Abstract
Exercise improves glucose metabolism and delays the onset and/or reverses insulin resistance in the elderly by an unknown mechanism. In the present study, we examined the effects of exercise training on glucose metabolism, abdominal adiposity, and adipocytokines in obese elderly. Sixteen obese men and women (age = 63 ± 1 yr, body mass index = 33.2 ± 1.4 kg/m2) participated in a 12-wk supervised exercise program (5 days/wk, 60 min/day, treadmill/cycle ergometry at 85% of heart rate maximum). Visceral fat (VF), subcutaneous fat, and total abdominal fat were measured by computed tomography. Fat mass and fat-free mass were assessed by hydrostatic weighing. An oral glucose tolerance test was used to determine changes in insulin resistance. Exercise training increased maximal oxygen consumption (21.3 ± 0.8 vs. 24.3 ± 1.0 ml·kg−1·min−1, P < 0.0001), decreased body weight (P < 0.0001) and fat mass (P < 0.001), while fat-free mass was not altered (P > 0.05). VF (176 ± 20 vs. 136 ± 17 cm2, P < 0.0001), subcutaneous fat (351 ± 34 vs. 305 ± 28 cm2, P < 0.03), and total abdominal fat (525 ± 40 vs. 443 ± 34 cm2, P < 0.003) were reduced through training. Circulating leptin was lower (P < 0.003) after training, but total adiponectin and tumor necrosis factor-α remained unchanged. Insulin resistance was reversed by exercise (40.1 ± 7.7 vs. 27.6 ± 5.6 units, P < 0.01) and correlated with changes in VF (r = 0.66, P < 0.01) and maximal oxygen consumption (r = −0.48, P < 0.05) but not adipocytokines. VF loss after aerobic exercise training improves glucose metabolism and is associated with the reversal of insulin resistance in older obese men and women.
Keywords: diabetes, obesity, aging, abdominal adiposity
ADIPOSE TISSUE ACCUMULATES primarily in the central regions of the body with advancing age. The increase in abdominal adiposity contributes to the development of metabolic abnormalities, such as hyperinsulinemia and insulin resistance (25, 40). Aerobic exercise training has been reported to decrease glucose-stimulated insulin concentrations in older individuals (23, 31). Progressive glucose intolerance and the development of insulin resistance as a consequence of abdominal fat accumulation with advancing age could, therefore, be avoided (25) or at least delayed with appropriate compliance to an increase in daily exercise. Regardless of age group or gender, even individuals with normal or modestly increased body weight can develop insulin resistance, if very high levels of abdominal fat are present (38). How exactly adiposity contributes toward the development of insulin resistance and the mechanism by which exercise increases cellular glucose uptake need to be fully clarified, especially considering the possibility of a delay in onset and a reversal of this serious metabolic condition.
Data generated from large or small cross-sectional studies of single- or mixed-gender groups with varying age and obesity levels add to the challenge of finding consistency as to how an increase in abdominal adiposity contributes to the development of metabolic abnormalities. The claim that both visceral (VF) and subcutaneous fat (SCF) influence obesity-related insulin resistance contrasts with reports of independent effects for both of these adipose subdivisions (3, 9, 13, 34).
An increase in adiposity is also accompanied by changes in the secretion of adipocyte-derived mediators of insulin resistance, particularly the adipocytokines leptin, tumor necrosis factor (TNF)-α, and adiponectin. Increased circulating leptin and TNF-α and lower levels of circulating adiponectin are associated with insulin resistance (22). While exercise training appears to lower leptin and TNF-α, the effects of exercise on adiponectin are equivocal (18, 26). To date, there are limited data on how exercise alters adipocytokine levels in obese elderly adults (10, 37).
The purpose of our study was to determine the important association between exercise-induced improvements in glucose metabolism, abdominal adiposity, and adipocytokines. The potential for age discrepancy differences between studies has been recognized and suggests that findings may not apply equally to all age groups (13). Therefore, our hypotheses were based on the premise that, by solely focusing on an elderly, obese population, clarity could be drawn as to whether visceral and/or subcutaneous abdominal adiposity could influence cellular glucose uptake and insulin resistance in this age group following compliance with a lengthy, supervised exercise intervention program.
