drug Initiation Year: 2011 | 4,279 | 11.1 | 12,785 | 15.5 | 11.1 | 2,379 | 11.9 | 16,635 | 13.2 | 11.8 | |||
| Drug Initiation Year: 2012 | 2,262 | 5.8 | 14,440 | 17.5 | 5.9 | 1,392 | 6.9 | 16,930 | 13.4 | 6.9 | |||
| Drug Initiation Year: 2013 | 2,419 | 6.3 | 14,876 | 18.0 | 6.3 | 1,532 | 7.6 | 19,040 | 15.1 | 7.6 | |||
| Drug Initiation Year: 2014 | 2,333 | 6.0 | 14,447 | 17.5 | 6.0 | 1,603 | 8.0 | 17,837 | 14.1 | 8.0 | |||
| BLADDER COMORBIDITIES DURING BASELINE § | |||||||||||||
| Bladder Stones | 103 | 0.3 | 219 | 0.3 | 0.3 | 52 | 0.3 | 386 | 0.3 | 0.3 | |||
| Kidney Stones | 1,231 | 3.2 | 3,202 | 3.9 | 3.2 | 671 | 3.3 | 4,577 | 3.6 | 3.3 | |||
| Urinary Tract Infection | 9,545 | 24.7 | 24,107 | 29.2 | 24.6 | 5,176 | 25.8 | 35,116 | 27.8 | 25.9 | |||
| DIABETES-RELATED COMPLICATIONS DURING BASELINE § | |||||||||||||
| Nephropathy | 3,176 | 8.2 | 8,628 | 10.5 | 8.3 | 1,539 | 7.7 | 9,414 | 7.5 | 7.8 | |||
| Neuropathy | 7,437 | 19.2 | 19,216 | 23.3 | 19.6 | 3,624 | 18.1 | 21,696 | 17.2 | 18.1 | |||
| Retinopathy | 6,593 | 17.0 | 14,293 | 17.3 | 17.1 | 2,991 | 14.9 | 15,060 | 11.9 | 14.9 | |||
| HEALTHCARE UTILIZATION OR SMOKING-RELATED ENCOUNTER DURING BASELINE § | |||||||||||||
| Any Admission | 9,954 | 25.7 | 25,816 | 31.3 | 25.4 | 5,127 | 25.5 | 44,433 | 35.2 | 25.6 | |||
| Any Long-term Admission | 1,029 | 2.7 | 2,465 | 3.0 | 2.6 | 537 | 2.7 | 4,761 | 3.8 | 2.7 | |||
| Any Short-term Admission | 9,447 | 24.4 | 24,776 | 30.0 | 24.2 | 4,879 | 24.3 | 42,301 | 33.5 | 24.4 | |||
| Any SNF Admission | 2,940 | 7.6 | 8,305 | 10.1 | 7.5 | 1,547 | 7.7 | 15,420 | 12.2 | 7.8 | |||
| Any Electrocardiogram | 19,935 | 51.5 | 49,468 | 59.9 | 51.3 | 10,550 | 52.6 | 73,158 | 58.0 | 52.5 | |||
| Any Office Visit | 36,672 | 94.8 | 79,327 | 96.1 | 95.0 | 18,970 | 94.5 | 117,367 | 93.1 | 94.4 | |||
| Any Influenza Shot | 20,308 | 52.5 | 46,497 | 56.3 | 53.1 | 10,657 | 53.1 | 66,792 | 53.0 | 53.0 | |||
| Any Lipid Panel | 32,584 | 84.2 | 71,134 | 86.2 | 84.6 | 16,795 | 83.7 | 98,445 | 78.1 | 83.7 | |||
| Any PSA Test (men) | 9,493 | 58.3 | 18,042 | 56.9 | 58.4 | 4,946 | 59.2 | 26,542 | 51.6 | 59.2 | |||
| Any Colonoscopy | 3,787 | 9.8 | 8,491 | 10.3 | 9.8 | 2,026 | 10.1 | 12,453 | 9.9 | 10.1 | |||
| Any Mammogram (women) | 7,583 | 33.8 | 17,525 | 34.5 | 34.3 | 4,008 | 34.2 | 23,445 | 31.4 | 34.0 | |||
| Any Pap Smear (women) | 3,367 | 8.7 | 7,403 | 9.0 | 8.7 | 1,776 | 8.8 | 10,324 | 8.2 | 8.9 | |||
| Any Blood Test | 2,017 | 9.0 | 4,417 | 8.7 | 9.1 | 1,146 | 9.8 | 5,565 | 7.5 | 9.8 | |||
| Smoking** | 3,334 | 8.6 | 10,533 | 12.8 | 8.5 | 1,834 | 9.1 | 16,977 | 13.5 | 9.2 | |||
| OTHER COMORBIDITIES DURING BASELINE § | |||||||||||||
| Congestive Heart Failure | 6,648 | 17.2 | 20,971 | 25.4 | 17.1 | 3,479 | 17.3 | 32,491 | 25.8 | 17.3 | |||
| Chronic Kidney Disease | 5,051 | 13.1 | 13,402 | 16.2 | 13.0 | 2,600 | 13.0 | 19,373 | 15.4 | 13.0 | |||
| Connective Tissue Disease | 11,778 | 30.4 | 29,046 | 35.2 | 30.4 | 6,454 | 32.1 | 40,520 | 32.1 | 32.2 | |||
| COPD | 6,246 | 16.1 | 16,169 | 19.6 | 16.0 | 3,499 | 17.4 | 26,752 | 21.2 | 17.4 | |||
| Depression | 5,802 | 15.0 | 15,189 | 18.4 | 14.8 | 3,234 | 16.1 | 23,535 | 18.7 | 16.1 | |||
| Gastrointestinal Disorders | 308 | 0.8 | 808 | 1.0 | 0.8 | 196 | 1.0 | 1,243 | 1.0 | 1.0 | |||
| Infections | 16,802 | 43.4 | 40,235 | 48.7 | 43.3 | 8,989 | 44.8 | 58,534 | 46.4 | 44.8 | |||
| Myocardial Infarction | 319 | 0.8 | 1,026 | 1.2 | 0.8 | 142 | 0.7 | 1,630 | 1.3 | 0.7 | |||
| Stroke | 4,429 | 11.4 | 10,804 | 13.1 | 11.2 | 2,367 | 11.8 | 17,576 | 13.9 | 11.8 | |||
| History of Cancer†† | 7,489 | 19.4 | 19,836 | 24.0 | 19.4 | 4,046 | 20.2 | 29,856 | 23.7 | 20.1 | |||
| ANTIDIABETIC USE DURING BASELINE § | |||||||||||||
| DPP | 2,219 | 11.1 | 12,343 | 9.8 | 8.7 | ||||||||
| GLP-1 | 625 | 1.6 | 1,202 | 1.5 | 2.0 | 330 | 1.6 | 1,279 | 1.0 | 1.7 | |||
| Insulin | 6,474 | 16.7 | 15,526 | 18.8 | 16.7 | 4,258 | 21.2 | 18,544 | 14.7 | 21.2 | |||
| Short-acting Insulin | 4,186 | 10.8 | 9,537 | 11.6 | 10.7 | 2,847 | 14.2 | 12,198 | 9.7 | 14.2 | |||
| Long-acting Insulin | 3,901 | 10.1 | 10,041 | 12.2 | 10.1 | 2,575 | 12.8 | 10,915 | 8.7 | 12.8 | |||