EXPERIMENTAL PROCEDURES
Subjects
A total of 16 subjects (5 men/11 women) who were weight stable (<2 kg/6 mo weight change), sedentary (<20 min exercise twice per week), and obese [body mass index (BMI), 30–40 kg/m2] were recruited to participate in a 12-wk supervised aerobic exercise program (Table 1). The subject mean age was 63 ± 1 yr. Exclusion criteria included evidence of overt Type 1 and Type 2 diabetes, acute or chronic disease (cardiovascular, cerebrovascular, liver, renal, hematological, thyroid, or cancer), smoking, medication affecting metabolism, and weight loss >2 kg/6 mo before study commencement. All women were postmenopausal for more than 1 yr and had not been on hormone replacement therapy for at least 1 yr before enrollment in the study. This study was conducted in conformity with the principles of the Declaration of Helsinki as well as Title 45, US Code of Federal Regulations, Part 46, Protection of Human Subjects, and the research protocol was approved by the Institutional Review Board at Metro Health Medical Center. All subjects gave their written, informed consent to participate in this study in accordance with MetroHealth Medical Center’s guidelines for the protection of human subjects.
Table 1.
Subject characteristics
| Variables | Pretraining | Posttraining |
|---|---|---|
| Age, yr | 63±1 | |
| Body weight, kg | 94.1±4.3 | 90.9±4.0* |
| BMI, kg/m2 | 33.2±1.4 | 32.1±1.3* |
| Fat mass, kg | 38.8±2.0 | 35.4±2.2† |
| Fat-free mass, kg | 55.4±3.3 | 55.6±3.2 |
| Caloric intake, kcal | 1,948±182 | 1,872±306 |
| Total abdominal fat, cm2 | 525.4±40.3 | 442.5±33.9‡ |
| Subcutaneous fat, cm2 | 351.4±34.1 | 304.8±27.7‡ |
| Visceral fat, cm2 | 175.6±20.2 | 136.2±16.9* |
Values are means ± SE; n = 16 subjects: 11 women, 5 men. BMI, body mass index.
P < 0.0001
P < 0.005
P < 0.05.
All study participants received a medical history and physical examination, including a resting electrocardiogram, an oral glucose tolerance test (OGTT), and a complete blood profile (glycosylated hemoglobin, lipid profile, liver, renal, and hematological function test). An incremental maximal graded exercise stress test was performed to evaluate cardiovascular function.
Exercise training program
All exercise training sessions were supervised by an exercise physiologist and conducted in the Exercise Physiology Laboratories at the MetroHealth General Clinical Research Center. Maximal oxygen consumption was determined by an incremental graded treadmill exercise stress test. The test was deemed to be maximal if at least three of the following criteria were satisfied: a respiratory exchange ratio of ≥1.10, a leveling off in oxygen consumption with increasing workloads, volitional fatigue, or a heart rate greater than or equal to age-predicted maximum. Intensity for the exercise training program was calculated from the maximum heart rate achieved during the test.
Exercise training intensity commenced at a level prescribed between 60 and 65% of the heart rate maximum and gradually increased so that, by week 4, the subjects were exercising at 80–85% of heart rate maximum (~70% ). The target heart rate ranges were monitored using heart rate monitors (Polar Electro, Woodbury, NY) during each exercise session. For the duration of the 12-wk period, subjects exercised 5 days/wk for 50–60 min with a 10-min warm-up and cool-down. The subjects performed cycling or treadmill exercise, with over 75% of their effort spent on the treadmill. They were also given stretching exercises for the quadriceps, hamstrings, and gastrocnemius before and after each session.
Intensity, duration, resting and exercise heart rates, and blood pressures were recorded for each session. tests were performed at baseline and repeated at 4, 8, and 12 wk of exercise to monitor progress. The exercise intensity was adjusted accordingly.
Diet monitoring
Participants were encouraged not to alter their dietary intake during the course of the study. Every 3 wk, subjects were instructed to complete 3-day dietary records, which were analyzed by the General Clinical Research Center dietitian. The nutritionist met weekly with participants to ensure that they maintained their preintervention diet.
OGTT
An OGTT was used to measure insulin resistance. A standardized 75-g oral glucose load was ingested following a 10- to 12-h overnight fast at baseline (week 0) and ~16–18 h after the last exercise bout at the postintervention stage (week 12). Blood samples were collected from an antecubital vein at fasting and at 30, 60, 90, 120, and 180 min after glucose ingestion. Areas under the glucose and insulin curves were determined using the trapezoid model. Insulin resistance was estimated as previously described (8).
Body composition
Body weight was measured in kilograms (to the nearest 0.1 kg), and height without shoes was measured to the nearest 1.0 cm. BMI was calculated as weight (kg) divided by height squared (m2). Body density was determined by hydrostatic weighing, and percent body fat was estimated using the general equation of Siri, as previously described (24). Fat-free mass was calculated in kilograms as body weight minus kilograms of fat mass. Computed axial tomography (Picker PQ6000 Scanner; Marconi/Picker, Highland Heights, OH) was used to measure the distribution of cross-sectional abdominal fat [total abdominal fat (AT), VF, and SCF] regions, as previously described (1). With subjects placed in a supine position, measurements were made at 120 kV with a slice thickness of 8 mm. Images were obtained without contrast at the fourth lumbar vertebrae (L4). The images were digitized by optical density to separate bone, muscle, and fat compartments using the National Institutes of Health IMAGE program on a Macintosh platform. Scans were standardized using distances from bony landmarks, and digitized images were analyzed in a blinded fashion. Hydrostatic weight and computed tomography scans were performed at baseline and postintervention.
Analytical procedures
All samples for TNF-α, leptin, adiponectin, lipids, insulin, and glucose were run in duplicate in a single assay for each variable. Fasting blood samples were centrifuged at 4°C, and the plasma was stored at −70°C for subsequent analysis. Plasma TNF-α concentrations were measured by ELISA (Quantikine HS; R&D Systems, Minneapolis, MN). The intra-assay coefficient of variation was 14%, and the minimum detectable limit of the assay was 0.18 pg/ml. Plasma leptin samples were measured by radioimmunoassay (Linco Research, St. Charles, MO). Adiponectin levels were measured by an ELISA (R&D Systems). Serum triglycerides and total cholesterol were measured using enzymatic-colorimetric procedures (Hitachi 747, Roche, Indianapolis, IN). Insulin levels were determined by a double-antibody radioimmunoassay (Linco Research, St. Charles, MO). Plasma glucose concentrations were measured by the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH).
Statistical analysis
This intervention trial was designed to compare pre- and postexercise intervention variables. Changes in variables from baseline to the end of the intervention were determined by the paired Student’s t-test and one-way analysis of variance. The relationship between dependent and independent variables was based on univariate correlation analysis. The data were analyzed using the StatView II statistical package (Abacus Concepts, Berkley, CA). Data are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
All subjects completed the 12-wk exercise intervention program. No significant alteration in kilocalorie intake was seen pre- and postintervention (1,948 ± 182 vs. 1,872 ± 306 kcal, P = 0.688).
Effects of exercise on anthropometric variables
The average body weight was 94.1 ± 4.3 kg at the preexercise intervention stage. A significant reduction in body weight (~3%) was observed following the 12-wk exercise program (90.9 ± 4.0 kg, P < 0.0001). No significant alteration in fat-free mass occurred during this time period (55.4 ± 3.3 vs. 55.6 ± 3.2 kg, P > 0.05). The observed body weight loss was attributable to the significant reduction in fat mass (38.8 ± 2.0 vs. 35.4 ± 2.2 kg, P < 0.001) (Table 1).