| Meglitinides | 909 | 2.3 | 2,557 | 3.1 | 2.5 | 672 | 3.3 | 3,001 | 2.4 | 3.4 | |||
| Metformin | 18,532 | 47.9 | 42,485 | 51.5 | 48.0 | 8,784 | 43.8 | 55,033 | 43.6 | 43.8 | |||
| Sulfonylureas | 18,959 | 49.0 | 38,750 | 46.9 | 49.7 | ||||||||
| Other Antidiabetic ‡‡ | 308 | 0.80 | 737 | 0.89 | 124 | 0.62 | 510 | 0.40 | |||||
| MEDICATIONS DURING BASELINE § | |||||||||||||
| ACE Inhibitors | 14,332 | 37.0 | 29,477 | 35.7 | 36.9 | 6,476 | 32.3 | 42,145 | 33.4 | 32.2 | |||
| Anti-Cholesterol Drugs | 649 | 1.7 | 1,335 | 1.6 | 1.7 | 352 | 1.8 | 2,436 | 1.9 | 1.7 | |||
| ARBs | 5,573 | 14.4 | 14,254 | 17.3 | 14.5 | 2,979 | 14.8 | 16,520 | 13.1 | 14.9 | |||
| Beta-2 Antagonists | 3,095 | 8.0 | 7,849 | 9.5 | 7.9 | 1,704 | 8.5 | 12,404 | 9.8 | 8.4 | |||
| Bile Acid sequestrants | 419 | 1.1 | 1,311 | 1.6 | 1.1 | 274 | 1.4 | 1,565 | 1.2 | 1.4 | |||
| Beta-Blockers | 15,708 | 40.6 | 39,780 | 48.2 | 40.8 | 7,810 | 38.9 | 56,550 | 44.8 | 38.8 | |||
| CAIs | 1,308 | 3.4 | 3,060 | 3.7 | 3.5 | 734 | 3.7 | 3,396 | 2.7 | 3.7 | |||
| Calcium Channel Blockers | 10,863 | 28.1 | 25,282 | 30.6 | 28.1 | 5,277 | 26.3 | 35,605 | 28.2 | 26.3 | |||
| Estrogen | 517 | 1.3 | 1,071 | 1.3 | 1.4 | 296 | 1.5 | 1,554 | 1.2 | 1.5 | |||
| Fibrates | 3,225 | 8.3 | 6,803 | 8.2 | 8.6 | 1,556 | 7.8 | 8,286 | 6.6 | 7.8 | |||
| Glycosides | 2,018 | 5.2 | 5,446 | 6.6 | 5.2 | 979 | 4.9 | 8,787 | 7.0 | 4.9 | |||
| Loop Diuretics | 7,615 | 19.7 | 22,028 | 26.7 | 19.8 | 3,689 | 18.4 | 32,470 | 25.7 | 18.4 | |||
| Niacin | 692 | 1.8 | 1,320 | 1.6 | 1.8 | 399 | 2.0 | 1,441 | 1.1 | 2.0 | |||
| Non-loop Diuretics | 12,621 | 32.6 | 31,537 | 38.2 | 32.8 | 6,054 | 30.2 | 47,217 | 37.4 | 30.2 | |||
| Statins | 20,500 | 53.0 | 41,348 | 50.1 | 53.3 | 10,127 | 50.4 | 54,091 | 42.9 | 50.4 | |||
ACE, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blockers; CAIs, Carbonic Anhydrase Inhibitors; COPD, Chronic Obstructive Pulmonary Disease; DPP, dipeptidyl-peptidase-4; GLP-1, glucagon-like peptide-1 receptor agonists; IQR, interquartile range; PIO, pioglitazone; PSA, Prostate-specific antigen; SMRW, Standardized morbidity ratio weighted; SNF, Skilled Nursing Facility; SU, sulfonylureas.
Number of people initiating PIO differs for each comparison due to exclusions of prior use of drugs included in comparison only.
Distribution of pseudo-population of people initiating SU or DPP treatment SMR-weighted to the distribution of covariates of those initiating PIO treatment, using the propensity score to balance covariates (and therefore control for confounding). N’s not reported.
Other race combines the following races as defined by Medicare: Other, Asian, Hispanic, or Native American
The baseline period was during the 12 months prior to drug initiation for comorbidities and healthcare utilization, and 6 months prior to drug initiation for medication history.
Smoking was defined using a previously validated algorithm that was a composite of tobacco use diagnosis codes or consultation CPT codes or prescription filled for smoking cessation. Although perfect specificity and PPV, this measure has poor sensitivity (27.9% [95% CI: 16.6–39.1%]31
History of cancer was evaluated during all available data prior to cohort entry.
Other antidiabetic medications included alpha-glucosidase inhibitors, amylin analogs, and sodium-glucose co-transporter 2 (SGLT2) inhibitors. These drugs were not included in the propensity score model as <1% combined in this study population.
Table 2 shows the bladder cancer incidence rates per 100,000 person-years (representing post-initiation years for the IT and treatment years for the AT analyses) and corresponding HRs (crude and fully adjusted) for initiators of pioglitazone versus DPP-4s or sulfonylureas. Median treatment duration (1.1–1.2 years) was similar in all AT analyses due to the frequent treatment changes among patients with type 2 diabetes, while available follow-up in the IT analysis was >1 year longer for pioglitazone than DPP-4s or sulfonylureas because more patients initiated with pioglitazone in the earlier years and with DPP-4 in later years.