Insulin sensitivity and glucose tolerance
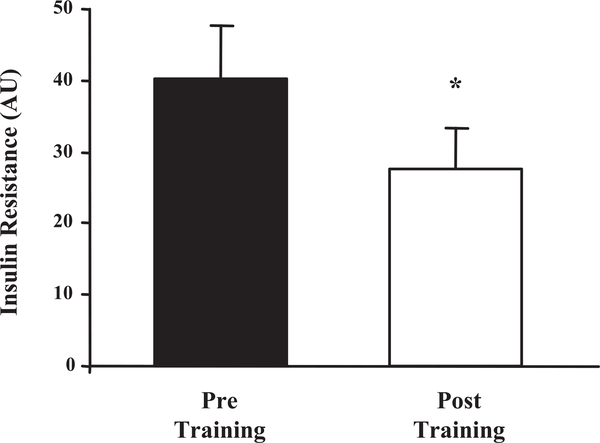
Changes in the OGTT-insulin areas were observed following the completion of the exercise intervention program (Table 2). It was observed that areas under the insulin curve (70,973 ± 9,291 vs. 55,760 ± 8,951 pM/3 h, P < 0.009) significantly decreased with exercise, highlighting an improvement in insulin response and increased insulin sensitivity. Likewise, a significant decrease in the OGTT-glucose areas was observed (547 ± 54 vs. 452 ± 53 mM/3 h, P < 0.03) when pre- and postintervention curves were compared. Insulin resistance was estimated from the product of the areas under the insulin and glucose response curves. A significant decrease in insulin resistance (40.1 ± 7.7 vs. 27.6 ± 5.6 units, P < 0.01) was found in participants after the exercise intervention program was completed (Fig. 1).
Table 2.
Blood parameters
| Variables | Pretraining | Posttraining |
|---|---|---|
| Fasting plasma glucose, mg/dl | 110±3 | 109±3 |
| Fasting plasma insulin, μU/ml | 20.8±2.7 | 16.7±1.8† |
| Glucose AUC | 547±54 | 452±53† |
| Insulin AUC | 70,973±9,291 | 55,760±8,951† |
| Cholesterol, mg/dl | 202±9 | 186±8† |
| Triglycerides, mg/dl | 195±26 | 164±19* |
| Leptin, ng/ml | 26.99±3.61 | 21.36±3.07 |
| TNF-α, pg/ml | 2.62±0.49 | 2.55±0.43 |
| Adiponectin, μg/ml | 6.32±0.90 | 6.05±1.23 |
Values are means ± SE; n = 16 subjects: 11 women, 5 men. AUC, area under the curve; TNF-α, tumor necrosis factor-α.
P < 0.005.
P < 0.05.
Fig. 1.
Insulin resistance before and after the 12-wk exercise training program. Values represent the means ± SE for 16 subjects. AU, arbitrary units, product of the areas under the glucose and insulin response curves (×107). *Posttraining significantly reduced compared with the pretraining data, P < 0.01.
Correlation of aerobic fitness and insulin resistance
The level of cardiovascular fitness improved with a 14% average increase in postintervention (21.3 ± 0.8 vs. 24.3 ± 1.0 ml·kg−1·min−1, P < 0.0001). The alteration in insulin resistance was negatively correlated with (r = −0.48, P < 0.05), indicating an increase in insulin sensitivity on improvement in participant fitness levels.
Adipose tissue distribution and insulin resistance correlation
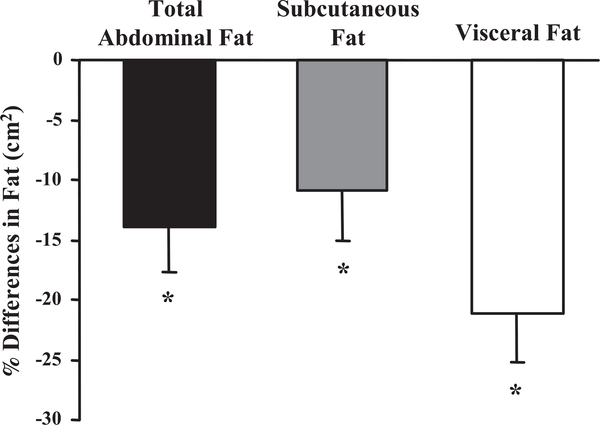
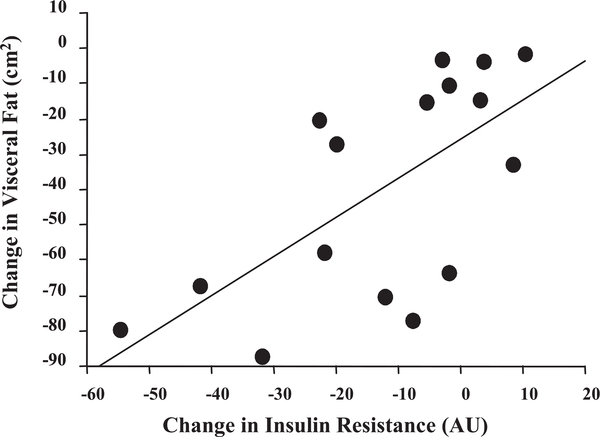
Changes in abdominal adiposity as measured by computed axial tomography showed that AT (visceral and subcutaneous) decreased significantly when pre- and postexercise intervention values were compared (525.4 ± 40.3 vs. 442.5 ± 33.9 cm2, P < 0.003). The separation of AT into visceral (176 ± 20 vs. 136 ± 17 cm2, P < 0.0001) and subcutaneous (351.4 ± 34.1 vs. 304.8 ± 27.7 cm2, P < 0.03) adipose tissue revealed that both depots were significantly reduced following the exercise intervention program (Fig. 2). Insulin resistance was not correlated with abdominal fat, SCF, or body weight (data not shown). However, the change in insulin resistance correlated with alterations in VF (r = 0.66, P < 0.006), indicating that insulin resistance was reversed when VF decreased (Fig. 3).
Fig. 2.
Percent change in total abdominal, subcutaneous, and visceral fat based on computed tomography measures performed pretraining and posttraining. Values represent means ± SE for 16 subjects. *The percent change in total abdominal, subcutaneous, and visceral fat was significantly lower compared with the pretraining measures, P < 0.03.
Fig. 3.
Correlation data showing the association between the change in insulin resistance and the change in visceral fat, based on pretraining to posttraining measures (r = 0.66, P < 0.01). Values are representative of 16 subjects.
Biomarkers of insulin resistance
As shown in Table 2, fasting insulin levels were lower (P < 0.02) after the exercise intervention, while glucose levels remained unchanged. Fasting leptin levels were reduced (P < 0.003), but TNF-α and adiponectin were unaffected by exercise training (P > 0.05). Dyslipidemia was significantly reversed, as evidenced by marked reductions in circulating triglycerides (P < 0.03) and cholesterol (P < 0.01).
DISCUSSION
Aging increases the susceptibility to insulin resistance as a consequence of obesity and/or a sedentary lifestyle, leading to potentially serious health risks (5, 25). The importance of body composition in determining insulin-resistant states cannot be overemphasized, as the abdominally obese are especially at risk of developing impaired glucose tolerance and reduced cellular responses to insulin (30). To determine whether resistance to insulin-mediated glucose disposal can be alleviated or even reversed, this investigation examined the affects of increased physical activity on inactive, abdominally obese, elderly individuals. The metabolic consequences of abdominal adiposity were shown to be alleviated following compliance with a supervised daily exercise intervention program of 12-wk duration. Glucose tolerance, insulin resistance, and physical fitness in all participants improved significantly when comparisons were made between pre- and postintervention levels. Even though AT, SCF, and VF were all found to be significantly reduced in size, it was the loss of VF within the abdominal region that proved to be the primary correlate with improved glucose control, which suggests it may be the main factor governing the reduction of insulin resistance in our obese, elderly participants. Therefore, by solely focusing on an elderly, obese population, visceral abdominal adiposity could be clearly seen to influence cellular glucose uptake and insulin resistance in this age group following compliance with a lengthy, supervised exercise intervention program.