Table 2.
Bladder Cancer Incidence among Initiators of Pioglitazone, Dipeptidyl-peptidase-4, or Sulfonylureas
| Comparison | Drug | Follow-Up (Years), Median (IQR)* | N† | Events | Person-Time (Years) | Rate‡ | Unadjusted HR (95% CI)§ | Fully Adjusted HR (95% CI) || |
|---|---|---|---|---|---|---|---|---|
| As-Treated Analyses | ||||||||
| Overall | ||||||||
| PIO vs. DPP | PIO | 1.15(0.67–2.16) | 29,651 | 147 | 47,766 | 307.8 | 1.50(1.21–1.86) | 1.57(1.23–2.00) |
| DPP | 1.11(0.59–2.12) | 61,438 | 193 | 94,426 | 204.4 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.11(0.65–2.09) | 15,198 | 73 | 23,830 | 306.3 | 1.32(1.02–1.69) | 1.32(1.02–1.70) |
| SU | 1.24(0.62–2.45) | 97,056 | 387 | 167,879 | 230.5 | 1 (reference) | 1 (reference) | |
| Duration of Treatment Restricted to 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.15(0.67–1.97) | 29,651 | 108 | 36,010 | 299.9 | 1.49(1.16–1.92) | 1.63(1.22–2.17) |
| DPP | 1.11(0.59–1.97) | 61,438 | 144 | 71,869 | 200.4 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.11(0.65–1.97) | 15,198 | 57 | 18,125 | 314.5 | 1.30(0.98–1.73) | 1.32(0.98–1.78) |
| SU | 1.24(0.62–1.97) | 97,056 | 287 | 119,458 | 240.3 | 1 (reference) | 1 (reference) | |
| Duration of Treatment After 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.14(0.47–2.08) | 8,367 | 39 | 11,756 | 331.7 | 1.53(1.00–2.32) | 1.45(0.93–2.26) |
| DPP | 1.01(0.41–1.97) | 16,894 | 49 | 22,557 | 217.2 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.11(0.46–2.07) | 4,110 | 16 | 5,706 | 280.4 | 1.37(0.81–2.32) | 1.29(0.76–2.18) |
| SU | 1.23(0.51–2.29) | 31,940 | 100 | 48,422 | 206.5 | 1 (reference) | 1 (reference) | |
| Initial-Treatment Analyses | ||||||||
| Overall | ||||||||
| PIO vs. DPP | PIO | 3.35(1.52–4.86) | 35,512 | 282 | 115,379 | 244.4 | 1.26(1.07–1.49) | 1.22(1.02–1.47) |
| DPP | 1.86(0.82–3.28) | 70,628 | 308 | 157,386 | 195.7 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 3.23(1.39–4.75) | 18,224 | 128 | 57,282 | 223.5 | 0.99(0.82–1.20) | 1.02(0.84–1.24) |
| SU | 2.05(0.86–3.73) | 108,593 | 594 | 262,177 | 226.6 | 1 (reference) | 1 (reference) | |
| Time Since Treatment Initiation Restricted to 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.97(1.52–1.97) | 35,512 | 152 | 58,351 | 260.5 | 1.35(1.09–1.66) | 1.38(1.08–1.77) |
| DPP | 1.86(0.82–1.97) | 70,628 | 194 | 99,661 | 194.7 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.97(1.39–1.97) | 18,224 | 71 | 29,399 | 241.5 | 1.04(0.81–1.34) | 1.05(0.80–1.36) |
| SU | 1.97(0.86–1.97) | 108,593 | 364 | 156,316 | 232.9 | 1 (reference) | 1 (reference) | |
| Time Since Treatment Initiation After 2 Years | ||||||||
| PIO vs. DPP | PIO | 2.28(1.28–3.36) | 24,488 | 130 | 57,028 | 228.0 | 1.16(0.90–1.49) | 1.08(0.82–1.41) |
| DPP | 1.38(0.61–2.64) | 33,748 | 114 | 57,725 | 197.5 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 2.22(1.25–3.33) | 12,187 | 57 | 27,883 | 204.4 | 0.93(0.70–1.24) | 0.99(0.73–1.32) |
| SU | 1.69(0.76–2.92) | 55,848 | 230 | 105,861 | 217.3 | 1 (reference) | 1 (reference) | |
| Initial-treatment Analyses -- Restricted to Patients with <=2 Years Duration of Treatment | ||||||||
| Time Since Treatment Initiation after 2 Years | ||||||||
| PIO vs. DPP | PIO | 2.15(1.22–3.24) | 16,121 | 74 | 36,042 | 205.3 | 1.02(0.72–1.44) | 0.89(0.61–1.28) |
| DPP | 1.35(0.57–2.62) | 16,854 | 57 | 28,404 | 200.7 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 2.10(1.20–3.21) | 8,077 | 32 | 17,807 | 179.7 | 0.74(0.50–1.10) | 0.82(0.55–1.23) |
| SU | 1.60(0.70–2.83) | 23,908 | 105 | 43,745 | 240.0 | 1 (reference) | 1 (reference) | |
CI, Confidence Interval; DPP, dipeptidyl-peptidase-4; HR, Hazard Ratio; IQR, interquartile range; PIO, pioglitazone; SU, sulfonylureas
Follow-up started on the cohort entry date (second dispensing) and was censored at first occurrence of outcome, death, end of study (December 2014), or end of enrollment. As-treated analyses additionally censored at treatment discontinuation (no subsequent fill of initiated drug class within days-supply plus a 90-day grace period). A 180-day induction period was imposed excluding time from the beginning of follow-up. The as-treated analyses additionally added a 180-day latency period to the end of follow-up when possible prior to death or end of patient data.
Number contributing with at least 180 days of follow-up. Those who initiated PIO differs for each comparison due to exclusions of prior use of drugs included in comparison only.