Our results support previous findings showing that weight loss, particularly due to increased daily exercise, improves insulin sensitivity in overweight and obese individuals (35). By attaining an ~3% weight loss, our participants greatly improved their insulin sensitivity postexercise intervention, as shown by the 31% reduction in insulin resistance, despite higher therapeutic recommendation weight loss levels previously reported (5–10%) to be necessary for improvements in insulin sensitivity (11). In other studies in which reductions in BMI and waist circumference measurements were controlled, the investigators still noted the beneficial effect to be partially due to the corresponding reduction in abdominal fat, particularly visceral tissue (19). Furthermore, studies examining the effects of therapeutic interventions (e.g., hormone replacement therapy and dehydroepiandrosterone replacement) independent of exercise in elderly populations have also reported that decreases in abdominal fat are inversely correlated with insulin sensitivity (8, 41). Thus, even in the absence of elevated physical activity, there is a strong relationship between decreased visceral adipose tissue and the reversal of insulin resistance in elderly adults.
There is no clear consensus on how increased VF contributes to insulin resistance. It has been suggested that VF is highly sensitive to lipolytic stimuli, which may, in turn, induce insulin resistance by increasing gluconeogenesis in the liver, thus promoting hyperglycemia and also facilitating an increase in free fatty acids (FFA) (32). While there is no direct evidence to support this mechanism in humans, recent data from dogs in which insulin resistance was induced by a high-fat diet show a concomitant increase in the hepatic gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (21). Furthermore, it is also known that increased fatty acid oxidation in the liver can stimulate gluconeogenesis (4). Alternative mechanisms may be acting through FFA-induced inhibition of hepatic insulin clearance (39). These mechanisms have been invoked in previous studies to explain the association between central obesity and the insulin resistance of aging (5, 25). More recent elegant studies by Nielsen et al. (29) have revealed that hepatic FFA delivery is raised in individuals in proportion to increasing amounts of VF. Thus it is likely that increased hepatic insulin resistance may be directly attributed to VF. Furthermore, individuals with greater amounts of VF release more FFA from the liver. It is now well established that elevated FFAs contribute to peripheral insulin resistance (2). Thus the observation that exercise can reduce VF is significant and suggest a mechanism whereby exercise may reverse both hepatic and peripheral insulin resistance in abdominally obese individuals.
Consequent to aerobic exercise training, the effects of body fat loss on insulin metabolism have been shown to be gender independent (12, 20). While there are distinct sex-dependent differences in the regional fat distribution, others found that, after controlling for tissue size, reductions in visceral adipose tissue were not different between the sexes (20). After weight loss, there were no apparent statistically significant sex-related effects in the improvement in insulin sensitivity in obese men and women (12). These results support our observations from a nonstratified mixed-gender sample group.
The subdivision of visceral into intra- and extraperitoneal tissue and SCF into deep and superficial depots was not carried out in this study. It was shown previously that subdivision of VF and subcutaneous tissue provided no additional insight into the relationship between abdominal obesity and metabolic risk in obesity. Subdivision did not alter the observation that VF alone correlated strongly with insulin resistance, independent of abdominal SCF depots (34).
Significant improvement in aerobic fitness (14%) was evident in our elderly, obese participants. The reduction in insulin resistance was associated with the increase in physical fitness, such that those who experienced the greatest improvement of also experienced the greatest decrease in insulin resistance. While a reduction in insulin sensitivity is commonly described during aging (6, 36), it has recently been shown that the age-related decline in insulin sensitivity may principally result from muscle deconditioning due to physical inactivity (33). Similar insulin sensitivity levels have been recorded for young and elderly individuals when matched for physical activity levels (33). Despite the lack of follow-up in our study, the long-term effects of sustained benefits from physical activity on insulin resistance are encouraged by findings such as ours involving regular daily exercise.