Incidence Rate reported per 100 000 Person-Years
Cox proportional hazards models
Cox proportional hazards models adjusted for all variables in Table 1, except for those excluded within each comparison by incident user design, using propensity-score weighting (standardized to PIO population)
Compared to DPP-4s initiators in the AT and IT analyses, pioglitazone initiators had an increased incidence of bladder cancer overall (307.8 vs 204.4 per 100 000 person-years; HR=1.57 [1.23–2.00] and 244.4 vs 195.7; HR=1.22 [1.02–1.47]). The increased risk emerged within the first 2 years of treatment (HR=1.63 [1.22–2.17] and 1.38 [1.08–1.77]) and remained after 2 years (HR=1.45 [0.93–2.26] and 1.08 [0.82–1.41]). If treatment was stopped within the first 2 years, the risk after 2 years post-initiation was attenuated (205.3 vs 200.7; HR=0.89 [0.61–1.28]).
Compared to sulfonylureas initiators, pioglitazone initiators had an increased incidence of bladder cancer overall in the AT analysis (306.3 vs 230.5; HR=1.32 [1.02–1.70]), but not in the IT analysis (223.5 vs 226.6; HR=1.02 [0.84–1.24]). However, this is likely an artifact of longer follow-up available for pioglitazone initiators, as there was some increased risk when follow-up was restricted to 2 years (241.5 vs 232.9; HR=1.05 [0.80–1.36]). If treatment was stopped within the first 2 years, the risk after 2 years post-initiation was attenuated (179.7 vs 240.0; HR=0.82 [0.55–1.23]).
HRs that adjusted for age, sex, and race were similar to crude (e-Table S1). Figure 1 displays Kaplan-Meier curves estimated via Cox proportional hazards models PS-weighted (standardized to pioglitazone population) for all variables in Table 1, unless otherwise specified. We identified increasing relative rates of bladder cancer over time associated with pioglitazone for all analyses except the comparison to sulfonylureas in the IT analysis.
Figure 1.
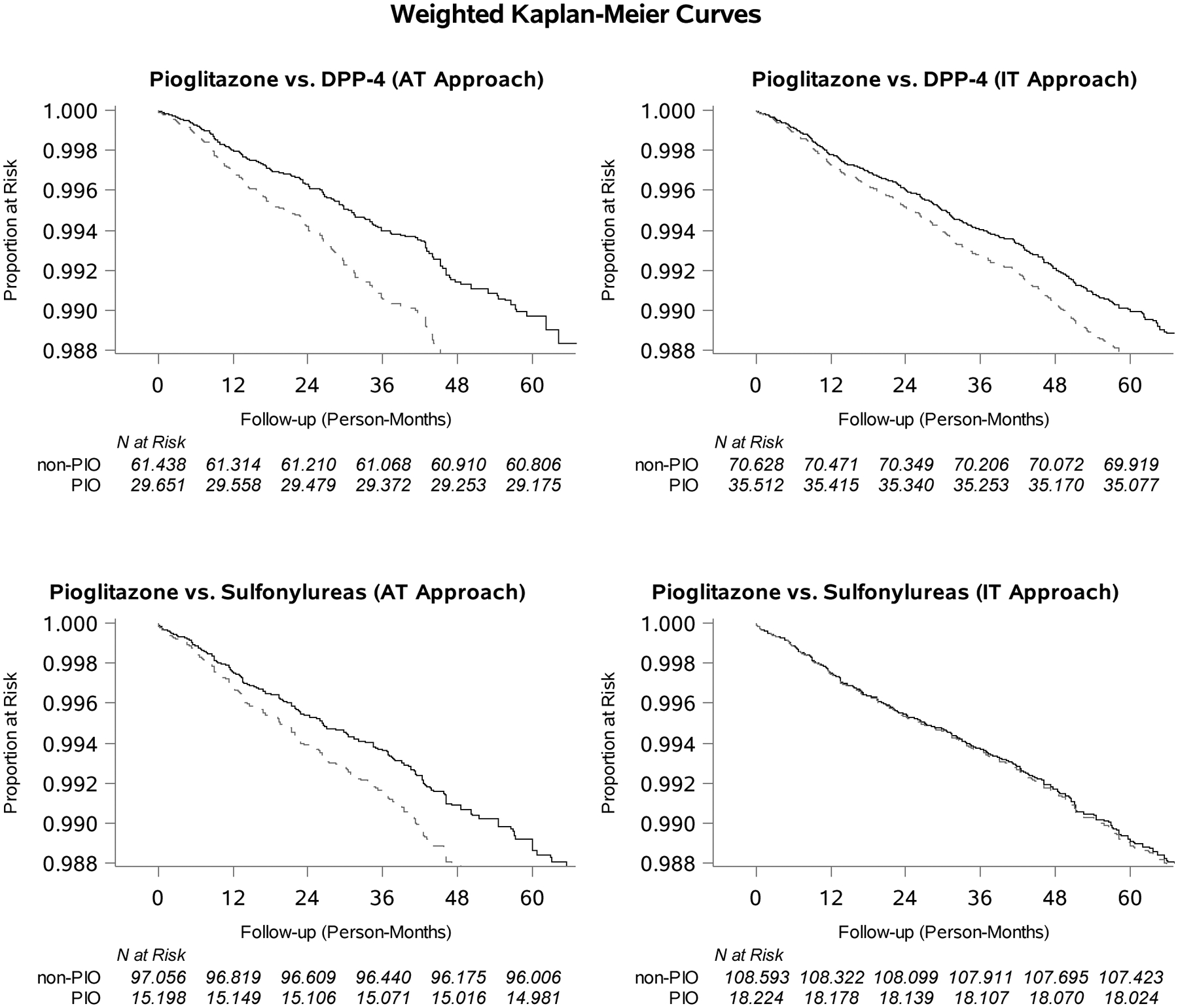
Weighted Kaplan-Meier Curves Identifying those Remaining without Bladder Cancer over Follow-up among Initiators of Pioglitazone (dotted line) or Active Comparator (solid line; Dipeptidyl-peptidase-4 or Sulfonylureas) with at Least 180 days of Follow-up.
Supplemental e-Table S1 reports the AT results from Table 2 with varied induction and latency periods. When no induction or latency periods were used, HRs were attenuated for pioglitazone compared to DPP-4s (1.14 [0.90–1.46]) or sulfonylureas (1.13 [0.87–1.46]). For both comparisons, similar results were found when induction was lengthened from 6 to 12 and 18 months. When latency was shortened from 6 to 3 months, HRs were attenuated for pioglitazone versus DPP-4.
Figure 2, which illustrates the relative rates of detection procedures estimated via Cox proportional hazards models PS-weighted (standardized to pioglitazone population) for all variables in Table 1, indicates no appreciable differences for 0–6 or >6–12-months after initiation. In our external validation study using MCBS data (e-Table S20), pioglitazone initiators were less likely to be ever smokers compared to DPP-4 initiators (48.6 vs. 56.2%) and sulfonylureas initiators (49.1 vs. 60.0%). Pioglitazone initiators were less likely to be obese (BMI≥30kg/m2) than DPP-4 initiators (38.1 vs. 54.3%) and SU initiators (42.1 vs. 42.8%).
Figure 2.
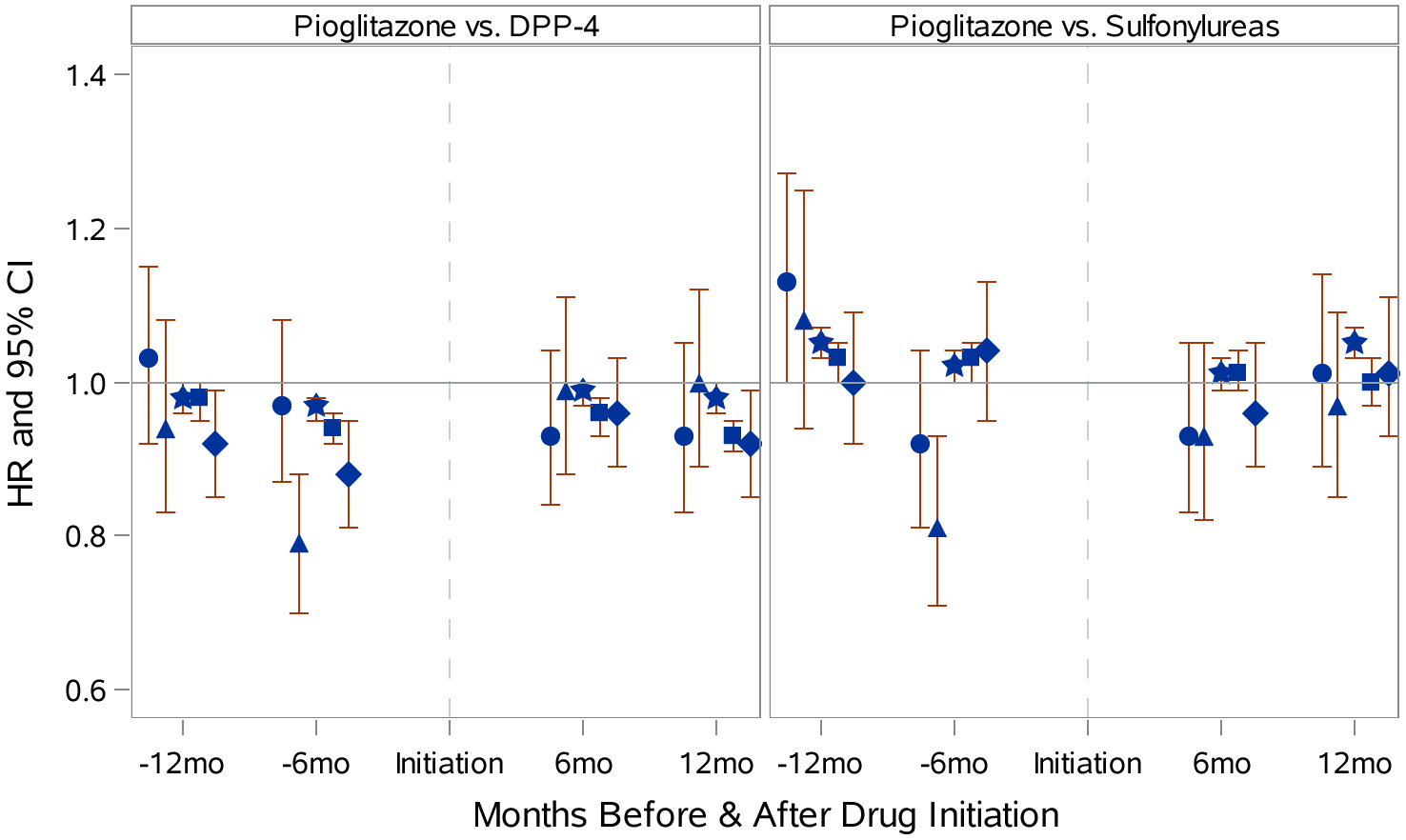
Relative Rates of Bladder-Cancer-Related Diagnostic Procedures 6 and 12 Months Before and After Initiation of Pioglitazone, Dipeptidyl-peptidase-4, and Sulfonylureas where Circle=Cystoscopy, Triangle=Cytology, Square=Non-dipstick Urinalysis, Star=Dipstick Urinalysis, Diamond=Urine Function Test
Multiple sensitivity analyses identified more pronounced HRs in the AT analyses for pioglitazone compared to DPP-4, and attenuated HRs compared to sulfonylureas. These included use of more stringent outcome definition (e-Table S4) and sensitive cohorts that excluded patients with the following: prevalent cancer, CHF, treatment contrary to PS in the 1% and 2% tails, and no concurrent metformin use (e-Table S6, S9, S13, and S15; Table 3). Otherwise, sensitivity analyses yielded similar HRs (Tables S3–S19).
Table 3.