Recent discoveries in fat cell biology have led to the suggestion that adipocytokines secreted from the adipocytes may act as mediators of insulin resistance (15). Although increased circulating TNF-α levels have been associated with insulin resistance in obesity, aging, and exercise-induced muscle damage, reports of change in TNF-α levels after exercise training are equivocal (7, 17, 24). Data from the present study suggest that circulating TNF-α may not change in obese elderly adults after exercise training. This does not preclude the possibility that changes in locally secreted TNF-α do not occur, and a decrease in TNF-α at the tissue level could have a profound effect on altering insulin sensitivity (14). Leptin was first identified as a product of the ob gene in adipose tissue and is thought to regulate energy balance through central hypothalamic pathways, but it may also play a role in insulin resistance by promoting lipid oxidation and inhibiting lipid synthesis (27, 43). There are several reports of decreased circulating leptin after exercise training, and the present study confirms these results. Despite the decrease in leptin, we did not see an association between the change from pre- to postintervention and improvements in insulin resistance. This suggests that, under the present study conditions, the decrease in leptin may not have been sufficiently robust and could have only contributed indirectly to the reversal of insulin resistance. Likewise, total adiponectin, which is another candidate biomarker of insulin resistance, did not change with exercise training, despite improvements in insulin sensitivity. However, in the case of adiponectin, it is likely that changes in the multimeric forms of the protein may be more important than changes in total adiponectin itself (42). Indeed, we recently presented a preliminary report in which exercise training led to increases in the adiponectin multimer ratio in obese elderly, even though the total adiponectin concentration was unchanged (28). Further studies are needed to examine the effects of exercise training on these adiponectin multimers and any consequent relationship this may have with changes in insulin resistance. Our observations of decreased triglycerides and cholesterol following exercise training are consistent with previous reports and reinforce the positive effect of exercise training on these important risk factors for metabolic syndrome, Type 2 diabetes, and cardiovascular disease (16).
To conclude, we report the novel observation that the loss of abdominal VF alone through exercise correlates with improved glucose control in an elderly, obese subject group. The influence of the SCF depot can be ruled out in this regard. In this way, clarity can be drawn as to whether visceral and/or subcutaneous abdominal adiposity could influence cellular glucose uptake and insulin resistance. The improvement in physical fitness, glucose tolerance, and body composition reduces the risk of developing Type 2 diabetes in this at-risk population. Our association data suggest that the physiological mechanisms that cause age-related insulin resistance include a low-exercise capacity and accumulation of visceral adipose tissue. Fortunately, exercise successfully targets and reduces VF and enhances glucose tolerance, making it a highly effective treatment strategy for insulin resistance in the elderly, obese.
ACKNOWLEDGMENTS
We thank the nursing/dietary staff of the General Clinical Research Center at MetroHealth Medical Center for supporting the implementation of the study, and Ashley Nardi for performing the adiponectin assay. Thanks also to the YMCA of Greater Cleveland and the research participants for their cooperation and commitment.
GRANTS
This research was supported by National Institutes of Health Grants AG-12834 and RR-00080, and the Diabetes Association of Greater Cleveland (467-R-01).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
O’Leary, Valerie B., Christine M. Marchetti, Raj K. Krishnan, Bradley P. Stetzer, Frank Gonzalez, and John P. Kirwan. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat.