Exclusion of Patients without History of Metformin: Bladder Cancer Incidence among Initiators of Pioglitazone, Dipeptidyl-peptidase-4, and Sulfonylureas
| Comparison | Drug | Follow-Up (Years), Median (IQR)* | N† | Events | Person-Time (Years) | Rate‡ | Unadjusted HR (95% CI)§ | Fully Adjusted HR (95% CI) || |
|---|---|---|---|---|---|---|---|---|
| As-Treated Analyses | ||||||||
| Overall | ||||||||
| PIO vs. DPP | PIO | 1.17(0.67–2.21) | 14,476 | 75 | 23,713 | 316.3 | 1.75(1.29–2.38) | 1.82(1.28–2.59) |
| DPP | 1.14(0.60–2.17) | 32,088 | 91 | 50,400 | 180.6 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.17(0.67–2.17) | 6,819 | 27 | 11,059 | 244.1 | 1.18(0.78–1.77) | 1.21(0.79–1.85) |
| SU | 1.31(0.65–2.58) | 43,281 | 161 | 77,736 | 207.1 | 1 (reference) | 1 (reference) | |
| Duration of Treatment Restricted To 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.17(0.67–1.97) | 14,476 | 53 | 17,768 | 298.3 | 1.69(1.18–2.43) | 1.80(1.17–2.76) |
| DPP | 1.14(0.60–1.97) | 32,088 | 67 | 38,016 | 176.2 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.17(0.67–1.97) | 6,819 | 18 | 8,315 | 216.5 | 1.02(0.62–1.68) | 1.03(0.62–1.73) |
| SU | 1.31(0.65–1.97) | 43,281 | 115 | 54,678 | 210.3 | 1 (reference) | 1 (reference) | |
| Duration of Treatment after 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.13(0.47–2.09) | 4,214 | 22 | 5,945 | 370.0 | 1.91(1.07–3.41) | 1.86(1.02–3.41) |
| DPP | 1.03(0.43–2.00) | 9,129 | 24 | 12,384 | 193.8 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.13(0.47–2.12) | 1,942 | <11 | NR | 328.0 | 1.65(0.81–3.38) | 1.65(0.81–3.37) |
| SU | 1.24(0.51–2.31) | 15,159 | 46 | 23,058 | 199.5 | 1 (reference) | 1 (reference) | |
| Initial-treatment Analyses | ||||||||
| Overall | ||||||||
| PIO vs. DPP | PIO | 3.41(1.57–4.86) | 17,081 | 133 | 56,079 | 237.2 | 1.40(1.10–1.79) | 1.31(1.00–1.72) |
| DPP | 1.90(0.84–3.31) | 36,450 | 140 | 81,932 | 170.9 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 3.32(1.46–4.80) | 8,022 | 51 | 25,684 | 198.6 | 1.07(0.79–1.45) | 1.11(0.81–1.52) |
| SU | 2.12(0.91–3.81) | 47,590 | 219 | 117,202 | 186.9 | 1 (reference) | 1 (reference) | |
| Time Since Treatment Initiation Restricted To 2 Years | ||||||||
| PIO vs. DPP | PIO | 1.97(1.57–1.97) | 17,081 | 71 | 28,215 | 251.6 | 1.43(1.05–1.96) | 1.38(0.96–1.99) |
| DPP | 1.90(0.84–1.97) | 36,450 | 91 | 51,710 | 176.0 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 1.97(1.46–1.97) | 8,022 | 23 | 13,063 | 176.1 | 0.87(0.56–1.36) | 0.88(0.56–1.38) |
| SU | 1.97(0.91–1.97) | 47,590 | 141 | 69,406 | 203.2 | 1 (reference) | 1 (reference) | |
| Time Since Treatment Initiation after 2 Years | ||||||||
| PIO vs. DPP | PIO | 2.27(1.29–3.32) | 11,993 | 62 | 27,865 | 222.5 | 1.37(0.93–2.00) | 1.24(0.84–1.84) |
| DPP | 1.39(0.62–2.65) | 17,644 | 49 | 30,222 | 162.1 | 1 (reference) | 1 (reference) | |
| PIO vs. SU | PIO | 2.21(1.27–3.29) | 5,506 | 28 | 12,621 | 221.9 | 1.34(0.87–2.06) | 1.43(0.92–2.23) |
| SU | 1.71(0.77–2.93) | 25,124 | 78 | 47,796 | 163.2 | 1 (reference) | 1 (reference) | |
CI, Confidence Interval; DPP, dipeptidyl-peptidase-4; HR, Hazard Ratio; IQR, interquartile range; NR, Not Reportable given number of events <11; PIO, pioglitazone; SU, sulfonylureas
Follow-up started on the cohort entry date (second dispensing) and was censored at first occurrence of outcome, death, end of study (December 2014), or end of enrollment. As-treated analyses additionally censored at treatment discontinuation (no subsequent fill of initiated drug class within days-supply plus a 90-day grace period). A 180-day induction period was imposed excluding time from the beginning of follow-up. The as-treated analyses additionally added a 180-day latency period to the end of follow-up when possible prior to death or end of patient data.
Number contributing with at least 180 days of follow-up. The number initiating PIO differs for each comparison due to exclusions of prior use of drugs included in comparison only.
Incidence Rate reported per 100 000 Person-Years
Cox proportional hazards models
Cox proportional hazards models adjusted for all variables in Table 1, except for metformin and those excluded within each comparison by incident user design, using propensity-score weighting (standardized to PIO population)
DISCUSSION
In this incident-user, active-comparator study of a national sample of older US adults, we identified a risk of bladder cancer associated with pioglitazone that increased with treatment duration compared to DPP-4s and sulfonylureas, clinical alternatives for the management of type-2 diabetes, which was consistent across a wide range of sensitivity analyses.
Weighted Kaplan-Meier curves identified an increasing rate of bladder cancer over time, which align with results reported for follow-up <1.5, 1.5–4, and >4 years by Lewis et al. (HR=0.88 [0.68–1.16], 1.03 [0.80–1.33], and 1.16 [0.87,1.54]),4 and ≤1, 1–2, and >2 years by Azoulay et al. (RR=0.56 [0.07–4.42), 3.03 [0.63–14.52), and 1.99 [1.14–3.45]),18 Tuccori et al. (HR=1.33 [0.73–2.40], 1.66 [0.97–2.84], and 1.78 [1.21–2.64])16 and Mackenzie et al., who evaluated both incident-user (HR=1.02 [0.81–1.28], 0.95 [0.62–1.44], and 1.24 [0.83–1.84]) and prevalent-user cohorts (HR=1.03 [0.93–1.14], 1.14 [0.98–1.31], and 1.16 [1.00–1.35]).14 Our overall results contradict those that found no evidence of an increased risk for multiple reasons, including differences of data source, comparator choice, whether or not prevalent users were included, and how the risk window was defined.
Data sources and inclusion criteria can create potential sources of selection bias. Three large observational studies assessed the association between pioglitazone and bladder cancer using US data.4,9,14 Lewis et al., who followed Kaiser Permanente Northern California diabetes registrants aged 40 years or older,4 and Vallarino et al., who followed United Healthcare beneficiaries aged 45 years or older,9 used cohorts of employer-based commercially insured individuals, which may under-represent patients at greatest risk of bladder cancer, given that the median age at diagnosis is 73.25 Mackenzie et al. used a cohort of Medicare beneficiaries similar to ours, but found no evidence of increased risk of bladder cancer for users of pioglitazone when compared to all other diabetic therapies.14
Our study is the first to compare pioglitazone to DPP-4s, a class of drugs prescribed to similar patients as pioglitazone, as demonstrated by the balance of measured covariates prior to adjustment and consistency across crude and adjusted HRs for pioglitazone versus DPP-4s. Sulfonylureas were chosen as an additional comparator as the most common therapy after metformin during the study period.33 A composite comparison of DPP-4s or sulfonylureas was considered but not included because combining therapies would mix effects. Comparator choice can strongly influence results, because treatment choices are routinely based on the underlying disease and its severity; confounding by severity threatens study validity when there are major differences in disease severity between those prescribed the exposure and the comparator.20,21 Vallarino et al. found a risk reduction of bladder cancer for pioglitazone when compared to insulin,9 which may be a poor comparator choice since insulin differed from pioglitazone in route of administration (injection vs. oral), cost (generally less expensive), and indication (typically given to patients with more severe diabetes) during much of the study period. Other studies included all non-pioglitazone antidiabetic users as the comparator,7,10,13–16,18,33,34 mixing therapies prescribed to patients with varying degrees of diabetes severity into one group, making it difficult to disentangle individual treatment effects and increasing the potential for biased estimates.
An active comparator is recommended for comparative effectiveness research to present the least biased estimates.20,21 Including untreated patients with diabetes in the comparator4,13 can threaten validity further due to confounding by indication, since these patients may be able to manage their diabetes without medical therapy (e.g., diet and exercise). Given the use of claims, we were unable to fully control for two known risk factors for bladder cancer, smoking and workplace exposures (i.e. industrial chemicals).25 A smoking algorithm with excellent specificity but poor sensitivity (27.9%)31 was added to identify smoking-related claims. Diagnosis of COPD was also included in the PS model as a smoking proxy. In our external validation study, pioglitazone initiators were less likely to be smokers and obese than comparator drug initiators. Assuming transportability of MCBS to our sample, the increased risk of bladder cancer we observed cannot be explained by residual confounding by smoking or BMI, and may actually be an underestimation of the true risk.
Our study has other limitations that should be considered when interpreting results. Since bladder cancer was defined using administrative claims without pathological confirmation, misclassification is possible but unlikely to be differential with respect to treatment choice. We used a previously validated algorithm for identifying solid tumors in administrative claims24 and varied the definition in sensitivity analyses to confirm similar results. Timing of outcome ascertainment is especially important for cancer outcomes given the unlikelihood for short-term treatment to have an immediate causal impact. For example, subsequent review of the 11 bladder neoplasms (8 pioglitazone and 3 placebo) reported within the first year of PROactive3 concluded that the events could not have been caused by short-term exposure.12 Since the actual risk period relevant for drug-associated cancers is poorly understood, evaluating multiple risk windows like we did minimizes the potential for protopathic bias or the identification of spurious events due to misclassification of the risk period.35,36 Furthermore, using both IT and AT approaches allowed us to present conservative estimates via the IT approach in addition to treatment-duration-specific estimates via the AT approach.
Detection bias was another potential concern for this study. Annual urinalysis is recommended for patients with diabetes given their increased risk of kidney disease.37,38 Edema, a common side-effect of pioglitazone,39 may lead to additional urological screening and diagnostic work-up, because it also can be an early sign of kidney disease.40 Our additional analyses did not find evidence of an increased rate of these procedures in pioglitazone initiators compared to DPP-4s or sulfonylureas, which eliminated detection bias as an explanation for our findings.
In summary, we identified an increased risk of bladder cancer associated with pioglitazone treatment, as suggested by some,15–18 but disputed by others.4,7–14 The risk emerged within the first 2 years of treatment and increased over time. Findings from our secondary and sensitivity analyses suggest that these results are unlikely to be explained by differential detection rates, cohort selection, outcome definitions, or censoring approaches. It is important to note that relative differences reflected when reporting HRs may distort clinical interpretation, as the crude absolute risk differences were incredibly small, requiring over 1000 person-years of treatment to observe one excess bladder cancer event for pioglitazone compared to DPP-4s or sulfonylureas. Therefore, pioglitazone’s tolerability and effectiveness in maintaining blood-glucose control relative to clinical alternatives41 should be weighed against a small absolute increase in risk of bladder cancer when considering which diabetic treatment to prescribe. Although rare, bladder cancer is the fifth most common cancer representing 4.6% of all new cancer cases in the US.25 With the continued development and marketing of new antidiabetic medications, evaluation of their safety using study design and analytic methods that minimize all threats to validity increasingly important.
Supplementary Material
ACKNOWLEDGEMENTS
Project Funding.
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL 200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine.
Footnotes
Conflict of Interest Statement.
E. M. G is a doctoral student at the University of North Carolina at Chapel Hill. She receives a Graduate Research Assistant stipend from the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111). She is also a consulting scientist for Aetion, Inc., a software company that has industry clients, but receives no direct financial benefit from industry clients; J. B. B. is supported by the NIH (UL1TR001111 and R01HL110380). He is an investigator and/or consultant without any direct financial benefit to him under contracts between his employer and the following companies: Amylin Pharmaceuticals, Inc., Andromeda, Astellas, AstraZeneca, Boehringer Ingelheim GmbH & Co. KG, Bristol-Myers Squibb Company, Dance Biopharm, Elcelyx Therapeutics, Inc., Eli Lilly and Company, GI Dynamics, GlaxoSmithKline, Halozyme Therapeutics, F. Hoffmann-La Roche, Ltd., Intarcia Therapeutics, Johnson & Johnson, Lexicon, LipoScience, Macrogenics, Medtronic, Merck, Metavention, Novo Nordisk, Orexigen Therapeutics, Inc., Osiris Therapeutics, Inc., Pfizer, Inc., PhaseBio Pharmaceuticals Inc., Quest Diagnostics, Sanofi, Scion neuroStim, Takeda, ToleRx, vTv Pharmaceuticals. He has stock options and has received payments from PhaseBio; J. L. L. is funded by the UNC Oncology Clinical Translational Research Training Program (5K12CA120780) and receives salary support and research support from the PhRMA Foundation for a Research Starter Award to the Department of Epidemiology, University of North Carolina-Chapel Hill; V.P. receives salary support from investigator-initiated grants from Merck and Amgen and from the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111); T. S. receives investigator-initiated research funding and support as Principal Investigator (R01/R56 AG023178) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214), National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk.