REFERENCES
- 1.Abrams HL and McNeil BJ. Medical implications of computed topography (“CAT scanning”). N Engl J Med 298: 255–261, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Boden G and Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32: 14–23, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Brochu M, Starling RD, Tchernof A, Matthews DE, Garcia-Rubi E, and Poehlman ET. Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab 85: 2378–2384, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Clore JN, Glickman PS, Helm ST, Nester JE, and Blackard WG. Evidence for dual control mechanism regulating hepatic glucose output in nondiabetic men. Diabetes 40: 1033–1040, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Coon PJ, Rogus EM, Drinkwater D, Muller DC, and Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin Endocrinol Metab 75: 1125–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA. Glucose intolerance and aging. Diabetes Care 4: 493–501, 1981. [DOI] [PubMed] [Google Scholar]
- 7.del Aguila LF, Krishnan RK, Ulbrecht JS, Farrell PA, Correll PH, Lang CH, Zierath JR, and Kirwan JP. Muscle damage impairs insulin stimulation of IRS-1, PI3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab 279: E206–E212, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Evans EM, Van Pelt RE, Binder EF, Williams DB, Ehhsani AA, and Kohrt WM. Effects of HRT and exercise training on insulin action, glucose tolerance, and body composition in older women. J Appl Physiol 90: 2033–2040, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Gan SK, Kriketos AD, Ellis BA, Thompson CH, and Kraegen EW. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 6: 1706–1713, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, and Kanaley JA. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism 54: 866–875, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16: 397–415, 1992. [PubMed] [Google Scholar]
- 12.Goodpaster BH, Kelley DE, Wing RR, Meier A, and Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48: 839–847, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Thaete FL, Simoneau JA, and Kelly DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46: 1579–1585, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, and Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor-α in frail elderly humans. FASEB J 15: 475–482, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13: 51–59, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Heath GW, Ehsani AA, Hagberg JM, Hinderliter JM, and Goldberg AP. Exercise training improves lipoprotein lipid profiles in patients with coronary artery disease. Am Heart J 105: 889–895, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, and Spiegelman BM. IRS-1 mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α and obesity-induced insulin resistance. Science 271: 665–668, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Hulver MW, Saleh O, MacDonald KG, Pories WJ, and Barakat HA. Ethnic differences in adiponectin levels. Metabolism 53: 1–3, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Fortier A, Hudson R, and Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 25: 431–438, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I and Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord 23: 1035–1046, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, and Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 288: E454–E461, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kern PA, Di Gregorio GB, Lu T, Rassouli N, and Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 52: 1779–1785, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, and Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year old men and women. J Gerontol 48: M84–M90, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Kirwan JP, Krishnan RK, Weaver JA, del Aguila LF, and Evans WJ. Human aging is associated with altered TNF-α production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab 281: E1137–E1143, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kohrt WM, Kirwan JP, King DS, Staten MA, and Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes 42: 273–281, 1993. [PubMed] [Google Scholar]
- 26.Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, and Mantzoros CS. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 11: 1048–1054, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Muoio DM, Dohm GL, Fiedorek FT Jr., Tapscott EB, and Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46: 1360–1363, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Nardi AE, Marchetti CM, Phillips SA, Ciaraldi TP, and Kirwan JP. Adiponectin multimer ratio is increased following exercise and diet treatment in impaired glucose tolerance (Abstract). Diabetes, Suppl 1: A267, 2005. [Google Scholar]
- 29.Nielsen S, Guo Z, Johnson CM, Hensrud DD, and Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiris AN, Mueller RA, Smith GA, Struve MF, and Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest 78: 1648–1657, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratley RE, Hagberg JM, Dengel DR, Rogus EM, Muller DC, and Goldberg AP. Aerobic exercise training-induced reductions in abdominal fat and glucose-stimulated insulin responses in middle-aged and older men. J Am Geriatr Soc 48: 1055–1061, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Rebuffe-Scrive M, Andersson B, Olbe L, and Bjorntorp P. Metabolism of adipose tissue in intra-abdominal depots of nonobese men and women. Metabolism 37: 453–458, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, and Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J 18: 737–739, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Aru J, Freeman J, Hudson R, and Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 282: E657–E663, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Ross R, Janssen I, Dawson J, Kungl A, Kuk JL, Wong SL, Nguyen-Duy T, Lee SJ, Kilpatrick K, and Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12: 789–798, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Rowe JW, Minaker KL, Pallotta JA, and Flier JS. Characterization of the insulin resistance of aging. J Clin Invest 71: 1581–1587, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan AS, Nicklas BJ, Berman DM, and Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord 27: 1066–1071, 2003. [DOI] [PubMed] [Google Scholar]
- 38.St.-Onge MP, Janssen I, and Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 27: 2222–2228, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Svedberg J, Bjorntorp P, Smith U, and Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes 39: 570–574, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Vague J La differenciation sexuelle, facteur determinant des formes de l’obesite. Presse Med 30: 339–340, 1947. [PubMed] [Google Scholar]
- 41.Villareal DT and Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 272: 2243–2248, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, and Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, and Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]