REFERENCES
- 1.Cohen SM. Effects of PPARgamma and combined agonists on the urinary tract of rats and other species. Toxicol Sci. 2005;87(2):322–327. Epub 2005 Jul 2027. [DOI] [PubMed] [Google Scholar]
- 2.Dominick MA, White MR, Sanderson TP, et al. Urothelial carcinogenesis in the urinary bladder of male rats treated with muraglitazar, a PPAR alpha/gamma agonist: Evidence for urolithiasis as the inciting event in the mode of action. Toxicol Pathol. 2006;34(7):903–920. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314(3):265–277. doi: 210.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 5.Federal Drug Administration. Actos (pioglitazone): Ongoing Safety Review - Potential Increased Risk of Bladder Cancer. MedWatch Safety Alerts for Human Medical Products: 2011 Safety Alerts for Human Medical Products. 2011. [Google Scholar]
- 6.Federal Drug Administration, ,. FDA Drug Safety Communication: Updated FDA review concludes that use of type 2 diabetes medicine pioglitazone may be linked to an increased risk of bladder cancer. 12/12/2016 2016. [Google Scholar]
- 7.Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35(2):278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto K, Hamamoto Y, Honjo S, et al. Possible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetes. Diabetes research and clinical practice. 2013;99(2):e21–23. [DOI] [PubMed] [Google Scholar]
- 9.Vallarino C, Perez A, Fusco G, et al. Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clinical drug investigation. 2013;33(9):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75(1):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo HW, Tiao MM, Ho SC, Yang CY. Pioglitazone use and the risk of bladder cancer. The Kaohsiung journal of medical sciences. 2014;30(2):94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. 2016;18(3):266–273. doi: 210.1111/dom.12608. Epub 12016 Jan 12608. [DOI] [PubMed] [Google Scholar]
- 13.Korhonen P, Heintjes EM, Williams R, et al. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. BMJ. 2016;354:i3903.(doi): 10.1136/bmj.i3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie TA, Zaha R, Smith J, Karagas MR, Morden NE. Diabetes Pharmacotherapies and Bladder Cancer: A Medicare Epidemiologic Study. Diabetes Ther. 2016;7(1):61–73. doi: 10.1007/s13300-13016-10152-13304. Epub 12016 Feb 13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55(7):1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuccori M, Filion KB, Yin H, Yu OH, Platt RW, Azoulay L. Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ. 2016;352:i1541.(doi): 10.1136/bmj.i1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374(14):1321–1331. doi: 1310.1056/NEJMoa1506930. Epub 1502016 Feb 1506917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. Bmj. 2012;344:e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampp C, Pippins J. Pioglitazone and bladder cancer: FDA’s assessment. Pharmacoepidemiol Drug Saf. 2017;26(2):117–118. doi: 110.1002/pds.4154. Epub 2017 Jan 1009. [DOI] [PubMed] [Google Scholar]
- 20.Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. Epub 2015 Sep 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. AHRQ Publication No. 12(13)-EHC099 Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 22.Research Data Assistance Center (ResDAC). Strengths and Limitations of CMS Administrative Data in Research. 2012; http://www.resdac.org/resconnect/articles/156. Accessed 20 August, 2014. [Google Scholar]
- 23.Ishibashi N, Maebayashi T, Aizawa T, et al. Refractory Haematuria Resulting From Peritoneal Dissemination of Metastatic Gastric Cancer: Radiation Therapy For A Nodule Infiltrating The Urinary Bladder. Urol J. 2017;14(1):2982–2984. [PubMed] [Google Scholar]
- 24.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer causes & control: CCC. 2007;18(5):561–569. [DOI] [PubMed] [Google Scholar]
- 25.American Cancer Society. Bladder Cancer: Detailed Guide. Last Medical Review: 2/26/2014 2014. [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 27.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. [DOI] [PubMed] [Google Scholar]
- 28.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. [DOI] [PubMed] [Google Scholar]
- 29.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010;172(7):843–854. doi: 810.1093/aje/kwq1198. Epub 2010 Aug 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokhale M, Buse JB, Mack C, Stürmer T. Calendar Time as an Instrumental Variable in Assessing the Risk of Heart Failure with Antihyperglycemic Drugs [abstract]. In: Abstracts of the 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management, The Convention Centre Dublin, Dublin, Ireland August 25–28, 2016. Abstract nr 712016;No Suppl 3:3–679. doi: 610.1002/pds.4070. Located at: Pharmacoepidemiol Drug Saf. [Google Scholar]
- 31.Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using Medicare data--a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf. 2016;25(4):472–475. doi: 410.1002/pds.3953. Epub 2016 Jan 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Medicare & Medicaid Services. Appendix A. Technical documentation for the Medicare Current Beneficiary Survey. 2003. 2003; https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/Downloads/OLD/HHC2003appendixA.pdf. Accessed May 04, 2017. [Google Scholar]
- 33.Lipska KJ, Yao X, Herrin J, et al. Trends in Drug Utilization, Glycemic Control, and Rates of Severe Hypoglycemia, 2006–2013. Diabetes Care. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34(6):1369–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothman KJ. Induction and latent periods. Am J Epidemiol. 1981;114(2):253–259. [DOI] [PubMed] [Google Scholar]
- 36.Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105(19):1456–1462. doi: 1410.1093/jnci/djt1211. Epub 2013 Aug 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Diabetes Association (ADA). Standards of medical care in diabetes––2013. Diabetes Care. 2013;36(Suppl 1):S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC). Proteinuria (NIH Publication No. 14–4732). April 2, 2014 2014. [Google Scholar]
- 39.Niemeyer NV, Janney LM. Thiazolidinedione-induced edema. Pharmacotherapy. 2002;22(7):924–929. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JD, Habel L, Quesenberry C, et al. Proteinuria testing among patients with diabetes mellitus is associated with bladder cancer diagnosis: potential for unmeasured confounding in studies of pioglitazone and bladder cancer. Pharmacoepidemiol Drug Saf. 2014;23(6):636–645. doi: 610.1002/pds.3619. Epub 2014 Apr 1025. [DOI] [PubMed] [Google Scholar]
- 41.Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006;66(1):85–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


